Abstract
Polycystic ovary syndrome (PCOS) is characterized by ovarian dysfunction and associated with ovarian theca-interstitial (T-I) cell hyperplasia, hyperinsulinemia, systemic inflammation and oxidative stress. This in vitro study tested whether rat T-I cell growth with or without insulin can be altered by resveratrol, a natural polyphenol with anti-carcinogenic, anti-inflammatory, anti-proliferative and antioxidant properties. Rat T-I cells were cultured with and without resveratrol and/or insulin, and the effects on DNA synthesis, number of viable cells and markers of apoptosis were evaluated. Resveratrol alone induced a potent concentration-dependent inhibition of cell growth by inhibiting DNA synthesis, decreasing the number of viable cells and increasing the activity of executioner caspases 3 and 7; these effects of resveratrol counteracted the pro-proliferative and anti-apoptotic effects of insulin. Immunofluorescence analysis of cells incubated with resveratrol showed concentration- and time-dependent morphological changes consistent with apoptosis. The present findings indicate that resveratrol promotes apoptosis to reduce rat T-I cell growth in vitro as well as inhibiting insulin-induced rat T-I cell growth. This suggests a possibility that resveratrol and/or mechanisms mediating its effect may be relevant to the development of novel treatments for PCOS, which is characterized by both excessive ovarian mesenchyma growth and hyperinsulinemia.
Keywords: apoptosis, insulin, ovarian theca-interstitial cells, proliferation, resveratrol
Introduction
Theca-interstitial (T-I) cells play a crucial role in the regulation of ovarian function. Under pathological conditions such as polycystic ovary syndrome (PCOS), the ovaries are typically enlarged, with the hyperplasia of T-I cells associated with ovarian dysfunction (Hughesdon, 1982). Furthermore, excessive growth of the T-I compartment may be induced by stimuli such as oxidative stress and insulin. This concept is supported by our previous in vitro studies, whereby insulin and moderate oxidative stress stimulated the proliferation of T-I cells (Duleba et al., 1997a, b, 2004). In addition, insulin also protected T-I cells from apoptosis (Spaczynski et al., 2005). In contrast, antioxidants inhibited the growth of T-I cells (Duleba et al., 2004). More recently, statins, which are inhibitors of 3-hydroxy-3-methylglutaryl co-enzyme A (HMG-CoA) reductase, were found to reduce T-I cell growth by inhibiting the mitogen-activated protein kinase (MAPK) pathway (Izquierdo et al., 2004; Kwintkiewicz et al., 2006a, b; Rzepczynska et al., 2009). In clinical trials, statins have been shown to improve a broad range of clinical, endocrine and metabolic aspects of PCOS (Banaszewska et al., 2007; Sathyapalan et al., 2009).
The study of natural compounds with pharmacological activity, such as polyphenols, has become an emerging trend in nutritional and pharmacologic research. Resveratrol (trans-3,5,4′-trihydroxystilbene) is a natural polyphenol synthesized by plants as a phytoalexin that protects against ultraviolet radiation and fungal infection. It is found in high concentrations in grapes, berries, nuts and red wine with potentially beneficial anti-carcinogenic, anti-inflammatory, antioxidant and cardioprotective properties (Jiang et al., 2005). Resveratrol has also been shown to exhibit profound in vitro and in vivo growth-inhibitory and apoptosis-inducing activities in several cancer cell lines and animal models of carcinogenesis (Joe et al., 2002; Jiang et al., 2005). These properties of resveratrol have been linked to the inhibition of proliferation in association with cell cycle arrest and apoptotic cell death typically observed in vitro at concentrations in the range of 25–400 µM (Joe et al., 2002; Sun et al., 2006; van Ginkel et al., 2007). In addition, resveratrol also exerts its effects by interacting with multiple cellular targets and modulating various signal transduction pathways, including the inhibition of the MAPK pathway (Athar et al., 2009). In a recent study, dietary resveratrol supplementation has been shown to reduce hepatic HMG-CoA reductase mRNA expression in hamsters fed a high-fat diet (Cho et al., 2008). Hence, resveratrol may share with statins the ability to inhibit the mevalonate pathway.
In view of the parallels between the actions of statins and resveratrol, we proposed to study the effects of resveratrol on T-I cells. In this report, for the first time, we demonstrate that resveratrol inhibits proliferation, induces apoptosis and alters morphology of T-I cells.
Materials and Methods
Animals
Immature (25-day-old) female Sprague–Dawley rats were obtained from Charles River Laboratories (Wilmington, MA, USA) and housed in an air-conditioned environment with a 12 h light: 12 h dark cycle. All animals received standard rat chow and water ad libitum. In order to stimulate folliculogenesis, the rats received daily injections of 17β-estradiol (1 mg/0.3 ml of sesame oil s.c.) for 3 days (from 28 to 30 days of age). At 31 days of age, the rats were anesthetized using ketamine and xylazine (i.p.) and sacrificed through intracardiac perfusion using 0.9% saline. All treatments and procedures were carried out in accordance with accepted standards of humane animal care as outlined in the NIH Guide for the Care and Use of Laboratory Animals with a protocol approved by the Institutional Animal Care and Use Committee at the University of California, Davis.
Cell culture and treatments
The ovaries were dissected and T-I cells purified through discontinuous Percoll gradient centrifugation as previously described (Magoffin and Erickson, 1988; Duleba et al., 1997a, b). The purity of this cell preparation has been verified using immunocytochemical assays (Duleba et al., 1997a, b). The cells were counted, with viability routinely in the 85–95% range. The purified T-I cells were cultured for up to 48 h at 37°C in an atmosphere of 5% CO2 in humidified air, in serum-free McCoy's 5A medium (supplemented with antibiotics, 0.1% BSA and 2 mM l-glutamine). The cells were incubated without (control) or with resveratrol (30–100 µM) and/or insulin (30 nM). Since resveratrol is not soluble in purely aqueous solutions, it was initially dissolved in ethanol and subsequently diluted in culture media; the final concentration of ethanol in cultures was 0.1%. Cultures not exposed to resveratrol received an identical concentration of ethanol. All the above chemicals were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Cell proliferation assays
The purified T-I cells were incubated for 48 h in 96-well culture plates with or without individual additives. The extent of DNA synthesis was determined through a thymidine incorporation assay. Radiolabeled [3H] thymidine (1 µCi/well) was added to the cells during the last 24 h of culture. At the end of the culture period, the cells were harvested with a multiwell cell harvester (PHD Harvester, Model 290; Cambridge Technology, Inc., Watertown, MA, USA). Radioactivity was measured in a liquid scintillation counter, Wallac 1409 (PerkinElmer, Shelton, CT, USA). Each treatment was carried out in at least eight replicates.
