Abstract
The structure of the oxygenated heme group of oxyhemoglobin may be formulated as [Hb(Heme d1/25)·OO-]. The heme iron atom is formally ferric, and the ligand is bound superoxide anion. When deoxyhemoglobin combines reversibly with oxygen a partial transfer of an electron occurs from the ferrous iron atom to the oxygen molecule. By surrendering its electron the iron atom has become ferric; in accepting an electron the ligated oxygen molecule has become a new species, the bound superoxide anion (·OO-).
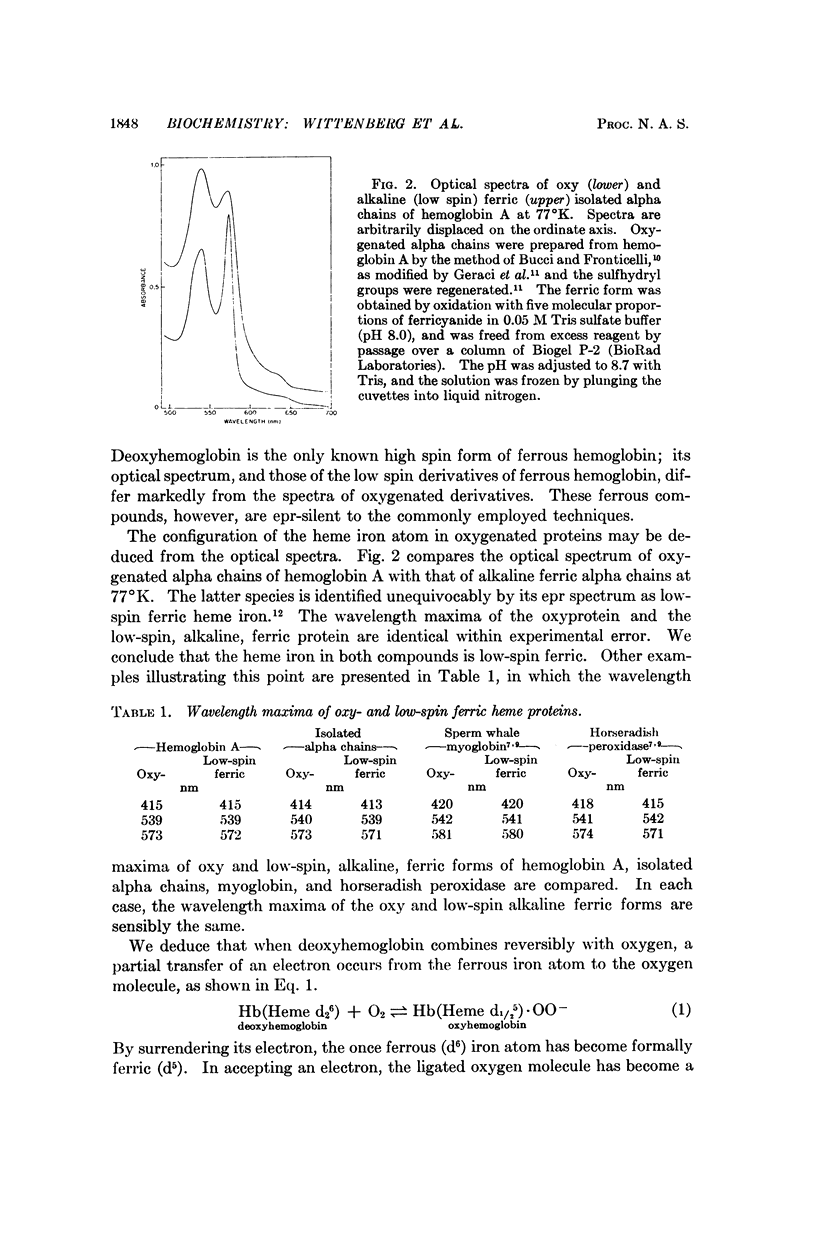
The configuration of the heme iron atom is deduced from comparison of the optical spectrum in the visible region of oxyhemoglobin with that of alkaline ferric hemoglobin whose configuration is established by electron paramagnetic resonance spectroscopy. The configuration of both species is low spin ferric heme iron (Heme d1/25). The configuration of the ligated oxygen molecule of oxyhemoglobin is not accessible to study by magnetic or optical probes. However it may be known by analogy with the configuration of the ligated oxygen molecule of reversibly oxygenated cobalt complexes whose structure has been proved by both electron paramagnetic resonance and x-ray diffraction analysis. It is bound superoxide anion (·OO-). Other physical studies bearing on the structure of the oxygenated heme group are discussed. Reasons are given for believing that the proposed formulation of the oxyhemoglobin structure is consistent with the known stability of oxyhemoglobin.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUCCI E., FRONTICELLI C. A NEW METHOD FOR THE PREPARATION OF ALPHA AND BETA SUBUNITS OF HUMAN HEMOGLOBIN. J Biol Chem. 1965 Jan;240:PC551–PC552. [PubMed] [Google Scholar]
- Bayston J. H., King N. K., Looney F. D., Winfield M. E. Superoxocobalamin, the first intermediate in the autoxidation of vitamin B12r. J Am Chem Soc. 1969 May 7;91(10):2775–2779. doi: 10.1021/ja01038a060. [DOI] [PubMed] [Google Scholar]
- Blumberg W. E., Peisach J., Wittenberg B. A., Wittenberg J. B. The electronic structure of protoheme proteins. I. An electron paramagnetic resonance and optical study of horseradish peroxidase and its derivatives. J Biol Chem. 1968 Apr 25;243(8):1854–1862. [PubMed] [Google Scholar]
- Crumbliss A. L., Basolo F. Monomeric cobalt-oxygen complexes. Science. 1969 Jun 6;164(3884):1168–1170. doi: 10.1126/science.164.3884.1168. [DOI] [PubMed] [Google Scholar]
- Geraci G., Parkhurst L. J., Gibson Q. H. Preparation and properties of alpha- and beta-chains from human hemoglobin. J Biol Chem. 1969 Sep 10;244(17):4664–4667. [PubMed] [Google Scholar]
