Abstract
Objective:
To explore how neonates with respiratory failure are selected for extracorporeal membrane oxygenation (ECMO) once severity of illness criteria are met, and to determine how conflicts between ECMO providers and parents over the initiation of ECMO are addressed.
Study Design:
A cross-sectional study was conducted using a data collection survey, which was sent to the directors of neonatal respiratory ECMO centers.
Result:
The lowest birth weight and gestational age at which respondents would consider placing a neonate on ECMO were frequently below recommended thresholds. There was wide variability in respondents' willingness to place neonates on ECMO in the presence of conditions such as intraventricular hemorrhage and hypoxic ischemic encephalopathy. The number of respondents who would never seek to override parental refusal of ECMO was equal to the number who would always do so.
Conclusion:
Significant variability exists in the selection criteria for neonatal ECMO and in how conflicts with parents over the provision of ECMO are resolved.
Keywords: ECMO, newborn, respiratory failure, ethics
Introduction
The first successful use of extracorporeal membrane oxygenation (ECMO) to treat a neonate with meconium aspiration syndrome was reported by Bartlett et al., in 1976.1 Since then ECMO has become an accepted treatment for neonates with respiratory failure due to a variety of causes that fail ‘conventional' management. More than 22 000 neonates with respiratory failure treated with ECMO have been reported to the Extracorporeal Life Support Organization (ELSO) International Registry as of January 2009.2
Determining which neonates are likely to benefit from ECMO and are appropriate candidates for ECMO is difficult. This is because of an expanding choice of non-ECMO therapies for respiratory failure in term neonates, significant advances in ECMO technology, and limited data regarding the relative risks and benefits of these therapies. Measures of the severity of illness such as the oxygenation index and the alveolar–arterial oxygen gradient are fairly well standardized as indicators for initiating ECMO after the failure of optimal non-ECMO support. Other criteria are also used in patient selection. These include, for example, birth weight and gestational age, markers of reversibility of the underlying disease process, indicators of the risk of bleeding while on ECMO and predictors of long-term neurodevelopmental impairment. The most recent guidelines for these other general criteria were published by ELSO in 20053 (Table 1).
Table 1. General neonatal ECMO criteria.
| Gestational age ⩾34 weeks or birth weight ⩾2000 g |
| No significant coagulopathy or uncontrolled bleeding |
| No major intracranial hemorrhage |
| Reversible lung disease with length of mechanical ventilation <10–14 days |
| No uncorrectable congenital heart disease |
| No lethal congenital anomalies |
| No evidence of irreversible brain damage |
Modified with permission from Van Meurs et al.3
These criteria are meant to serve only as a guide, and the specific selection criteria used by individual ECMO centers are unknown. We investigated how ECMO centers in the United States and abroad are determining patient eligibility for neonatal ECMO once severity of illness criteria have been met. We also investigated how disagreements between families and ECMO providers regarding the initiation of ECMO support are approached by providers.
Methods
An electronic survey instrument was designed to explore the patient selection process for ECMO to treat neonatal respiratory failure. The survey consisted of 14 questions (including three multipart questions) related to patient selection and nine demographic questions about the respondents and their ECMO centers.
The survey was sent to all active neonatal ECMO centers that were members of ELSO, which includes all international centers that have submitted data from any neonatal respiratory subjects over the past 5 years. Surveys were addressed to the medical director of the ECMO program, or to the assistant or associate director specifically responsible for the neonatal ECMO program. The survey was initially distributed through the ELSO Monthly Newsletter in February 2008, followed by two additional electronic invitations.4 Surveys were received through May 2008.
Data were imported into a separate database and analyzed using SPSS 15.0 (SPSS Inc., Chicago, IL, USA). Owing to the categorical nature of the variables collected in the survey, histograms and frequency distributions were used to describe each variable. Categorical variables with under-represented levels were collapsed such that some of the ordinal categorical variables became dichotomous. Study results were summarized with frequencies, percents, pie-charts and bar graphs. We conducted bivariate analyses for categorical variables using χ2 or Fisher's exact test. A two-sided α of 0.05 was used to establish the statistical significance.
The study protocol was submitted to the Yale University School of Medicine Human Investigation Committee and met the criteria for exemption from committee review.
