Abstract
Rationale
L-type (Cav1.2) Ca2+ channels are critical regulators of muscle and neural function. Although Cav1.2 channel activity varies regionally, little is known about the mechanisms underlying this heterogeneity.
Objective
To test the hypothesis that Cav1.2 channels can gate coordinately.
Methods and Results
We used optical and electrophysiological approaches to record Cav1.2 channel activity in cardiac, smooth muscle, and tsA-201 cells expressing Cav1.2 channels. Consistent with our hypothesis, we found that small clusters of Cav1.2 channels can open and close in tandem. FRET and electrophysiological studies suggest that this coupling of Cav1.2 channels involves transient interactions between neighboring channels via their C-termini. The frequency of coupled gating events increases in hypertensive smooth muscle and in cells expressing a mutant Cav1.2 channel that causes arrhythmias and autism in humans with Timothy syndrome (LQT8).
Conclusions
Coupled gating of Cav1.2 channels may represent a novel mechanism for the regulation of Ca2+ influx and excitability in neurons, cardiac, and arterial smooth muscle under physiological and pathological conditions.
Keywords: Cav1.2 channels, PKC, calmodulin, arrhythmias
Introduction
Voltage-gated L-type (Cav1.2) Ca2+ channels are expressed in the surface membrane of neurons and muscle cells, where they regulate multiple processes including excitability, contraction, gene expression, and memory storage1–4. Recent studies have revealed an unexpected feature of Cav1.2 channels: their activity (i.e. open probability, Po) varies within the cell membrane5–7. Low activity Cav1.2 channels open randomly and infrequently at rest, producing brief elevations in intracellular Ca2+ concentration ([Ca2+]i) called “Ca2+ sparklets”8. In contrast, small clusters of Cav1.2 channels can function in a high open probability mode that generates localized zones of relatively high Ca2+ influx (“persistent Ca2+ sparklets”).
Although targeting of PKCα to the surface membrane by the kinase anchoring protein AKAP150 (the rodent ortholog of human AKAP79) increases the probability of persistent Cav1.2 channel activity9, the mechanisms by which small clusters of these channels open concertedly are unknown. Here, we employed FRET approaches in combination with optical and electrophysiological recordings of Ca2+ influx via Cav1.2 channels to investigate this important issue. Our data indicate that activators of PKCα, calmodulin antagonists, or a specific Cav1.2 channel mutation that causes arrhythmias and autism in humans with the Timothy syndrome move the ubiquitous Ca2+-binding protein calmodulin from the IQ domain in the C-terminal tail of the channel. This induces functional interactions between nearby Cav1.2 channel via their C-termini that could lead to coupled gating of these channels.
Methods
An expanded Methods section is available in the online Supplement Material at http://circres.ahajournals.org.
Isolation of arterial and adult and neonatal cardiac myocytes
Mice and rats were euthanized as approved by the University of Washington Institutional Animal Care and Use Committee. Adult arterial myocytes and neonatal cardiac myocytes were prepared as described previously5, 10.
Cav1.2 and calmodulin constructs and inducible PKCα translocation system and their expression in tsA-201 cells
We transfected tsA-201 cells using JetPEI (Polyplus Transfection) with plasmids encoding calcium channel pore-forming and accessory subunits as well as calmodulin. We generated the rabbit homolog of the human Timothy syndrome Cav1.2 (G436R, Rabbit; G406R Human)11. Wild type calmodulin and the Cav1.2 mutant with a stop codon at amino acid 1670 (Cav1.2Δ1670X) were kindly provided by Dr. David T. Yue. We generated a rapamycin-induced PKCα translocation system similar to the one developed by Liberles et al12 and Suh et al13. Reagents for this system were obtained from Agilent Technologies and Dr. John Sexton (PKCα).
Coupled Markov chain model
We implemented a coupled Markov chain model in Matlab® to determine the coupling coefficient (κ) among Cav1.2 channels underlying membrane currents and Ca2+ sparklet sites. The model was originally developed by Chung and Kennedy14 to analyze individual records of partially coupled GABA-activated chloride channels.
Electrophysiology, confocal and total internal reflection fluorescence microscopy
Voltage-clamp experiments were performed using standard patch-clamp techniques. Total internal reflection fluorescence (TIRF) images were acquired using a through-the-lens TIRF microscope. Images were acquired at 100–200 Hz. Ca2+ sparklets were detected and defined for analysis using an automated algorithm5. Some experiments involved a Nikon swept field confocal system.
Statistics
Data are presented as mean ± SEM. A p value of less than 0.05 was considered significant. The asterisk (*) symbol is used in the figures to illustrate a significant difference between groups.
Results
Optical recordings of coupled L-type Ca2+ channels
A TIRF microscope was used to image Ca2+ sparklets in arterial smooth muscle cells, neonatal ventricular myocytes, and tsA-201 cells expressing wild type Cav1.2 channels. All Ca2+ sparklet experiments were performed in voltage clamped cells using the whole-cell configuration of the patch-clamp technique. Ca2+ sparklets were recorded in cells treated with the SERCA pump inhibitor thapsigagin (1 µmol/L) to eliminate Ca2+ release from intracellular Ca2+ stores. To image Ca2+ sparklets, cells were dialyzed with a patch pipette solution containing the fluorescent Ca2+ indicator fluo-5F and EGTA. The inclusion of the relatively slow Ca2+ buffer EGTA (on rate ≈100-fold slower than fluo-5F) in the intracellular solution serves an important purpose: it restricts fluo-5F fluorescence to the site of Ca2+ entry (≈ 1 µm). This occurs because with EGTA in the cytosol, Ca2+ entering the cell initially interacts with the faster fluo-5F, producing a fluorescence signal, but then quickly (≈ 2 ms) binds to the more abundant and non-fluorescent EGTA. Thus, in our TIRF experiments, [Ca2+]i signals are limited to the sub-membrane space near the mouth of surface membrane Ca2+ channels.
Figure 1A shows representative Ca2+ sparklets from arterial smooth muscle cells, neonatal ventricular myocytes, and tsA-201 cells expressing Cav1.2 channels. These Ca2+ sparklets were recorded while cells were held at −70 mV to increase the driving force for Ca2+ influx and maintain a low L-type Ca2+ channel activity, which increased contrast and hence our ability to detect discrete Ca2+ entry sites. A Δ[Ca2+]i amplitude histogram of these records revealed two discrete peaks corresponding to closed channels (Δ[Ca2+]i = 0 nmol/L) and openings of ≈38 nmol/L, which as demonstrated previously, represents the amplitude of quantal Ca2+ sparklet events in tsA-201 cells expressing Cav1.2 and arterial myocytes5, 6, 15. A previous study6 suggested that quantal Ca2+ sparklets are likely produced by the opening of a single Ca2+ channel.
Figure 1. Optical recordings of coupled gating of Cav1.2 channels.
