Abstract
Transforming growth factor-β (TGF-β) is a potent inhibitor of cell proliferation. This study investigated whether overexpression of Smad7, which blocks TGF-β–induced activation of Smad2/3, could prevent the suppression of regeneration of small-for-size liver grafts. Rats were intravenously given adenoviruses (2 × 109 pfu/rat) carrying the LacZ gene or the Smad7 gene (Ad-Smad7) 3 days prior to liver harvesting. Half-size livers were implanted into recipients of the same weight or twice the donor weight, and this resulted in half-size or quarter-size liver grafts. Cell proliferation, detected by 5-bromo-2′-deoxyuridine (BrdU) incorporation, increased to 23% in half-size grafts at 38 hours after implantation but was only 4% in quarter-size grafts. Graft weight did not increase after 38 hours in full-size and quarter-size grafts but increased 28% in half-size grafts. Ad-Smad7 restored BrdU labeling to 32%, and the graft weight increased to 43% in quarter-size grafts. Serum total bilirubin increased approximately 30-fold after the implantation of quarter-size grafts. Ad-Smad7 blunted hyperbilirubinemia by 80%. The basal hepatic TGF-β1 level was 7 ng/g of liver wet weight, and this increased to 30 ng/g at 1.5 hours after the transplantation of full-size grafts but decreased rapidly afterwards. After the transplantation of quarter-size grafts, however, TGF-β1 progressively increased to 159 ng/g in 38 hours. Nuclear phosphorylated Smad2/3 was barely detectable, and p21Cip1 expression was negligible in full-size grafts but increased markedly in quarter-size grafts. Ad-Smad7 blocked Smad2/3 activation and expression of p21Cip1. Together, these data show that TGF-β is responsible, at least in part, for the defective liver regeneration in small-for-size grafts by activating the Smad signaling pathway.
Living donor and split liver transplantation (LT) has become more widely used in recent years to alleviate the mortality resulting from the scarcity of suitable liver grafts for transplantation.1,2 Donor-recipient graft size disparity leading to small-for-size graft dysfunction and failure is an important issue limiting the wider use of partial LT for adults.3 The mechanisms underlying the dysfunction and failure of small-for-size grafts remain unclear. Previous studies have indicated that liver regeneration is markedly suppressed in small-for-size liver grafts, and this appears to contribute to graft failure.4–6
Growth factors such as hepatic growth factor (HGF), transforming growth factor-α (TGF-α), epidermal growth factor (EGF), and vascular endothelial growth factor and the cytokines interleukin-6 (IL-6) and tumor necrosis factor-α (TNFα) stimulate liver regeneration.7–9 TGF-β is a potent inhibitor of hepatocyte proliferation that counterbalances the stimulatory effects of mitogens during liver regeneration.10–12 In many cell types, TGF-β is the most potent growth-inhibitory polypeptide currently known.13 However, whether TGF-β plays a role in the suppression of small-for-size liver graft regeneration remains unclear.
TGF-β elicits its biological effects by signaling through a heteromeric receptor complex consisting of type I and type II receptors. The binding of TGF-β to type II receptors leads to the recruitment, phosphorylation, and activation of type I receptors, which subsequently phosphorylate proteins of the Smad family, particularly Smad2 and Smad3.14,15 Phosphorylated Smad2 and Smad3 form a complex with Smad4, move into the nucleus, and activate target genes expressing regulatory proteins for cell proliferation, differentiation, and cell death.14,16 TGF-β also regulates mitogen-activated protein kinase–mediated signaling pathways and other signaling proteins, such as protein kinase A, protein kinase C, phospholipase C, and nuclear factor-κB, in different cell types.17,18
Another Smad family member, Smad7, associates stably with the TGF-β receptor complex, which inhibits TGF-β–induced phosphorylation of Smad2 and Smad3 and blocks TGF-β–dependent signaling.19 This study examined the effect of the adenoviral delivery of Smad7 on liver regeneration after LT with small-for-size grafts. Our results show that TGF-β1 increases sharply after the transplantation of small-for-size liver grafts in association with Smad2/3 phosphorylation and nuclear translocation. Smad7 adenoviral expression blocks Smad2/3 activation, promotes liver regeneration, and improves graft function.
MATERIALS AND METHODS
Animals and LT
A recombinant adenovirus containing the transgene for either β-galactosidase (Ad-LacZ) or human Smad7 (Ad-Smad7) was prepared at the University of North Carolina as described previously.20 Ad-LacZ, which contains the β-galactosidase gene driven by the cytomegalovirus promoter, was used as a control viral vector. The Ad-Smad7 virus expresses a C-terminal hemagglutinin (HA)-tagged human Smad7 protein. The human Smad7 complementary DNA was obtained from Dr. Wrana (Hospital for Sick Children, Toronto, Canada) and cloned into the pGI-AdCMV5 transfer vector (Qbiogene, Carlsbad, CA). Viral amplification was performed in 293 cells and cesium chloride purified by the standard methodology. Viral titer estimates were performed by optical density measurements. The virus (2 × 109 pfu) was diluted in 0.5 mL of normal saline and injected into the tail vein of donor rats (male Lewis rats, 170–200 g). In preliminary studies from this laboratory, the infection rates of liver cells were over 80% when doses of adenovirus ranging from 1 to 3 × 109 pfu were used.
Our previous studies showed that adenoviral protein expression peaks in 2 to 3 days and persists for about 3 weeks.21 Therefore, 3 days after the viral infection, livers were explanted and reduced in size by the removal of the left lateral lobe, the left portion of the median lobe, and the anterior and posterior caudate lobes ex vivo as described.5 This procedure decreased liver mass by approximately 50%.22 Explants were stored in University of Wisconsin solution at 0 to 1°C for 6 hours and rinsed with lactated Ringer’s solution (Abbott Laboratories, North Chicago, IL) just prior to implantation.
Reduced-size liver explants were implanted into recipients of similar (170–200 g) or greater body weights (350–430 g), and this resulted in graft weight/standard liver weight (defined as 4% of body weight) values of approximately 50% (half-size) and approximately 25% (quarter-size), respectively.23 Unreduced livers were implanted into recipients of similar body weights (170–200 g) as full-size controls. The hepatic artery and the bile duct were anastomosed with intraluminal splints. Implantation procedures usually took about 50 minutes. For sham operations, the abdominal wall was opened, and ligaments around the liver were freed. The abdominal wall was then closed with running sutures after 50 minutes. Under these conditions, survival was 100% for full-size grafts, 80% for half-size grafts, and 30% for quarter-size grafts.23 The graft weight/standard liver weight values were on average 24.9% ± 6% in the quarter-size group pretreated with saline, 26.1% ± 4% in the quarter-size group treated with Ad-LacZ, and 26.0% ± 3% for the quarter-size grafts treated with Ad-Smad7 (P > 0.10 versus the saline-pretreated and Ad-LacZ–pretreated quarter-size groups). The graft weight was measured just prior to cold storage and after harvesting at various times after implantation. All animals were given humane care in compliance with institutional guidelines using protocols approved by the Institutional Animal Care and Use Committee.
Bilirubin
Blood samples were collected from the tail vein at 18, 24, and 38 hours after implantation. Total bilirubin in sera was determined with analytical kits from Pointe Scientific (Uncoln Park, MI) to assess liver function.
Immunohistochemical Staining for 5-Bromo-2′-Deoxyuridine (BrdU), Proliferating Cell Nuclear Antigen (PCNA), Phosphorylated Smad2/3, and β-Galactosidase
BrdU was injected intraperitoneally (100 mg/kg) 1 hour prior to liver harvesting to detect cells synthesizing DNA. At 38 hours after implantation, livers were harvested, and BrdU incorporation and expression of PCNA, another marker of cell proliferation, were determined immunohistochemically in liver sections as described elsewhere.5 Cells positive and negative for BrdU and PCNA were counted in 10 randomly selected fields under a light microscope with a 20× objective lens. Immunohistochemistry of phosphorylated Smad2/3, Ski-like oncogene (SnoN), and β-galactosidase was performed with primary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) at a concentration of 1:200 for 1 hour, 1 hour, and 20 minutes at room temperature, respectively. Phosphorylated Smad2/3-positive and Smad2/3-negative cells were counted in 10 randomly selected fields under a light microscope with a 40× objective lens.
