Abstract
Bioassay-guided separation of an extract of the wings from a Taiwan butterfly, Byasa polyeuctes termessa, allowed isolation of a new cancer cell growth inhibitor designated papilistatin (1a). The structure was determined by analysis of 1D and 2D NMR spectra and by HRMS. Against a panel of six human and the murine P388 leukemia cancer cell lines, papilistatin exhibited cancer cell growth inhibition with GI50s of 0.093–3.5 μg/mL. Papilistatin was also found to have antibacterial activity.
Insects are now believed to number some four to six million species,2 with butterflies and moths (order Lepidoptera) comprising over 100,000 species.3 Such an enormous reservoir of as yet unexplored compounds that could include biologically active structures is most compelling. Recent examples that provide some insight into the scope of this potential reservoir include the pheromones of the giant white butterfly Idea leuconoe,4a spiroacetals from a parasitoid wasp (order Hymenoptera),4b the defensive spray of a walkingstick insect (order Phasmatodea),4c and the anticancer drug pancratistatin from a grasshopper (order Saltatoria).4d The medically important toxins of certain Brazilian centipede (class Chilopoda) venoms present another dimension.5 Investigation of other Arthropoda classes such as the arachnids, comprising nearly 40,000 species, are leading to discoveries of new and potentially useful constituents.6 Illustrative are the p53-MDM2/MDMX inhibitor, derived from a toxin of an Asian scorpion,6a and the sulfated nucleoside constituents of a spider venom.6b
In 1965–66, we began to pioneer the first systematic investigation of arthropods for antineoplastic components and summarized the initial promising results in 1968.3 Our most recent advance in that area was the isolation from a Texas grasshopper of pancratistatin,4d a preclinical anticancer drug that had in the meantime been isolated by us from an African plant. Another class Insecta metabolite investigation that was delayed until recently pertains to the papilionid butterfly Byasa polyeuctes termessa (Atrophaneura polyeuctes termessus Fruhstorfer), which was collected in Taiwan in 1967. We now report the isolation and structure of a new cancer cell growth inhibitor from the butterfly, designated papilistatin (1a).
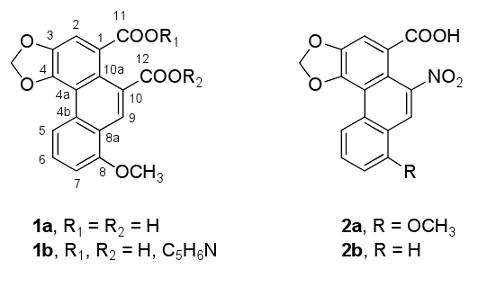
To our knowledge, no prior chemical studies of B. polyeuctes have been reported, nor of the genus, except that one member, Byasa alcinous (Atrophaneura alcinous Klug), was investigated for papilochromes7 and butyric acids8 with negative results. In addition, it was reported that B. alcinous is attracted to Aristolochia debilis,9 one of many Aristolochiaceae species that are used as herbal folk remedies. The active compounds that have been isolated from these plants10,11 include the aristolochic acids (AA), of which aristolochic acid I (2a) and aristolochic acid II (2b) are the major components; also in the active extracts are aristolactams and terpenoids. Kupchan and Doskotch10a reported in 1962 that a sample of 2a from A. indica was active in the US National Cancer Institute's in vivo adenocarcinoma 755 evaluation system, and two years later its first phase 1 human cancer clinical trial was reported.12 Subsequently, 2a (or the AA mixture) was linked to the development of tumors in mice and rats, and AA was associated with the carcinogenicity and nephrotoxicity of certain Chinese and Balkan herbs.13a-d The evidence now indicates that 2a and 2b form DNA adducts that are genotoxic mutagens, following metabolic activation via reduction of the nitro group.13a-c
Results and Discussion
A 1967 collection of Byasa polyeuctes termessa was dissected and the anticancer activity was located in the wings. In an assay at the U.S. National Cancer Institute using random-bred albino rats bearing the Walker 256 carcinoma (subcutaneous), a 95% ethanol extract of the wings led to a 68-78% (at 400 mg/kg) reduction in tumor growth by day 10 and was judged a confirmed active. A solution of the ethanol extract (4.8 g) in CH3OH–CH2Cl2 (9:1) was filtered to collect the solid phase (0.11 g). The solution was concentrated to dryness and taken up in CH3OH. A yellow amorphous powder (48 mg) that separated was removed, and the remaining solution was fractionated on a Sephadex LH-20 column, with CH3OH as eluent. The P388-active fractions were further separated by successive column chromatographic procedures using Sephadex LH-20. Final separation and purification was achieved by repeated reversed-phase high-performance liquid chromatography (CH3CN–H2O or CH3OH–H2O) to afford papilistatin (1a, 15 mg) as a yellow powder.
