Abstract
Purpose
IBTR! version 1.0 is a web-based tool that uses literature-derived relative risk ratios for seven clinicopathologic variables to predict ipsilateral breast tumor recurrence (IBTR) after breast-conserving therapy (BCT). Preliminary testing demonstrated over-estimation in high-risk subgroups. This study uses two independent population-based datasets to create and validate a modified nomogram, IBTR! version 2.0.
Methods
Cox regression modeling was performed on 7,811 patients treated with BCT at the British Columbia Cancer Agency (median follow-up, 9.4 years). Population-based hazard ratios were generated for the seven variables in the original nomogram. A modified nomogram was then tested against 664 patients from Massachusetts General Hospital (median follow-up, 9.3 years). The mean predicted and observed 10-year estimates were compared for the entire cohort and for four groups predefined by nomogram-predicted risks: group 1: less than 3%; group 2: 3% to 5%; group 3: 5% to 10%; and group 4: more than 10%.
Results
IBTR! version 2.0 predicted an overall 10-year IBTR estimate of 4.0% (95% CI, 3.8 to 4.2), while the observed estimate was 2.8% (95% CI, 1.6 to 4.7; P = .10). The predicted and observed IBTR estimates were: group 1 (n = 283): 2.2% versus 1.3%, P = .40; group 2 (n = 237): 3.8% versus 3.5%, P = .80; group 3 (n = 111): 6.7% versus 3.2%, P = .05; and group 4 (n = 33): 12.5% versus 8.7%, P = .50.
Conclusion
IBTR! version 2.0 is accurate in the majority of patients with a low to moderate risk of in-breast recurrence. The nomogram still overestimates risk in a minority of patients with higher risk features. Validation in a larger prospective data set is warranted.
INTRODUCTION
Breast-conserving therapy (BCT), with breast-conserving surgery (BCS) and adjuvant breast radiation therapy (RT), is standard local management for the majority of patients with early-stage breast cancer. Multiple randomized trials have shown that adjuvant RT significantly decreases the risk of local recurrence,1–7 but the absolute risk reduction from RT varies depending on individual patient, tumor, and treatment characteristics. A web-based predictive nomogram, IBTR!, was developed to estimate individualized risk of ipsilateral breast tumor recurrence (IBTR) after BCT.8
The methods of creating IBTR! version 1.0 have been previously described.8 At the time of initial model development, the investigators at Tufts University did not have access to population-based data on which to build the model. It was therefore developed based on analyses of the published randomized trials of BCS with and without RT and institutional studies that evaluated prognostic factors that were not well assessed in the randomized trials. The IBTR! model contained seven prognostic factors: age, tumor size, tumor grade, margin status, lymphovascular invasion, use of chemotherapy, and use of hormone therapy. In IBTR! version 1.0, literature-derived relative risk ratios for each of the seven variables were used to generate a 10-year IBTR predicted estimate for individual subjects, both with and without RT after partial mastectomy. Subsequent to the version 1.0 development, collaborative research with the Massachusetts General Hospital (MGH) and the British Columbia Cancer Agency (BCCA) was undertaken to validate the nomogram.
In the MGH preliminary validation testing, the nomogram was tested among 1,138 patients treated with BCT (median follow-up, 7 years). IBTR! version 1.0 performed well in the overall data set (predicted recurrence rate 4.3% v observed 3.4%) and in favorable risk patients. There was a trend toward overestimation of in-breast recurrence for intermediate-risk patients. Performance in the highest risk group of patients (predicted risk > 20%) was difficult to assess because of small patient numbers.9
In the BCCA preliminary validation testing, conducted separately from the MGH analysis, IBTR! version 1.0 was evaluated against a randomly selected subset of 2,071 women treated with BCT at the BCCA with at least 10 years of follow-up. The entire BCCA data set was not used during this early phase to permit the possibility of population-based model refinement and retesting. In the preliminary analysis, the difference between 10-year predicted (10.5%; SE, 0.3) and observed (6.2%; SE, 0.5) was significant (P < .001). IBTR! version 1.0 performed well in patients with favorable risk factors but the model overestimated risk in patients with higher risk characteristics.10
The demonstration of overestimation in high-risk subgroups during preliminary testing9,10 led to this study designed to modify and retest the model, capitalizing on the availability of two independent datasets to develop IBTR! version 2.0.