The total number of viable cells was estimated with the use of a CellTiter-Blue® Cell Viability Assay (Promega, Madison, WI, USA). This assay involves the conversion of resazurin to resorufin by metabolically active cells, resulting in the generation of a fluorescent product at the excitation wavelength 544 nm and the emission wavelength 590 nm that is proportional to the number of viable cells. Fluorescence was determined with the use of a microplate reader (Fluostar Omega, BMG, Durham, NC, USA). To validate the assay, a standard curve with a known number of cells was generated and a linear correlation was verified (r2 = 0.99; P < 0.001).
Caspase-3/7 activity assay
The measurement of apoptosis in T-I cells was made using the Apo-ONE® Homogeneous Caspase-3/7 Assay kit (Promega, Madison, WI, USA), following the manufacturer's instructions. The cells were incubated overnight in 96-well culture plates, replaced with fresh media and then treated with various concentrations of resveratrol (30–100 µM) at different time points (3, 6, 12, 24 and 48 h) in the absence or presence of insulin (30 nM). Caspase-3/7 activity was measured in a microplate reader (Fluostar Omega, BMG, Durham, NC, USA) at excitation wavelength 485 nm and emission wavelength 520 nm.
TUNEL assay
The detection of DNA fragmentation in T-I cells was determined using the HT TiterTACS™ Assay kit (Trevigen, Gaithersburg, MD, USA), following the manufacturer's instructions. The cells were incubated overnight in 96-well culture plates, replaced with fresh media, and then treated with various concentrations of resveratrol (30–100 µM) for 48 h. Briefly, the cells were fixed with 3.7% buffered formaldehyde solution for 7 min, washed with PBS, permeabilized with 100% methanol for 20 min, washed twice with PBS, digested with proteinase K for 15 min, quenched with 3% hydrogen peroxide, washed with distilled water, labeled with the TdT reaction mix and incubated at 37°C for 1 h in a humidified chamber and the reaction terminated with stop buffer. Then the cells were incubated with Strep-HRP for 10 min, washed four times with PBS, followed by the addition of TACS-Sapphire™ substrate and the colorimetric reaction was stopped with 0.2 N HCl after 30 min. Negative controls were labeled without the TdT enzyme and positive controls were generated with TACS-Nuclease™ to create DNA breaks. The colorimetric reaction was measured in a microplate reader (Fluostar Omega, BMG, Durham, NC, USA) at absorbance 450 nm.
DAPI nuclear and F-actin staining
The T-I cells were treated with resveratrol (50 and 100 µM) at various time points (6, 12, 24 and 48 h) and then subjected to DAPI and F-actin staining to observe nuclear and cellular morphological changes. Approximately 16 000 T-I cells per well were seeded in duplicate in 8-well culture slides (BD Biosciences, Bedford, MA, USA). Briefly, the cells were fixed with 4% paraformaldehyde in PBS for 30 min, washed three times with PBS, blocked with 1% BSA in PBS for 30 min, washed twice with PBS and stained with Texas red-phalloidin and DAPI (Molecular Probes, Carlsbad, CA, USA). Slides were then examined under an Olympus BX61 fluorescent microscope at 40× magnification (Olympus America, Melville, NY, USA). Apoptotic cells were morphologically defined by nuclear shrinkage and chromatin condensation or fragmentation, whereas cellular morphological features were defined by disrupted or disorganized F-actin filaments.
Statistical analysis
Statistical analysis was performed with JMP 7.0 software (SAS, Cary, NC, USA), using analysis of variance followed by post hoc pairwise comparisons using Bonferroni's correction. Baseline data are expressed as means (±SEM). Normality of distribution was assessed by the Shapiro–Wilk test. When appropriate, data were logarithmically transformed. A value of P < 0.05 was considered statistically significant.
Results
Resveratrol inhibits T-I DNA synthesis in a dose-dependent fashion
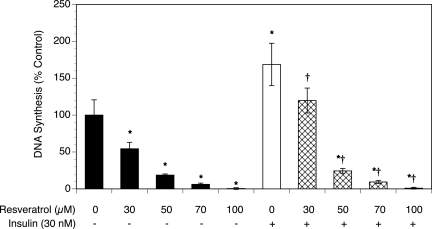
To determine whether resveratrol affects T-I cell proliferation, the extent of DNA synthesis was measured using a radiolabeled thymidine incorporation assay. As shown in Fig. 1, resveratrol significantly inhibited DNA synthesis in a dose-dependent manner compared with the control, reducing thymidine incorporation by 99% at the highest concentration of resveratrol (P < 0.001).
Figure 1.
Effect of resveratrol (30–100 µM) on proliferation in the absence and presence of insulin (30 nM).
Ovarian T-I cells were cultured for 48 h in chemically defined media. Proliferation was evaluated by determination of DNA synthesis (by thymidine incorporation). Each bar represents mean ± SEM (N = 8); * denotes means significantly different from control in the absence of insulin (P < 0.001); † denotes means significantly different from insulin alone (P < 0.001; applies only to comparison among cultures containing insulin).
