Abstract
Within primary visual cortex (V1), BDNF signaling through its high affinity receptor TrkB is important for normal development and experience-dependent plasticity. TrkB is expressed in several alternatively spliced isoforms, including full length TrkB (TrkB.FL), and several truncated isoforms (TrkB.T1, TrkB.T2 and TrkB.T4) that lack the intracellular tyrosine kinase domain. These isoforms are important components of BDNF signaling, yet little is known about the developmental or experience-dependent regulation of their expression. Using immunohistochemistry, we found TrkB.FL and TrkB.T1 expressed in interneurons and pyramidal neurons within V1, but not in cortical astrocytes. We used real-time PCR to quantify changes in mRNA expression of BDNF, the four TrkB isoforms, and the low affinity receptor P75NTR during normal development, and in response to visual deprivation at two different ages. BDNF expression increased between postnatal days 10 (P10) and P30, and was rapidly down-regulated by 3 days of visual deprivation during both the pre-critical period (P14–P17) and the critical period (P18–P21). Over the same developmental period expression of each TrkB isoform was regulated independently; TrkB.T1 increased, TrkB.FL and TrkB.T2 decreased, and TrkB.T4 showed transient changes. Neither brief visual deprivation nor prolonged dark-rearing induced changes in TrkB.FL or TrkB.T1 expression. However, TrkB.T4 expression was reduced by brief visual deprivation, while TrkB.T4, TrkB.T2 and P75NTR were up-regulated by prolonged dark-rearing into the critical period. Our data indicate that TrkB isoform expression can be selectively regulated by visual experience, and may contribute to experience-dependent cortical plasticity.
Keywords: TrkB, BDNF, visual deprivation, critical period, P75NTR
INTRODUCTION
In mammals, there are distinct windows, or “critical periods” in development during which visual experience is essential for generating proper wiring and function of cortical circuits (Wiesel and Hubel, 1963; Hubel and Wiesel, 1964; Stafford, 1984; Prusky et al., 2000; Feller and Scanziani, 2005; Hensch, 2005; Tagawa et al., 2005; Hooks and Chen, 2007). The neurotrophin brain derived neurotrophic factor (BDNF) is expressed in an activity-dependent manner within visual cortex (Bozzi et al., 1995; Capsoni et al., 1999; Rossi et al., 1999; Majdan and Shatz, 2006), and is known to play a number of important roles in cortical development and plasticity. BDNF signaling influences dendritic growth (McAllister et al., 1996, 1997; Cheung et al., 2007), excitatory and inhibitory synapse function (Desai et al., 2002; Gomes et al., 2006), homeostatic plasticity (Rutherford et al., 1998), and the induction (Akaneya et al., 1997) and maintenance (Sermasi et al., 2000) of LTP. In mice that over-express BDNF, inhibitory drive in visual cortex matures earlier, visual acuity develops earlier, and the critical period begins and ends earlier (Huang et al., 1999).
BDNF signaling within cortex is complex and is mediated through a number of receptors that can have antagonistic effects. The high affinity receptor for BDNF, TrkB, is expressed in several alternatively spliced isoforms (Middlemas et al., 1991; Forooghian et al., 2001; Stoilov et al., 2002). Among them are full length TrkB (TrkB.FL), and three truncated forms (TrkB.T1, TrkB.T2, and TrkB.T4). All three of the truncated forms of the receptor lack the intracellular tyrosine kinase domain of the full length receptor, and are thought to antagonize the effects of TrkB.FL (Biffo et al., 1995; Eide et al., 1996). TrkB.T1 and TrkB.T2 have very short intracellular tails; however TrkB.T4, which has also been described as TrkB-T-ShC (Stoilov et al., 2002), is much longer than the other two truncated domains, and contains a putative internalization sequence (Forooghian et al., 2001) as well as an Shc binding domain (Stoilov et al., 2002). In addition to TrkB, BDNF can also signal through the low affinity receptor P75NTR, which is responsive to all the neurotrophins (Huang and Reichardt, 2003). While BDNF expression within cortex is known to be regulated by visual experience (Bozzi et al., 1995; Capsoni et al., 1999; Rossi et al., 1999; Majdan and Shatz, 2006), little is known about the developmental regulation of TrkB receptor isoforms, or whether their expression is also regulated by visual experience.
Rodent visual cortex has several distinct plasticity periods, among them a “pre-critical period” (pre-CP) initiated around eye opening and lasting about a week, and the classical critical period (CP) beginning just after the pre-CP and lasting until approximately postnatal day 35 (P35) (Feller and Scanziani, 2005). These distinct developmental stages are characterized by different forms of visual system plasticity (Maffei et al., 2004, 2006), but it is not known whether the ability of visual experience to regulate BDNF signaling through changes in ligand or receptor expression is itself developmentally regulated. Here we used real-time PCR to quantitatively measure mRNA expression levels of BDNF, the four TrkB isoforms, and P75NTR in rodent visual cortex during normal development and in response to visual deprivation during the pre-CP and the CP. We found that BDNF expression could be reduced by even very brief (3 day) periods of visual deprivation at both developmental time periods. During normal postnatal development the ratio of TrkB.FL to TrkB.T1 decreased developmentally. While TrkB.FL and TrkB.T1 expression were unaffected by visual deprivation, TrkB.T2, TrkB.T4 and P75NTR expression were changed in a manner that depended upon deprivation protocol and developmental period. These data indicate that visual experience can influence BDNF signaling in cortex through changes in both ligand and receptor expression, and suggest that changes in the relative abundance of TrkB isoforms could play important roles in both pre-CP and CP plasticity.
METHODS
General Methods
Experiments were perfomed on Long-Evans rats, obtained from Charles River Associates. All animals were kept on a 12 hour light/dark cycle (with the exception of the dark-reared animals), with food and water available ad libitum. All procedures were approved by the Brandeis IACUC and were in accordance with NIH guidelines.
Visual Deprivation
Monocular eyelid sutures were performed as reported previously (Maffei et al., 2004; Maffei et al., 2006; Maffei and Turrigiano, 2008a). Suturing was performed early on the appropriate day for the experiment (P14 or P18), and then three days (on P17 or P21) or six days (on P24) later the animals were anaesthetized by isofluorane inhalation, and sacrificed. The brain was removed, hemispheres separated, and slices were cut using a Leica VT 1000s. Monocular and binocular portions of V1 were dissected under a dissecting microscope, using known landmarks as a guide (Paxinos and Watson, 1998).
Dark-reared litters were placed in a ventilated light-tight box at P10 with food and water available ad libitum. At P21, animals were removed, anaesthetized in the dark by isofluorane inhalation, and immediately sacrificed. Slices were cut, and V1 was dissected in the same way as MD animals.
Immunohistochemistry
Rats were anaesthetized with a mixture of 70mg/Kg ketamine, 3.5mg/Kg xylazine hycrochloride, and .7mg/Kg acepromazine maleate intraperitoneally. Animals were then perfused with 4% paraformaldehyde (PFA) for 30 minutes. Brains were removed, left in 4% paraformaldehyde for 1 hour, and mounted in 5% agar/phosphate buffered saline (PBS). 50µm slices were cut on a Leica 1000s vibratome, then blocked in 1% normal goat serum (NGS) + 0.03% Triton X in PBS for 1 hour at room temperature. Slices were incubated overnight at 4°C in antibody solution (0.1% NGS, 0.01% Triton X, in PBS) with the appropriate primary antibodies: rabbit anti-TrkB.T1 (1:50, Santa Cruz Biotech), rabbit anti-TrkB.FL (1:250, Santa Cruz Biotech), biotin conjugated NeuN (1:500, Chemicon), chicken anti-GFAP (1:500, Abcam), mouse anti-Parvalbumin (1:500, Chemicon), mouse anti-Map1 (1:500, Chemicon), rabbit anti- P75NTR (1:500, Promega). Slices were then washed 3×30 minutes in wash solution (PBS containing 0.01% Triton X), and incubated for 2 hours at room temperature in antibody solution containing the appropriate secondary antibodies at 1:500. Slices were then washed 3×30 minutes and mounted onto slides. Images were acquired on a Leica Confocal microscope using LCS software. Z-sections were converted to 2D image stacks and images from the two channels were overlaid using ImageJ software (NIH).
Specificity of TrkB antibodies was checked by pre-incubation with a peptide consisting of the amino acid sequence specific for the appropriate antibody (10x peptide concentration by weight, Santa Cruz Biotech). Pre-incubation significantly reduced intensity of both TrkB.FL and TrkB.T1 staining, to apparent background levels.
Real Time RT-PCR
After dissection, tissue samples from monocular and binocular V1 were immediately placed in tubes of extraction buffer from a Picopure RNA isolation kit (Arcturus) on ice. As per kit instructions, samples were incubated at 42°C for 30 minutes, and stored at −80°C at least over night until use. RNA was extracted using a Picopure RNA isolation kit (Arcturus), and cDNA was immediately synthesized using a thermoscript RT-PCR system (Invitrogen), including RNase treatment. Sybr Green mixture (Invitrogen) was used for real time RT-PCR reactions. Primers were designed through Integrated DNA Technologies and purified by standard desalting. The following primer sequences were used: BDNF forward ACTGGAACTCGCAATGCCGAACTA, BDNF reverse TCTATCCTTATGAACCGCCAGCCA; TrkB.FL forward AACCACACACAAGGAAGAACATCAA, TrkB.FL reverse TGACATCAGCGGCAGTTAAGAGGT; TrkB.T1 forward AACTCCTGGGACTACTGTTGCCTA, TrkB.T1 reverse AACAAGCAGGCTGCGGACATCTTT; TrkB.T2 forward AGGATTACTCAGCCTTGCCCACTT, TrkB.T2 reverse CATGATTGCCACAGGACCACTCAA; TrkB.T4 forward TCACTGCAGGAACTAATGGAGGCT, TrkB.T4 reverse AACTCCGGACTTGGATGTTGCTGA; P75NTR forward AGTGTGCAGATGTGCCTATGGCTA, P75NTR reverse ACGTGGTTGGCTTCGTCTGAGTAT; GAPDH forward TCCCAGAGCTGAATGGGAAACTCA, GAPDH reverse AGCATCAAAGGTGGAAGAGTGGGA. PCR products were sequenced and/or analyzed by gel electrophoresis for verification.