(A) Representative records of Ca2+ influx via Cav1.2 channels in neonatal cardiac myocytes (top), tsA-201 cells (middle), and arterial myocytes (bottom). (B) All-points histogram of Ca2+ sparklet traces in panel A. The solid black line is the fit (χ2 = 2.1) to the data with a multi-component Gaussian function with centers at 0 and 38 nmol/L. (C) Δ[Ca2+]i from a Ca2+ sparklet site in an arterial myocyte with coupled Cav1.2 channels. (D) All-points histogram of the Ca2+ sparklet trace in panel C. The solid black line is the best-fit (χ2 = 2.5) to the data with a three-component Gaussian function with centers at 0, 120, and 160 nmol/L. (E) Histogram of the coupling coefficients (κ) of multiple Ca2+ sparklet records from cardiac, arterial, and tsA-201 cells. (F) Ca2+ sparklet records with varied κ values.
It is important to note that, consistent with previous work5, 6, Ca2+ sparklet activity in smooth muscle cells, neonatal ventricular myocytes, and tsA-201 cells transfected with Cav1.2 was rapidly eliminated by the application of the L-type Ca2+ channel inhibitor nifedipine (10 µmol/L) or by superfusion of a Ca2+-free external solution (data not shown). Importantly, and as reported recently5, Ca2+ sparklets were never observed in untransfected tsA-201 cells. Furthermore, Ca2+ sparklets in arterial and neonatal ventricular myocytes have the same amplitude of quantal event, activity, and pharmacology (e.g. insensitive to 1 µmol/L thapsigargin, eliminated by 10 µmol/L nifedipine) as Ca2+ sparklets in tsA-201 cells expressing Cav1.2 channels5, 15. Collectively these observations strongly suggest that Ca2+ sparklets in arterial smooth muscle cells, neonatal ventricular myocytes, and tsA-201 cells expressing Cav1.2 channels are produced by Ca2+ influx via L-type Ca2+ channels.
Although most Ca2+ sparklets recorded in these cells were similar to those in Figure 1A, we noticed that in a number of Ca2+ sparklet sites (191 of 491 sites) small clusters of Cav1.2 channels opened and closed simultaneously to promote greater elevation in subcellular [Ca2+]i (Figure 1C). The Δ[Ca2+]i histogram of the trace in Figure 1D had three distinct peaks at a Δ[Ca2+]i of 0, 120, and 160 nmol/L. The peaks at 120 and 160 nmol/L likely resulted from the simultaneous activation of 3 and 4 Cav1.2 channels, respectively. Note the absence of a peak near the quantal amplitude of Ca2+ influx (i.e. 38 nmol/L) in this record. Examples of records with sequential quantal and multi-channel openings are shown in Figure 4A and Figure 5A below.
Figure 4. Displacing CaM from the IQ domain of Cav1.2 increases coupled gating of WT and TS Cav1.2 channels.
(A) Ca2+ sparklet records from tsA-201 and arterial myocytes showing induction of coupled gating by W7 (100 µmol/L). κ values are shown above each trace. (B) Number of coupled sites per cell before and after W7. (C) Cartoon of Cav1.2-EGFP and CaM-tRFP. Right, images of Cav1.2-EGFP in tsA-201 cells under control conditions, during W7 treatment, and following CaM-tRFP photobleaching. (D) Cav1.2-EGFP fluorescence intensity at an example cross section of the membrane marked by the white line in each of the images in panel C. Dotted lines show the FDA and FA values used to calculate effective FRET=1-(FDA/FA). (E) FRET in specific sites before and after W7 (center, lower row) or PDBu (right, lower row) application. Yellow bars are mean values. (F) Ca2+ sparklet records with coupled Cav1.2 channels from tsA-201 cells expressing Cav1.2-TS and hypertensive smooth muscle. (G) Representative Cav1.2 channel currents evoked by depolarization from −80 to −30 mV in patches (cell-attached) from tsA-201 cells expressing WT (upper trace) and Cav1.2-TS channels (lower trace). The close state is denoted by a “c” and the open state by an “o”. (H) Number of sites with coupled Cav1.2 channels per cell in cells expressing WT and TS Cav1.2 and in normotensive (NT) and hypertensive (HTN) smooth muscle. (I) FRET data between EGFP-tagged WT or TS Cav1.2 and CaM-tRFP.
Figure 5. AKAP150, but not PKCα, is required for coupled gating of Cav1.2 channels.
(A) Representative Δ[Ca2+]i records from Ca2+ sparklet sites in AKAP150−/− and PKCα smooth muscle cells under control conditions (right) and after exposure to 100 µmol/L W7. The coupling coefficient (κ) of these Cav1.2 channels is shown above each trace. Dotted lines show the amplitude of quantal levels. (B) Bar plot of the mean number of coupled sites per cell.
The observation of Ca2+ sparklets resulting from the simultaneous openings of small clusters of Cav1.2 channels is surprising because the Po of a single Cav1.2 channel is only ≈10−6 at −70 mV16. The probability of independently gating channels opening simultaneously at this potential should be very low: ≈10−12, 10−18, and 10−24 for the random, coincident opening of 2, 3, or 4 channels. Thus, the probability of observing simultaneous openings of Ca2+ channels should be progressively lower as the number of participating channels increase, which should result in a histogram with peaks of decreasing amplitudes as multichannel events become rarer. On the basis of that analysis, Ca2+ sparklet events like the one in Figure 1C should be very rare. Yet, Ca2+ sparklets resulting from the coincident activation of 2 or more Cav1.2 channels were frequently observed. This suggests that this is not simply the consequence of overlapping stochastic openings of independently activated Cav1.2 channels. Rather, our data provide evidence for the coordinated activation of a small cluster of these channels.
To test this hypothesis, we implemented a coupled Markov chain model to determine the coupling coefficient (κ) among Cav1.2 channels within Ca2+ sparklet sites (Figures 1E and 1F). The model was originally developed by Chung and Kennedy14 and used to analyze individual records of partially coupled GABA-activated chloride channels. Briefly, this model is a simplified Markov chain model with only 3 free parameters that takes the form of a set of binary chains that are interdependent according to a lumped coupling coefficient parameter called κ. The other two parameters (called ρ and ς) describe the intrinsic characteristics of the binary chains. The κ value could range from 0 for independently gating channels to 1 for “tightly” coupled channels that open and close simultaneously all the time. A detailed description of this model is provided in the Methods section of the online Supplemental Material.
Using this analysis, we found that although the majority of Ca2+ sparklets (59%) were likely the result of Ca2+ influx via independently gating Cav1.2 channels (i.e. κ = 0), numerous Ca2+ sparklets were produced by Cav1.2 channels with a κ > 0. Indeed, the coupling coefficient of non-independent events (i.e. κ > 0) ranged from 0.1 to 1 with a median value of 0.2. Together with the Ca2+ sparklet data above, this Markov analysis suggested that a subpopulation of Cav1.2 channels was capable of gating coordinately.
Electrophysiological recordings of coupled L-type Ca2+ channels
We used electrophysiological techniques to record L-type Ca2+ channel currents (Figure 2). In these experiments, we recorded elementary L-type Ca2+ channel currents from cell-attached patches from neonatal ventricular myocytes. L-type Ca2+ channel currents were evoked by a 2 s step depolarization to −30 mV from the holding potential of −80 mV. The patch pipette solution contained 20 mmol/L Ca2+ (charge carrier), 10 µmol/L tetrodotoxin (TTX), 130 mmol/L TEA+ and no Na+ or K+ to eliminate Na+ and K+ channel currents. The L-type Ca2+ channel opener BayK-8644 (500 nmol/L) was included in the pipette solution to increase the mean open time and Po of these channels and the probability of PKCα-dependent, persistent L-type Ca2+ channel activity5.