- Hoffman B. M., Petering D. H. Coboglobins: oxygen-carrying cobalt-reconstituted hemoglobin and myoglobin. Proc Natl Acad Sci U S A. 1970 Oct;67(2):637–643. doi: 10.1073/pnas.67.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang G., Marshall W. Mössbauer effect in some haemoglobin compounds. J Mol Biol. 1966 Jul;18(3):385–404. doi: 10.1016/s0022-2836(66)80032-3. [DOI] [PubMed] [Google Scholar]
- Pauling L., Coryell C. D. The Magnetic Properties and Structure of Hemoglobin, Oxyhemoglobin and Carbonmonoxyhemoglobin. Proc Natl Acad Sci U S A. 1936 Apr;22(4):210–216. doi: 10.1073/pnas.22.4.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peisach J., Blumberg W. E., Wittenberg B. A., Wittenberg J. B., Kampa L. Hemoglobin A: an electron paramagnetic resonance study of the effects of interchain contacts on the heme symmetry of high-spin and low-spin derivatives of ferric alpha chains. Proc Natl Acad Sci U S A. 1969 Jul;63(3):934–939. doi: 10.1073/pnas.63.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peisach J., Blumberg W. E., Wittenberg B. A., Wittenberg J. B. The electronic structure of protoheme proteins. 3. Configuration of the heme and its ligands. J Biol Chem. 1968 Apr 25;243(8):1871–1880. [PubMed] [Google Scholar]
- Perutz M. F., Muirhead H., Cox J. M., Goaman L. C. Three-dimensional Fourier synthesis of horse oxyhaemoglobin at 2.8 A resolution: the atomic model. Nature. 1968 Jul 13;219(5150):131–139. doi: 10.1038/219131a0. [DOI] [PubMed] [Google Scholar]
- Simplicio J., Wilkins R. G. The kinetics of the rapid interaction of bis(histidinato)cobalt (II) with oxygen. J Am Chem Soc. 1967 Nov 22;89(24):6092–6095. doi: 10.1021/ja01000a017. [DOI] [PubMed] [Google Scholar]
- VIALE R. O., MAGGIORA G. M., INGRAHAM L. L. MOLECULAR ORBITAL EVIDENCE FOR WEISS'S OXYHAEMOGLOBIN STRUCTURE. Nature. 1964 Jul 11;203:183–184. doi: 10.1038/203183b0. [DOI] [PubMed] [Google Scholar]
- WEISS J. J. NATURE OF THE IRON-OXYGEN BOND IN OXYHAEMOGLOBIN. Nature. 1964 Apr 4;202:83–84. doi: 10.1038/202083b0. [DOI] [PubMed] [Google Scholar]
- WEISS J. J. NATURE OF THE IRON-OXYGEN BOND IN OXYHAEMOGLOBIN. Nature. 1964 Jul 11;203:182–183. [PubMed] [Google Scholar]
- WEISS J. J. NATURE OF THE IRON-OXYGEN BOND IN OXYHAEMOGLOBIN. Nature. 1964 Jul 11;203:182–183. [PubMed] [Google Scholar]
- Wang B. C., Schaefer W. P. Structure of an oxygen-carrying cobalt complex. Science. 1969 Dec 12;166(3911):1404–1406. doi: 10.1126/science.166.3911.1404. [DOI] [PubMed] [Google Scholar]
- Wittenberg B. A., Kampa L., Wittenberg J. B., Blumberg W. E., Peisach J. The electronic structure of protoheme proteins. II. An electron paramagnetic resonance and optical study of cytochrome c peroxidase and its derivatives. J Biol Chem. 1968 Apr 25;243(8):1863–1870. [PubMed] [Google Scholar]