Analysis was done using the 2−ΔΔC T method (Livak and Schmittgen, 2001). The average cycle threshold (reactions were performed in triplicate) for each gene of interest was first normalized to GAPDH in order to control for any differences in total RNA extracted (ΔC T). Then the ΔC T for the deprived condition (the contralateral hemisphere for mV1, both hemispheres for bV1, or the DR animal) was normalized to the ΔC T for the control condition (ipsilateral hemisphere for monocular V1, control animal for binocular V1, or normally reared animal for dark-rearing experiments). All primers had an efficiency value between 1.8 and 2. All receptor data were obtained in parallel in the same PCR runs from the same samples. Significance for developmental data was determined with an ANOVA, followed by the post-hoc Tukey test. For the remaining data, Student’s t-tests, corrected for multiple comparisons as appropriate, were used to determine significance. P values of 0.05 or lower were considered significant.
RESULTS
Developmental and experience-dependent changes in BDNF expression
It is known that BDNF mRNA expression within primary visual cortex (V1) increases during development (Patz and Wahle, 2006), but while many experimenters examine V1 as a whole, rodent V1 can be broken down into two distinct regions. The monocular portion of V1 (mV1) contains neurons that are driven by only the contralateral eye, but binocular V1 (bV1) contains neurons that are driven by both eyes; thus the effects of visual deprivation in these two regions is not equivalent, and it is not known whether this generates differential regulation of BDNF expression. To test for regional differences we microdisected mV1 and bV1 and examined BDNF mRNA expression within both regions using real-time PCR. Cycle thresholds of BDNF relative to GAPDH were similar in mV1 and bV1 at P10, the youngest age examined, indicating that BDNF expression at this age did not differ between these two cortical regions. To investigate developmental changes in BDNF expression, mRNA expression at each age was normalized to expression at P10. BDNF mRNA expression increased significantly between P10 and P60 in both regions of visual cortex (Fig. 1A; mV1, n = 16–18 at each time point, ANOVA p < 0.0001; bV1, n = 15–21 at each time point, ANOVA p < 0.0001). However, the pattern of change was different in the two regions (Fig. 1A; P17 t test p < 0.01, n = 16–17; P30 t-test p < 0.05, n = 18). In bV1, BDNF expression increased steeply between P10 and P17, and then reached a plateau that persisted into adulthood. In contrast BDNF expression in mV1 increased more gradually and did not reach peak levels until P24 (n=17). These data suggest that BDNF expression is developmentally accelerated in the binocular portion of V1.
Figure 1.
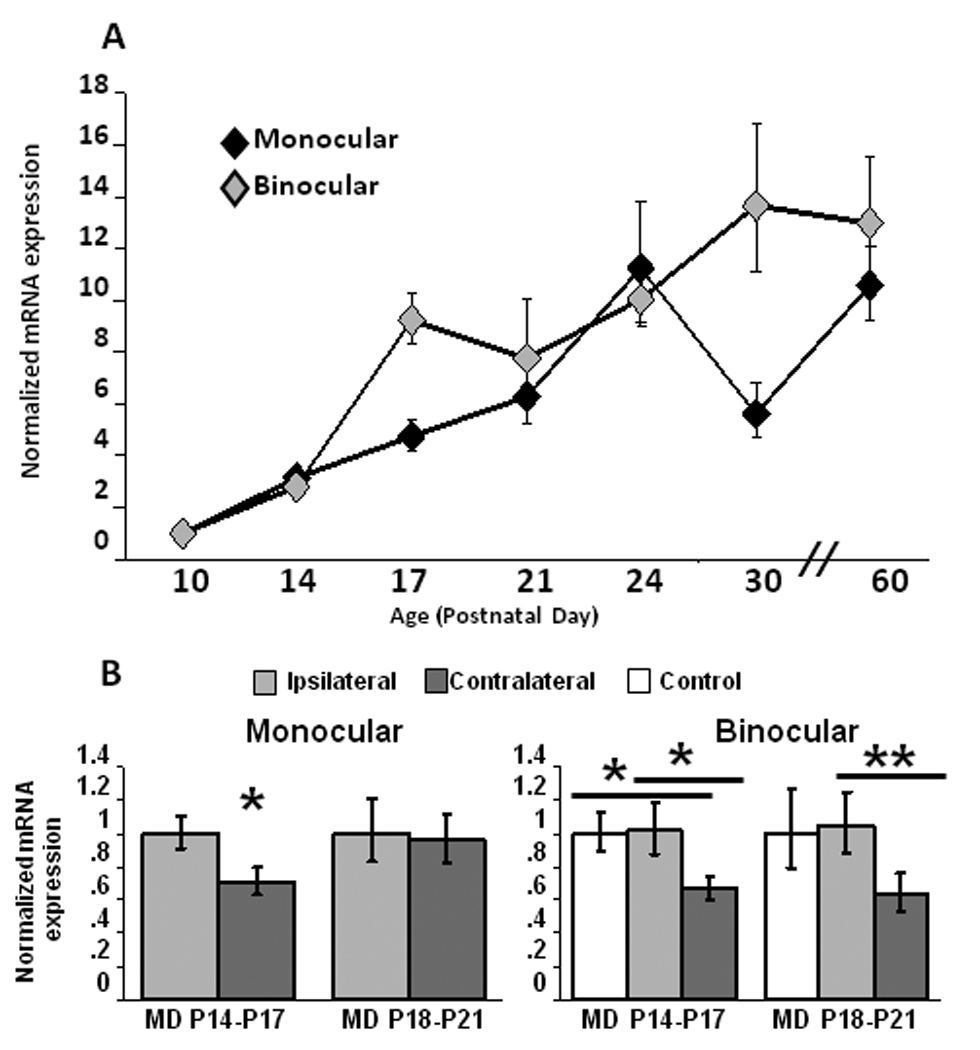
Developmental and experience-dependent regulation of BDNF expression in monocular and binocular regions of primary visual cortex (V1). (A) BDNF expression increased during early postnatal development in both monocular (ANOVA p < 0.0001) and binocular (ANOVA p < 0.0001) zones of V1. mRNA expression at each developmental time-point was normalized to values at P10. (B) Two days of monocular deprivation (MD) during the pre-CP (P14–P17) down-regulated BDNF expression in both mV1 and bV1, while MD at the beginning of the CP (P18–P21) down-regulated BDNF mRNA only in bV1. mRNA expression in MD animals was normalized in mV1 to ipsilateral (non-deprived) cortex, and in bV1 to a sham operated littermate control animal. *p < 0.05, **p < 0.01
Experience-dependent plasticity in the visual system can be triggered by brief visual deprivation, both during the pre-CP and the CP (Frenkel and Bear, 2004; Maffei et al., 2004; Tagawa et al., 2005; Maffei et al., 2006). While BDNF expression is known to be regulated by longer visual deprivation protocols (ranging from 4-days to several weeks) during the CP (Bozzi et al., 1995; Capsoni et al., 1999; Rossi et al., 1999; Majdan and Shatz, 2006), whether BDNF mRNA levels can change rapidly in response to brief visual deprivation, and whether this differs during the pre-CP and CP, has not been determined. To examine this we deprived one set of animals from P14 until P17 (the pre-CP), and a second set of animals from P18 until P21 (the beginning of the CP), and examined BDNF expression in both mV1 and bV1. We normalized mV1 data to the cortex ipsilateral to the deprived eye. Neurons within bV1 are driven by both eyes, with a strong contralateral bias, so that MD results in strong deprivation in contralateral cortex and weaker deprivation in ipsilateral cortex. Consequently for bV1 we normalized data from both hemispheres to a littermate sham deprived “control” animal that received the same anaesthetization and handling, but no lid suture.
In mV1, three days of MD from before eye opening (P14–P17) modestly but significantly decreased BDNF mRNA expression (Fig. 1B; n=15, p < 0.05). In contrast, the same deprivation performed at the beginning of the classical visual system CP (P18–P21) had no effect on BDNF expression (Fig. 1B; n=19). In bV1 MD decreased BDNF expression in the contralateral hemisphere at both developmental periods, relative to the ipsilateral hemisphere, and to the control animal (Fig. 1C; pre-CP n=13, CP n=19). These data indicate that BDNF expression can be rapidly regulated by experience, and that during the CP binocular cortex is the main locus of this regulation.
Cell-type specific TrkB protein expression
Cell-type specific expression of TrkB isoforms appears to vary by brain region. In hippocampus mRNA of TrkB.FL and TrkB.T2 is expressed only in neurons, while TrkB.T1 is expressed both in neurons and glia (Armanini et al., 1995). TrkB.T1 protein is expressed in neurons but not in glia in motor cortex, but in prefrontal cortex, both neurons and glia express TrkB.T1 protein (Ohira et al., 2005). It is unknown which cell types within V1 express truncated TrkB receptor protein, or how receptor expression is regulated by activity. To address cell-type specific expression we used double-label immunohistochemistry against various cell-type markers and either TrkB.FL or TrkB.T1, (the isoforms for which selective antibodies are available), in slices derived from P21 rats. We found no obvious region-specific differences in staining patterns between mV1 and bV1 for either TrkB.FL or TrkB.T1. Both TrkB receptor isoforms could be detected in the soma and proximal dendrites of cortical neurons, both in parvalbumin positive interneurons (Fig. 2 and Figs. 3 A–C), and in putative pyramidal neurons as evidenced by the high degree of overlap between the neuron-specific marker NeuN and both receptor isoforms (Fig. 2 and Figs. 3 D–F), as well as the pyramidal-like morphology that is clear from MAP co-labeling (Fig. 2 and Figs. 3 G–I). There was no overlap between staining for either TrkB isoform and the astrocyte marker GFAP (data not shown). These data indicate that TrkB.FL and TrkB.T1 protein is present in both inhibitory and excitatory neurons within visual cortex, and at lower levels (if at all) in visual cortical astrocytes.
