Abstract
Multidrug resistant tuberculosis is now thought to afflict between 1 and 2 million patients annually. Although significant regional variability in the distribution of disease has been recorded, surveillance data are limited by several factors. The true burden of disease is likely underestimated. Nevertheless, the estimated burden is substantial enough to warrant concerted action. A range of approaches is possible, but all appropriate interventions require scale-up of laboratories and early treatment with regimens containing a sufficient number of second-line drugs. Ambulatory treatment for most patients, and improved infection control, can facilitate scale-up with decreased risk of nosocomial transmission. Several obstacles have been considered to preclude worldwide scale-up of treatment, mostly attributable to inadequate human, drug, and financial resources. Further delays in scale-up, however, risk continued generation and transmission of resistant tuberculosis, as well as associated morbidity and mortality.
Keywords: Tuberculosis, drug resistance, surveillance, management, epidemiology
An estimated 489,139, or nearly 5% of all new cases of tuberculosis (TB) diagnosed in 2006 were multidrug resistant (MDR), that is resistant to isoniazid and rifampicin, the two most effective anti-TB agents. This represents an increase of 12% since 2004 and 56% since 2000.1 An additional 1 to 1.5 million prevalent cases of MDR-TB were estimated in 2006, resulting in as many as 2 million people with active disease.2 The successful treatment of MDR-TB requires the use of second-line drugs, which historically presented an insurmountable cost barrier in resource-poor settings. To alleviate this gap, the World Health Organization (WHO) established the Green Light Committee (GLC) in 2000 to facilitate access to and strictly supervise the use of second-line agents for TB control.
Even with the inception of the GLC and a significant reduction in cost of second-line drugs, drug resistant TB continues to grow and challenge the current capacity in most settings. Recognition of the magnitude of the MDR-TB problem and its associated morbidity and mortality has motivated recent calls for increased research and scaled-up treatment.3,4 Several recent, excellent articles have reviewed the molecular mechanisms of resistance, risk factors for drug resistant TB (DR-TB), DR-TB and HIV, and global epidemiology of TB.5–21 This article highlights the gaps in knowledge of the global epidemiology of MDR-TB, illustrates the elements of a programmatic response and confronts some of the perceived obstacles to scale-up of programmatic management of MDR-TB.
EPIDEMIOLOGY OF MDR-TB
Drug Resistance and Multidrug Therapy
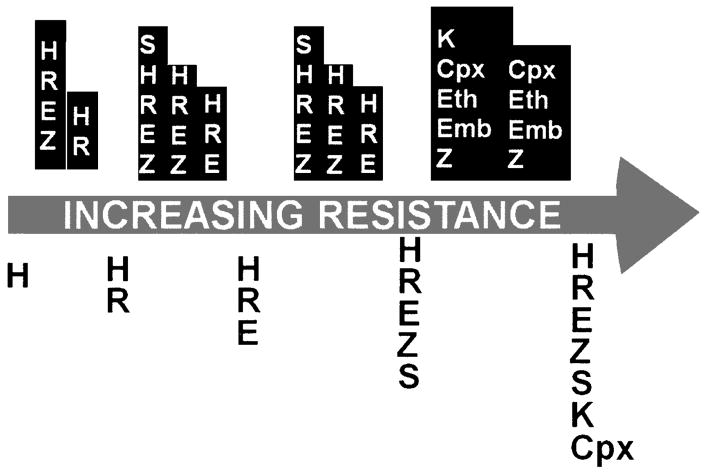
DR-TB is defined as tuberculosis caused by a strain of Mycobacterium tuberculosis that grows, in vitro, in the presence of one or more antimycobacterial drugs. Spontaneous mutations leading to resistance occur at random in large populations of M. tuberculosis at a rate per cell division of 10−10 for rifampin (RIF), 10−8 for isoniazid (INH) and streptomycin, 10−6 to 10−8 for fluoroquinolones, 10−7 for ethambutol, and 10−3 for pyrazinamide. 22–24 Resistance can be engendered through inadequate treatment (Fig. 1). Exposure to drugs kills susceptible organisms, selecting for resistant mutants that become responsible for persistent disease, a phenomenon known as acquired resistance. Resistant organisms are also transmitted; the consequent TB episode is considered to have been caused by primary resistance.