METHODS
Construction of IBTR! Version 2.0 Using BCCA Data
The BCCA Breast Cancer Outcomes Unit database was used to identify 7,811 women diagnosed between 1989 and 1999 with newly diagnosed invasive breast cancer, pT1-3, all N stages, M0, treated with BCS, and adjuvant RT with either 50 Gy in 25 fractions or 42.5 Gy in 16 fractions with or without a boost.11 Cox regression modeling,12–14 adjusting for the effects of demographic, pathologic, and treatment factors, was performed on these 7,811 subjects to generate population-based hazard ratios (HR) and 95% CIs for each of the seven variables in the IBTR! nomogram. These revised HRs formed the basis for a modified IBTR! version 2.0.
Validation Testing of IBTR! Version 2.0 Using MGH Data
The MGH cohort used for model validation was comprised of 664 patients with nonmetastatic invasive breast cancer diagnosed between 1990 and 1999, all treated with BCS and adjuvant RT. This time interval was selected to allow sufficient follow-up time to observe 10-year events. All patients received RT to the whole breast, median dose 50 Gy in 25 fractions with a boost when indicated. Patients treated with BCS but without RT were excluded from both datasets.
Follow-up data regarding disease status was obtained from the most updated information available in the hospital electronic record or radiation oncology chart.
The end point used in this analysis was IBTR as the first event, defined in both data sets as any first recurrence involving the ipsilateral breast without simultaneous regional or distant recurrence occurring within 4 months after the IBTR diagnosis. This definition was adopted to ensure consistency between the two data sets and with published National Surgical Adjuvant Breast Projects (NSABP) and Eastern Cooperative Oncology Group (ECOG) studies.15,16
The validation testing methodology in this study was guided by the published experience of British Columbia investigators validating Adjuvant! Online, a web-based risk prediction model to estimate survival outcomes with and without adjuvant systemic therapy,17 and consultative team discussions with the biostatistician coinvestigators at MGH (A.N.) and BCCA (M.L.). Anonymized patient data from MGH were entered by investigators blinded to patient outcomes into the modified IBTR! version 2.0 to generate 10-year predicted IBTR estimates for each patient. Mean IBTR!-predicted and MGH observed IBTR estimates were compared using t-tests for the entire cohort and for four groups predefined by nomogram-predicted risks: group 1: less than 3%; group 2: 3% to 5%; group 3: 5% to 10%; and group 4: higher than 10%. These groups represented clinically meaningful risk cohorts. Competing events were treated as censored observations in the analysis of both BCCA and MGH data. The discriminatory ability of the model to distinguish low-risk from high-risk groups was evaluated using Harrell's concordance statistics.18,19 The c index, or Harrell's concordance index, was derived from the Wilcoxon-Mann-Whitney two-sample rank test.19
Statistical analysis was performed using SPSS software (version 17.0; SPSS Inc, Chicago, IL) and R 2.8.1 I(http://www.r-project.org/) with Hmisc and Design packages. All statistical tests were two-tailed with significance established at P < .05.
This study was approved by the research ethics boards of the University of British Columbia and MGH.
RESULTS
The clinical characteristics of the BCCA cohort (n = 7,811) used in Cox modeling to modify the IBTR! nomogram and the MGH cohort (n = 664) used for validation testing are summarized in Table 1. The median age in both groups was 58 years. The median follow-up time was 9.4 years in the BCCA cohort and 9.3 years in the MGH cohort.
Table 1.