Figure 2.
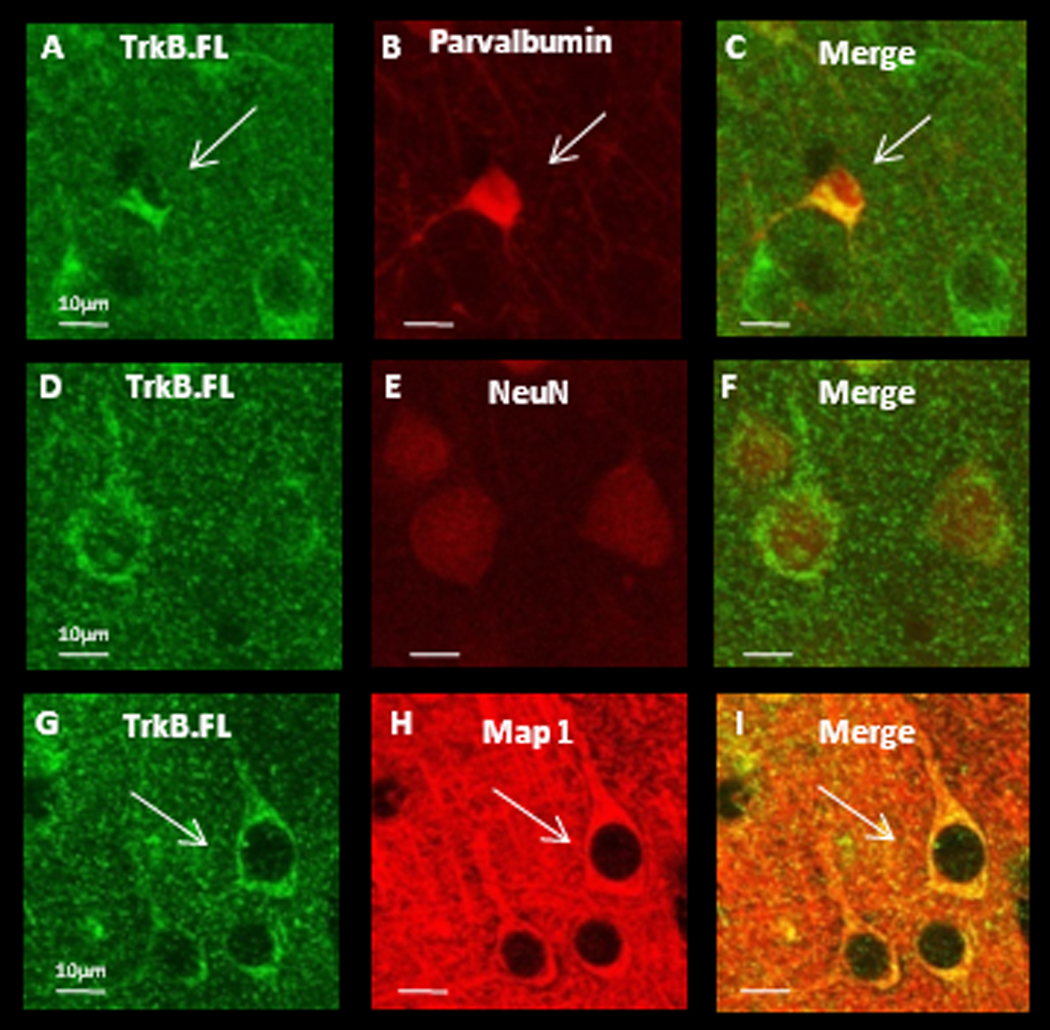
TrkB.FL protein is detectible in soma and proximal dendrites of both interneurons and putative pyramidal neurons within V1. (A–C) Double labeling against TrkB.FL and parvalbumin. White arrows indicate an interneuron. (D–F) Double labeling against TrkB.FL and NeuN, a marker for neuronal nuclei. (G–I) Double labeling against TrkB.FL and MAP1 to label dendritic processes. White arrows indicate a putative pyramidal neuron. Scale bars = 10µm.
Figure 3.
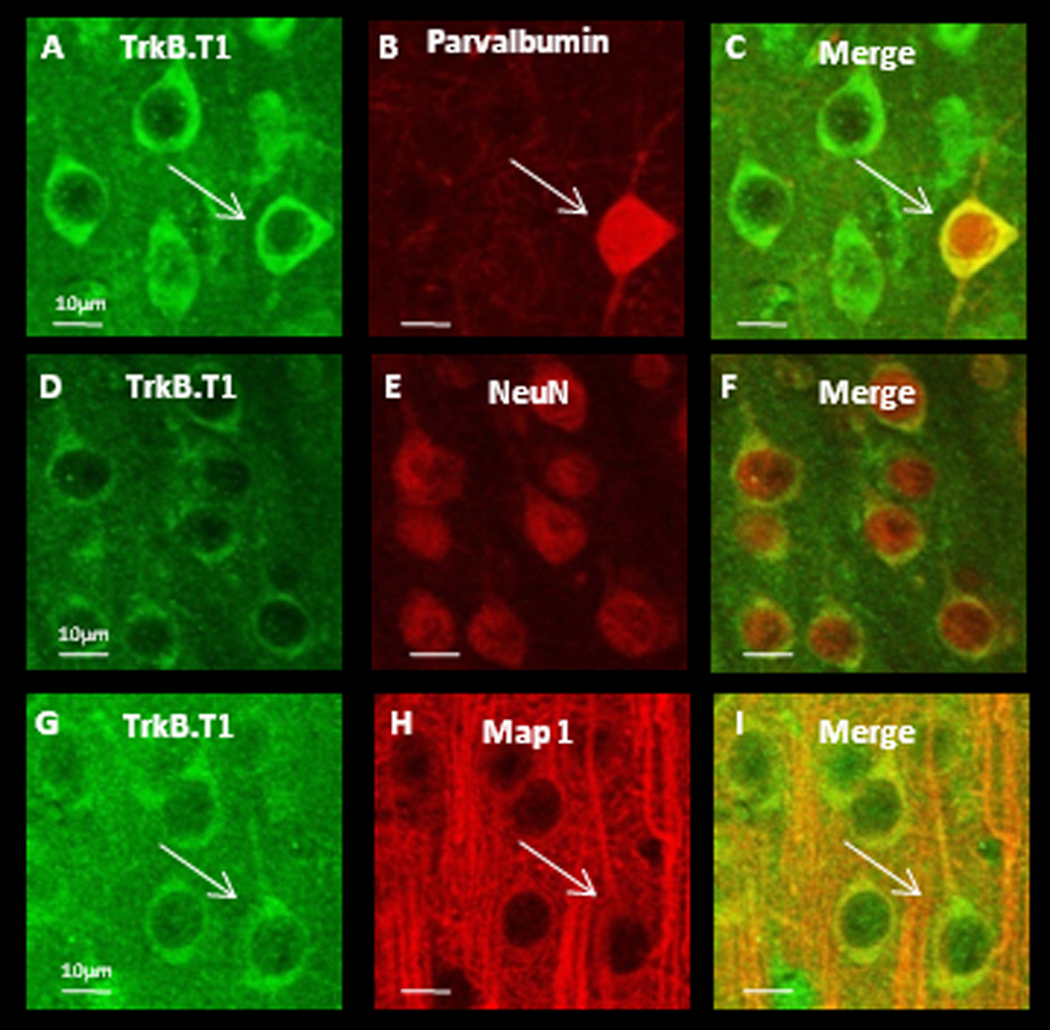
TrkB.T1 protein is detectible in soma and proximal dendrites of both interneuron’s and putative pyramidal neurons. (A–C) Double labeling against TrkB.T1 and parvalbumin. White arrows indicate an interneuron. (D–F) Double labeling against TrkB.T1 and NeuN, a marker for neuronal nuclei. (G–I) Double labeling against TrkB.T1 and MAP1 to label dendritic processes. White arrows indicate a putative pyramidal neuron. Scale bars = 10µm.
Developmental regulation of TrkB receptor isoforms
Visual experience could regulate BDNF signaling through changes in expression levels of TrkB isoforms, yet almost nothing is known about the developmental regulation of different splice variants of TrkB. To examine this we used real-time PCR to quantify developmental changes in the expression of four TrkB receptor isoforms in mV1 and bV1. P10 was again the earliest age examined so the data for each age were normalized to P10. Over this developmental period TrkB.FL expression decreased slightly (Figs. 4A and B; n=14–21 at each time point, ANOVA, p < 0.01), while TrkB.T1 increased steadily from P14 on in both mV1 and bV1 (Figs. 4A and B; n=15–19 at each time point, ANOVA, p < 0.0001). As a consequence, the ratio of full length to truncated TrkB decreased dramatically after eye opening in both regions of visual cortex. In mV1 TrkB.T2 decreased from P10 (Fig. 4C; n=15–17 at each time point, ANOVA p < 0.01), while in bV1 there was no significant change in expression (Fig. 4D). Finally, in mV1 TrkB.T4 expression was stable until P24 when it increased dramatically (Fig. 4C; n=14–16 at each time point, ANOVA p < 0.05), then returned to previous levels; in contrast there was no developmental change in TrkB.T4 in bV1 (Fig. 4D). Because of the caveats of the real-time PCR method, such as differences in primer and product length and different amplification efficiencies, these data cannot offer a measure of absolute expression ratio, however cycle threshold values of TrkB isoforms relative to GAPDH at P10 suggest that TrkB.FL expression is the highest (mV1 = 2.93; bV1 = 2.17), followed by TrkB.T1 (mV1 = 4.95, bV1 = 4.68), with TrkB.T2 expression (mV1 = 9.38, bV1 = 10.41) and TrkB.T4 expression (mV1 = 12.66, bV1 = 9.54) being much lower. (Note that a higher cycle threshold indicates less mRNA). By P60, the relative expression levels have changed so that TrkB.T1 (mV1 = 3.21, bV1 = 2.50) expression is higher than TrkB.FL (mV1 = 3.41, bV1 = 3.18), with TrkB.T2 (mV1 = 10.62, bV1 = 10.00) and TrkB.T4 (mV1 = 12.30, bV1 = 9.42) remaining much lower. Because the intracellular changes that result from BDNF binding to TrkB receptors depends on the receptor isoform that is expressed (Biffo et al., 1995; Eide et al., 1996; Baxter et al., 1997; Yacoubian and Lo, 2000), these developmental changes in the ratio of full length to truncated receptors are likely to have important consequences for BDNF signaling within visual cortex.