Results
Description of the study sample
Responses were received from 81 of 124 (65%) active neonatal respiratory ECMO centers. Of the respondents, 53% identified themselves as neonatologists, 23% as pediatric intensive care physicians and 18% as surgeons. A total of 52% of respondents were male and 48% were female. A total of 20% of respondents were 40 years old or younger, 49% were 41 to 50 years old and 27% were 51 to 60 years old. The majority of respondents came from the North American (84%) and the European (12%) ECMO centers. The majority of respondents described their institution as an academic medical center (70%) or a hospital affiliated with an academic medical center (20%). Most ECMO centers (87%) had been in operation for >10 years. Regarding patient volume, 78% of respondents reported their center's current average yearly number of non-congenital diaphragmatic hernia (CDH) neonatal respiratory patients as ⩽10, and 43% as ⩽5. A total of 96% of respondents reported their center's current average yearly number of CDH patients as ⩽10, and 70% as ⩽5.
General method for making decisions regarding ECMO candidacy
When confronted with patients for whom ECMO candidacy is unclear, 87% of respondents usually or always rely on a consensus opinion of the ECMO team members to make their decisions. A total of 54% of respondents utilize phone consultation with other ECMO centers at least sometimes. Only one-quarter of respondents stated that determining ECMO candidacy in this setting is usually or always an individual physician's decision (Table 2).
Table 2. Approach to neonatal ECMO decision making.
| Response to survey question, ‘When confronted with patients for whom ECMO candidacy is unclear, how often have you used the following approaches in decision making?' | |||||
|---|---|---|---|---|---|
| Never | Rarely | Sometimes | Usually | Always | |
| Individual ECMO physician's decision | 18 | 33 | 24 | 16 | 9 |
| ECMO/Neonatology team group consensus | 0 | 1 | 12 | 58 | 30 |
| Ethics consultation | 42 | 36 | 21 | 1 | 0 |
| Phone consultation with other ECMO centers | 15 | 31 | 41 | 9 | 4 |
Values expressed as percentages.
ECMO candidacy and gestational age/birth weight
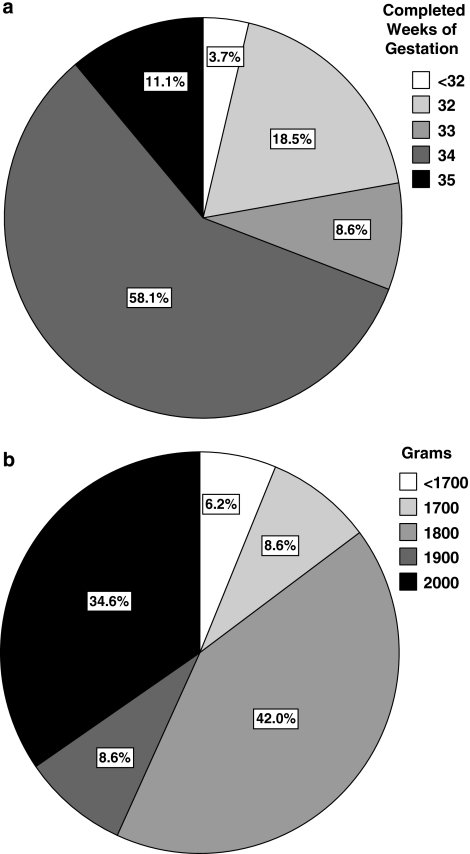
Respondents were asked the lowest gestational age and birth weight at which they would consider placing a neonate on ECMO. A total of 31% of respondents set 33 completed weeks of gestation or lower as their lowest gestational age (Figure 1a). A total of 23% of respondents reported that they had actually placed a neonate with a gestational age <34 weeks on ECMO and 5% of respondents reported having placed a neonate with a gestational age <32 weeks on ECMO. A total of 65% of respondents would consider neonates with birth weights <2000 g as potential ECMO candidates, and 15% would consider neonates with birth weights <1800 g as potential ECMO candidates (Figure 1b).
Figure 1.