Clinical Characteristics of the BCCA Cohort Used in Cox Regression Modeling to Create the Modified IBTR! Version 2.0 Nomogram and the MGH Cohort Used for Validation Testing
| Characteristic | BCCA |
MGH |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| No. of patients | 7811 | 664 | ||
| Median follow-up time, years | 9.4 | 9.3 | ||
| Range | 0.1-17 | 0.3-17.2 | ||
| Median age, years | 58 | 58 | ||
| Range | 17-88 | 28-89 | ||
| ≤ 40 | 670 | 8.6 | 50 | 7.5 |
| 41-45 | 804 | 10.3 | 44 | 6.6 |
| 46-50 | 1,050 | 13.4 | 96 | 14.5 |
| 51-55 | 985 | 12.6 | 94 | 14.2 |
| 56-60 | 919 | 11.8 | 90 | 13.6 |
| 61-65 | 933 | 11.9 | 65 | 9.8 |
| 66-70 | 1,012 | 13.0 | 94 | 14.2 |
| > 70 | 1,438 | 18.4 | 131 | 19.7 |
| Margin | ||||
| Positive | 788 | 10.1 | 52 | 7.8 |
| Close (< 2 mm) | 15 | 0.2 | 71 | 10.7 |
| Negative (> 2 mm) | 6,730 | 86.2 | 541 | 81.5 |
| Unknown | 278 | 3.6 | 0 | 0 |
| Lymphovascular invasion | ||||
| Present | 2,025 | 25.9 | 118 | 17.8 |
| Absent | 5,516 | 70.6 | 546 | 82.2 |
| Unknown | 270 | 3.5 | 0 | 0 |
| Tumor size, cm | ||||
| ≤ 1 | 2,096 | 25.9 | 263 | 39.6 |
| 1.1-2 | 3,675 | 47.0 | 280 | 42.1 |
| > 2 | 2,040 | 26.1 | 121 | 18.2 |
| Grade | ||||
| I | 1,359 | 17.4 | 152 | 22.9 |
| II | 3,593 | 46.0 | 331 | 49.8 |
| III | 2,544 | 32.6 | 181 | 27.3 |
| Unknown | 315 | 4.0 | 0 | 0 |
| Hormone therapy | ||||
| Yes | 3,080 | 39.4 | 404 | 60.8 |
| No | 4,731 | 60.6 | 260 | 39.2 |
| Chemotherapy | ||||
| Yes | 2,275 | 29.1 | 219 | 33.0 |
| No | 5,536 | 70.9 | 445 | 67.0 |
Abbreviations: BCCA, British Columbia Cancer Agency; MGH, Massachusetts General Hospital.
In the BCCA data set, the overall rate of IBTR was 348 (4.5%) of 7,811. The KM IBTR estimate at 10 years was 4.9% (95% CI 4.3% to 5.4%). The BCCA-derived Cox regression hazard ratios and associated 95% CIs for each of the seven variables in the modified IBTR! nomogram are summarized in Table 2.
Table 2.
BCCA Population-Derived Cox Regression Hazard Ratios for the Revised IBTR! Version 2.0 Nomogram
| Characteristic | Hazard Ratio | 95% CIs |
|---|---|---|
| Age, years | ||
| ≤ 40 | 2.03 | 1.36 to 3.01 |
| 41-45 | 1.47 | 0.99 to 2.18 |
| 46-50 | 0.86 | 0.56 to 1.31 |
| 51-55 | 1.00 | |
| 56-60 | 0.71 | 0.45 to 1.13 |
| 61-65 | 0.74 | 0.47 to 1.15 |
| 66-70 | 0.74 | 0.47 to 1.14 |
| > 70 | 0.53 | 0.33 to 0.83 |
| LVI | ||
| Positive | 1.12 | 0.86 to 1.46 |
| Negative | 1.00 | |
| Unknown | 0.92 | 0.47 to 1.77 |
| Margin, mm | ||
| Positive | 2.19 | 1.65 to 2.91 |
| Close (< 2 mm) | 0.97 | 0.13 to 6.94 |
| Negative (> 2 mm) | 1.00 | |
| Unknown | 1.83 | 1.19 to 2.81 |
| Hormone therapy | ||
| Yes | 0.73 | 0.56 to 0.95 |
| No | 1.00 | |
| Chemotherapy | ||
| Yes | 0.79 | 0.59 to 1.07 |
| No | 1.00 | |
| Tumor grade | ||
| I | 0.70 | 0.48 to 1.01 |
| II | 1.00 | |
| III | 1.55 | 1.22 to 1.97 |
| Unknown | 1.20 | 0.69 to 2.08 |
| Tumor size, cm | ||
| ≤1 | 1.40 | 1.08 to 1.82 |
| 1.1-2 | 1.00 | |
| > 2 | 1.07 | 0.81 to 1.41 |
Abbreviations: BCCA, British Columbia Cancer Agency; IBTR, ipsilateral breast tumor recurrence; LVI, lymphovascular invasion.