We have previously reported that insulin and moderate oxidative stress stimulated the proliferation of rat T-I cells (Duleba et al., 1997a, b; Spaczynski et al., 2005; Kwintkiewicz et al., 2006a, b). In other biological systems, resveratrol has also been shown to increase insulin sensitivity and protect against insulin resistance (Baur et al., 2006; Lagouge et al., 2006). To evaluate whether resveratrol could modulate insulin-induced stimulation of cell proliferation, T-I cells were treated without (control) or with insulin (30 nM) and/or resveratrol at a concentration between 30 and 100 µM. As shown in Fig. 1, insulin alone stimulated thymidine incorporation by 69% above control levels (P < 0.01). In the presence of insulin, resveratrol also induced a dose-dependent inhibition of DNA synthesis, virtually eliminating thymidine incorporation at the highest concentration of resveratrol (P < 0.001).
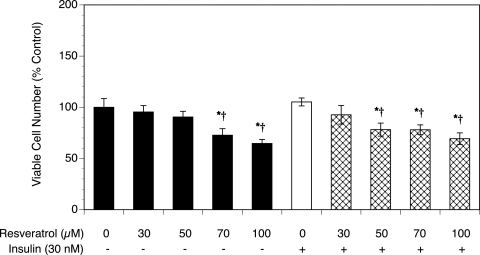
Resveratrol reduces the number of viable T-I cells
As shown in Fig. 2, resveratrol treatment resulted in a dose-dependent decrease in the number of cells, with a 35% reduction in the viable cell number at the highest concentration of resveratrol (P < 0.01). Similarly, resveratrol induced a dose-dependent decrease of the viable cell number in the presence of insulin (P < 0.05). Notably, the effects of individual treatments on the total viable cell number are typically less pronounced than the effects on DNA synthesis, especially in short-term cultures. This difference is due to the fact that DNA synthesis is a more sensitive marker of change in the proliferation rate, although the total viable cell number represents the sum of both proliferating and non-proliferating cells.
Figure 2.
Effect of resveratrol (30–100 µM) on cell viability in the absence or presence of insulin (30 nM).
Ovarian T-I cells were cultured as described in Fig. 1. Proliferation was evaluated by estimation of the number of viable cells using MTS assay. Each bar represents mean ± SEM (N = 8); * denotes means significantly different from control in the absence of insulin (P < 0.05); † denotes means significantly different from insulin alone (P < 0.05; applies only to comparison among cultures containing insulin).
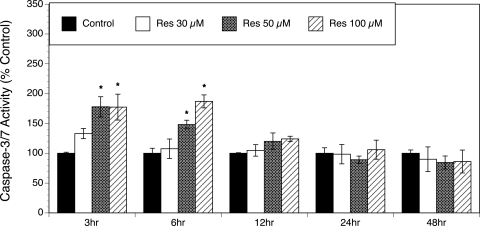
Resveratrol-induced apoptosis is mediated by caspase activation
Resveratrol has been shown to induce apoptosis in various cell lines (Joe et al., 2002; Jiang et al., 2005). To determine whether the inhibitory effect of resveratrol on the growth of T-I cells was, at least in part, due to the induction of apoptosis, caspase-3/7 activity was measured in the absence and presence of resveratrol at concentrations ranging from 30 to 100 µM and at various time points (3, 6, 12, 24 and 48 h). Figure 3 indicates that resveratrol induced a concentration-dependent increase in caspase-3/7 activation, as observed after 3 and 6 h of resveratrol treatment (P < 0.005). This caspase activation was no longer evident after 12, 24 or 48 h of exposure to resveratrol.
Figure 3.
Effect of resveratrol (30–100 µM) on caspase-3/7 activation.
Ovarian T-I cells were exposed to resveratrol (Res) for 3, 6, 12, 24 and 48 h in chemically defined media. Caspase-3/7 activity was determined by the Apo-ONE® Homogeneous Caspase-3/7 Assay (Promega, Madison, WI, USA). Each bar represents mean ± SEM (N = 4); * denotes means significantly different from control (P < 0.005).
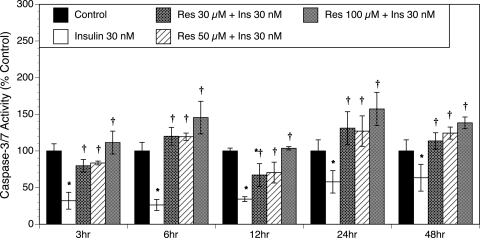
Our previous in vitro study revealed that insulin protected T-I cells from apoptosis (Spaczynski et al., 2005). The ability of insulin to modulate resveratrol-induced apoptosis was also studied. Figure 4 demonstrates that insulin alone at all of the tested time points (3, 6, 12, 24 and 48 h) protected T-I cells from caspase-3/7 activation (P < 0.01). However, resveratrol effectively counteracted the actions of insulin, as shown by a concentration-dependent increase in caspase-3/7 activity (P < 0.01).
Figure 4.
Effect of resveratrol (30–100 µM) on caspase-3/7 activity in the presence of insulin (30 nM).
Ovarian T-I cells were stimulated with 30 nM insulin (Ins) for 2 h and then incubated with different concentrations of resveratrol (Res) for 3, 6, 12, 24 and 48 h in chemically defined media. Caspase-3/7 activity was measured as indicated in Fig. 4. Each bar represents mean ± SEM (N = 4); * denotes means significantly different from control (P < 0.01); † denotes means significantly different from insulin alone (P < 0.01; applies only to comparison among cultures containing insulin).
Resveratrol induces DNA fragmentation
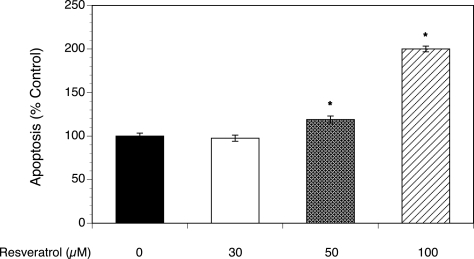
To further verify that resveratrol indeed induces apoptosis in T-I cells, an additional method of apoptosis identification, the TUNEL assay, was used to detect DNA fragmentation. As shown in Fig. 5, resveratrol induced a dose-dependent increase in DNA fragmentation (P < 0.001).
Figure 5.
Effect of resveratrol (30–100 µM) on DNA fragmentation.