(a) Responses to the survey question, ‘What is the lowest gestational age that you would consider placing a neonate on ECMO?' Answer choices are identified with associated shading. Percentages of responses are shown in the pie-chart. (b) Responses to the survey question, ‘What is the lowest birth weight, assuming that the patient meets your minimum gestational age requirements, that you would consider placing a neonate on ECMO?' Answer choices are identified with associated shading. Percentages of responses are shown in the pie-chart.
Respondents from centers with 15 or fewer non-CDH cases per year were more likely to set 33 completed weeks of gestation or lower as their lowest gestational age for ECMO candidacy than were respondents from centers with more than 15 non-CDH cases per year, although the difference was not statistically significant (34 vs 11%, P=0.26). Of the respondents from centers with more than 15 non-CDH cases per year, one identified 33 weeks, six identified 34 weeks and two identified 35 weeks gestation as the lowest gestational age for ECMO candidacy. There was no significant difference between the percentage of neonatologists and non-neonatologists who identified 33 weeks gestation or lower as the lowest gestational age for ECMO candidacy. Respondents were significantly more likely to pick a lowest birth weight below 2000 g than they were to pick a lowest gestational age below 34 weeks for ECMO candidacy (65 vs 31%, P<0.0001).
ECMO candidacy and specific co-morbidities
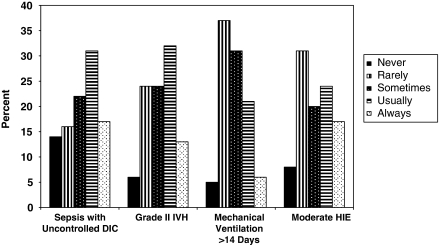
Respondents were asked how often neonates who met severity of illness criteria would be offered ECMO in the presence of specific co-morbidities, such as sepsis with uncontrolled disseminated intravascular coagulation, grade II intraventricular hemorrhage, mechanical ventilation for more than 14 days and moderate hypoxic ischemic encephalopathy. In the presence of any one of these conditions, at least one-quarter of respondents stated that they would never or rarely offer ECMO and at least one-quarter of respondents stated that they would always or usually offer ECMO (Figure 2). Other conditions were considered absolute contraindications to ECMO by a large percentage of respondents. For patients who otherwise meet ECMO criteria, 91% of respondents would never offer ECMO to a neonate with Trisomy 13, 90% would never offer ECMO to a neonate with Trisomy 18, 73% would never offer ECMO to a neonate with grade III or grade IV intraventricular hemorrhage and 48% would never offer ECMO to a neonate with severe hypoxic ischemic encephalopathy.
Figure 2.
Responses to the survey question, ‘Assuming a patient has failed conventional therapy, meets your criteria for ECMO, and has no other potential contraindications, how often would you offer ECMO if the following conditions were present?' Answer choices are ‘Never', ‘Rarely', ‘Sometimes', ‘Usually', or ‘Always'. Answer choices for each of four conditions are presented on the x axis, percentage of responses within each condition on the y axis.
Trisomy 21 was considered an absolute contraindication to ECMO by only 3% of respondents; 68% of respondents would usually or always offer ECMO to neonates with Trisomy 21 who otherwise meet ECMO criteria.
ECMO candidacy and conflicts in decision making between family members and healthcare providers
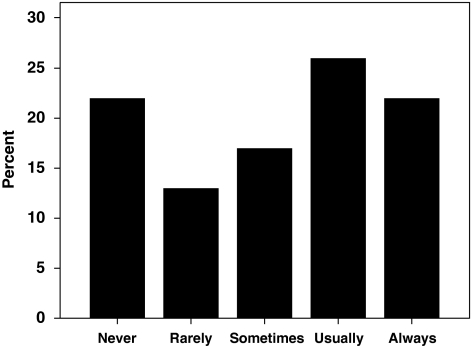
When presented with a scenario in which parents refused to give consent for ECMO for a neonate with respiratory failure whom the respondent felt was likely to survive bypass with a favorable neurodevelopmental outcome, 22% of respondents stated they would never seek to override parental refusal of ECMO, whereas an equal percentage said they would always do so (Figure 3). In fact, 15% of respondents reported that they had initiated ECMO at least once despite parental objection. Respondents were significantly more likely to override parental refusal of ECMO for meconium aspiration syndrome than they were for CDH (60 vs 16%, P<0.0001). Respondents who are neonatologists were significantly more likely to override parental refusal of ECMO for meconium aspiration syndrome than were other respondents (82 vs 41%, P=0.0003).