The structure of 1a was determined by detailed spectroscopic analyses. The IR spectrum showed hydroxyl group (3400 cm−1) and aromatic ring (1590 cm−1, 1395 cm−1) absorptions. Resonances in the 1H NMR spectrum were indicative of an OCH3 group (δ 3.89), a 1,2,3-substituted aromatic ring (doublets at δ 7.13 and δ 8.73 and a triplet at δ 7.75), and another two aromatic protons (singlets at δ 8.21 and δ 8.96). In the HMBC NMR spectrum, the carbon signal at δ 156.9 (s) showed long-range correlations with protons at δ 3.89 (3H, s), δ 7.13 (1 H, d), δ 7.75 (1 H, dd), and δ 8.96 (1H, s), supporting the placement of a methoxy group at C-8, which was confirmed by the ROESY correlations between the methyl protons at δ 3.89 and the two protons at δ 7.13 and δ 8.96 (Figure 1). The HMBC correlation between the two protons at δ 6.34 and the carbons at δ 146.5 (C-3) and δ 146.3 (C-4) supported the placement of the methylene group. The structures of 1a and 2a are closely related, suggesting the possibility that B. polyeuctes acquires 1a by feeding on an Aristolochia species. Compounds 2a and 2b have been found in other southeast Asian swallowtail butterflies that feed on AA-containing Aristolochia species, and their assumed role in chemical defense is under investigation.14 Presumably, the Byasa species concentrates papilistatin (1a) from such a plant source, possibly for a variety of defensive purposes, which should stimulate future biological studies.
Figure 1.

Key HMBC and ROE correlations for papilistatin (1a).
When screened against a panel of six human cancer cell lines and the P388 lymphocytic leukemia, 1a exhibited strong selective activity against the colon KM20L-2 and pancreas BXPC-3 lines, with GI50 values of 0.096 and 0.093 μg/mL, respectively (see Experimental). Against a panel of bacteria and fungi, 1a had primarily anti-Gram-positive antibacterial activity (see Experimental), inhibiting growth of Streptococcus pneumoniae (minimum inhibitory concentration [MIC] 32 μg/mL), Micrococcus luteus (MIC 16–32 μg/mL), Staphylococcus aureus (MIC 64 μg/mL), and Enterococcus faecalis (MIC 16–32 μg/mL). Compound 1a also inhibited growth of the pathogenic Gram-negative bacterium Neisseria gonorrhoeae (MIC 8–16 μg/mL).
Experimental Section
General Experimental Procedures
Solvents used for column chromatography were distilled. Sephadex LH-20 (particle size 25–100 μm) used in gel permeation and partition column chromatographic separations was obtained from Pharmacia Fine Chemicals AB, Uppsala, Sweden. The TLC plates were viewed under shortwave UV light and then developed by 10% H2SO4 spray reagent followed by heating at approximately 150 °C. For HPLC separations, a Phenomenex Luna (particle size 5 μm, 250 mm × 10.0 mm) C18 column and a Phenomenex Luna (particle size 5 μm, 250 mm × 4.6 mm) C18 column were used in reversed-phase mode with a Waters Delta (model 600E) solvent metering pump and a Waters 2487 Dual λ absorbance detector. IR spectra were recorded using an Avatar 360 FT-IR instrument with the sample prepared in a CH2Cl2 film. The UV spectrum was recorded with a Perkin-Elmer 336. The NMR experiments were conducted using a Varian Unity Inova 500 spectrometer operating at 500 and 120 MHz for 1H and 13C NMR, respectively.
Byasa polyeuctes termessa
The papilionid butterfly was obtained in various areas of Taiwan in 1967 by collectors employed by the Butterfly Company of New York, New York, under the supervision of professional entomologists, and supplied to our Institute. Such collections usually involved several thousand specimens of each species, and voucher specimens of this Byasa species have been maintained in the ASU Cancer Research Institute.
Isolation of Papilistatin (1a)
Isolation of an active cancer cell growth inhibitor from the Byasa species was conveniently realized as follows. The butterfly was first dissected into a cross section of anatomical parts, principally the heads, abdomens, thoraxes, and wings. At that time (1967–1969), the initial anticancer activity was detected in 95% ethanol extracts of the wings using the Walker 256 carcinoma (subcutaneous) in random-bred albino rats under the auspices of the U.S. National Cancer Institute.3 The extract was given intraperitoneally (in saline) on the first day of tumor transplant and repeated over each of five days, with growth of the tumor measured on day 10. The result was 68-78% (at 400 mg/kg) growth inhibition, a confirmed active level.
