Abstract
Purpose
The After Mapping of the Axilla: Radiotherapy or Surgery? (AMAROS) phase III study compares axillary lymph node dissection (ALND) and axillary radiation therapy (ART) in early breast cancer patients with tumor-positive sentinel nodes. In the ART arm, the extent of nodal involvement remains unknown, which could have implications on the administration of adjuvant therapy. In this preliminary analysis, we studied the influence of random assignment to ALND or ART on the choice for adjuvant treatment.
Patients and Methods
In the first 2,000 patients enrolled in the AMAROS trial, we analyzed the administration of adjuvant systemic therapy. Multivariate analysis was used to assess variables affecting the administration of adjuvant chemotherapy. Adjuvant therapy was applied according to institutional guidelines.
Results
Of 2,000 patients, 566 patients had a positive sentinel node and were treated per random assignment. There was no significant difference in the administration of adjuvant systemic therapy. In the ALND and ART arms, 58% (175 of 300) and 61% (162 of 266) of the patients, respectively, received chemotherapy. Endocrine therapy was administered in 78% (235 of 300) of the patients in the ALND arm and in 76% (203 of 266) of the patients in the ART arm. Treatment arm was not a significant factor in the decision, and no interactions between treatment arm and other factors were observed. Multivariate analysis showed that age, tumor grade, multifocality, and size of the sentinel node metastasis significantly affected the administration of chemotherapy. Within the ALND arm, the extent of nodal involvement remained not significant in a sensitivity multivariate analysis.
Conclusion
Absence of knowledge regarding the extent of nodal involvement in the ART arm appears to have no major impact on the administration of adjuvant therapy.
INTRODUCTION
The primary site of lymphatic drainage of the breast is the axillary region. Hence, the axillary lymph nodes are often the first site of regional metastatic disease in breast cancer. Axillary clearance provides knowledge on the presence or absence of dissemination to the axillary lymph nodes—important information for prognosis and staging.1 It also ensures regional tumor control and may in some cases improve survival.2 Therefore, axillary lymph node dissection (ALND) of the axilla was standard of care in patients with breast cancer for many years.
With the widespread introduction of the sentinel node biopsy procedure, the question was raised about how to deal with potential additional lymph node metastases in case of a positive sentinel node. After ALND, morbidity including lymph edema and decreased arm and shoulder function is seen in 5% to 39% of the patients.3–5 A less invasive alternative for ALND in the case of positive sentinel nodes might be axillary radiation therapy (ART).
In the 1960s and 1970s, the value of ART was analyzed in a series of randomized clinical trials.6–11 The original objective was to test the hypothesis of improving survival by maintaining an immunologic barrier in the axillary lymph nodes. With respect to the end point of survival, no difference was found between ALND and ART except in a British trial that showed worse survival due to lower axillary control rates after ART.9 Conversely, the only trial focusing on patients with clinically negative lymph nodes showed a low axillary recurrence rate of 4% in the ART arm after 25 years.12
To investigate differences in regional control, survival, and long-term morbidity between ALND and ART, an international multicenter phase III trial was initiated in 2001 by the European Organisation for Research and Treatment of Cancer (EORTC). This study is called the After Mapping of the Axilla: Radiotherapy or Surgery? (AMAROS) trial (EORTC 10981-22023 trial) and is ongoing. Patients with clinically negative lymph nodes are randomly assigned between ALND and ART in case of a tumor-positive sentinel node biopsy. The main objective of the trial is to show noninferiority of the radiotherapy arm (ART) compared with the ALND treatment arm with respect to axillary recurrence-free rate in sentinel node–positive patients. In total, 4,766 patients have to be included. Up to December 2008, more than 4,000 patients have been enrolled.
Besides survival and locoregional control, another concern is the extent of nodal involvement, which remains unknown when ALND is replaced by ART. More extensive nodal involvement is associated with a higher TNM classification and poorer prognosis.13 Hence, these patients are likely to have a larger absolute benefit from adjuvant systemic and radiation therapy. Therefore, nodal status is commonly used to select patients for adjuvant treatment. The aim of this preliminary analysis was to analyze the influence of ART, and consequently an unknown extent of nodal involvement, on decisions concerning adjuvant systemic treatment.
PATIENTS AND METHODS
After obtaining permission from the EORTC Independent Data Monitoring Committee, the first 2,000 patients with operable unifocal invasive breast cancer (5 to 30 mm) and clinically negative lymph nodes who had enrolled in the AMAROS trial were analyzed. The CONSORT diagram and study design of the AMAROS trial are shown in Figures 1 and 2, respectively. Quality assurance for the sentinel node procedure and axillary radiotherapy was described previously.14,15 Exclusion criteria were metastatic disease, previous treatment of the axilla by surgery or radiotherapy, previous treatment of cancer (except basal cell carcinoma of the skin and in situ carcinoma of the cervix), or pregnancy. Between 2001 and 2005, 2,000 patients were entered onto the AMAROS trial from 26 institutions in Europe. Before the sentinel node biopsy (SNB) procedure, patients were randomly assigned between ALND and ART. This allowed the application of breast surgery and axillary surgery simultaneously when positive sentinel nodes were found by analyzing frozen sections. Random assignment was accomplished centrally by the EORTC headquarters, and patients were stratified according to institution. The AMAROS trial was approved by the institutional ethical committees, and informed consent was obtained for all patients.