Figure 3.
Responses to the survey question, ‘How often would you seek to override parental refusal of ECMO for respiratory failure if you believe the neonate is likely to survive ECMO with a favorable neurodevelopmental outcome?' Answer choices are presented on the x axis, percentage of responses on the y axis.
On the other side of the potential conflict between physicians and parents regarding the use of ECMO, 57% of respondents reported that they had refused to provide ECMO to a child whose parents requested this therapy. A total of 46% of respondents reported that they had treated a child with ECMO whom they felt was not an appropriate candidate, because of strong parental request to do so.
Interpretation of the literature regarding the efficacy of ECMO
When asked why they use ECMO to treat non-CDH patients who fail conventional therapy, 54% of respondents stated that the literature has shown with a high degree of certainty, and 44% with a moderate degree of certainty that ECMO significantly improves the survival of these patients. When asked why they use ECMO to treat CDH patients who fail conventional therapy, 5% of respondents stated that the literature has shown with a high degree of certainty, and 34% with a moderate degree of certainty that ECMO significantly improves the survival of these patients. A total of 61% of respondents stated that the literature provides weak evidence that ECMO improves the survival of neonates with CDH, but use ECMO because they believe it improves survival or that it should be offered as a treatment of ‘last resort'.
Discussion
The selection of appropriate neonates with respiratory failure for ECMO support is based on the severity of illness and other criteria. Three small-randomized trials conducted in the United States5, 6, 7 and one larger trial conducted in the United Kingdom8 showed that ECMO, or a policy of referral for ECMO, improves survival of neonates with respiratory failure. Patients were enrolled in these studies if they were felt to have a high likelihood of mortality with continued non-ECMO management, and if they met other ECMO eligibility criteria. A high likelihood of mortality was usually based on measures of illness severity such as the ratio of arterial to alveolar partial pressure of oxygen, the alveolar–arterial oxygen gradient or the oxygenation index. The oxygenation index and other severity of illness criteria are limited in their ability to predict mortality,9 particularly as the non-ECMO management of neonates with respiratory failure has evolved. These severity of illness criteria for ECMO may need to be updated,10, 11, 12, 13 particularly for neonates with CDH,14, 15, 16 but at the moment are fairly well standardized.
Our survey addressed other criteria used to determine ECMO eligibility. We found that there is significant variation between ECMO centers, and significant deviation from the published ELSO guidelines, regarding patient selection on the basis of these criteria. This variability is not surprising and potentially has many explanations. Neonates with respiratory failure are a heterogeneous group of patients, and ECMO is provided by a variety of caregivers, in diverse settings, and at centers with varying ECMO experience. A total of 43% of the ECMO centers participating in our survey treat an average of five or fewer non-CDH cases per year. Finally, ECMO and its alternative therapies are continuously evolving, and there are limited and at times conflicting data regarding the relative efficacy, complications and long-term outcomes associated with these therapies.
The willingness of many survey respondents to offer ECMO to smaller and less-mature babies, for example, comes in the face of conflicting data regarding the added risk these babies face on ECMO,17, 18, 19, 20, 21, 22 and is fostered by improvements in ECMO technology that decrease the risk of bleeding and allow cannulation of smaller vessels. Different birth weight and gestational age thresholds between respondents may reflect differences in adoption of these technologies or different levels of comfort and experience. Note, however, that centers with higher ECMO case loads tended to maintain higher minimum gestational age thresholds.
The wide range in respondents' willingness to provide ECMO in the face of relative contraindications, such as coagulopathy, intraventricular hemorrhage, prolonged ventilation and hypoxic ischemic encephalopathy may similarly be due to different levels of comfort and experience between ECMO centers. Other potential factors are the difficulty and variability in defining several conditions (such as ‘irreversible' lung disease), the uncertain prognostic significance of other conditions (such as grade II intraventricular hemorrhage or moderate hypoxic ischemic encephalopathy) and the varying degrees of severity and potential reversibility of other conditions (such as sepsis and disseminated intravascular coagulation). However, even co-morbidities that are easily defined, convey a consistently poor prognosis, and are irreversible, such as Trisomy 18 and Trisomy 13, do not seem to be absolute contraindications to ECMO among respondents: ∼10% of respondents stated that they would offer ECMO in at least some circumstances for patients with these conditions.