Prior to preparation of the ethanol extract, the wings (83 g) were given a pretreatment with petroleum ether and 1:1 ethanol–H2O3 to yield petroleum ether (3.5 g), 50% ethanol (11.0 g) and 95% ethanol (4.8 g) extracts. By means of bioassay-guided separation using the murine P388 lymphocytic leukemia, the isolation of papilistatin (1a) proceeded as follows. The 95% ethanol extract (4.8 g) was taken up in 9:1 CH3OH–CH2Cl2, and the less soluble material (0.11 g) was removed. Removal of solvent from the solution left a residue that was dissolved in CH3OH (48 mg of insoluble material was removed) and subjected to gel permeation chromatography on Sephadex LH-20. A total of eight cancer-inhibitory fractions (4.7 g altogether) were recovered. Two of these fractions were combined (1.2 g) and subjected to successive chromatographic separations on Sephadex LH-20 in CH3OH–CH2Cl2 (2:3), hexane–2-propanol–CH3OH (8:1:1), and hexane–CH2Cl2–acetone (4:3:3) as eluents. Final purification by employment of repeated HPLC in 5:95 to 4:1 CH3CN–H2O afforded papilistatin (1a) as a yellow amorphous powder (15 mg): UV (EtOH) λmax 322, 285 nm; IR (CH2Cl2) νmax 3400, 2960, 2880, 1731, 1590, 1395, 1350, 1275, 1050, 820, 780 cm-1; 1H NMR (C5D5N, 500 MHz) δ 8.96 (1H, s, H-9), 8.73 (1H, d, J = 8.5 Hz, H-5), 8.21 (1H, s, H-2), 7.75 (1H, dd, J = 8.5, 8.0 Hz, H-6), 7.13 (1H, d, J = 8.0 Hz, H-7), 6.34 (2H, s, OCH2O), 3.89 (3H, s, OCH3); 13C NMR (C5D5N, 120 MHz) δ 169.8 (s, C-11), 156.9 (s, C-8), 147.3 (s, C-12), 146.5 (s, C-3), 146.3 (s, C-4), 131.2 (d, C-6), 131.0 (s, C-4b), 126.6 (s, C-1*), 120.3 (d, C-9), 120.1 (s, C-4a), 119.4 (d, C-5), 118.6 (s, C-8a), 118.4 (s, C-10a), 113.1 (d, C-2), 108.4 (d, C-7), 103.0 (t, OCH2O), 97.5 (s, C-10*), 56.0 (q, OCH3) (assignments marked with an asterisk are interchangeable); HRMS (APCI+) m/z 324.0615 (calcd for C18H12O6 + 2H − H2O, 324.0634).
From a pyridine–hexane solution, yellow crystals of papilistatin pyridinium salt 1b were obtained: mp 262.1 °C (dec).
Cancer Cell Line Evaluations
The GI50 (μg/mL) results for papilistatin (1a) against six human cancer cell lines and the murine P388 lymphocytic leukemia are as follows: breast MCF-7 (3.5), CNS-28 (>1), colon KM20L-2 (0.096), lung NCI-H460 (>1), pancreatic BXPC-3 (0.093), prostate DU-145 (>1), and P388 leukemia (3.4). These experiments were conducted as previously described.15
Antimicrobial Susceptibility Testing
Compound 1a was screened against the bacteria Stenotrophomonas maltophilia ATCC 13637, Micrococcus luteus Presque Isle 456, Staphylococcus aureus ATCC 29213, Escherichia coli ATCC 25922, Enterobacter cloacae ATCC 13047, Enterococcus faecalis ATCC 29212, Streptococcus pneumoniae ATCC 6303, and Neisseria gonorrhoeae ATCC 49226, and against the fungi Candida albicans ATCC 90028 and Cryptococcus neoformans ATCC 90112, according to established broth microdilution susceptibility assays.16,17
Supplementary Material
Acknowledgments
We are pleased to acknowledge financial support provided by grants RO1 CA90441-01-05, 2R56 CA090441-06A1, and 5RO1 CA 090441-07 awarded by the Division of Cancer Treatment and Diagnosis, National Cancer Institute, DHHS; the Arizona Disease Control Research Commission; the Robert B. Dalton Endowment Fund; Dr. A. D. Keith; Dr. W. Crisp; and Mrs. A. Crisp. For other assistance we thank Drs. J.-C. Chapuis, D. Doubek, and J. L. Hartwell, as well as B. Abbott, J. F. Day, E. Glanz, C. Weber, and L. Williams.
Footnotes
Dedicated to the memory of Professor Georgy B. Elyakov (1929-2005), a pioneering expert in the chemistry of naturally occurring substances, especially from marine organisms.
Supporting Information Available: NMR spectra of compound 1a. This material is available free of charge via the Internet at http://pubs.acs.org.
References and Notes
- 1.Contribution 573 of the series Antineoplastic Agents. For part 572, refer to Smith AB, III, Razler TM, Meis RM, Pettit GR. J Org Chem. 2008;73:1201–1208. doi: 10.1021/jo701816h.