Western Blotting
Livers were harvested, snap-frozen in liquid nitrogen, homogenized in a buffer containing 40 mM trishydroxymethylaminomethane (pH 7.6), 140 mM NaCl, and 1% protease and phosphatase inhibitor cocktails (Sigma, St. Louis, MO), and centrifuged at 1000g for 10 minutes at 4°C. The supernatant was analyzed for HA protein, Smad7, SnoN, p21Cip1, p27Kip1, p15Ink4B, p16Ink4A, and tumor protein p53 (p53) by western blotting6 using specific antibodies against the proteins of interest (Santa Cruz Biotechnology) at a dilution of 1:300 to 1:500 at 4°C overnight followed by incubation with an appropriate secondary antibody at 1:1000 to 1:3000 for 1 hour. Chemiluminescence was detected with an ECL Plus western blotting detection system (Amersham Biosciences, Little Chalfont, United Kingdom). To confirm equal loading, blots were reprobed with anti-actin primary antibody at 1:3000 for 1 hour (ICN, Costa Mesa, CA). The protein concentration was determined with the Bio-Rad protein assay (Bio-Rad, Hercules, CA).
Detection of TGF-β1 in Liver Tissue
Liver tissue was collected into liquid nitrogen, stored at −80°C, and homogenized after thawing in a buffer (pH 7.5) containing 20 mM trishydroxymethylaminomethane, 0.25 M sucrose, 2 mM ethylene diamine tetraacetic acid, 10 mM ethylene glycol tetraacetic acid, 1% Triton X-100, and 1% protease inhibitor cocktail (Sigma). The homogenates were centrifuged at 100,000g at 4°C for 1 hour. Total TGF-β1 in the supernatant, including both precursor and biologically active cleaved forms, was determined with a TGF-β1 Emax immunoassay system from Promega (Madison, WI) according to the manufacturer’s instructions.
Statistical Analysis
All groups were compared with an analysis of variance or Kruskal-Wallis test as appropriate. Numbers in each group for different parameters are shown in the figure legends. Differences were considered significant at P < 0.05.
RESULTS
TGF-β1 Increased in Small-For-Size Liver Grafts
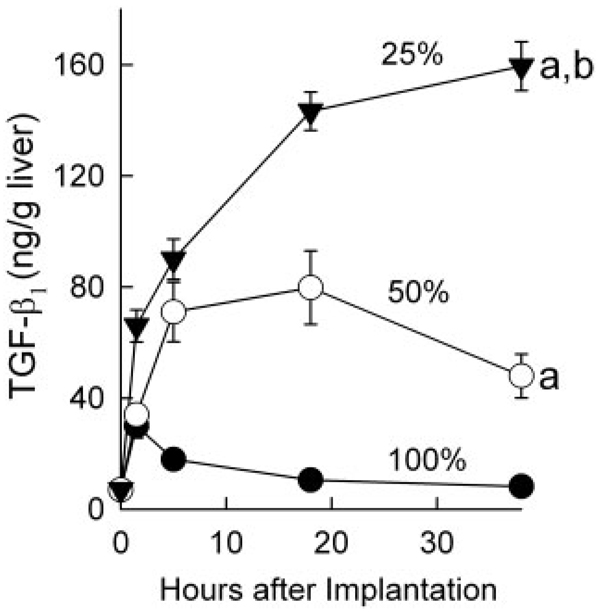
TGF-β is a potent inhibitor of cell proliferation. Accordingly, we measured total TGF-β1 in livers before and after LT. Before LT, TGF-β1 was 7 ng/g of liver wet weight. After full-size LT, TGF-β1 increased to 30 ng/g at 1.5 hours but decreased afterwards to close to pre-transplant levels at 38 hours after the operation (Fig. 1). After the transplantation of half-size grafts, a progressive increase of TGF-β1 occurred with a maximum of 79 ng/g of liver at 18 hours, and it then declined gradually. An even larger sustained increase occurred after the transplantation of quarter-size grafts, with TGF-β1 levels rising to 143 ng/g after 18 hours and to 159 ng/g after 38 hours (Fig. 1).
Figure 1.
TGF-β1 production increased after partial liver transplantation. Livers were harvested at 1.5, 5, 18, and 38 hours after full-size (100%, n = 4), half-size (50%, n = 4), and quarter-size (25%, n = 5) liver transplantation, and total TGF-β1 was determined. Values are means and standard errors of the mean (P < 0.001 by 2-way analysis of variance). aP < 0.001 versus full-size grafts and bP < 0.001 versus half-size grafts.
Expression of β-Galactosidase and HA Protein in the Liver After Viral Gene Delivery
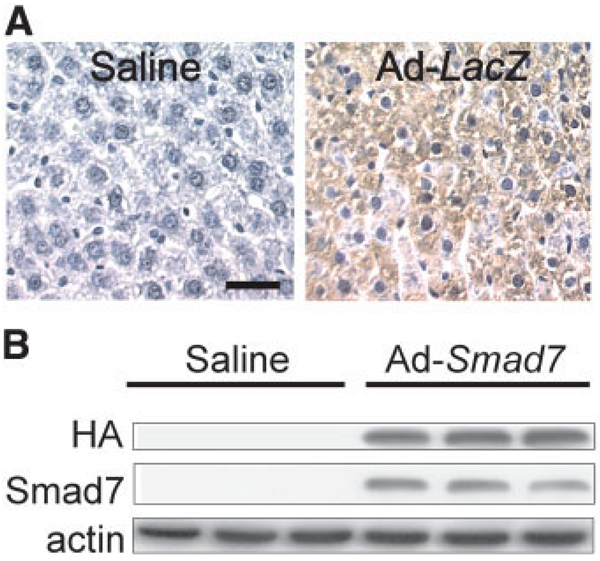
Rats were infected with Ad-LacZ, which carries the gene for β-galactosidase. At 3 days after infection with Ad-LacZ, over 80% of hepatocytes expressed β-galactosidase (Fig. 2A, right). By contrast, β-galactosidase was not detectable in livers from rats receiving normal saline (Fig. 2A, left). These results indicate that the adenovirus effectively infects liver cells.
Figure 2.
Efficiency of adenoviral transfection. Livers were harvested 3 days after the injection of normal saline, Ad-LacZ, or Ad-Smad7. (A) β-Galactosidase was detected immunohistochemically (n = 4 per group). The left panel shows a liver from a saline-treated rat; the right panel shows a liver from an Ad-LacZ–treated rat. The bar represents 20 µm. (B) The HA tag of Smad7 and Smad7 were detected by western blotting in comparison with actin (n = 3 per group).
The Ad-Smad7 virus expresses a C-terminal, HA-tagged human Smad7 protein. The HA tag is a general epitope tag in expression vectors and does not interfere with the bioactivity or biodistribution of recombinant proteins. The HA tag is present only in the Smad7 proteins encoded by the Smad7 gene carried by the adenoviral vector. Therefore, detection of the HA tag indicates expression of the Smad7 gene transfected by adenoviral vectors. The HA tag was not detectable by western blotting in livers from rats given saline (Fig. 2B). By contrast, livers from rats infected with Ad-Smad7 showed high levels of the HA tag, indicating Smad7 protein expression (Fig. 2B). We also detected Smad7 expression by western blotting. Smad7 was barely detectable in livers from saline-treated rats but increased overtly in livers from rats infected with Ad-Smad7 (Fig. 2B). Therefore, this Smad7 expression is possibly mainly due to gene transfection.