Figure 1.
Amplifier effect of repeated, standardized regimens. Example of how selective pressure of repeated standardized regimens (represented by white letters on dark background over arrow) can result in serial acquisition of resistance (represented by dark letters on light background under arrow), ultimately XDR-TB.
The phenomenon of acquired resistance was first observed with the introduction of streptomycin (SM) in 1945. After initial bacteriologic and radiographic response, resistance to SM emerged in 85% of patients tested,25 and monotherapy with SM afforded no long-term survival benefit.26 Combination therapy was introduced to prevent the development of resistance27 and remains one of the cornerstones of anti-TB therapy, which usually comprises four drugs, including INH and RIF.28,29 The DOTS (directly observed therapy, short-course chemotherapy) strategy, introduced by the WHO in 1993, incorporates direct observation of multidrug therapy and several other elements to minimize the acquisition of drug resistance and facilitate TB control.30
The emergence of increasingly resistant strains of M. tuberculosis, however, is evidence that the DOTS strategy alone cannot successfully prevent drug resistance in all settings.31,32 Of particular importance is MDR-TB, which severely compromises treatment outcomes. 33–38 Extensively drug resistant TB (XDR-TB) refers to MDR-TB isolates with further resistance to a second-line injectable agent and a fluoroquinolone. Treatment success among these patients has been reported to be worse than among patients with MDR-TB.39–42
The Global Epidemiology of Drug Resistant TB: Knowledge and Gaps
Considerable effort has been expended to estimate the number of cases of DR-TB and the percent of TB cases caused by resistant organisms. Through a standardized, four-survey, 14-year effort, the Global Project Anti-tuberculosis Drug Resistance Surveillance (GPADRS) reported the burden of DR-TB in 138 settings in 114 countries.2,31,43,44 Based on reported figures and data on nine epidemiologic indicators, global estimates were derived for 2006: 489,139 (95% CI: 455,093 to 614,215) cases of MDR-TB, representing 4.8% (4.6 to 6.0) of all TB cases.2
These survey data, although extremely valuable, suffer from several limitations described in a recent article.45 A few are highlighted here. First, the MDRTB burden was estimated to be lower in nonsurveyed places than in regions with survey data. Because poor TB control is an important risk factor for drug resistance, and some infrastructure is required to implement a survey of antituberculosis drug resistance, settings that have not conducted surveys may, in fact, have higher rates of MDR-TB.23,43 Second, surveys provide, at most, limited information on important subpopulations that can have higher risks of MDR-TB. These include previously treated patients, who are often enrolled, but not in sufficient numbers to generate accurate estimates. Patients treated in the private sector46–49 are generally not included in survey samples. Furthermore, HIV-infected TB patients may be underrepresented in surveys that require sputum-smear positivity for inclusion.50 Finally, a substantial pool of chronic patients with MDR-TB suffers at home, with substantial delays in seeking care51; these patients, therefore, are likely to be excluded from health facility–based samples. These omissions may result in an underestimate of the burden of resistant disease and preclude design of effective standardized regimens.52
The foregoing summary results also mask significant variability (Table 1 53–64).* In some parts of the world, like the former Soviet Union and eastern Europe, the percent of new and previously treated TB cases with resistant disease is alarmingly high: in Baku, Azerbaijan, multidrug resistance was reported in more than 20% and 55% of new and previously treated patients, respectively, in 2006. The absolute MDR-TB burden, however, is relatively low at 431 cases. In contrast, in the Indian states surveyed, DR-TB among new patients remains relatively infrequent (0.5 to 3.4%). However, high frequency of MDR-TB among previously treated patients (e.g., 17.4% in Gujarat State in 2006), combined with high TB incidence, yield a disturbing estimate of the absolute number of MDR-TB cases: 110,132 (95% CI: 79,975 to 142,386). In China, MDR-TB incidence was estimated at 5%, resulting in 130,548 cases (95% CI: 97,663 to 164,900) in 2006. These examples, in which percent of TB cases that are MDR and absolute number of MDR-TB patients provide very different impressions of the magnitude of the problem, highlight the importance of deriving and comparing population incidence or prevalence to make policy decisions.65
Table 1.
