Abstract
Purpose
To evaluate the safety, maximum-tolerated dose (MTD), pharmacokinetics (PKs), pharmacodynamics, and preliminary anticancer activity of ramucirumab (IMC-1121B), a fully human immunoglobulin G1 monoclonal antibody targeting the vascular endothelial growth factor receptor (VEGFR)-2.
Patients and Methods
Patients with advanced solid malignancies were treated once weekly with escalating doses of ramucirumab. Blood was sampled for PK studies throughout treatment. The effects of ramucirumab on circulating vascular endothelial growth factor-A (VEGF-A), soluble VEGFR-1 and VEGFR-2, tumor perfusion, and vascularity using dynamic contrast-enhanced magnetic resonance imaging were assessed.
Results
Thirty-seven patients were treated with 2 to 16 mg/kg of ramucirumab. After one patient each developed dose-limiting hypertension and deep venous thrombosis at 16 mg/kg, the next lower dose (13 mg/kg) was considered the MTD. Nausea, vomiting, headache, fatigue, and proteinuria were also noted. Four (15%) of 27 patients with measurable disease had a partial response (PR), and 11 (30%) of 37 patients had either a PR or stable disease lasting at least 6 months. PKs were characterized by dose-dependent elimination and nonlinear exposure consistent with saturable clearance. Mean trough concentrations exceeded biologically relevant target levels throughout treatment at all dose levels. Serum VEGF-A increased 1.5 to 3.5 times above pretreatment values and remained in this range throughout treatment at all dose levels. Tumor perfusion and vascularity decreased in 69% of evaluable patients.
Conclusion
Objective antitumor activity and antiangiogenic effects were observed over a wide range of dose levels, suggesting that ramucirumab may have a favorable therapeutic index in treating malignancies amenable to VEGFR-2 inhibition.
INTRODUCTION
Angiogenesis is regulated principally by interactions between vascular endothelial growth factors (VEGFs) and VEGF receptors (VEGFRs) and plays a key role in cancer growth and metastasis.1–5 VEGF-A is the central regulator of tumor angiogenesis, endothelial proliferation, permeability, and survival.1,6,7 VEGF-A binds with high affinity to two structurally similar tyrosine kinase receptors, VEGFR-1 and VEGFR-2, both expressed on tumor vasculature.8,9 Blockade of the VEGF-A/VEGFR-2 interaction inhibits tumor angiogenesis and growth in preclinical studies and is a promising approach to anticancer treatment.10–20
Few anticancer therapeutics that directly and specifically inhibit VEGFR-2 have been evaluated.21,22 Ramucirumab (IMC-1121B; ImClone Systems, New York, NY) is a fully human immunoglobulin G1 monoclonal antibody (MAb) that binds with high affinity (approximately 50 pM) to the extracellular VEGF-binding domain of VEGFR-2. Both ramucirumab and its murine version, DC-101, were designed to bind to a VEGFR-2 epitope involved in ligand binding and block VEGF ligands from binding this site and activating the receptor.23,24 Inhibition of VEGF-stimulated VEGFR-2 activation by ramucirumab or DC-101 conferred significant antitumor activity in a range of malignancies in animal models as single agents and in combination with other therapeutics.25–28 In nonclinical toxicology studies, ramucirumab was well tolerated, and a no observable effect level was not established.
The principal objectives of the present study were to establish the safety profile and maximum-tolerated dose (MTD) of ramucirumab administered weekly to patients with advanced solid malignancies; characterize the pharmacokinetics (PK), immunogenicity, and pharmacodynamic (PD) effects on serum VEGF-A, soluble (s) VEGFR-1, and sVEGFR-2; assess changes in tumor perfusion and vascularity evaluated by dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI); and preliminarily evaluate antitumor activity.
PATIENTS AND METHODS
Patient Selection
Patients with advanced solid malignancies refractory to treatment or lacking standard therapeutic options were eligible. Other eligibility criteria included the following: age ≥ 18 years; adequate hematologic, hepatic, and renal function; and an Eastern Cooperative Oncology Group performance status ≤ 2. Key exclusion criteria were as follows: centrally located pulmonary lesions adjacent to or invading large blood vessels as assessed by the investigator; serious nonhealing active wound, ulcer, or bone fracture; deep venous thrombosis (DVT) within 6 months of entry; proteinuria ≥ 1+; prior left chest wall radiotherapy; anthracycline dose ≥ 300 mg/m2 with abnormal left ventricular ejection fraction; prior treatment with VEGF or VEGFR inhibitors or any MAb (amended to within 6 weeks of entry); hypersensitivity reaction to a therapeutic protein; and use of thrombolytic agents, full-dose heparin or warfarin, aspirin, and nonsteroidal anti-inflammatory drugs. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and Good Clinical Practice. The protocol was approved by the institutional review boards of the participating institutions. Written informed consent was obtained in accordance with federal and institutional guidelines.
Study Design
Ramucirumab was administered at escalating doses as a 1-hour intravenous infusion at a rate of ≤ 25 mg/min. Cycles consisted of four weekly ramucirumab infusions followed in the first cycle by a 2-week, treatment-free PK sampling period (eliminated in an amendment after preliminary PK assessment).
The initial ramucirumab dose level (2 mg/kg) was based on the results of PK and toxicology studies in nonhuman primates. The dose was increased sequentially by 100% (4 mg/kg), 50% (6 mg/kg), and 33% (8 mg/kg); thereafter, the dose escalation increment was fixed at 25% with no intrapatient dose escalation. Dose level assignment used a standard 3 + 3 design with at least three evaluable patients per cohort. If no dose-limiting toxicities (DLTs) occurred during cycle 1, three new patients were treated at the next higher level.
The MTD was defined as the highest dose level at which less than two patients experienced a DLT in cycle 1. DLT was defined as any grade 4 neutropenia lasting more than 7 days; grade ≥ 3 febrile neutropenia, thrombocytopenia, or anemia; or any grade ≥ 3 nonhematologic toxicity considered drug related by the investigator. Up to two dose reductions per patient were permitted. Toxicity was graded using the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3).
Ramucirumab was supplied by ImClone Systems in 20-mL glass vials containing 100 mg of product formulated at a concentration of 5.0 mg/mL in phosphate-buffered saline. The total dose was calculated based on body weight.
Pretreatment and Follow-Up Studies
Clinical and laboratory evaluations (CBC, serum chemistries, and urinalysis) were performed before treatment, weekly in cycles 1 and 2, and every other week thereafter. An ECG was obtained before treatment. Disease assessments by modified Response Evaluation Criteria in Solid Tumors (RECIST) were performed before treatment, after the 2-week treatment-free period in cycle 1, and every other cycle thereafter. Patients continued treatment until disease progression or intolerable toxicity.
Serum PK Sampling and Assay
In preclinical xenograft studies, ramucirumab serum concentrations ≥ 20 μg/mL were associated with anticancer activity; accordingly, PK analyses were directed toward identifying doses for subsequent studies capable of producing trough concentrations more than 20 μg/mL.