Figure 4.
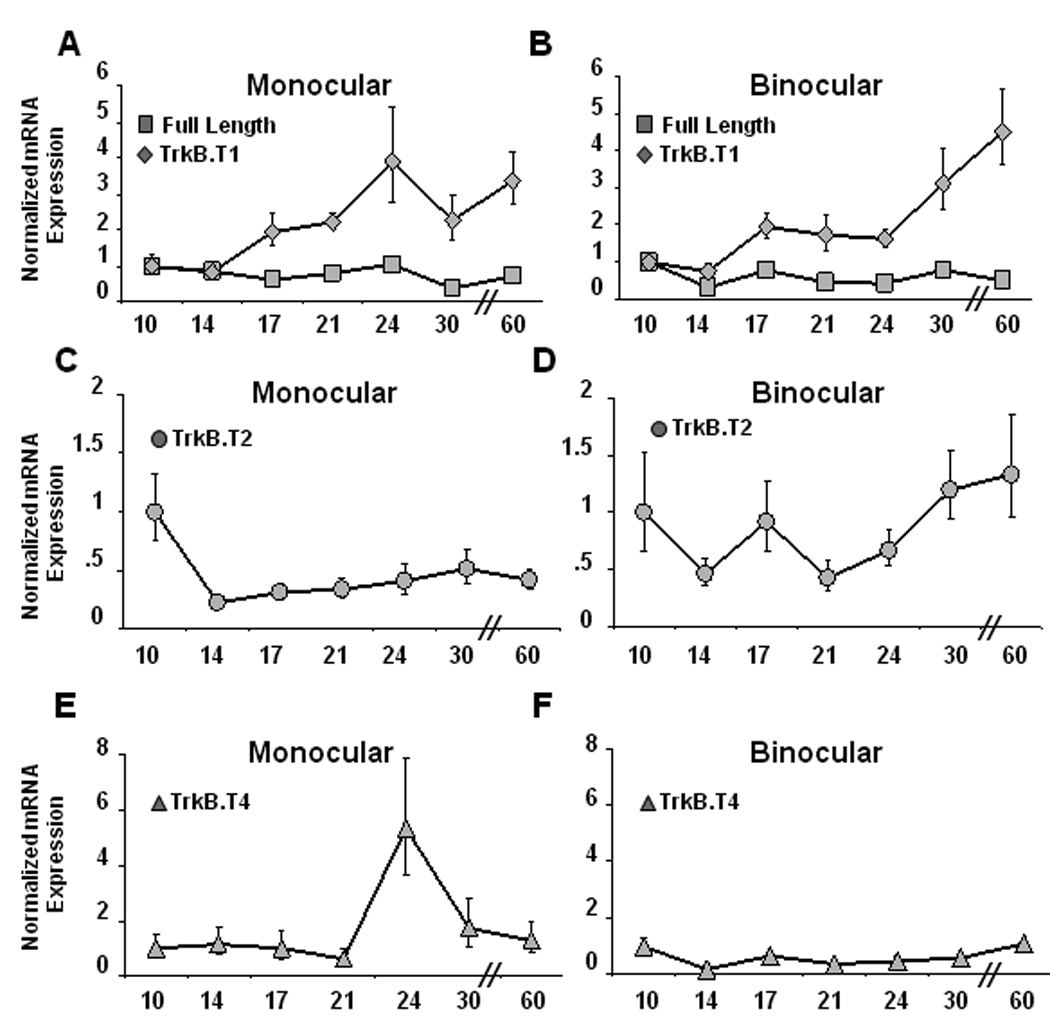
Developmental changes in expression levels of TrkB isoforms in mV1 and bV1. (A–B) Between postnatal days 10 and 60, TrkB.FL expression decreased (ANOVA p < 0.01) and TrkB.T1 expression increased (ANOVA p < 0.001) in both mV1 (A) and bV1 (B). (C–D) TrkB.T2 expression decreased before eye opening (ANOVA p < 0.01) in mV1 (C) but not in bV1 (D). (E–F) In mV1 TrkB.T4 expression was stable until P24, when it increased dramatically before falling again (ANOVA p < 0.05), whereas in bV1 TrkB.T4 expression levels remained relatively stable (F). mRNA expression at each developmental time-point was normalized to values at P10.
Regulation of TrkB isoforms by visual experience
In the retina, TrkB.FL and TrkB.T1 are differentially regulated in response to light (Asai et al., 2007), but it is unknown whether TrkB isoform expression changes within V1 in response to visual deprivation. We quantified mRNA expression of the four TrkB isoforms in response to brief (3 day) MD during the pre-CP and CP as described for BDNF above. MD had no significant effect on the expression of TrkB.FL, TrkB.T1, or TrkB.T2 in either mV1 or bV1 at either developmental time (Fig. 5). In contrast, MD during the pre-CP significantly reduced the expression of TrkB.T4 in both mV1 (Fig. 5A; n=11, p < 0.05) and bV1 (Fig. 5B; n=14, p < 0.05). This effect on TrkB.T4 expression was selective for the pre-CP, as the same deprivation performed from P18–P21 produced no change in TrkB.T4 expression (Fig. 5C and D). To test for the effects of a slightly longer MD on TrkB isoform expression during the CP, we doubled the deprivation period. MD from P18 until P24 again had no significant effect on the expression of TrkB.FL and TrkB.T1. However, 6 day MD significantly reduced expression of TrkB.T4 in bV1 (Fig 5F; n=9, p < 0.05), and of TrkB.T2 in both mV1 (Fig 5E; n=10 p < 0.05) and in bV1 (Fig 5F; n=12, p < 0.05). Brief visual deprivation thus has highly selective effects on TrkB isoform expression, affecting only one of the four isoforms during the pre-CP, and only two of the four isoforms during the CP.
Figure 5.
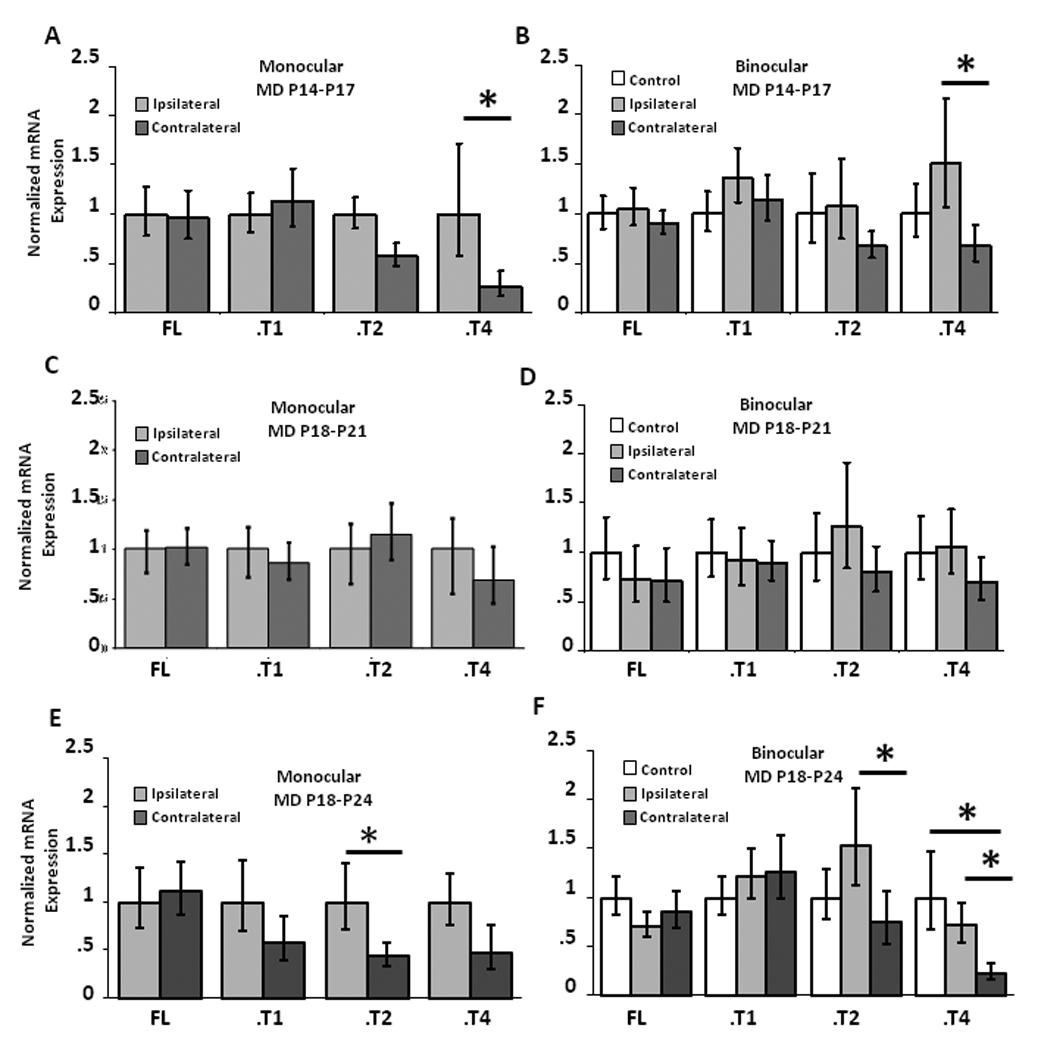
Experience-dependent regulation of TrkB isoforms by brief MD during the pre-CP and CP. (A–B) TrkB.T4 expression decreased in response to 3 day MD during the pre-CP in both mV1 (A) and bV1 (B), while the expression of the other TrkB isoforms was not significantly affected. (C–D) Expression of TrkB isoforms was unaffected by 3 day MD during the CP in either mV1 or bV1. mRNA expression was normalized in mV1 to ipsilateral cortex, and in bV1 to a sham operated littermate control animal. *p < 0.05
To test for the effects of much more prolonged visual deprivation on TrkB isoform expression, we dark reared animals from P10 until P21, and normalized expression to animals raised during the same time period in the normal 12 hr light/12 hr dark condition. In mV1 (Fig. 6A) and bV1 (Fig. 6B) DR resulted in a large (5–6-fold) increase in TrkB.T4 expression (LR n=10, DR n=14, p < 0.001, and p < 0.01 respectively), and had no significant effect on TrkB.FL or TrkB.T1 expression. DR also induced a 3-fold increase in TrkB.T2, which was selective for bV1 (Fig. 6A and B; n=15, p < 0.05). Interestingly, both brief MD during the pre-CP and prolonged DR into the CP selectively affected TrkB.T4 expression, but in opposite directions.