Global Burden of MDR-TB (from Surveys, Surveillance, Convenience Samples, or Estimates) by Country
| MDR** |
Drug-Resistant (Non-MDR) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Country/Region | Reference† | Period | Source Population; Sampling Strategy‡ | N Tested± | N | % | N | % |
| Afghanistan | Report #4 | 2006 | Estimated | 2139 (671,8802) | 4.9 (1.6,19.5) | |||
| Albania | Report #4 | 2006 | Estimated | 14 (4,67) | 2.1 (0.7,10.3) | |||
| Algeria* | Report #4 | 2001 | National; proportionate cluster | 518 | 6 | 1.2 | 26 | 5 |
| Andorra | Report #4 | 2005 | National; 100% cases | 9 | 0 | 0 | 9 | 11.1 |
| Angola | Report #4 | 2006 | Estimated | 1665 (547,7144) | 3.2 (1.1,13.1) | |||
| Antigua and Barbuda | Report #4 | 2006 | Estimated | 0 | 1.3 (0.7,9.1) | |||
| Argentina | Report #4 | 2005 | National; proportionate cluster | 819 | 36 | 4.4 | 66 | 8.1 |
| Armenia | Report #4 | 2007 | National; 100% diagnostic units | 892 | 199 | 22.3 | 261 | 29.3 |
| Australia | Report #4 | 2005 | National; 100% cases | 808 | 12 | 1.5 | 69 | 8.6 |
| Austria | Report #4 | 2005 | National; 100% cases | 609 | 13 | 2.1 | 59 | 9.7 |
| Azerbaijan | Report #4 | 2007 | Subnational; Baku; 100% diagnostic units | 1103 | 431 | 39.1 | 345 | 31.3 |
| Bahamas | Report #4 | 2006 | Estimated | 3 (1,12) | 1.9 (0.7,8.5) | |||
| Bahrain | Report #4 | 2006 | Estimated | 11 (4,43) | 3.5 (1.1,13.4) | |||
| Bangladesh | Van Deun et al, 199953 | 1994 | Subnational; cluster; 5 health centers | 0.02 | 18.6 | |||
| Belarus | Report #4 | 2006 | Estimated | 1,096 (371,3,272) | 15.7 (5.4,46.5) | |||
| Belgium | Report #4 | 2005 | National; 100% cases | 758 | 11 | 1.5 | 36 | 4.8 |
| Belize | Report #4 | 2006 | Estimated | 4 (1,15) | 2.3 (0.8,10.2) | |||
| Benin* | Report #1 | 1997 | National; proportionate cluster | 333 | 10 | 0.3 | 27 | 8.1 |
| Bhutan | Report #4 | 2006 | Estimated | 28 (8119) | 4.2 (1.3,17.5) | |||
| Bolivia | Report #1 | 1996 | National; proportionate cluster | 605 | 11 | 1.8 | 160 | 26.5 |
| Bosnia and Herzegovina | Report #4 | 2005 | National; 100% cases | 1141 | 11 | 1 | 30 | 2.6 |
| Botswana | Report #3 | 2002 | National; 100% diagnostic units | 1288 | 21 | 1.6 | 126 | 9.8 |
| Brazil | Report #1 | 1996 | Nearly countrywide; proportionate cluster | 2888 | 62 | 2.1 | 234 | 8.1 |
| Brunei | Report #4 | 2006 | Estimated | 11 (3,47) | 3.3 (1.1,13.8) | |||
| Bulgaria | Report #4 | 2006 | Estimated | 451 (143,1563) | 13.2 (4.2,44.1) | |||
| Burkina Faso | Report #4 | 2006 | Estimated | 1170 (369,5402) | 2.9 (1.0,13.1) | |||
| Burundi* | Sanders et al, 200654 | 2002–2003 | Subnational; 7 diagnostic units in Bujumbura; 100% cases | 2.7 | 17.9 | |||
