Abstract
Homeostatic synaptic scaling is a form of synaptic plasticity that adjusts the strength of all of a neuron’s excitatory synapses up or down to stabilize firing. Current evidence suggests that neurons detect changes in their own firing rates through a set of calcium-dependent sensors that then regulate receptor trafficking to increase or decrease the accumulation of glutamate receptors at synaptic sites. Additional mechanisms may allow local or network-wide changes in activity to be sensed through parallel pathways, generating a nested set of homeostatic mechanisms that operate over different temporal and spatial scales.
Introduction
“Somehow the unstable stuff of which we are composed has learned the trick of maintaining stability”
- Walter Cannon, The Wisdom of the Body
If you are like me, your car probably needs a trip to the shop. A mechanic will spend hours mucking about under the hood and will charge you a lot of money, and for a little while your car will be fine—until, inevitably, it needs to go back to the shop again. In contrast, you have made it through your life thus far without bringing your brain in for a tune-up. This is remarkable, given that your brain is far more complex than the internal combustion engine. With billions of neurons each forming up to 100,000 synaptic connections, the mammalian brain is the most complex system in the known universe. Also unlike your car or your computer, your brain is not hard-wired but is constantly undergoing modifications to store information and adapt to changes in the environment. Nervous systems are thus faced with a fundamental problem: how to allow plastic mechanisms to shape their output and function, without compromising the stability and integrity of the underlying circuits that drive behavior. In other words, if brains are to work at all, they must be capable of assessing their own function and doing tune-ups on the fly.
This remarkable feat is accomplished through a set of “homeostatic” plasticity mechanisms that allow neurons to sense how active they are and to adjust their properties to maintain stable function (Davis and Bezprozvanny, 2001; Marder and Prinz, 2003; Turrigiano, 1999; Turrigiano and Nelson, 2004). Loosely defined, a homeostatic form of plasticity is one that acts to stabilize the activity of a neuron or neuronal circuit in the face of perturbations, such as changes in cell size or in synapse number or strength, that alter excitability. A large number of plasticity phenomena have now been identified in a wide range of systems that conform to this definition of homeostatic plasticity (Davis and Bezprozvanny, 2001; Marder and Prinz, 2003; Turrigiano, 1999; Turrigiano and Nelson, 2004). This review focuses on one form of homeostatic plasticity displayed by central glutamatergic neurons, called synaptic scaling, and its cellular and molecular mechanisms. A picture is beginning to emerge of how a cellular negative feedback system under the control of neuronal activity is implemented. Understanding when, where, and how homeostatic plasticity operates in the central nervous system is likely to generate important insights into how circuits adapt during experience-dependent plasticity, as well as the genesis of aberrant states, such as addiction or epilepsy, that involve adaptive plasticity or imbalances in synaptic excitation and inhibition.
The Stability Problem
Given the complexity of most central neural circuits, maintaining stability in function is a problem that permeates nearly every aspect of circuit development and plasticity. For example, setting excitation and inhibition to the proper levels so that activity can propagate through a network without either dying out or increasing uncontrollably into an epileptic-like state is not trivial (Turrigiano and Nelson, 2004). A second kind of stability problem arises in circuits that have plastic synapses (Figure 1A). Learning-related adaptations require neural networks to detect correlations between events in the environment and store these as changes in synaptic strength or other cellular properties (Abbott and Nelson, 2000). Examples of such adaptations include long-term potentiation (LTP) and long-term depression (LTD) (Abbott and Nelson, 2000; Malenka and Bear, 2004), which strengthen synaptic inputs that are effective at depolarizing the postsynaptic neuron and weaken inputs that are not, thus reinforcing useful pathways in the brain. Despite their utility these mechanisms have a dark side that was appreciated as soon as theoreticians tried to use such rules to store information in simulated neural networks: synapses that are strengthened become more effective at depolarizing the postsynaptic neuron and will continue to be strengthened in an unconstrained positive feedback cycle, eventually driving neuronal activity to saturation (Abbott and Nelson, 2000; Miller, 1996; Miller and MacKay, 1994). In addition, because of this positive feedback the synapse-specificity of these synaptic plasticity mechanisms breaks down. As correlated activity of presynaptic and postsynaptic neurons drives strengthening of specific synapses, the postsynaptic neuron will be driven more strongly, and so presynaptic inputs that were initially only poorly correlated with postsynaptic firing will be better able to trigger firing of the postsynaptic neuron, and they too can become strengthened even without a triggering environmental stimulus (Figure 1B). The many forms of plasticity based on correlated activity of presynaptic and postsynaptic neurons that have now been described biologically are each likely to carry with them their own unique destabilizing influences (Abbott and Nelson, 2000). This implies that nervous systems must have a matching set of plasticity mechanisms that counteract these destabilizing forces.
Figure 1. Mechanisms of Synaptic Plasticity Are Potentially Destabilizing.
A) Correlated presynaptic and postsynaptic firing induces long-term potentiation (LTP), which then allows the presynaptic neuron to drive the postsynaptic neuron more strongly. This increases the correlation between presynaptic and postsynaptic activation, which drives more LTP, and so on in an unconstrained positive feedback cycle. B) Unconstrained LTP will lose synapse-specificity, because when one input undergoes LTP and drives the postsynaptic neuron more strongly, it makes it easier for other inputs to make the postsynaptic neuron fire, and they begin to undergo LTP as well. C) Homeostatic synaptic scaling prevents this runaway potentiation. When LTP of one input increases postsynaptic firing, synaptic scaling will reduce the strength of all synaptic inputs until the firing rate returns to control levels. Note that synaptic strengths are reduced proportionally, so that the relative strength of the potentiated synapse remains the same.
Fortunately, there is a simple and very general solution to many aspects of this stability problem. Theoretical work has suggested that if each neuron can sense how active it is and adjust its synaptic weights up or down to keep this activity close to some set-point value, network activity will remain stable in the face of correlation-based changes in synaptic strength or developmental changes in synaptic connectivity (Figure 1C; Miller, 1996; Sullivan and de Sa, 2006). A decade ago, just such a mechanism was discovered in neocortical neurons and dubbed “synaptic scaling” because it was observed to globally scale all of a neuron’s synapses up or down in strength in the correct direction to stabilize neuronal firing (Turrigiano et al., 1998). This observation suggested that average neuronal activity, like many other physiological variables, is subject to classical homeostatic feedback control that stabilizes it around some set-point value (Cannon, 1932). There are various ways to build such a negative feedback system, but at the minimum it likely requires that neurons sense some aspect of activity, integrate this measure over a time-step that is long (minutes to hours) relative to the time scale of information transfer (milliseconds to minutes), and adjust synaptic properties to minimize the difference between this value and an activity set-point (Davis, 2006). In the discussion below I describe our current state of understanding about what aspect of neuronal or network activity is sensed by neurons during homeostatic synaptic scaling, the signaling pathways that are triggered by this change in activity, and how changes in synaptic strength are implemented.
Synaptic Scaling: the Phenomenon
Synaptic scaling was first described in cultured neocortical neurons, where it was observed that perturbing network activity generated compensatory changes in synaptic strength that returned average firing rates back to control values (Turrigiano et al., 1998). These cultures form networks of excitatory pyramidal and inhibitory GABAergic neurons that generate robust spontaneous activity. Blocking a fraction of inhibition initially increases firing rates, but over a time-scale of many hours firing rates return to control values. Similarly, when cultured hippocampal neurons are transfected with an inwardly rectifying potassium channel to hyperpolarize them and reduce firing, over time firing rates recover despite the continued expression of the channel (Burrone et al., 2002). These experiments lend support to the notion that cortical and hippocampal pyramidal neurons have a target firing rate, and synaptic strengths are regulated to maintain these rates relatively constant in the face of perturbations in input. As discussed above, this provides a robust mechanism for generating stability in network function in the face of developmental or learning-related changes in synaptic input. An important issue is that such “firing rate homeostasis” has not yet been directly demonstrated in the intact central nervous system.
In principle, neurons could maintain stable firing rates through homeostatic regulation of many aspects of neuronal excitability. These possibilities include balancing inward and outward voltage-dependent conductances that determine firing properties [generally called “intrinsic excitability”, (Desai, 2003; Marder and Goaillard, 2006; Zhang and D.J., 2003)], regulating inhibitory and/or excitatory synaptic strength (Turrigiano and Nelson, 2004) or synapse number (Kirov et al., 2004), or by adjusting the ease with which other forms of plasticity can be induced (so-called “metaplasticity”, Abraham and Bear, 1996). Evidence suggests that all of these mechanisms can contribute to the homeostatic regulation of neuronal firing rates in central circuits, although not all mechanisms operate in all neurons at all developmental times (Desai et al., 2002; Maffei and Turrigiano, 2008; Turrigiano and Nelson, 2004).
Arguably the best understood form of homeostatic plasticity in the central nervous system is synaptic scaling of excitatory synapses, which has been demonstrated both in vitro and in vivo, including spinal neurons and neocortical and hippocampal pyramidal neurons (Lissin et al., 1998; O’Brien et al., 1998; Thiagarajan et al., 2005; Turrigiano et al., 1998). Pharmacological manipulations of activity induce bidirectional compensatory changes in the unit strength of glutamatergic synapses, over a time scale of many hours (Ibata et al., 2008; Sutton et al., 2006; Turrigiano et al., 1998). This can be measured by recording miniature excitatory postsynaptic currents (mEPSCs, or “minis”), which represent the postsynaptic response to release of individual vesicles of neurotransmitter, and can be considered a measure of the unit strength of a synapse. By measuring minis arising from many synapses onto the same neuron, it was observed that modulating network activity increased or decreased the entire amplitude distribution of mEPSCs uniformly, in effect scaling synaptic strength up or down (Turrigiano et al., 1998). Such a scaling process has the attractive property of allowing neurons to normalize firing without changing the relative strength of synaptic inputs, thus avoiding disrupting information storage or processing mechanisms that rely on differences in synaptic weights. Interestingly, the rules for synaptic scaling depend on the synapse type: inhibitory synapses onto pyramidal neurons are scaled in the opposite direction from excitatory synapses, suggesting that firing rate is regulated through reciprocal changes in excitation and inhibition (Kilman et al., 2002; Swanwick et al., 2006).