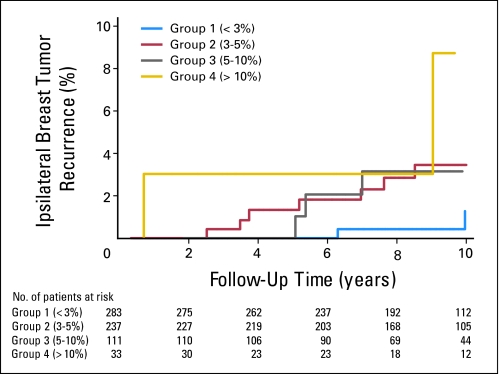
Overall, there were only 22 cases of IBTR as a first event in the MGH validation data set. IBTR! version 2.0 predicted an overall 10-year IBTR estimate of 4.0% (95% CI, 3.8 to 4.2), while the observed estimate was 2.8% (95% CI, 1.6 to 4.7; P = .10). Figure 1 depicts Kaplan-Meier IBTR in the four risk groups defined by predicted estimates. Within each group, the predicted and observed IBTR estimates were: group 1 (n = 283): 2.2% versus 1.3%, P = .40; group 2 (n = 237): 3.8% versus 3.5%, P = .80; group 3 (n = 111): 6.7% versus 3.2%, P = .05; and group 4 (n = 33): 12.5% versus 8.7%, P = .50 (Table 3).
Fig 1.
Kaplan-Meier observed in-breast tumor recurrence in four risk groups based on predicted estimates less than 3%, 3% to 5%, 5% to 10%, and higher than 10%.
Table 3.
Ten-Year Comparisons of Predicted and Observed IBTR Estimates
| Group | No. of Patients | No. of IBTR Events | Predicted 10-Year IBTR! Version 2.0 Estimates (SE) |
95% CI Predicted | Observed 10-Year IBTR Estimates (SE) |
95% CI Observed | P (t test) | ||
|---|---|---|---|---|---|---|---|---|---|
| % | SE | % | SE | ||||||
| 1 IBTR < 3% | 283 | 2 | 2.2 | 0.03 | 2.1 to 2.2 | 1.3 | 1.0 | 0.3 to 5.5 | .4 |
| 2 IBTR 3%-5% | 237 | 11 | 3.8 | 0.04 | 3.7 to 3.8 | 3.5 | 1.3 | 1.7 to 7.2 | .8 |
| 3 IBTR 5%-10% | 111 | 6 | 6.7 | 0.1 | 6.5 to 7.0 | 3.2 | 1.8 | 1.0 to 9.5 | .05 |
| 4 IBTR > 10% | 33 | 3 | 12.5 | 0.5 | 11.4 to 13.6 | 8.7 | 6.2 | 2.1 to 32.5 | .5 |
| Overall | 664 | 22 | 4.0 | 1.0 | 3.8 to 4.2 | 2.8 | 0.8 | 1.6 to 4.7 | .1 |
Abbreviations: IBTR, ipsilateral breast tumor recurrence; SE, standard error.
In the calculation of the Harrell's concordance statistics, a Cox model was fitted with nomogram-calculated probabilities as a sole predictor, yielding a P value of .008 and a Harrell's concordance of 0.66.