Liver Regeneration Was Suppressed After the Transplantation of Small-for-Size Liver Grafts: Reversal by Smad7
Liver regeneration was evaluated by BrdU incorporation, expression of PCNA, and increases in graft weight. Our previous studies showed that after the implantation of half-size livers, BrdU labeling first increased at about 18 hours postoperatively in both periportal and midzonal regions of the liver lobule and was maximal after 38 hours. Proliferating cells were predominantly hepatocytes.5
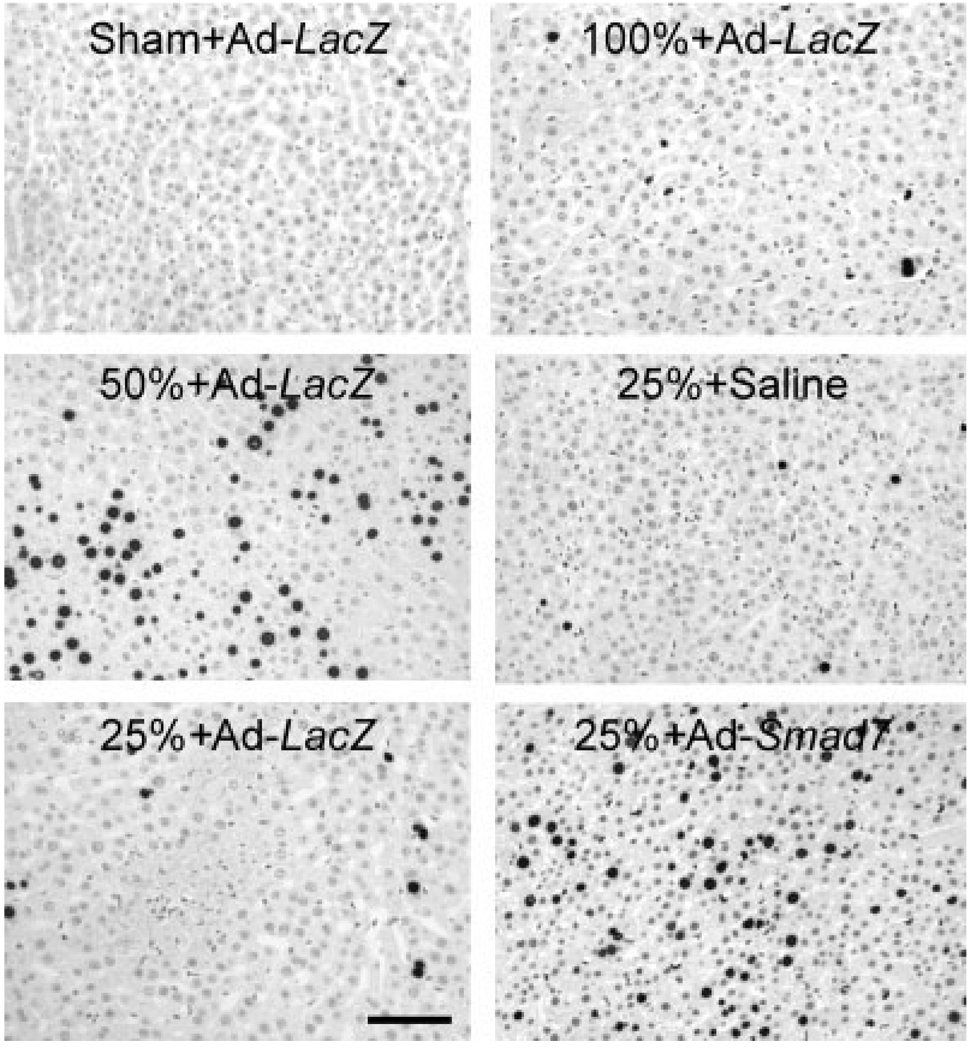
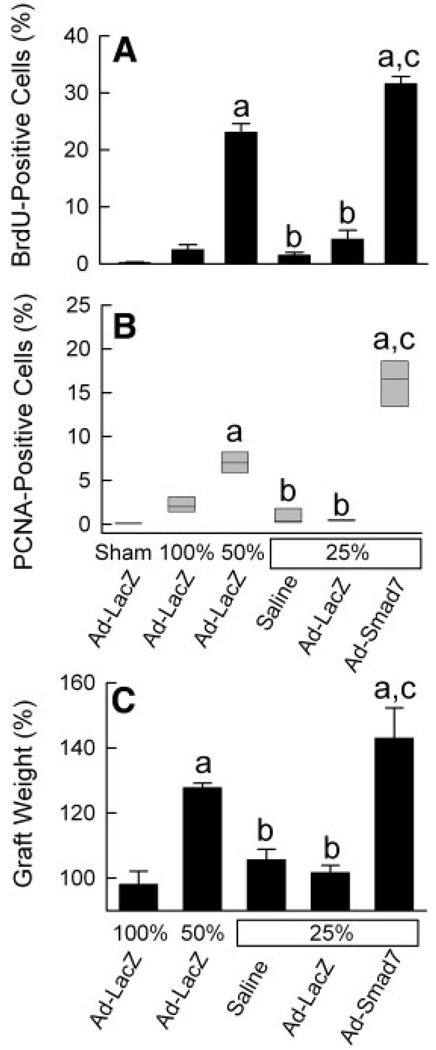
In this study, cell proliferation was assessed at 38 hours after implantation. The concentration of BrdU-positive cells was about 0.2% in livers from Ad-LacZ–treated mice after a sham operation (Fig. 3, upper left, and Fig. 4A), which was not different from that for saline-treated rats after a sham operation (data not shown). After Ad-LacZ treatment and LT, BrdU labeling increased to 2.5% and 23%, respectively, in full-size and half-size grafts (Fig. 3, upper right and middle left, and Fig. 4A). By contrast, in quarter-size grafts, BrdU labeling was only 2% and 4% after saline and Ad-LacZ treatment, respectively, and this indicated suppression of cell proliferation in small-for-size liver grafts (P < 0.001 versus half-size grafts; Fig. 3, middle right and lower left, and Fig. 4A). Adenoviral expression of Smad7, which blocks the TGF-β/Smad signaling, restored cell proliferation in quarter-size grafts to 32% (Fig. 3, lower right, and Fig. 4A).
Figure 3.
Ad-Smad7 reverses the suppression of cell proliferation in small-for-size liver grafts. Livers were transplanted 3 days after adenoviral infection, and they were harvested 38 hours after implantation or a sham operation for immunohistochemical detection of BrdU, as described in the Materials and Methods section. The upper left panel shows a liver from a sham-operated rat treated with Ad-LacZ, the upper right panel shows a full-size graft (100%) from a donor rat treated with Ad-LacZ, the middle left panel shows a half-size graft (50%) from a donor rat treated with Ad-LacZ, the middle right panel shows a quarter-size graft (25%) from a donor rat treated with saline, the lower left panel shows a quarter-size graft from a donor rat treated with Ad-LacZ, and the lower right panel shows a quarter-size graft from a donor rat treated with Ad-Smad7. Note the marked increase of BrdU incorporation (black nuclear labeling) in the half-size graft (middle left) compared to the sham-operated liver or full-size liver grafts (upper left and right); this was suppressed in quarter-size grafts after Ad-LacZ or saline treatment (lower left and middle right). Donor treatment with Ad-Smad7 markedly increased BrdU incorporation after quarter-size transplantation (lower right). The bar represents 50 µm. n = 4 per group.
Figure 4.