How do neurons accomplish this global negative feedback control of synaptic strength? The best current evidence suggests that neurons can detect changes in their own firing rates through a set of calcium-dependent sensors that then regulate receptor trafficking to increase or decrease the accumulation of glutamate receptors at synaptic sites (Figure 2). Additional mechanisms may allow local or network-wide changes in activity to be sensed through parallel pathways, generating a nested set of homeostatic mechanisms that operate over different temporal and spatial scales.
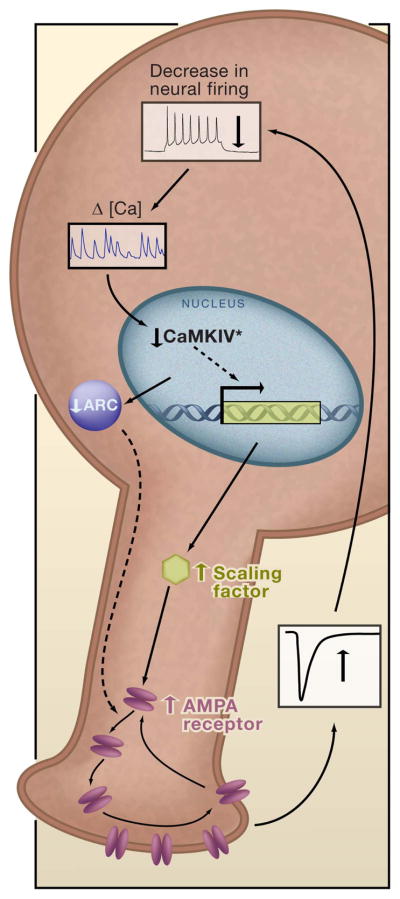
Figure 2. A Feedback Mechanism for Regulating Synaptic Strength.
A drop in neuronal firing leads to a drop in somatic calcium. This decreases the amount of activated CaMKIV (CaMKIV*) in the nucleus, and increases transcription of a “Scaling Factor” that enhances AMPA receptor accumulation at synapses through an unknown mechanism. This increases excitatory synaptic strength and raises firing rates back to target levels. There may be several signaling pathways that can act in parallel to generate synaptic scaling (dashed arrows); for example, decreased neuronal excitation will decrease the immediate early gene product Arc; reduced Arc levels increase AMPA receptor accumulation by reducing endocytosis.
Induction of Synaptic Scaling: Local Versus Global Mechanisms
Homeostatic adjustments in excitatory synaptic strength require that neurons sense some aspect of “activity” and translate changes in this activity into compensatory changes in synaptic strength, but the nature of the activity signal that controls synaptic scaling, has been a matter of debate. Neurons could sense changes in their own firing rate and globally scale synaptic weights up or down to compensate (Turrigiano and Nelson, 2004); alternatively, local changes in synaptic signaling could induce local homeostatic changes in synaptic transmission (Hou et al., 2008; Sutton et al., 2006); and finally, synaptic scaling could require widespread changes in network activity, perhaps through activity-dependent release of a soluble factor by many neurons or glia simultaneously (Rutherford et al., 1998; Stellwagen and Malenka, 2006). The standard paradigms for inducing synaptic scaling postsynaptically, including blockade or enhancement of network activity in culture (Lissin et al., 1998; O’Brien et al., 1998; Shepherd et al., 2006; Thiagarajan et al., 2005; Turrigiano et al., 1998) and sensory deprivation in the intact animal (Desai et al., 2002; Goel and Lee, 2007; Maffei et al., 2004). These approaches modify the activity of every neuron in the network, and so have not been able to distinguish between these various possibilities.
Differentiating between these possibilities is technically difficult. For example, to test the sufficiency of changes in postsynaptic firing in the induction of synaptic scaling it is necessary to manipulating firing in an individual neuron without affecting the activity of other neurons in the network, while monitoring changes in synaptic strength. A major expression mechanism of synaptic scaling is changes in the accumulation of synaptic glutamate receptors. Central synapses typically cluster both AMPA receptors and NMDA receptors. AMPA receptors are ionotropic and carry the majority of excitatory synaptic current in the central nervous system; NMDA receptors are also ionotropic but open as a function of voltage, flux calcium, and mediate a number of calcium-dependent forms of synaptic plasticity (Malenka and Bear, 2004). Synaptic scaling results in postsynaptic changes in both types of glutamate receptors (Lissin et al., 1998; O’Brien et al., 1998; Shepherd et al., 2006; Turrigiano et al., 1998; Stellwagen and Malenka, 2006; Watt et al., 2000; Wierenga et al., 2005), and can therefore be monitored by measuring changes in receptor accumulation at synapses.
In hippocampal cultures, chronic hyperpolarization of individual neurons through expression of an inwardly rectifying K channel (Kir) was observed to induce a presynaptic form of homeostatic plasticity (Burrone et al., 2002; Murthy et al., 2001). However, because this manipulation hyperpolarizes both somatic and dendritic regions of the neuron, this approach cannot distinguish cleanly between a critical role for somatic versus local dendritic depolarization in the induction of homeostatic plasticity. To circumvent this problem, a recent study used microperfusion of neuronal somata to pharmacologically block postsynaptic firing while leaving presynaptic firing intact, and monitored synaptic scaling optically using fluorescently-tagged AMPA-type glutamate receptors (Figure 3A, Ibata et al., 2008). Inhibiting postsynaptic spikes with local perfusion at the soma of tetrodotoxin (which blocks voltage-gated sodium channels) was sufficient to increase synaptic accumulation of AMPA receptors throughout the dendrites; in contrast acute pharmacological blockade of either presynaptic release or postsynaptic glutamate receptor activation at a subset of synapses did not induce local scaling at the blocked synapses (Ibata et al., 2008). The effects of somatic block of spikes and of global block of network activity with tetrodotoxin were similar, strongly suggesting that blocking postsynaptic spikes is sufficient to induce full-blown scaling up of synaptic strengths. Whether scaling down of synaptic strengths during elevated activity is also controlled by postsynaptic firing has not been investigated.
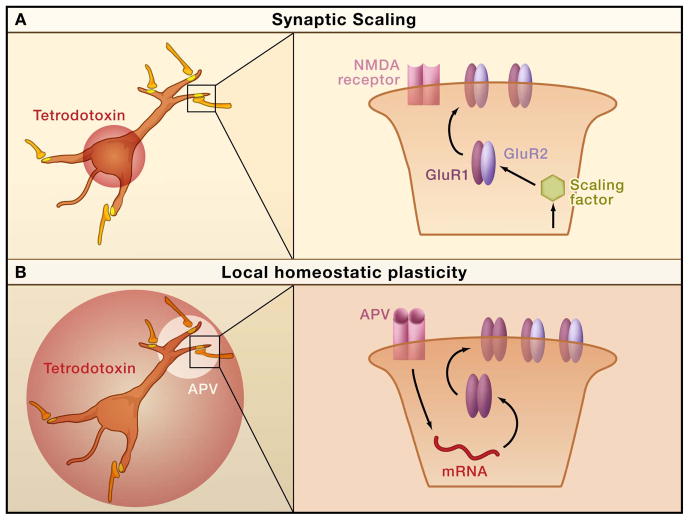
Figure 3. Distinct Mechanisms of Global and Local Changes in Synaptic Strength.
A. Blocking postsynaptic firing while leaving presynaptic and network activity intact scales up synaptic strengths in the dendrites, via a mechanism that results in increased accumulation of both GluR1 and GluR2 subunits of AMPA receptors. B. When action potential firing is blocked and NMDA receptor activation is locally blocked with the antagonist APV, there is a local increase in synaptic GluR1 accumulation that requires local dendritic protein synthesis.
Several recent studies have used genetic disruption of synaptic transmission to test the role of local glutamate receptor signaling in homeostatic plasticity. In keeping with the results of local pharmacological synaptic blockade described above, two studies found that prolonged disruption of presynaptic release (leaving other synapses intact and thus the postsynaptic neuron active) did not locally increase accumulation of glutamate receptors, and in fact reduced accumulation relative to nearby unblocked synapses (Ehlers et al., 2007; Harms and Craig, 2005). However, a third study found that a reduction in neurotransmitter release due to chronic hyperpolarization of presynaptic neurons did induce a local increase in receptor accumulation (Hou et al., 2008); it is not clear why these studies differ. Interestingly, when global application of tetrodotoxin is combined with local pharmacological blockade of glutamate receptors, there is a local acceleration of AMPA receptor accumulation that requires local protein synthesis in the dendrites (Sutton et al., 2006; Sutton et al., 2007), suggesting that miniature synaptic transmission acts as a local brake on the accumulation of AMPA receptors under conditions when action potentials are also blocked (Figure 3B). Taken together, these studies suggest the coexistence of two distinct plasticity mechanisms: a global mechanism that is triggered by changes in postsynaptic firing (synaptic scaling), and a local mechanism that is triggered when action potentials and glutamate receptors are blocked simultaneously. It is not entirely clear when this second mechanism for locally increasing glutamate receptor accumulation would come into play. The combined absence of action potential firing and presynaptic quantal release might occur during early development as synapses are just forming, during pathological conditions if inputs have been lost or networks have been completely silenced, or perhaps in response to neuromodulators.
Global and local homeostatic plasticity are likely to interact with forms of plasticity such as LTP and LTD rather differently. A global process that scales synaptic strength up or down proportionally allows neurons to stabilize firing without changing the relative strength of synaptic inputs, so that information stored by changes in synaptic weight induced by synapse-specific forms of plasticity like LTP an LTD are not disrupted. How a local homeostatic mechanism will interact with LTP and LTD depends on just how local it is. A very local mechanism (operating on individual synapses) would tend to erase the effects of LTP or LTD; such a “forgetting” mechanism might be useful if it could be selectively turned on or off (for example, by neuromodulators), but otherwise would be counterproductive. On the other hand, a “quasi-local” mechanism that operates on groups of synapses distributed along a dendritic branch could have useful computational properties, as it would allow one dendritic branch to act relatively independently of other branches (Rabinowitch and Segev, 2008). Whether blocking action potentials and glutamate receptor activation simultaneously produces local or quasi-local homeostatic synaptic plasticity is not known.