To measure ramucirumab serum concentrations, heparinized blood samples were collected before, immediately after, and at 0.5, 1, 2, 4, 8, 24, 48, 96, and 168 hours after the first and final infusion in C1; 264 and 336 hours after the final infusion in C1; before and 1 hour after the final infusion in each cycle; at study completion; and on the 45-day follow-up visit, whenever possible. A validated bridging enzyme-linked immunosorbent assay measured ramucirumab serum concentrations. The assay was linear for ramucirumab in the range of 1 ng/mL (lower limit of quantitation) to 200 ng/mL (upper limit of quantitation), with an inter- and intra-assay precision of coefficient of variation of ≤ 10%. Bioanalytical assay methods are provided in the Appendix (online only). Noncompartmental PK analyses were performed on samples obtained in cycle 1 using WinNonlin software version 3.3 (Pharsight, Mountain View, CA).
PD Studies
Circulating concentrations of VEGF-A, sVEGFR-1, and sVEGFR-2 were measured from blood samples obtained after the first ramucirumab infusion in cycle 1 and before and 1 hour after the last infusion in subsequent cycles. PD markers were measured using commercial enzyme-linked immunosorbent assay kits (R&D Systems, Minneapolis, MN). Further details are provided in the Appendix.
Detection of Human Antihuman Antibodies to Ramucirumab
Blood sampling to detect human antihuman antibodies to ramucirumab was performed before the last infusion of each cycle, at the end of therapy, and at the 45-day follow-up visit. Analytic methods are provided in the Appendix.
DCE-MRI
DCE-MRI was performed in patients with appropriate liver lesions to minimize methodologic variability. Specific image acquisition protocols are provided in the Appendix.
Perceptive Informatics (Waltham, MA) performed postprocessing kinetic modeling using the compartmental Tofts model for calculating the following three quantitative end points: initial area under the signal-intensity curve for the first 60 seconds after gadolinium injection, uptake rate constant as a volume transfer constant between blood and extravascular extracellular space, and volume of extravascular extracellular space for interstitial leakage space.29 Results of DCE-MRI were pooled with data obtained from another phase I study of ramucirumab administered once every 2 to 3 weeks30; studies had identical entry criteria.
RESULTS
Patient Demographics and Treatment
Thirty-seven patients, whose relevant demographic and disease characteristics are listed in Table 1, received 888 total infusions of ramucirumab, given weekly at seven dose levels ranging from 2 to 16 mg/kg. The median number of infusions administered was 11 (range, one to 141 infusions). The dose-escalation scheme, numbers of cycles, and DLTs in cycle 1 by dose level are listed in Table 2. At the time of data analysis for this publication, one patient (16 mg/kg) was receiving treatment after 85 weeks.
Table 1.
Patient Demographics and Clinical Characteristics
| Demographic or Clinical Characteristic | No. of Patients (N = 37) |
|---|---|
| Age, years | |
| Median | 56 |
| Range | 36-76 |
| ECOG performance status | |
| 0 | 11 |
| 1 | 26 |
| Sex | |
| Male | 23 |
| Female | 14 |
| Previous therapy | |
| Chemotherapy | 30 |
| Radiotherapy | 20 |
| Hormonal | 7 |
| VEGF-targeting agents (antiligand, TKI) | 5 |
| Immunotherapy | 2 |
| Investigational agent | 15 |
| Other | 12 |
| Primary malignancy | |
| Colorectal | 6 |
| Head and neck | 4 |
| Hepatocellular | 3 |
| Pancreas | 3 |
| Carcinoid, esophagus, neuroendocrine, ovary, prostate, melanoma, stomach, uterine (leiomyosarcoma) | 16* |
| Bladder, breast, thyroid (medullary), thyroid (papillary), biliary tract | 5† |
| Tumor response assessment | |
| Measurable disease | 27 |
| Evaluable disease | 10 |
| Prostate | 2 |
| Neuroendocrine | 2 |
| Esophagus | 2 |
| Liver | 1 |
| Colorectal | 1 |
| Thyroid | 1 |
| Melanoma | 1 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; VEGF, vascular endothelial growth factor; TKI, tyrosine kinase inhibitor.
Two patients had each of the malignancies listed.
One patient had each of the malignancies listed.
Table 2.
Dose-Escalation Scheme
| Cohort No. | Ramucirumab Dose Level (mg/kg) | No. of New Patients | No. of Doses |
No. of Patients With DLT (cycle 1) | |
|---|---|---|---|---|---|
| Median | Range | ||||
| 1 | 2 | 6 | 5 | 1-26 | 0 |
| 2 | 4 | 4 | 29.5 | 28-105 | 0 |
| 3 | 6 | 4 | 16.5 | 4-99 | 0 |
| 4 | 8 | 5 | 22 | 2-141 | 0 |
| 5 | 10 | 7 | 11 | 4-34 | 1* |
| 6 | 13 | 5 | 16 | 5-69 | 0 |
| 7 | 16 | 6 | 4 | 1-81 | 2† |
Abbreviation: DLT, dose-limiting toxicity.
Grade 3 hypertension.
One patient with grade 3 deep venous thrombosis and one patient with grade 3 hypertension in cycle 2 (after protocol-defined DLT window).
Toxicity
Two patients experienced DLTs in cycle 1. The first DLT occurred in a patient who developed grade 3 hypertension after receiving the fourth dose of ramucirumab (10 mg/kg), resulting in expansion of this dose level without further DLTs. This event resolved rapidly with antihypertensive medication; however, the patient developed disease progression and was taken off study. The second DLT in cycle 1 was a DVT that occurred in a patient after the fourth 16 mg/kg dose of ramucirumab. Although this event resolved within 2 weeks during treatment suspension, the patient discontinued treatment for disease progression. Another patient treated with ramucirumab 16 mg/kg developed grade 3 hypertension after the first dose of cycle 2. Although this particular DLT was not considered preclusive for dose escalation because the event occurred after cycle 1, its temporal proximity to cycle 1, together with the other cycle 1 DLT, contributed to the determination of 13 mg/kg as the MTD of ramucirumab on a weekly schedule.
Twenty-two patients (60%) experienced grade 3 to 5 adverse events, irrespective of potential drug-related attribution. The most frequently reported serious events included hypertension (13.5%), abdominal pain (10.8%), and anorexia, vomiting, increased blood alkaline phosphatase, headache, proteinuria, dyspnea, and DVT (each in 5.4% of patients).
Hypertension seemed to be related to both dose and cumulative therapy at higher doses. Five patients experienced grade 3 hypertension at doses ranging from 8 to 16 mg/kg. Hypertension was generally noted in the peritreatment period and occasionally associated with other symptoms, particularly headache. Hypertension generally resolved after administration of common oral antihypertensive medications, enabling further ramucirumab treatment. However, a patient with a history of sunitinib-related hypertension developed grade 3 hypertension and headache after receiving two 8 mg/kg doses. Hypertension did not respond to antihypertensives, eventually resulting in study discontinuation and resolution of events.
Other DLTs included grade 3 proteinuria in cycle 7 (4 mg/kg) and grade 3 vomiting in cycle 2 (2 mg/kg). The most frequently reported (≥ 25% of all patients) adverse events, regardless of relation to ramucirumab, included fatigue (51.4%), headache (51.4%), peripheral edema (35.1%), diarrhea (35.1%), nausea (32.4%), upper respiratory tract infection (32.4%), abdominal pain (29.7%), anorexia (29.7%), constipation (29.7%), epistaxis (29.7%), proteinuria (29.7%), arthralgia (27.0%), cough (27.0%), and dyspnea (27.0%).