DISCUSSION
Many studies have examined independent risk factors for IBTR, but due to the complex interactions of different prognostic factors, individualized estimates of recurrence risk and absolute benefits with RT use can be complex and difficult to predict. As local recurrence is associated with an increased risk of distant metastasis and mortality,1,20 accurate prediction of IBTR risks based on patient-specific risk factors and accurate estimates of the absolute benefit of RT are of clinical value to assist patients and their care providers in making informed decisions regarding adjuvant local therapy.
Distinct from Adjuvant!Online, which was based on Surveillance Epidemiology and End Results data that lacks local recurrence outcomes information, IBTR! was initially constructed based on a comprehensive review of available randomized trials, meta-analyses, and institutional reports. The seven variables selected for inclusion in IBTR! were those that were deemed to be associated with the most consistent and most significant impact on IBTR as reported by multiple studies.8 Factors that were judged to be less consistently supported by the literature, and thus were not included in the model, were tumor histology and an extensive in situ component.8 To avoid confounding and redundancy, hormone receptor status was also excluded as an independent risk factor because of its close association with the use of hormone suppression therapy.8 There is emerging data suggesting that the biologic subtypes of breast cancer, which are closely associated with estrogen receptor/progesterone receptor and HER-2 receptor status, may impact local recurrence.21 Due to the short follow-up time and lack of consistency in the available data,22 biologic subtype is not part of IBTR! version 2.0. However, similar to Adjuvant! Online, the IBTR! model will continue to be modified as new data with mature follow-up emerges.
Despite the effort to synthesize the literature, the initial nomogram solely based on interpretation of the published literature was found on preliminary testing to be limited by significant overestimations in patients with unfavorable risk characteristics.9,10 The reasons for the overestimation are multifactorial, but may include different definitions of IBTR and varying methods of capturing these events across different studies. In addition, competing risks of distant metastasis and death in patients with high-risk characteristics such as very young age, grade 3 histology, and the presence of lymphovascular invasion impairs the ability of a predictive model to accurately estimate IBTR risks over a long time interval.
To address these caveats, this study adopted the definition of IBTR as a first event and ensured consistency in the coding of this end point in the two reference data sets. The larger BCCA data set was used to generate population-based HRs for the modified nomogram, and this was tested against the MGH data set. The overall 1.2% difference between predicted and observed estimates of IBTR and nonsignificant P value on t-testing suggests that the nomogram was fairly consistent within the validation data set. In this analysis, the predicted 10-year estimates of IBTR were accurate to within less than 1% in groups 1 and 2, patients with a predicted 10-year IBTR rate of lower than 6%. These patients comprised a large majority (78%) of the entire validation population.
The differences between predicted and observed IBTR estimates in groups 3 and 4 are difficult to interpret. The absolute differences were 3.5% and 3.8%, respectively, and the group comparisons were not statistically different using t-testing (P ≥ .05). However, the IBTR event rates in these groups were relatively low, and this study could be under-powered to detect significant differences. Consequently, it may be prudent to pursue confirmatory testing of the nomogram on larger data sets before its introduction to widespread clinical use.
Since RT after BCS results in a very consistent 0.7 relative risk reduction across multiple randomized trials,1 IBTR! might best be used to derive the absolute risk of local recurrence after adjuvant RT among women with a low risk of breast recurrence who may be considering not undergoing RT after BCS. Although the risk of recurrence without RT could not be addressed in this prospective validation study because of low numbers of patients in the reference data sets who had not received RT, this risk could be estimated by dividing the IBTR!-derived estimate by 0.3. This study suggests that this is a reasonable use of IBTR! version 2.0 if the predicted 10-year breast recurrence risk following BCS plus RT is 5% or lower.
The development of new tools to assist clinicians and patients in individualizing adjuvant treatment decision-making in breast cancer continues to be a high research and clinical priority. Progress in molecular analysis and genetic profiling techniques have advanced in recent years, not only in the setting of systemic recurrence and mortality risk prediction, but also in locoregional risk assessment.23–25 Until these novel techniques can be implemented in the clinical setting, oncologists will continue to primarily rely on clinicopathologic characteristics to make decisions regarding adjuvant local management.