Homeostatic Regulation of AMPA Receptor Trafficking
A straightforward way to regulate glutamatergic synaptic strength is to increase or decrease the accumulation of glutamate receptors in the postsynaptic membrane (Figure 2, 3). Synaptic glutamate receptors are highly dynamic, and are constantly trafficking between extrasynaptic and synaptic compartments, where they can be tethered through association with synaptic scaffolding proteins. This process is highly regulated and many forms of synaptic plasticity, including some forms of LTP and LTD, are induced through changes in one or more of these trafficking and tethering steps that results in changes in receptor number at the synapse (Malinow and Malenka, 2002; Newpher and Ehlers, 2008). There is consensus across studies from cultured hippocampal, cortical, and spinal neurons that a major expression mechanism of synaptic scaling is changes in the accumulation of postsynaptic AMPA receptors (Lissin et al., 1998; O’Brien et al., 1998; Shepherd et al., 2006; Turrigiano et al., 1998; Stellwagen and Malenka, 2006; Wierenga et al., 2005) and NMDA receptors (Mu et al., 2003; Rao and Craig, 1997; Watt et al., 2000). Additionally, in cortical neurons AMPA and NMDA receptors are modified in a proportional manner by scaling up or down, through a mechanism that is not understood (Watt et al., 2004; Watt et al., 2000). Under some circumstances these postsynaptic changes may be accompanied by additional presynaptic changes in synaptic function (Branco et al., 2008; Burrone et al., 2002; De Gois et al., 2005; Erickson et al., 2006; Thiagarajan et al., 2005; Wierenga et al., 2006), discussed in the following section.
Most work on synaptic scaling has focused on changes in AMPA receptor accumulation. AMPA receptors are heterotetramers composed of subunits GluR1-4 (Greger et al., 2007), and each of these subunits confer different properties on the receptor (Isaac et al., 2007) and can be regulated differently by interactions with scaffolding and signaling molecules (Bredt and Nicoll, 2003). Most synapses onto cortical pyramidal neurons contain GluR1 and GluR2 (Jonas et al., 1994; Kumar et al., 2002; Wenthold et al., 1996; Wierenga et al., 2005) but the extent to which synaptic receptors are homomers or heteromers of these two subunits, and the degree to which subunit composition can be regulated by various forms of activity-dependent plasticity, is currently under debate (Adesnik and Nicoll, 2007; Gray et al., 2007; Plant et al., 2006). There is general agreement that synaptic scaling is induced by changes in AMPA receptor accumulation, whereas there is less agreement on the subunit composition of the newly accumulated receptors. Several studies have reported that synaptic scaling operates on GluR2-containing AMPA receptors (Cingolani et al., 2008; O’Brien et al., 1998; Wierenga et al., 2005), but several others have reported enhanced GluR1 accumulation with smaller or absent changes in GluR2 (Ju et al., 2004; Thiagarajan et al., 2005; Sutton et al., 2006).
This discrepancy between studies likely reflects the existence of two mechanistically distinct forms of homeostatic plasticity, a global mechanism (synaptic scaling) and a local mechanism, triggered by different methods of activity-deprivation. Synaptic scaling can be induced by changes in postsynaptic firing alone without requiring changes in NMDA receptor activation (Leslie et al., 2001; Turrigiano et al., 1998), and activity-deprivation through the application of tetrodotoxin induces a coordinated change in GluR1 and GluR2 (Figure 3A, Wierenga et al., 2005). In contrast, a local enhancement of homeostatic plasticity is induced when postsynaptic firing and NMDA receptors are blocked simultaneously (Sutton et al., 2006), and treatment with tetrodotoxin (or CNQX, an AMPA receptor antagonist which also lowers firing) and NMDA receptor blockers together induces relatively selective increases in GluR1 (Figure 3B, Ju et al., 2004; Sutton et al., 2006; Thiagarajan et al., 2005). This suggests that these two forms of plasticity occur via distinct trafficking steps that result in synaptic accumulation of distinct AMPA receptor types. The GluR2 content affects the rectification, kinetics, and calcium permeability of AMPA receptors. As a consequence activity-dependent changes in this composition could have interesting effects on the function and plasticity of central synapses (Isaac et al., 2007; Thiagarajan et al., 2007). Understanding how and when these two forms of plasticity are activated within neural circuits in vivo is an important goal.
These studies highlight the interesting observation that several distinct forms of synaptic plasticity occur through changes in the trafficking and tethering of AMPA receptors at synaptic sites, and may target receptors with distinct compositions. For example LTP at Schaffer collateral synapses onto hippocampal CA1 neurons is mediated through rapid calcium-dependent insertion and synaptic accumulation of AMPA receptors, through a process that requires regulatory sequences on the GluR1, but not the GluR2, subunit (Hayashi et al., 2000; Malinow and Malenka, 2002; Shi et al., 2001). It is currently controversial whether the initial steps of LTP involve insertion of AMPA receptors lacking GluR2 into the postsynaptic density (Adesnik and Nicoll, 2007; Gray et al., 2007; Isaac et al., 2007; Plant et al., 2006). Local homeostatic plasticity induced by tetrodotoxin and NMDA receptors also targets GluR1, but the pathway linking altered activity to increased receptor insertion is mechanistically distinct from that underlying LTP in the CA1 region of the hippocampus, given that this form of LTP requires activation of NMDA receptors, whereas local homeostatic plasticity requires blockade of NMDA receptors. The receptor trafficking mechanisms underlying synaptic scaling are currently unknown, but are independent of NMDA receptor activation (Leslie et al., 2001; O’Brien et al., 1998; Turrigiano et al., 1998), and will likely involve mechanisms that target GluR1/2 heteromeric receptors.
In addition to these molecular distinctions, the temporal characteristics of LTP and synaptic scaling differ in important ways that are likely to have interesting mechanistic implications. Some forms of LTP and LTD are the result of rapid (within minutes) insertion or removal of synaptic AMPA receptors that result in relatively stable changes in synaptic strength at particular synapses (Malinow and Malenka, 2002). This initial rapid insertion does not require transcription. Synaptic scaling is fundamentally different: it appears to be a slow, cumulative, and dynamic form of plasticity where the number of synaptic AMPA receptors is continuously adjusted up or down to stabilize firing (Ibata et al., 2008; O’Brien et al., 1998; Turrigiano et al., 1998). Chronic changes in activity regulate the synaptic content of a large array of synaptic proteins in addition to glutamate receptors, suggesting that the overall protein composition of the postsynaptic density is adjusted by ongoing activity (Ehlers, 2003). Surprisingly, even the earliest phases of synaptic scaling (within the first 4 hours) are dependent on transcription (Ibata et al., 2008). These studies suggest that synaptic scaling operates through graded, transcription-dependent changes in the receptor trafficking and/or scaffolding machinery in a way that continuously adjusts the steady-state number of receptors in the synaptic membrane (Figure 2). This could scale synaptic strength up or down through changes in synaptic delivery, turnover, or tethering of AMPA receptors in the synaptic membrane, or possibly all of the above. How much of the receptor trafficking machinery is shared between LTP, local homeostatic plasticity, and synaptic scaling and how much is unique to each form of plasticity is currently an open question, but clearly there is more than one way to regulate synaptic AMPA receptor accumulation.
Presynaptic homeostatic plasticity
Synaptic strength is determined by a number of factors in addition to the number of receptors in the postsynaptic membrane. In particular, the number of presynaptic neurotransmitter release sites, and the probability that neurotransmitter vesicles will be released following an action potential (“release probability”), are also major determinants of synaptic strength. At the neuromuscular junction there is extensive evidence for homeostatic regulation of presynaptic function (Davis, 2006), but in central neurons this has been more contentious. Several studies have found that under some conditions changes in postsynaptic glutamate receptor number and in presynaptic release may cooperate to homeostatically regulate synaptic transmission (Burrone et al., 2002; Murthy et al., 2001; Thiagarajan et al., 2005; Wierenga et al., 2006), although these processes can clearly be dissociated and likely work via different mechanisms (Burrone et al., 2002; Wierenga et al., 2006; Tokuoka and Goda, 2008). Some differences between studies likely reflect differences in culture conditions; in younger cortical cultures (less than 3 weeks in vitro), chronic blockade of activity has no apparent effect on presynaptic function, whereas in older cultures activity-deprivation increases postsynaptic receptor accumulation, synapse number, and the probability of synaptic vesicle release from the presynaptic terminal (Wierenga et al., 2006). At the moment it is not clear why cortical neurons transition from a purely postsynaptic form of synaptic homeostasis to mixed pre and postsynaptic expression, or how these two forms of homeostatic plasticity interact. Presynaptic expression of homeostatic plasticity in cortical circuits has not yet been reported in vivo, whereas postsynaptic forms have (Desai et al., 2002; Maffei et al., 2004; Goel and Lee, 2006). Hence, it remains to be seen what contribution these presynaptic mechanisms make to experience-dependent plasticity.
Currently, not much is known about the signaling mechanisms that regulate presynaptic homeostasis at central synapses. Chronic hyperpolarization of the postsynaptic neuron can induce an increase in mEPSC frequency, suggesting that the relevant activity signal for these presynaptic changes is postsynaptic depolarization (Burrone et al., 2002). A recent study went a step further and showed that presynaptic inputs onto the same dendritic region have similar release probabilities, and that locally increasing dendritic depolarization produces a relatively rapid (within 2 hours) and local presynaptic reduction in release probability (Branco et al., 2008). This suggests that, as at the neuromuscular junction (Davis, 2006), retrograde or transsynaptic signaling of some sort is important for presynaptic homeostasis of central synapses. Such signaling could induce a “quasi-local” form of presynaptic homeostasis, with the spatial characteristics dependent on how far the retrograde signal spreads (Branco et al., 2008).
Signaling Pathways Underlying Synaptic Scaling
How are changes in neuronal activity translated into homeostatic changes in synaptic AMPA receptor number? Neurons must have internal sensors that can detect a perturbation in activity, and then modulate a signal transduction cascade that regulates AMPA receptor accumulation. A number of molecular pathways have now been proposed to mediate synaptic scaling (Table 1). These studies have used pharmacological manipulations to disrupt synaptic scaling by targeting particular molecules or pathways. In principle a target molecule could participate in synaptic scaling in several ways: 1) the target could itself be the “activity signal” that is correlated with a change in activity and triggers downstream signaling pathways; 2) the target could be a central component of a signaling cascade triggered by the activity signal; 3) the target could be part of the basic receptor trafficking machinery needed to express the plasticity; and 4) the target could modulate a pathway necessary for induction or expression of the plasticity. By “mediate”, workers generally mean category 1 or 2, but given the complexity of the synaptic machinery, many perturbations will likely fall into categories 3, and could reflect disruptions of general receptor trafficking and tethering process, rather than pathways specific to synaptic scaling. In addition, there is extensive cross-talk between signaling pathways, making it likely that many perturbations could be due to disruptions of molecules that are modulators rather than mediators of synaptic scaling. For example, complete loss of function or overexpression of a target could disrupt plasticity by changing the state of another core signaling pathway, even if the target does not normally participate in the induction or expression of plasticity under physiological conditions. This is not to imply that determining the molecular mechanism(s) of synaptic scaling is a hopeless enterprise, but simply that the function of a given target needs to be probed in multiple ways and the results carefully interpreted before concluding where it falls in the scheme above.