Perhaps the most striking variability among respondents was in their willingness to initiate ECMO for neonates who meet ECMO criteria, are expected to survive ECMO with a favorable neurodevelopmental outcome, but whose parents refuse ECMO support. This variability may be because of differences in clinical experience, differences in personal values with regard to parental authority versus the newborn's right to treatment or other factors. The fact that neonatologists were more than twice as likely as non-neonatologists to state that they would override parental refusal of ECMO for neonates with meconium aspiration syndrome who were considered appropriate ECMO candidates may reflect some of these differences.
The willingness of respondents to initiate ECMO over parental objection was also shown to depend on the cause of the respiratory failure. Koogler and Lantos23 have noted that in the past physicians' reticence to override parental refusal of ECMO may have been appropriate because of uncertain ECMO survival rates, the perceived burdens of ECMO, and the uncertain long-term sequelae of ECMO, but that the 94% survival rate for babies in the ELSO Registry with meconium aspiration who received ECMO may ‘…create a moral obligation for doctors to seek court approval to override parental refusals.' A total of 60% of respondents stated that they would override parental refusal of ECMO for meconium aspiration syndrome; respondents' willingness to override parental refusal may be mitigated by their interpretation of the literature regarding ECMO's efficacy compared with ‘conventional' therapy. The clinical trials of neonatal ECMO conducted in the United States randomized a total of 59 patients, most of who were treated before 1990, and two of the studies used non-conventional randomization strategies. The United Kingdom ECMO trial enrolled 185 patients using a conventional randomization strategy, and included follow-up to age of 7 years.24 However, relatively few patients receiving conventional management in that study were treated with inhaled nitric oxide, high-frequency ventilation or surfactant (12, 15 and 40%, respectively), and it is not clear whether the study's results would be replicated in the setting of current non-ECMO care. Only 54% of respondents to our survey felt that the literature has shown with a high degree of certainty that ECMO significantly improves survival in non-CDH patients.
There have been no randomized trials showing that ECMO improves survival of neonates with CDH, and only 5% of respondents felt that the literature has shown with a high degree of certainty that ECMO significantly improves survival of these patients. This may partly explain why only 16% of respondents would initiate ECMO over parental refusal for neonates with CDH. Not surprisingly, respondents who felt that the literature has shown with a high or moderate degree of certainty that ECMO significantly improves survival of patients with CDH were more likely to initiate ECMO over parental refusal than were respondents who felt the literature provided weak evidence of this benefit (27 vs 9%, P=0.055). Differences in the perceived amount of suffering associated with treatment, and in short- and long-term morbidities, may also explain the difference in approach to parental refusal of ECMO support for neonates with meconium aspiration versus those with CDH.
The results of this survey provide insight into the practices of two-thirds of the ECMO centers that are members of ELSO regarding the selection of neonates for ECMO once it is determined that severity of illness criteria have been met. The difficulty in determining ECMO eligibility is likely reflected in the small percentage of respondents who stated that difficult decisions regarding ECMO candidacy are usually or always an individual ECMO physician's decision. Most often these decisions are made by a consensus of ECMO team members, and not infrequently after telephone consultation with other ECMO centers.
The results of this survey allow ECMO centers to determine how their criteria to determine neonatal ECMO eligibility compare with those of other centers. The results may also help physicians at institutions that do not provide ECMO decide which patients should be referred to an ECMO center. The results of this survey provide a ‘snapshot' of respondents' views regarding the ECMO eligibility in 2008, but do not provide insight into how these perceptions may have changed over time. For example, the current willingness of respondents to consider patients with Trisomy 21 eligible for ECMO probably developed over time as attitudes regarding the care of patients with Trisomy 21 evolved and experience placing these patients on ECMO was gained.25
This survey is limited in that respondents had to answer questions about clinical scenarios without being provided additional information that may have modified their responses. There may have been differences in how respondents interpreted some questions. In addition, each respondent was asked to answer for him or herself, and responses may not reflect the opinions or practice of other members of the ECMO center. Finally, one-third of ECMO centers contacted did not participate in the survey, and thus their practices are not reflected in these data. Although the geographic distribution of respondents to our survey was very similar to the distribution of ELSO member centers, we cannot be sure how representative our sample was of the entire ELSO population regarding other parameters. Nonetheless, our survey reflects the opinions of over 80 ECMO clinicians practicing in the United States and abroad in a variety of clinical settings.