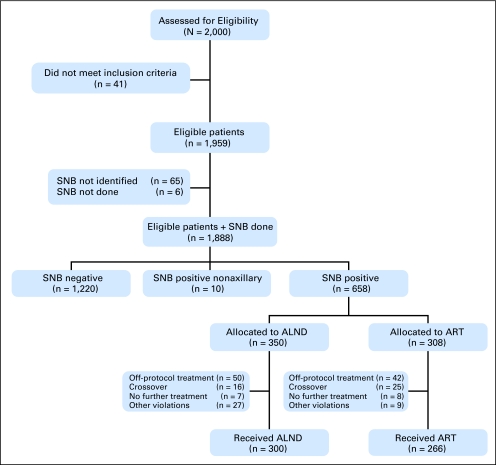
Fig 1.
CONSORT flow chart. The final 566 patients with positive sentinel nodes and treated according their random assignment (axillary lymph node dissection [ALND] v axillary radiation therapy [ART]) form the basis of this study. SNB, sentinel node biopsy.
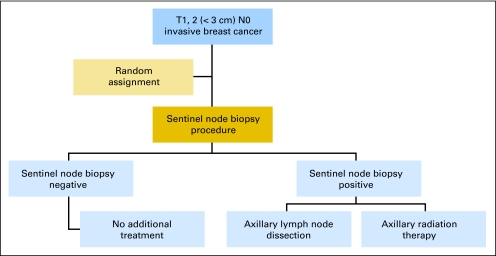
Fig 2.
Study design. Patients with clinically negative lymph nodes and tumors less than 3 cm are randomly assigned between an axillary lymph node dissection or axillary radiation therapy before the sentinel node biopsy procedure.
Surgery
In 1,744 patients, SNB was performed using the combined method of blue dye and isotope with intraoperative detection using a gamma probe. A minority of SNBs were performed with isotope (n = 181) or blue dye (n = 19) only. Lymphoscintigrams were recommended although not mandatory. Radioactive and blue-stained nodes were removed together with nodes that were highly suggestive of metastatic cancer on palpation. Subsequently, mastectomy or breast-conserving surgery was carried out. Patients with tumor-positive sentinel nodes who were allocated to the ALND arm underwent level I and II ALNDs within 12 weeks. Removal of at least eight lymph nodes was mandatory.
Radiotherapy
Sentinel node–positive patients allocated to the ART arm were irradiated within 12 weeks after surgery. All three levels of the axilla together with the medial part of the supraclavicular fossa were considered clinical target volume. The prescribed dose to the axilla was 50 Gy in 25 fractions of 2 Gy, 5 days a week. Postoperative axillary irradiation in patients undergoing ALND was allowed in patients with four or more tumor-positive nodes (pN2 or pN3) and applied according to the institutional protocols.
Pathology
As a minimal requirement, three histologic levels (500-micron distance) for each sentinel node were examined. On each level, two parallel sections were performed: one for immunohistochemistry and one for hematoxylin and eosin staining. immunohistochemistry staining was performed for markers containing at least cytokeratin 8 and 18 (eg, CAM 5.2). Immunohistochemical staining was required only when hematoxylin and eosin staining was negative. Tumor deposits were categorized as isolated tumor cells (< 0.2 mm), micrometastases (0.2 to 2 mm), or macrometastases (> 2 mm).
Adjuvant Systemic Therapy
The indications to offer systemic therapy were not fixed in the protocol. The actual chemotherapies and endocrine therapies were given according to the local guidelines. To obtain an objective criterion for the administration of adjuvant chemotherapy, we used the clinicopathologic risk as predicted by Adjuvant! and a predefined cutoff value of clinical high- and low-risk patients, as previously described by Mook et al.16
The Adjuvant! software version 8.0 (www.adjuvantonline.com) calculated 10-year survival probability based on patient's age, comorbidities, tumor size, tumor grade, estrogen receptor (ER) status, and number of positive axillary lymph nodes.17,18 Since the ER status was not collected, we used the administration of endocrine therapy as a surrogate for ER status. Patients were considered to have low clinical risk when the 10-year breast cancer–specific survival as predicted by Adjuvant! was more than 88% for ER-positive tumors and more than 92% for ER-negative tumors.19 We analyzed the difference when these patients were considered sentinel node–positive (categorized as one to three positive lymph nodes) instead of their actual number of involved nodes (categorized as four to nine, or more than nine positive lymph nodes).
Statistical Analysis
In this study, we tested the hypothesis that the administration of adjuvant systemic therapy was similar in both treatment arms. Among the first 2,000 patients enrolled in the AMAROS trial, 92 patients with a positive sentinel node were not treated according to their random assignment and were excluded. All 566 patients with positive sentinel nodes who underwent ALND or ART according to their random assignment were included in this per protocol analysis. The proportions of patients treated with adjuvant systemic therapy in each treatment arm were compared. Associations between the administration of adjuvant chemotherapy with regard to patient- and tumor-related factors were evaluated using univariate and multivariate logistic regression analysis. We used Fisher's exact tests and a forward stepwise selection method with likelihood ratios to analyze associated variables. All P values were two-tailed, with a value of .05 or lower considered significant. To investigate whether the treatment arm had any influence on systemic treatment, interaction terms with all other factors were tested using a cutoff P value of .10 for significance.