Table 1.
A sample of the molecular pathways that influence synaptic scaling
| Role | Scaling up or down | Citations | |
|---|---|---|---|
| Soluble released factors | |||
| BDNF | Released in activity-dependent manner | Reduced BDNF scales synapses up; changes in BDNF signaling cannot account for scaling down | Rutherford et al 1998 |
| TNFα | Released by glia when activity falls | Increased TNFα scales synapses up; scaling up | Stellwagen et al 2006, Kaneko et al., 2008 |
| Transsynaptic signaling molecules and cell adhesion molecules | |||
| β3 integrin | Surface levels of β3 integrin regulated by activity; regulates GluR2 endocytosis | β3 integrin is necessary for scaling up; scaling down not tested | Cingolani et al., 2008 |
| MHC1 | Synaptic localization; mRNA regulated by activity | Reduced levels of MHC1 attenuate scaling up; scaling down not tested | Goddard et al., 2007 |
| Intracellular signaling pathways | |||
| Intracellular Calcium | Somatic calcium levels reflect level of action potential firing | Blocking calcium influx scales up synaptic strengths and occludes the effects of activity-blockade | Ibata et al., 2008; Thiagarajan et al., 2005 |
| CaMKIV, CaMKs | CaMK activation levels regulated by calcium | Reduced activation of CaMKs and CaMKIV scales synapses up; scaling down not tested. | Ibata et al., 2008; Thiagarajan et al., 2002 |
| Arc | Activity-dependent changes in Arc protein regulates AMPA receptor endocytosis | Reduced Arc scales synapses up and occludes scaling; not necessary for scaling down. | Rial Verde et al., 2006; Shepherd et al., 2006 |
| Plk2, CDK5 | Increased signaling involved in synaptic scaling down | Activity-dependent degradation of Spar to regulate synaptic strength; involved in scaling down, scaling up not tested. | Seeburg et al., 2008 |
The first molecule suggested to be a homeostatic activity signal for synaptic scaling was brain-derived neurotrophic factor (BDNF, Rutherford et al., 1998). BDNF is thought to be released by cortical pyramidal neurons in an activity-dependent manner, and is important for cortical development and for expression of many forms of plasticity (Lu, 2003). Exogenous BDNF prevents the scaling up of synaptic strengths normally induced by chronic blockage of activity in cortical cultures. Further, preventing activation of endogenous BDNF receptors (by incubating cultures with a soluble form of the high-affinity BDNF receptor TrkB) mimics the effects of activity blockade, and scales synaptic strengths up (Rutherford et al., 1998). Together these data suggest that one pathway through which activity-blockade increases excitatory synaptic strengths is through a reduction in the amount of BDNF released by pyramidal neurons. Chronic treatment with exogenous BDNF under control conditions, however, does not decrease the amplitude of mEPSC onto cultured cortical pyramidal neurons (although it does increase mEPSC amplitude onto inhibitory interneurons, Rutherford et al., 1998), and blocking BDNF signaling does not prevent scaling down of mEPSCs by chronic depolarization (Leslie et al., 2001), arguing that enhanced BDNF release is not essential for homeostatic down-scaling of synaptic currents. This reflects an important theme that will be revisited below: currently most of the signaling pathways implicated in scaling up of synaptic strengths appear to be unnecessary for scaling down synaptic strengths.
An issue with this model is that in hippocampal culture systems chronic treatment with exogenous BDNF enhances mEPSC amplitude onto excitatory neurons (Copi et al., 2005; McLean Bolton et al., 2000), which appears inconsistent with a role in homeostatic scaling up of excitatory synapses. Although these studies did not examine the effects of chronic block of BDNF signaling, or of BDNF in the presence of tetrodotoxin, they do raise the issue of how general BDNF-dependent synaptic scaling is, and whether it might differ between cell types or developmental stages. Interestingly chronic elevation of BDNF enhances inhibitory synapse number and strength onto both cortical and hippocampal pyramidal neurons in culture (McLean Bolton et al., 2000; Rutherford et al., 1997; Swanwick et al., 2006). It also enhances the development of inhibition in intact cortex (Huang et al., 1999), suggesting the attractive possibility that the same signal reciprocally regulates excitation and inhibition to stabilize neuronal activity.
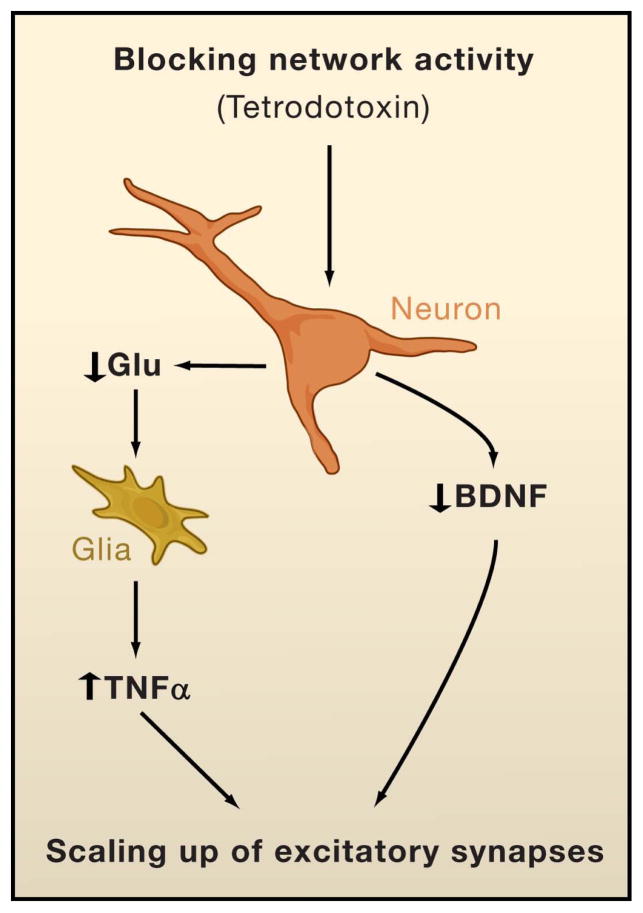
A second molecule suggested to serve as an activity signal for synaptic scaling is the cytokine tumor necrosis factor α (TNFα). TNFα was first characterized as an immune molecule that is part of the inflammatory response to pathological states such as injury, and can initiate cell death (Bessis et al., 2007). Application of TNFα to hippocampal or cortical cultures rapidly (within 15 minutes) increases mEPSC amplitude and surface number of AMPA receptors (Beattie et al., 2002; Stellwagen et al., 2005; Stellwagen and Malenka, 2006). Adding a soluble TNFα receptor to the medium to sop up endogenous TNFα blocks the ability of prolonged (48 hours) treatment with tetrodotoxin to scale up mEPSC amplitude, and TNFα conditioned medium from cultures treated for 2 days with tetrodotoxin increases mEPSC amplitude through a TNFα-dependent mechanism (Stellwagen and Malenka, 2006). Scaling down of synaptic strengths by enhanced activity is not prevented by blocking TNFα signaling; again suggesting that scaling up and scaling down are mediated through different pathways. Interestingly, the TNFα involved in scaling up synaptic strengths in response to tetrodotoxin originates from glia rather than neurons. Wild-type neurons grown on glia deficient in TNFα expression do not scale up synaptic strengths in response to 48 hours of tetrodotoxin treatment, whereas neurons lacking TNFα grown on wild-type glia do scale up (Stellwagen and Malenka, 2006). This indicates that the effects of TNFα on synaptic scaling are not cell-autonomous but require some interaction between neurons and glia (although which class of glial cell is not clear). The model proposed is that tetrodotoxin reduces glutamate release from neurons, which is sensed by glia and leads to increased glial release of TNFα that then acts on neurons to increase mEPSC amplitude. Blocking TNFα signaling also prevents scaling of miniature inhibitory postsynaptic currents (mIPSC) (Stellwagen and Malenka, 2006); thus increased TNFα and reduced BDNF in response to activity-blockade both work to reciprocally regulate the amplitudes of mEPSCs and mIPSCs (Figure 4).
Figure 4. Role of BDNF and TNFα in Synaptic Scaling.
Blocking network activity with tetrodotoxin reduces release of the brain-derived neurotrophic factor (BDNF) from neurons, and the cytokine tumor necrosis factor α (TNFα) from glial cells. A chronic (several days) reduction in BDNF and increase in TNFα both scale up excitatory synapses onto pyramidal neurons.
The early phase of synaptic scaling is fully expressed following a drop in postsynaptic firing, and does not require changes in the activity of entire networks (or even nearby groups of neurons) (Ibata et al., 2008). This raises a problem for both the BDNF and the TNFα models of synaptic scaling, as both models make the implicit assumption that synaptic scaling is induced by altered release of a soluble factor by many cells at once, which then acts broadly in the network to influence AMPA receptor accumulation and thus synaptic strength. It is possible that BDNF and TNFα normally function locally; pyramidal neurons could respond to their own BDNF in an autocrine fashion (Horch, 2004), and it is possible that interactions between individual neurons and glia might affect TNFα release locally, although this kind of interaction has not been demonstrated.
Another major issue with the model for TNFα mediated synaptic scaling is the time course of the enhancement of synaptic strength that is dependent on glial-derived TNFα. Tetrodotoxin treatment did not significantly increase levels of TNFα in culture medium until after 48 hours, and conditioned medium from tetrodotoxin treated cultures was not effective at increasing mEPSC amplitude prior to 48 hours of activity-blockade. In contrast, synaptic scaling is robustly observed after 4–24 hours of activity-blockade in the majority of studies, is a graded process that produces measurable changes in synaptic accumulation of AMPA receptors within a few hours, and synaptic strength continues to increase as the duration of activity-deprivation is prolonged (Ibata et al., 2008; Sutton et al., 2006; Thiagarajan et al., 2005; Turrigiano et al., 1998). TNFα can acutely enhance mEPSC amplitude within a few minutes, so the requirement for prolonged activity-blockade to induce TNFα dependent synaptic scaling is not due to slow effects of TNFα, but rather to delayed build-up of TNFα in the culture medium. This suggests that changes in TNFα release cannot account for the early phase of synaptic scaling, and raises the possibility that TNFα is not the “activity signal” that mediates synaptic scaling, but is instead necessary for maintenance or expression of synaptic scaling during prolonged activity-blockade.