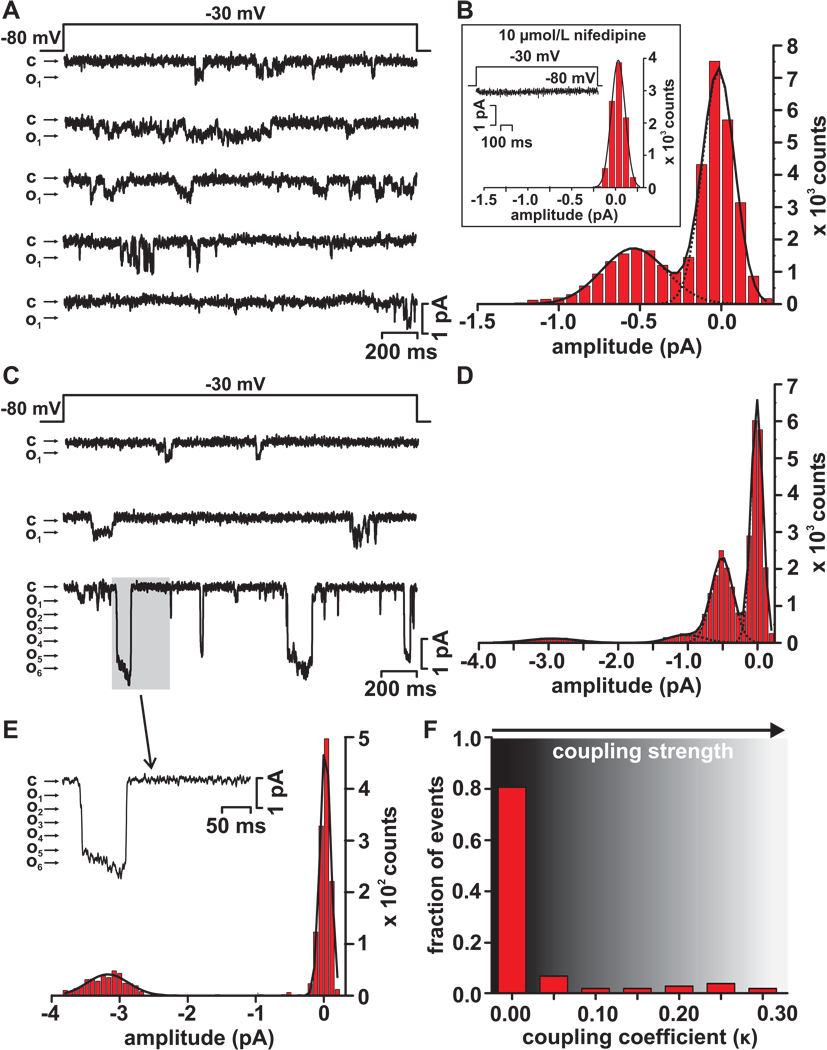
Figure 2. Electrical recordings of coupled L-type Ca2+ channels.
(A) Representative elementary L-type Ca2+ channel records obtained during a step depolarization (2 s) from −80 mV to −30 mV. Openings are shown as downward deflections, with the close state denoted by “c”, and the open state by “o”. (B) Histogram of L-type Ca2+ currents traces in panel A. The solid black line is the fit to the data with a multi-component Gaussian function with centers at 0.0 and 0.5 pA. The inset shows a histogram from a representative record obtained in the presence of 10 µmol/L nifedipine. (C) Representative single and coupled L-type Ca2+ channel current records obtained during step depolarizations from −80 mV to −30 mV. (D) Histogram of the L-type Ca2+ currents records in panel C. The solid black line is the fit to the data with a multi-component Gaussian function with centers at 0.0, −0.5, −1.0, and −2.9 pA. (E) Trace shows a portion (gray box) of the record in panel C showing a L-type Ca2+ current resulting from the simultaneous opening and closing of multiple channels. A histogram from this portion of the trace is shown below. The histogram was fitted with a Gaussian function with centers at 0.0 and −3.1 pA. (F) Histogram of the coupling coefficients (κ) of multiple L-type Ca2+ current records from cell-attached patches from neonatal ventricular myocytes.
As expected from our Ca2+ sparklet studies, depolarization to −30 mV evoked relatively low levels of Ca2+ channel activity in most (112 out of 134 sweeps, or 84%; from 5 cells) of the sweeps examined (Figure 2A). A current amplitude histogram from these records show two distinct peaks with centers at 0 mV (i.e. closed channel) and 0.53 pA, which corresponds to the expected single channel current level for an L-type Ca2+ channel at −30 mV with 20 mmol/L Ca2+16. The κ value of the Cav1.2 channels in these patches was 0, suggesting that these currents were produced by independently gating channels. At this point it is important to note that in control experiments inclusion of 10 µmol/L nifedipine prevented all Ca2+ channel activity, indicating that L-type Ca2+ channels produced these currents (inset in Figure 2B).
Consistent with our Ca2+ sparklet data, we found that in a minority of the records (22 out 134 sweeps or 16%; from 5 cells) membrane depolarization to −30 mV evoked elementary as well as relatively large currents that were likely produced by the simultaneous opening and closing of multiple L-type Ca2+ channels (Figure 2C). The amplitude histogram of the current records in Figure 2C had four prominent peaks at 0.0, 0.5, 1.0, and 2.9 pA, likely resulting from the activation of a single or simultaneous activation of two or six L-type Ca2+ channels, respectively. Closer examination (Figure 2E) of one of the large current events highlighted by the gray box in the bottom sweep in Figure 2D, indicates that in this patch 5–6 channel likely opened and then closed simultaneously multiple times. Three additional multichannel current events of similar amplitude were observed in this sweep. As noted above, the prominence of the peak with a center at 2.9 pA in the histogram in Figure 2C and absence of peaks of larger amplitude between this peak and the peak at 1 pA suggests coupled gating between L-type Ca2+ channels. Consistent with this, the coupling coefficient of the L-type Ca2+ channels in this section was 0.22, suggesting this current was produced by partially coupled channels. Indeed, analysis of all L-type Ca2+ channel records indicates that the vast majority of the currents were likely the result of independent openings of L-type Ca2+ channels (i.e. κ = 0; Figure 2F). In combination with the Ca2+ sparklet data above, these findings strongly support to the hypothesis that small clusters of Cav1.2 channels can open and close coordinately.
Activation of PKCα increases coupled gating of Cav1.2 channels
Activation of PKCα has been linked to elevated persistent Ca2+ sparklet activity in arterial myocytes5, 6, 17. Thus, we investigated whether membrane translocation and activation of this kinase increases coupled gating of Cav1.2 channels. To do this, we used two complementary approaches. First, we generated a chemically-induced PKCα translocation system12, 13. Briefly, we created an adenoviral vector expressing a FKBP12-rapamycin-binding element (FRB) that is anchored to the plasma membrane via the myristoylation and palmitoylation modification sequence Lyn11. This adenoviral vector also expressed PKCα fused to the enhanced green fluorescent protein (EGFP) and a FK506 binding protein. Application of rapamycin promoted heterodimerization of protein domains from FKBP-PKCα-EGFP and the membrane anchored FRB. This recruited FKBP-PKCα-EGFP to the membrane from the cytosol upon addition of rapamycin (Figure 3A). We used this inducible PKCα in neonatal ventricular myocytes and tsA-201 cells expressing Cav1.2. We found that induction of membrane translocation of PKCα (Figure 3A) increased Ca2+ sparklet activity and coupled gating of Cav1.2 channels in cardiac myocytes (n = 5 cells, Figure 3B) and tsA-201 cells (n = 6 cells, Online Figure I).