The detection of apoptosis through DNA fragmentation was determined by the terminal dUTP nick-end labeling (TUNEL) assay. Ovarian T-I cells were exposed to resveratrol (Res) for 48 h in chemically defined media, fixed and labeled according to the HT TiterTACS™ (Trevigen, Gaithersburg, MD, USA) protocol prior to colorimetric analysis. Each bar represents mean ± SEM (N = 8); * denotes means significantly different from control (P < 0.001).
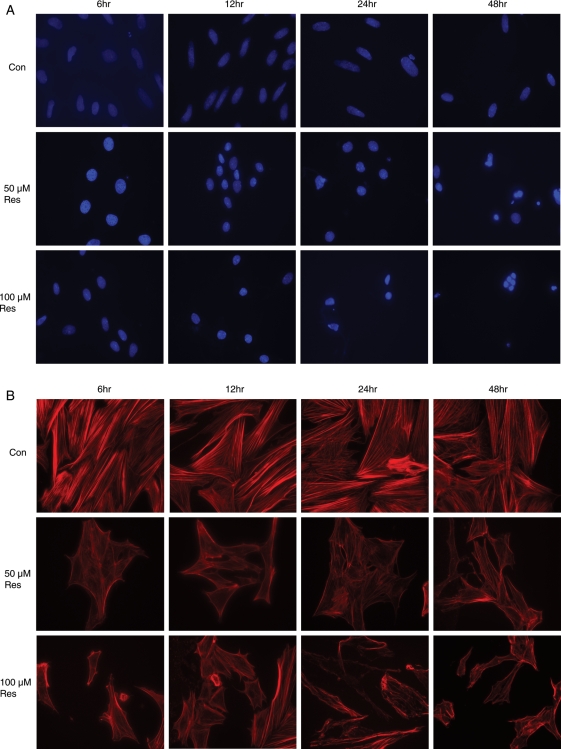
Resveratrol induces morphological changes in T-I cells
The effects of resveratrol on the nuclear morphology and cytoskeleton of T-I cells were observed under a fluorescent microscope following DAPI staining of the nucleus and F-actin staining with Texas red-phalloidin. The morphological features of apoptosis include nuclear shrinkage with condensed chromatin and cytoskeleton degradation/fragmentation. As shown in Fig. 6A, untreated control cells had large oval-shaped nuclei, although cells exposed to resveratrol had small spherical nuclei with condensed chromatin. These effects were progressive, with the most prominent changes observed after 48 h of exposure to resveratrol. As can be seen in Fig. 6B, resveratrol treatment also resulted in progressive time- and concentration-dependent degradation of the F-actin cytoskeleton, compared with the untreated control cells, which displayed linear strands of actin filaments that traversed the entire length of the cells.
Figure 6.
Effect of resveratrol (50 and 100 µM) on morphology.
Ovarian T-I cells were treated without (Con) or with 50 and 100 µM of resveratrol (Res) for 6, 12, 24 and 48 h, then fixed, stained and visualized under a fluorescent microscope (40× magnification) as described in Materials and Methods. Nuclear staining with DAPI (A) and F-actin (B) were used to observe morphological changes. Apoptotic cells were defined as chromatin condensation and nuclear fragmentation or formation of apoptotic bodies, membrane blebbing and cytoskeleton degradation.
Discussion
The present study demonstrates that resveratrol inhibits the cell proliferation and promotes the apoptosis of ovarian T-I cells by: (i) limiting DNA synthesis and cell viability, (ii) increasing caspase-3/7 activity and DNA fragmentation and (iii) inducing nuclear and cytoskeletal morphological changes consistent with apoptosis. To our knowledge, this study is the first report evaluating the effects of resveratrol on ovarian T-I cells.
Over the past decade, resveratrol has emerged as a very promising natural compound with immense therapeutic potential. In several experimental models of carcinogenesis, resveratrol inhibits cancer initiation, promotion and progression (Jang et al., 1997). However, to date, the studies evaluating the effects of resveratrol on ovarian function are limited, with data indicating that resveratrol exerts predominantly estrogenic effects (Henry and Witt, 2002, 2006). Recent studies have indicated that resveratrol counteracts oxidative stress and exerts hepatoprotective effects against acute and chronic liver damage in rodents (Kasdallah-Grissa et al., 2007, Ajmo et al., 2008). Resveratrol effectively scavenges hydroxyls and superoxides and protects against lipid peroxidation in cell membranes and DNA damage caused by reactive oxygen species (Leonard et al., 2003). In addition, resveratrol has been demonstrated to increase intracellular antioxidant levels and phase II enzymes in cultured cardiomyocytes and aortic smooth muscle cells (Cao and Li, 2004; Li et al., 2006). Our previous studies have shown that moderate oxidative stress promotes the proliferation of T-I cells while antioxidants have the opposite effects (Duleba et al., 2004; Kwintkiewicz et al., 2006a, b). Hence, it is tempting to speculate that the effects of resveratrol observed in the present study may be, at least in part, due to its antioxidant properties.
More recently, reports on the potential for resveratrol to extend lifespan in cell culture and animal models have continued to generate scientific interest. It has been reported that resveratrol mimics caloric restriction and interferes with the aging process by activating sirtuins (Howitz et al., 2003). Sirtuins are a conserved family of NAD+-dependent histone deacetylases involved in gene silencing processes related to aging and the promotion of cell survival. Indeed, resveratrol administration (and activation of SIRT1) has increased the lifespans of yeast (Howitz et al., 2003), fruit flies and worms (Wood et al., 2004), fish (Valenzano et al., 2006), and mice that are fed a high-calorie diet (Baur et al., 2006). However, not all actions of resveratrol are related to the activation of SIRT1. For example, resveratrol protects HepG2 cells from oxidative stress-induced apoptosis by mediating the downstream activation of AMP-activated protein kinase and poly(ADP-ribose) polymerase (Shin et al., 2009). In another study, resveratrol inhibited platelet-derived growth factor-stimulated proliferation of mesangial cells by inhibiting Akt and Erk1/2 independently of SIRT1 (Venkatesan et al., 2008). In several cell lines and primary rat hepatocytes, resveratrol also inhibited insulin-signaling pathways in a SIRT1-independent pathway (Zhang, 2006).