Figure 6.
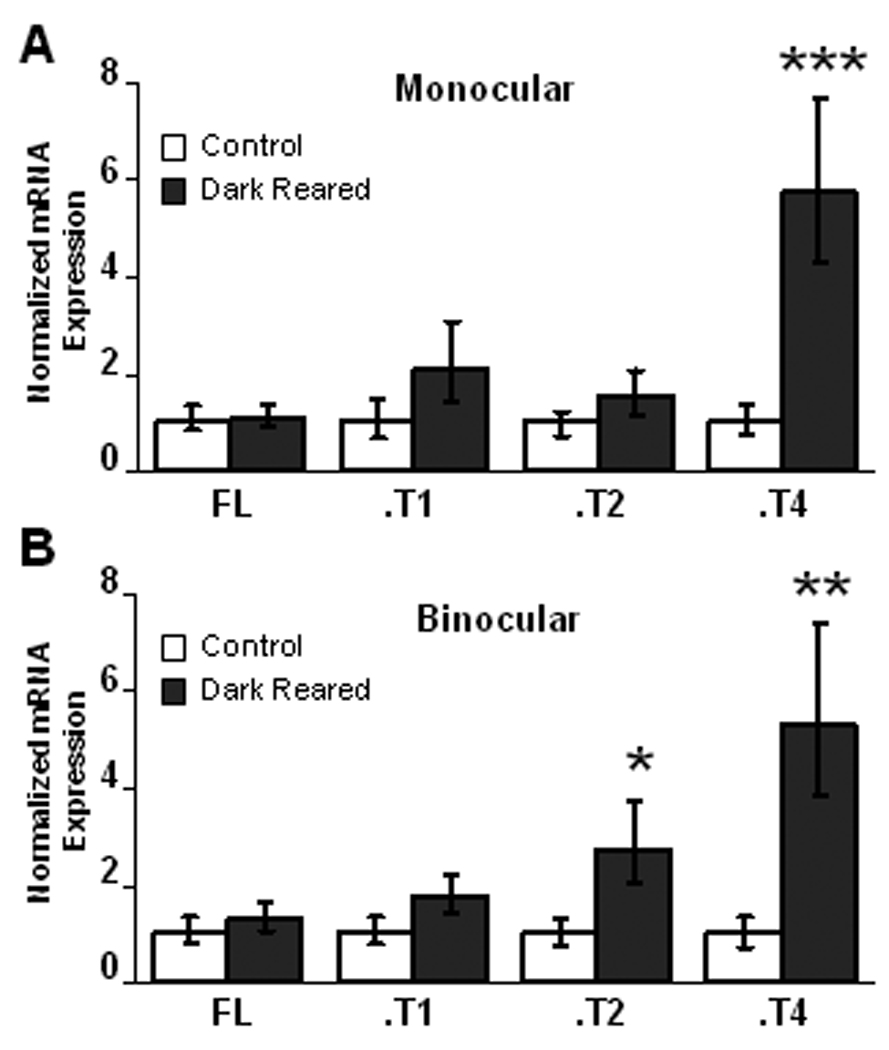
Regulation of TrkB isoforms by dark-rearing between P10 and P21. (A) DR resulted in a 6-fold increase in TrkB.T4 expression in mV1, without affecting expression of the other TrkB isoforms. (B) DR resulted in a 5-fold increase in TrkB.T4 expression in bV1, and a 3-fold increase in TrkB.T2 expression in bV1. mRNA expression in DR animals was normalized to expression in animals that were raised during the same time period in a 12 hour light/12 hour dark environment. *p < 0.05, **p < 0.01, ***p < 0.001
Regulation of P75NTR both during development in response to visual experience
In addition to signaling through TrkB, BDNF can also signal through the lower affinity receptor, P75NTR. We used double label immunohistochemistry on visual cortical slices from P21 rats to determine cell-type specific expression of P75NTR as in the TrkB receptor experiments outlined above. P75NTR expression could be detected in the soma and proximal dendrites of cortical neurons as evidenced by the high degree of overlap between the neuron-specific marker NeuN and P75NTR (Figs. 7 D–F). Unlike the TrkB isoforms that were expressed in most or all parvalbumin-positive interneurons, P75NTR was expressed in only a subset of parvalbumin positive interneurons (Fig. 7 A–C). We also found P75NTR expression in putative pyramidal neurons, made clear by the pyramidal-like morphology from MAP co-labeling (Figs. 7 G–I). There was no overlap between staining for P75NTR and the astrocyte marker GFAP (data not shown).
Figure 7.
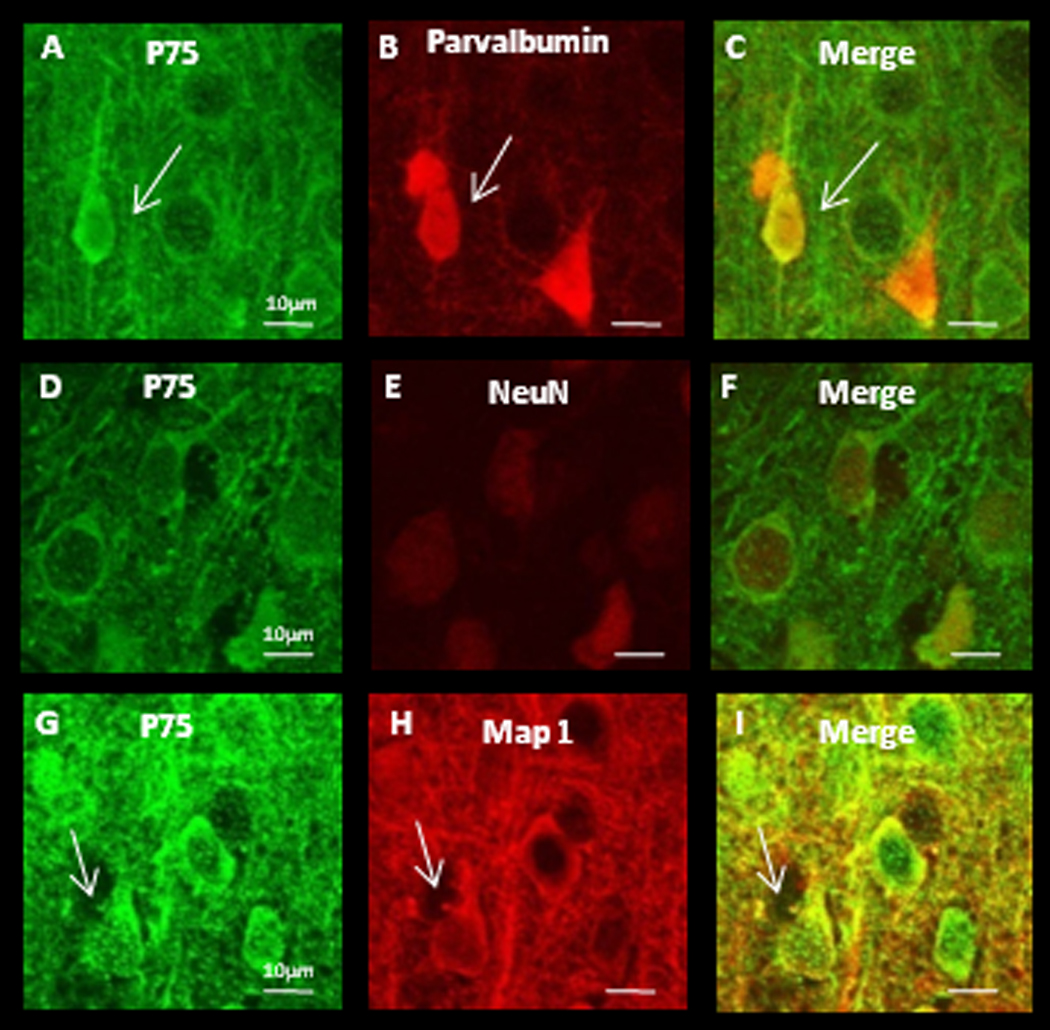
P75NTR protein is detectible in soma and proximal dendrites of both interneuron’s and putative pyramidal neurons. (A–C) Double labeling against P75NTR and parvalbumin. White arrows indicate an interneuron. (D–F) Double labeling against P75NTR and NeuN, a marker for neuronal nuclei. (G–I) Double labeling against P75NTR and MAP1 to label dendritic processes. White arrows indicate a putative pyramidal neuron. Scale bars = 10µm.