PKs
Mean PK parameters derived from noncompartmental analyses of the ramucirumab concentration versus time data after the first and final doses in cycle 1 are listed in Table 3. The clearance rate of ramucirumab decreased disproportionately as dose increased from 2 to 16 mg/kg. This nonlinear effect, suggesting saturation of ramucirumab clearance mechanism(s), was less evident at doses exceeding 8 mg/kg. After the initial and final infusions in cycle 1, clearance rate decreased in a manner suggesting that the steady-state was being approached. Furthermore, maximum concentration and area under the concentration-time curve from zero extrapolated to infinity, after the first and last infusions of ramucirumab in cycle 1, and half-life values increased disproportionately as dose increased from 2 to 16 mg/kg. Ramucirumab half-life at steady-state ranged from approximately 200 to 300 hours at doses of 8 to 16 mg/kg. A large interpatient variability was also noted with respect to PK parameters.
Table 3.
Noncompartmental Ramucirumab PK Parameters
| Dose Level and Cycle 1 Infusion | No. of Patients | Half-Life (hours) |
PK Parameters in Cycle 1* |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Trough Cmin (μg/mL) |
Cmax (μg/mL) |
AUC0-∞ (h · μg/mL) |
Cl (mL/h/kg) |
||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| 2 mg/kg | |||||||||||
| First infusion | 6 | 68.4 | 10.7 | 6.83 | 1.60 | 43.7 | 6.71 | 3,914 | 534 | 0.519 | 0.075 |
| Last infusion | 3 | 104 | 22.0 | 104 | 21.8 | 21.3 | 7.37 | 78 | 27 | 0.222 | 0.076 |
| 4 mg/kg | |||||||||||
| First infusion | 6 | 92.5 | 34.5 | 18.8 | 10.7 | 80.3 | 11.7 | 9,143 | 4,192 | 0.508 | 0.220 |
| Last infusion | 4 | 200 | 51.1 | 55.5 | 24.6 | 155 | 77.7 | 25,839 | 9,174 | 0.179 | 0.092 |
| 6 mg/kg | |||||||||||
| First infusion | 4 | 86.8 | 22.4 | 41.7 | 1.53 | 183 | 25.3 | 19,099 | 2,331 | 0.318 | 0.0.041 |
| Last infusion | 4 | 136 | 89.6 | 118 | 26.3 | 364 | 124 | 45,850 | 15,040 | 0.140 | 0.045 |
| 8 mg/kg | |||||||||||
| First infusion | 5 | 123 | 34.9 | 93.4 | 21.0 | 325 | 62.2 | 43,824 | 10,341 | 0.190 | 0.038 |
| Last infusion | 5 | 318 | 140 | 176 | 57.9 | 497 | 168 | 132,789 | 47,746 | 0.067 | 0.025 |
| 10 mg/kg | |||||||||||
| First infusion | 7 | 110 | 30.4 | 166 | 138 | 406 | 152 | 40,333 | 9,134 | 0.264 | 0.085 |
| Last infusion | 7 | 205 | 61.3 | 313 | 157 | 616 | 100 | 156,840 | 49,016 | 0.069 | 0.022 |
| 13 mg/kg | |||||||||||
| First infusion | 5 | 90.1 | 25.2 | 103 | 44.0 | 429 | 134 | 45,710 | 25,058 | 0.399 | 0.275 |
| Last infusion | 5 | 300 | 136 | 328 | 73.8 | 883 | 225 | 236,290 | 89,906 | 0.061 | 0.019 |
| 16 mg/kg | |||||||||||
| First infusion | 6 | 120 | 34.7 | 177 | 42.1 | 558 | 132 | 67,871 | 13,361 | 0.245 | 0.059 |
| Last infusion | 5 | 283 | 87.0 | 280 | 98.1 | 934 | 508 | 190,592 | 68,357 | 0.096 | 0.046 |
Abbreviations: PK, pharmacokinetic; Cmin, minimum concentration; Cmax, maximum concentration; AUC0-∞, area under the concentration-time curve from zero extrapolated to infinity; Cl, clearance; SD, standard deviation.
All PK parameters (first infusion) were calculated over 168 hours after the first infusion in cycle 1. All PK parameters (final infusion) were calculated over 336 hours after the final infusion in cycle 1 except Cmin, which was calculated 168 hours after the final infusion of cycle 1.
Mean ramucirumab trough concentrations (Cmin) were measured 7 days after treatment. In cycle 1, target Cmin values (≥ 20 μg/mL) were achieved at all doses, except for some patients after their first infusion at 2 to 4 mg/kg. Mean Cmin and maximum concentration values as functions of ramucirumab dose and cumulative cycle number are shown in Figures 1A and 1B. Cmin values measured in cycle 2 to cycle 32 averaged 29, 105, 222, 247, 353, 369, and 519 μg/mL after treatment with ramucirumab doses of 2, 4, 6, 8, 10, 13, and 16 mg/kg, respectively. There was negligible accumulation evident over at least 32 cycles of treatment (Figs 1A and 1B).
Fig 1.
Scatterplots depicting mean values for (A) maximum concentration and (B) minimum concentration as a function of cycle number and at various ramucirumab dose levels (error bars indicate standard deviations).
PDs
Serum biomarkers.
The effects of ramucirumab on serum concentrations of VEGF-A, sVEGFR-2, and sVEGFR-1 after the first infusion in cycle 1 are shown in Figures 2A to 2F. Serum VEGF-A concentrations increased almost immediately after treatment, remaining elevated until the next treatment. At 7 days after treatment, VEGF-A concentrations were 1.5- to 3.5-fold higher than pretreatment concentrations. VEGF-A levels remained elevated through extended cycles (months for several patients) as long as ramucirumab was present (data not shown). Acute post-treatment elevations in VEGF-A concentrations were observed in all patients; the magnitude of this effect did not seem to be dose related. In contrast to VEGF-A concentrations, sVEGFR-1 and sVEGFR-2 concentrations generally decreased immediately after ramucirumab treatment and recovered to near-pretreatment levels. Similar to VEGF-A, the effects on sVEGFR-1 and sVEGFR-2 were not related to dose.
Fig 2.
Scatterplots depicting (A, C, and E) raw data and (B, D, and F) mean percentage changes from pretreatment values in the following pharmacodynamic assessments over time after first infusion of ramucirumab: (A and B) serum vascular endothelial growth factor (VEGF)-A; (C and D) serum soluble VEGF receptor (sVEGFR)-1; and (E and F) serum sVEGFR-2.
DCE-MRI.
Post-treatment changes in tumor perfusion and vascularity were assessed using DCE-MRI parameters (initial area under the signal-intensity curve, volume of extravascular extracellular space, and uptake rate constant). Twenty-two patients had sequential DCE-MRI performed. Kinetic modeling was completed for 13 patients (Appendix Table A1, online only).