A posteriori, this analysis of 566 patients has approximately 80% power to detect an odds ratio of 1.65 between the randomized arms (or any other covariate with two levels). To quantify such an odds ratio further, this translates to differences in percentages of patients treated with chemotherapy of 55% versus 67% or of 60% versus 71%. This analysis was not preplanned and therefore there is no a priori expected magnitude of differences.
RESULTS
The patient flow is outlined in Figure 1lists basic patient, tumor, and treatment characteristics. There were no significant differences between the ALND and ART arms. The median ages in the ALND and ART arms were 56 and 55 years, respectively. Most patients had a tumor size between 10 and 20 mm (54%, ALND arm; 53%, ART arm) and had macrometastases in their sentinel nodes (61%, ALND arm; 66%, ART arm). In the ALND arm, 87% of patients had nodal involvement in one to three nodes (pN1), 8% in four to nine nodes (pN2), and 4% in more than nine nodes (pN3). Table 2. Of the first 2,000 patients, 41 patients were ineligible because of patient refusal or because they did not meet the inclusion criteria. In six of the eligible 1,959 patients, the SNB procedure was not performed. In 65 patients, the sentinel node could not be identified. As a result, the sentinel node identification rate was 97% (1,888 of 1,953). Sixty-five percent of the patients (n = 1,220) were sentinel node–negative and underwent no further axillary treatment. Ten patients (0.5%) had only nonaxillary tumor-positive sentinel nodes. In these patients, no further axillary treatment was performed. Thirty-five percent of the patients (n = 658) were sentinel node–positive and were to be treated according to earlier random assignment between ALND and ART. Treatment noncompliance was 14% (n = 92). These 92 patients were excluded from further analysis (Appendix Table A1, online only). In 41 patients, crossover to the other treatment arm was seen. The crossovers were mainly due to patient refusal or were decided on by local treating physicians on the basis of patient and tumor characteristics. No further treatment was given in 15 patients. The majority of these patients had only isolated tumor cells. Other severe protocol deviations, such as inaccurate lymph node dissections and treatment delay, were seen in 36 patients. Of the 658 sentinel node–positive patients, 300 were treated per random assignment with ALND and 266 patients were treated with ART. These 566 patients form the study cohort of this report.
Table 2.
Administration of Adjuvant Therapy
| Therapy | ALND Arm (n = 300) |
ART Arm (n = 266) |
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| None | 23 | 8 | 24 | 9 |
| Endocrine therapy | 95 | 32 | 80 | 30 |
| Chemotherapy | 35 | 12 | 39 | 15 |
| Chemotherapy plus endocrine therapy | 140 | 47 | 123 | 46 |
| Missing | 7 | 2 | 0 | 0 |
| Radiotherapy (breast/chest wall) | 257 | 86 | 237 | 89 |
| Axillary radiotherapy | 15 | 5 | 266 | 100 |
Abbreviations: ALND, axillary lymph node dissection; ART, axillary radiation therapy.
Table 1 shows the administration of adjuvant therapy in both treatment arms.
Table 1.
Basic Characteristics of Patients in the ALND and ART Arms
| Characteristic | ALND Arm (n = 300) |
ART Arm (n = 266) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| Age, years | ||||
| Median | 56 | 55 | ||
| Range | 24-83 | 32-82 | ||
| Menopausal status | ||||
| Pre | 88 | 29 | 93 | 35 |
| Peri | 19 | 6 | 20 | 8 |
| Post | 176 | 59 | 142 | 53 |
| Unknown | 17 | 6 | 11 | 4 |
| pT stage, cm | ||||
| ≤ 1 | 30 | 10 | 33 | 12 |
| 1-< 2 | 162 | 54 | 141 | 53 |
| 2-< 3 | 90 | 30 | 84 | 32 |
| 3-< 5 | 17 | 6 | 7 | 3 |
| > 5 | 1 | 0.3 | 1 | 0.4 |
| SNB results* | ||||
| Macro | 182 | 61 | 175 | 66 |
| Micro | 78 | 26 | 59 | 22 |
| ITCs | 32 | 11 | 31 | 12 |
| No. of positive sentinel nodes | ||||
| 1 | 215 | 72 | 187 | 70 |
| 2 | 67 | 22 | 62 | 23 |
| 3 | 14 | 5 | 15 | 6 |
| 4 | 3 | 1 | 2 | 1 |
| 13 | 1 | 0.3 | 0 | 0 |
| pN stage, nodes | ||||
| 1-3 | 261 | 87 | NA | |
| 4-9 | 25 | 8 | NA | |
| 10+ | 12 | 4 | NA | |
| Missing | 2 | 1 | NA | |
| Histology | ||||
| Ductal | 216 | 72 | 198 | 74 |
| Lobular | 39 | 13 | 31 | 12 |
| Other | 45 | 15 | 37 | 14 |
| Grade | ||||
| I | 66 | 22 | 64 | 24 |
| II | 141 | 47 | 120 | 45 |
| III | 85 | 28 | 77 | 29 |
| Missing | 8 | 3 | 5 | 2 |
| Surgery | ||||
| BCS | 263 | 88 | 238 | 89 |
| Mastectomy | 37 | 12 | 28 | 11 |
Abbreviations: ALND, axillary lymph node dissection; ART, axillary radiation therapy; SNB, sentinel node biopsy; ITC, isolated tumor cell; NA, not applicable; BCS, breast-conserving surgery.