One of the most ubiquitous and important “activity signals” in the nervous system is changes in intracellular calcium mediated by voltage-dependent channels, and this influx is crucial for induction of many forms of synaptic and intrinsic plasticity (Lisman et al., 2002; Malenka and Bear, 2004; Zhang and Linden, 2003). Modeling studies have shown that an efficient way to design a homeostatic feedback loop is to use an integrator of intracellular calcium, and adjust neuronal properties to keep this integrated calcium signal close to some “calcium set-point” (LeMasson et al., 1993; Liu et al., 1998; Marder and Prinz, 2002). Consistent with this notion, a drop in calcium influx through voltage-gated calcium channels triggers scaling up of synaptic strengths (Ibata et al., 2008; Thiagarajan et al., 2005). Somatic spikes trigger large transient increases in calcium in the soma, and blockade of these somatic calcium transients through somatic perfusion of calcium channel blockers was sufficient to induce synaptic scaling (Ibata et al., 2008). This suggest that a major pathway mediating the early stages of synaptic scaling is a drop in action potential firing that leads to reduced somatic calcium influx (Figure 2). This drop in somatic calcium influx generates an intracellular signal that leads to a gradual and cumulative increase in AMPA receptors at synaptic sites. This suggests that synaptic scaling does not act to stabilize average firing rate, but instead to stabilize somatic calcium. It is currently an unresolved issue whether the three potential activity signals described above (somatic calcium, BDNF, and TNFα) activate three different pathways that mediate or modulate synaptic scaling over different temporal and spatial scales, or whether these three signals intersect at some step in a single pathway for synaptic scaling (see below).
Once a change in activity is sensed, signaling through one or more transduction cascades must be increased or decreased, and changes in signaling through this cascade must result in altered AMPA receptor accumulation at synaptic sites. Calcium influx can be sensed by multiple calcium-dependent kinases, among them the calcium/calmodulin dependent protein kinase (CaMK) family. Pharmacological blockade of CaMKs with KN93 (which blocks CaMKI, II, and IV) prevents the effects of activity blockade on mEPSCs (Thiagarajan et al., 2002). Interestingly, chronic manipulations of activity modify the ratio of CaMKIIα and CaMKIIβ isoforms in hippocampal neurons, and changing this ratio through overexpression of one or the other isoform has complicated homeostatic effects on mEPSC amplitude, frequency, and kinetics. Changes in the ratio of CaMKIIα to CaMKIIβ can account for the effects of activity blockade on mEPSC frequency observed in some studies, but not on amplitude, suggesting that another CaMK is the critical player (Thiagarajan et al., 2002). A recent study suggested that CaMKIV may be the CaMK family member most intimately involved in postsynaptic scaling (Figure 2, Ibata et al., 2008). Like CaMKII, CaMKIV must autophosphorylate after calcium/calmodulin binding to become active, but must also be phosphorylated by CaM kinase kinase (CaMKK) (Soderling, 1999). CaMKIV has a strong nuclear localization, and there are high levels of activated CaMKIV under normal activity conditions (Ibata et al., 2008). This pool of active kinase is reduced by brief treatment with tetrodotoxin, whereas transfection with a dominant negative form of CaMKIV (or pharmacological inhibition of CaMKK) increases mEPSC amplitude and occludes the effects of tetrodotoxin (Ibata et al., 2008). CaMKIV is a transcriptional regulator, and transcription inhibitors block synaptic scaling downstream of CaMKIV activation. This study suggests that in neocortical neurons a drop in somatic calcium influx is sensed through a drop in activated nuclear CaMKIV, which then triggers a transcription-dependent homeostatic increase in synaptic AMPA receptor accumulation and mEPSC amplitude. In this model, CaMKIV acts as a transcriptional repressor (although not necessarily directly) to dampen expression of factors that enhance synaptic accumulation of AMPA receptors.
Yet another pathway recently implicated in synaptic scaling is activity-dependent expression of the immediate early gene Arc (Figure 2). Arc protein levels are bi-directionally regulated by the chronic changes in activity used to induce synaptic scaling in vitro (Shepherd et al., 2006). In cultured neurons or slices from mice lacking Arc AMPA receptor-mediated currents are larger (Rial Verde et al., 2006; Shepherd et al., 2006), although Plath et al., 2006 reported no change in baseline transmission. Scaling up of mEPSCs by tetrodotoxin is occluded by Arc knockout, whereas scaling down is not (Shepherd et al., 2006). The effects of Arc overexpression on AMPA-mediated synaptic transmission are more variable, with no effect in one study (Shepherd et al., 2006) and reduced transmission in another (Rial Verde et al., 2006). The effects of Arc appear to be mediated through an effect on AMPA receptor endocytosis, as Arc can directly interact with and influence the endocytic machinery involved in AMPA receptor recyling. However, there is disagreement on which subunits (GluR1 or GluR2/3) are targeted (Chowdhury et al., 2006; Rial Verde et al., 2006). These studies suggest that a drop in Arc protein could contribute to the build-up of synaptic AMPA receptors during synaptic scaling in response to activity-blockade. Conversely, environmental stimuli that strongly activate neurons and thus generate strong Arc expression should reduce surface AMPA receptor number and generate a homeostatic reduction in synaptic strength; but given that loss of Arc expression does not block synaptic scaling down by enhanced activity (Shepherd et al., 2006) this cannot be the exclusive pathway that mediates removal of AMPA receptors during synaptic scaling down. Whether Arc is an essential element of the signaling pathway coupling altered activity to synaptic scaling up, or extreme manipulations of Arc gum up the receptor recycling machinery necessary for synaptic scaling up, is not yet clear.
Several molecules important for synapse formation and structure have now been implicated in synaptic scaling. In mice bearing mutations that cause loss of function in the major histocompatibility complex class I, scaling up of mEPSC amplitude with tetrodotoxin is reduced (Goddard et al., 2007). Postsynaptic loss of the cell adhesion molecule β-catenin also greatly attenuates bidirectional synaptic scaling (Okuda et al., 2007). Finally, postsynaptic β3 integrins affect GluR2-dependent endocytosis and are necessary for synaptic scaling; treatment with tetrodotoxin increases surface levels of β3 integrin, over-expression of β3 integrin increases mEPSC amplitude, and β3 integrin knockout prevents tetrodotoxin-induced synaptic scaling (Cingolani et al., 2008). These effects could be due to rapid signaling roles for these various adhesion and immune molecules, or to disruption of synaptic structure or postsynaptic density scaffolds when these molecules are lost or reduced.
Most of the molecules or pathways described above are required during scaling up of synaptic strengths, but are not required for scaling down (although a few, such as CaMKIV and β3 integrin have not yet been testing during scaling down). This suggests that the signaling pathways and receptor trafficking machinery involved in scaling up and scaling down are different. A recent study found that activity-dependent induction of polo-like kinase 2 (Plk2) is important for scaling down of synaptic strengths during elevated activity (Seeburg et al., 2008). Plk2 is calcium-dependent, and when primed by CDK5 phosphorylation can bind to the scaffolding protein Spar and trigger its degradation. Plk2 is required for synaptic scaling down, dominant-negative CKD5 constructs also block scaling down, as do overexpressed Spar mutants that cannot be degraded by Plk2 phosphorylation (Seeburg et al., 2008). Together these data suggest that calcium influx during heightened neuronal activity triggers Plk2 and CDK5 activation, which phosphorylates Spar and leads to a reduction in mEPSC amplitude. Whether this pathway is also important in synaptic scaling up was not tested. It seems likely that the homeostatic control of synaptic strength is constructed as a push-pull mechanism, where the level of neuronal firing titrates the relative magnitude of two (or more) processes that have opposing effects on synaptic strength.
It is difficult to fit all of the data described above into a unified view of the signaling pathways underlying synaptic scaling. Some of these signaling pathways have known interactions; to give a few examples, BDNF can induce Arc expression under some conditions (Rao et al., 2006), and can activate CaMKIV (Minichiello et al., 2002, Spencer et al., 2008), suggesting that the ability of reduced BDNF signaling to scale up synaptic strengths in cortical cultures (Rutherford et al., 1998) could be due to reduced activation of CaMKIV and Arc. Integrin signaling can activate CDK5 in neuron-derived cell lines (Li et al, 2000), and one of the effects of TNFα is to rapidly increase surface levels of β3 integrin (Cingolani et al., 2008), raising the possibility that TNFα works through the integrin pathway to influence AMPA receptor accumulation. CaMKIV is necessary for calcium-dependent expression of TNFα in lymphocytes (Lobo et al., 1999), but whether this is a cell-autonomous interaction, or plays a similar role in neurons and/or glia, is unclear. There are undoubtedly many more such interactions between these various pathways that are yet to be described.
The study of homeostatic synaptic plasticity is still relatively young, and it is likely that this cast of molecular players will rapidly increase over the next few years. Rather than thinking of all of these potential signaling pathways as linear and independent, a more useful analogy may be the principle of “six degrees of separation”, where every element in a network is connected to every other element by way of only a few intermediaries. In this view, manipulating any element in a network can have far-reaching effects on a large number of other signaling elements, and ultimately on synaptic function and plasticity. It remains to be seen whether BDNF, TNFα, Arc, CaMKIV, Plk2, and others prove to be part of a core set of push-pull signaling pathways for synaptic scaling, or are situated such that manipulating their function modulates the function of other core signaling or trafficking elements.
