Abstract
Purpose
Prior trials have shown that surgery followed by hepatic artery infusion (HAI) of floxuridine (FUDR) alternating with systemic fluorouracil improves survival rates. Oxaliplatin combined with capecitabine has demonstrated activity in advanced colorectal cancer. Based on this observation a trial was conducted to assess the potential benefit of systemic oxaliplatin and capecitabine alternating with HAI of FUDR. The primary end point was 2-year survival.
Patients and Methods
Patients with liver-only metastases from colorectal cancer amenable to resection or cryoablation were eligible. HAI and systemic therapy was initiated after metastasectomy. Alternating courses of HAI consisted of 0.2 mg/m2/d FUDR and dexamethasone, day 1 through 14 weeks 1 and 2. Systemic therapy included oxaliplatin 130 mg/m2 day 1 with capecitabine at 1,000 mg/m2 twice daily, days 1 through 14, weeks 4 and 5. Two additional 3-week courses of systemic therapy were given. Capecitabine was reduced to 850 mg/m2 twice daily after interim review of toxicity.
Results
Fifty-five of 76 eligible patients were able to initiate protocol-directed therapy and completed median of six cycles (range, one to six). Three postoperative or treatment-related deaths were reported. Overall, 88% of evaluable patients were alive at 2 years. With a median follow-up of 4.8 years, a total of 30 patients have had disease recurrence, 11 involving the liver. Median disease-free survival was 32.7 months.
Conclusion
Alternating HAI of FUDR and systemic capecitabine and oxaliplatin met the prespecified end point of higher than 85% survival at 2 years and was clinically tolerable. However, the merits of this approach need to be established with a phase III trial.
INTRODUCTION
In patients with liver-only metastases from colorectal cancer, approximately 15% to 25% will have initially resectable disease.1 Consideration of resection in this setting is supported by a growing body of literature. With more than 50 years of case series, it is now quite evident that surgery provides an opportunity for long-term survival.2,3 When used alone survival after surgical resection is estimated to be 30% at 5 years and 20% at 10 years.4–6 Despite this success the liver is the most common site of recurrence after metastasectomy.7
Given the high rate of liver recurrence there has been interest in the use of regional chemotherapy after surgical resection as one method of providing adjuvant treatment. Several trials evaluating hepatic artery infusion (HAI) therapy after resection have reported an improved survival as well as a decrease in hepatic recurrence compared to patients receiving systemic fluorouracil therapy. In a trial from Memorial Sloan-Kettering Cancer Center (MSKCC), patients were randomly assigned to systemic chemotherapy alone versus systemic chemotherapy combined with HAI floxuridine (FUDR).8,9 A significant benefit was seen in patients receiving combined therapy. The median survival in the group receiving combined therapy was 72.2 months compared to 59.3 months for those receiving systemic therapy alone. At 2 years the rate of survival free of hepatic recurrence was 90% in the combined therapy group compared to 60% in the systemic therapy only group (P < .001). However, recurrence outside the liver appeared similar in both groups. In a separate trial, patients with two to four resected hepatic metastases were randomized to resection alone versus HAI FUDR combined with systemic infusional fluorouracil (FU).10 This trial also showed a marked decrease in hepatic recurrence with HAI as well as a significant improvement in recurrence-free survival.
Based on these observations a phase II trial was undertaken to assess the feasibility and potential benefit of using a more active systemic therapy combination, consisting of capecitabine and oxaliplatin, together with HAI FUDR.
PATIENTS AND METHODS
Eligibility Criteria
Patients were potentially eligible for participation in the trial if they had either a previously resected colon or rectal cancer or presented with potentially resectable synchronous colon or rectal cancer with metastases limited to the liver. The liver metastases were required to be potentially resectable as determined by a surgeon with experience in liver surgery. Cryoablation was allowed as an alternative to surgical resection and radiofrequency ablation as an adjunct to surgical resection. Prior resection of liver metastases was permitted if intraoperative ultrasound had not been used. However, prior resection of extra-hepatic metastases was not permitted.