| Cambodia | Report #3 | 2001 | National; proportionate cluster | 734 | 3 | 0.4 | 80 | 10.9 |
| Cameroon | Report #4 | 2006 | Estimated | 786 (227,4036) | 2.1 (0.6,11.0) | |||
| Canada | Report #4 | 2005 | National; 100% cases | 1203 | 23 | 1.9 | 123 | 10.2 |
| Cape Verde | Report #4 | 2006 | Estimated | 22 (7,102) | 2.3 (0.8,10.7) | |||
| Central African Republic | Report #2 | 1998 | Subnational; 100% diagnostic units in Bangui | 495 | 11 | 2.2 | 77 | 15.5 |
| Chad | Report #4 | 2006 | Estimated | 807 (230,4297) | 2.4 (0.7,13.3) | |||
| Chile | Report #3 | 2001 | National; proportionate cluster | 1158 | 17 | 1.5 | 132 | 11.4 |
| China | Report #4 | 2006 | Estimated | 130548 (97633,164900) | 8.3 (7.0,10.2) | |||
| Guandong Province | Report #2 | 1999 | Proportionate cluster | 524 | 24 | 4.6 | 60 | 11.4 |
| Beijing Municipality | Report #4 | 2004 | 100% diagnostic units | 1197 | 42 | 3.5 | 199 | 16.6 |
| Shandong Province | Report #2 | 1997 | Proportionate cluster | 1229 | 72 | 5.9 | 215 | 17.5 |
| Henan Province | Report #3 | 2001 | Proportionate cluster | 1487 | 192 | 12.9 | 333 | 22.4 |
| Liaoning Province | Report #3 | 1999 | Proportionate cluster | 904 | 106 | 11.7 | 287 | 31.7 |
| Heilongjiang Province | Report #4 | 2005 | Proportionate cluster | 1995 | 241 | 12.1 | 612 | 30.7 |
| Hubei Province | Report #3 | 1999 | Proportionate cluster | 1097 | 70 | 6.4 | 185 | 16.9 |
| Zhejiang Province | Report #2 | 1999 | Proportionate cluster | 942 | 85 | 9 | 117 | 12.4 |
| Shanghai Municipality | Report #4 | 2005 | 100% diagnostic units | 964 | 55 | 5.7 | 118 | 12.2 |
| Inner Mongolia Autonomous region | Report #4 | 2002 | Proportionate cluster | 1114 | 188 | 16.9 | 310 | 27.8 |
| China, Hong Kong SAR | Report #4 | 2005 | 100% cases | 4350 | 41 | 0.9 | 439 | 10.1 |
| China, Macao SAR | Report #4 | 2005 | 100% cases | 284 | 9 | 3.2 | 38 | 13.3 |
| Colombia* | Report #3 | 2000 | National; proportionate cluster | 1087 | 16 | 1.5 | 152 | 14 |
| Comoros | Report #4 | 2006 | Estimated | 9 (3,45) | 2.3 (0.7,11.9) | |||
| Congo | Report #4 | 2006 | Estimated | 321 (90,1737) | 2.1 (0.6,11.0) | |||
| Costa Rica | Report #3 | 2006 | National; 100% diagnostic units | 284 | 5 | 1.8 | 15 | 5.2 |
| Côte d’Ivoire* | Report #4 | 2006 | National; proportionate cluster | 320 | 8 | 2.5 | 68 | 21.3 |
| Croatia | Report #4 | 2005 | National; 100% cases | 647 | 6 | 0.9 | 16 | 2.5 |
| Cuba | Report #4 | 2005 | National; proportionate cluster | 198 | 1 | 0.5 | 20 | 10.1 |
| Cyprus | Report #4 | 2006 | Estimated | 1 (0,3) | 1.3 (0.4,7.6) | |||
| Czech Republic | Report #4 | 2005 | National; 100% cases | 582 | 13 | 2.2 | 38 | 6.6 |