Furthermore, resveratrol has also gained prominence as a potent chemopreventive agent that interferes with signaling pathways regulating cell death and survival. Resveratrol has been reported to inhibit the proliferation of numerous human cancer cell lines, including MCF-7, HL60, SW480 and prostate LNCaP (Joe et al., 2002; Benitez et al., 2007), vascular smooth muscle cells (Mnjoyan and Fujise, 2003) and hepatic stellate cells (Souza et al., 2008). Similarly, our results show that resveratrol also dose-dependently inhibits cell proliferation by limiting DNA synthesis in T-I cells. The anti-proliferative activities of resveratrol may arise from its ability to interfere with nuclear factor-κB (NF-κB), p38 MAPK and phosphatidylinositol 3-kinase/Akt survival pathways (Fulda and Debatin, 2006), resulting in the suppression of DNA synthesis and cell proliferation, the inhibition of cell cycle progression and the induction of apoptosis. It is also important to note that the effects of resveratrol on cellular growth are not universally inhibitory and, in several biological systems, resveratrol has been shown to protect cells from death (Brito et al., 2006; Gong et al., 2007; Anekonda and Adamus, 2008; Cao et al., 2008; Rubiolo et al., 2008; Shin et al., 2009).
Apoptosis is a key regulator of tissue homeostasis during normal development and disease states. One hallmark of apoptosis is caspase activation. Indeed, numerous studies have documented that resveratrol-mediated apoptosis involves the activation of caspase 3, the executer of apoptotic cell death, in several human cancer cell lines, including U251 glioma, malignant B cells and MDA231 cells (Jiang et al., 2005; Shimizu et al., 2006; Alkhalaf et al., 2008). Our data are in agreement with these observations and indicate that, in ovarian T-I cells, resveratrol induces the activation of executioner caspases 3/7. In addition, the apoptotic effects of resveratrol were further confirmed by increased DNA fragmentation using the TUNEL assay.
We also observed that resveratrol induced progressive dose- and time-dependent morphological changes that are consistent with apoptosis, such as cell and nuclei shrinkage and nuclear fragmentation. Under physiological conditions, the actin cytoskeleton contributes to the maintenance of plasma membrane integrity and cell shape. There is increasing evidence for an association between actin filament disruption and the initiation of apoptosis, as actin may act as an early modulator of apoptotic commitment (White et al., 2001; Bando et al., 2002). For example, in some cell types, cells with fragmented nuclei also displayed completely disorganized F-actin (Van de Water et al., 1996; Atencia et al., 2000). In another study, the cytoskeletal disruption in apoptotic cells potentiated mitochondrial membrane permeability, thereby enhancing cytochrome c release and the subsequent activation of the caspase cascade (Yamazaki et al., 2000). In the same study, it was also found that actin degradation accelerated caspase 3 activation (Yamazaki et al., 2000). Our findings on resveratrol-induced F-actin cytoskeletal changes are also in agreement with observations from other biological in vitro systems, indicating that resveratrol alters cell morphology (Bruder et al., 2001; Azios and Dharmawardhane, 2005; Azios et al., 2007).
In the present study, the effects of resveratrol were detected at concentrations ranging from 30 to 100 µM, which were comparable to those used in other studies, whereby resveratrol inhibited the proliferation of various cell types at concentrations in the range of 25–400 µM (Sun et al., 2006; Tang et al., 2006; Bhardwaj et al., 2007; Hwang et al., 2007; van Ginkel et al., 2007). These in vitro observations may be of clinical relevance as the bioavailability of resveratrol in human and in rodent models is in the micromolar range (Walle et al., 2004; van Ginkel et al., 2007). For example, in a phase I pharmacokinetic study of healthy volunteers, the peak plasma levels of resveratrol were above 2 µM and the peak levels of conjugated metabolites of resveratrol were up to 8-fold higher (Boocock et al., 2007). Interestingly, the effects of resveratrol in vivo may be detected at lower concentrations than those observed in vitro. For example, in the nude mouse model of neuroblastoma, resveratrol remained remarkably effective in inhibiting tumor growth, with serum levels in the 2–10 µM range within 30 min of oral gavage (van Ginkel et al., 2007). In the same study, resveratrol induced marked growth inhibition and subsequent loss of cultured human neuroblastoma cell viability, with IC50 values in the range of 70–120 µM (van Ginkel et al., 2007). Thus, the concentration of resveratrol (in the micromolar range) required to inhibit proliferation and to induce apoptosis in our model agrees with those used in other in vitro studies (Joe et al., 2002; Sun et al., 2006; van Ginkel et al., 2007), indicating that resveratrol, at pharmacological concentrations, may be effective in limiting excessive ovarian T-I cell growth.
Our present findings may have potential clinical applications. In PCOS, the typically enlarged ovaries are characterized by thecal and stromal hyperplasia (Hughesdon, 1982). This ovarian enlargement is associated with excessive ovarian androgen production and the disruption of menstrual cyclicity. Improvement of ovarian function, with restoration of ovulation and fertility, was observed with surgical reduction of ovarian size and/or partial destruction of ovarian tissues by procedures such as wedge resection and laparoscopic ovarian drilling (Donesky and Adashi, 1995; Duleba et al., 2003). It is tempting to speculate that the reduction in the growth of T-I cells as a result of resveratrol treatment may be of similar benefit. However, in view of the significant differences between the ovarian physiology of human and rodent, the present findings should be interpreted with caution and the present observations should be validated with human T-I cells.
In summary, our results suggest that resveratrol exerts its anti-proliferative and pro-apoptotic actions by mediating caspase-3/7 activation and inducing morphologic changes in cultured ovarian T-I cells. These findings are of potential translational relevance to conditions associated with excessive growth of T-I cells, such as PCOS, whereby resveratrol may represent a novel therapeutic agent.
Authors' Roles
D.H.W. planned and ran the experiments, conducted statistical analysis and wrote the manuscript. J.A.V. assisted with experiments and reviewed the manuscript. A.B.C. assisted with experiments and reviewed the manuscript. A.J.D. planned the experiments, supervised and contributed to data interpretation and writing the manuscript.
