Abstract
Heart failure (HF) is common, results in poor clinical outcomes, and is associated with large health-care costs. The incidence of HF continues to rise, with approximately 670,000 new cases per year and a 20% lifetime risk of HF for persons 40 years and older in the United States. Risk factors for HF have been identified and thus preventative strategies should have a positive effect on disease burden, morbidity, and mortality. Although coronary artery disease and hypertension have traditionally been considered among the most important modifiable risk factors for the development of HF, recent studies have highlighted the importance of increasingly prevalent metabolic risk factors – glucose, diabetes, obesity, and the metabolic syndrome. This paper will present evidence for the link between glucose, diabetes, obesity, metabolic syndrome and incident HF. Furthermore, we will discuss how risk factor modification and other preventive therapies may help curb the rising incidence of HF.
Keywords: Glucose, Obesity, Heart Failure, Metabolic Syndrome, Diabetes
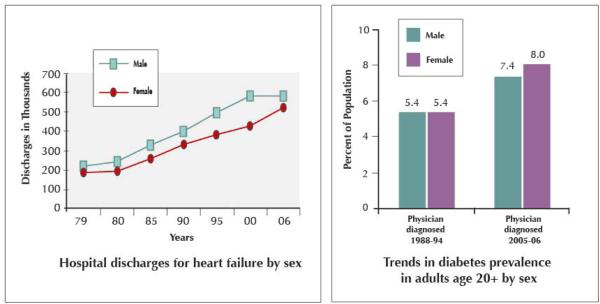
Heart failure (HF) continues to be a major public health concern, as HF incidence, hospitalizations, and cost continue to rise. There are approximately 670,000 new cases of HF per year in the United States (US) in persons over age 45 years. Incidence of HF increases in older age, with an incidence rate (per 1000 person-years) of 9.2 and 4.7 for men and women ages 65 – 74, 22.3 and 14.8 for men and women ages 75 – 84, and 41.9 and 32.7 for men and women 85 years and older (1). Lifetime risk of developing HF is approximately 20% for persons at age 40 years, and remains at 20% for persons reaching 80 years of age. HF hospitalizations have tripled in the time period between 1979 and 2004, due in part to the aging population and advanced cardiovascular therapeutics which prolong survival (2-4). Age-adjusted hospitalization rates for primary diagnoses of HF increased from 1979 to 2004 from 219 / 100,000 to 390 / 100, respectively (Figure 1) (3). The estimated cost burden of HF in the US in 2009 is $37.2 billion (1).
Figure 1. Age-Adjusted Hospitalization Rates for Heart Failure.
National Hospital Discharge Survey, 1979–2004Trends of age-adjusted heart failure hospitalization rate (per 100,000) from 1979 to 2004 among patients with heart failure as the first-listed or additional (2nd to 7th) diagnosis for men and women. From Fang, J., G. A. Mensah, et al. (2008). “Heart Failure-Related Hospitalization in the U.S., 1979 to 2004.” Journal of the American College of Cardiology 52(6): 428.
Risk Factors for Incident Heart Failure – Initial Studies
There are numerous established and hypothesized risk factors for the development of HF (Table 1). Large epidemiologic studies have helped delineate risk factors for HF in the general population. A study of a 1970 Framingham cohort followed for 20 years found that hypertension had the greatest population-attributable risk for HF, accounting for 39% of HF cases in men and 59% in women. History of myocardial infarction had the second highest population-attributable risk 34% in men and 13% in women. Other risk factors for HF included diabetes, left ventricular hypertrophy, and valvular heart disease (5). Another large prospective cohort study, the First National Health and Nutrition Examination Survey Epidemiologic Follow-up Study (NHANES I), also initiated in the 1970's, found that coronary artery disease was the largest independent risk factor for HF, in addition to hypertension, smoking, physical inactivity, overweight status, low educational level, diabetes, and valvular heart disease (6). Since diabetes, obesity, and the metabolic syndrome have become increasingly prevalent in the US population over the past decades since the inception of the above studies (1) (Figure 1), the impact of these risk factors on the growing epidemic of HF warrants additional attention.
Table 1.
Established and Hypothesized Risk Factors for Heart Failure
Major Clinical Risk Factors
|
Toxic Risk Precipitants
|
Minor Clinical Risk Factors
|
Genetic Risk Predictors
|
IL-6, CRP
|
Morphological Risk Predictors
|
5-FU indicates 5-fluorouracil; SNP, single-nucleotide polymorphism; LVID, left ventricular internal dimension; LVH, left ventricular hypertrophy; NSAIDs, nonsteroidal antiinflammatory drugs; IGF, insulinlike growth factor; TNF, tumor necrosis factor; IL, interleukin; CRP, C-reactive protein; and HR, heart rate.
(From Schocken DD, Benjamin EJ, Fonarow GC, et al. Prevention of Heart Failure: A Scientific Statement From the American Heart Association Councils on Epidemiology and Prevention, Clinical Cardiology, Cardiovascular Nursing, and High Blood Pressure Research; Quality of Care and Outcomes Research Interdisciplinary Working Group; and Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation 2008;117:2544-2565.)
Diabetes, Glucose, and Incident Heart Failure
The prevalence of diabetes in the US, the vast majority of which is type II diabetes, has increased by more than 60% since 1990. Diabetes is now diagnosed in approximately 10% of those ≥ 20 years and 15% of those ≥ 65 years of age; furthermore, undiagnosed diabetes is present in an additional approximate 7% and 3% of these age groups respectively (1). The total prevalence of diabetes is expected to more than double by 2050 (1).
The Framingham study initially established that a clinical history of diabetes was independently associated with risk of developing HF, with a two-fold increased risk in men and a five-fold increased risk in women (7). More recent studies (8,9) have reported higher annual incidences of HF in diabetics however, for example, 31/1000 person-years in a cohort study using a Kaiser Permanente Northwest database (9), compared to 9-14 cases / 1000 reported in Framingham (7). Although diabetes predicts risk of developing HF independent of age, gender, or race/ethnicity, it confers a relatively higher relative risk of HF in populations of women and young people (9,10). For example, among 2391 women with coronary artery disease but no HF at baseline followed in the Heart and Estrogen/progestin Replacement Study (HERS), diabetes was the strongest independent risk factor for development of HF, with an adjusted hazard ratio of 3.1 (2.3–4.2) (10).