Synaptic Scaling in the Visual Cortex
Homeostatic plasticity appears to stabilize circuit function in vivo in a number of organisms and brain areas (Davis and Bezprozvanny, 2001; Marder and Goaillard, 2006; Turrigiano, 1999). Synaptic scaling has been most thoroughly studied in vivo in the visual system, using standard visual deprivation paradigms to generate an analog in vivo of activity-blockade in culture. Visual cortical microcircuitry can be modified in an activity-dependent manner in response to changes in sensory experience (Katz and Shatz, 1996), and there is now mounting evidence that synaptic scaling plays important roles during various critical periods of visual system development. For example, during the second and third postnatal weeks when synaptogenesis is high, there is an inverse relationship between the frequency and amplitude of mEPSCs onto principal neurons in layer 4 of primary visual cortex (Layer 4 is the primary input layer to cortex, where the majority of thalamic inputs terminate). This suggests that as the number of excitatory synapses rises (therefore increasing mEPSC frequency) and visual input increases, synaptic strength is reduced through an activity-dependent homeostatic mechanism. This interpretation is strengthened by the observation that raising animals in the dark prevents the developmental decrease in mEPSC amplitude (Desai et al., 2002). Further, blocking activity in the optic nerve with tetrodotoxin for two days scales up mEPSC amplitude onto pyramidal neurons, and this increase in mEPSC amplitude is reversed when optic nerve activity is allowed to resume. This phenomenon looks phenotypically identical to the synaptic scaling induced in culture following activity-blockade (Desai et al., 2002), suggesting that is it indeed the same process. Interestingly, synaptic scaling in layer 4 turns off after the first postnatal week, and turns on in layer 2/3 (Desai et al., 2002), where it can be induced into adulthood (Goel and Lee, 2007; Maffei and Turrigiano, 2008). Visual cortex therefore is endowed with layer-specific critical periods for homeostatic plasticity.
In rodents, most of primary visual cortex is driven only by the contralateral eye (monocular cortex), but about 1/3 is binocular, and receives input from both eyes. When one eye is deprived (generally through eyelid suture) during the classical critical period there is a change in the ability of the two eyes to drive neurons within binocular cortex: cortical neurons rapidly (within two or three days) lose responsiveness to the deprived eye, and then (over the next several days) gain responsiveness to the non-deprived eye (Shatz, 1990). These changes have been ascribed to LTD and LTP (respectively) of excitatory inputs to cortical neurons (Malenka and Bear, 2004) but it has been suggested that the potentiation of responses to the non-deprived eye might arise instead through homeostatic mechanisms that boost the excitability of cortical neurons in response to a drop in sensory input (Kaneko et al., 2008; Mrsic-Flogel et al., 2007). A recent study used in vivo calcium imaging to monitor eye-specific activation of individual neurons within binocular layer 2/3, and found that binocularly-driven neurons maintained their overall level of responsiveness to the two eyes, so that the drop in the responsiveness to the deprived eye stimulation was compensated by an increase in responsiveness to non-deprived eye stimulation. Thus the net sensory drive to these neurons remained relatively constant, indicating overall response homeostasis (Mrsic-Flogel et al., 2007). A very similar response homeostasis following visual deprivation has been reported in the superior colliculus, a region of the midbrain that receives visual input (Chandrasekaran et al., 2007). Interestingly, in visual cortex, the population of neurons that were driven only by the deprived eye had stronger responses after deprivation, as did all neurons after binocular deprivation, both of which are more consistent with a global homeostatic mechanism than with an LTP-like mechanism (Mrsic-Flogel et al., 2007).
In support of the notion that synaptic scaling underlies gain of responsiveness to the non-deprived eye, blocking TNFα signaling in visual cortex (either in mice lacking TNFα or by infusion of a soluble TNFα receptor into cortex) had no effect on the loss of responsiveness to the deprived eye, but prevented the gain of responsiveness to the non-deprived eye (Kaneko et al., 2008). Ascribing this effect to impaired synaptic scaling rests on the assumption that loss of TNFα in vivo selectively blocks synaptic scaling without affecting the myriad other forms of synaptic plasticity that could contribute to response potentiation. TNFα is required for synaptic scaling but not for one form of cortical LTP in vitro (Kaneko et al., 2008), but it is not possible to rule out effects on the many other forms of Hebbian or homeostatic plasticity that have been identified in cortical neurons (Abbott and Nelson, 2000; Desai, 2003; Maffei and Turrigiano, 2008; Sjostrom et al., 2006). In addition it remains to be verified that lid suture induces synaptic scaling onto cortical pyramidal neurons within binocular cortex, and that this is prevented by blocking TNFα signaling in vivo.
Complicating the interpretation of these studies is the recent report that the mode of homeostatic plasticity within layer 2/3 during the critical period depends strongly on the method of visual deprivation. Most studies of synaptic scaling have been carried out in monocular cortex, where it has been observed that lowering visual drive through intraocular injections of tetrodotoxin and by rearing animals in the dark both induce synaptic scaling (Desai et al., 2002; Goel and Lee, 2007; Maffei and Turrigiano, 2008). In contrast, lowering visual drive through eyelid suture does not induce synaptic scaling in layer 2/3, but instead induces a homeostatic increase in the intrinsic excitability of layer 2/3 pyramidal neurons (Maffei and Turrigiano, 2008). Both forms of visual deprivation produce a compensatory increase in the spontaneous firing of layer 2/3 pyramidal neurons in acute slices derived from monocular visual cortex, suggesting that the microcircuit in layer 2/3 can use different forms of homeostatic plasticity to compensate for the loss of visual drive, depending on the mode of deprivation. Intraocular tetrodotoxin blocks both visually-driven and spontaneous retinal activity, whereas lid suture leaves spontaneous activity and some general light responsiveness intact and is very effective at driving LTD of excitatory synapses (Maffei and Turrigiano, 2008; Rittenhouse et al., 1999). One explanation for the difference between the effects of tetrodotoxin and lid suture is that synaptic scaling is unable to overcome the very strong LTD induced at layer 2/3 synapses during lid suture, and so other mechanisms, notably homeostatic regulation of intrinsic excitability, must be recruited to maintain response homeostasis (Maffei and Turrigiano, 2008). If a similar process occurs in binocular visual cortex, then the response homeostasis observed following lid suture (Mrsic-Flogel et al., 2007) is likely due to homeostatic intrinsic plasticity, rather than synaptic scaling. An interesting question is whether TNFα signaling is necessary for the expression of homeostatic intrinsic plasticity as well as synaptic scaling.
These studies highlight the notion that experience-dependent plasticity is unlikely to be explained by a single form of synaptic plasticity, but rather arises through a complex interplay between many forms of excitatory, inhibitory, and intrinsic mechanisms occurring at many sites within the cortical microcircuit. Understanding experience-dependent plasticity will likely require an integrated view of how these various forms of plasticity cooperate to modify microcircuit function. The existence of redundant forms of homeostatic plasticity may ensure that network compensation can be achieved in response to a wide range of sensory perturbations. A more complete mechanistic understanding of synaptic scaling will likely generate methods of selectively interfering with synaptic scaling without compromising other forms of plasticity, which should greatly facilitate parsing the particular role of synaptic scaling and other forms of plasticity in central nervous system function.
Concluding Remarks
Neurons use homeostatic synaptic scaling to stabilize their firing rates in the face of developmental or learning-induced changes in drive, and this contributes to the ability of central neuronal networks to “tune themselves up” and maintain stable function throughout life. From considerable work a rough outline is emerging of how such a biological homeostatic negative feedback system is designed. Several negative feedback pathways have been identified that could contribute to the stabilization of synaptic weights. One such pathway is outlined in Figure 2, and works in the following manner: a drop in neuronal firing leads to a decrease in somatic calcium ions, which reduces the activation of CaMKIV. Reduced CaMKIV activation in turn increases the transcription of one or more factors that regulate the steady-state number of AMPAR at synaptic sites. An increase in the amount of this factor leads to an increase in AMPAR accumulation, which then increases excitatory synaptic drive. This boosts neuronal firing, brings somatic calcium back into the desired range, and increases CaMKIV activation back to baseline levels. Scaling down could work either as the reciprocal of this (that is, too much activated CaMKIV scales synapses down), or could be achieved through an alternative pathway (such as the Plk2 pathway) that preferentially senses elevated activity and converges onto the same machinery.
This rough picture leaves many issues unresolved. For example, how does modulating activation of intracellular signaling molecules lead to a precise homeostatic adjustment in neuronal firing, without overshooting, oscillations, or drift in the system? From an engineering perspective, homeostatic negative feedback systems are most robust when implemented as an “integral feedback system”, where homeostatic compensation is controlled by an error signal that is the integral over some time-step of the difference between the set point and the actual value (see Davis, 2006; van Rossum et al., 2000). Given the dependence of synaptic scaling on somatic calcium, it is reasonable to suppose that the “difference” neurons are measuring is the deviation of somatic calcium from a target value; if and how neurons compute an integral of this difference is unclear.
A second important issue is how the activity “set-point” of a neuron is determined. One possibility is that the set-point is determined by the sensitivity of a calcium sensor (or sensors), so that only deviations away from the target internal calcium value trigger these sensors to generate homeostatic compensation. This would require the properties and amounts of these sensors to be precisely regulated, and such a system might not be terribly robust. Alternatively, the set point could arise from an equilibrium between several opposing forces regulating receptor accumulation. If lowering activity increases the amount of a factor that enhances receptor accumulation (Factor+), and raising activity increases the amount of a factor that reduces receptor accumulation (Factor−), then the firing rate at which these two forces equalize will be the activity set point (Figure 5). This model makes the interesting prediction that modulating the strength of one of these signaling pathways will shift the activity-set point to more positive or negative values. This could provide a simple means to “customize” the activity set point, so that different classes of neuron, or neurons at different developmental stages, can have different set points. It also suggests that disease states that involve disregulation of cortical activity, such as some forms of epilepsy, might be caused by changes in the balance between opposing signaling pathways for synaptic scaling that result in a shift in the activity set-point. Finally, building an activity set point in this way would benefit from the existence of multiple sensors and pathways. Adding additional pathways will only increase the robustness of the system, and make it less sensitive to variation in the expression of any given sensor. This may explain some of the apparent redundancy in signaling pathways that have been postulated to contribute to synaptic scaling.
Figure 5. Establishing a Set Point for the Rate of Neuronal Firing.
A firing rate set-point can be constructed out of two opposing synaptic scaling factors. Factor+ (solid red line) increases in activation as activity rises, and scales down the strengths of excitatory synapses strengths. Factor- (solid blue line) increases in activation as activity falls, and scales up synaptic strengths. The firing rate at which these two opposing forces equalize is the activity set-point (solid black circle). If one of these factors is reduced or increased in magnitude, the activity set-point will shift: for example, if Factor+ is reduced in magnitude (dashed blue line), the activity set-point will shift to a more negative value (solid gray circle).