Sequential DCE-MRI demonstrated decreased tumor perfusion and vascularity in nine (69%) of 13 patients, including five of seven patients in this study and four of six patients in the study with dosing every 2 to 3 weeks. A representative sequential DCE-MRI, demonstrating decreased perfusion and permeability in a liver metastasis after treatment with ramucirumab, is depicted in Appendix Figure A3 (online only). In patients for whom more than one tumor was assessed, decrements in tumor perfusion and vascularity consistently occurred in all target lesions.
Detection of Antibodies to Ramucirumab
Twenty-six patients with pre- and post-treatment samples were evaluated for development of antiramucirumab antibodies; none were detected.
Anticancer Activity
Twenty-seven patients had measurable disease, and 10 patients had evaluable disease. Table 4 lists the characteristics of patients who experienced confirmed partial response (PR) or stable disease (SD) lasting at least 12 weeks. Four patients, 11% of all patients and 15% of patients with measurable disease, had confirmed PRs, including two patients treated at 4 mg/kg (melanoma and gastric carcinoma, with PR lasting 31 and 103 weeks, respectively) and one patient each treated at 13 mg/kg (uterine leiomyosarcoma, with PR lasting 70 weeks) and 16 mg/kg (ovarian carcinoma, with PR lasting 86+ weeks). All patients received multiple prior therapies. Of 23 patients (62%) with SD as their best response, 11 patients with measurable disease had reductions in the sum of target lesions. Eleven patients (30%) experienced a confirmed PR or SD lasting ≥ 24 weeks across a range of doses. Other preliminary evidence of potential clinical benefit includes substantial reduction in tumor pain with reduced analgesic requirements (four patients) and significant reduction in refractory pleural effusions and frequency of palliative thoracenteses in a patient with papillary thyroid carcinoma.
Table 4.
Patients With PR and SD (≥ 12 weeks)
| Dose Level and Primary Malignancy | Best Response (≥ 12 weeks) | Duration of Response (weeks) |
|---|---|---|
| 2 mg/kg | ||
| Colorectal | SD | 30 |
| Breast | SD | 15 |
| Ovarian | SD | 15 |
| 4 mg/kg | ||
| Melanoma | PR | 31 |
| Gastric | PR | 103 |
| Colorectal | SD | 31 |
| 6 mg/kg | ||
| Head and neck | SD | 105 |
| Head and neck | SD | 29 |
| 8 mg/kg | ||
| Neuroendocrine (nasopharynx) | SD | 151 |
| Prostate | SD | 41 |
| Cholangiocarcinoma | SD | 21 |
| 10 mg/kg | ||
| Pancreatic | SD | 37 |
| Colorectal | SD | 15* |
| Prostate | SD | 14 |
| Uterine leiomyosarcoma | SD | 13 |
| 13 mg/kg | ||
| Head and neck | SD | 21 |
| Uterine leiomyosarcoma | PR | 70 |
| Neuroendocrine | SD | 23 |
| 16 mg/kg | ||
| Ovarian | PR | 86+* |
Abbreviations: PR, partial response; SD, stable disease.
Patient received prior anti vascular endothelial growth factor therapy.
DISCUSSION
In contrast to other agents directed against the VEGFR-2/VEGF axis, ramucirumab binds a specific epitope on the extracellular domain of VEGFR-2, thereby blocking all VEGF ligands from binding to this therapeutically validated target.23 Although a number of tyrosine kinas inhibitors are being used, their biochemical promiscuity and potential for off-target toxicities present potential limitations in cancer therapy. Because ramucirumab binds to VEGFR-2 specifically and with high affinity, it may offer a rational modulation advantage. Moreover, in contrast to bevacizumab, which binds to VEGF-A only, ramucirumab blocks all known VEGFs from binding to VEGFR-2. The combined effects of high specificity and more complete target inhibition could lead to a more complete blockade of angiogenesis.
In the present study, ramucirumab was well tolerated as a weekly 1-hour infusion. The principal toxicities encountered, including hypertension, vascular thrombotic events, and proteinuria, were similar qualitatively to those observed with other therapeutics targeting the VEGFR-2/VEGF axis.31–41 Although some serious adverse effects occurred early at the 16 mg/kg dose, these events were uncommon and did not hinder ramucirumab administration for protracted periods. On the basis of the development of grade 3 hypertension associated with severe headache and a grade 3 DVT early into treatment at 16 mg/kg, the MTD on a weekly schedule was determined to be 13 mg/kg.
The ramucirumab PK profile was characterized by dose-dependent elimination and nonlinear exposure consistent with saturable clearance, similar to PK profiles exhibited by other antireceptor antibodies.42–44 The large interindividual variability in PK parameters may be a result of the limited number of patients or unknown host effects. The ramucirumab PK profile served as a strong translational tool from preclinical experiments, supporting the notion that weekly doses are biologically relevant. The minimal target trough level (≥ 20 μg/mL), selected a priori based on PD and efficacy data in human tumor xenografts implanted in mice, was achieved in all treated patients (Fig 1B).
The primary elimination pathway for ramucirumab is likely receptor-mediated clearance; although target trough levels were reached at all doses, if drug clearance is rapid or not saturated, patients could have unblocked VEGFR-2 available to bind circulating VEGF and support tumor vascularity and growth. At doses more than 8 mg/kg, clearance is saturated, leading to a high probability that all VEGFR-2s are blocked by ramucirumab.
The affinity of ramucirumab for the VEGF-binding epitope on the extracellular domain of VEGFR-2 (dissociation constant = 50 pM) is much higher than the natural VEGF-A ligand.6,26 Therefore, VEGF-A concentrations would be expected to increase after treatment as a result of displacement of the receptor-bound natural VEGF-A ligand. Indeed, VEGF-A concentrations increased 1.5- to 3.5-fold across dose levels, particularly evident at doses ≥ 8 mg/kg; such elevations were sustained for protracted periods, likely because of the slow clearance of ramucirumab. The magnitude and duration of VEGF-A elevations after ramucirumab treatment may be useful PD indices to gauge adequacy of VEGFR-2 blockade. Some assay interference caused by the presence of ramucirumab was noted; however, the net effect decreased the sensitivity of the biomarker assay, suggesting that the results reflect a somewhat conservative presentation of the presence of these biomarkers. Early decrements in sVEGFR-1 and sVEGFR-2 receptors were also observed. These effects may be a result of ramucirumab binding to sVEGFR-2 and/or depletion of circulating VEGF-A binding sites as a result of the avid binding of both VEGFR-2 and sVEGFR-2 by ramucirumab. The magnitude of these effects, particularly the degree of elevated VEGF-A concentrations, did not seem to be dose related. These observations complement the results of PK studies in which ramucirumab target concentrations were exceeded at all dose levels, coupled with evidence of anticancer activity. The consistent and robust decrements in tumor perfusion and vascularity observed provide further preliminary support that the ramucirumab doses evaluated in this study, regardless of the small number of patients, are biologically relevant. However, it would not be prudent to relate these biomarker changes to anticancer activity because of the wide dosing range, the heterogeneous nature of patients with regard to tumor type and prior therapy, and the small patient population.
The apparent disease control rate, tabulated as the percentage of patients with PR or SD after ramucirumab monotherapy, was approximately 73%. Four patients had confirmed PRs with several types of refractory malignancies. Furthermore, 11 patients (30%) with an array of primary tumor types and dose ranges (2 to 16 mg/kg) experienced either PR or SD lasting ≥ 6 months.