Nine patients had positive nonsentinel nodes (size unknown).
There was no significant difference in the number of patients who were treated with adjuvant chemotherapy or hormonal therapy. In the ALND arm, 58% (n = 175) of the patients received adjuvant chemotherapy and 78% (n = 235) received adjuvant endocrine therapy. In the ART arm, 61% (n = 162) of the patients received adjuvant chemotherapy and 76% (n = 203) received adjuvant endocrine therapy. The overall administration of taxane-based regimens was low, and there was no clear difference between the two treatment arms (Appendix Table A2, online only). In the ALND arm, four of the 10 patients who were treated with a taxane-based regimen had at least four positive lymph nodes. We analyzed the variables affecting the administration of adjuvant chemotherapy (Table 3). Univariate analysis showed that age, menopausal status, pathologic T stage, multifocality, the size of sentinel node metastasis, tumor grade, and nodal involvement (in the ALND arm only) affected the administration of adjuvant chemotherapy. In multivariate analysis, variables significantly affecting the use of adjuvant chemotherapy were age, tumor grade, the size of the sentinel node metastasis, and multifocality (Table 4). Axillary treatment (ALND or radiotherapy), number of positive sentinel nodes, tumor size, hormonal treatment, radiation to the breast, and menopausal status were not retained in the model. The extent of nodal involvement (analyzed as both a continuous and a categoric variable) did not significantly affect adjuvant chemotherapy administration in multivariate analysis of the ALND arm separately (Table 5). When added to this model, the number of positive nodes has a P value of .47.
Table 3.
Variables Affecting the Use of Adjuvant Chemotherapy
| Variable | ALND Arm* |
ART Arm |
||||
|---|---|---|---|---|---|---|
| No. of Patients With CT | Total No. of Patients in the Group | % CT | No. of Patients With CT | Total No. of Patients in the Group | % CT | |
| No. of patients with CT | 175 | 293 | 162 | 266 | ||
| Age, years | ||||||
| Median | 51 in CT group | 51 in CT group | ||||
| 64 in non-CT group | 63 in non-CT group | |||||
| Menopausal status | ||||||
| Pre | 77 | 86 | 90 | 84 | 93 | 90 |
| Peri | 13 | 19 | 68 | 15 | 20 | 75 |
| Post | 72 | 172 | 42 | 55 | 142 | 39 |
| pT stage, cm | ||||||
| ≤ 1 | 15 | 29 | 52 | 14 | 33 | 42 |
| 1-< 2 | 86 | 158 | 54 | 81 | 141 | 57 |
| 2-< 3 | 63 | 89 | 71 | 61 | 84 | 73 |
| 3-< 5 | 10 | 16 | 63 | 5 | 7 | 71 |
| > 5 | 1 | 1 | 100 | 1 | 1 | 100 |
| SNB results | ||||||
| ITCs | 10 | 31 | 32 | 10 | 31 | 32 |
| Micro | 42 | 77 | 55 | 33 | 59 | 56 |
| Macro | 119 | 177 | 67 | 118 | 175 | 67 |
| pN stage | ||||||
| 1-3 | 148 | 256 | 58 | NA | ||
| 4-9 | 7 | 11 | 64 | NA | ||
| > 10 | 19 | 25 | 76 | NA | ||
| Histology | ||||||
| Ductal | 126 | 212 | 59 | 122 | 198 | 62 |
| Lobular | 24 | 37 | 65 | 16 | 31 | 52 |
| Other | 25 | 44 | 57 | 24 | 37 | 65 |
| Grade | ||||||
| I | 29 | 65 | 45 | 29 | 64 | 45 |
| II | 75 | 137 | 55 | 68 | 120 | 57 |
| III | 66 | 83 | 80 | 64 | 77 | 83 |
| Multifocality | ||||||
| Yes | 28 | 33 | 85 | 28 | 33 | 85 |
| No | 147 | 260 | 57 | 134 | 233 | 58 |
| Endocrine therapy | ||||||
| Yes | 35 | 58 | 60 | 39 | 63 | 62 |
| No | 140 | 235 | 60 | 80 | 203 | 65 |
Abbreviations: ALND, axillary lymph node dissection; ART, axillary radiation therapy; CT, chemotherapy; SNB, sentinel node biopsy; ITC, isolated tumor cell; NA, not applicable because the final nodal status remains unknown, although two patients in the ART group had at least pN2 since four tumor-positive sentinel nodes were removed.
In seven patients, information about the adjuvant treatment is missing.
Table 4.
Results of Multivariate Analysis Including Variables Affecting the Administration of Adjuvant Chemotherapy
| Covariate* | Odds Ratio for Receiving CT | 95% CI | P |
|---|---|---|---|
| Age | |||
| Per additional year of age | 0.85 | 0.83 to 0.88 | < .0001 |
| Grade | |||
| I† | 1 | < .0001 | |
| II | 1.73 | 0.99 to 3.01 | |
| III | 7.05 | 3.56 to 13.96 | |
| Size of sentinel node metastasis | |||
| Single ITC† | 1 | .0001 | |
| Clusters of ITCs | 1.85 | 0.27 to 12.49 | |
| Micro | 4.90 | 0.80 to 29.98 | |
| Macro | 9.83 | 1.65 to 58.79 | |
| Multifocality | |||
| Yes v no | 4.91 | 2.02 to 11.90 | .0004 |
Abbreviations: CT, chemotherapy; ITC, isolated tumor cell.