We measured P75NTR mRNA expression both during normal development and in response to the same visual deprivation protocols as in the TrkB experiments. P75NTR expression decreased steeply right around eye opening (at P14) in both mV1 (Fig. 8A; n=16–18 at each time point, ANOVA p < 0.0001) and bV1 (Fig. 8A; n=15–20 at each time point, ANOVA p < 0.0001), and remained low. When animals were subjected to MD we found no significant changes in P75NTR at either age in either section of V1 (data not shown). In contrast, DR between P10 and P21 induced a 3-fold increase in P75NTR expression in bV1 relative to the normally reared condition (Fig. 8B; n=14 p < 0.05). In DR animals P75NTR expression at P21 was comparable to that at P10, suggesting that DR prevents the normal developmental down-regulation of P75NTR within visual cortex. Taken together our data demonstrate that visual experience produces highly selective changes in BDNF receptor expression, that depend on developmental stage, cortical area, and mode of visual deprivation.
Figure 8.
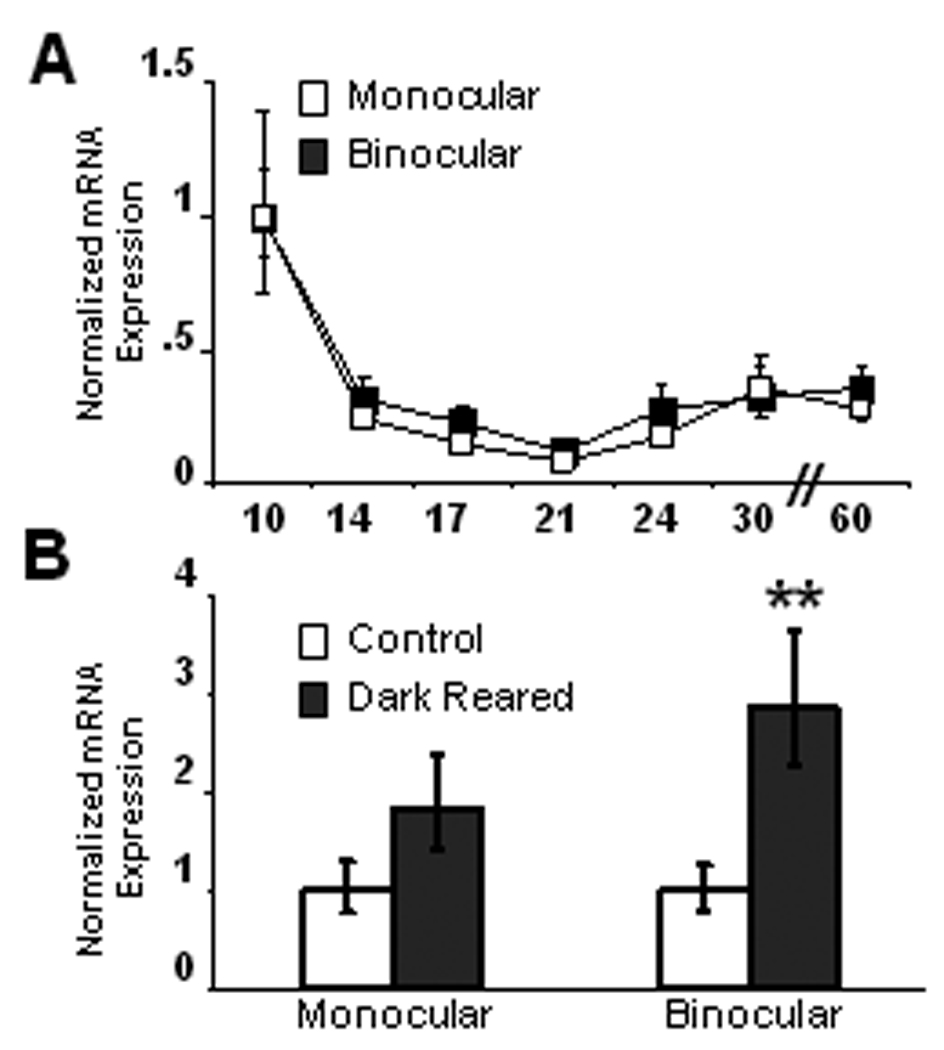
Developmental and experience-dependent regulation of the low affinity neurotrophins receptor, P75NTR, in mV1 and bV1. (A) P75NTR mRNA expression decreased right around eye opening in both mV1 and bV1(ANOVA p < 0.001). (B) DR resulted in a 3-fold increase in P75NTR expression in bV1, but no significant change in mV1. mRNA expression at each developmental time-point was normalized to values at P10. mRNA expression in DR animals was normalized to expression in animals that were raised during the same time period in a 12 hour light/12 hour dark environment. **p < 0.01
DISCUSSION
The relative abundance of full length and truncated TrkB is thought to have important consequences for BDNF signaling (Yacoubian and Lo, 2000; Klau et al., 2001; Chakravarthy et al., 2006). Here we show for the first time that the expression of truncated TrkB isoforms in rodent visual cortex can be regulated by visual experience. TrkB.T2 and the recently identified TrkB.T4 were regulated in response to both brief MD and prolonged DR, but in opposite directions. This regulation was highly specific, as neither TrkB.FL nor TrkB.T1, the two most studied TrkB isoforms, were not affected by either form of visual deprivation. Our data indicate that visual deprivation can change the relative abundance of full length and truncated TrkBs within visual cortex, which in turn may contribute to the effects of visual deprivation on cortical development and plasticity.
BDNF signaling plays a number of critical roles in cortical development, including the maturation of inhibition (Huang et al., 1999) and the modulation of ocular dominance plasticity (Cabelli et al., 1997; Lein and Shatz, 2000; Gianfranceschi et al., 2003). How the relative abundance of different TrkB isoforms affects BDNF signaling is incompletely understood, but the ratio of TrkB.FL to TrkB.T1 in culture is known to affect BDNF-dependent dendritic outgrowth (Yacoubian and Lo, 2000) and synapse formation (Klau et al., 2001; Chakravarthy et al., 2006; Gomes et al., 2006). Some of these effects are likely mediated through dominant-negative effects of truncated TrkBs on signaling through TrkB.FL (Biffo et al., 1995; Eide et al., 1996; Cheung et al., 2007), but there is also evidence that BDNF can activate signaling cascades through binding to TrkB.T1 and TrkB.T2 (Baxter et al., 1997; Yacoubian and Lo, 2000). The intracellular tails of TrkB.T1 and TrkB.T2 are similar in length, and do not contain any putative signaling domains (Middlemas et al., 1991), so these two isoforms were though to very similar functions. However, we have shown that TrkB.T1 and TrkB.T2 are differentially regulated both during normal development and in response to activity deprivation. These data argue that these two isoforms which contain c-tails of similar length could play distinct roles on BDNF signaling within V1. TrkB.T4 was only recently identified. Its expression is restricted to the brain in both mice and humans (Stoilov et al., 2002) and has been found to be highly expressed in cat visual cortex (Forooghian et al., 2001), but little is known about its distribution or function. Like the other truncated isoforms TrkB.T4 lacks an intracellular tyrosine kinase domain, but unlike TrkB.T1 and TrkB.T2, TrkB.T4 has a longer intracellular tail with a possible signaling domain (Forooghian et al., 2001; Stoilov et al., 2002).
We found that each of the TrkB isoforms was regulated differently during visual cortical development. In support of previous studies (Allendoerfer et al., 1994; Patz and Wahle, 2006) we found that expression of TrkB.FL was relatively stable during development, while expression of TrkB.T1 was strongly up-regulated in both mV1 and bV1. In contrast to TrkB.T1, TrkB.T2 was down-regulated during development in mV1 but not in bV1, while TrkB.T4 increased in mV1 but not bV1 during the classical visual system critical period. These data indicate that there are highly selective changes in expression of the four identified TrkB isoforms within visual cortex, suggesting that BDNF signaling will depend strongly on developmental stage.
Activity-dependent BDNF expression has been extensively studied (Bozzi et al., 1995; Capsoni et al., 1999; Rossi et al., 1999; Lein and Shatz, 2000; Ichisaka et al., 2003), but little is known about the activity- or experience-dependent regulation of TrkB. In keeping with published studies that used prolonged visual deprivation (Bozzi et al., 1995; Rossi et al., 1999), we found that even brief periods of visual deprivation (3-day MD) result in a decrease in BDNF expression. Importantly, we also found that TrkB isoform expression can be selectively regulated by visual experience. Both TrkB.FL and TrkB.T1 expression were insensitive to either brief MD or prolonged DR. In contrast, TrkB.T4 expression was highly dependent on visual experience. TrkB.T4 expression was down-regulated in both mV1 and bV1 in response to 3 days of MD during the pre-CP, and was down-regulated in bV1 during the CP in response to 6 days of MD. In addition, TrkB.T2 expression decreased in both mV1 and bV1 in response to 6 days of MD during the CP. Prolonged DR (from before eye opening into the beginning of the critical period) strongly up-regulated TrkB.T4 expression, and induced a weaker but significant increase in TrkB.T2 expression. These two deprivation protocols result in very distinct visual experiences. MD blocks only patterned visual input through one eye, leaving luminance cues intact through the deprived eye, and total visual experience unchanged through the non-deprived eye. DR however, blocks all visual experience through both eyes. Thus these two protocols have very different functional consequences for neuronal connectivity and excitability in V1 (Gordon and Stryker, 1996). Critical period timing remains intact after MD, but is changed by DR (Hensch, 2005), and the direction of the shift in the cortical balance between excitation and inhibition is of the opposite sign for MD than for DR (Morales et al., 2002; Maffei et al., 2006; Maffei and Turrigiano, 2008b). The differential regulation of TrkB isoforms by MD and DR suggests that BDNF signaling within V1 depends on what deprivation protocol is used, which could contribute to the distinct effects on cortical plasticity of these two forms of visual deprivation.