Patients were required to be at least 18 years and to have an Eastern Cooperative Oncology Group performance score of 0 to 1. Hematologic and chemistry parameters were to be in acceptable ranges including: absolute neutrophil count ≥ 1.2 × 109/L, direct bilirubin ≤ 1.5 times the institutional upper normal limit (UNL), AST ≤ 2.5 times the UNL, consistent with values used in previously published trials.8,10 The creatinine needed to be at or below the upper limit of normal. If the creatinine was above the UNL then a measured creatinine clearance of higher than 60 mL/min/1.73 m2 was required.
Patients were allowed to have prior adjuvant therapy with FU with or without irinotecan, leucovorin, or levamisole, but not capecitabine or oxaliplatin. Prior adjuvant or neoadjuvant radiation for rectal cancer was allowed. Prior use of HAI therapy was not permitted.
This trial was approved by the Mayo institutional review board (IRB) and the IRBs of the participating institutions. A signed written informed consent was obtained from all patients before initiating therapy. Adverse events were monitored by the IRB and a data safety monitoring board, in accord with Mayo policy.
Treatment
At the time of surgery, multiple wedge resections or a combination of lobectomy or segmentectomy with wedge resections was permissible. Intraoperative ultrasound of the liver was recommended before resection, but not required. It was expected that all metastatic lesions identified by preoperative computed tomography (CT) scan and that found by intraoperative palpation or intraoperative ultrasound would be resected unless otherwise contraindicated. Radiofrequency ablation (RFA) was not allowed to be used as the sole modality for surgical ablation of all metastatic lesions, but could be used in conjunction with resection. In general, it was recommended that RFA be reserved for situations in which the planned surgical approach would be significantly altered (eg, a trisegmentectomy in place of a lobectomy) without the use of RFA. Cryoablation was allowed as the sole modality of treatment.
In patients who were found to have anatomy appropriate for placement of a HAI catheter, it was recommended that all side branches distal to the site of catheter placement should be ligated and divided to prevent misperfusion of the distal stomach and proximal duodenum and therefore lessen the risk for chemical gastritis and duodenitis. It was also recommended that ligation of side branches proximally along the common hepatic artery to the celiac axis should also be done to prevent back flow of chemotherapy and perfusion of the upper gastrointestinal tract. To prevent chemical cholecystitis, a cholecystectomy was advised. Once the catheter was in place, intraoperative assessment of perfusion was required by infusion of 5 mL of fluorescein via the catheter or port. Postoperatively, a technetium sulfur colloid radionuclide liver scan was required to assess liver perfusion.
After recovery from surgery, the planned chemotherapy treatment was started as outlined in Table 1. FUDR was held if a grade 1 or 2 adverse event (National Cancer Institute Common Toxicity Criteria version 2) felt to be related to the FUDR occurred. In the event of FUDR adverse event, treatment was discontinued until there had been clinical resolution of adverse event, return of direct bilirubin to ≤ 1.5 × UNL, and return of AST and alkaline phosphatase to ≤ 2.5 × UNL. If those criteria were not met within 4 weeks of onset of FUDR adverse event, FUDR was permanently discontinued. The FUDR dose was reduced if moderate chemical hepatitis or evidence of gastroduodenitis was noted. Dose reduction was based on the most abnormal liver function tests during the preceding treatment cycle. FUDR administration was permanently discontinued if severe chemical hepatitis or biliary stricture was noted.
Table 1.