Liver regeneration is suppressed in quarter-size liver grafts: reversal by Ad-Smad7. The conditions were the same as those for Fig. 3. (A) BrdU labeling, (B) PCNA labeling, and (C) the increase in the graft weight at 38 hours after transplantation were determined as described in the Materials and Methods section. (A,C) The values are means and standard errors of the mean, and differences between groups were tested by 1-way analysis of variance (P < 0.001 for panels A and C, n = 4 per group). aP < 0.001 versus full-size grafts (100%), bP < 0.001 versus half-size grafts (50%), and cP < 0.001 versus quarter-size grafts (25%) treated with saline or Ad-LacZ. (B) The values are 25th percentiles, medians, and 75th percentiles, and differences between groups were tested by the Kruskal-Wallis test (P < 0.001, n = 4 per group). aP < 0.05 versus full-size grafts (100%), bP < 0.05 versus half-size grafts (50%), and cP < 0.05 versus quarter-size grafts (25%) treated with saline or Ad-LacZ. Differences in BrdU incorporation, PCNA expression, and graft weight between quarter-size grafts treated with saline and Ad-LacZ were not statistically significant.
PCNA was used as another indicator of cell proliferation. PCNA increased from 0.1% after a sham operation to 2.0% and 7.0% after the transplantation of full-size and half-size grafts, respectively, from Ad-LacZ–treated donors (Fig. 4B). By contrast, PCNA expression was 0.2% and 0.4% in quarter-size grafts after saline and Ad-LacZ treatment, respectively (Fig. 4B). Ad-Smad7 donor treatment increased PCNA expression markedly in quarter-size grafts to 16.6% (Fig. 4B).
Increases in the graft weight reflect both the proliferation and hypertrophy of liver cells. Thirty-eight hours after implantation, the graft weight did not increase in full-size grafts infected with Ad-LacZ but increased by 28% in half-size grafts infected with Ad-LacZ (Fig. 4C). After quarter-size transplantation, the graft weight did not increase in grafts pretreated with either saline or Ad-LacZ (Fig. 4C). However, after infection with Ad-Smad7, the weight of quarter-size grafts increased by 43%, and this indicates that inhibition of TGF-β/Smad signaling reverses the suppression of regeneration of small-for-size liver grafts (Fig. 4C).
Smad7 Expression Improved the Function of Small-for-Size Liver Grafts
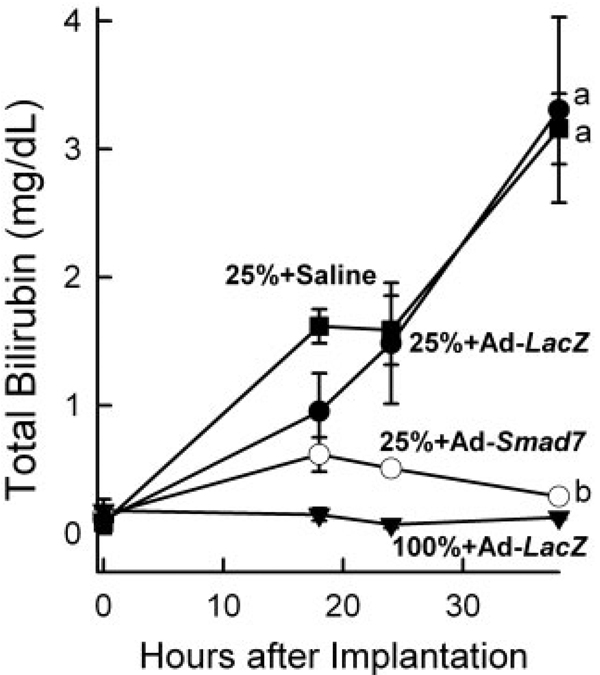
The improvement of liver regeneration should enhance the recovery of liver function. Hyperbilirubinemia indicates poor liver function. Therefore, total bilirubin was measured after LT. Prior to LT, the serum bilirubin level was on average approximately 0.1 mg/dL. At 38 hours after full-size and half-size LT, bilirubin did not increase significantly (Fig. 5 and data not shown). By contrast, bilirubin increased more than 30-fold in rats receiving quarter-size liver grafts pretreated with saline or Ad-LacZ, and this indicated poor liver function (Fig. 5). Pretreatment of liver donors with Ad-Smad7 decreased peak bilirubin by 80% after quarter-size LT (Fig. 5). With Ad-Smad7 treatment, the total bilirubin level after the transplantation of quarter-size grafts was not statistically different from that of full-size grafts (Fig. 5).
Figure 5.
Ad-Smad7 protects against hyperbilirubinemia after the transplantation of quarter-size liver grafts. Blood samples were collected at 18, 24, and 38 hours after implantation for bilirubin measurement. Values are means and standard errors of the mean. Differences among groups were tested by 2-way analysis of variance (P < 0.001, n = 4 per group). aP < 0.001 versus full-size (100%) grafts from Ad-LacZ–treated donor rats and bP < 0.001 versus quarter-size (25%) grafts from donor rats treated with Ad-LacZ. Differences in total bilirubin between recipients of quarter-size grafts treated with saline and Ad-LacZ were not statistically significant.
Ad-Smad7 Blocked Nuclear Translocation of Phosphorylated Smad2/3 in Small-for-Size Liver Grafts
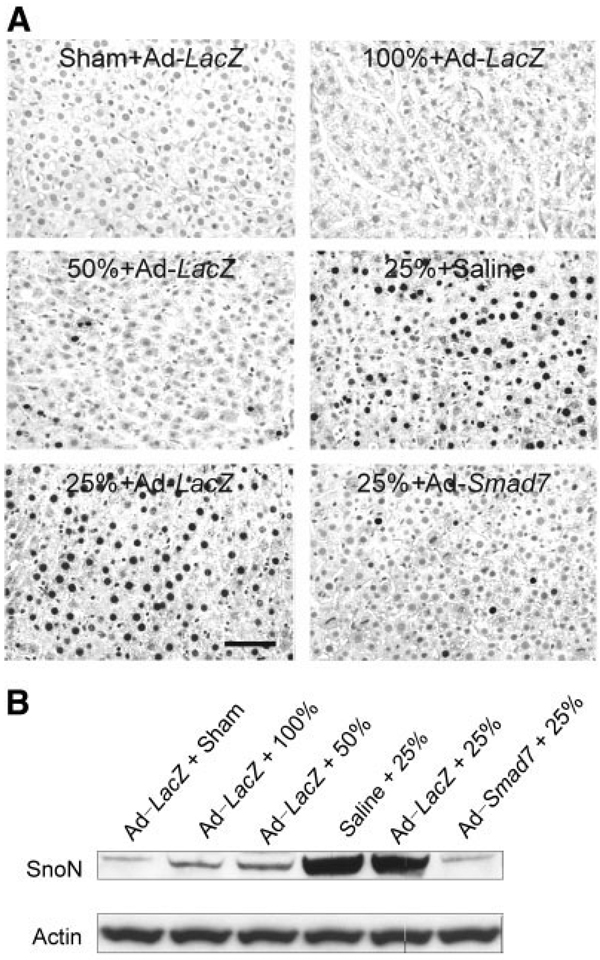
Activation of TGF-β receptors leads to phosphorylation and nuclear translocation of Smad2 and Smad3, thus activating target genes that negatively regulate the cell cycle.14,16 Therefore, nuclear translocation of phosphorylated Smad2/3 was assessed. After a sham operation and full-size LT, phosphorylated Smad2/3 in nuclei of hepatocytes was barely detectable (median = 0.43% and median = 0.42%, respectively, n = 4 per group) by immunocytochemistry (Fig. 6A, upper left and right). In half-size grafts, a few hepatocytes (median = 2.4%, n = 4, P < 0.05 versus full-size grafts by the Kruskal-Wallis test) showed phosphorylated Smad2/3 in their nuclei (Fig. 6A, middle left). By contrast, after the transplantation of saline-treated or Ad-LacZ–treated quarter-size liver grafts, phosphorylated Smad2/3 increased markedly in nuclei of many hepatocytes at 18 hours (data not shown) and 38 hours after the operation (Fig. 6A, middle right and lower left; median = 33.8% and median = 33.1%, respectively, n = 4 per group, P < 0.05 versus full-size grafts by the Kruskal-Wallis test). Importantly after the pretreatment of donors with Ad-Smad7, nuclear phosphorylated Smad2/3 decreased substantially to 1.8% in hepatocytes (Fig. 6A, lower right; n = 4 per group, P < 0.05 versus quarter-size grafts with Ad-LacZ treatment by the Kruskal-Wallis test).