In patients with diabetes, the degree of glycemic control as indexed by glycosylated hemoglobin (HbA1c) is associated with magnitude of HF risk (11-13). In a cohort study of 48,858 adult patients with (predominantly type 2) diabetes, each 1% increase in HbA1c was associated with an 8% increased risk of HF hospitalization or HF death, even after adjusting for demographics, medical history, medications, and other risk factors (11). Among 1827 participants in the Atherosclerosis Risk in Communities (ARIC) study with diabetes and no evidence of HF at baseline, risk of HF increased proportionally with HbA1c; even in those without coronary disease, HbA1c of 6.1-7%, 7.1 – 8%, and >8% (compared to HbA1c<6%), corresponded to relative risks of developing HF of 1.7, 2.5, and 3.1, respectively.
Not only HbA1c but elevated glucose level is a risk factor for HF, independent of the presence of diagnosed or treated diabetes. In a prospective cohort study of 31,546 subjects at high risk of cardiovascular disease, fasting plasma glucose was an independent predictor of hospitalization for HF; per mmol increase in fasting plasma glucose, relative risk was 1.23 (1.03–1.47) in subjects without diabetes and 1.04 (1.01–1.07) for those with diabetes (14). Impaired fasting glucose and/or impaired glucose tolerance were also linked to the incidence of HF in a non-diabetic population of over 20,000 subjects living in the Portland, Oregon area (15).
Not surprisingly, insulin resistance without overt diabetes is also significantly linked to the development of HF. In the Uppsala Longitudinal Study of Adult Men older than 70 years, insulin resistance and insulin sensitivity, as measured by pro-insulin levels and euglycemic insulin clamp testing respectively, were independently associated with reduced risk of HF; relative risk per 1 standard deviation increase in pro-insulin level was 1.29 (1.02 – 1.64) and relative risk per 1 standard deviation decrease in clamp glucose disposal rate relative risk was 0.66 (0.51 – 0.86) (16). Furthermore, in men at age 50 years of age, insulin resistance and pro-insulin levels were shown to be predictive of both left ventricular systolic and diastolic dysfunction 20 years later, at age 70 years (17,18).
There are numerous potential pathophysiologic mechanisms underlying the relationship between diabetes, glucose, insulin resistance and HF, in addition to the well-known diabetes-associated risk of coronary atherosclerosis (Figure 2). The heart, which requires enormous energy for daily function (5kg ATP/day), may become less energy efficient in the setting of insulin resistance, with decreased glucose utilization and increased free fatty acid utilization. This metabolic dysregulation may increase susceptibility to injury such as pressure overload or ischemia and thus promote deleterious renin-angiotensin-aldosterone system activation. Experimental data links both hyperglycemia and hyperinsulinemia with increased sympathetic nervous system activation, a key pathophysiologic mechanism in HF. Morphologic changes in diabetic myocardium include myocyte hypertrophy, perivascular fibrosis, and increased collagen deposition; these histologic changes are likely a function of accumulation of advanced glycation end products in the myocardium, increased fatty acid deposition, and oxidative stress related to hyperglycemia and hyperinsulinemia. Endomyocardial biopsy specimens from non-ischemic systolic HF patients have shown increased deposition of advanced glycation end-products and increased myocardial collagen volume fraction in diabetic vs. non-diabetic patients; both advanced glycation end products and collagen volume were associated with higher diastolic left ventricular stiffness (19). Furthermore, the increased intramyocardial triglyceride content in patients with both impaired glucose tolerance and diabetes, may lead to lipotoxicity and cardiomyocyte apoptosis, ultimately leading to cardiac dysfunction (20-26).
Figure 2.
Multiple Pathogenetic Mechanisms Involved in the Relationship Between Diabetes, Metabolic Disease, and Heart Failure.
Obesity and Incident Heart Failure
Strongly correlated to insulin resistance, obesity is another emerging risk factor for HF. Although the concept of a cardiomyopathy relating to obesity had previously been described (27), the strong independent, and incremental relationship between body mass index (BMI, kg/m2) and HF incidence was recently established. In a study of 5881 participants in the Framingham Heart Study, increase in the risk of HF per unit BMI increase was 5% for men and 7% for women, even after adjustment for demographics and known risk factors of myocardial infarction, diabetes, hypertension, and cholesterol (28). The positive correlation between BMI and risk of HF was confirmed in a larger prospective cohort study of 21,094 men (mean age 53 years) without known coronary heart disease enrolled in the Physicians' Health Study; in this study both overweight (BMI 25.0 – 29.9) and obesity (BMI ≥ 30) were independently associated with risk of new onset HF, with adjusted relative risks compared to lean (BMI<25) men of 1.49 (1.32–1.69) and 2.80 (2.24–3.50), respectively (Figure 3). In addition to BMI, level of physical activity was also associated with HF risk, with the highest relative risk of HF seen in obese men who were also physically inactive (3.9 [2.6 – 6.0]) compared to men who were lean and physically active. Obese, active men 2.7 (2.1 – 3.5), overweight, inactive men 1.8 (1.4 – 2.2), as well as overweight and active men 1.5 (1.3 – 1.7) were all at higher risk compared to lean, active men (29). In addition to BMI, other anthropometric indices of obesity, including waist circumference and waist to hip ratio, have also been associated with incident HF (30).
Figure 3. Cumulative Incidence of HF According to Categories of BMI.
From Kenchaiah S, Sesso HD, Gaziano JM. Body Mass Index and Vigorous Physical Activity and the Risk of Heart Failure Among Men. Circulation 2009;119:44-52.
Although the relationship between overweight / obesity and incident HF may be related to hemodynamic and anatomic cardiac changes related to excess body mass (31), recent evidence suggests that the relationship is also mediated by obesity-related metabolic, inflammatory, and hormonal changes. Obesity is highly correlated with insulin resistance, which may in part potentiate the link between obesity and HF. In the Uppsala study, insulin sensitivity (clamp glucose disposal rate) but not anthropometric indices of obesity, was independently predictive of HF risk when the two were entered together in a fully adjusted model (16). Analyses from the Multi-Ethnic Study of Atherosclerosis (MESA) study suggest that inflammation may potentiate the link between obesity and risk of developing HF. Although risk of HF was 83% higher in obese compared to non-obese subjects after adjustment for traditional risk factors, the relationship between obesity and incident HF was no longer significant after adjustment for the inflammatory biomarkers interleukin-6 or C-reactive protein, which are known to associated with risk of HF (32,33). Most recently, the adipokine resistin, expressed by adipocytes and associated with insulin resistance and inflammation, was associated with risk of developing HF independent of coronary heart disease and other risk factors in a Framingham offspring analysis, providing an additional clue to mechanisms underlying obesity and the development of HF (34). Other adipokines associated with obesity, including leptin and adiponectin, have not been found to independently predict new onset HF (34,35).