Protocol Directed HAI and Systemic Therapy After Resection of Colorectal Liver Metastases
| Type and Agent | Dose | Route | Week(s) of Cycle | Day(s) of Week of Cycle | Rest Week | ReRx |
|---|---|---|---|---|---|---|
| Cycles 1-4 | ||||||
| HAI | ||||||
| FUDR | 0.2 mg/kg/d | HAI continuous | 1-2 | 1-14 | 3 | Every 6 weeks |
| DXM | 1 mg/24 h | Added to FUDR 2-week infusion | ||||
| Heparin | 1,000 U/24 h | Added to FUDR 2-week infusion | ||||
| Systemic therapy | ||||||
| OXAL | 130 mg/m2 | IV over 2 hours | 4-5 | 1 | 6 | |
| CAPCIT | 1,700 mg/m2/d* | PO BID, given in the morning and evening | 1-14 | |||
| Cycles 5-6 | ||||||
| Systemic therapy | ||||||
| OXAL | 130 mg/m2 | IV over 2 h | 1-2 | 1 | 3 | Every 3 weeks |
| CAPCIT | 1,700 mg/m2/d* | PO BID, given in the morning and evening | 1-14 |
Abbreviations: HAI, hepatic artery infusion; FUDR, floxuridine; DXM, dexamethasone; OXAL, oxaliplatin; CAPCIT, capecitabine; IV, intravenous; PO, orally; BID, twice per day.
The first 32 patients enrolled received 2,000 mg/m2/day.
Evaluation, Disease Assessment, and Follow-Up
Once chemotherapy was started, patients were seen at the start of each cycle of treatment. A CBC and chemistries were obtained weekly during treatment and at the start of each cycle of therapy. A CT scan was obtained following the completion of FUDR and then every 3 months for 1 year after the completion of all planned therapy. CT scans were then obtained every 6 months until 2.5 years from the end of therapy. Patients experiencing disease progression were followed every 6 months, until 5 years postregistration or death.
Definitions of Key End Points
Time to recurrence (ie, disease-free interval) was defined as the time from surgery to documentation of disease recurrence. If a patient died without a documentation of disease recurrence, the patient was considered to have had a recurrence of their disease at the time of their death unless there was sufficient documented evidence to conclude recurrence did not occur before death. In the case of a patient never returning for any evaluations postsurgery, the patient was censored and counted as a recurrence on day 1, postsurgery. Disease-free survival was calculated as the time from registration to death or recurrence, whichever was earlier. Hepatic disease-free survival was calculated at the time from registration to death or hepatic recurrence, whichever was earlier. Survival was defined as the time from registration to death as a result of any cause. Patients lost to follow-up were censored for these end points at the date of last contact (or disease assessment), as applicable.
Statistical Considerations
The primary end point of this trial was 2-year survival where success was defined as an evaluable patient living at least 2 years from the date of surgery. Since 2-year survival was expected to be 70% in patients with multiple hepatic metastases who underwent surgical resection without adjuvant chemotherapy, the aggressive combined modality treatment outlined in this protocol was considered clinically beneficial if the 2-year survival was at least 85%. Hence, the null hypothesis (Ho) tested that the true success proportion was at most 70% (ie, Ho: P ≤ .70) versus the alternative hypothesis (Ha) that the true success proportion was at least 85% (ie, Ha: P ≥ .85). This hypothesis required a total of 45 eligible patients who initiated protocol directed therapy and were observed for 2.5 years. A single-stage, phase II design with a significance level of .09 and power of 87% for detecting a true-success proportion of 85% was used. CIs for the estimate of the primary end point were calculated via the method of Duffy and Santner.11
The percent of targeted dose administered to each patient for a given cycle was calculated as the total dose administered divided by the protocol-specified dose targeted for the cycle. Patterns of treatment failure and toxicity, including complications associated with the intra-arterial catheter, were summarized. Summary statistics (eg, mean, median, standard deviation) and graphical methods were used to display continuous variables. Frequency tables with χ2 and Fisher's exact tests were used to explore the relationships between dichotomous variables. The distributions of time to recurrence, time to hepatic recurrence, disease-free survival, hepatic disease-free survival, and survival were estimated using the Kaplan-Meier method.12 Cox proportional hazards models were used to explore the associations of covariates with time to event end points. The statistical package used to perform analyses was SAS, version 9.0 (SAS Institute, Cary, NC).