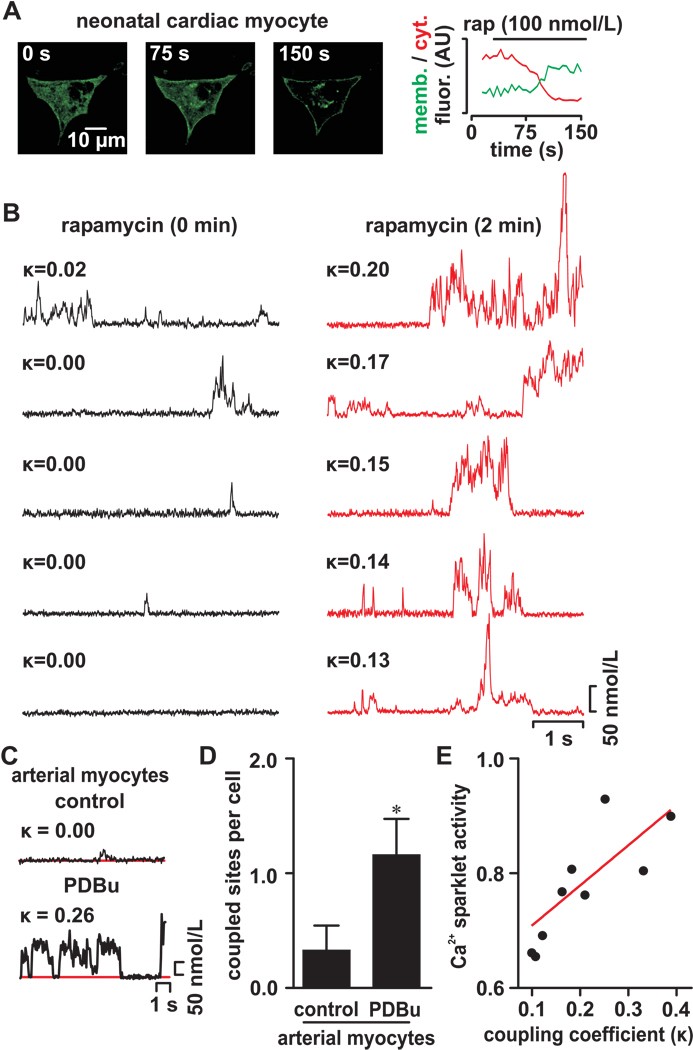
Figure 3. Activation of PKCα increases coupled gating between Cav1.2 channels.
(A) Confocal images of neonatal ventricular cells expressing the inducible PKCα translocation system before (0 s) and after (75 and 150 s) application of 0.1 µmol/L rapamycin to induce membrane translocation of this kinase. The graph on the right side shows the time course of PKCα fluorescence intensity in the membrane and cytosol in this cardiac myocyte. Δ[Ca2+]i records from multiple Ca2+ sparklet sites before (left) and after (right) induction of PKCα-EGFP translocation are shown below the confocal images. (B) Δ[Ca2+]i records from a Ca2+ sparklet site before and after application of PDBu. The κ of the Cav1.2 channels in this site is shown above each record. (C) Ca2+ sparklet records from an arterial myocyte before and after PDBu (500 nmol/L). (D) Bar plot of the mean number of coupled sites per cell in arterial myocytes under control condition and after application of 500 nmol/L PDBu. (E) Relationship between Ca2+ sparklet activity and coupling coefficient. Red line is a linear fit to the data (r2 = 0.64).
Our second approach involved the activation of endogenous PKC using the phorbol 12,13-dibutyrate (PDBu; 500 nmol/L). Activation of PKC with PDBu had a similar effect in arterial myocytes (n = 17 cells, Figure 3C and 3D). Taken together, these data suggest that membrane translocation and activation of PKCα increases the probability of coupled gating between Cav1.2 channels.
Movement of calmodulin away from the IQ domain in the C-tail of Cav1.2 channels increases coupled gating of these channels
We observed that the activity of Ca2+ sparklets produced by coupled Cav1.2 channels was higher than those produced by independently gating channels (Figure 3E). This raised the intriguing possibility that the probability of coupled gating of Cav1.2 channels might be enhanced by deficient Ca2+-dependent inactivation (CDI) of these channels. Calmodulin (CaM) mediates CDI of Cav1.2 channels18, 19. Thus, we tested the hypothesis that inhibition of CaM increases the probability of coupled gating of Cav1.2 channels.
Consistent with this hypothesis, we found that application of the CaM antagonist W7 (100 µmol/L) increased the number of sites with coupled Cav1.2 channels ≈7-fold and 27-fold in tsA-201 cells (n =4 cells) and myocytes (n = 7 cells), respectively (p < 0.05; Figures 4A and 4B). Similar findings were obtained in myocytes injected with the CaM-inhibitory peptide (1 µmol/L; n = 4 cells; Supplement materials and Online Figure II). Application of either CaM antagonist increased the number of Ca2+ sparklet sites with coupled Cav1.2 channels, but not the median κ value (0.2) of coupled channels (p > 0.05).
To examine the dynamics of this phenomenon, we used fluorescence resonance energy transfer (FRET) to visualize the interaction between Cav1.2 channels and CaM. The C-terminal of Cav1.2 was tagged with the enhanced green fluorescent protein (Cav1.2- ) and CaM was fused with tag-RFP® (CaM-
) and CaM was fused with tag-RFP® (CaM- ) (Figures 4C and 4D). CaM associates with the IQ domain in the C-terminal of Cav1.2, which is critical for CDI of these channels18, 20. Accordingly, FRET was relatively high (0.32 ± 0.02, n = 10 cells) between Cav1.2-
) (Figures 4C and 4D). CaM associates with the IQ domain in the C-terminal of Cav1.2, which is critical for CDI of these channels18, 20. Accordingly, FRET was relatively high (0.32 ± 0.02, n = 10 cells) between Cav1.2- and CaM-
and CaM- under control conditions. Application of the CaM antagonist W7 (100 µmol/L) or the PKC activator PDBu (500 nmol/L) decreased FRET between Cav1.2-
under control conditions. Application of the CaM antagonist W7 (100 µmol/L) or the PKC activator PDBu (500 nmol/L) decreased FRET between Cav1.2- and CaM-
and CaM- from 0.21 ± 0.04 to 0.08 ± 0.03 (n = 10 cells) and from 0.33 ± 0.02 to 0.12 ± 0.10 (n = 11 cells), respectively (Figure 4E) (p < 0.05). Note, however, that the magnitude of the decrease in FRET induced by W7 and PKCα activation varied within cells. The relative change in FRET ranged from 0 in some sites (
from 0.21 ± 0.04 to 0.08 ± 0.03 (n = 10 cells) and from 0.33 ± 0.02 to 0.12 ± 0.10 (n = 11 cells), respectively (Figure 4E) (p < 0.05). Note, however, that the magnitude of the decrease in FRET induced by W7 and PKCα activation varied within cells. The relative change in FRET ranged from 0 in some sites ( line in Figure 4E) to 100% in others (
line in Figure 4E) to 100% in others ( line in Figure 4E). A decrease in FRET suggests an increase in the distance between Cav1.2-
line in Figure 4E). A decrease in FRET suggests an increase in the distance between Cav1.2- and CaM-
and CaM- . This could result from two potential scenarios. First, CaM-
. This could result from two potential scenarios. First, CaM- dissociates from the IQ domain of Cav1.2-
dissociates from the IQ domain of Cav1.2- channels. Second, CaM-
channels. Second, CaM- changes in position within the C-tail of Cav1.2 channels, moving away from the
changes in position within the C-tail of Cav1.2 channels, moving away from the  fused to the C-terminal of these channel enough to decrease or even eliminate FRET. Although our FRET experiments cannot distinguish between these two possibilities, they evidently suggest that displacement of CaM away from the IQ domain of Cav1.2 channels may be a critical step in the induction of coupled gating of these channels.