The Metabolic Syndrome and Incident Heart Failure
The metabolic syndrome, a cluster of risk factors for cardiovascular disease and type II diabetes, is exceedingly prevalent in the US with an estimated prevalence of over 75 million in those ≥ 20 years. The presence of metabolic syndrome is associated with risk of cardiovascular disease, although whether its prognostic value is equal to or better than the Framingham risk score, is subject to debate (1).
Since obesity, insulin resistance, and high blood pressure are components of the metabolic syndrome, it is not surprising that the metabolic syndrome has been linked to incident HF. Butler et al. investigated the impact of metabolic syndrome on cardiovascular outcomes in 3031 subjects age 70-79; metabolic syndrome was defined by ATP-III criteria (Third report of the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults) as any three of the following risk factors: increased waist circumference (men >101.6 cm and women >88.9 cm), high triglycerides (≥150 mg/dl), low high density lipoprotein (men <40 mg/dl, women <50 mg/dl), high blood pressure (≥130/≥85 mm Hg), and high fasting glucose level (≥110mg/dL). Metabolic syndrome was independently associated not only with incident myocardial infarction and coronary events, but also with incident HF hospitalization (RR 1.5, p=0.009) in this cohort of older adults. However, when the subgroup of subjects without past history of coronary heart disease or HF was analyzed, the relationship was no longer statistically significant (36). However, a study of 2314 middle-aged men without baseline HF or coronary heart disease found metabolic syndrome, defined by ATP-III criteria with BMI substituted for waist circumference, to be a significant, independent predictor of subsequent HF, even after adjusting for interim MI (hazard ratio 1.80, 95% CI 1.11 to 2.91) (37).
HF risk may not be associated with the metabolic syndrome per se but rather with individual risk factors reflected by metabolic syndrome. In the analysis of MESA (32), metabolic syndrome as defined by ATPIII criteria predicted two-fold increased risk of HF in unadjusted analyses; however, after adjustment for known risk factors, only two specific components of the metabolic syndrome – abdominal adiposity and high plasma glucose – were strong, independent predictors of HF, while metabolic syndrome itself was not. Li et al, analyzing NHANES data, found the metabolic syndrome also to be associated with two-time higher risk of incident HF; in this analysis, insulin resistance, as quantified the homoeostasis model assessment (HOMA), accounted for >90% of the association between metabolic syndrome and HF (38).
Does Risk Factor Modification Help Prevent Heart Failure?
It is clear from the multitude of epidemiologic data described that diabetes, insulin resistance, glucose, and obesity are risk factors for the development of HF. Furthermore, it has been shown that the absence of risk factors, or having “optimal risk factors”, is associated with extremely low incidence of HF; the vast majority of incident HF, close to 90%, is attributable to the modifiable risk factors of diabetes, obesity, smoking, blood pressure, and hypercholesterolemia (39). In light of the rapidly increasing prevalence of obesity, diabetes, and pre-diabetes in the US, it is imperative HF prevention strategies include modification of these risk factors.
Diabetes
For patients with diabetes (type I or type II), the American College of Cardiology (ACC), American Heart Association (AHA), and American Diabetes Association recommend glycemic control to HbA1c ≤ 7% for reduction of cardiovascular risk (ACC/AHA Class IIb recommendation, level of evidence A). These recommendations are based on long-term follow-up from the Diabetes Control and Complications Trial (DCCT) and the UK Prospective Diabetes Study (UKPDS), which show a reduction in cardiovascular risk at or around HbA1c of 7% (40).
However, more recent studies of more intensive glucose control have not yielded uniformly beneficial results. The Glucose Control and Vascular Complications in Veterans with Type 2 Diabetes (VADT), in 1791 patients with mean Hba1c at baseline of 9.4%, randomized subjects to intensive glucose control with metformin, thiazolidinedione (glitazone), and / or sulfonylurea vs. usual care; although HbA1c was lower by a mean of 1.5% at an average follow-up of 5.6 years in the intensive therapy group, there was no significant difference in any cardiovascular outcomes or all cause mortality (41). The ADVANCE collaborative study group randomized 11,140 diabetic patients with mean HbA1c of 7.5% to intensive glucose control defined as the use of gliclazide (modified release) plus other drugs as required to achieve a glycated hemoglobin value of 6.5% or less vs. usual care; this study also showed no significant effect on macrovascular events (42). Another recent large, randomized,controlled clinical trial (The Action to Control Cardiovascular Risk in Diabetes Study Group, ACCORD), including 10,251 patients with type II diabetes and mean HbA1c of 8.1% at baseline, compared intensive treatment with a variety of oral and insulin-based medications and goal HbA1c<6% to usual therapy; this trial was stopped early due to excess mortality in the intensive treatment arm, although the primary composite outcome of cardiovascular events was similar between the two groups (43). And most recently, a meta-analysis including the above three trials plus UKPDS and PROspectivepioglitAzoneClinical Trial In macrovVascular Events (PROactive) studied the effect of intensive control of glucose on cardiovascular outcomes and death; this meta-analysis demonstrated an overall, significant reduction in non-fatal myocardial infarction and coronary heart disease events with intensive treatment compared to standard treatment. The overall effects of intensive glucose control on HF events and all cause mortality were neutral. However, there was significant heterogeneity between subgroups of studies separated by glitazone use, suggesting that glitazone use was associated with excess risk of HF (44).
In light of strong association between diabetes, hyperglycemia, and HF incidence, it is surprising that intensive glucose control has not been more clearly linked with decreased HF incidence or improved cardiovascular outcomes. There are several potential explanations for this lack of benefit. It may be that only patients early in the course of their diabetes rather than those with severe long-standing disease are able to reap the benefits of intense glucose control; only the UKPDS study, which included patients with a recent diagnosis of diabetes, showed decreased risk of myocardial infarction with intensive glucose control (45). The excellent background risk-reducing therapy in recent diabetes trials, including over 86% of subjects in VADT and ACCORD on statins, may have made it more difficult to show incremental risk reduction with glucose lowering (46). Furthermore, more frequent episodes of severe hypoglycemia were associated with more intensive glucose control in these clinical studies, and hypoglycemia may trigger adverse cardiovascular events (46). Another detrimental effect of intensive glucose control seen in the majority of clinical trials was significant weight gain (45).