RESULTS
Patient Enrollment
A total of 123 patients were preregistered and proceeded to surgery. Of those patients, 47 were not able to start the planned therapy for reasons including unresectable disease (n = 13), positive margins (n = 9), and extrahepatic disease (n = 8). The remaining 76 patients, registered by 25 sites, achieved complete surgical resection of their disease (71 resected, four cryoablation, 12 RFA), placement of a catheter, and proceeded to enrollment. However, 21 of 76 patients were unable to begin planned therapy because of elevated liver function tests (n = 2), disease progression (n = 1), deaths felt not to be surgically related (n = 2), death due to surgical complications (n = 1), declared ineligible after surgery was completed (n = 3), or other reasons (n = 12). Two deaths occurred between surgery and treatment because of renal failure and myocardial infarction with a pulmonary embolism. Therefore, only 55 (45%) of 123 patients initiated protocol-directed therapy within the planned 21 to 56 days of metastasectomy.
Between February 22, 2002, and April 8, 2005, a total of 55 patients initiated protocol-directed therapy (Table 2). Early during the course of the trial, the North Central Cancer Treatment Group real-time toxicity monitoring program13 prompted a review of adverse event data based on the rate of grade 3 to 4 toxicity exceeding what was considered acceptable. In particular, 47% of patients had grade 3 capecitabine-induced diarrhea, as compared with 22% in a prior NCCTG trial using HAI FUDR alone.14 For the remainder of the trial, the dose for capecitabine was reduced to 1,700 mg/m2/d.
Table 2.
Characteristics of Patients Initiating Post-Operative Protocol-Directed Therapy
| Characteristic | Capecitabine (mg/m2/d) |
P | |||
|---|---|---|---|---|---|
| 2,000 |
1,700 |
||||
| No. | % | No. | % | ||
| No. of patients | 32 | 23 | |||
| Age | 55 | 60 | .42 | ||
| Range | 34-79 | 41-69 | |||
| No. of hepatic metastases | |||||
| Median resected | 1 | 1 | .07 | ||
| Range | 0-7 | 0-3 | |||
| Median cryoablated | 0 | 0 | .23 | ||
| Range | 0-1 | ||||
| Median RFA | 0 | 0 | .92 | ||
| Range | 0-2 | 0-1 | |||
| Race | .82 | ||||
| African American | 2 | 6 | 1 | 4 | |
| Native Hawaiian/Pacific Islander | 1 | 3 | 1 | 4 | |
| White | 27 | 87 | 21 | 92 | |
| Not reported | 1 | 3 | 0 | 0 | |
| Sex | .25 | ||||
| Female | 9 | 28 | 11 | 48 | |
| Male | 22 | 69 | 12 | 52 | |
| Not reported | 1 | 3 | 0 | 0 | |
| Performance score | .85 | ||||
| 0 | 21 | 68 | 15 | 65 | |
| 1 | 10 | 32 | 8 | 35 | |
| Anomalous hepatic arterial circulation | .65 | ||||
| Yes | 7 | 22 | 6 | 27 | |
| No | 25 | 78 | 16 | 73 | |
| Postoperative complications | .70 | ||||
| Yes | 4 | 13 | 2 | 9 | |
| No | 28 | 88 | 20 | 91 | |
| Complications related to catheter or pump | .14 | ||||
| Yes | 3 | 9 | 0 | 0 | |
| No | 29 | 91 | 22 | 100 | |
| Extent of metastases | .55 | ||||
| Bilobar | 8 | 25 | 5 | 22 | |
| > 2 unilobar | 9 | 28 | 4 | 17 | |
| Single | 15 | 47 | 14 | 51 | |
Abbreviation: RFA, radiofrequency ablation.