- 2.Trowell S. The Futurist. 2003:17–19. [Google Scholar]
- 3.Pettit GR, Hartwell JL, Wood HB. Cancer Res. 1968;28:2168–2169. [PubMed] [Google Scholar]
- 4.(a) Sabitha G, Fatima N, Reddy EV, Yadav JS. Tetrahedron Lett. 2008;49:6087–6089. [Google Scholar]; (b) Schwartz BD, Moore CJ, Rahm F, Hayes PY, Kitching W, De Voss JJ. J Am Chem Soc. 2008;130:14853–14860. doi: 10.1021/ja8036433. [DOI] [PubMed] [Google Scholar]; (c) Dossey AT, Walse SS, Conle OV, Edison AS. J Nat Prod. 2007;70:1335–1338. doi: 10.1021/np070151g. [DOI] [PubMed] [Google Scholar]; (d) Pettit GR, Meng Y, Herald DL, Knight JC, Day JF. J Nat Prod. 2005;68:1256–1258. doi: 10.1021/np0402367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malta MB, Lira MS, Soares SL, Rocha GC, Knysak I, Martins R, Guizze SPG, Santoro ML, Barbaro KC. Toxicon. 2008;52:255–263. doi: 10.1016/j.toxicon.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 6.(a) Li C, Liu M, Monbo J, Zou G, Li C, Yuan W, Zella D, Lu WY, Lu W. J Am Chem Soc. 2008;130:13546–13548. doi: 10.1021/ja8042036. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Taggi AE, Meinwald J, Schroeder FC. J Am Chem Soc. 2004;126:10364–10369. doi: 10.1021/ja047416n. [DOI] [PubMed] [Google Scholar]
- 7.Umebachi Y. Sci Rep Kanazawa U. 1977;22:187–195. [Google Scholar]
- 8.Oshima K, Honda H, Yamamoto I. Tokyo Nogyo Diagaku Nogaku Shuho. 1975;20:117–120. [Google Scholar]
- 9.Hayashi N, Sugiyama Y, Komae H, Sakao T. J Nat Prod. 1987;50:769–770. [Google Scholar]
- 10.(a) Kupchan SM, Doskotch RW. J Med Pharm Chem. 1962;91:657–659. doi: 10.1021/jm01238a029. [DOI] [PubMed] [Google Scholar]; (b) Kupchan SM, Merianos JJ. J Org Chem. 1968;33:3735–3738. doi: 10.1021/jo01274a011. [DOI] [PubMed] [Google Scholar]
- 11.For reviews of the Aristolochiaceae compounds and their activities, see Wu TS, Damu AG, Su CR, Kuo PC. Nat Prod Rep. 2004;21:594–624. doi: 10.1039/b401950d.Kumar V, Poonam, Prasad AK, Parmar VS. Nat Prod Rep. 2003;20:565–583. doi: 10.1039/b303648k.Mix DB, Guinadeau H, Shamma M. J Nat Prod. 1982;45:657–666.
- 12.Jackson L, Kofman S, Weiss A, Brodovsky H. Cancer Chemother Reports. 1964;42:35–37. [PubMed] [Google Scholar]
- 13.(a) Chen T. J Food Drug Anal. 2007;15:387–399. [Google Scholar]; (b) Arlt VM, Stiborová M, Schmeiser HH. Mutagenesis. 2002;48:81–95. doi: 10.1093/mutage/17.4.265. [DOI] [PubMed] [Google Scholar]; (c) Arlt VM, Stiborová M, vom Brocke J, Simões ML, Lord GM, Nortier JL, Hollstein M, Phillips DH. Carcinogenesis. 2007;28:2253–2261. doi: 10.1093/carcin/bgm082. [DOI] [PubMed] [Google Scholar]; (d) Yuan J, Nie L, Zeng D, Luo X, Tang F, Ding L, Liu Q, Guo M, Yao S. Talanta. 2007;73:644–650. doi: 10.1016/j.talanta.2007.04.042. [DOI] [PubMed] [Google Scholar]
- 14.Mebs D, Schneider M. Chemoecology. 2002;12:11–13. [Google Scholar]
- 15.Monks A, Scudiero D, Skehan P, Shoemaker R, Paull K, Vistica D, Hose C, Langley J, Cronise P, Vaigro-Wolff A, Gray-Goodrich M, Campbell H, Mayo J, Boyd M. J Natl Cancer Inst. 1991;83:757–766. doi: 10.1093/jnci/83.11.757. [DOI] [PubMed] [Google Scholar]
- 16.NCCLS. NCCLS document M7-A5. 5th. NCCLS; Wayne, PA: 2000. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard. [Google Scholar]
- 17.NCCLS. NCCLS document M27-A2. 2nd. NCCLS; Wayne, PA: 2002. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved Standard. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