Figure 6.
Ad-Smad7 prevents Smad2/3 nuclear translocation in quarter-size liver grafts. The conditions were the same as those for Fig. 3. (A) Phosphorylated Smad2/3 was detected immunohistochemically (n = 4 per group), as described in the Materials and Methods section. The upper left panel shows a liver from a sham-operated donor rat treated with Ad-LacZ, the upper right panel shows a full-size graft (100%) from a rat treated with Ad-LacZ, the middle left panel shows a half-size graft (50%) from a rat treated with Ad-LacZ, the middle right panel shows a quarter-size graft (25%) from a rat treated with saline, the lower left panel shows a quarter-size graft from a rat treated with Ad-LacZ, and the lower right panel shows a quarter-size graft from a rat treated with Ad-Smad7. Note that black immunoreactivity for phosphorylated Smad2/3 in nuclei increased in quarter-size grafts (25%) compared to sham-operated livers and full-size (100%) and half-size (50%) liver grafts. Ad-Smad7 treatment decreased phosphorylated Smad2/3 labeling in quarter-size grafts. The bar represents 50 µm. (B) SnoN and actin in liver tissue were detected by western blotting (n = 4 per group), and representative images are shown.
SnoN exerts negative control over TGF-β signaling by interaction with Smads. Overexpression of SnoN inhibits certain TGF-β–inducible signals.24 Therefore, we also investigated SnoN expression by western blotting. A basal level of SnoN expression was observed in livers from sham-operated rats (Fig. 6B, n = 4). After transplantation, SnoN expression was slightly increased in full-size and half-size grafts (n = 4 per group) but dramatically increased in quarter-size grafts treated with saline or transfected with Ad-LacZ (n = 4 per group). In quarter-size grafts transfected with Ad-Smad7 (n = 4), expression of SnoN was decreased to almost the basal level, possibly because of inhibition of TGF-β–induced up-regulation of SnoN. Detection of nuclear SnoN by immunohistochemical staining showed a similar pattern of alterations (data not shown). Therefore, Ad-Smad7 does not exert its effects by increasing nuclear SnoN.
p21Cip1 Expression Increased After the Transplantation of Small-For-Size Liver Grafts: Prevention by Ad-Smad7
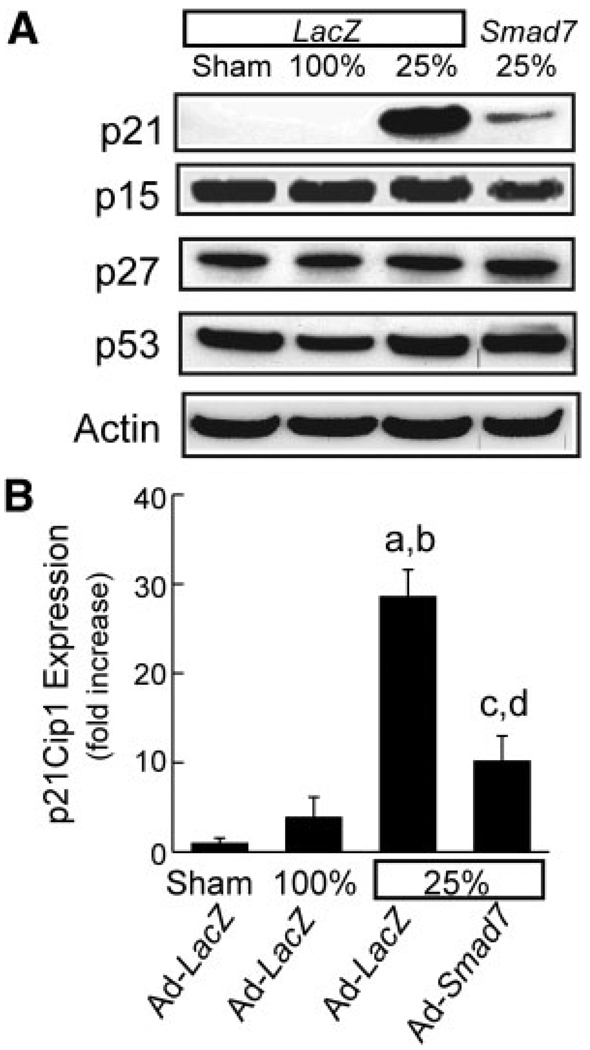
Cyclins regulate the cell cycle in association with cyclin-dependent kinases (CDKs), which are under the inhibitory control of cyclin-dependent kinase inhibitors (CDKIs).25,26 Antiproliferative effects of TGF-β in some cell types are mediated by CDKIs.18,27,28 Therefore, we investigated whether CDKIs are involved in Smad2/3-mediated TGF-β’s antiproliferative effects in small-for-size liver grafts. p21Cip1, a universal inhibitor of CDKs, was barely detectable by western blotting in sham-operated livers and full-size liver grafts (Fig. 7A,B). After the transplantation of quarter-size grafts, p21Cip1 increased markedly at 18 hours (data not shown) and remained more than 28-fold–elevated at 38 hours (Fig. 7A,B). After inhibition of TGF-β/Smad signaling by Ad-Smad7, expression of p21Cip1 was blunted by 60% (Fig. 7A,B). By contrast, the CDKIs p27Kip1 and p15Ink4B were not significantly different between sham-operated livers, full-size grafts, and quarter-size grafts (Fig. 7A), and the CDKI p16Ink4A was not detectable in any group (data not shown).
Figure 7.
p21Cip1 expression is suppressed after transplantation of quarter-size liver grafts: reversal by Ad-Smad7. Liver grafts were harvested at 38 hours after implantation, and p21Cip1, p27Kip1, p15Ink4B, p53, and actin were detected by western blotting, as described in the Materials and Methods sections. (A) Representative gels (n = 4 per group). (B) Plots of p21Cip1/actin ratios (means and standard errors of the mean) determined by densitometry (P < 0.001 by 1-way analysis of variance, n = 4 per group). aP < 0.001 versus the sham operation, bP < 0.001 versus the full-size grafts (100%) from Ad-LacZ–treated donors, cP < 0.001 versus the quarter-size grafts (25%) from Ad-LacZ–treated donors, and dP = 0.042 versus the sham operation.
In some cells, p21Cip1 can be induced by a p53-dependent mechanism following stress.18 Accordingly, we measured p53 after LT. Although p21Cip1 was markedly higher in quarter-size grafts compared to full-size grafts, p53 was not different between sham-operated livers, full-size grafts, and quarter-size grafts (Fig. 7A). Therefore, p21Cip1 changes in small-for-size grafts did not appear to be occurring in a p53-dependent manner.
DISCUSSION
TGF-β/Smad Signaling Plays an Important Role in the Suppression of Liver Regeneration in Small-for-Size Liver Grafts
Survival and functional recovery of liver grafts after partial LT depend highly on liver regeneration. Unfortunately, liver regeneration is suppressed in small-for-size liver grafts,4–6,29 and this prevents or delays the recovery of liver mass and function after partial LT. As a result, the small grafts can experience excessive metabolic burden and eventually fail. Therefore, promotion of liver regeneration is of clear benefit for improving the outcome of small-for-size LT. Why small-for-size liver grafts fail to regenerate is not fully understood. Our previous study showed that repopulation of stem cells appears not to play an important role in liver regeneration after partial LT in rats, at least in early stages, and thus the mechanisms suppressing regeneration after quarter-size LT are acting on hepatocytes rather than stem cells.5
Liver regeneration is a complex, multistep process. In the early stage of regeneration, a variety of cytokines (eg, IL-6 and TNFα) and hormones are released, transcription factors and kinases are activated, and immediate-early genes are expressed.7,8 This activation of the immediate-early genes and transcription factors is usually neither specific nor sufficient to cause liver regeneration. However, they prepare the liver for regeneration. Such priming makes hepatocytes responsive to growth factors (eg, HGF, EGF, heparin-binding EGF, and amphiregulin)7,30,31 which stimulate progression of the cell cycle.7–9 Early signaling events lead to activation of secondary or delayed gene responses (eg, expression of cyclins and activation of cyclin kinases) and progression of the cell cycle. Other cytokines and factors, such as TGF-β, CDKIs, retinoblastoma proteins, suppressor of cytokine signaling-3, and the p53 gene, inhibit proliferation responses.7 Appropriate regeneration requires orchestrated functions of different elements not only in appropriate amounts but also at the appropriate location and time.