Outcome
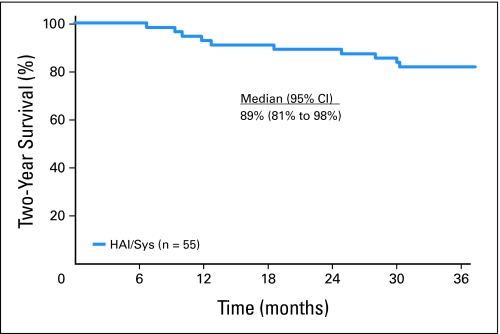
With a median follow-up of 4.8 years a total of 30 patients (55%) have had disease recurrence, 11 involving the liver, meaning that 44 patients (80%) had control of their liver. Median time to recurrence is 2.7 years (95% CI, 1.7 to upper limit not reached). At 2 years after surgery, 88% of patients were still alive, exceeding the preset level of success. Table 3 presents the Kaplan-Meier estimates of time to recurrence, disease-free survival, hepatic disease-free survival, and survival, at various time points of interest. A total of 18 patients have died. Median overall survival (Fig 1) has not been reached (95% CI, 5.0 to upper limit not reached).
Table 3.
Outcome of Patients Initiating Post-Operative Protocol-Directed Therapy (N = 55)
| Event Type | Estimate* |
|---|---|
| Recurrence (any) | |
| Events | 30 |
| Median, months | 32.7 |
| 2 years† | 59.7 |
| 95% CI | 48.0 to 74.3 |
| Hepatic recurrence | |
| Events | 11 |
| Median, months | Did not reach median |
| 2 years | 80.5 |
| 69.7 to 92.9 | |
| Disease-free survival | |
| Events | 30 |
| Median, months | 32.7 |
| 2 years | 59.7 |
| 95% CI | 48.0 to 74.3 |
| Hepatic disease-free survival | |
| Events | 20 |
| Median, months | Did not reach median |
| 2 years | 75.5 |
| 64.7 to 88.1 | |
| Survival | |
| Events | 18 |
| Median, months | Did not reach median |
| 1 year | 92.7 |
| 95% CI | 86.1 to 99.9 |
| 2 years | 89.1 |
| 95% CI | 81.2 to 97.7 |
Kaplan-Meier methodology.
Estimate of event-free rate at specified time point.
Fig 1.
Overall survival in patients initiating protocol-directed post-operative therapy. HAI, hepatic artery infusion; Sys, systemic therapy.
Adverse Events
Thirty-two of 55 evaluable patients experienced at least one grade 3 adverse event (National Cancer Institute Common Toxicity Criteria, version 2.0) considered at least possibly related to study treatment (Table 4). No treatment-related fatalities were reported at either capecitabine dose level.
Table 4.
Summary of Serious Adverse Events (n = 54 patients)
| Grade 3* Adverse Event (National Cancer Institute Common Toxicity Criteria version 2) | No. for Capecitabine Dose Level (mg/m2/d) |
|
|---|---|---|
| 2,000 (n = 32) | 1,700 (n = 23) | |
| Hematologic | ||
| Neutropenia | 1 | 0 |
| Thrombocytopenia | 0 | 0 |
| Hepatic | ||
| AST | 1 | 4 |
| Bilirubin | 2 | 1 |
| Alkaline phosphatase | 1 | 1 |
| ALT | 3 | 6 |
| Gastrointestinal | ||
| Abdominal pain | 6 | 1 |
| Nausea | 6 | 2 |
| Vomiting | 7 | 3 |
| Diarrhea | 11 | 4 |
| Neurologic | ||
| Paresthesia | 6 | 4 |
| Laryngeal dysesthesia | 1 | 0 |
| Neuro/sensory | 0 | 1 |
| Pulmonary | ||
| Dyspnea | 1 | 2 |
| Constitutional | ||
| Fatigue | 5 | 1 |
| Dermatology/skin | ||
| Hand/foot | 1 | 0 |
Events reported at least possibly related to study treatment.
Fourteen patients experienced a complication related to their HAI catheter or pump. Ten patients were able to receive HAI therapy, but experienced catheter occlusion (n = 6), pump malfunction (n = 3), infection (n = 1), or other problems (n = 7) that did not interfere with the protocol-directed therapy. Four patients were unable to receive therapy because of infection, catheter occlusion, catheter malfunction, and an unspecified reason.