Acknowledgments
I thank all current and former members of my lab and many collaborators and colleagues for their important contributions to these ideas. GGT is supported by NS 36853 and an NIH Director’s Pioneer Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott LF, Nelson SB. Synaptic plasticity: taming the beast. Nat Neurosci. 2000;3:1178–1183. doi: 10.1038/81453. [DOI] [PubMed] [Google Scholar]
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends In Neurosciences. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- Adesnik H, Nicoll RA. Conservation of glutamate receptor 2-containing AMPA receptors during long-term potentiation. J Neurosci. 2007;27:4598–4602. doi: 10.1523/JNEUROSCI.0325-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- Bessis A, Bechade C, Bernard D, Roumier A. Microglial control of neuronal death and synaptic properties. Glia. 2007;55:233–238. doi: 10.1002/glia.20459. [DOI] [PubMed] [Google Scholar]
- Branco T, Staras K, Goda Y. Local dendritic activity sets release probability at hippocampal synapses. Neuron. 2008;59:475–485. doi: 10.1016/j.neuron.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron. 2003;40:361–379. doi: 10.1016/s0896-6273(03)00640-8. [DOI] [PubMed] [Google Scholar]
- Burrone J, O’Byrne M, Murthy VN. Multiple forms of synaptic plasticity triggered by selective suppression of activity in individual neurons. Nature. 2002;420:414–418. doi: 10.1038/nature01242. [DOI] [PubMed] [Google Scholar]
- Cannon WB. The Wisdom of the Body. New York: W.W. Norton Co., Inc; 1932. [Google Scholar]
- Chandrasekaran AR, Shah RD, Crair MC. Developmental homeostasis of mouse retinocollicular synapses. J Neurosci. 2007;27:1746–1755. doi: 10.1523/JNEUROSCI.4383-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S, Shepherd JD, Okuno H, Lyford G, Petralia RS, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron. 2006;52:445–459. doi: 10.1016/j.neuron.2006.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cingolani LA, Thalhammer A, Yu LM, Catalano M, Ramos T, Colicos MA, Goda Y. Activity-dependent regulation of synaptic AMPA receptor composition and abundance by beta3 integrins. Neuron. 2008;58:749–762. doi: 10.1016/j.neuron.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copi A, Jungling K, Gottmann K. Activity- and BDNF-induced plasticity of miniature synaptic currents in ES cell-derived neurons integrated in a neocortical network. J Neurophysiol. 2005;94:4538–4543. doi: 10.1152/jn.00155.2005. [DOI] [PubMed] [Google Scholar]
- Davis GW. Homeostatic control of neural activity: from phenomenology to molecular design. Annu Rev Neurosci. 2006;29:307–323. doi: 10.1146/annurev.neuro.28.061604.135751. [DOI] [PubMed] [Google Scholar]
- Davis GW, Bezprozvanny I. Maintaining the stability of neural function: a homeostatic hypothesis. Annu Rev Physiol. 2001;63:847–869. doi: 10.1146/annurev.physiol.63.1.847. [DOI] [PubMed] [Google Scholar]
- De Gois S, Schafer MK, Defamie N, Chen C, Ricci A, Weihe E, Varoqui H, Erickson JD. Homeostatic scaling of vesicular glutamate and GABA transporter expression in rat neocortical circuits. J Neurosci. 2005;25:7121–7133. doi: 10.1523/JNEUROSCI.5221-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai NS. Homeostatic plasticity in the CNS: synaptic and intrinsic forms. J Physiol Paris. 2003;97:391–402. doi: 10.1016/j.jphysparis.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Desai NS, Cudmore RH, Nelson SB, Turrigiano GG. Critical periods for experience-dependent synaptic scaling in visual cortex. Nat Neurosci. 2002;5:783–789. doi: 10.1038/nn878. [DOI] [PubMed] [Google Scholar]
- Ehlers MD. Activity level controls postsynaptic composition and signaling via the ubiquitin-proteasome system. Nat Neurosci. 2003;6:231–242. doi: 10.1038/nn1013. [DOI] [PubMed] [Google Scholar]
- Ehlers MD, Heine M, Groc L, Lee MC, Choquet D. Diffusional trapping of GluR1 AMPA receptors by input-specific synaptic activity. Neuron. 2007;54:447–460. doi: 10.1016/j.neuron.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson JD, De Gois S, Varoqui H, Schafer MK, Weihe E. Activity-dependent regulation of vesicular glutamate and GABA transporters: a means to scale quantal size. Neurochemistry international. 2006;48:643–649. doi: 10.1016/j.neuint.2005.12.029. [DOI] [PubMed] [Google Scholar]
- Goddard CA, Butts DA, Shatz CJ. Regulation of CNS synapses by neuronal MHC class I. Proc Natl Acad Sci U S A. 2007;104:6828–6833. doi: 10.1073/pnas.0702023104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel A, Lee HK. Persistence of experience-induced homeostatic synaptic plasticity through adulthood in superficial layers of mouse visual cortex. J Neurosci. 2007;27:6692–6700. doi: 10.1523/JNEUROSCI.5038-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray EE, Fink AE, Sarinana J, Vissel B, O’Dell TJ. Long-term potentiation in the hippocampal CA1 region does not require insertion and activation of GluR2-lacking AMPA receptors. J Neurophysiol. 2007;98:2488–2492. doi: 10.1152/jn.00473.2007. [DOI] [PubMed] [Google Scholar]
- Greger IH, Ziff EB, Penn AC. Molecular determinants of AMPA receptor subunit assembly. Trends Neurosci. 2007;30:407–416. doi: 10.1016/j.tins.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Harms KJ, Craig AM. Synapse composition and organization following chronic activity blockade in cultured hippocampal neurons. J Comp Neurol. 2005;490:72–84. doi: 10.1002/cne.20635. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Horch HW. Local effects of BDNF on dendritic growth. Rev Neurosci. 2004;15:117–129. doi: 10.1515/revneuro.2004.15.2.117. [DOI] [PubMed] [Google Scholar]
- Hou Q, Zhang D, Jarzylo L, Huganir RL, Man HY. Homeostatic regulation of AMPA receptor expression at single hippocampal synapses. Proc Natl Acad Sci U S A. 2008;105:775–780. doi: 10.1073/pnas.0706447105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang ZJ, Kirkwood A, Pizzorusso T, Porciatti V, Morales B, Bear MF, Maffei L, Tonegawa S. BDNF regulates the maturation of inhibition and the critical period of plasticity in mouse visual cortex. Cell. 1999;98:739–755. doi: 10.1016/s0092-8674(00)81509-3. [DOI] [PubMed] [Google Scholar]
- Ibata K, Sun Q, Turrigiano GG. Rapid synaptic scaling induced by changes in postsynaptic firing. Neuron. 2008;57:819–826. doi: 10.1016/j.neuron.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Isaac JT, Ashby M, McBain CJ. The Role of the GluR2 Subunit in AMPA Receptor Function and Synaptic Plasticity. Neuron. 2007;54:859–871. doi: 10.1016/j.neuron.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Jonas P, Racca C, Sakmann B, Seeburg PH, Monyer H. Differences in Ca2+ permeability of AMPA-type glutamate receptor channels in neocortical neurons caused by differential GluR-B subunit expression. Neuron. 1994;12:1281–1289. doi: 10.1016/0896-6273(94)90444-8. [DOI] [PubMed] [Google Scholar]
- Ju W, Morishita W, Tsui J, Gaietta G, Deerinck TJ, Adams SR, Garner CC, Tsien RY, Ellisman MH, Malenka RC. Activity-dependent regulation of dendritic synthesis and trafficking of AMPA receptors. Nat Neurosci. 2004 doi: 10.1038/nn1189. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Stellwagen D, Malenka RC, Stryker MP. Tumor necrosis factor-alpha mediates one component of competitive, experience-dependent plasticity in developing visual cortex. Neuron. 2008;58:673–680. doi: 10.1016/j.neuron.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilman V, van Rossum MC, Turrigiano GG. Activity deprivation reduces miniature IPSC amplitude by decreasing the number of postsynaptic GABA(A) receptors clustered at neocortical synapses. J Neurosci. 2002;22:1328–1337. doi: 10.1523/JNEUROSCI.22-04-01328.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirov SA, Goddard CA, Harris KM. Age-dependence in the homeostatic upregulation of hippocampal dendritic spine number during blocked synaptic transmission. Neuropharmacology. 2004;47:640–648. doi: 10.1016/j.neuropharm.2004.07.039. [DOI] [PubMed] [Google Scholar]
- Kumar SS, Bacci A, Kharazia V, Huguenard JR. A developmental switch of AMPA receptor subunits in neocortical pyramidal neurons. J Neurosci. 2002;22:3005–3015. doi: 10.1523/JNEUROSCI.22-08-03005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie KR, Nelson SB, Turrigiano GG. Postsynaptic depolarization scales quantal amplitude in cortical pyramidal neurons. J Neurosci. 2001;21:RC170. doi: 10.1523/JNEUROSCI.21-19-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Lissin DV, Gomperts SN, Carroll RC, Christine CW, Kalman D, Kitamura M, Hardy S, Nicoll RA, Malenka RC, von Zastrow M. Activity differentially regulates the surface expression of synaptic AMPA and NMDA glutamate receptors. Proc Natl Acad Sci U S A. 1998;95:7097–7102. doi: 10.1073/pnas.95.12.7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo FM, Zanjani R, Ho N, Chatila TA, Fuleihan RL. Calcium-dependent activation of TNF family gene expression by Ca2+/calmodulin kinase type IV/Gr and calcineurin. J Immunol. 1999;162:2057–2063. [PubMed] [Google Scholar]
- Lu B. BDNF and activity-dependent synaptic modulation. Learn Mem. 2003;10:86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A, Nelson SB, Turrigiano GG. Selective reconfiguration of layer 4 visual cortical circuitry by visual deprivation. Nat Neurosci. 2004;7:1353–1359. doi: 10.1038/nn1351. [DOI] [PubMed] [Google Scholar]
- Maffei A, Turrigiano GG. Multiple modes of network homeostasis in visual cortical layer 2/3. J Neurosci. 2008;28:4377–4384. doi: 10.1523/JNEUROSCI.5298-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Marder E, Goaillard JM. Variability, compensation and homeostasis in neuron and network function. Nat Rev Neurosci. 2006;7:563–574. doi: 10.1038/nrn1949. [DOI] [PubMed] [Google Scholar]