Funding
This study was supported by grant R01-HD050656 from the Eunice Shriver National Institute of Child Health and Human Development (to A.J.D.).
References
- Ajmo JM, Liang X, Rogers CQ, Pennock B, You M. Resveratrol alleviates alcoholic fatty liver in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295:G833–G842. doi: 10.1152/ajpgi.90358.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkhalaf M, El-Mowafy A, Renno W, Rachid O, Ali A, Al-Attyiah R. Resveratrol-induced apoptosis in human breast cancer cells is mediated primarily through the caspase-3-dependent pathway. Arch Med Res. 2008;39:162–168. doi: 10.1016/j.arcmed.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Anekonda TS, Adamus G. Resveratrol prevents antibody-induced apoptotic death of retinal cells through upregulation of Sirt1 and Ku70. BMC Res Notes. 2008;1:122. doi: 10.1186/1756-0500-1-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atencia R, Asumendi A, Garcia-Sanz M. Role of cytoskeleton in apoptosis. Vitam Horm. 2000;58:267–297. doi: 10.1016/s0083-6729(00)58028-5. [DOI] [PubMed] [Google Scholar]
- Athar M, Back JH, Kopelovich L, Bickers DR, Kim AL. Multiple molecular targets of resveratrol: Anti-carcinogenic mechanisms. Arch Biochem Biophys. 2009;486:95–102. doi: 10.1016/j.abb.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azios NG, Dharmawardhane SF. Resveratrol and estradiol exert disparate effects on cell migration, cell surface actin structures, and focal adhesion assembly in MDA-MB-231 human breast cancer cells. Neoplasia. 2005;7:128–140. doi: 10.1593/neo.04346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azios NG, Krishnamoorthy L, Harris M, Cubano LA, Cammer M, Dharmawardhane SF. Estrogen and resveratrol regulate Rac and Cdc42 signaling to the actin cytoskeleton of metastatic breast cancer cells. Neoplasia. 2007;9:147–158. doi: 10.1593/neo.06778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banaszewska B, Pawelczyk L, Spaczynski RZ, Dziura J, Duleba AJ. Effects of simvastatin and oral contraceptive agent on polycystic ovary syndrome: prospective, randomized, crossover trial. J Clin Endocrinol Metab. 2007;92:456–461. doi: 10.1210/jc.2006-1988. [DOI] [PubMed] [Google Scholar]
- Bando M, Miyake Y, Shiina M, Wachi M, Nagai K, Kataoka T. Actin cytoskeleton is required for early apoptosis signaling induced by anti-Fas antibody but not Fas ligand in murine B lymphoma A20 cells. Biochem Biophys Res Commun. 2002;290:268–274. doi: 10.1006/bbrc.2001.6199. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitez DA, Pozo-Guisado E, Alvarez-Barrientos A, Fernandez-Salguero PM, Castellon EA. Mechanisms involved in resveratrol-induced apoptosis and cell cycle arrest in prostate cancer-derived cell lines. J Androl. 2007;28:282–293. doi: 10.2164/jandrol.106.000968. [DOI] [PubMed] [Google Scholar]
- Bhardwaj A, Sethi G, Vadhan-Raj S, Bueso-Ramos C, Takada Y, Gaur U, Nair AS, Shishodia S, Aggarwal BB. Resveratrol inhibits proliferation, induces apoptosis, and overcomes chemoresistance through down-regulation of STAT3 and nuclear factor-kappaB-regulated antiapoptotic and cell survival gene products in human multiple myeloma cells. Blood. 2007;109:2293–2302. doi: 10.1182/blood-2006-02-003988. [DOI] [PubMed] [Google Scholar]
- Boocock DJ, Faust GE, Patel KR, Schinas AM, Brown VA, Ducharme MP, Booth TD, Crowell JA, Perloff M, Gescher AJ, et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol Biomarkers Prev. 2007;16:1246–1252. doi: 10.1158/1055-9965.EPI-07-0022. [DOI] [PubMed] [Google Scholar]
- Brito PM, Mariano A, Almeida LM, Dinis TC. Resveratrol affords protection against peroxynitrite-mediated endothelial cell death: A role for intracellular glutathione. Chem Biol Interact. 2006;164:157–166. doi: 10.1016/j.cbi.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Bruder JL, Hsieh T, Lerea KM, Olson SC, Wu JM. Induced cytoskeletal changes in bovine pulmonary artery endothelial cells by resveratrol and the accompanying modified responses to arterial shear stress. BMC Cell Biol. 2001;2:1. doi: 10.1186/1471-2121-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z, Li Y. Potent induction of cellular antioxidants and phase 2 enzymes by resveratrol in cardiomyocytes: protection against oxidative and electrophilic injury. Eur J Pharmacol. 2004;489:39–48. doi: 10.1016/j.ejphar.2004.02.031. [DOI] [PubMed] [Google Scholar]
- Cao C, Lu S, Kivlin R, Wallin B, Card E, Bagdasarian A, Tamakloe T, Wang WJ, Song X, Chu WM, et al. SIRT1 confers protection against UVB- and H(2)O(2)-induced cell death via modulation of p53 and JNK in cultured skin keratinocytes. J Cell Mol Med. 2008 doi: 10.1111/j.1582-4934.2008.00453.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho IJ, Ahn JY, Kim S, Choi MS, Ha TY. Resveratrol attenuates the expression of HMG-CoA reductase mRNA in hamsters. Biochem Biophys Res Commun. 2008;367:190–194. doi: 10.1016/j.bbrc.2007.12.140. [DOI] [PubMed] [Google Scholar]
- Donesky BW, Adashi EY. Surgically induced ovulation in the polycystic ovary syndrome: wedge resection revisited in the age of laparoscopy. Fertil Steril. 1995;63:439–463. doi: 10.1016/s0015-0282(16)57408-1. [DOI] [PubMed] [Google Scholar]