One important reason for the surprising lack of effect of intense glucose control on HF incidence may be related to medication choice. In patients with diabetes, the type of anti-diabetic medication used in HF may impact the future development of HF. In a retrospective cohort study of 5631 patients with new onset diabetes started on oral monotherapy, sulfonyolurea use was associated with significantly higher risk of developing HF than metformin use; incidence was 4.4 vs. 3.3 / 1000 persons, respectively, and adjusted hazard ratio was 1.24 (1.01–1.54) for sulfonylurea compared to metformin (47). Another observational study showed that diabetics treated with an insulin-containing regimen were at highest risk of HF while those treated with a metformin-containing regimen were at lowest risk (48). Of note, metformin compared to other anti-diabetic therapies was also associated with decreased macrovascular risk in overweight diabetic subjects studied in UKPDS (49). These data suggest that metformin or perhaps other insulin-sensitizing regimens for subjects with diabetes may be preferable to those that increase insulin levels, although prospective studies in this arena are needed.
Glitazones, although insulin-sensitizers, have been associated with edema and weight gain, and may increase the risk of new onset HF. A retrospective cohort study of type II diabetics identified by insurance claims (n = 33,544) found that TZD use was associated with a higher adjusted incidence of new onset HF compared to no TZD use (8.8% vs. 5.5% at 40 months) (50). The association between TZDs and new onset HF was confirmed in analyses of PROactive, which evaluated macrovascular morbidity and mortality in subjects with cardiovascular or peripheral vascular disease but without baseline HF. Although subjects randomized to pioglitazone had a non-significant trend towards reduced mortality / vascular events in the primary analysis (51), higher rates of both serious and non-serious HF were seen in the pioglitazone group (adjusted relative risk 1.53 [confidence interval 1.18-1.98]). Among those who developed HF, however, all cause mortality and HF deaths were similar between the two randomization groups, suggesting that perhaps TZDs are merely increasing signs of HF in patients at risk (52). Echocardiography studies have shown no change in cardiac function with TZD therapy (53).
Lifestyle Modification and Prevention
An essential component of HF prevention is reducing the incidence of type II diabetes. Lifestyle interventions, as definitively shown by the Diabetes Prevention Program Research Group, can effectively reduce the incidence of type II diabetes. This study randomized 3234 subjects with overweight BMI (≥24) and impaired fasting glucose to standard lifestyle recommendations plus placebo, standard lifestyle recommendations plus metformin, or an intensive program of lifestyle modification including diet and exercise; resulting diabetes incidence rates were 11.0, 7.8, and 4.8 per 100 person-years, respectively (p<0.0001). Reduction in incidence of diabetes was highest for the lifestyle intervention group (58% risk reduction compared to placebo group and 39% risk reduction compared to metformin group) (54).
Medical therapy aimed at preventing the onset of diabetes has been explored, but the success seen with lifestyle interventions has not been replicated. The Diabetes REduction Assessment with ramipril and rosiglitazone Medication (DREAM) trial investigated whether medical therapy could prevent new onset diabetes in patients with high fasting glucose or impaired glucose tolerance but without diabetes or cardiovascular disease; metabolic syndrome characteristics were common in this cohort, in which mean waist circumference was 101 cm (men) and 96 cm (women) and mean blood pressure 135.9 / 83.3 mmHg(55,56). Ramipril did not reduce risk of new onset diabetes, rate of cardiovascular events, or new onset HF in this population (57). Rosiglitazone did prevent the onset of diabetes in this high risk group (relative risk 0.38 [0.33–0.44]). However, rosiglitazone did not alter risk of cardiovascular events and furthermore significantly increased risk of HF (7.04 [1.60–31.0])(57). Thus, lifestyle modifications (diet and exercise) should be recommended as a primary intervention for those with hyperglycemia and/or metabolic syndrome, at risk of developing HF.
In light of clearly increased risk of HF with overweight and obesity, lifestyle interventions should be geared towards attaining a BMI ≤ 25. Diets aimed at weight loss may not only reduce the incidence of overweight and obesity but also reduce insulin resistance, diabetes, and the metabolic syndrome(58,59). Reduced-calorie diets are effective at cardiovascular risk reduction regardless of the macronutrient components of the diet, as shown in a recent dietary intervention study of 811 overweight adults randomized to one of four diets with variable emphasis on protein, fat, and carbohydrate intake; diets resulted in similar weight loss, decrease in waist circumference, lowered cholesterol levels, as well as reduction in fasting insulin levels (59). However, prospective studies demonstrating that weight loss results in lower risk of incident HF are needed.
Prevention of Heart Failure: What is Established
Patients at high risk of HF may be classified as having “Stage A” HF; stage A patients are those without symptoms of HF and without any structural heart disease who have risk factors for HF such as diabetes, hypertension, obesity, or coronary artery disease (4). For those with Stage A disease, evidence-based, guideline-recommended interventions should be implemented to decrease risk of HF (Table 2, Figure 4).
Table 2.
Class I Recommendations for the Treatment of Stage A Heart Failure from the ACC / AHA Guidelines
|
From American College of Cardiology Foundation/American Heart Association Task Force on Practice G, International Society for Heart and Lung T, Hunt SA, et al. 2009 Focused Update Incorporated Into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults. J Am Coll Cardiol 2009:j.jacc.2008.11.013.
Figure 4. Recommended Therapies by Stage of Heart Failure Algorithm.
ACEI indicates angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; EF, ejection fraction; FHx CM, family history of cardiomyopathy; HF, heart failure; LV, left ventricular; LVH, left ventricular hypertrophy; and MI, myocardial infarction. From American College of Cardiology Foundation/American Heart Association Task Force on Practice G, International Society for Heart and Lung T, Hunt SA, et al. 2009 Focused Update Incorporated Into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults. J Am Coll Cardiol 2009:j.jacc.2008.11.013.
An integral component of prevention in patients with diabetes is systolic and diastolic blood pressure control, particularly with angiotensin converting enzyme inhibitors or angiotensin receptor blockers (60-62). UKPDS clearly demonstrated that tight (< 150/85 mm Hg) with less tight (< 180/105 mm Hg) blood pressure control significantly reduces risk of HF; a 10-mm Hg decrease in systolic blood pressure was associated with a 56% decreased risk of incident HF (63). Captopril and atenolol therapy were equally efficacious in reducing risk of HF and other diabetes-related complications (64). A diabetes substudy (n = 3577) of the Heart Outcomes Prevention Evaluation (HOPE) also demonstrated a 20% relative risk reduction in any HF events for those randomized to ramipril vs. placebo (65).
In another subset of Stage A patients, those with coronary artery disease, treatment of hypercholesterolemia with statin therapy is effective at reducing the risk of HF, as shown in a substudy of the Scandinavian Simvastatin Survival Study (4S); new onset HF was 10.3% in the placebo group compared to 8.3% in the simvastatin group (p<0.015) (66). Established medical therapies to reduce risk of HF in those at risk are outlined in Table 3.
Table 3.