- Marder E, Prinz AA. Current compensation in neuronal homeostasis. Neuron. 2003;37:2–4. doi: 10.1016/s0896-6273(02)01173-x. [DOI] [PubMed] [Google Scholar]
- McLean Bolton M, Pittman AJ, Lo DC. Brain-derived neurotrophic factor differentially regulates excitatory and inhibitory synaptic transmission in hippocampal cultures. J Neurosci. 2000;20:3221–3232. doi: 10.1523/JNEUROSCI.20-09-03221.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KD. Synaptic economics: competition and cooperation in synaptic plasticity. Neuron. 1996;17:371–374. doi: 10.1016/s0896-6273(00)80169-5. [DOI] [PubMed] [Google Scholar]
- Miller KD, MacKay DJC. The role of constraints in Hebbian learning. Neural Computation. 1994;6:100–124. [Google Scholar]
- Mrsic-Flogel TD, Hofer SB, Ohki K, Reid RC, Bonhoeffer T, Hubener M. Homeostatic regulation of eye-specific responses in visual cortex during ocular dominance plasticity. Neuron. 2007;54:961–972. doi: 10.1016/j.neuron.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Mu Y, Otsuka T, Horton AC, Scott DB, Ehlers MD. Activity-dependent mRNA splicing controls ER export and synaptic delivery of NMDA receptors. Neuron. 2003;40:581–594. doi: 10.1016/s0896-6273(03)00676-7. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Schikorski T, Stevens CF, Zhu Y. Inactivity produces increases in neurotransmitter release and synapse size. Neuron. 2001;32:673–682. doi: 10.1016/s0896-6273(01)00500-1. [DOI] [PubMed] [Google Scholar]
- Newpher TM, Ehlers MD. Glutamate receptor dynamics in dendritic microdomains. Neuron. 2008;58:472–497. doi: 10.1016/j.neuron.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien RJ, Kamboj S, Ehlers MD, Rosen KR, Fischbach GD, Huganir RL. Activity-dependent modulation of synaptic AMPA receptor accumulation. Neuron. 1998;21:1067–1078. doi: 10.1016/s0896-6273(00)80624-8. [DOI] [PubMed] [Google Scholar]
- Okuda T, Yu LM, Cingolani LA, Kemler R, Goda Y. beta-Catenin regulates excitatory postsynaptic strength at hippocampal synapses. Proc Natl Acad Sci U S A. 2007;104:13479–13484. doi: 10.1073/pnas.0702334104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant K, Pelkey KA, Bortolotto ZA, Morita D, Terashima A, McBain CJ, Collingridge GL, Isaac JT. Transient incorporation of native GluR2-lacking AMPA receptors during hippocampal long-term potentiation. Nat Neurosci. 2006;9:602–604. doi: 10.1038/nn1678. [DOI] [PubMed] [Google Scholar]
- Plath N, Ohana O, Dammermann B, Errington ML, Schmitz D, Gross C, Mao X, Engelsberg A, Mahlke C, Welzl H, et al. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52:437–444. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Pratt KG, Watt AJ, Griffith LC, Nelson SB, Turrigiano GG. Activity-dependent remodeling of presynaptic inputs by postsynaptic expression of activated CaMKII. Neuron. 2003;39:269–281. doi: 10.1016/s0896-6273(03)00422-7. [DOI] [PubMed] [Google Scholar]
- Rabinowitch I, Segev I. Two opposing plasticity mechanisms pulling a single synapse. TINS. 2008;31:377–383. doi: 10.1016/j.tins.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Rao A, Craig AM. Activity regulates the synaptic localization of the NMDA receptor in hippocampal neurons. Neuron. 1997;19:801–812. doi: 10.1016/s0896-6273(00)80962-9. [DOI] [PubMed] [Google Scholar]
- Rao VR, Pintchovski SA, Chin J, Peebles CL, Mitra S, Finkbeiner S. AMPA receptors regulate transcription of the plasticity-related immediate-early gene Arc. Nat Neurosci. 2006;9:887–895. doi: 10.1038/nn1708. [DOI] [PubMed] [Google Scholar]
- Rial Verde EM, Lee-Osbourne J, Worley PF, Malinow R, Cline HT. Increased expression of the immediate-early gene arc/arg3.1 reduces AMPA receptor-mediated synaptic transmission. Neuron. 2006;52:461–474. doi: 10.1016/j.neuron.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenhouse CD, Shouval HZ, Paradiso MA, Bear MF. Monocular deprivation induces homosynaptic long-term depression in visual cortex. Nature. 1999;397:347–350. doi: 10.1038/16922. [DOI] [PubMed] [Google Scholar]
- Rutherford LC, DeWan A, Lauer HM, Turrigiano GG. Brain-derived neurotrophic factor mediates the activity-dependent regulation of inhibition in neocortical cultures. J Neurosci. 1997;17:4527–4535. doi: 10.1523/JNEUROSCI.17-12-04527.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford LC, Nelson SB, Turrigiano GG. BDNF has opposite effects on the quantal amplitude of pyramidal neuron and interneuron excitatory synapses. Neuron. 1998;21:521–530. doi: 10.1016/s0896-6273(00)80563-2. [DOI] [PubMed] [Google Scholar]
- Seeburg DP, Feliu-Mojer M, Gaiottino J, Pak DT, Sheng M. Critical role of CDK5 and Polo-like kinase 2 in homeostatic synaptic plasticity during elevated activity. Neuron. 2008;58:571–583. doi: 10.1016/j.neuron.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatz CJ. Impulse activity and the patterning of connections during CNS development. Neuron. 1990;5:745–756. doi: 10.1016/0896-6273(90)90333-b. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Rumbaugh G, Wu J, Chowdhury S, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron. 2006;52:475–484. doi: 10.1016/j.neuron.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105:331–343. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- Sjostrom PJ, Turrigiano GG, Nelson SB. Multiple forms of long-term plasticity at unitary neocortical layer 5 synapses. Neuropharmacology. 2006 doi: 10.1016/j.neuropharm.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Soderling TR. The Ca-calmodulin-dependent protein kinase cascade. Trends Biochem Sci. 1999;24:232–236. doi: 10.1016/s0968-0004(99)01383-3. [DOI] [PubMed] [Google Scholar]
- Stellwagen D, Beattie EC, Seo JY, Malenka RC. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J Neurosci. 2005;25:3219–3228. doi: 10.1523/JNEUROSCI.4486-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-alpha. Nature. 2006;440:1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- Sullivan TJ, de Sa VR. Homeostatic synaptic scaling in self-organizing maps. Neural Netw. 2006;19:734–743. doi: 10.1016/j.neunet.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Ito HT, Cressy P, Kempf C, Woo JC, Schuman EM. Miniature neurotransmission stabilizes synaptic function via tonic suppression of local dendritic protein synthesis. Cell. 2006;125:785–799. doi: 10.1016/j.cell.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Taylor AM, Ito HT, Pham A, Schuman EM. Postsynaptic decoding of neural activity: eEF2 as a biochemical sensor coupling miniature synaptic transmission to local protein synthesis. Neuron. 2007;55:648–661. doi: 10.1016/j.neuron.2007.07.030. [DOI] [PubMed] [Google Scholar]
- Swanwick CC, Murthy NR, Kapur J. Activity-dependent scaling of GABAergic synapse strength is regulated by brain-derived neurotrophic factor. Molecular and cellular neurosciences. 2006;31:481–492. doi: 10.1016/j.mcn.2005.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiagarajan TC, Lindskog M, Malgaroli A, Tsien RW. LTP and adaptation to inactivity: overlapping mechanisms and implications for metaplasticity. Neuropharmacology. 2007;52:156–175. doi: 10.1016/j.neuropharm.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Thiagarajan TC, Lindskog M, Tsien RW. Adaptation to synaptic inactivity in hippocampal neurons. Neuron. 2005;47:725–737. doi: 10.1016/j.neuron.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Thiagarajan TC, Piedras-Renteria ES, Tsien RW. alpha- and betaCaMKII. Inverse regulation by neuronal activity and opposing effects on synaptic strength. Neuron. 2002;36:1103–1114. doi: 10.1016/s0896-6273(02)01049-8. [DOI] [PubMed] [Google Scholar]
- Tokuoka H, Goda Y. Activity-dependent coordination of presynaptic release probability and postsynaptic GluR2 abundance at single synapses. PNAS. 2008;105:14656–14661. doi: 10.1073/pnas.0805705105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrigiano GG. Homeostatic plasticity in neuronal networks: the more things change, the more they stay the same. Trends Neurosci. 1999;22:221–227. doi: 10.1016/s0166-2236(98)01341-1. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG. More than a sidekick: glia and homeostatic synaptic plasticity. Trends in molecular medicine. 2006;12:458–460. doi: 10.1016/j.molmed.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–896. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- van Rossum MC, Bi GQ, Turrigiano GG. Stable Hebbian learning from spike timing-dependent plasticity. J Neurosci. 2000;20:8812–8821. doi: 10.1523/JNEUROSCI.20-23-08812.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt AJ, Sjostrom PJ, Hausser M, Nelson SB, Turrigiano GG. A proportional but slower NMDA potentiation follows AMPA potentiation in LTP. Nat Neurosci. 2004 doi: 10.1038/nn1220. [DOI] [PubMed] [Google Scholar]
- Watt AJ, van Rossum MC, MacLeod KM, Nelson SB, Turrigiano GG. Activity coregulates quantal AMPA and NMDA currents at neocortical synapses. Neuron. 2000;26:659–670. doi: 10.1016/s0896-6273(00)81202-7. [DOI] [PubMed] [Google Scholar]
- Wenthold RJ, Petralia RS, Blahos J, II, Niedzielski AS. Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J Neurosci. 1996;16:1982–1989. doi: 10.1523/JNEUROSCI.16-06-01982.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga CJ, Ibata K, Turrigiano GG. Postsynaptic expression of homeostatic plasticity at neocortical synapses. J Neurosci. 2005;25:2895–2905. doi: 10.1523/JNEUROSCI.5217-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga CJ, Walsh MF, Turrigiano GG. Temporal Regulation of the Expression Locus of Homeostatic Plasticity. J Neurophysiol. 2006 doi: 10.1152/jn.00107.2006. [DOI] [PubMed] [Google Scholar]
- Zhang W, Linden DJ. The other side of the engram: experience-driven changes in neuronal intrinsic excitability. nature reviews neuroscience. 2003;4:885–900. doi: 10.1038/nrn1248. [DOI] [PubMed] [Google Scholar]