fused to the C-terminal of these channel enough to decrease or even eliminate FRET. Although our FRET experiments cannot distinguish between these two possibilities, they evidently suggest that displacement of CaM away from the IQ domain of Cav1.2 channels may be a critical step in the induction of coupled gating of these channels.
Increased coupled gating of Cav1.2 channels during hypertension and Timothy syndrome
Increased Cav1.2 channel activity underlies lethal cardiac arrhythmias and autism in humans with Timothy syndrome (TS)21. In TS, a single amino acid substitution (Gly-436-Arg) in Cav1.2 (Cav1.2-TS) increases the open time and Po of these channels11, 21. We recorded Ca2+ sparklets (−70 mV) and depolarization-evoked currents in tsA-201 cells expressing WT and Cav1.2-TS channels (Figure 4F and 4G, respectively). We found a higher frequency of Ca2+ sparklets with coupled Cav1.2 channels in tsA-201 cells expressing Cav1.2-TS (n = 10 cells) than WT channels (n = 10 cells; Figure 4H; p < 0.05).
Next, we used experimental conditions similar to those used to record Ca2+ currents in neonatal cardiac myocytes above. Membrane depolarization to −30 mV activated WT and Cav1.2-TS channels in tsA-201 cells. Voltage-activated Ca2+ currents were never recorded in untransfected tsA-201 cells. As in neonatal myocytes, the amplitude of the elementary Ca2+ currents activated by a voltage pulse to −30 mV was similar (0.49 ± 0.01 pA) to that in neonatal ventricular myocytes (0.53 ± 0.01 pA; p > 0.05). This observation gives further support to the view that Cav1.2 channels produced the currents recorded in neonatal myocytes. Consistent with our Ca2+ sparklet studies, we recorded a number of Ca2+ currents resulting from the simultaneous opening and closing (i.e. coupled gating) of channels in cells expressing Cav1.2-TS than WT Cav1.2 (Figures 4G).
Increased L-type Ca2+ channel activity in arterial myocytes has been implicated in the chain of events that leads to vascular dysfunction during hypertension22, 23. In hypertensive arterial myocytes, higher Cav1.2 channel activity is due, at least in part, to increased local PKCα activity. We found a higher frequency of coupled Cav1.2 channel gating events both in arterial myocytes from an animal model of angiotensin II-induced hypertension and in tsA-201 cells expressing Cav1.2-TS channels (Figures 4H).
We investigated whether, as with WT Cav1.2 channels, the higher frequency of coupled gating events of Cav1.2-TS channels was associated with displacement of CaM from the IQ domain in the C terminal of these channels. Accordingly, FRET between Cav1.2-TS- and CaM-
and CaM- was undetectable (n = 13 cells; Figure 4I). These data suggest that coupled gating of Cav1.2 channels is associated with displacement of CaM away from the IQ domain in the C-terminal tail and contributes to enhanced channel function and Ca2+ influx during hypertension and TS.
was undetectable (n = 13 cells; Figure 4I). These data suggest that coupled gating of Cav1.2 channels is associated with displacement of CaM away from the IQ domain in the C-terminal tail and contributes to enhanced channel function and Ca2+ influx during hypertension and TS.
AKAP150 increases the probability of coupled gating between Cav1.2 channels
The scaffolding protein AKAP150 targets PKCα to the cell membrane24 and interacts with the C-tail of Cav1.2 channels25. Therefore, we tested the hypothesis that PKCα activation and its targeting to the membrane by AKAP150 is necessary to induce coupled gating of Cav1.2 channels during CaM inhibition (Figure 5). Application of W7 increased the number of coupled Cav1.2 channel events in PKCα null (PKCα−/−; n = 5 cells), but not in AKAP150 null myocytes (AKAP150−/−; n = 9 cells; p < 0.05). Because the expression of PKCα is similar in WT and AKAP150−/− myocytes9, the lack of effect of W7 on Cav1.2 channels in AKAP150−/− was not due to decreased PKCα expression. These data suggest that CaM antagonists work downstream of PKCα and that AKAP150 participates in the molecular events that facilitate coupled gating of Cav1.2 channels.
Coupling of Cav1.2 channels involves interactions between neighboring channels via their C-termini
We investigated the role of the C-terminal of Cav1.2 channels in coupled gating. To do this, a Cav1.2 construct lacking amino acids 1670-2171 (Cav1.2Δ670X), which eliminates a large section of the C-terminal tail, was expressed in tsA-201 cells (Figure 6A). These channels lack the putative AKAP-binding region25. Unlike WT channels, Cav1.2Δ1670X channels showed no coupled gating activity under either control conditions or after the application of W7 (n = 5 cells), indicating that residues 1670-2171 of Cav1.2 are critical for coupled gating activity.
Figure 6. Coupled gating involves the C-tail of Cav1.2 channels.
A) Bar plot of number of sites showing coupling of Cav1.2 channels in cells expressing a truncated Cav1.2 channel (Cav1.2Δ1670X) or WT Cav1.2 channels before and after application of 100 µmol/L W7. (B) Bar plot of effective FRET between Cav1.2-TS-EGFP and Cav1.2-TS-tRFP or WT Cav1.2-EGFP and Cav1.2-tRFP before and after PDBu or W7. The inset shows a cartoon of Cav1.2-EGFP and Cav1.2-tRFP. (C) Proposed model for coupled gating of Cav1.2 channels.