After living donor LT, serum HGF and TGF-β increase.32,33 HGF, TNFα, and IL-6 also increase after partial LT in rodents.5,34 These major regenerative cytokines and growth factors are higher in quarter-size liver grafts that fail to regenerate than in half-size grafts that regenerate rapidly.5 Therefore, inhibition of regeneration in quarter-size grafts is unlikely due to insufficiency of proregenerative HGF, TNFα, and IL-6 formation.5 Rather, suppression of regeneration of small-for-size liver grafts is associated with inhibition of proliferative c-Jun N-terminal kinase and activating protein-1 activation at an early stage after LT.5 Because the speed of the regenerative responses depends on a balance of proliferative and inhibitory factors, in this study, we further investigated whether TGF-β signaling is involved in the suppression of small-for-size liver graft regeneration.
TGF-β is a potent growth inhibitor in a variety of cell types.8,10,18,35 After the transplantation of quarter-size grafts, TGF-β1 increased dramatically at 18 hours after the operation and remained elevated for at least 38 hours (Fig. 1). This higher and sustained increase of TGF-β1 was associated with suppression of regeneration, and this suggests that TGF-β may play an important role in the inhibition of regeneration (Fig. 3 and Fig. 4). Reactive oxygen species, which increase dramatically in failing small-for-size liver grafts,23 enhance the synthesis and activation of TGF-β.36,37 Therefore, it is not surprising that TGF-β increases to a greater extent in small-for-size liver grafts.
Two major signaling cascades mediate the biological and pathological effects of TGF-β, namely, the Smad and Ras/mitogen-activated protein kinase pathways.18 Smads can be divided into 3 groups on the basis of their structure and function: the receptor-activated Smads, including Smads 1 to 3, 5, and 8; the common-partner Smads, including Smad4, Medea, and Sma-4; and the inhibitory Smads, including Smads 6/7 and Dad.14,16,38–41 Smads mediate the signaling of several different members of the TGF-β superfamily.14,15,42 Activation of TGF-β receptor-I leads to phosphorylation of receptor-activated Smads (Smad2 and Smad3), which form a complex with Smad4, a common-partner Smad. The Smad2/3-Smad4 complex translocates into the nucleus and activates target genes that negatively regulate the cell cycle.16 Smad7, an inhibitory Smad, associates stably with the TGF-β receptor complex and inhibits TGF-β–dependent phosphorylation of Smad2 and Smad3.14,19
In the present study, we delivered the Smad7 gene by an adenoviral vector to overexpress Smad7 in the liver (Fig. 2). Without Smad7 gene delivery, liver regeneration was inhibited in quarter-size liver grafts, as shown by suppression of BrdU incorporation, PCNA expression, and graft weight gain (Fig. 3 and Fig. 4). Suppression of liver regeneration was associated with translocation of phosphorylated Smad2/3 to the nucleus (Fig. 6). Gene delivery of Smad7 largely blocked Smad2/3 activation and nuclear translocation (Fig. 6) and markedly improved the regeneration and functional recovery of quarter-size liver grafts (Fig. 3, Fig. 4, and Fig. 5). These results are consistent with the conclusion that TGF-β/Smad signaling plays an important role in the suppression of regeneration of small-for-size liver grafts.
SnoN is a negative regulator of TGF-β signaling. Therefore, we investigated whether Ad-Smad7 exerts it effects by increasing SnoN. To the contrary, we observed an increase in SnoN in small-for-size grafts that was decreased by Ad-Smad7. Therefore, prevention of the suppression of small-for-size liver graft regeneration by Ad-Smad7 is not mediated by increasing nuclear SnoN. The relation of SnoN and TGF-β is complex. In the absence of TGF-β, SnoN interacts directly with Smad2/Smad3-Smad4 complexes and recruits the nuclear hormone receptor corepressor/mSin3A/histone deacetylase complex to Smads, thus repressing TGF-β signaling.24,43 With TGF-β treatment, SnoN is rapidly degraded via the ubiquitin-proteasome pathway,44,45 and this leads to the dissociation of SnoN from the Smads, thus allowing the TGF-β signal to pass through. However, a longer TGF-β treatment induces SnoN messenger RNA and increases SnoN expression46; this indicates that TGF-β signaling also controls SnoN expression. This may exert a negative feedback to limit TGF-β’s effects. Increased SnoN expression in small-for-size grafts may reflect a response of the liver to increased TGF-β levels in an attempt to limit TGF-β’s effects. Because Smad7 blocks TGF-β signaling, it likely also decreases TGF-β–dependent induction of SnoN.
Role of CDKIs in the Suppression of Regeneration of Small-for-Size Liver Grafts
Progress through the cell cycle is controlled by cyclins and protein kinase complexes of CDKs, which phosphorylate their downstream targets on serines and threonines.47,48 Cyclin/CDKs hyperphosphorylate retinoblastoma gene products, leading to the transcription of a number of genes required for cell cycle progression.49,50 CDKIs inhibit cyclin/CDKs, leading to cell cycle arrest. In some cells, TGF-β up-regulates the expression of the CDKIs p15Ink4B, p27Kip1, and p21Cip1.36,51 p21Cip1, a potent universal growth inhibitor, forms complexes with cyclin D–Cdk4/6, cyclin E–Cdk2, and cyclin A–Cdk2 to inhibit their activities.52,53 Expression of p21Cip1 depends on p53 in some cell lines but is independent of p53 in some other cell lines.54–57
In this study, we investigated the effects of Ad-Smad7 on CDKI expression after LT. CDKIs p27Kip1, p15Ink4B, and p16Ink4A were not different between sham-operated livers, full-size liver grafts, and quarter-size grafts (Fig. 7A). By contrast, p21Cip1was barely detectable in sham-operated livers and full-size grafts but increased markedly in quarter-size grafts (Fig. 7). After treatment with Ad-Smad7 to block TGF-β/Smad signaling, expression of p21Cip1 was blunted (Fig. 7A,B). Expression of p53 was not altered in all groups studied. These results indicate that TGF-β inhibits regeneration of small-for-size liver grafts, most likely by up-regulating CDKI p21Cip1 in a p53-independent manner. This up-regulation of p21Cip1 by TGF-β is mediated by the Smad signaling pathway.
Taken together, our results indicate that TGF-β increases after the transplantation of small-for-size liver grafts and likely plays an important role in the suppression of liver regeneration. Failure of liver regeneration is likely mediated by activation of the Smad signaling pathway that up-regulates CDKI p21Cip1, leading to cell cycle arrest. Therefore, anti–TGF-β therapy holds promise as a new strategy for improving the regeneration of small-for-size grafts clinically. However, TGF-β is a cytokine that has a variety of physiological and pathophysiological effects. Although inhibition of TGF-β could be therapeutic for some situations in which overproduction of TGF-β leads to diseases (eg, liver fibrosis and suppressed liver regeneration after massive liver resection and small-for-size LT), caution should be paid to the potential adverse effects of overexpression of Smad7 related to the beneficial effects of TGF-β, such as wound healing and suppression of tumor growth, especially in small-for-size LT patients with a previous history of hepatic carcinoma because a previous study showed that small-for-size LT increases the risk of tumor invasion and migration.58 In our short-term studies, we did not observe adverse effects. However, long-term survival studies would be needed in the future to investigate any potential adverse effects of overexpression of Smad7.