These results provide proof that VEGFR-2 blockade with an MAb may be an effective anticancer strategy. Although ramucirumab is well tolerated at the MTD of 13 mg/kg, it may be reasonable to use ramucirumab doses as low as 8 mg/kg in disease-directed evaluations for several reasons. Ramucirumab PK clearance appears to be saturated at 8 mg/kg, suggesting receptor saturation because receptor-mediated clearance is likely the predominant clearance mechanism. Ramucirumab also has a tolerable safety profile, trough concentrations at least an order of magnitude higher than biologically relevant targets, favorable PD effects, preliminary evidence of decreased perfusion and vascularity on DCE-MRI, and anticancer activity. On the basis of these results, subsequent disease-directed development of ramucirumab is clearly warranted.
Acknowledgment
We are grateful to the patients who participated in this trial, the physicians who enrolled them, and the many clinical research associates, nurses, and data managers for their help in patient recruitment, data management, and patient care during the conduct of this trial.
Appendix
Full Eligibility Criteria
Patients ≥ 18 years old with advanced solid malignancies refractory to or lacking standard therapy were eligible to participate. Other eligibility criteria included the following: life expectancy ≥ 3 months; adequate hematologic, hepatic, and renal function (absolute neutrophils ≥ 1,500/μL, hemoglobin ≥ 10 gm/dL, platelets ≥ 100,000/μL, total bilirubin ≤ 1.5× upper limit of normal [ULN], AST and ALT ≤ 2.5× ULN or ≤ 5× ULN if known liver metastases, and serum creatinine ≤ 1.5× ULN); Eastern Cooperative Oncology Group performance status of 0 to 2; and chemotherapy, radiation therapy, or surgery completion ≥ 28 days from enrollment. Exclusion criteria included the following: large, centrally located pulmonary lesions adjacent to or invading large blood vessels; nonhealing wound; ulcer or fracture; history or evidence of thrombosis within 6 months of study entry; current or recent use of thrombolytic agent, full-dose warfarin, heparin, aspirin (> 325 mg/d) or nonsteroidal anti-inflammatory; and/or major surgical procedure, open biopsy, or injury within 28 days of study treatment. Other exclusion criteria included patients with proteinuria ≥ 1+, concurrent active malignancy, prior left chest radiotherapy or cumulative anthracycline dose of ≥ 300 mg/m2, prior exposure to bevacizumab or other agents targeting the vascular endothelial growth factor (VEGF) ligand or receptor, other monoclonal antibody within 6 weeks of study entry, or a history of allergic reactions to monoclonal antibodies. The study was approved by the institutional review boards of participating institutions. Patients gave written informed consent according to federal and institutional guidelines.
Pretreatment and Follow-Up Studies
Clinical and laboratory evaluations (CBC, electrolytes, blood urea nitrogen, creatinine, glucose, total protein, albumin, calcium phosphate, uric acid, lactate dehydrogenase, total cholesterol, alkaline phosphatase, total and direct bilirubin, transaminases, clotting times, and urinalysis) were performed before treatment and weekly for cycles 1 and 2 and every other week thereafter. Patients had a baseline ECG. Creatine kinase was obtained before treatment, in week 1 of cycle 2 and subsequent cycles, and at the end of therapy. Disease assessments by modified Response Evaluation Criteria in Solid Tumors (RECIST) occurred before treatment, at the end of the 2-week observation period in cycle 1, and every other cycle thereafter. Patients continued treatment in the absence of disease progression or intolerable toxicity.
The original protocol stipulated a lead-in dose of ramucirumab to be given followed by a 2-week break for pharmacokinetic (PK) sampling and safety assessments; however, this was dropped in an amendment after safety was demonstrated. Patients were replaced if they were unable to complete the initial 4-week treatment cycle and 2-week dose-limiting toxicity observation period for non–drug-related reasons.
Plasma PK Sampling and Assay
Ramucirumab PK blood samples were collected in cycle 1, before, immediately after, and at 0.5, 1, 2, 4, 8, and 24 hours after the first (day 1) and last (day 22) ramucirumab infusions. Additional PK samples were drawn 48 hours (day 3), 96 hours (day 5), and 168 hours (day 8, before the second infusion) after the first infusion and 48, 96, 168, 264, and 336 hours after the final (day 22) ramucirumab infusion of cycle 1. For cycles 2 and beyond, blood samples were drawn before and 1 hour after the final ramucirumab infusion of each treatment cycle (day 22). Where possible, samples were collected at study completion and at a 45-day follow-up visit.
Blood samples were collected in tubes containing no anticoagulant, and samples were allowed to clot for 30 to 60 minutes at room temperature. Samples were then centrifuged at 1,500 rpm for 15 minutes, divided equally into three aliquots, and immediately stored at −20°C to −80°C. A validated enzyme-linked immunosorbent assay was used to quantify levels of ramucirumab. Briefly, a kinase insert domain-containing receptor (KDR) protein construct was immobilized to a 96-well microtiter plate by incubating in 1× phosphate-buffered saline (PBS; pH = 7.2). Plates were then blocked with 10% horse serum in 1× PBS-0.05% Tween-20 buffer at room temperature. After washing in 1× PBS-0.05% Tween-20, ramucirumab standard, ramucirumab controls, and test samples were incubated in the wells. Ramucirumab, with a KDR binding domain, was captured by the KDR bound to the microwells. After a thorough washing of the wells to remove all unbound antibody, antihuman Fc-specific immunoglobulin G1 horseradish peroxidase conjugated antibody was added. The conjugate binds to the captured ramucirumab. Excess, unbound conjugate was removed by further washing. The bound conjugate was then visualized by adding the substrate tetramethylbenzidine, which gives a blue reaction product that turns yellow on addition of the stopping solution. The intensity of the color produced is dependent on the concentration of ramucirumab in the sample. A noncompartmental analysis was conducted on clinical trial samples to determine the pharmacokinetic parameters describing the disposition of ramucirumab after weekly dosing.
Pharmacodynamic Marker Collection and Assays
Blood samples collected before initial ramucirumab infusion, immediately after the infusion, and at 1, 4, 8, 24, 48 (day 3), 96 (day 5), and 168 (day 8) hours were assayed for circulating levels of VEGF-A, soluble VEGF receptor (sVEGFR) -1, and sVEGFR-2. Serum samples were also collected before and 1 hour after the final ramucirumab infusion of each treatment cycle.
One sample of approximately 2 to 2.5 mL of blood was collected in a PaxGene (PreAnalytiX, Franklin Lakes, NJ) tube (red top); a separate sample of approximately 2 to 2.5 mL was collected in an EDTA tube (lavender top). Tubes were then immediately inverted eight to 10 times and stored at −20°C.
Detection of Human Antihuman Antibodies to Ramucirumab
Approximately 7 mL of blood was collected in two tubes containing no anticoagulant. The tubes were allowed to clot for 30 to 60 minutes at room temperature and then centrifuged at 1,500 rpm for 15 minutes. Serum was then removed and distributed into three 2-mL aliquots and stored at −20 degrees to −80°C. Ramucirumab immunogenicity was examined using a non–species-specific, double-antigen radiometric assay. Briefly, ramucirumab was immobilized onto a polystyrene bead. Unbound antibodies were removed by washing. Serum antiramucirumab antibodies were detected by incubating a mixture of ramucirumab-coated beads and serum or diluted serum samples. Antiramucirumab antibody present in the sample attached to bead-bound ramucirumab to form an antiramucirumab antibody–ramucirumab complex. Exogenously added iodine-125–ramucirumab then bound to the complex in proportion to the amount of antiramucirumab antibody present.