| Democratic Republic of the Congo | Report #3 | 1999 | Subnational; Kinshasa, proportionate cluster | 710 | 41 | 5.8 | 236 | 33.2 |
| Denmark | Report #4 | 2005 | National; 100% cases | 325 | 5 | 1.5 | 16 | 5 |
| Djibouti | Report #4 | 2006 | Estimated | 449 (150,1489) | 6.2 (2.1,20.1) | |||
| Dominica | Report #4 | 2006 | Estimated | 0 (0,1) | 2.2 (0.8,10.2) | |||
| Dominican Republic | Report #1 | 1995 | National; proportionate cluster | 420 | 43 | 10.2 | 141 | 33.6 |
| East Timor | Kelly et al, 200555 | 2000–2001 | National; re-treatment failures | 64.3 | 28.6 | |||
| Ecuador | Report #3 | 2002 | National; 100% diagnostic units | 997 | 85 | 8.5 | 160 | 16 |
| Egypt | Report #3 | 2002 | National; proportionate cluster | 849 | 97 | 11.4 | 245 | 28.8 |
| El Salvador | Report #3 | 2001 | National; 100% diagnostic units | 711 | 9 | 1.3 | 48 | 6.7 |
| Equatorial Guinea | Tudó et al, 200456 | 1999–2001 | Subnational; nearly 100% in 5 of 18 districts | 3.4 | 14.8 | |||
| Eritrea | Report #4 | 2006 | Estimated | 127 (36,681) | 2.7 (0.8,14.1) | |||
| Estonia | Report #4 | 2005 | National; 100% cases | 387 | 79 | 20.4 | 57 | 14.7 |
| Ethiopia | Report #4 | 2005 | National; proportionate cluster | 880 | 22 | 2.5 | 231 | 26.3 |
| Fiji | Report #4 | 2006 | National; random cluster | 38 | 0 | 0 | 0 | 0 |
| Finland | Report #4 | 2005 | National; 100% cases | 315 | 3 | 1 | 11 | 3.4 |
| France | Report #4 | 2005 | National; 100% cases | 1501 | 24 | 1.6 | 119 | 7.9 |
| Gabon | Report #4 | 2006 | Estimated | 98 (31,460) | 1.9 (0.6,9.1) | |||
| Gambia | Report #3 | 2000 | National; 100% diagnostic units | 225 | 1 | 0.4 | 8 | 3.6 |
| Georgia | Report #4 | 2006 | National; 100% diagnostic units | 1422 | 219 | 15.4 | 586 | 41.2 |
| Germany | Report #4 | 2005 | National; 100% cases | 3886 | 105 | 2.7 | 373 | 9.6 |
| Ghana | Report #4 | 2006 | Estimated | 1090 (288,6169) | 2.2 (0.6,12.1) | |||
| Greece | Report #4 | 2006 | Estimated | 45 (15,186) | 2.0 (0.7,8.5) | |||
| Guam | Report #4 | 2002 | National; random cluster | 47 | 0 | 0 | 2 | 4.3 |
| Guatemala | Report #4 | 2002 | National; proportionate cluster | 823 | 61 | 7.4 | 257 | 31.2 |
| Guinea | Report #2 | 1998 | SubNational; sentinel sites; random cluster | 571 | 12 | 2.1 | 83 | 14.5 |
| Guinea-Bissau | Report #4 | 2006 | Estimated | 109 (32,545) | 2.8 (0.8,13.9) | |||
| Guyana | Menner, Gunther, Orawa et al, 200557 | 2001 | Convenience; 36 patients from Georgetown Chest Clinic | 11.1 | 22.2 | |||
| Haiti | Ferdinand et al, 200358 | 2000 | Convenience; GHESKIO HIV VCT center in Port au Prince | 8.9 | 20.4 | |||