- Duleba AJ, Spaczynski RZ, Olive DL, Behrman HR. Effects of insulin and insulin-like growth factors on proliferation of rat ovarian theca-interstitial cells. Biol Reprod. 1997a;56:891–897. doi: 10.1095/biolreprod56.4.891. [DOI] [PubMed] [Google Scholar]
- Duleba AJ, Spaczynski RZ, Olive DL, Behrman HR. Effects of insulin and insulin-like growth factors on proliferation of rat ovarian theca-interstitial cells. Biol Reprod. 1997b;56:891–897. doi: 10.1095/biolreprod56.4.891. [DOI] [PubMed] [Google Scholar]
- Duleba AJ, Banaszewska B, Spaczynski RZ, Pawelczyk L. Success of laparoscopic ovarian wedge resection is related to obesity, lipid profile, and insulin levels. Fertil Steril. 2003;79:1008–1014. doi: 10.1016/s0015-0282(02)04848-3. [DOI] [PubMed] [Google Scholar]
- Duleba AJ, Foyouzi N, Karaca M, Pehlivan T, Kwintkiewicz J, Behrman HR. Proliferation of ovarian theca-interstitial cells is modulated by antioxidants and oxidative stress. Hum Reprod. 2004;19:1519–1524. doi: 10.1093/humrep/deh299. [DOI] [PubMed] [Google Scholar]
- Fulda S, Debatin KM. Resveratrol modulation of signal transduction in apoptosis and cell survival: a mini-review. Cancer Detect Prev. 2006;30:217–223. doi: 10.1016/j.cdp.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Gong QH, Wang Q, Shi JS, Huang XN, Liu Q, Ma H. Inhibition of caspases and intracellular free Ca2+ concentrations are involved in resveratrol protection against apoptosis in rat primary neuron cultures. Acta Pharmacol Sin. 2007;28:1724–1730. doi: 10.1111/j.1745-7254.2007.00666.x. [DOI] [PubMed] [Google Scholar]
- Henry LA, Witt DM. Resveratrol: phytoestrogen effects on reproductive physiology and behavior in female rats. Horm Behav. 2002;41:220–228. doi: 10.1006/hbeh.2001.1754. [DOI] [PubMed] [Google Scholar]
- Henry LA, Witt DM. Effects of neonatal resveratrol exposure on adult male and female reproductive physiology and behavior. Dev Neurosci. 2006;28:186–195. doi: 10.1159/000091916. [DOI] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Hughesdon PE. Morphology and morphogenesis of the Stein-Leventhal ovary and of so called ‘hyperthecosis’. Obstet Gynecol Surv. 1982;37:59–77. doi: 10.1097/00006254-198202000-00001. [DOI] [PubMed] [Google Scholar]
- Hwang JT, Kwak DW, Lin SK, Kim HM, Kim YM, Park OJ. Resveratrol induces apoptosis in chemoresistant cancer cells via modulation of AMPK signaling pathway. Ann N Y Acad Sci. 2007;1095:441–448. doi: 10.1196/annals.1397.047. [DOI] [PubMed] [Google Scholar]
- Izquierdo D, Foyouzi N, Kwintkiewicz J, Duleba AJ. Mevastatin inhibits ovarian theca-interstitial cell proliferation and steroidogenesis. Fertil Steril. 2004;82:1193–1197. doi: 10.1016/j.fertnstert.2004.03.037. [DOI] [PubMed] [Google Scholar]
- Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–220. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- Jiang H, Zhang L, Kuo J, Kuo K, Gautam SC, Groc L, Rodriguez AI, Koubi D, Hunter TJ, Corcoran GB, et al. Resveratrol-induced apoptotic death in human U251 glioma cells. Mol Cancer Ther. 2005;4:554–561. doi: 10.1158/1535-7163.MCT-04-0056. [DOI] [PubMed] [Google Scholar]
- Joe AK, Liu H, Suzui M, Vural ME, Xiao D, Weinstein IB. Resveratrol induces growth inhibition, S-phase arrest, apoptosis, and changes in biomarker expression in several human cancer cell lines. Clin Cancer Res. 2002;8:893–903. [PubMed] [Google Scholar]
- Kasdallah-Grissa A, Mornagui B, Aouani E, Hammami M, El May M, Gharbi N, Kamoun A, El-Fazaa S. Resveratrol, a red wine polyphenol, attenuates ethanol-induced oxidative stress in rat liver. Life Sci. 2007;80:1033–1039. doi: 10.1016/j.lfs.2006.11.044. [DOI] [PubMed] [Google Scholar]
- Kwintkiewicz J, Foyouzi N, Piotrowski P, Rzepczynska I, Duleba AJ. Mevastatin inhibits proliferation of rat ovarian theca-interstitial cells by blocking the mitogen-activated protein kinase pathway. Fertil Steril. 2006a;86:1053–1058. doi: 10.1016/j.fertnstert.2006.04.020. [DOI] [PubMed] [Google Scholar]
- Kwintkiewicz J, Spaczynski RZ, Foyouzi N, Pehlivan T, Duleba AJ. Insulin and oxidative stress modulate proliferation of rat ovarian theca-interstitial cells through diverse signal transduction pathways. Biol Reprod. 2006b;74:1034–1040. doi: 10.1095/biolreprod.105.049908. [DOI] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Leonard SS, Xia C, Jiang BH, Stinefelt B, Klandorf H, Harris GK, Shi X. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem Biophys Res Commun. 2003;309:1017–1026. doi: 10.1016/j.bbrc.2003.08.105. [DOI] [PubMed] [Google Scholar]
- Li Y, Cao Z, Zhu H. Upregulation of endogenous antioxidants and phase 2 enzymes by the red wine polyphenol, resveratrol in cultured aortic smooth muscle cells leads to cytoprotection against oxidative and electrophilic stress. Pharmacol Res. 2006;53:6–15. doi: 10.1016/j.phrs.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Magoffin DA, Erickson GF. Purification of ovarian theca-interstitial cells by density gradient centrifugation. Endocrinology. 1988;122:2345–2347. doi: 10.1210/endo-122-5-2345. [DOI] [PubMed] [Google Scholar]