Because protein expression of adenoviral gene delivery peaks at 2 to 3 days, whereas TGF-β increases within 18 hours after small-for-size LT, delivery of Ad-Smad7 at the same time as LT or after small-for-size syndrome develops would likely not achieve protection as satisfactory as that achieved by predelivery of the gene. Nonetheless, our study illustrates the important role played by TGF-β in the suppression of regeneration of small-for-size liver grafts. On the basis of this observation, TGF-β inhibitors and neutralizing antibodies may prove to be effective as therapy against small-for-size liver syndrome. Future studies will be needed to determine the appropriate dose and time frame for such therapeutic use of TGF-β inhibitors.
Acknowledgments
This study was supported in part by grants DK70844 and DK037034 from the National Institutes of Health.
Abbreviations
- Ad-LacZ
adenovirus carrying LacZ
- Ad-Smad7
adenovirus carrying human Smad7
- BrdU
5-bromo-2′-deoxyuridine
- CDK
cyclin-dependent kinase
- CDKI
cyclin-dependent kinase inhibitor
- EGF
epidermal growth factor
- HA
hemagglutinin
- HGF
hepatic growth factor
- IL-6
interleukin 6
- LT
liver transplantation
- p53
tumor protein p53
- PCNA
proliferating cell nuclear antigen
- SnoN
Ski-like oncogene
- TGF
transforming growth factor
- TNFα
tumor necrosis factor-α
REFERENCES
- 1.Renz JF, Emond JC, Yersiz H, Ascher NL, Busuttil RW. Split-liver transplantation in the United States: outcomes of a national survey. Ann Surg. 2004;239:172–181. doi: 10.1097/01.sla.0000109150.89438.bd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Strong RW. Living-donor liver transplantation: an over-view. J Hepatobiliary Pancreat Surg. 2006;13:370–377. doi: 10.1007/s00534-005-1076-y. [DOI] [PubMed] [Google Scholar]
- 3.Sugawara Y, Makuuchi M, Takayama T, Imamura H, Dowaki S, Mizuta K, et al. Small-for-size grafts in living-related liver transplantation. J Am Coll Surg. 2001;192:510–513. doi: 10.1016/s1072-7515(01)00800-6. [DOI] [PubMed] [Google Scholar]
- 4.Tian Y, Graf R, Jochum W, Clavien PA. Arterialized partial orthotopic liver transplantation in the mouse: a new model and evaluation of the critical liver mass. Liver Transpl. 2003;9:789–795. doi: 10.1053/jlts.2003.50170. [DOI] [PubMed] [Google Scholar]
- 5.Zhong Z, Schwabe RF, Kai Y, He L, Yang L, Bunzendahl H, et al. Liver regeneration is suppressed in small-for-size liver grafts after transplantation: involvement of JNK, cyclin D1 and defective energy supply. Transplantation. 2006;82:241–250. doi: 10.1097/01.tp.0000228867.98158.d2. [DOI] [PubMed] [Google Scholar]
- 6.Rehman H, Connor HD, Ramshesh VK, Theruvath TP, Mason RP, Wright GL, et al. Ischemic preconditioning prevents free radical production and mitochondrial depolarization in small-for-size rat liver grafts. Transplantation. 2008;85:1322–1331. doi: 10.1097/TP.0b013e31816de302. [DOI] [PubMed] [Google Scholar]
- 7.Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213:286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–S53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 9.Streetz KL, Luedde T, Manns MP, Trautwein C. Interleukin 6 and liver regeneration. Gut. 2000;47:309–312. doi: 10.1136/gut.47.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scotté M, Masson S, Lyoumi S, Hiron M, Ténière P, Lebreton JP, Daveau M. Cytokine gene expression in liver following minor or major hepatectomy in rat. Cytokine. 1997;9:859–867. doi: 10.1006/cyto.1997.0273. [DOI] [PubMed] [Google Scholar]
- 11.Bissell DM, Wang S, Jarnagin WR, Roll FJ. Cell-specific expression of transforming growth factor-beta in rat liver. Evidence for autocrine regulation of hepatocyte proliferation. J Clin Invest. 1995;96:447–455. doi: 10.1172/JCI118055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakamura T, Tomita Y, Hirai R, Yamaoka K, Kaji K, Ichihara A. Inhibitory effect of transforming growth factor-beta on DNA synthesis of adult rat hepatocytes in primary culture. Biochem Biophys Res Commun. 1985;133:1042–1050. doi: 10.1016/0006-291x(85)91241-0. [DOI] [PubMed] [Google Scholar]
- 13.Barnard JA, Lyons RM, Moses HL. The cell biology of transforming growth factor beta. Biochim Biophys Acta. 1990;1032:79–87. doi: 10.1016/0304-419x(90)90013-q. [DOI] [PubMed] [Google Scholar]
- 14.Lonn P, Moren A, Raja E, Dahl M, Moustakas A. Regulating the stability of TGFbeta receptors and Smads. Cell Res. 2009;19:21–35. doi: 10.1038/cr.2008.308. [DOI] [PubMed] [Google Scholar]
- 15.Derynck R. TGF-beta-receptor-mediated signaling. Trends Biochem Sci. 1994;19:548–553. doi: 10.1016/0968-0004(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 16.Nakao A, Imamura T, Souchelnytskyi S, Kawabata M, Ishisaki A, Oeda E, et al. TGF-beta receptor-mediated signalling through Smad2, Smad3 and Smad4. EMBO J. 1997;16:5353–5362. doi: 10.1093/emboj/16.17.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang YE. Non-Smad pathways in TGF-beta signaling. Cell Res. 2009;19:128–139. doi: 10.1038/cr.2008.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yue J, Mulder KM. Transforming growth factor-beta signal transduction in epithelial cells. Pharmacol Ther. 2001;91:1–34. doi: 10.1016/s0163-7258(01)00143-7. [DOI] [PubMed] [Google Scholar]
- 19.Nakao A, Afrakhte M, Morén A, Nakayama T, Christian JL, Heuchel R, et al. Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta signalling. Nature. 1997;389:631–635. doi: 10.1038/39369. [DOI] [PubMed] [Google Scholar]
- 20.Tsukada S, Westwick JK, Ikejima K, Sato N, Rippe RA. SMAD and p38 MAPK signaling pathways independently regulate alpha 1 (I) collagen gene expression in unstimulated and transforming growth factor-beta-stimulated hepatic stellate cells. J Biol Chem. 2005;280:10055–10064. doi: 10.1074/jbc.M409381200. [DOI] [PubMed] [Google Scholar]
- 21.Zhong Z, Froh M, Wheeler MD, Smutney O, Lehmann TG, Thurman RG. Viral gene delivery of superoxide dismutase attenuates experimental cholestasis-induced liver fibrosis in the rat. Gene Ther. 2002;9:183–191. doi: 10.1038/sj.gt.3301638. [DOI] [PubMed] [Google Scholar]
- 22.Omura T, Ascher NL, Emond JC. Fifty-percent partial liver transplantation in the rat. Transplantation. 1996;62:292–293. doi: 10.1097/00007890-199607270-00023. [DOI] [PubMed] [Google Scholar]
- 23.Zhong Z, Connor HD, Froh M, Bunzendahl H, Lind H, Lehnert M, et al. Free radical-dependent dysfunction of small-for-size rat liver grafts: prevention by plant polyphenols. Gastroenterology. 2005;129:652–664. doi: 10.1016/j.gastro.2005.05.060. [DOI] [PubMed] [Google Scholar]
- 24.Sun Y, Liu X, Eaton EN, Lane WS, Lodish HF, Weinberg RA. Interaction of the Ski oncoprotein with Smad3 regulates TGF-β signaling. Mol Cell. 1999;4:499–509. doi: 10.1016/s1097-2765(00)80201-4. [DOI] [PubMed] [Google Scholar]