Dose Intensity
At the initial dose of capecitabine (2,000 mg/m2/d) patients received a median of 6 cycles of treatment (range, 1 to 6). Reasons for prematurely discontinuing protocol directed therapy included refusal (n = 5), adverse events (n = 4), disease recurrence (n = 4), and other reasons (n = 2). By the end of the 4 cycles of FUDR, patients were receiving 75% of their targeted dose of FUDR. Patients received an average of 78% to 84% of their targeted capecitabine dose and 75% to 100% of their oxaliplatin dose. Oxaliplatin dose levels (ie, 130 mg/m2) were generally sustained throughout trial participation, with a few 25% reductions occurring near the final course of treatment. The most common reason for oxaliplatin dose adjustment was neurotoxicity (11 of 137 cycles). Capecitabine was most often adjusted due to diarrhea (11 of 137 cycles). Delays were rare, happening in only 10 cycles.
At the reduced dose of capecitabine (1,700 mg/m2/d) patients received a median of 6 cycles of treatment (range, 2 to 6). Reasons for premature discontinuation of treatment included adverse events (n = 1), alternative treatment (n = 1), and other reasons (n = 1). By the fourth cycle of treatment, patients were receiving 79% of the targeted dose for FUDR (Table 3). Patients received an average of 88% to 96% of their targeted capecitabine dose and 93% to 100% of their oxaliplatin dose during treatment, with the exception of oxaliplatin at cycle 6 (74%). Dose levels of oxaliplatin stabilized at 98% of targeted dose, at cycle 2. The most common reason for oxaliplatin dose adjustment was neurotoxicity (five of 132 cycles). Capecitabine was most often adjusted due to diarrhea (three of 132 cycles). Delays were more common, happening in 17 cycles and most often because of hematologic toxicity.
DISCUSSION
Recent advances in chemotherapy for metastatic colorectal cancer with the introduction of oxaliplatin and irinotecan have led to higher response rates and improvements in overall survival. Given the potential promising results of HAI FUDR and systemic FU after resection of liver-only metastases from colorectal cancer, we undertook a phase II clinical trial to assess the potential benefit of a more active systemic chemotherapy regimen consisting of capecitabine and oxaliplatin alternating with HAI FUDR. In a randomized phase III trial, the combination of capecitabine and oxaliplatin in the first-line setting has provided a 19.8-month median overall survival, equivalent to that seen with FOLFOX.15
Two randomized trials from North America of HAI FUDR alternating with systemic FU have been reported and are available for comparison.8–10 Of the patients that started protocol-directed therapy in our trial 88% were alive at 2 years, exceeding the planned level of success for this trial. This compares to an 86% 2-year survival with HAI FUDR and systemic FU in the single institution trial reported from MSKCC. Median overall survival in our trial has not been reached compared to 72.2 months in long-term follow-up reported for the MSKCC trial and 63.7 months in the Eastern Cooperative Oncology Group/Southwest Oncology Group trial.
In our trial the 2-year disease-free survival rate was 59.7% compared with 57% in the MSKCC trial. The hepatic disease-free survival rate in our trial, however, was lower than that seen in the MSKCC trial (75.5% v 90%). The reason for the higher rate of hepatic recurrence in our trial is unclear. Possibilities would include inability to give planned therapy, inadequate resection of liver metastases, antagonistic interaction between HAI FUDR and systemic capecitabine/oxaliplatin, inadequate dose of capecitabine in patients receiving the reduced dose, improved methods of detection (positron emission tomography scanning and widespread use of specific liver CT scanning for metastatic disease protocols), or a higher risk population. The latter possible explanation does not appear to apply. The median number of liver metastases resected in this trial appeared comparable to that of the other reported adjuvant trials, including the MSKCC trial.