Medical Therapies to Reduce Risk of Heart Failure
| Clinical Trial | No. of Patients* |
Patient Inclusion Criteria | HF Incidence | Relative Risk Reduction, % |
|
|---|---|---|---|---|---|
| Randomized, placebo- controlled trials |
|||||
| ACEI | HOPE (ramipril, 2.5 or 10 mg) |
9297 | Vascular disease (CAD, PVD, or stroke) or diabetes plus cardiac risk factor; creatinine <2.4 mg/dL |
Ramipril vs placebo, 9% vs 11% |
23 |
| ACEI | EUROPA (perindopril, 8 mg) |
12 218 | Documented stable CAD |
Perindopril vs placebo, 1.0% vs 1.7% |
39 |
| ACEI | SAVE (captopril, target 50 mg, TID) |
2231 | After acute MI; LVEF ≤40%; without HF symptoms |
Captopril vs placebo, 11% vs 16% |
37 |
| Antiplatelet (ADP inhibitor) | CURE (clopidogrel 300- mg load, then 75 mg) |
12 562 | Acute coronary syndrome: non–ST- segment elevation ECG changes or elevated cardiac enzymes |
Clopidogrel vs placebo, 3.7% vs 4.4% |
18 |
| ARB | RENAAL (losartan, 50–100 mg) |
1513 | Type 2 diabetes mellitus, nephropathy |
Losartan vs placebo, 11.9% vs 16.7% |
32 |
| ARB | IDNT (irbesartan, 300 mg) |
1715 | Hypertension, type 2 diabetes, nephropathy |
Irbesartan vs placebo, N/A |
23 |
| Statin | 4S (simvastatin 20–40 mg) |
4444 | History of MI or angina, cholesterol 213–309 mg/dL, triglycerides <221 mg/dL |
Simvastatin vs placebo, 8.3% vs 10.3% |
19 |
| Randomized, active- controlled trials |
|||||
| β-Blocker or ACEI, with tight BP control | UKPDS (captopril or atenolol, goal BP <150/85 mm Hg) |
1148 | Type 2 diabetes mellitus, hypertension |
Captopril or atenolol (BP <150/85 mm Hg) vs other drugs (BP <180/105 mm Hg), 3.6% vs 8.1% |
56 |
| Retrospective studies |
|||||
| β-Blocker | SOLVD (subanalysis of prevention trial) |
2107 | Asymptomatic LV dysfunction, ejection fraction <35% |
Enalapril plus β- blocker vs enalapril plus no β-blocker, N/A |
36 |
| β-Blocker | SAVE (subanalysis) |
2231 | Ejection fraction <40%, no overt HF, post-MI patients |
β-Blocker vs no β- blocker, 16.5% vs 22.6% |
32 |
ACEI indicates ACE inhibitor; PVD, peripheral vascular disease; SAVE, Survival And Ventricular Enlargement Trial; TID, 3 times per day; LVEF, LV ejection fraction; ADP, adenosine diphosphate; ARB, angiotensin receptor blocker; ECG, electrocardiogram; RENAAL, Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan; IDNT, Irbesartan in Diabetic Nephropathy; N/A, not applicable; and BP, blood pressure.
Including whites, blacks, and Hispanics.
From Schocken DD, Benjamin EJ, Fonarow GC, et al. Prevention of Heart Failure: A Scientific Statement From the American Heart Association Councils on Epidemiology and Prevention, Clinical Cardiology, Cardiovascular Nursing, and High Blood Pressure Research; Quality of Care and Outcomes Research Interdisciplinary Working Group; and Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation 2008;117:2544-2565.
Caveat: Patients with Established Heart Failure
Although obesity and dysglycemia are clear risk factors for the development of HF, they are not risk factors for poor prognosis in patients with chronic, established HF. In fact, numerous studies have demonstrated a “reverse epidemiology” of obesity in HF; higher body mass index (BMI) has been associated with improved rather than impaired outcomes in chronic HF(28,67,68). Surprisingly, high compared to low HbA1c has also been associated with better outcomes in one cohort of patients with advanced systolic HF(69). Malnutrition, cachexia, and inflammation, all of which associated with severe HF and poor HF prognosis, may help explain these paradoxical associations in chronic HF (70). Evidence based, guideline recommended therapies for patients with established HF (Stage C and D) are shown in Figure 4.
Future Directions
Further research is needed to understand the mechanisms involved in progression from diabetes, obesity, and metabolic syndrome to incident HF and identify additional therapeutic targets for preventing HF. Prospective clinical trials to establish optimal glycemic control targets for reducing the risk of incident HF are needed as well as comparative effectiveness studies to determine which glycemic control medications result in the lowest risk of new onset HF. Whether novel targets of therapy, such as adipokines/resistin, inflammatory cytokines, or oxidative markers may prove useful in preventing progression to HF in high risk patients needs further investigation. There is an important need for research to better understand the contribution of genetic factors to metabolic risk factors in the risk of incident HF. There should also be pursuit of pharmacogenetic research to maximize the efficacy and minimize the toxicity of medications for HF prevention.
Importantly there is a need for further behavioral research on improving compliance and adherence to proven therapies for managing HF risk factors. It is also essential there be implementation of effective systems to ensure use of evidence-based, guideline-recommended therapies for prevention of HF in those with metabolic risk factors. Of utmost importance, is a greater awareness by both medical professionals and the public in the US of 1) the national and global impact of HF, 2) the importance of diabetes, obesity, and metabolic syndrome as modifiable risk factors for HF, and 3) that evidence-based strategies which can reduce the incidence of HF. Initial studies in Europe have shown a low level of awareness among the public as well as a low level of adherence to guideline recommended therapies by physicians; interventional educational programs are being undertaken (71-73).
Conclusions
The emergence of HF as a global public health problem with high prevalence, high morbidity, and extraordinary cost underscores the urgency of efforts to identify and modify risk factors for incident HF. The vast majority of incident HF is due to preventable risk factors. Focus on the growing, inter-related epidemics of diabetes, obesity, and metabolic syndrome is warranted, since it is established that lifestyle interventions can decrease the risk of these syndromes. Furthermore, appropriate medical treatment of hypertension, dyslipidemia, and hyperglycemia in those at risk of HF is an essential component of prevention. Further studies are needed to better understand the inter-relationship of these metabolic risk factors and incident HF and to develop more effective strategies to prevent HF in those at risk.
Acknowledgments
Funding/Support: Dr. Horwich is a recipient of NIH / NHLBI 1K23HL085097. Dr Fonarow holds the Eliot Corday Chair in Cardiovascular Medicine and Science and is also supported by the Ahmanson Foundation.