In the initial model, the following covariates were attempted: age, grade, size of sentinel node metastasis, multifocality, pathologic tumor size, menopausal status, number of positive sentinel nodes, adjuvant hormonal treatment, breast irradiation, and axillary treatment (axillary lymph node dissection or axillary radiation therapy). Variables not shown were not retained in the model.
Reference level.
Table 5.
Results of Multivariate Analysis Including Variables Affecting the Administration of Adjuvant Chemotherapy in the ALND Arm
| Covariate* | Odds Ratio for Receiving CT | 95% CI | P |
|---|---|---|---|
| Age | |||
| Per additional year of age | 0.86 | 0.82 to 0.89 | < .0001 |
| Grade | |||
| I† | 1 | .0004 | |
| II | 1.38 | 0.64 to 2.98 | |
| III | 5.55 | 2.21 to 13.95 | |
| Size of sentinel node metastasis | |||
| Single ITC† | 1 | .005 | |
| Clusters of ITCs | 1.91 | 0.13 to 28.45 | |
| Micro | 4.10 | 0.33 to 51.49 | |
| Macro | 10.76 | 0.87 to 133.00 | |
| Multifocality | |||
| Yes v no | 5.53 | 1.51 to 20.26 | .010 |
Abbreviations: ALND, axillary lymph node dissection; CT, chemotherapy; ITC, isolated tumor cell.
In the initial model, the following covariates were attempted: age, grade, size of sentinel node metastasis, number of positive sentinel nodes, nodal involvement (continuous), multifocality, pathologic tumor size, menopausal status, adjuvant hormonal treatment, and breast irradiation. Variables not shown were not retained in the model.
Reference level.
To analyze whether clinicians would unjustifiably withhold chemotherapy in the 37 patients with at least four positive lymph nodes, we assessed the clinicopathologic risk as predicted by Adjuvant! as if only the SNB results were known. Adjuvant! classified all 37 patients with four or more positive lymph nodes as clinical high risk, using the predefined cutoff. When the patients were considered as sentinel node–positive (categorized as one to three positive lymph nodes), Adjuvant! classified two patients (5%) as clinical low risk and 35 patients as clinical high risk. Strikingly, in practice these two patients were not treated with chemotherapy even though according to the number of positive lymph nodes they were classified as high risk.
DISCUSSION
This study shows no difference in the administration of adjuvant systemic therapy between the two treatment arms of this trial, which randomly assigned patients with a tumor-positive sentinel node between ALND and ART. In an overall multivariate analysis, the treatment arm did not influence the administration of systemic therapy, and within the ALND treatment arm, the number of involved nodes was not retained in the multivariate model. Furthermore, we showed that the proportion of patients having four or more positive lymph nodes in the ALND arm is low (12%), and that the majority of these patients (95%) are classified as clinically high risk, even without knowledge of the actual number of involved lymph nodes. Therefore, we suggest that the absence of knowledge regarding the extent of nodal involvement in the ART arm does not affect the administration of adjuvant systemic therapy.
Nonetheless, the extent of nodal involvement is an important prognostic factor that is taken into account in the International Union Against Cancer (UICC) TNM classification and, therefore, used in many guidelines to select patients for adjuvant treatment.13,17,20,21 The categorization of patients into groups with one to three (pN1) versus four or more positive nodes (pN2 and pN3) originated in the early days of adjuvant chemotherapy for breast cancer.22 Only patients with four or more nodes appeared to benefit from adjuvant chemotherapy. Later studies proved the benefit of chemotherapy in patients with one to three positive nodes and even subgroups of patients with tumor-negative nodes.23
At present, hormone responsiveness is the most important discriminator for adjuvant therapy.23 The additional effect of adjuvant chemotherapy beyond the effect of endocrine therapy reduces with increasing age. This may lead to the choice for endocrine treatment alone, without chemotherapy, in older patients with a relatively low a priori risk. Patients with more than four tumor-positive lymph nodes, indicating a worse prognosis, are more likely to receive additional chemotherapy.21 According to the current St. Gallen criteria, hormone receptor–positive patients with one to three positive nodes are at intermediate risk. Patients with four or more positive nodes are at high risk.
When ALND is omitted in patients with positive sentinel nodes, the extent of nodal involvement will remain unknown and cannot be used to select patients for adjuvant systemic therapy. This study shows that, in clinical practice, nodal extent does not appear to affect the number of patients receiving adjuvant chemotherapy. Multivariate analysis shows that the administration of chemotherapy is mainly based on age, tumor grade, multifocality, and the size of sentinel node metastasis and not by the nodal extent. This suggests that pN classification is only of limited additional importance compared with these other variables.
Recently, systematic analysis of gene expression patterns using microarray technology has led to the discovery of prognostic gene expression signatures, such as the 70-gene profile (MammaPrint) or the 21-gene profile (Oncotype DX).24,25 These prognostic gene signatures outperformed the current guidelines and are being validated in two large clinical trials: the EORTC Microarray in Node-Negative and 1 to 3 Positive Lymph Node Disease May Avoid Chemotherapy (MINDACT) trial and the Trial Assigning Individualized Options for Treatment (Rx) (TAILORx) trial.26 In the future, these prognostic tools may be increasingly used to select patients for adjuvant systemic therapy.