The C-termini of Cav1.2 channels could be involved in coupled gating by coming into close proximity. To examine this possibility, we expressed WT and TS Cav1.2 channels with  or
or  fused to their C-temini and determined FRET between channels (Figure 6B). Under control conditions, FRET between WT Cav1.2-
fused to their C-temini and determined FRET between channels (Figure 6B). Under control conditions, FRET between WT Cav1.2- and Cav1.2-
and Cav1.2- was low (0.02 ± 0.02; n = 10 cells). This is consistent with the observation of a relatively low frequency of coupled Cav1.2 events under these experimental conditions (see Figure 1 above). Application of W7 or PDBu, which increases coupled gating of Cav1.2 channels, increased FRET between WT Cav1.2-
was low (0.02 ± 0.02; n = 10 cells). This is consistent with the observation of a relatively low frequency of coupled Cav1.2 events under these experimental conditions (see Figure 1 above). Application of W7 or PDBu, which increases coupled gating of Cav1.2 channels, increased FRET between WT Cav1.2- and Cav1.2-
and Cav1.2- channels to 0.16 ± 0.02 (n = 8 cells) and 0.23 ± 0.03 (n = 10 cells), respectively (p < 0.05). Interestingly, under control conditions, FRET was higher in cells expressing
channels to 0.16 ± 0.02 (n = 8 cells) and 0.23 ± 0.03 (n = 10 cells), respectively (p < 0.05). Interestingly, under control conditions, FRET was higher in cells expressing  - and
- and  -tagged Cav1.2-TS channels (0.10 ± 0.01; n = 10 cells) than between WT Cav1.2 channels (p < 0.05; Figure 6B). These data suggest that the C-termini of Cav1.2 channels come into close proximity under conditions that favor coupled gating.
-tagged Cav1.2-TS channels (0.10 ± 0.01; n = 10 cells) than between WT Cav1.2 channels (p < 0.05; Figure 6B). These data suggest that the C-termini of Cav1.2 channels come into close proximity under conditions that favor coupled gating.
Discussion
In this study, we demonstrated that a cluster of Cav1.2 channel proteins can be organized to facilitate their coordinated opening and closing. A cartoon of the proposed model for coupled gating of Cav1.2 channels is shown in Figure 6C. Our data suggest that CaM antagonists (e.g. W7 and CaM inhibitory peptide), activators of PKCα, or a specific Cav1.2 channel mutation (e.g. Timothy syndrome), that move CaM away from the IQ domain in the C-terminal tail of the channel promote coupled gating between Cav1.2 channels. Although at present the exact mechanisms underlying coupled gating between Cav1.2 are unclear, our FRET analysis of  - and
- and  -tagged Cav1.2 channels suggests the possibility that coupled gating between these channels may involve transient interactions between a variable number (2–6) of adjacent channels via their C-termini. A recent report suggesting that the C-terminal of Cav1.2 channels dimerizes in vitro27 gives additional credence to this view.
-tagged Cav1.2 channels suggests the possibility that coupled gating between these channels may involve transient interactions between a variable number (2–6) of adjacent channels via their C-termini. A recent report suggesting that the C-terminal of Cav1.2 channels dimerizes in vitro27 gives additional credence to this view.
An important feature of our model is that AKAP150 increases the probability of coupled gating presumably by facilitating interactions between the C-termini of Cav1.2. Because AKAP150 interacts with only a subpopulation of Cav1.2 channels9, this scaffolding protein promotes coupled gating in a fraction of channels, resulting in subcellular variations in Cav1.2 activity. This represents a novel function of AKAP150 that is independent of its ability to target proteins to specific regions of the cell.
In our model, the level of expression of AKAP150 is a critical determinant of coupled Cav1.2 channel activity in cardiac and smooth muscle cells for multiple reasons. We demonstrated that PKCα increases the frequency of coupled gating events. Interestingly, in the Timothy syndrome, the Gly-436-Arg substitution in Cav1.2 creates a putative phosphorylation site for CaM-dependent kinase II (CaMKII)11. The actions of PKCα and CaMKII on WT and Cav1.2-TS are opposed by the Ca2+/CaM-dependent phosphatase calcineurin. Note, however, that AKAP150 targets PKCα and calcineurin to the surface membrane. Accordingly, the level of coupled gating events between Cav1.2 channels may not be simply limited by AKAP150 expression, but also by the relative activities of nearby calcineurin, CaMKII, and PKCα.
These conditions for coupled gating between Cav1.2 channels impose technical challenges for the recording and analysis of the molecular mechanisms underlying these events. For example, since only a subpopulation of Cav1.2 channels could undergo coupled gating, the use of patch-clamp approaches to record currents from relatively small areas of the membrane is likely to yield limited results in terms of the number of couple gating events per trial. However, our study suggests that optical recordings of Cav1.2 channels from relatively large portions of the surface may represent a more efficient approach to study coupled gating between these channels. For the same reasons, conventional biochemical approaches (e.g. co-IP) broadly used to examine direct, stable protein-protein interactions could be difficult to implement to study the molecular mechanisms of coupled gating between Cav1.2 channels. FRET could be used to circumvent these limitations. However, although powerful, this technique has its limitations because it reports the proximity of two fluorescent proteins (in our case  and
and  ) and not necessarily the specific sections of the channel to which they are fused. Thus, currently, whether coupled gating between Cav1.2 channels involves direct physical interactions between the C-tails of adjacent channels or to an intermediary protein such as AKAP150 and how this translates into the simultaneous opening and closing of their pores is unclear. Future experiments should address this critical issue.
) and not necessarily the specific sections of the channel to which they are fused. Thus, currently, whether coupled gating between Cav1.2 channels involves direct physical interactions between the C-tails of adjacent channels or to an intermediary protein such as AKAP150 and how this translates into the simultaneous opening and closing of their pores is unclear. Future experiments should address this critical issue.
Our data provide insights into the mechanisms by which PKCα could induce coupled gating between Cav1.2 channels. We found that PKC activation moves CaM away from the IQ domain of Cav1.2 channels. Yet, although PKCα increases coupled Cav1.2 channel activity, this kinase was not required for the induction of coupled activity since application of W7 induced coupled gating of Cav1.2 channels in PKCα−/− arterial myocytes. Together, these findings suggest that PKCα activation promotes coupled gating between Cav1.2 channels by inducing the movement of CaM away from the IQ domain in the C-tail of Cav1.2. However, our data indicate that phosphorylation of Cav1.2 channel by this kinase per se is not necessary to induce coupled gating. Subsequent studies should examine if a similar mechanism is involved in coupled gating between Cav1.2-TS channels.
An intriguing observation in this study is that coupling between Cav1.2 channels is transient, with the number of channels opening and closing simultaneously varying with time and between sites. In many instances, we saw sets of 2, 3, 4, 5 and 6 channels opening and closing together. We did not detect a particular pattern in terms of the number of channels undergoing coupled gating. This coupling mechanism is fundamentally different from that of ryanodine receptors (RyRs) in cardiac muscle28, in which tetramers of tightly coupled (i.e. κ ≈ 1) channels undergo stable openings under physiological conditions. At present, the precise mechanisms that control the coupling strength between Cav1.2 channels and how it changes under physiological (e.g. changes in membrane potential) and pathological conditions is unclear.
Although the effects of coupled gating of Cav1.2 channels on excitation-contraction (EC) coupling in cardiac and smooth muscle were not examined in this study, previous studies suggest coupling of these channels could have important implications in this process. In ventricular myocytes, Cav1.2 channels and RyRs in nearby junctional sarcoplasmic reticulum (jSR) form a functional unit called a “couplon”29. Ca2+ influx via Cav1.2 activates small clusters of RyRs via the mechanism of Ca2+-induced Ca2+ release, producing Ca2+ sparks. In these cells, activation of multiple couplons during an action potential results in a cell-wide increase in [Ca2+]i that activates contraction. Because the probability of Ca2+ spark occurrence (PSpark) is proportional to the Cav1.2 current and local [Ca2+]i30, it is intriguing to speculate that PSpark is likely to be higher in couplons with coupled Cav1.2 channels than in couplons with independently gating channels. Unlike ventricular myocytes, in smooth muscle, Cav1.2 channels and RyRs are not tightly coupled. In these cells, activation of Cav1.2 channels results in an increase in [Ca2+]i that directly activates contractile proteins. Thus, an increase in coupled gating of Cav1.2 channels could result in an increase in Ca2+ influx and thus global [Ca2+]i. Subsequent studies should test these intriguing hypotheses.