Continued efforts to refine and establish validity of clinical prediction tools across the entire spectrum of low- and high-risk patients are thus needed to realize the goal of assisting women and their care providers in appraising risks and making individualized treatment choices.
In conclusion, the IBTR! version 2.0 nomogram is acceptably accurate in the majority of patients with a low to moderate risk of in-breast recurrence. The model still overestimates risk in the minority of patients with higher risk features. Although this overestimation was not statistically significant in this study, validation testing of this promising tool in a larger prospective data set is warranted.
Appendix
Table A1.
Clinical Characteristics of Patients in the Four Risk Groups Predefined by Nomogram-Predicted Risks
| Characteristic | Group 1: IBTR < 3% |
Group 2: IBTR 3%-5% |
Group 3: IBTR 5%-10% |
Group 4: IBTR > 10% |
||||
|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | |
| No. of patients | 283 | 237 | 111 | 33 | ||||
| Median age, years | 63.5 | 58.1 | 47.6 | 37.9 | ||||
| ≤ 40 | 0 | 0 | 3 | 6 | 23 | 46 | 24 | 48 |
| 41-45 | 0 | 0 | 11 | 25 | 29 | 66 | 4 | 9 |
| 46-50 | 42 | 44 | 43 | 45 | 10 | 10 | 1 | 1 |
| 51-55 | 30 | 32 | 39 | 41 | 24 | 26 | 1 | 1 |
| 56-60 | 47 | 52 | 33 | 37 | 10 | 11 | 0 | 0 |
| 61-65 | 31 | 48 | 29 | 45 | 4 | 6 | 1 | 2 |
| 66-70 | 42 | 45 | 45 | 48 | 5 | 5 | 2 | 2 |
| > 70 | 91 | 69 | 34 | 26 | 6 | 5 | 0 | 0 |
| Margin | ||||||||
| Positive | 2 | 4 | 14 | 27 | 26 | 50 | 10 | 19 |
| Close (< 2 mm) | 41 | 58 | 18 | 25 | 9 | 13 | 3 | 4 |
| Negative (> 2 mm) | 240 | 44 | 205 | 38 | 76 | 14 | 20 | 4 |
| Unknown | 0 | 0 | 0 | 0 | ||||
| Lymphovascular invasion | ||||||||
| Present | 44 | 37 | 35 | 30 | 33 | 28 | 6 | 5 |
| Absent | 239 | 44 | 202 | 37 | 78 | 14 | 27 | 5 |
| Unknown | 0 | 0 | 0 | 0 | ||||
| Tumor size, cm | ||||||||
| ≤ 1 | 78 | 30 | 123 | 47 | 48 | 18 | 14 | 5 |
| 1.1-2 | 158 | 56 | 78 | 28 | 35 | 12 | 9 | 3 |
| > 2 | 47 | 39 | 36 | 30 | 28 | 23 | 10 | 8 |
| Grade | ||||||||
| I | 112 | 74 | 31 | 20 | 9 | 6 | 0 | 0 |
| II | 151 | 46 | 127 | 38 | 46 | 14 | 7 | 2 |
| III | 20 | 11 | 79 | 44 | 56 | 31 | 26 | 14 |
| Unknown | 0 | 0 | 0 | 0 | ||||
| Hormone therapy | ||||||||
| Yes | 235 | 58 | 118 | 29 | 47 | 12 | 4 | 1 |
| No | 48 | 18 | 119 | 46 | 64 | 25 | 29 | 11 |
| Chemotherapy | ||||||||
| Yes | 82 | 37 | 67 | 31 | 51 | 23 | 19 | 9 |
| No | 201 | 45 | 170 | 38 | 60 | 13 | 14 | 3 |
Abbreviation: IBTR, ipsilateral breast cancer tumor recurrence.