The ability to give the planned course of therapy and the frequency of adverse events were clinically acceptable. Overall the protocol-directed therapy was well-tolerated with 67% of patients receiving all 6 cycles of therapy. At the initial dose of capecitabine approximately 75% of patients received the planned dose whereas at the reduced dose nearly 100% of patients received the planned dose. Although our trial used a higher dose of FUDR than that given in the MSKCC trial, 80% of patients received the planned 4 cycles of FUDR. Furthermore, a median of 77% the planned dose was given to patients receiving the fourth cycle of FUDR. The ability to deliver FUDR and the limited amount of any long-term associated biliary or hepatic toxicity from FUDR therefore does not appear to indicate any synergistic toxicity between HAI FUDR and systemic capecitabine and oxaliplatin as measured by the ability to give the planned therapy on schedule and at the planned dose.
Given the phase II nature of this trial, the benefit of adding HAI FUDR to systemic capecitabine and oxaliplatin for this patient population remains unclear. An attempt was made to make a formal comparison in the phase III trial NSABP C-09 in which patients were randomly assigned between capecitabine and oxaliplatin with or without HAI FUDR. However, this trial closed early given difficulties with adequate accrual, likely related to a marked decline in the use of HAI FUDR in the United States. Given this difficulty it is unlikely that any future formal phase III comparison will be made between multidrug systemic therapy with or without FUDR.
In conclusion, this phase II trial showed that therapy with systemic oxaliplatin and capecitabine alternating with HAI FUDR can be safely given. Given the improvement in systemic therapy for metastatic colorectal cancer and the decline in use of HAI FUDR in the United States other approaches need to be investigated such as the use of biologic agents as a component of systemic therapy without HAI FUDR.
Appendix
Additional participating institutions include Siouxland Hematology-Oncology Associates, Sioux City, IA 51105 (Donald B. Wender, MD); Medcenter One Health Systems, Bismarck, ND 58506 (Edward J. Wos, DO); Cedar Rapids Oncology Project CCOP, Cedar Rapids, IA 52403 (Martin Wiesenfeld, MD); Geisinger Clinic & Medical Center CCOP, Danville, PA 17822 (Albert M. Bernath, Jr MD); Mayo Clinic Florida, Jacksonville, FL 32224 (Edith A. Perez, MD); Mayo Clinic Arizona, Scottsdale, AZ 85259 (Tom R. Fitch, MD); Sioux Community Cancer Consortium, Sioux Falls, SD 57105 (Loren K. Tschetter, MD).
Footnotes
This study was conducted as a collaborative trial of the North Central Cancer Treatment Group, Mayo Clinic, and National Surgical Adjuvant Breast and Bowel Project and was supported in part by Public Health Service Grants No. CA-25224, U10-CA-12027, CA-37404, CA-35101, CA-35272, CA-52352, CA-35448, CA-35103 from the National Cancer Institute, Department of Health and Human Services; Sanofi-Aventis US; Sanofi-Synthelabo; Roche Laboratories, Inc; and by Grant No. CA25224-18 from the National Institutes of Health.
The content is solely the responsibility of the authors and does not necessarily represent the views of the National Cancer Institute or the National Institute of Health.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical Trial Registration: NCT00026234.