In conclusion, the results of this survey show that there is significant variability between respondents in how neonates are selected for ECMO once severity of illness criteria have been met. There is also variability in how respondents address conflicts between parents and the ECMO team regarding the initiation of ECMO. It is hoped that these findings will be helpful to ECMO and non-ECMO clinicians, and stimulate further discussion and continued investigations regarding patient selection for neonatal ECMO. Patient eligibility criteria for ECMO can be expected to evolve as advances are made in ECMO technology and in non-ECMO care, as additional information regarding patient outcomes becomes available, and as ethical issues relevant to patient selection are addressed.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
We thank Veronika Northrup, MPH, for assistance with the statistical analysis and for review of the paper.
References
- Bartlett RH, Gazzaniga AB, Jeffries MR, Huxtable RF, Haiduc NJ, Fong SW. Extracorporeal membrane oxygenation (ECMO) cardiopulmonary support in infancy. Trans Am Soc Artif Intern Organs. 1976;22:80–93. [PubMed] [Google Scholar]
- ECMO Registry of the Extracorporeal Life Support Organization (ELSO) Ann Arbor, MI; January2009 [Google Scholar]
- Van Meurs K, Lally KP, Peek G, Zwischenberger JB.ECMO Extracorporeal Cardiopulmonary Support in Critical Care3rd edn. Extracorporeal Life Support Organization: Ann Arbor, MI; 2005 [Google Scholar]
- Survey Monkey . www.surveymonkey.com ; accessed 1/9/08.
- Bartlett RH, Roloff DW, Cornell RG, Andrews AF, Dillon PW, Zwischenberger JB. Extracorporeal circulation in neonatal respiratory failure: a prospective randomized study. Pediatrics. 1985;76:479–487. [PubMed] [Google Scholar]
- O'Rourke PP, Crone RK, Vacanti JP, Ware JH, Lillehei CW, Parad RB, et al. Extracorporeal membrane oxygenation and conventional medical therapy in neonates with persistent pulmonary hypertension of the newborn: a prospective randomized study. Pediatrics. 1989;84:957–963. [PubMed] [Google Scholar]
- Bifano EM, Hakanson DO, Hingre RV, Gross SJ.Prospective randomized controlled trial of conventional treatment or transport for ECMO in infants with persistent pulmonary hypertension (PPHN) [abstract] Pediatr Res 199231196A1542552 [Google Scholar]
- UK Collaborative ECMO Trial Group UK collaborative randomised trial of neonatal extracorporeal membrane oxygenation. Lancet. 1996;348:75–82. [PubMed] [Google Scholar]
- Dworetz AR, Moya FR, Sabo B, Gladstone I, Gross I. Survival of infants with persistent pulmonary hypertension without extracorporeal membrane oxygenation. Pediatrics. 1989;84:1–6. [PubMed] [Google Scholar]
- Kössel H, Bauer K, Kewitz G, Karaca S, Versmold H. Do we need new indications for ECMO in neonates pretreated with high-frequency ventilation and/or inhaled nitric oxide. Intensive Care Med. 2000;26:1489–1495. doi: 10.1007/s001340000603. [DOI] [PubMed] [Google Scholar]
- Radhakrishnan RS, Lally PA, Lally KP, Cox CS., Jr ECMO for meconium aspiration syndrome: support for relaxed entry criteria. ASAIO J. 2007;53 4:489–491. doi: 10.1097/MAT.0b013e318063c602. [DOI] [PubMed] [Google Scholar]
- Karimova A, Brown K, Ridout D, Beierlein W, Cassidy J, Smith J, et al. Neonatal extracorporeal membrane oxygenation: practice patterns and predictors of outcome in the UK Arch Dis Child Fetal Neonatal Ed 2008. e-pub ahead of print 1 October 2008, doi:10.1136/adc.2008.141051. [DOI] [PubMed]