Footnotes
See accompanying editorial on page 709
Supported in part by the Jane Mailloux Research Fund, Blanche Montesi Fund, Tim Levy Fund for breast cancer research, Grants No. NIH CA21239 and CA50628 from the National Institutes of Health, and the Canadian Breast Cancer Foundation, British Columbia/Yukon Chapter.
Presented in part at the Annual Conference of the American Society of Therapeutic Radiation Oncology, September 21-24, 2008, Boston, MA.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Mona Sanghani, Pauline T. Truong, Andrzej Niemierko, Mary Lesperance, David E. Wazer, Alphonse G. Taghian
Administrative support: Pauline T. Truong, Rita Abi Raad, Andrzej Niemierko, Ivo A. Olivotto, David E. Wazer, Alphonse G. Taghian
Provision of study materials or patients: Mona Sanghani, Pauline T. Truong, Rita Abi Raad, Andrzej Niemierko, Mary Lesperance, Alphonse G. Taghian
Collection and assembly of data: Mona Sanghani, Pauline T. Truong, Rita Abi Raad, Andrzej Niemierko, Mary Lesperance, David E. Wazer, Alphonse G. Taghian
Data analysis and interpretation: Mona Sanghani, Pauline T. Truong, Rita Abi Raad, Andrzej Niemierko, Mary Lesperance, Ivo A. Olivotto, David E. Wazer, Alphonse G. Taghian
Manuscript writing: Mona Sanghani, Pauline T. Truong, David E. Wazer, Alphonse G. Taghian
Final approval of manuscript: Mona Sanghani, Pauline T. Truong, Rita Abi Raad, Andrzej Niemierko, Mary Lesperance, Ivo A. Olivotto, David E. Wazer, Alphonse G. Taghian
REFERENCES
- 1.Early Breast Cancer Trialists' Collaborative Group. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 3.Veronesi U, Marubini E, Mariani L, et al. Radiotherapy after breast-conservation surgery in small breast carcinoma: Long-term results of a randomized trial. Ann Oncol. 2001;12:997–1003. doi: 10.1023/a:1011136326943. [DOI] [PubMed] [Google Scholar]
- 4.Clark R, Whelan T, Levine M, et al. Randomized clinical trial of breast irradiation following lumpectomy and axillary dissection for node negative breast cancer: An update. J Natl Cancer Inst. 1996;88:1659–1664. doi: 10.1093/jnci/88.22.1659. [DOI] [PubMed] [Google Scholar]
- 5.Forrest A, Stewart H, Everington D, et al. Randomised controlled trial of conservation therapy for breast cancer: 6-year analysis of the Scottish trial. Scottish Cancer Trials Breast Group. Lancet. 1996;348:708–713. doi: 10.1016/s0140-6736(96)02133-2. [DOI] [PubMed] [Google Scholar]
- 6.Liljegren G, Holmberg L, Bergh J, et al. 10-year results after sector resection with or without postoperative radiotherapy for stage I breast cancer: A randomized trial. J Clin Oncol. 1999;17:2326–2333. doi: 10.1200/JCO.1999.17.8.2326. [DOI] [PubMed] [Google Scholar]
- 7.Malmstrom P, Holmberg L, Anderson H, et al. Breast conservation surgery, with and without radiotherapy, in women with lymph node-negative breast cancer: A randomised clinical trial in a population with access to public mammography screening. Eur J Cancer. 2003;39:1690–1697. doi: 10.1016/s0959-8049(03)00324-1. [DOI] [PubMed] [Google Scholar]
- 8.Sanghani M, Balk E, Cady B, et al. Predicting the risk of local recurrence in patients with breast cancer: An approach to a new computer-based predictive tool. Am J Clin Oncol. 2007;30:473–480. doi: 10.1097/COC.0b013e31805c13d9. [DOI] [PubMed] [Google Scholar]