To conclude, we propose that coupled gating of Cav1.2 channels could translate into a larger increase in L-type Ca2+ channel activity than by an increase in independent gating activity. The simultaneous activation of a small cluster of channels would enhance Ca2+ influx per gating event. Under physiological conditions, this could increase the activation probability of Ca2+-dependent proteins involved in contraction, excitability, memory, and gene expression. In TS, coupled gating of Cav1.2 channels could increase the probability of Ca2+-dependent arrhythmias and increase Ca2+-dependent neurotoxicity. In hypertensive smooth muscle, coupled gating of Cav1.2 channels could increase myogenic tone and hence blood pressure. We propose that coupled gating of Cav1.2 channels may represent a general mechanism that contributes to Ca2+ influx and excitability under physiological and pathological conditions.
Novelty and Significance
What is known?
Increased calcium influx via dihydropyridine-sensitive L-type calcium channels has been identified in the pathogenesis of diseases such as cardiac arrhythmias, autism, and hypertension.
Blockade of L-type calcium channels is currently widely used in the treatment of arrhythmias, angina, and hypertension.
Not all L-type calcium channels are equal; some sites of channel activity allow more calcium influx than others.
What new information does this article contribute?
Instead of operating independently, clusters of L-type calcium channels can be formed spontaneously and transiently, to allow these clusters of channels to open concertedly in a “coupled” manner.
The observed frequency of these “coupled” L-type calcium channel openings is significantly increased in diseases such as Timothy syndrome (LQT8) and hypertension.
When calmodulin has reduced affinity for the L-type calcium channel, L-type calcium channels couple more frequently in the presence of AKAP150.
While it has become clear recently that not all L-type calcium channels are functionally equal and differences in local calcium concentration can play an important role in excitation-contraction coupling and calcium signaling, we wanted to know if clusters of L-type calcium channels can open in a “coupled” fashion as to maximize local calcium elevation. Using optical and electrophysiological approaches, we observed that coupled calcium channel openings and clustering do occur, and that their frequency is significantly increased with a Timothy syndrome (LQT8) mutation and in arterial myocytes from hypertensive animals. Furthermore, we described a possible model for these coupled openings. When calmodulin has decreased affinity for the C-terminal tail of the L-type calcium channel, coupled openings and physical clustering of L-type calcium channels occur more frequently, as long as A-kinase anchoring protein (AKAP) 150 is present. Therefore, our proposed model is that coupling occurs when calmodulin moves away from the C-terminal, allowing for the C-terminal tails to physically interact with the aid of AKAP150. This is a completely novel model that for the first time describes how L-type calcium channels can act together to bring about greater calcium influx than independent channels. Clinically, our model also suggests potential pharmacologic targets for the treatment of arrhythmia and hypertension.
Supplementary Material
Acknowledgements
We thank Drs. Bertil Hille, Carmen A. Ufret-Vincenty, and Madeline Nieves-Cintrón for reading this manuscript.
Source of funding
Supported by grants from NIH (HL085870; HL85686 ;HL088366), Leducq Foundation (cycAMP ObCDV), and the AHA (0735251N; 0840094N).
Abbreviations
- AKAP150
A-kinase anchoring protein 150
- CaM
calmodulin
- CaMKII
Ca2+/calmodulin-dependent kinase II
- EGFP
enhanced green fluorescent protein
- FRET
fluorescence resonance energy transfer
- jSR
junctional sarcoplasmic reticulum
- PDBu
phorbol 12,13-dibutyrate
- PKCα
protein kinase C α
- RyR
ryanodine receptor
- SR
sarcoplasmic reticulum
- TS
Timothy syndrome
- tRFP
tag red fluorescent protein
- TIRF
total internal reflection fluorescence
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Subject codes: 136, 138, 149, 150, 152
Disclosures
None.
Contributor Information
Manuel F. Navedo, Department of Physiology & Biophysics, University of Washington, Seattle, WA 98195.
Edward P. Cheng, Department of Physiology & Biophysics, University of Washington, Seattle, WA 98195.
Can Yuan, Department of Physiology & Biophysics, University of Washington, Seattle, WA 98195.
Scott Votaw, Department of Physiology & Biophysics, University of Washington, Seattle, WA 98195.
Jeffery D. Molkentin, Howard Hughes Medical Institute and Children's Hospital Medical Center for Molecular Cardiovascular Biology, 3333 Burnet Ave, Cincinnati, OH 45229.
John D. Scott, Howard Hughes Medical Institute and Department of Pharmacology, University of Washington, Seattle, WA 98195.
Luis F. Santana, Department of Physiology & Biophysics, University of Washington, Seattle, WA 98195.