| Honduras | Report #3 | 2004 | National; proportionate cluster | 530 | 17 | 3.2 | 66 | 12.5 |
| Hungary | Report #4 | 2006 | Estimated | 69 (23,258) | 3.0 (1.0,11.1) | |||
| Iceland | Report #4 | 2005 | National; 100% cases | 8 | 0 | 0 | 0 | 0 |
| India | Report #4 | 2006 | Estimated | 110132 (79975, 142386) | 4.9 (3.9,6.2) | |||
| Mayhurbhanj District, Orissa State* | Report #4 | 2001 | 100% diagnostic units | 282 | 2 | 0.7 | 13 | 4.6 |
| Wardha District, Maharashtra State* | Report #3 | 2001 | 100% diagnostic units | 197 | 1 | 0.5 | 38 | 19.3 |
| Delhi State | Report #1 | 1995 | 100% diagnostic units | 2240 | 298 | 13.3 | 428 | 19.1 |
| Raichur District, Karnataka State* | Report #3 | 1999 | 100% diagnostic units | 278 | 7 | 2.5 | 54 | 19.4 |
| North Arcot District, Tamil Nadu State* | Report #3 | 1999 | 100% diagnostic units | 282 | 8 | 2.8 | 70 | 24.9 |
| Ernakulam district, Kerala State* | Report #4 | 2004 | 100% diagnostic units | 305 | 6 | 2 | 79 | 25.9 |
| Gujarat State | Report #4 | 2006 | Proportionate cluster | 2618 | 219 | 8.4 | 600 | 22.9 |
| Tamil Nadu State* | Report #2 | 1997 | Proportionate cluster | 384 | 13 | 3.4 | 59 | 15.4 |
| Hoogli district, West Bengal State* | Report #4 | 2001 | 100% diagnostic units | 263 | 8 | 3 | 36 | 13.7 |
| Indonesia* | Report #4 | 2004 | Subnational; Mimika district, Papua Province; 100% diagnostic units | 101 | 2 | 2 | 12 | 11.9 |
| Iran | Report #2 | 1998 | National; random cluster | 722 | 60 | 8.3 | 78 | 10.8 |
| Iraq | Report #4 | 2006 | Estimated | 969 (334,3246) | 5.6 (2.0,18.6) | |||
| Ireland | Report #4 | 2005 | National; 100% cases | 273 | 3 | 1.1 | 10 | 3.7 |
| Israel | Report #4 | 2005 | National; 100% cases | 217 | 12 | 5.5 | 34 | 15.7 |
| Italy | Report #4 | 2005 | Subnational; 100% cases in ½ the country | 585 | 22 | 3.8 | 56 | 10 |
| Jamaica | Report #4 | 2006 | Estimated | 4 (1,20) | 1.8 (0.5,9.4) | |||
| Japan | Report #4 | 2002 | National; 100% diagnostic units | 3122 | 60 | 1.9 | 278 | 8.9 |
| Jordan | Report #4 | 2004 | National; 100% diagnostic units | 141 | 18 | 12.8 | 43 | 30.5 |
| Kazakhstan | Report #3 | 2001 | National; 100% diagnostic units | 678 | 231 | 34.1 | 234 | 34.8 |
| Kenya | Report #1 | 1995 | Nearly countrywide; proportionate cluster | 491 | 0 | 0 | 45 | 9.2 |
| Republic of Korea | Report #4 | 2004 | National; proportionate cluster | 2914 | 110 | 3.8 | 288 | 9.9 |
| Kuwait | Mokaddas et al, 200859 | 1996–2005 | National surveillance | 0.9 | 12.5 | |||
| Kyrgyzstan | Report #4 | 2006 | Estimated | 1368 (443,4026) | 18.2 (6.2,51.5) | |||
| Latvia | Report #4 | 2005 | National; 100% cases | 1055 | 160 | 15.2 | 249 | 23.6 |