- Mugford M, Elbourne D, Field D.Extracorporeal membrane oxygenation for severe respiratory failure in newborn infants Cochrane Database Syst Rev 2008. Issue 3, Art. No.: CD001340. DOI:10.1002/14651858.CD001340.pub2. [DOI] [PubMed]
- Staak FHJ, Thiesbrummel A, de Haan AFJ, Oeseburg B, Geven WB, Festen C. Do we use the right entry criteria for extracorporeal membrane oxygenation in congenital diaphragmatic hernia. J Pediatr Surg. 1993;28:1003–1005. doi: 10.1016/0022-3468(93)90502-c. [DOI] [PubMed] [Google Scholar]
- Boloker J, Bateman DA, Wung J-T, Stolar CJH. Congenital diaphragmatic hernia in 120 infants treated consecutively with permissive hypercapnea/spontaneous respiration/elective repair. J Pediatr Surg. 2002;37:357–366. doi: 10.1053/jpsu.2002.30834. [DOI] [PubMed] [Google Scholar]
- Harrington KP, Goldman AP. The role of extracorporeal membrane oxygenation in congenital diaphragmatic hernia. Semin Pediatr Surg. 2005;14:72–76. doi: 10.1053/j.sempedsurg.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Revenis ME, Glass P, Short BL. Mortality and morbidity rates among lower birth weight infants (2000–2500 grams) treated with extracorporeal membrane oxygenation. J Pediatr. 1992;121:452–458. doi: 10.1016/s0022-3476(05)81804-9. [DOI] [PubMed] [Google Scholar]
- Hirschl RB, Schumacher RE, Snedecor SN, Bui KC, Bartlett RH.The efficacy of extracorporeal life support in premature and low birth weight newborns J Pediatr Surg 1993281336–1340.discussion 1341. [DOI] [PubMed] [Google Scholar]
- De Sanctis JT, Bramson RT, Blickman JG. Can clinical parameters help reliably predict the onset of acute intracranial hemorrhage in infants receiving extracorporeal membrane oxygenation. Radiology. 1996;199:429–432. doi: 10.1148/radiology.199.2.8668789. [DOI] [PubMed] [Google Scholar]
- Hardart GE, Fackler JC. Predictors of intracranial hemorrhage during neonatal extracorporeal membrane oxygenation. J Pediatr. 1999;134:156–159. doi: 10.1016/s0022-3476(99)70408-7. [DOI] [PubMed] [Google Scholar]
- Hardart GE, Hardart KM, Arnold JH. Intracranial hemorrhage in premature neonates treated with extracorporeal membrane oxygenation correlates with conceptional age. J Pediatr. 2004;145:184–189. doi: 10.1016/j.jpeds.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Rozmiarek AJ, Qureshi FG, Cassidy L, Ford HR, Gaines BA, Rycus P, et al. How low can you go? Effectiveness and safety of extracorporeal membrane oxygenation in low-birth-weight neonates. J Pediatr Surg. 2004;39:845–847. doi: 10.1016/j.jpedsurg.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Koogler TK, Lantos J.ECMO Ethics in the Twenty-first CenturyIn: Van Meurs K, Lally KP, Peek G, Zwischenberger JB (eds)ECMO Extracorporeal Cardiopulmonary Support in Critical Care3rd edn. Extracorporeal Life Support Organization: Ann Arbor, MI; 2005197 [Google Scholar]
- McNally H, Bennett CC, Elbourne D, Field DJ. United Kingdom collaborative randomized trial of neonatal extracorporeal membrane oxygenation: follow-up to age 7 years. Pediatrics. 2006;117:e845–e854. doi: 10.1542/peds.2005-1167. [DOI] [PubMed] [Google Scholar]
- Southgate WM, Annibale DJ, Husley TC, Purohit DM. International experience with trisomy 21 infants placed on extracorporeal membrane oxygenation. Pediatrics. 2001;107:549–552. doi: 10.1542/peds.107.3.549. [DOI] [PubMed] [Google Scholar]