Dynamic Contrast-Enhanced Magnetic Resonance Imaging Acquisition
One patient in each cohort was required to have two baseline dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) scans (to correct for intrapatient variability) completed within 7 days of initiating ramucirumab. Scans were performed on Siemens (Berlin, Germany) or General Electric (Fairfield, CT) 1.5-T MRI scanners using a whole-body coil. Each region of interest was analyzed for signal intensities for each of the 29 DCE-MRI phases. The criteria suggested for choosing lesions to observe included the ability to replicate slice selection on separate days and a lesion that had the following criteria: least affected by motion, allowing for inclusion of a major artery in the image volume, toward the center of the image volume and plane, at least 2 cm in diameter if superficial and 3 cm in diameter if deep, ≤ 8 cm, well-defined on standard computed tomography or MRI, and not cystic, necrotic, or previously irradiated.
After obtaining initial localizer and T1-weighted gradient echo scans with flip angles of 5, 15, and 30 degrees for precise calculations of T1 relaxation time, a fast series of T1-weighted gradient echo scans were performed with a simultaneous intravenous injection of gadolinium-DTPA contrast agent (Magnevist; Berlex, Montville, NJ). Contrast medium was injected at a dose of 0.1 mmol/kg, injection rate of 4 mL/sec, followed by 20-mL saline flush at a rate of 2 mL/sec using a power injector. The power injector was preset with a delay of 8 seconds after initiation of fast series DCE-MRI to obtain a precontrast baseline scan. The following acquisition parameters were used: TR/TE = 3.14/1.16 milliseconds (Siemens) and 4.50/1.92 milliseconds (General Electric); flip angle of 13 degrees (Siemens) and 15 degrees (General Electric); field of view 420 mm; slice thickness 8 mm (Siemens) and 4 mm (General Electric); no slice gaps; total number of slices per phase of 16 (Siemens) and 32 (General Electric); 8-second acquisition time per slice; and a total number of phases of 29. Intrapatient protocol modifications for baseline and cycle 1 DCE-MRI were not allowed. All images were analyzed by institutional radiologists to determine regions of interest for tumor, liver, muscle, and hepatic artery. Postprocessing kinetic modeling was performed by Perceptive Informatics (Waltham, MA) using the compartmental Tofts model for calculation of the following three quantitative end points: initial area under the signal-intensity curve for the first 60 seconds after gadolinium injection, the uptake rate constant, and the volume of extravascular extracellular space. Changes for each of these parameters were compared within each patient at baseline and in cycle 1.
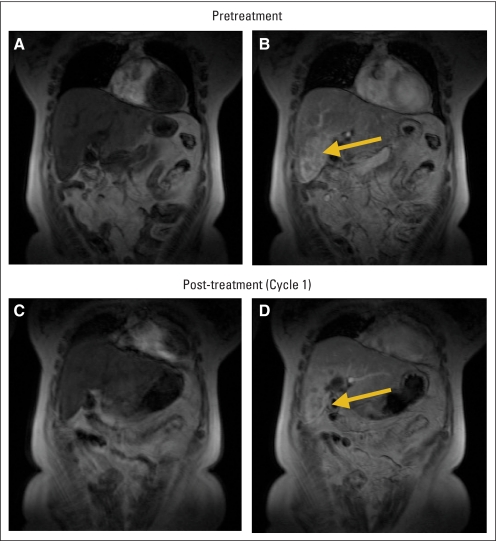
Fig A1.
Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) of a target hepatic lesion (arrow) in a patient with a metastatic cholangiocarcinoma. Pretreatment imaging (A) before contrast and (B) 16 seconds after injection of gadolinium, and (C) precontrast and (D) postcontrast scans after treatment with ramucirumab 8 mg/kg in cycle 1. After ramucirumab treatment, DCE-MRI indicated notable reductions in both tumor permeability and vascularity.
Table A1.
Changes in DCE-MRI Parameters Indicative of Perfusion and Vascularity (cycle 1)
| Dose (mg/kg) | Target Tumor No. | % Change From Pretreatment |
Interpretation | Clinical Correlates | ||
|---|---|---|---|---|---|---|
| Ktrans | IAUC | Ve | ||||
| Present study (weekly administration) | ||||||
| 8 | 1 | 997 | 50 | −32 | Increase in perfusion | Off study as a result of grade 3 hypertension in cycle 1; received prior VEGFR-2 tyrosine kinase inhibitor |
| 8 | 1 | −60 | −56 | −43 | Decrease in perfusion and vascularity | Stable disease for 21 weeks |
| >10 | 1 | 933 | −10 | 37 | Increase in perfusion and vascularity | Stable disease for 13 weeks |
| 2 | 2,678 | 166 | 125 | |||
| 10 | 1 | −8 | −9 | −9 | Decrease in perfusion and vascularity | Stable disease for 9 weeks |
| 2 | −19 | −13 | −1 | |||
| 13 | 1 | −71 | −80 | 17 | Decrease in perfusion | Stable disease for 21 weeks |
| 2 | −64 | −21 | −3 | |||
| 16 | 1 | −15 | −21 | −9 | Decrease in perfusion and vascularity | Stable disease for 7 weeks; off study as a result of grade 3 hypertension in cycle 2 |
| 16 | 1 | −11 | −8 | −12 | Decrease in perfusion and vascularity | Partial response for 86+ weeks; r3eceived prior anti-VEGF therapy |
| Study 0402 (administered every 2 or 3 weeks) | ||||||
| 8 | 1 | −7 | −39 | −5 | Decrease in perfusion and vascularity | Stable disease for 15 weeks; received prior anti-VEGF therapy |
| 2 | −30 | −42 | 41 | |||
| 3 | −46 | −58 | −18 | |||
| 8 | 1 | 181 | 6 | 119 | Increase in perfusion and vascularity | Stable disease for 7 weeks; received prior VEGFR-2 tyrosine kinase inhibitor |
| 10 | 1 | −63 | −51 | 124 | Decrease in perfusion | Stable disease for 63 weeks |
| 15 | 1 | 541 | −39 | 28 | Increase in vascularity | |
| 2 | 1,165 | 35 | 29 | |||
| 15 | 1 | −58 | −47 | −40 | Decrease in perfusion and vascularity | |
| 20 | 1 | 9 | −36 | −47 | Decrease in perfusion and vascularity | Stable disease for 18 weeks |
| 2 | 3 | −60 | −49 | |||
Abbreviations: DCE-MRI, dynamic contrast-enhanced magnetic resonance imaging; Ktrans, uptake rate constant; IAUC, initial area under the signal-intensity curve; Ve, volume of extravascular extracellular space; VEGFR-2, vascular endothelial growth factor receptor-2; VEGF, vascular endothelial growth factor.