Abbreviations List
- BMI
body mass index
- HF
heart failure
- ACEI
angiotensin converting enzyme inhibitor
- ARB
angiotensin receptor blocker
- LVSD
left ventricular systolic dysfunction
- LVEF
left ventricular ejection fraction
- MESA
Multi-Ethnic Study of Atherosclerosis
- TZD
thiazolidinedione
- UKPDS
UK Prospective Diabetes Study
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The following relationships related to this manuscript exist: TBH (none), GCF (consulting and/or honorarium from GlaxoSmithKline, Pfizer, Novartis, BristolMyersSquibb, Sanofi-Aventis, and Merck)
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, et al. Heart Disease and Stroke Statistics--2009 Update. A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008 doi: 10.1161/CIRCULATIONAHA.108.191261. CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- 2.Braunwald E. Cardiovascular Medicine at the Turn of the Millennium: Triumphs, Concerns, and Opportunities. N Engl J Med. 1997;337:1360–1369. doi: 10.1056/NEJM199711063371906. [DOI] [PubMed] [Google Scholar]
- 3.Fang J, Mensah GA, Croft JB, Keenan NL. Heart Failure-Related Hospitalization in the U.S., 1979 to 2004. Journal of the American College of Cardiology. 2008;52:428. doi: 10.1016/j.jacc.2008.03.061. [DOI] [PubMed] [Google Scholar]
- 4.Schocken DD, Benjamin EJ, Fonarow GC, et al. Prevention of Heart Failure: A Scientific Statement From the American Heart Association Councils on Epidemiology and Prevention, Clinical Cardiology, Cardiovascular Nursing, and High Blood Pressure Research; Quality of Care and Outcomes Research Interdisciplinary Working Group; and Functional Genomics and Translational Biology Interdisciplinary Working Group. Circulation. 2008;117:2544–2565. doi: 10.1161/CIRCULATIONAHA.107.188965. [DOI] [PubMed] [Google Scholar]
- 5.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. Jama. 1996;275:1557–62. [PubMed] [Google Scholar]
- 6.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med. 2001;161:996–1002. doi: 10.1001/archinte.161.7.996. [DOI] [PubMed] [Google Scholar]
- 7.Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29–34. doi: 10.1016/0002-9149(74)90089-7. [DOI] [PubMed] [Google Scholar]
- 8.Bertoni AG, Hundley WG, Massing MW, Bonds DE, Burke GL, Goff DC., Jr. Heart Failure Prevalence, Incidence, and Mortality in the Elderly With Diabetes. Diabetes Care. 2004;27:699–703. doi: 10.2337/diacare.27.3.699. [DOI] [PubMed] [Google Scholar]
- 9.Nichols GA, Gullion CM, Koro CE, Ephross SA, Brown JB. The incidence of congestive heart failure in type 2 diabetes: an update. Diabetes Care. 2004;27:1879–84. doi: 10.2337/diacare.27.8.1879. [DOI] [PubMed] [Google Scholar]
- 10.Bibbins-Domingo K, Lin F, Vittinghoff E, et al. Predictors of Heart Failure Among Women With Coronary Disease. Circulation. 2004;110:1424–1430. doi: 10.1161/01.CIR.0000141726.01302.83. [DOI] [PubMed] [Google Scholar]
- 11.Iribarren C, Karter AJ, Go AS, et al. Glycemic control and heart failure among adult patients with diabetes. Circulation. 2001;103:2668–73. doi: 10.1161/01.cir.103.22.2668. [DOI] [PubMed] [Google Scholar]
- 12.Stratton IM, Adler AI, Neil HAW, et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pazin-Filho A, Kottgen A, Bertoni A, et al. HbA1c as a risk factor for heart failure in persons with diabetes: the Atherosclerosis Risk in Communities (ARIC) study. Diabetologia. doi: 10.1007/s00125-008-1164-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Held C, Gerstein HC, Yusuf S, et al. Glucose levels predict hospitalization for congestive heart failure in patients at high cardiovascular risk. Circulation. 2007;115:1371–5. doi: 10.1161/CIRCULATIONAHA.106.661405. [DOI] [PubMed] [Google Scholar]
- 15.Nichols GA, Arondekar B, Herman WH. Complications of dysglycemia and medical costs associated with nondiabetic hyperglycemia. Am J Manag Care. 2008;14:791–8. [PubMed] [Google Scholar]
- 16.Ingelsson E, Sundstrom J, Arnlov J, Zethelius B, Lind L. Insulin Resistance and Risk of Congestive Heart Failure. JAMA. 2005;294:334–341. doi: 10.1001/jama.294.3.334. [DOI] [PubMed] [Google Scholar]
- 17.Arnlov J, Lind L, Zethelius B, et al. Several factors associated with the insulin resistance syndrome are predictors of left ventricular systolic dysfunction in a male population after 20 years of follow-up. Am Heart J. 2001;142:720–4. doi: 10.1067/mhj.2001.116957. [DOI] [PubMed] [Google Scholar]
- 18.Ärnlöv J, Lind L, Sundström J, Andrén B, Vessby B, Lithell H. Insulin resistance, dietary fat intake and blood pressure predict left ventricular diastolic function 20 years later. Nutrition, Metabolism and Cardiovascular Diseases. 2005;15:242. doi: 10.1016/j.numecd.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 19.van Heerebeek L, Hamdani N, Handoko ML, et al. Diastolic Stiffness of the Failing Diabetic Heart: Importance of Fibrosis, Advanced Glycation End Products, and Myocyte Resting Tension. Circulation. 2008;117:43–51. doi: 10.1161/CIRCULATIONAHA.107.728550. [DOI] [PubMed] [Google Scholar]
- 20.Neubauer S. The Failing Heart -- An Engine Out of Fuel. N Engl J Med. 2007;356:1140–1151. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 21.Taegtmeyer H, McNulty P, Young ME. Adaptation and Maladaptation of the Heart in Diabetes: Part I: General Concepts. Circulation. 2002;105:1727–1733. doi: 10.1161/01.cir.0000012466.50373.e8. [DOI] [PubMed] [Google Scholar]
- 22.Witteles RM, Fowler MB. Insulin-Resistant Cardiomyopathy: Clinical Evidence, Mechanisms, and Treatment Options. Journal of the American College of Cardiology. 2008;51:93. doi: 10.1016/j.jacc.2007.10.021. [DOI] [PubMed] [Google Scholar]
- 23.Szczepaniak LS, Victor RG, Orci L, Unger RH. Forgotten but Not Gone: The Rediscovery of Fatty Heart, the Most Common Unrecognized Disease in America. Circ Res. 2007;101:759–767. doi: 10.1161/CIRCRESAHA.107.160457. [DOI] [PubMed] [Google Scholar]