Clinical trial information can be found for the following: NCT00026234.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Lawrence Wagman, Medwaves (C) Stock Ownership: None Honoraria: Lawrence Wagman, Angiodynamics; Roderich E. Schwarz, sanofi-aventis Research Funding: Steven R. Alberts, sanofi-aventis Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Steven R. Alberts, Mark S. Roh, Michelle R. Mahoney, Michael J. O'Connell, David M. Nagorney, Lawrence Wagman, John S. Bolton
Provision of study materials or patients: Steven R. Alberts, Mark S. Roh, David M. Nagorney, Lawrence Wagman, Timothy L. Weiland, Lily Lau Lai, Roderich E. Schwarz, Roy Molina, Todor Dentchev, John S. Bolton
Collection and assembly of data: Steven R. Alberts, Michelle R. Mahoney, Thomas C. Smyrk
Data analysis and interpretation: Steven R. Alberts, Michelle R. Mahoney
Manuscript writing: Steven R. Alberts, Michelle R. Mahoney, Michael J. O'Connell, Lawrence Wagman
Final approval of manuscript: Steven R. Alberts, Mark S. Roh, Michelle R. Mahoney, Michael J. O'Connell, David M. Nagorney, Lawrence Wagman, Thomas C. Smyrk, Timothy L. Weiland, Lily Lau Lai, Roderich E. Schwarz, Roy Molina, Todor Dentchev, John S. Bolton
REFERENCES
- 1.Fusai G, Davidson BR. Management of colorectal liver metastases. Colorectal Dis. 2003;5:2–23. doi: 10.1046/j.1463-1318.2003.00410.x. [DOI] [PubMed] [Google Scholar]
- 2.Ballantyne GH. Surgical treatment of liver metastases in patients with colorectal cancer. Cancer. 1993;71:4252–4266. doi: 10.1002/1097-0142(19930615)71:12+<4252::aid-cncr2820711815>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 3.Wilson SM, Adson MA. Surgical treatment of hepatic metastases from colorectal cancer. Arch Surg. 1976;111:330–334. doi: 10.1001/archsurg.1976.01360220026004. [DOI] [PubMed] [Google Scholar]
- 4.Fong Y, Salo J. Surgical therapy of hepatic colorectal metastasis. Semin Oncol. 1999;26:514–523. [PubMed] [Google Scholar]
- 5.Poston GJ, Adam R, Alberts S, et al. OncoSurge: A strategy for improving resectability with curative intent in metastatic colorectal cancer. J Clin Oncol. 2005;23:7125–7134. doi: 10.1200/JCO.2005.08.722. [DOI] [PubMed] [Google Scholar]
- 6.Adam R, Lucidi V, Bismuth H. Hepatic colorectal metastases: Methods of improving resectability. Surg Clin North Am. 2004;84:659–671. doi: 10.1016/j.suc.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Topal B, Kaufman L, Aerts R, et al. Patterns of failure following curative resection of colorectal liver metastases. Eur J Surg Oncol. 2003;29:248–253. doi: 10.1053/ejso.2002.1421. [DOI] [PubMed] [Google Scholar]
- 8.Kemeny N, Huang Y, Cohen AM, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med. 1999;341:2039–2048. doi: 10.1056/NEJM199912303412702. [DOI] [PubMed] [Google Scholar]
- 9.Kemeny NE, Gonen M. Hepatic arterial infusion after liver resection. N Engl J Med. 2005;352:734–735. doi: 10.1056/NEJM200502173520723. [DOI] [PubMed] [Google Scholar]
- 10.Kemeny MM, Adak S, Gray B, et al. Combined-modality treatment for resectable metastatic colorectal carcinoma to the liver: Surgical resection of hepatic metastases in combination with continuous infusion of chemotherapy–An intergroup study. J Clin Oncol. 2002;20:1499–1505. doi: 10.1200/JCO.2002.20.6.1499. [DOI] [PubMed] [Google Scholar]
- 11.Duffy D, Santner T. Confidence intervals for a binomial parameter based on multistage tests. Biometrics. 1987;43:81–93. [Google Scholar]
- 12.Kaplan E, Meier P. Nonparametric estimation for incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 13.Goldberg RM, Sargent DJ, Morton RF, et al. Early detection of toxicity and adjustment of ongoing clinical trials: The history and performance of the North Central Cancer Treatment Group's real-time toxicity monitoring program. J Clin Oncol. 2002;20:4591–4596. doi: 10.1200/JCO.2002.03.039. [DOI] [PubMed] [Google Scholar]
- 14.Bolton JS, O'Connell MJ, Mahoney MR, et al. Final results of hepatic arterial infusion (HAI) plus systemic (SYS) chemotherapy after multiple metastasectomy in patients with colorectal carcinoma metastatic (M-CRC) to the liver: A North Central Cancer Treatment Group (NCCTG) phase II study (abstract 3527) J Clin Oncol. 2004;22:251s. doi: 10.1016/j.clcc.2011.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cassidy J, Clarke S, Diaz-Rubio E, et al. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol. 2008;26:2006–2012. doi: 10.1200/JCO.2007.14.9898. [DOI] [PubMed] [Google Scholar]