Footnotes
Supported in part by ImClone Systems, a wholly owned subsidiary of Eli Lilly, and by Grant No. P30 CA006927 from the National Cancer Institute to Fox Chase Cancer Center. J.L.S., a senior fellow mentored by S.G.E., received funding from the National Cancer Institute of Canada through the Terry Fox Foundation and the Alberta Heritage Foundation for Medical Research.
Presented in part at the 20th Annual European Organisation for Research and Treatment of Cancer–National Cancer Institute–American Association for Cancer Research (EORTC-NCI-AACR) Symposium on Molecular Targets and Cancer Therapeutics, October 21-24, 2008, Geneva, Switzerland; 19th Annual EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics, October 22-26, 2007, San Francisco, CA; 18th Annual EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics, November 7-10, 2006, Prague, Czech Republic; 42nd Annual Meeting of the American Society of Clinical Oncology, June 2-6, 2006, Atlanta, GA.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00793975.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Floyd Fox, ImClone Systems (C); Hagop Youssoufian, ImClone Systems (C); Eric K. Rowinsky, ImClone Systems (C) Consultant or Advisory Role: Roger B. Cohen, ImClone Systems (C) Stock Ownership: Hagop Youssoufian, ImClone Systems Honoraria: Roger B. Cohen, ImClone Systems; E. Gabriela Chiorean, Speaking honoraria, ImClone Systems Research Funding: Roger B. Cohen, ImClone Systems; D. Ross Camidge, ImClone Systems; E. Gabriela Chiorean, ImClone Systems; S. Gail Eckhardt, ImClone Systems Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Jennifer L. Spratlin, Roger B. Cohen, Neal J. Meropol, Floyd Fox, Hagop Youssoufian, Eric K. Rowinsky, S. Gail Eckhardt
Provision of study materials or patients: Jennifer L. Spratlin, Roger B. Cohen, Lia Gore, D. Ross Camidge, Sami Diab, Stephen Leong, Cindy O'Bryant, Laura Q.M. Chow, Neal J. Meropol, Nancy L. Lewis, E. Gabriela Chiorean, Hagop Youssoufian, S. Gail Eckhardt
Collection and assembly of data: Jennifer L. Spratlin, Roger B. Cohen, Matthew Eadens, Floyd Fox, Hagop Youssoufian
Data analysis and interpretation: Jennifer L. Spratlin, Roger B. Cohen, D. Ross Camidge, Natalie J. Serkova, Neal J. Meropol, Floyd Fox, Hagop Youssoufian, Eric K. Rowinsky, S. Gail Eckhardt
Manuscript writing: Jennifer L. Spratlin, Roger B. Cohen, Matthew Eadens, Lia Gore, D. Ross Camidge, Natalie J. Serkova, Neal J. Meropol, Floyd Fox, Hagop Youssoufian, Eric K. Rowinsky, S. Gail Eckhardt
Final approval of manuscript: Jennifer L. Spratlin, Roger B. Cohen, Matthew Eadens, Lia Gore, D. Ross Camidge, Sami Diab, Stephen Leong, Cindy O'Bryant, Laura Q.M. Chow, Natalie J. Serkova, Neal J. Meropol, Nancy L. Lewis, E. Gabriela Chiorean, Floyd Fox, Hagop Youssoufian, Eric K. Rowinsky, S. Gail Eckhardt
REFERENCES
- 1.Klagsbrun M, D'Amore PA. Vascular endothelial growth factor and its receptors. Cytokine Growth Factor Rev. 1996;7:259–270. doi: 10.1016/s1359-6101(96)00027-5. [DOI] [PubMed] [Google Scholar]
- 2.Neufeld G, Cohen T, Gengrinovitch S, et al. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- 3.Ferrara N. Molecular and biological properties of vascular endothelial growth factor. J Mol Med. 1999;77:527–543. doi: 10.1007/s001099900019. [DOI] [PubMed] [Google Scholar]
- 4.Aguayo A, Kantarjian H, Manshouri T, et al. Angiogenesis in acute and chronic leukemias and myelodysplastic syndromes. Blood. 2000;96:2240–2245. [PubMed] [Google Scholar]
- 5.Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- 6.Leung DW, Cachianes G, Kuang WJ, et al. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 7.Inoue M, Hager JH, Ferrara N, et al. VEGF-A has a critical, nonredundant role in angiogenic switching and pancreatic beta cell carcinogenesis. Cancer Cell. 2002;1:193–202. doi: 10.1016/s1535-6108(02)00031-4. [DOI] [PubMed] [Google Scholar]
- 8.de Vries C, Escobedo JA, Ueno H, et al. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science. 1992;255:989–991. doi: 10.1126/science.1312256. [DOI] [PubMed] [Google Scholar]
- 9.Shibuya M, Yamaguchi S, Yamane A, et al. Nucleotide sequence and expression of a novel human receptor-type tyrosine kinase gene (flt) closely related to the fms family. Oncogene. 1990;5:519–524. [PubMed] [Google Scholar]
- 10.Witte L, Hicklin DJ, Zhu Z, et al. Monoclonal antibodies targeting the VEGF receptor-2 (Flk1/KDR) as an anti-angiogenic therapeutic strategy. Cancer Metastasis Rev. 1998;17:155–161. doi: 10.1023/a:1006094117427. [DOI] [PubMed] [Google Scholar]
- 11.Zhu Z, Witte L. Inhibition of tumor growth and metastasis by targeting tumor-associated angiogenesis with antagonists to the receptors of vascular endothelial growth factor. Invest New Drugs. 1999;17:195–212. doi: 10.1023/a:1006314501634. [DOI] [PubMed] [Google Scholar]
- 12.Zhu Z, Bohlen P, Witte L. Clinical development of angiogenesis inhibitors to vascular endothelial growth factor and its receptors as cancer therapeutics. Curr Cancer Drug Targets. 2002;2:135–156. doi: 10.2174/1568009023333881. [DOI] [PubMed] [Google Scholar]
- 13.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 14.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 15.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 16.Escudier B, Eisen T, Stadler WM, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 17.Giantonio BJ, Catalano PJ, Meropol NJ, et al. Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. [DOI] [PubMed] [Google Scholar]
- 18.Miller KD. E2100: A phase III trial of paclitaxel versus paclitaxel/bevacizumab for metastatic breast cancer. Clin Breast Cancer. 2003;3:421–422. doi: 10.3816/CBC.2003.n.007. [DOI] [PubMed] [Google Scholar]
- 19.Miller KD, Chap LI, Holmes FA, et al. Randomized phase III trial of capecitabine compared with bevacizumab plus capecitabine in patients with previously treated metastatic breast cancer. J Clin Oncol. 2005;23:792–799. doi: 10.1200/JCO.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 20.Adams VR, Leggas M. Sunitinib malate for the treatment of metastatic renal cell carcinoma and gastrointestinal stromal tumors. Clin Ther. 2007;29:1338–1353. doi: 10.1016/j.clinthera.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 21.Posey JA, Ng TC, Yang B, et al. A phase I study of anti-kinase insert domain-containing receptor antibody, IMC-1C11, in patients with liver metastases from colorectal carcinoma. Clin Cancer Res. 2003;9:1323–1332. [PubMed] [Google Scholar]