- 24.Van De Borne P, Hausberg M, Hoffman RP, Mark AL, Anderson EA. Hyperinsulinemia produces cardiac vagal withdrawal and nonuniform sympathetic activation in normal subjects. Am J Physiol. 1999;276:R178–83. doi: 10.1152/ajpregu.1999.276.1.R178. [DOI] [PubMed] [Google Scholar]
- 25.Hoffman RP, Hausberg M, Sinkey CA, Anderson EA. Hyperglycemia without hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Diabetes Complications. 1999;13:17–22. doi: 10.1016/s1056-8727(98)00019-1. [DOI] [PubMed] [Google Scholar]
- 26.Biondi-Zoccai GGL, Abbate A, Liuzzo G, Biasucci LM. Atherothrombosis, inflammation, and diabetes. Journal of the American College of Cardiology. 2003;41:1071–1077. doi: 10.1016/s0735-1097(03)00088-3. [DOI] [PubMed] [Google Scholar]
- 27.Alexander JK. Obesity and the heart. Heart Dis Stroke. 1993;2:317–21. [PubMed] [Google Scholar]
- 28.Kenchaiah S, Evans JC, Levy D, et al. Obesity and the Risk of Heart Failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 29.Kenchaiah S, Sesso HD, Gaziano JM. Body Mass Index and Vigorous Physical Activity and the Risk of Heart Failure Among Men. Circulation. 2009;119:44–52. doi: 10.1161/CIRCULATIONAHA.108.807289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loehr LR, Rosamond WD, Poole C, et al. Association of Multiple Anthropometrics of Overweight and Obesity With Incident Heart Failure: The Atherosclerosis Risk in Communities Study. Circ Heart Fail. 2009;2:18–24. doi: 10.1161/CIRCHEARTFAILURE.108.813782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morricone L, Malavazos AE, Coman C, Donati C, Hassan T, Caviezel F. Echocardiographic Abnormalities in Normotensive Obese Patients: Relationship with Visceral Fat. Obesity. 2002;10:489. doi: 10.1038/oby.2002.67. [DOI] [PubMed] [Google Scholar]
- 32.Bahrami H, Bluemke DA, Kronmal R, et al. Novel Metabolic Risk Factors for Incident Heart Failure and Their Relationship With Obesity: The MESA (Multi-Ethnic Study of Atherosclerosis) Study. Journal of the American College of Cardiology. 2008;51:1775. doi: 10.1016/j.jacc.2007.12.048. [DOI] [PubMed] [Google Scholar]
- 33.Vasan RS, Sullivan LM, Roubenoff R, et al. Inflammatory Markers and Risk of Heart Failure in Elderly Subjects Without Prior Myocardial Infarction: The Framingham Heart Study. Circulation. 2003;107:1486–1491. doi: 10.1161/01.cir.0000057810.48709.f6. [DOI] [PubMed] [Google Scholar]
- 34.Frankel DS, Vasan RS, D'Agostino RB, Sr, et al. Resistin, Adiponectin, and Risk of Heart Failure: The Framingham Offspring Study. Journal of the American College of Cardiology. 2009;53:754. doi: 10.1016/j.jacc.2008.07.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lieb W, Sullivan LM, Harris TB, et al. Plasma leptin levels and incidence of heart failure, cardiovascular disease and total mortality in the elderly. Diabetes Care. 2008 doi: 10.2337/dc08-1596. dc08-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Butler J, Rodondi N, Zhu Y, et al. Metabolic Syndrome and the Risk of Cardiovascular Disease in Older Adults. Journal of the American College of Cardiology. 2006;47:1595–1602. doi: 10.1016/j.jacc.2005.12.046. [DOI] [PubMed] [Google Scholar]
- 37.Ingelsson E, Arnlov J, Lind L, Sundstrom J. Metabolic syndrome and risk for heart failure in middle-aged men. Heart. 2006;92:1409–1413. doi: 10.1136/hrt.2006.089011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li C, Ford ES, McGuire LC, Mokdad AH. Association of metabolic syndrome and insulin resistance with congestive heart failure: findings from the Third National Health and Nutrition Examination Survey. J Epidemiol Community Health. 2007;61:67–73. doi: 10.1136/jech.2006.048173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Folsom AR, Yamagishi K, Hozawa A, Chambless LE. Atherosclerosis Risk in Communities Study I: Absolute and Attributable Risks of Heart Failure Incidence in Relation to Optimal Risk Factors. Circ Heart Fail. 2009;2:11–17. doi: 10.1161/CIRCHEARTFAILURE.108.794933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Skyler JS, Bergenstal R, Bonow RO, et al. Intensive Glycemic Control and the Prevention of Cardiovascular Events: Implications of the ACCORD, ADVANCE, and VA Diabetes Trials: A Position Statement of the American Diabetes Association and a Scientific Statement of the American College of Cardiology Foundation and the American Heart Association. Journal of the American College of Cardiology. 2009;53:298. doi: 10.1016/j.jacc.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 41.Duckworth W, Abraira C, Moritz T, et al. Glucose Control and Vascular Complications in Veterans with Type 2 Diabetes. N Engl J Med. 2009;360:129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 42.The ACG Intensive Blood Glucose Control and Vascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 43.The Action to Control Cardiovascular Risk in Diabetes Study G Effects of Intensive Glucose Lowering in Type 2 Diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ray KK, Seshasai SRK, Wijesuriya S, et al. Effect of intensive control of glucose on cardiovascular outcomes and death in patients with diabetes mellitus: a meta-analysis of randomised controlled trials. The Lancet. 373:1765–1772. doi: 10.1016/S0140-6736(09)60697-8. [DOI] [PubMed] [Google Scholar]
- 45.Montori VM, Fernandez-Balsells M. Glycemic Control in Type 2 Diabetes: Time for an Evidence-Based About-Face? Ann Intern Med. 2009;150:803–808. doi: 10.7326/0003-4819-150-11-200906020-00008. [DOI] [PubMed] [Google Scholar]
- 46.Del Prato S. Megatrials in type 2 diabetes. From excitement to frustration? Diabetologia. 2009;52:1219–26. doi: 10.1007/s00125-009-1352-5. [DOI] [PubMed] [Google Scholar]
- 47.McAlister FA, Eurich DT, Majumdar SR, Johnson JA. The risk of heart failure in patients with type 2 diabetes treated with oral agent monotherapy. European Journal of Heart Failure. 2008;10:703. doi: 10.1016/j.ejheart.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 48.Nichols GA, Koro CE, Gullion CM, Ephross SA, Brown JB. The incidence of congestive heart failure associated with antidiabetic therapies. Diabetes Metab Res Rev. 2004 doi: 10.1002/dmrr.480. [DOI] [PubMed] [Google Scholar]