- 9.Sanghani M, Abi-Raad R, Niemierko A, et al. Validation of a web-based predictive nomogram for ipsilateral breast tumor recurrence after breast conserving therapy. Int J Radiat Oncol Biol Phys. 2008;72(suppl):S91. doi: 10.1200/JCO.2009.22.6662. abstr 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Truong PT, Lesperance M, Sanghani M, et al. Independent validation of IBTR! A computer-based tool to predict ipsilateral breast recurrence in women with invasive breast cancer treated with breast conserving surgery. Int J Radiat Oncol Biol Phys. 2008;72(suppl):S91–S92. abstr 203. [Google Scholar]
- 11.Whelan T, MacKenzie R, Julian J, et al. Randomized trial of breast irradiation schedules after lumpectomy for women with lymph node-negative breast cancer. J Natl Cancer Inst. 2002;94:1143–1150. doi: 10.1093/jnci/94.15.1143. [DOI] [PubMed] [Google Scholar]
- 12.Lawless JF. Statistical Models and Methods for Lifetime Data. ed 2. Hoboken, NJ: Wiley; 2003. [Google Scholar]
- 13.Klein JP, Moeschberger ML. Survival Analysis: Techniques for Censored and Truncated Data. ed 2. New York: NY Springer; 2003. [Google Scholar]
- 14.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. ed 2. Hoboken, NJ: Wiley; 2002. [Google Scholar]
- 15.Taghian A, Jeong JH, Mamounas E, et al. Patterns of local failure in patients with operable breast cancer treated by mastectomy and adjuvant chemotherapy with or without tamoxifen and without radiotherapy: Results from five National Surgical Adjuvant Breast and Bowel Project randomized clinical trials. J Clin Oncol. 2004;22:4247–4254. doi: 10.1200/JCO.2004.01.042. [DOI] [PubMed] [Google Scholar]
- 16.Recht A, Gray R, Davidson NE, et al. Locoregional failure 10 years after mastectomy and adjuvant chemotherapy with or without tamoxifen without irradiation: Experience of the Eastern Cooperative Oncology Group. J Clin Oncol. 1999;17:1689–1700. doi: 10.1200/JCO.1999.17.6.1689. [DOI] [PubMed] [Google Scholar]
- 17.Olivotto IA, Bajdik CD, Ravdin PM, et al. Population-based validation of the prognostic model Adjuvant! for early breast cancer. J Clin Oncol. 2005;23:2716–2725. doi: 10.1200/JCO.2005.06.178. [DOI] [PubMed] [Google Scholar]
- 18.Pepe M. New York, NY: Oxford Statistical Science Series, Oxford University Press; 2003. The Statistical Evaluation of Medical Tests for Classification and Prediction. [Google Scholar]
- 19.Harrell FE, Califf RM, Pryor DB, et al. Evaluating the yield of medical tests. J Am Med Ass. 1982;247:2543–2546. [PubMed] [Google Scholar]
- 20.Anderson SJ, Wapnir I, Dignam JJ, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in patients treated with breast-conserving therapy in five National Surgical Adjuvant Breast and Bowel Project protocols of node-negative breast cancer. J Clin Oncol. 2009;27:2466–2473. doi: 10.1200/JCO.2008.19.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen P, Taghian A, Katz M, et al. Breast cancer subtype approximated by estrogen receptor, progesterone receptor, and HER-2 is associated with local and distant recurrence after breast-conserving therapy. J Clin Oncol. 2008;26:2372–2378. doi: 10.1200/JCO.2007.14.4287. [DOI] [PubMed] [Google Scholar]
- 22.Haffty BG, Yang Q, Reiss M, et al. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol. 2006;25:5652–5657. doi: 10.1200/JCO.2006.06.5664. [DOI] [PubMed] [Google Scholar]
- 23.Van't Veer LJ, Dai H, Van de Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 24.Nuyten DSA, Kreike B, Hart AAM, et al. Predicting a local recurrence after breast-conserving therapy by gene expression profiling. Breast Cancer Res. 2006;8:R62. doi: 10.1186/bcr1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng SH, Horng CF, West M, et al. Genomic prediction of locoregional recurrence after mastectomy y in breast cancer. J Clin Oncol. 2006;28:4594–4602. doi: 10.1200/JCO.2005.02.5676. [DOI] [PubMed] [Google Scholar]