Apart from its use to select patients for adjuvant systemic therapy, the extent of further nodal involvement is also used to select patients for postoperative locoregional radiotherapy. The risk of locoregional recurrences increases with the number of tumor-positive nodes. Therefore, patients with more than three positive nodes (pN2 and pN3) have the largest benefit in both locoregional control and survival. These patients are considered candidates for postoperative locoregional radiotherapy worldwide, also following ALND. Considering intermediate-risk patients with one to three positive nodes (pN1), there is more discussion. The Danish and Canadian studies27–29 showed that patients with one to three positive nodes and patients with more than four positive nodes have a similar absolute survival benefit of 9% after 15 years. One explanation is that patients with less nodal involvement may have less systemic disease and therefore a better life expectancy. It is assumed that, especially in these patients, preservation of locoregional control may result in a survival benefit. These studies are criticized for the relatively high locoregional recurrence rates, which may be less in similar patient categories nowadays. In summary, controversy still exists concerning postmastectomy radiotherapy for pN1 (intermediate-risk) patients.30 Furthermore, biologic tumor classification and molecular features of a tumor may better provide discriminators from which patients are likely to benefit. We hope the Selective Use of Postoperative Radiotherapy After Mastectomy (SUPREMO) trial (www.supremo-trial.com) will provide these results.
A few comments on this study are warranted. First, the expression of hormonal receptors is not collected. We used the administration of endocrine therapy as a surrogate for endocrine responsiveness. Since endocrine therapy is recommended only in estrogen- or progesterone-positive tumors, we assumed that these data would be comparable.
Second, the overall use of taxane-based regimens in this study is low. At present, the use of taxanes in early-stage breast cancer has increased and might be influenced by the number of involved nodes. Similarly, the influence on the administration of trastuzumab in HER2-positive patients could not be assessed because the patients in this study were treated between 2000 and 2005. Furthermore, systemic treatment recommendations differ between the United States and Europe.
In conclusion, this analysis shows that in patients with a tumor-positive sentinel node, treating the axilla with radiation instead of lymph node dissection, and thus performing an incomplete axillary staging, does not appear to significantly influence the prescription of adjuvant systemic therapy. These results support the hypothesis that the administration of adjuvant chemotherapy is mainly based on tumor and patient characteristics and SNB status and that knowledge of further nodal involvement is redundant. However, these results will be validated in a consecutive subset of patients accrued to the AMAROS study.
Acknowledgment
We thank the women who participated in this study and to the doctors, nurses, and data managers from the participating hospitals in Europe who enrolled patients in the AMAROS trial.
Appendix
These principal investigators and coinvestigators of the AMAROS trial entered patients onto the study, participated in the study, or both (number of accrued patients is in parentheses): The Netherlands: W. Bouma, Gelre Hospital, Apeldoorn (144); P. de Graaf, Reinier de Graaf Hospital, Delft (85); G. Nieuwenhuyzen, Catharina Hospital, Eindhoven (114); J. Merkus, Rode Kruis Hospital, Den Haag (39); E. Rutgers and N. Russell, Antoni van Leeuwenhoek Hospital, Amsterdam (267); M. Albregts and T. van Dalen, Universitair Medisch Centrum Utrecht and Diakonessen Hospital, Utrecht (13); C. van de Velde, Leiden University Medical Center, Leiden (59); A. Marinelli and H. Struikmans, Medisch Centrum Haaglanden (38); O. Guicherit, Bronovo Hospital, Den Haag (11); H. Rijna, Kennemer Gasthuis, Haarlem (39); W. Steup, Leyenburg Hospital, Den Haag (1); J. de Vries, University Medical Centre Groningen, Groningen (19); J. Debet, Laurentius Hospital, Roermond (28); G. van Tienhoven and B. Kluit, Academic Medical Center, Amsterdam (62); R. Tobon Morales, St. Jansdal Hospital, Harderwijk, and Helen Westenberg, Arnhem Radiotherapy Institution, Arnhem (46); J. Klinkenbijl, Rijnstate Hospital, and Helen Westenberg, ARTI, Arnhem (228); H. van der Mijle, Nijsmellinghe, Drachten (198); S. Veltkamp, Amstelveen Hospital, Amstelveen (114). France: M. Bolla, Centre Hospitalier Universitaire de Grenoble, Grenoble (19); Y. Belkacemi, Centre Oscar Lambret, Lille (43). Switzerland: M. Kohlik, Hopital Cantonal Universitaire Geneve, Geneve (2). Poland: J. Jassem and J. Jaskiewicz, Medical University of Gdansk, Gdansk (2). Italy: M. Mano, Ospedale San Giovanni Antica Seda, Torino (52); L. Cataliotti, Universita Degli Studi di Firenze (293). Slovenia: M. Snoj, Insitute of Oncology, Ljubljana (82). Israel: H. Goldberg, Rambam Medical Center, Haifa (2).
Table A1.