- 49.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-Year Follow-up of Intensive Glucose Control in Type 2 Diabetes. N Engl J Med. 2008;359:1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 50.Delea TE, Edelsberg JS, Hagiwara M, Oster G, Phillips LS. Use of Thiazolidinediones and Risk of Heart Failure in People With Type 2 Diabetes: A retrospective cohort study. Diabetes Care. 2003;26:2983–2989. doi: 10.2337/diacare.26.11.2983. [DOI] [PubMed] [Google Scholar]
- 51.Dormandy JA, Charbonnel B, Eckland DJA, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. The Lancet. 366:1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 52.Erdmann E, Charbonnel B, Wilcox RG, et al. Pioglitazone Use and Heart Failure in Patients With Type 2 Diabetes and Preexisting Cardiovascular Disease: Data from the PROactive Study (PROactive 08) Diabetes Care. 2007;30:2773–2778. doi: 10.2337/dc07-0717. [DOI] [PubMed] [Google Scholar]
- 53.Dargie HJ, Hildebrandt PR, Riegger GAJ, et al. A Randomized, Placebo-Controlled Trial Assessing the Effects of Rosiglitazone on Echocardiographic Function and Cardiac Status in Type 2 Diabetic Patients With New York Heart Association Functional Class I or II Heart Failure. Journal of the American College of Cardiology. 2007;49:1696. doi: 10.1016/j.jacc.2006.10.077. [DOI] [PubMed] [Google Scholar]
- 54.Diabetes Prevention Program Research G Reduction in the Incidence of Type 2 Diabetes with Lifestyle Intervention or Metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.The DTI Effect of Ramipril on the Incidence of Diabetes. N Engl J Med. 2006;355:1551–1562. doi: 10.1056/NEJMoa065061. [DOI] [PubMed] [Google Scholar]
- 56.Effect of rosiglitazone on the frequency of diabetes in patients with impaired glucose tolerance or impaired fasting glucose: a randomised controlled trial. The Lancet. 368:1096–1105. doi: 10.1016/S0140-6736(06)69420-8. [DOI] [PubMed] [Google Scholar]
- 57.The DTI Effects of Ramipril and Rosiglitazone on Cardiovascular and Renal Outcomes in People With Impaired Glucose Tolerance or Impaired Fasting Glucose: Results of the Diabetes REduction Assessment with ramipril and rosiglitazone Medication (DREAM) trial. Diabetes Care. 2008;31:1007–1014. doi: 10.2337/dc07-1868. [DOI] [PubMed] [Google Scholar]
- 58.Shai I, Schwarzfuchs D, Henkin Y, et al. Weight Loss with a Low-Carbohydrate, Mediterranean, or Low-Fat Diet. N Engl J Med. 2008;359:229–241. doi: 10.1056/NEJMoa0708681. [DOI] [PubMed] [Google Scholar]
- 59.Sacks FM, Bray GA, Carey VJ, et al. Comparison of Weight-Loss Diets with Different Compositions of Fat, Protein, and Carbohydrates. N Engl J Med. 2009;360:859–873. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.American College of Cardiology Foundation/American Heart Association Task Force on Practice G, International Society for Heart. Lung T, Hunt SA, et al. 2009 Focused Update Incorporated Into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults. J Am Coll Cardiol. 2009 doi: 10.1016/j.jacc.2008.11.013. j.jacc.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 61.Arnold JMO, Yusuf S, Young J, et al. Prevention of Heart Failure in Patients in the Heart Outcomes Prevention Evaluation (HOPE) Study. Circulation. 2003;107:1284–1290. doi: 10.1161/01.cir.0000054165.93055.42. [DOI] [PubMed] [Google Scholar]
- 62.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of Losartan on Renal and Cardiovascular Outcomes in Patients with Type 2 Diabetes and Nephropathy. N Engl J Med. 2001;345:861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 63.Group UKPDS Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- 64.Group UKPDS Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. BMJ. 1998;317:713–720. [PMC free article] [PubMed] [Google Scholar]
- 65.Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. The Lancet. 2000;355:253. [PubMed] [Google Scholar]
- 66.Kjekshus J, Pedersen TR, Olsson AG, Faergeman O, Pyorala K. The effects of simvastatin on the incidence of heart failure in patients with coronary heart disease. J Card Fail. 1997;3:249–54. doi: 10.1016/s1071-9164(97)90022-1. [DOI] [PubMed] [Google Scholar]
- 67.Horwich TB, Fonarow GC, Hamilton MA, MacLellan WR, Woo MA, Tillisch JH. The relationship between obesity and mortality in patients with heart failure. J Am Coll Cardiol. 2001;38:789–95. doi: 10.1016/s0735-1097(01)01448-6. [DOI] [PubMed] [Google Scholar]
- 68.Kalantar-Zadeh K, Block G, Horwich T, Fonarow GC. Reverse epidemiology of conventional cardiovascular risk factors in patients with chronic heart failure. J Am Coll Cardiol. 2004;43:1439–44. doi: 10.1016/j.jacc.2003.11.039. [DOI] [PubMed] [Google Scholar]
- 69.Eshaghian S, Horwich TB, Fonarow GC. An unexpected inverse relationship between HbA1c levels and mortality in patients with diabetes and advanced systolic heart failure. Am Heart J. 2006;151:91. doi: 10.1016/j.ahj.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 70.Tamara B, Horwich GCF. Reverse Epidemiology beyond Dialysis Patients: Chronic Heart Failure, Geriatrics, Rheumatoid Arthritis, COPD, and AIDS. Seminars in Dialysis. 2007;20:549–553. doi: 10.1111/j.1525-139X.2007.00346.x. [DOI] [PubMed] [Google Scholar]
- 71.Remme W, Boccanelli A, Cline C, et al. Increasing awareness and perception of heart failure in Europe and improving care--rationale and design of the SHAPE Study. Cardiovasc Drugs Ther. 2004;18:153–9. doi: 10.1023/B:CARD.0000029033.83282.d0. [DOI] [PubMed] [Google Scholar]
- 72.Remme WJ, McMurray JJ, Hobbs FD, et al. Awareness and perception of heart failure among European cardiologists, internists, geriatricians, and primary care physicians. Eur Heart J. 2008;29:1739–52. doi: 10.1093/eurheartj/ehn196. [DOI] [PubMed] [Google Scholar]
- 73.Remme WJ, McMurray JJ, Rauch B, et al. Public awareness of heart failure in Europe: first results from SHAPE. Eur Heart J. 2005;26:2413–21. doi: 10.1093/eurheartj/ehi447. [DOI] [PubMed] [Google Scholar]