- 22.Jayson GC, Ton C, Parker GJ, et al. Phase I and DCE-MRI evaluation of CDP791, a di-Fab PEG conjugate that inhibits VEGFR2. J Clin Oncol. 2007;25(suppl):143s. abstr 3523. [Google Scholar]
- 23.Lu D, Jimenez X, Zhang H, et al. Selection of high affinity human neutralizing antibodies to VEGFR2 from a large antibody phage display library for antiangiogenesis therapy. Int J Cancer. 2002;97:393–399. doi: 10.1002/ijc.1634. [DOI] [PubMed] [Google Scholar]
- 24.Rockwell P, Neufeld G, Glassman A, et al. In vitro neutralization of vascular endothelial growth factor activation Flk-1 by a monoclonal antibody. Mol Cell Differ. 1995;3:91–109. [Google Scholar]
- 25.Lu D, Shen J, Vil MD, et al. Tailoring in vitro selection for a picomolar affinity human antibody directed against vascular endothelial growth factor receptor 2 for enhanced neutralizing activity. J Biol Chem. 2003;278:43496–43507. doi: 10.1074/jbc.M307742200. [DOI] [PubMed] [Google Scholar]
- 26.Zhu Z, Hattori K, Zhang H, et al. Inhibition of human leukemia in an animal model with human antibodies directed against vascular endothelial growth factor receptor 2: Correlation between antibody affinity and biological activity. Leukemia. 2003;17:604–611. doi: 10.1038/sj.leu.2402831. [DOI] [PubMed] [Google Scholar]
- 27.Prewett M, Huber J, Li Y, et al. Antivascular endothelial growth factor receptor (fetal liver kinase 1) monoclonal antibody inhibits tumor angiogenesis and growth of several mouse and human tumors. Cancer Res. 1999;59:5209–5218. [PubMed] [Google Scholar]
- 28.Bruns CJ, Liu W, Davis DW, et al. Vascular endothelial growth factor is an in vivo survival factor for tumor endothelium in a murine model of colorectal carcinoma liver metastases. Cancer. 2000;89:488–499. [PubMed] [Google Scholar]
- 29.Tofts PS, Brix G, Buckley DL, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: Standardized quantities and symbols. J Magn Reson Imaging. 1999;10:223–232. doi: 10.1002/(sici)1522-2586(199909)10:3<223::aid-jmri2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 30.Chiorean E, Sweeney C, Hurwitz H, et al. Phase I dose-escalation study of the anti-VEGFR-2 recombinant human IgG1 monoclonal antibody (MAb) IMC-1121B, administered every other week (q2w) or every 3 weeks (q3w) in patients (pts) with advanced cancers. 19th Annual EORTC-NCI-AACR Symposium on Molecular Targets and Cancer Therapeutics; October 22-26, 2007; San Francisco, CA. abstr B15. [Google Scholar]
- 31.Gordon MS, Margolin K, Talpaz M, et al. Phase I safety and pharmacokinetic study of recombinant human anti-vascular endothelial growth factor in patients with advanced cancer. J Clin Oncol. 2001;19:843–850. doi: 10.1200/JCO.2001.19.3.843. [DOI] [PubMed] [Google Scholar]
- 32.Faivre S, Delbaldo C, Vera K, et al. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J Clin Oncol. 2006;24:25–35. doi: 10.1200/JCO.2005.02.2194. [DOI] [PubMed] [Google Scholar]
- 33.Britten CD, Kabbinavar F, Hecht JR, et al. A phase I and pharmacokinetic study of sunitinib administered daily for 2 weeks, followed by a 1-week off period. Cancer Chemother Pharmacol. 2007;61:515–524. doi: 10.1007/s00280-007-0498-4. [DOI] [PubMed] [Google Scholar]
- 34.Wilhelm SM, Carter C, Tang L, et al. BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res. 2004;64:7099–7109. doi: 10.1158/0008-5472.CAN-04-1443. [DOI] [PubMed] [Google Scholar]
- 35.Clark JW, Eder JP, Ryan D, et al. Safety and pharmacokinetics of the dual action Raf kinase and vascular endothelial growth factor receptor inhibitor, BAY 43-9006, in patients with advanced, refractory solid tumors. Clin Cancer Res. 2005;11:5472–5480. doi: 10.1158/1078-0432.CCR-04-2658. [DOI] [PubMed] [Google Scholar]
- 36.Awada A, Hendlisz A, Gil T, et al. Phase I safety and pharmacokinetics of BAY 43-9006 administered for 21 days on/7 days off in patients with advanced, refractory solid tumours. Br J Cancer. 2005;92:1855–1861. doi: 10.1038/sj.bjc.6602584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moore M, Hirte HW, Siu L, et al. Phase I study to determine the safety and pharmacokinetics of the novel Raf kinase and VEGFR inhibitor BAY 43-9006, administered for 28 days on/7 days off in patients with advanced, refractory solid tumors. Ann Oncol. 2005;16:1688–1694. doi: 10.1093/annonc/mdi310. [DOI] [PubMed] [Google Scholar]
- 38.Drevs J, Siegert P, Medinger M, et al. Phase I clinical study of AZD2171, an oral vascular endothelial growth factor signaling inhibitor, in patients with advanced solid tumors. J Clin Oncol. 2007;25:3045–3054. doi: 10.1200/JCO.2006.07.2066. [DOI] [PubMed] [Google Scholar]
- 39.Ryan CJ, Stadler WM, Roth B, et al. Phase I dose escalation and pharmacokinetic study of AZD2171, an inhibitor of the vascular endothelial growth factor receptor tyrosine kinase, in patients with hormone refractory prostate cancer (HRPC) Invest New Drugs. 2007;25:445–451. doi: 10.1007/s10637-007-9050-y. [DOI] [PubMed] [Google Scholar]
- 40.Tamura T, Minami H, Yamada Y, et al. A phase I dose-escalation study of ZD6474 in Japanese patients with solid, malignant tumors. J Thorac Oncol. 2006;1:1002–1009. [PubMed] [Google Scholar]
- 41.Holden SN, Eckhardt SG, Basser R, et al. Clinical evaluation of ZD6474, an orally active inhibitor of VEGF and EGF receptor signaling, in patients with solid, malignant tumors. Ann Oncol. 2005;16:1391–1397. doi: 10.1093/annonc/mdi247. [DOI] [PubMed] [Google Scholar]
- 42.Dirks NL, Nolting A, Kovar A, et al. Population pharmacokinetics of cetuximab in patients with squamous cell carcinoma of the head and neck. J Clin Pharmacol. 2008;48:267–278. doi: 10.1177/0091270007313393. [DOI] [PubMed] [Google Scholar]
- 43.Kuester K, Kloft C. Pharmacokinetics of Monoclonal Antibodies. Weinheim, Germany: Wiley-VCH; 2006. pp. 45–91. [Google Scholar]
- 44.Fracasso PM, Burris H, 3rd, Arquette MA, et al. A phase 1 escalating single-dose and weekly fixed-dose study of cetuximab: Pharmacokinetic and pharmacodynamic rationale for dosing. Clin Cancer Res. 2007;13:986–993. doi: 10.1158/1078-0432.CCR-06-1542. [DOI] [PubMed] [Google Scholar]