Administration of Systemic Therapy in Noncompliant Patients (n = 92)
| Adjuvant Chemotherapy | ALND Arm (n = 50) |
RT Arm (n = 42) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| No | 16 | 32.0 | 10 | 23.8 |
| Yes | 30 | 60.0 | 22 | 52.4 |
| Missing data | 4 | 8.0 | 10 | 23.8 |
Abbreviations: ALND, axillary lymph node dissection; RT, radiation therapy.
Table A2.
Type of Systemic Therapy
| Adjuvant Therapy | ALND Arm (n = 175) |
ART Arm (n = 162) |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| CMF (6×) | 14 | 8 | 8 | 5 |
| AC (4× or 6× or 2×) | 91 | 53 | 76 | 47 |
| FEC (6× or 5×) | 38 | 22 | 27 | 17 |
| CAF (6× or 5×) | 3 | 2 | 4 | 2 |
| EPI (4×) + CMF (4×) | 12 | 7 | 40 | 25 |
| Taxane based | 10 | 6 | 2 | 1 |
| Other | 7 | 4 | 4 | 2 |
| Missing | 0 | 0 | 1 | 0.6 |
Abbreviations: ALND, axillary lymph node dissection; ART, axillary radiation therapy; CMF, cyclophosphamide, methotrexate, and fluorouracil; AC, doxorubicin and cyclophosphamide; FEC, fluorouracil, epirubicin, and cyclophosphamide; CAF, cyclophosphamide, doxorubicin, and fluorouracil; EPI, epirubicine.
Footnotes
Supported by Grants No. 2U10 CA11488-28 through 5U10 CA011488-38 from the National Cancer Institute, Bethesda, MD, and by a donation from the Kankerbestrijding/ Koningin Wilhelmina Fonds from the Netherlands through the European Organisation for Research and Treatment of Cancer Charitable Trust.
Presented at the American Society of Clinical Oncology Breast Cancer Symposium, September 5, 2008, Washington, DC, and the Annual Meeting of the European Society of Surgical Oncology, September 10, 2008, The Hague, The Netherlands.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Marieke E. Straver, Geertjan van Tienhoven, Cornelis J.H. van de Velde, Robert E. Mansel, Jan Bogaerts, Gaston Demonty, Emiel J.T. Rutgers
Administrative support: Nicole Duez
Provision of study materials or patients: Geertjan van Tienhoven, Cornelis J.H. van de Velde, Robert E. Mansel, Luigi Cataliotti, Jean Klinkenbijl, Helen A. Westenberg, Huub van der Mijle, Emiel J.T. Rutgers
Collection and assembly of data: Marieke E. Straver, Philip Meijnen, Jan Bogaerts, Gaston Demonty, Nicole Duez
Data analysis and interpretation: Marieke E. Straver, Jan Bogaerts, Gaston Demonty, Emiel J.T. Rutgers
Manuscript writing: Marieke E. Straver, Philip Meijnen, Geertjan van Tienhoven, Jan Bogaerts, Gaston Demonty, Helen A. Westenberg, Coen Hurkmans, Emiel J.T. Rutgers
Final approval of manuscript: Marieke E. Straver, Philip Meijnen, Geertjan van Tienhoven, Cornelis J.H. van de Velde, Robert E. Mansel, Jan Bogaerts, Gaston Demonty, Nicole Duez, Luigi Cataliotti, Jean Klinkenbijl, Helen A. Westenberg, Huub van der Mijle, Coen Hurkmans, Emiel J.T. Rutgers
REFERENCES
- 1.Carter CL, Allen C, Henson DE. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer. 1989;63:181–187. doi: 10.1002/1097-0142(19890101)63:1<181::aid-cncr2820630129>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 2.Orr RK. The impact of prophylactic axillary node dissection on breast cancer survival: A Bayesian meta-analysis. Ann Surg Oncol. 1999;6:109–116. doi: 10.1007/s10434-999-0109-1. [DOI] [PubMed] [Google Scholar]
- 3.Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: The ALMANAC Trial. J Natl Cancer Inst. 2006;98:599–609. doi: 10.1093/jnci/djj158. [DOI] [PubMed] [Google Scholar]
- 4.Olson JA, Jr, McCall LM, Beitsch P, et al. Impact of immediate versus delayed axillary node dissection on surgical outcomes in breast cancer patients with positive sentinel nodes: Results from American College of Surgeons Oncology Group Trials Z0010 and Z0011. J Clin Oncol. 2008;26:3530–3535. doi: 10.1200/JCO.2007.15.5630. [DOI] [PubMed] [Google Scholar]
- 5.Petrek JA, Heelan MC. Incidence of breast carcinoma-related lymphedema. Cancer. 1998;83:2776–2781. doi: 10.1002/(sici)1097-0142(19981215)83:12b+<2776::aid-cncr25>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 6.Cancer research campaign (King's/Cambridge) trial for early breast cancer. A detailed update at the tenth year. Cancer Research Campaign Working Party. Lancet. 1980;2:55–60. [PubMed] [Google Scholar]
- 7.Bruce J. Operable cancer of the breast. A controlled clinical trial. Cancer. 1971;28:1443–1452. doi: 10.1002/1097-0142(197112)28:6<1443::aid-cncr2820280617>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 8.Forrest AP, Roberts MM, Preece P, et al. The Cardiff-St Mary's trial. Br J Surg. 1974;61:766–769. doi: 10.1002/bjs.1800611005. [DOI] [PubMed] [Google Scholar]