The effects of altered TrkB receptor isoform expression on visual cortical function will depend critically on which cell types express the different forms of the receptor. Using immunohistochemistry we found that TrkB.FL and TrkB.T1 are both widely expressed in neurons, including pyramidal and parvalbumin positive inhibitory interneurons, but not in astrocytes. Although TrkB.T1 mRNA has previously been found to be highly expressed in cultured hippocampal astrocytes (Rudge et al., 1994), slice experiments indicate that in cortical regions and hippocampus TrkB.T1 and TrkB.FL are expressed in white matter tracts, but not in astrocytes (Fryer et al., 1996). The co-expression of TrkB.FL and TrkB.T1 protein in excitatory and inhibitory cortical neurons suggests that truncated TrkB receptors can influence BDNF signaling by antagonizing the effects of TrkB.FL receptors (Armanini et al., 1995). In mouse embryonic cortex, TrkB.T4 mRNA is expressed in neurons, but not astrocytes (Stoilov et al., 2002); however, due to lack of specific antibodies it remains unknown which cell types express TrkB.T2 and TrkB.T4 protein. Because these isoforms are regulated by visual activity, determining the localization and function of these isoforms is an important goal. It is also important to point out that if the experience-dependent expression of different TrkB isoforms is cell-type specific, we may have missed changes due to up-regulation in some cell types and down-regulation in others. Gene-chip experiments examining cell-type specific expression in cortex (for review see Nelson et al., 2006b) are not yet designed to detect these splice variants of TrkB, so the extent to which different neuronal populations express different TrkB isoforms is unknown.
While TrkB is the cognate receptor for BDNF, the low affinity neurotrophin receptor P75NTR, can bind BDNF as well. We used immunohistochemistry to investigate cell-type specific expression of P75NTR, and found expression in a subset of parvalbumin positive inhibitory neurons, and in putative pyramidal neurons, but not in astrocytes. As expected, P75NTR labeling is much less ubiquitous than TrkB receptor labeling. Basal levels of P75NTR in cortex are thought to be low, and as described previously (McQuillen et al., 2002), we found that P75NTR mRNA expression decreases developmentally. Interestingly, we found that P75NTR expression can be up-regulated by prolonged visual deprivation. This is likely functionally relevant, as P75NTR expression plays an important role in BDNF signaling. P75NTR can physically interact with TrkB (Huang and Reichardt, 2003), and when P75NTR is present TrkB becomes more specific for BDNF, and less likely to bind NT 3 (Zaccaro et al., 2001) and NT 4/5 (Huang and Reichardt, 2003). It has been shown by (Singh et al., 2008) that BDNF signaling through P75NTR is required for axon pruning during development, and in the degeneration of axons as a result of competition. According to their model, neuronal activity causes release of BDNF, which binds to P75NTR on neighboring inactive neurons. P75NTR is up-regulated in these neurons causing axonal degeneration. In this way, the active axon survives, while the inactive axon is lost (Singh et al., 2008). Further, it has been shown that P75NTR can influence neurotrophin signaling specifically within V1, as the effects of nerve growth factor perfusion are significantly reduced in visual cortex when P75NTR signaling is blocked (Sala et al., 1998). This suggests that the increase in P75NTR expression induced by visual deprivation will affect BDNF signaling in visual cortex.
The strong activity-dependent regulation of TrkB isoforms that we show raises a number of interesting questions. The circuit rewiring that takes place as a result of activity deprivation is occurring in a cell-type specific manner, with an increase in the strengths of synapses onto certain cell types, and a decrease in the strengths of synapses onto other cell types (Maffei et al., 2004; Maffei and Turrigiano, 2008a). It is possible that TrkB isoform expression is also regulated in a cell-type specific manner, with increasing expression in certain cell types, and decreasing expression in others. Visual deprivation also has distinct effects on circuit rewiring that are dependent on which cortical layer is examined (Maffei et al., 2004; Maffei et al., 2006). It is possible that activity-dependent expression of TrkB isoforms is also differing in a layer-specific manner. Because our samples contained a homogenous population of cells from all cortical layers, we were not able to address either of these questions here, but with advances in cell sorting techniques and transgenic mouse lines expressing markers for distinct cell types (Nelson et al., 2006a), these questions may soon be addressable.
Taken together our results indicate that the experience-dependent regulation of BDNF signaling pathways within visual cortex is more complicated than previously appreciated. The expression of each TrkB isoform can be independently regulated, both during normal cortical development, and in response to activity deprivation. BDNF signaling likely depends on the relative abundance of the four TrkB isoforms and P75NTR, so that these developmental and experience-driven changes in BDNF receptor isoforms could have profound effects on BDNF-dependent forms of cortical plasticity. In particular, we found that TrkB.T4 is strongly regulated by visual experience, in a manner that depends on deprivation protocol. This raises the interesting possibility that experience-dependent regulation of TrkB.T4 expression contributes to the differential effects of visual deprivation on cortical plasticity.
Acknowledgments
We thank Arianna Maffei, Chris Hempel, Annette Gray and Roman Pavlyuk for advice and technical assistance. Supported by EY 014429.
REFERENCES
- Akaneya Y, Tsumoto T, Kinoshita S, Hatanaka H. Brain-derived neurotrophic factor enhances long-term potentiation in rat visual cortex. J Neurosci. 1997;17:6707–6716. doi: 10.1523/JNEUROSCI.17-17-06707.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allendoerfer KL, Cabelli RJ, Escandon E, Kaplan DR, Nikolics K, Shatz CJ. Regulation of neurotrophin receptors during the maturation of the mammalian visual system. J Neurosci. 1994;14:1795–1811. doi: 10.1523/JNEUROSCI.14-03-01795.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armanini MP, McMahon SB, Sutherland J, Shelton DL, Phillips HS. Truncated and catalytic isoforms of trkB are co-expressed in neurons of rat and mouse CNS. Eur J Neurosci. 1995;7:1403–1409. doi: 10.1111/j.1460-9568.1995.tb01132.x. [DOI] [PubMed] [Google Scholar]
- Asai N, Abe T, Saito T, Sato H, Ishiguro S, Nishida K. Temporal and spatial differences in expression of TrkB isoforms in rat retina during constant light exposure. Exp Eye Res. 2007;85:346–355. doi: 10.1016/j.exer.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Baxter GT, Radeke MJ, Kuo RC, Makrides V, Hinkle B, Hoang R, Medina-Selby A, Coit D, Valenzuela P, Feinstein SC. Signal transduction mediated by the truncated trkB receptor isoforms, trkB.T1 and trkB.T2. J Neurosci. 1997;17:2683–2690. doi: 10.1523/JNEUROSCI.17-08-02683.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biffo S, Offenhauser N, Carter BD, Barde YA. Selective binding and internalisation by truncated receptors restrict the availability of BDNF during development. Development. 1995;121:2461–2470. doi: 10.1242/dev.121.8.2461. [DOI] [PubMed] [Google Scholar]
- Bozzi Y, Pizzorusso T, Cremisi F, Rossi FM, Barsacchi G, Maffei L. Monocular deprivation decreases the expression of messenger RNA for brain-derived neurotrophic factor in the rat visual cortex. Neuroscience. 1995;69:1133–1144. doi: 10.1016/0306-4522(95)00321-9. [DOI] [PubMed] [Google Scholar]
- Cabelli RJ, Shelton DL, Segal RA, Shatz CJ. Blockade of endogenous ligands of trkB inhibits formation of ocular dominance columns. Neuron. 1997;19:63–76. doi: 10.1016/s0896-6273(00)80348-7. [DOI] [PubMed] [Google Scholar]
- Capsoni S, Tongiorgi E, Cattaneo A, Domenici L. Differential regulation of brain-derived neurotrophic factor messenger RNA cellular expression in the adult rat visual cortex. Neuroscience. 1999;93:1033–1040. doi: 10.1016/s0306-4522(99)00240-7. [DOI] [PubMed] [Google Scholar]
- Chakravarthy S, Saiepour MH, Bence M, Perry S, Hartman R, Couey JJ, Mansvelder HD, Levelt CN. Postsynaptic TrkB signaling has distinct roles in spine maintenance in adult visual cortex and hippocampus. Proc Natl Acad Sci U S A. 2006;103:1071–1076. doi: 10.1073/pnas.0506305103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung ZH, Chin WH, Chen Y, Ng YP, Ip NY. Cdk5 is involved in BDNF-stimulated dendritic growth in hippocampal neurons. PLoS Biol. 2007;5:e63. doi: 10.1371/journal.pbio.0050063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai NS, Cudmore RH, Nelson SB, Turrigiano GG. Critical periods for experience-dependent synaptic scaling in visual cortex. Nat Neurosci. 2002;5:783–789. doi: 10.1038/nn878. [DOI] [PubMed] [Google Scholar]