- 25.Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366:701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 26.Grana X, Reddy EP. Cell cycle control in mammalian cells: role of cyclins, cyclin dependent kinases (CDKs), growth suppressor genes and cyclin-dependent kinase inhibitors (CKIs) Oncogene. 1995;11:211–219. [PubMed] [Google Scholar]
- 27.Moustakas A, Pardali K, Gaal A, Heldin CH. Mechanisms of TGF-beta signaling in regulation of cell growth and differentiation. Immunol Lett. 2002;82:85–91. doi: 10.1016/s0165-2478(02)00023-8. [DOI] [PubMed] [Google Scholar]
- 28.Robson CN, Gnanapragasam V, Byrne RL, Collins AT, Neal DE. Transforming growth factor-beta 1 up-regulates p15, p21 and p27 and blocks cell cycling in G1 in human prostate epithelium. J Endocrinol. 1999;160:257–266. doi: 10.1677/joe.0.1600257. [DOI] [PubMed] [Google Scholar]
- 29.Zhong Z, Theruvath TP, Currin RT, Waldmeier PC, Lemasters JJ. NIM811, a mitochondrial permeability transition inhibitor, prevents mitochondrial depolarization in small-for-size rat liver grafts. Am J Transplant. 2007;7:1103–1111. doi: 10.1111/j.1600-6143.2007.01770.x. [DOI] [PubMed] [Google Scholar]
- 30.Webber EM, Godowski PJ, Fausto N. In vivo response of hepatocytes to growth factors requires an initial priming stimulus. Hepatology. 1994;19:489–497. [PubMed] [Google Scholar]
- 31.Fitzgerald MJ, Webber EM, Donovan JR, Fausto N. Rapid DNA binding by nuclear factor kappa B in hepatocytes at the start of liver regeneration. Cell Growth Differ. 1995;6:417–427. [PubMed] [Google Scholar]
- 32.Asakura T, Ohkohchi N, Satomi S. Changes of serum cytokines associated with hepatic regeneration after living-related liver transplantation. Transplant Proc. 2000;32:2199–2203. doi: 10.1016/s0041-1345(00)01634-1. [DOI] [PubMed] [Google Scholar]
- 33.Ninomiya M, Harada N, Shiotani S, Hiroshige S, Minagawa R, Soejima Y, et al. Hepatocyte growth factor and transforming growth factor beta 1 contribute to regeneration of small-for-size liver graft immediately after transplantation. Transpl Int. 2003;16:814–819. doi: 10.1007/s00147-003-0628-9. [DOI] [PubMed] [Google Scholar]
- 34.Tian Y, Jochum W, Georgiev P, Moritz W, Graf R, Clavien PA. Kupffer cell-dependent TNF-alpha signaling mediates injury in the arterialized small-for-size liver transplantation in the mouse. Proc Natl Acad Sci U S A. 2006;103:4598–4603. doi: 10.1073/pnas.0600499103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276:60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 36.Biasi F, Mascia C, Poli G. TGFbeta 1 expression in colonic mucosa: modulation by dietary lipids. Genes Nutr. 2007;2:233–243. doi: 10.1007/s12263-007-0053-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koli K, Myllarniemi M, Keski-Oja J, Kinnula VL. Transforming growth factor-beta activation in the lung: focus on fibrosis and reactive oxygen species. Antioxid Redox Signal. 2008;10:333–342. doi: 10.1089/ars.2007.1914. [DOI] [PubMed] [Google Scholar]
- 38.Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- 39.Piek E, Heldin CH, Ten DP. Specificity, diversity, and regulation in TGF-beta superfamily signaling. FASEB J. 1999;13:2105–2124. [PubMed] [Google Scholar]
- 40.Heldin CH, Miyazono K, Ten DP. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 41.Massague J, Gomis RR. The logic of TGFbeta signaling. FEBS Lett. 2006;580:2811–2820. doi: 10.1016/j.febslet.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 42.Attisano L, Wrana JL. Signal transduction by members of the transforming growth factor-beta superfamily. Cytokine Growth Factor Rev. 1996;7:327–339. doi: 10.1016/s1359-6101(96)00042-1. [DOI] [PubMed] [Google Scholar]
- 43.Akiyoshi S, Inoue H, Hanai J, Kusanagi K, Nemoto N, Miyazono K, Kawabata M. c-Ski acts as a transcriptional co-repressor in transforming growth factor-beta signaling through interaction with Smads. J Biol Chem. 1999;274:35269–35277. doi: 10.1074/jbc.274.49.35269. [DOI] [PubMed] [Google Scholar]
- 44.Wan Y, Liu X, Kirschner MW. The anaphase-promoting complex mediates TGF-β signaling by targeting SnoN for destruction. Mol Cell. 2001;8:1027–1039. doi: 10.1016/s1097-2765(01)00382-3. [DOI] [PubMed] [Google Scholar]
- 45.Akiyoshi S, Inoue H, Hanai J, Kusanagi K, Nemoto N, Miyazono K, Kawabata M. TGF-β induces assembly of a Smad2-Smurf2 ubiquitin ligase complex that targets SnoN for degradation. Nat Cell Biol. 2001;3:587–595. doi: 10.1038/35078562. [DOI] [PubMed] [Google Scholar]
- 46.Stroschein SL, Wang W, Zhou S, Zhou Q, Luo K. Negative feedback regulation of TGF-β signaling by the SnoN onco-protein. Science. 1999;286:771–774. doi: 10.1126/science.286.5440.771. [DOI] [PubMed] [Google Scholar]
- 47.Roberts JM, Koff A, Polyak K, Firpo E, Collins S, Ohtsubo M, Massagué J. Cyclins, Cdks, and cyclin kinase inhibitors. Cold Spring Harb Symp Quant Biol. 1994;59:31–38. doi: 10.1101/sqb.1994.059.01.006. [DOI] [PubMed] [Google Scholar]
- 48.Morgan DO. Principles of CDK regulation. Nature. 1995;374:131–134. doi: 10.1038/374131a0. [DOI] [PubMed] [Google Scholar]
- 49.Sherr CJ, Roberts JM. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 50.Nelsen CJ, Rickheim DG, Timchenko NA, Stanley MW, Albrecht JH. Transient expression of cyclin D1 is sufficient to promote hepatocyte replication and liver growth in vivo. Cancer Res. 2001;61:8564–8568. [PubMed] [Google Scholar]
- 51.Hannon GJ, Beach D. p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature. 1994;371:257–261. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- 52.Sherr CJ, Roberts JM. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 1999;13:1501–1512. doi: 10.1101/gad.13.12.1501. [DOI] [PubMed] [Google Scholar]
- 53.Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–816. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 54.el-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, et al. WAF1, a potential mediator of p53 tumor suppression. Cell. 1993;75:817–825. doi: 10.1016/0092-8674(93)90500-p. [DOI] [PubMed] [Google Scholar]
- 55.el-Deiry WS, Harper JW, O’Connor PM, Velculescu VE, Canman CE, Jackman J, et al. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res. 1994;54:1169–1174. [PubMed] [Google Scholar]
- 56.Alpan RS, Pardee AB. p21WAF1/CIP1/SDI1 is elevated through a p53-independent pathway by mimosine. Cell Growth Differ. 1996;7:893–901. [PubMed] [Google Scholar]
- 57.Michieli P, Chedid M, Lin D, Pierce JH, Mercer WE, Givol D. Induction of WAF1/CIP1 by a p53-independent pathway. Cancer Res. 1994;54:3391–3395. [PubMed] [Google Scholar]
- 58.Man K, Lo CM, Xiao JW, Ng KT, Sun BS, Ng IO, et al. The significance of acute phase small-for-size graft injury on tumor growth and invasiveness after liver transplantation. Ann Surg. 2008;247:1049–1057. doi: 10.1097/SLA.0b013e31816ffab6XXX. [DOI] [PubMed] [Google Scholar]