- 9.Hayward JL. New surgical and radiotherapeutic techniques. In: Harris, et al., editors. Philadelphia, PA: JB Lippincott Company; 1983. [Google Scholar]
- 10.Langlands AO, Prescott RJ, Hamilton T. A clinical trial in the management of operable cancer of the breast. Br J Surg. 1980;67:170–174. doi: 10.1002/bjs.1800670304. [DOI] [PubMed] [Google Scholar]
- 11.Lythgoe JP, Palmer MK. Manchester regional breast study: 5 and 10 year results. Br J Surg. 1982;69:693–696. doi: 10.1002/bjs.1800691202. [DOI] [PubMed] [Google Scholar]
- 12.Fisher B, Jeong JH, Anderson S, et al. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med. 2002;347:567–575. doi: 10.1056/NEJMoa020128. [DOI] [PubMed] [Google Scholar]
- 13.Sobin LH, Wittekind C, editors. International Union Against Cancer. TNM Classification of Malignant Tumours. ed 6. New York, NY: Wiley-Blackwell; 2008. [Google Scholar]
- 14.Hurkmans CW, Borger JH, Rutgers EJ, et al. Quality assurance of axillary radiotherapy in the EORTC AMAROS trial 10981/22023: The dummy run. Radiother Oncol. 2003;68:233–240. doi: 10.1016/s0167-8140(03)00194-4. [DOI] [PubMed] [Google Scholar]
- 15.Rutgers EJ, Meijnen P, Bonnefoi H. Clinical trials update of the European Organization for Research and Treatment of Cancer Breast Cancer Group. Breast Cancer Res. 2004;6:165–169. doi: 10.1186/bcr906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mook S, Schmidt MK, Viale G, et al. The 70-gene prognosis-signature predicts disease outcome in breast cancer patients with 1-3 positive lymph nodes in an independent validation study. Breast Cancer Res Treat. 2009;116:295–302. doi: 10.1007/s10549-008-0130-2. [DOI] [PubMed] [Google Scholar]
- 17.Ravdin PM, Siminoff LA, Davis GJ, et al. Computer program to assist in making decisions about adjuvant therapy for women with early breast cancer. J Clin Oncol. 2001;19:980–991. doi: 10.1200/JCO.2001.19.4.980. [DOI] [PubMed] [Google Scholar]
- 18.Olivotto IA, Bajdik CD, Ravdin PM, et al. Population-based validation of the prognostic model ADJUVANT! for early breast cancer. J Clin Oncol. 2005;23:2716–2725. doi: 10.1200/JCO.2005.06.178. [DOI] [PubMed] [Google Scholar]
- 19.Buyse M, Loi S, van't Veer L, et al. Validation and clinical utility of a 70-gene prognostic signature for women with node-negative breast cancer. J Natl Cancer Inst. 2006;98:1183–1192. doi: 10.1093/jnci/djj329. [DOI] [PubMed] [Google Scholar]
- 20.Eifel P, Axelson JA, Costa J, et al. National Institutes of Health Consensus Development Conference Statement: Adjuvant therapy for breast cancer, November 1-3, 2000. J Natl Cancer Inst. 2001;93:979–989. doi: 10.1093/jnci/93.13.979. [DOI] [PubMed] [Google Scholar]
- 21.Goldhirsch A, Wood WC, Gelber RD, et al. Progress and promise: Highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann Oncol. 2007;18:1133–1144. doi: 10.1093/annonc/mdm271. [DOI] [PubMed] [Google Scholar]
- 22.Fisher B, Ravdin RG, Ausman RK, et al. Surgical adjuvant chemotherapy in cancer of the breast: Results of a decade of cooperative investigation. Ann Surg. 1968;168:337–356. doi: 10.1097/00000658-196809000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 24.Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. doi: 10.1056/NEJMoa041588. [DOI] [PubMed] [Google Scholar]
- 25.van't Veer LJ, Dai H, van de Vijver MJ, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. doi: 10.1038/415530a. [DOI] [PubMed] [Google Scholar]
- 26.Cardoso F, van't Veer L, Rutgers E, et al. Clinical application of the 70-gene profile: The MINDACT trial. J Clin Oncol. 2008;26:729–735. doi: 10.1200/JCO.2007.14.3222. [DOI] [PubMed] [Google Scholar]
- 27.Ragaz J, Olivotto IA, Spinelli JJ, et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-Year results of the British Columbia randomized trial. J Natl Cancer Inst. 2005;97:116–126. doi: 10.1093/jnci/djh297. [DOI] [PubMed] [Google Scholar]
- 28.Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med. 1997;337:949–955. doi: 10.1056/NEJM199710023371401. [DOI] [PubMed] [Google Scholar]
- 29.Overgaard M, Jensen MB, Overgaard J, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet. 1999;353:1641–1648. doi: 10.1016/S0140-6736(98)09201-0. [DOI] [PubMed] [Google Scholar]
- 30.Kunkler IH, Canney P, van Tienhoven G, et al. Elucidating the role of chest wall irradiation in ‘intermediate-risk’ breast cancer: The MRC/EORTC SUPREMO trial. Clin Oncol (R Coll Radiol) 2008;20:31–34. doi: 10.1016/j.clon.2007.10.004. [DOI] [PubMed] [Google Scholar]