- Eide FF, Vining ER, Eide BL, Zang K, Wang XY, Reichardt LF. Naturally occurring truncated trkB receptors have dominant inhibitory effects on brain-derived neurotrophic factor signaling. J Neurosci. 1996;16:3123–3129. doi: 10.1523/JNEUROSCI.16-10-03123.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feller MB, Scanziani M. A precritical period for plasticity in visual cortex. Curr Opin Neurobiol. 2005;15:94–100. doi: 10.1016/j.conb.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Forooghian F, Kojic L, Gu Q, Prasad SS. Identification of a novel truncated isoform of trkB in the kitten primary visual cortex. J Mol Neurosci. 2001;17:81–88. doi: 10.1385/JMN:17:1:81. [DOI] [PubMed] [Google Scholar]
- Frenkel MY, Bear MF. How monocular deprivation shifts ocular dominance in visual cortex of young mice. Neuron. 2004;44:917–923. doi: 10.1016/j.neuron.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Fryer RH, Kaplan DR, Feinstein SC, Radeke MJ, Grayson DR, Kromer LF. Developmental and mature expression of full-length and truncated TrkB receptors in the rat forebrain. J Comp Neurol. 1996;374:21–40. doi: 10.1002/(SICI)1096-9861(19961007)374:1<21::AID-CNE2>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Gianfranceschi L, Siciliano R, Walls J, Morales B, Kirkwood A, Huang ZJ, Tonegawa S, Maffei L. Visual cortex is rescued from the effects of dark rearing by overexpression of BDNF. Proc Natl Acad Sci U S A. 2003;100:12486–12491. doi: 10.1073/pnas.1934836100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes RA, Hampton C, EL-Sabeawy F, Sabo SL, McAllister AK. The dynamic distribution of TrkB receptors before, during, and after synapse formation between cortical neurons. J Neurosci. 2006;26:11487–11500. doi: 10.1523/JNEUROSCI.2364-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon JA, Stryker MP. Experience-dependent plasticity of binocular responses in the primary visual cortex of the mouse. J Neurosci. 1996;16:3274–3286. doi: 10.1523/JNEUROSCI.16-10-03274.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- Hooks BM, Chen C. Critical periods in the visual system: changing views for a model of experience-dependent plasticity. Neuron. 2007;56:312–326. doi: 10.1016/j.neuron.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. Trk receptors: roles in neuronal signal transduction. Annu Rev Biochem. 2003;72:609–642. doi: 10.1146/annurev.biochem.72.121801.161629. [DOI] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Hubel DH, Wiesel TN. Effects of Monocular Deprivation in Kittens. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1964;248:492–497. doi: 10.1007/BF00348878. [DOI] [PubMed] [Google Scholar]
- Ichisaka S, Katoh-Semba R, Hata Y, Ohshima M, Kameyama K, Tsumoto T. Activity-dependent change in the protein level of brain-derived neurotrophic factor but no change in other neurotrophins in the visual cortex of young and adult ferrets. Neuroscience. 2003;117:361–371. doi: 10.1016/s0306-4522(02)00771-6. [DOI] [PubMed] [Google Scholar]
- Klau M, Hartmann M, Erdmann KS, Heumann R, Lessmann V. Reduced number of functional glutamatergic synapses in hippocampal neurons overexpressing full-length TrkB receptors. J Neurosci Res. 2001;66:327–336. doi: 10.1002/jnr.10007. [DOI] [PubMed] [Google Scholar]
- Lein ES, Shatz CJ. Rapid regulation of brain-derived neurotrophic factor mRNA within eye-specific circuits during ocular dominance column formation. J Neurosci. 2000;20:1470–1483. doi: 10.1523/JNEUROSCI.20-04-01470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Maffei A, Turrigiano GG. Multiple modes of network homeostasis in visual cortical layer 2/3. J Neurosci. 2008a;28:4377–4384. doi: 10.1523/JNEUROSCI.5298-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A, Turrigiano G. Chapter 12 The age of plasticity: Developmental regulation of synaptic plasticity in neocortical microcircuits. Prog Brain Res. 2008b;169:211–223. doi: 10.1016/S0079-6123(07)00012-X. [DOI] [PubMed] [Google Scholar]
- Maffei A, Nelson SB, Turrigiano GG. Selective reconfiguration of layer 4 visual cortical circuitry by visual deprivation. Nat Neurosci. 2004;7:1353–1359. doi: 10.1038/nn1351. [DOI] [PubMed] [Google Scholar]
- Maffei A, Nataraj K, Nelson SB, Turrigiano GG. Potentiation of cortical inhibition by visual deprivation. Nature. 2006;443:81–84. doi: 10.1038/nature05079. [DOI] [PubMed] [Google Scholar]
- Majdan M, Shatz CJ. Effects of visual experience on activity-dependent gene regulation in cortex. Nat Neurosci. 2006;9:650–659. doi: 10.1038/nn1674. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Neurotrophin regulation of cortical dendritic growth requires activity. Neuron. 1996;17:1057–1064. doi: 10.1016/s0896-6273(00)80239-1. [DOI] [PubMed] [Google Scholar]
- McAllister AK, Katz LC, Lo DC. Opposing roles for endogenous BDNF and NT-3 in regulating cortical dendritic growth. Neuron. 1997;18:767–778. doi: 10.1016/s0896-6273(00)80316-5. [DOI] [PubMed] [Google Scholar]
- McQuillen PS, DeFreitas MF, Zada G, Shatz CJ. A novel role for p75NTR in subplate growth cone complexity and visual thalamocortical innervation. J Neurosci. 2002;22:3580–3593. doi: 10.1523/JNEUROSCI.22-09-03580.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middlemas DS, Lindberg RA, Hunter T. trkB, a neural receptor protein-tyrosine kinase: evidence for a full-length and two truncated receptors. Mol Cell Biol. 1991;11:143–153. doi: 10.1128/mcb.11.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales B, Choi SY, Kirkwood A. Dark rearing alters the development of GABAergic transmission in visual cortex. J Neurosci. 2002;22:8084–8090. doi: 10.1523/JNEUROSCI.22-18-08084.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson SB, Hempel C, Sugino K. Probing the transcriptome of neuronal cell types. Curr Opin Neurobiol. 2006a;16:571–576. doi: 10.1016/j.conb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Nelson SB, Sugino K, Hempel CM. The problem of neuronal cell types: a physiological genomics approach. Trends Neurosci. 2006b;29:339–345. doi: 10.1016/j.tins.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Ohira K, Shimizu K, Yamashita A, Hayashi M. Differential expression of the truncated TrkB receptor, T1, in the primary motor and prefrontal cortices of the adult macaque monkey. Neurosci Lett. 2005;385:105–109. doi: 10.1016/j.neulet.2005.05.033. [DOI] [PubMed] [Google Scholar]
- Patz S, Wahle P. Developmental changes of neurotrophin mRNA expression in the layers of rat visual cortex. Eur J Neurosci. 2006;24:2453–2460. doi: 10.1111/j.1460-9568.2006.05126.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Prusky GT, West PW, Douglas RM. Experience-dependent plasticity of visual acuity in rats. Eur J Neurosci. 2000;12:3781–3786. doi: 10.1046/j.1460-9568.2000.00236.x. [DOI] [PubMed] [Google Scholar]
- Rossi FM, Bozzi Y, Pizzorusso T, Maffei L. Monocular deprivation decreases brain-derived neurotrophic factor immunoreactivity in the rat visual cortex. Neuroscience. 1999;90:363–368. doi: 10.1016/s0306-4522(98)00463-1. [DOI] [PubMed] [Google Scholar]
- Rudge JS, Li Y, Pasnikowski EM, Mattsson K, Pan L, Yancopoulos GD, Wiegand SJ, Lindsay RM, Ip NY. Neurotrophic factor receptors and their signal transduction capabilities in rat astrocytes. Eur J Neurosci. 1994;6:693–705. doi: 10.1111/j.1460-9568.1994.tb00981.x. [DOI] [PubMed] [Google Scholar]
- Rutherford LC, Nelson SB, Turrigiano GG. BDNF has opposite effects on the quantal amplitude of pyramidal neuron and interneuron excitatory synapses. Neuron. 1998;21:521–530. doi: 10.1016/s0896-6273(00)80563-2. [DOI] [PubMed] [Google Scholar]
- Sala R, Viegi A, Rossi FM, Pizzorusso T, Bonanno G, Raiteri M, Maffei L. Nerve growth factor and brain-derived neurotrophic factor increase neurotransmitter release in the rat visual cortex. Eur J Neurosci. 1998;10:2185–2191. doi: 10.1046/j.1460-9568.1998.00227.x. [DOI] [PubMed] [Google Scholar]
- Sermasi E, Margotti E, Cattaneo A, Domenici L. Trk B signalling controls LTP but not LTD expression in the developing rat visual cortex. Eur J Neurosci. 2000;12:1411–1419. doi: 10.1046/j.1460-9568.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- Singh KK, Park KJ, Hong EJ, Kramer BM, Greenberg ME, Kaplan DR, Miller FD. Developmental axon pruning mediated by BDNF-p75NTR-dependent axon degeneration. Nat Neurosci. 2008;11:649–658. doi: 10.1038/nn.2114. [DOI] [PubMed] [Google Scholar]
- Stafford CA. Critical period plasticity for visual function: definition in monocularly deprived rats using visually evoked potentials. Ophthalmic Physiol Opt. 1984;4:95–100. [PubMed] [Google Scholar]
- Stoilov P, Castren E, Stamm S. Analysis of the human TrkB gene genomic organization reveals novel TrkB isoforms, unusual gene length, and splicing mechanism. Biochem Biophys Res Commun. 2002;290:1054–1065. doi: 10.1006/bbrc.2001.6301. [DOI] [PubMed] [Google Scholar]
- Tagawa Y, Kanold PO, Majdan M, Shatz CJ. Multiple periods of functional ocular dominance plasticity in mouse visual cortex. Nat Neurosci. 2005;8:380–388. doi: 10.1038/nn1410. [DOI] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Single-Cell Responses in Striate Cortex of Kittens Deprived of Vision in One Eye. J Neurophysiol. 1963;26:1003–1017. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Yacoubian TA, Lo DC. Truncated and full-length TrkB receptors regulate distinct modes of dendritic growth. Nat Neurosci. 2000;3:342–349. doi: 10.1038/73911. [DOI] [PubMed] [Google Scholar]
- Zaccaro MC, Ivanisevic L, Perez P, Meakin SO, Saragovi HU. p75 Co-receptors regulate ligand-dependent and ligand-independent Trk receptor activation, in part by altering Trk docking subdomains. J Biol Chem. 2001;276:31023–31029. doi: 10.1074/jbc.M104630200. [DOI] [PubMed] [Google Scholar]