References
- 1.Gomez-Ospina N, Tsuruta F, Barreto-Chang O, Hu L, Dolmetsch R. The C terminus of the L-type voltage-gated calcium channel Ca(V)1.2 encodes a transcription factor. Cell. 2006;127:591–606. doi: 10.1016/j.cell.2006.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fourcaudot E, Gambino F, Casassus G, Poulain B, Humeau Y, Luthi A. L-type voltage-dependent Ca2+ channels mediate expression of presynaptic LTP in amygdala. Nat Neurosci. 2009;12:1093–1095. doi: 10.1038/nn.2378. [DOI] [PubMed] [Google Scholar]
- 3.Cannell MB, Berlin JR, Lederer WJ. Effect of membrane potential changes on the calcium transient in single rat cardiac muscle cells. Science. 1987;238:1419–1423. doi: 10.1126/science.2446391. [DOI] [PubMed] [Google Scholar]
- 4.Marrion NV, Tavalin SJ. Selective activation of Ca2+-activated K+ channels by co-localized Ca2+ channels in hippocampal neurons. Nature. 1998;395:900–905. doi: 10.1038/27674. [DOI] [PubMed] [Google Scholar]
- 5.Navedo MF, Amberg GC, Nieves M, Molkentin JD, Santana LF. Mechanisms Underlying Heterogeneous Ca2+ Sparklet Activity in Arterial Smooth Muscle. Journal of General Physiology. 2006;127:611–622. doi: 10.1085/jgp.200609519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Navedo MF, Amberg G, Votaw SV, Santana LF. Constitutively active L-type Ca2+ channels. Proc Natl Acad Sci U S A. 2005;102:11112–11117. doi: 10.1073/pnas.0500360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tour O, Adams SR, Kerr RA, Meijer RM, Sejnowski TJ, Tsien RW, Tsien RY. Calcium Green FlAsH as a genetically targeted small-molecule calcium indicator. Nature chemical biology. 2007;3:423–431. doi: 10.1038/nchembio.2007.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang SQ, Song LS, Lakatta EG, Cheng H. Ca2+ signalling between single L-type Ca2+ channels and ryanodine receptors in heart cells. Nature. 2001;410:592–596. doi: 10.1038/35069083. [DOI] [PubMed] [Google Scholar]
- 9.Navedo MF, Nieves-Cintrón M, Amberg GC, Yuan C, Votaw VS, Lederer WJ, McKnight GS, Santana LF. AKAP150 Is Required for Stuttering Persistent Ca2+ Sparklets and Angiotensin II Induced Hypertension. Circ Res. 2008;102:e1–e11. doi: 10.1161/CIRCRESAHA.107.167809. [DOI] [PubMed] [Google Scholar]
- 10.Devic E, Xiang Y, Gould D, Kobilka B. Beta-adrenergic receptor subtype-specific signaling in cardiac myocytes from beta(1) and beta(2) adrenoceptor knockout mice. Mol Pharmacol. 2001;60:577–583. [PubMed] [Google Scholar]
- 11.Erxleben C, Liao Y, Gentile S, Chin D, Gomez-Alegria C, Mori Y, Birnbaumer L, Armstrong DL. Cyclosporin and Timothy syndrome increase mode 2 gating of CaV1.2 calcium channels through aberrant phosphorylation of S6 helices. Proc Natl Acad Sci U S A. 2006;103:3932–3937. doi: 10.1073/pnas.0511322103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liberles SD, Diver ST, Austin DJ, Schreiber SL. Inducible gene expression and protein translocation using nontoxic ligands identified by a mammalian three-hybrid screen. Proc Natl Acad Sci U S A. 1997;94:7825–7830. doi: 10.1073/pnas.94.15.7825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suh BC, Inoue T, Meyer T, Hille B. Rapid chemically induced changes of PtdIns(4,5)P2 gate KCNQ ion channels. Science. 2006;314:1454–1457. doi: 10.1126/science.1131163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung SH, Kennedy RA. Coupled Markov chain model: characterization of membrane channel currents with multiple conductance sublevels as partially coupled elementary pores. Math Biosci. 1996;133:111–137. doi: 10.1016/0025-5564(95)00084-4. [DOI] [PubMed] [Google Scholar]
- 15.Navedo MF, Amberg GC, Westenbroek RE, Sinnegger-Brauns MJ, Catterall WA, Striessnig J, Santana LF. Cav1.3 channels produce persistent calcium sparklets, but Cav1.2 channels are responsible for sparklets in mouse arterial smooth muscle. Am J Physiol Heart Circ Physiol. 2007;293:H1359–H1370. doi: 10.1152/ajpheart.00450.2007. [DOI] [PubMed] [Google Scholar]
- 16.Rubart M, Patlak JB, Nelson MT. Ca2+ currents in cerebral artery smooth muscle cells of rat at physiological Ca2+ concentrations. J Gen Physiol. 1996;107:459–472. doi: 10.1085/jgp.107.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navedo MF, Nieves-Cintron M, Amberg GC, Yuan C, Votaw VS, Lederer WJ, McKnight GS, Santana LF. AKAP150 is required for stuttering persistent Ca2+ sparklets and angiotensin II-induced hypertension. Circ Res. 2008;102:e1–e11. doi: 10.1161/CIRCRESAHA.107.167809. [DOI] [PubMed] [Google Scholar]
- 18.Zuhlke RD, Pitt GS, Deisseroth K, Tsien RW, Reuter H. Calmodulin supports both inactivation and facilitation of L-type calcium channels. Nature. 1999;399:159–162. doi: 10.1038/20200. [DOI] [PubMed] [Google Scholar]
- 19.Imredy JP, Yue DT. Mechanism of Ca2+-sensitive inactivation of L-type Ca2+ channels. Neuron. 1994;12:1301–1318. doi: 10.1016/0896-6273(94)90446-4. [DOI] [PubMed] [Google Scholar]
- 20.Erickson MG, Alseikhan BA, Peterson BZ, Yue DT. Preassociation of calmodulin with voltage-gated Ca2+ channels revealed by FRET in single living cells. Neuron. 2001;31:973–985. doi: 10.1016/s0896-6273(01)00438-x. [DOI] [PubMed] [Google Scholar]
- 21.Splawski I, Timothy KW, Sharpe LM, Decher N, Kumar P, Bloise R, Napolitano C, Schwartz PJ, Joseph RM, Condouris K, Tager-Flusberg H, Priori SG, Sanguinetti MC, Keating MT. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell. 2004;119:19–31. doi: 10.1016/j.cell.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 22.Pesic A, Madden JA, Pesic M, Rusch NJ. High Blood Pressure Upregulates Arterial L-Type Ca2+ Channels. Is Membrane Depolarization the Signal? Circ Res. 2004 doi: 10.1161/01.RES.0000131495.93500.3c. [DOI] [PubMed] [Google Scholar]
- 23.Nieves-Cintron M, Amberg GC, Navedo MF, Molkentin JD, Santana LF. The control of Ca2+ influx and NFATc3 signaling in arterial smooth muscle during hypertension. Proc Natl Acad Sci U S A. 2008;105:15623–15628. doi: 10.1073/pnas.0808759105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klauck TM, Faux MC, Labudda K, Langeberg LK, Jaken S, Scott JD. Coordination of three signaling enzymes by AKAP79, a mammalian scaffold protein. Science. 1996;271:1589–1592. doi: 10.1126/science.271.5255.1589. [DOI] [PubMed] [Google Scholar]
- 25.Oliveria SF, Dell'Acqua ML, Sather WA. AKAP79/150 anchoring of calcineurin controls neuronal L-type Ca2+ channel activity and nuclear signaling. Neuron. 2007;55:261–275. doi: 10.1016/j.neuron.2007.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao T, Yatani A, Dell'Acqua ML, Sako H, Green SA, Dascal N, Scott JD, Hosey MM. cAMP-dependent regulation of cardiac L-type Ca2+ channels requires membrane targeting of PKA and phosphorylation of channel subunits. Neuron. 1997;19:185–196. doi: 10.1016/s0896-6273(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 27.Fallon JL, Baker MR, Xiong L, Loy RE, Yang G, Dirksen RT, Hamilton SL, Quiocho FA. Crystal structure of dimeric cardiac L-type calcium channel regulatory domains bridged by Ca2+* calmodulins. Proc Natl Acad Sci U S A. 2009;106:5135–5140. doi: 10.1073/pnas.0807487106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marx SO, Gaburjakova J, Gaburjakova M, Henrikson C, Ondrias K, Marks AR. Coupled gating between cardiac calcium release channels (ryanodine receptors) Circ Res. 2001;88:1151–1158. doi: 10.1161/hh1101.091268. [DOI] [PubMed] [Google Scholar]
- 29.Franzini-Armstrong C, Protasi F, Ramesh V. Shape, size, and distribution of Ca2+ release units and couplons in skeletal and cardiac muscles. Biophys J. 1999;77:1528–1539. doi: 10.1016/S0006-3495(99)77000-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Santana LF, Cheng H, Gomez AM, Cannell MB, Lederer WJ. Relation between the sarcolemmal Ca2+ current and Ca2+ sparks and local control theories for cardiac excitation-contraction coupling. Circ Res. 1996;78:166–171. doi: 10.1161/01.res.78.1.166. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.