- Mnjoyan ZH, Fujise K. Profound negative regulatory effects by resveratrol on vascular smooth muscle cells: a role of p53-p21(WAF1/CIP1) pathway. Biochem Biophys Res Commun. 2003;311:546–552. doi: 10.1016/j.bbrc.2003.10.023. [DOI] [PubMed] [Google Scholar]
- Rubiolo JA, Mithieux G, Vega FV. Resveratrol protects primary rat hepatocytes against oxidative stress damage: activation of the Nrf2 transcription factor and augmented activities of antioxidant enzymes. Eur J Pharmacol. 2008;591:66–72. doi: 10.1016/j.ejphar.2008.06.067. [DOI] [PubMed] [Google Scholar]
- Rzepczynska IJ, Piotrowski PC, Wong DH, Cress AB, Villanueva J, Duleba AJ. Role of Isoprenylation in Simvastatin-Induced Inhibition of Ovarian Theca-Interstitial Growth in the Rat. Biol Reprod. 2009;81:850–855. doi: 10.1095/biolreprod.109.078667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyapalan T, Kilpatrick ES, Coady AM, Atkin SL. The effect of atorvastatin in patients with polycystic ovary syndrome: a randomized double-blind placebo-controlled study. J Clin Endocrinol Metab. 2009;94:103–108. doi: 10.1210/jc.2008-1750. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Nakazato T, Xian MJ, Sagawa M, Ikeda Y, Kizaki M. Resveratrol induces apoptosis of human malignant B cells by activation of caspase-3 and p38 MAP kinase pathways. Biochem Pharmacol. 2006;71:742–750. doi: 10.1016/j.bcp.2005.12.018. [DOI] [PubMed] [Google Scholar]
- Shin SM, Cho IJ, Kim SG. Resveratrol protects mitochondria against oxidative stress through AMP-activated protein kinase-mediated glycogen synthase kinase-3beta inhibition downstream of poly(ADP-ribose)polymerase-LKB1 pathway. Mol Pharmacol. 2009;76:884–895. doi: 10.1124/mol.109.058479. [DOI] [PubMed] [Google Scholar]
- Souza IC, Martins LA, Coelho BP, Grivicich I, Guaragna RM, Gottfried C, Borojevic R, Guma FC. Resveratrol inhibits cell growth by inducing cell cycle arrest in activated hepatic stellate cells. Mol Cell Biochem. 2008;315:1–7. doi: 10.1007/s11010-008-9781-x. [DOI] [PubMed] [Google Scholar]
- Spaczynski RZ, Tilly JL, Mansour A, Duleba AJ. Insulin and insulin-like growth factors inhibit and luteinizing hormone augments ovarian theca-interstitial cell apoptosis. Mol Hum Reprod. 2005;11:319–324. doi: 10.1093/molehr/gah168. [DOI] [PubMed] [Google Scholar]
- Sun C, Hu Y, Liu X, Wu T, Wang Y, He W, Wei W. Resveratrol downregulates the constitutional activation of nuclear factor-kappaB in multiple myeloma cells, leading to suppression of proliferation and invasion, arrest of cell cycle, and induction of apoptosis. Cancer Genet Cytogenet. 2006;165:9–19. doi: 10.1016/j.cancergencyto.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Tang HY, Shih A, Cao HJ, Davis FB, Davis PJ, Lin HY. Resveratrol-induced cyclooxygenase-2 facilitates p53-dependent apoptosis in human breast cancer cells. Mol Cancer Ther. 2006;5:2034–2042. doi: 10.1158/1535-7163.MCT-06-0216. [DOI] [PubMed] [Google Scholar]
- Valenzano DR, Terzibasi E, Genade T, Cattaneo A, Domenici L, Cellerino A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol. 2006;16:296–300. doi: 10.1016/j.cub.2005.12.038. [DOI] [PubMed] [Google Scholar]
- Van de Water B, Kruidering M, Nagelkerke JF. F-actin disorganization in apoptotic cell death of cultured rat renal proximal tubular cells. Am J Physiol. 1996;270:F593–F603. doi: 10.1152/ajprenal.1996.270.4.F593. [DOI] [PubMed] [Google Scholar]
- van Ginkel PR, Sareen D, Subramanian L, Walker Q, Darjatmoko SR, Lindstrom MJ, Kulkarni A, Albert DM, Polans AS. Resveratrol inhibits tumor growth of human neuroblastoma and mediates apoptosis by directly targeting mitochondria. Clin Cancer Res. 2007;13:5162–5169. doi: 10.1158/1078-0432.CCR-07-0347. [DOI] [PubMed] [Google Scholar]
- Venkatesan B, Ghosh-Choudhury N, Das F, Mahimainathan L, Kamat A, Kasinath BS, Abboud HE, Choudhury GG. Resveratrol inhibits PDGF receptor mitogenic signaling in mesangial cells: role of PTP1B. FASEB J. 2008;22:3469–3482. doi: 10.1096/fj.08-109488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walle T, Hsieh F, DeLegge MH, Oatis JE, Jr, Walle UK. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32:1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- White SR, Williams P, Wojcik KR, Sun S, Hiemstra PS, Rabe KF, Dorscheid DR. Initiation of apoptosis by actin cytoskeletal derangement in human airway epithelial cells. Am J Respir Cell Mol Biol. 2001;24:282–294. doi: 10.1165/ajrcmb.24.3.3995. [DOI] [PubMed] [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Tsuruga M, Zhou D, Fujita Y, Shang X, Dang Y, Kawasaki K, Oka S. Cytoskeletal disruption accelerates caspase-3 activation and alters the intracellular membrane reorganization in DNA damage-induced apoptosis. Exp Cell Res. 2000;259:64–78. doi: 10.1006/excr.2000.4970. [DOI] [PubMed] [Google Scholar]
- Zhang J. Resveratrol inhibits insulin responses in a SirT1-independent pathway. Biochem J. 2006;397:519–527. doi: 10.1042/BJ20050977. [DOI] [PMC free article] [PubMed] [Google Scholar]