| Lebanon | Report #4 | 2003 | National; 100% diagnostic units | 206 | 12 | 5.8 | 37 | 18 |
| Lesotho | Report #1 | 1995 | National; proportionate cluster | 383 | 6 | 1.6 | 41 | 10.7 |
| Libyan Arab Jamahiriya | Report #4 | 2006 | Estimated | 33 (8,166) | 3.1 (0.8,15.2) | |||
| Lithuania | Report #4 | 2005 | National; 100% cases | 1739 | 338 | 19.4 | 243 | 14 |
| Luxembourg | Report #4 | 2005 | National; 100% cases | 37 | 4 | 10.8 | 0 | 0 |
| Macedonia | Report #4 | 2006 | Estimated | 19 (6,79) | 2.8 (0.9,11.4) | |||
| Madagascar | Report #4 | 2007 | National; proportionate cluster | 865 | 6 | 0.7 | 51 | 5.9 |
| Malawi | Salaniponi, Nyirenda, Kemp et al 200360 | 1999–2000 | Convenience; all 43 nonprivate hospitals in Malawi | 4 | 15 | |||
| Malaysia | Report #2 | 1997 | Subnational; peninsular Malaysia; proportionate cluster | 1017 | 1 | 0.1 | 50 | 4.9 |
| Maldives | Report #4 | 2006 | Estimated | 5 (2,24) | 3.7 (1.1,16.4) | |||
| Mali | Report #4 | 2006 | Estimated | 756 (177,4,363) | 2.2 (0.5,12.8) | |||
| Malta | Report #4 | 2005 | National; 100% cases | 11 | 0 | 0 | 2 | 18.2 |
| Mexico | Report #2 | 1997 | Subnational; Baja California, Sinaloa, Oaxaca; 100% diagnostic units | 441 | 32 | 7.3 | 59 | 13.3 |
| Republic of Moldova | Report #4 | 2006 | National; 100% diagnostic units | 2879 | 1204 | 41.8 | 599 | 20.8 |
| Mongolia* | Report #3 | 1999 | National; 100% diagnostic units | 405 | 4 | 1 | 115 | 28.4 |
| Morocco | Report #4 | 2006 | National; proportionate cluster | 1238 | 28 | 2.3 | 84 | 6.8 |
| Mozambique | Report #2 | 1999 | National; proportionate cluster | 1150 | 40 | 3.5 | 229 | 19.9 |
| Burma/Myanmar | Report #4 | 2003 | National; proportionate cluster | 849 | 47 | 5.5 | 61 | 7.2 |
| Namibia | Report #4 | 2006 | Estimated | 342 (103,1716) | 2.0 (0.6,9.8) | |||
| Nepal | Report #4 | 2007 | National; proportionate cluster | 930 | 41 | 4.4 | 113 | 12.2 |
| Netherlands | Report #4 | 2005 | National; 100% cases | 841 | 7 | 0.8 | 67 | 8 |
| New Caledonia | Report #4 | 2005 | National; random cluster | 5 | 0 | 0 | 1 | 20 |
| New Zealand | Report #4 | 2006 | National; 100% cases | 255 | 1 | 0.4 | 25 | 9.8 |
| Nicaragua | Report #4 | 2006 | National; proportionate cluster | 423 | 10 | 2.4 | 69 | 16.3 |
| Niger | Report #4 | 2006 | Estimated | 750 (233,3667) | 2.9 (0.9,13.5) | |||
| Nigeria | Kehinde et al, 200761 | 2005–2006 | Convenience; network of clinical sites in Ibadan | 53.6 | NA | |||
| Northern Mariana Islands* | Report #4 | 2006 | National; 100% cases | 18 | 2 | 11.1 | 2 | 11.1 |
| Norway | Report #4 | 2005 | National; 100% cases | 214 | 3 | 1.4 | 41 | 19.2 |
| Oman | Report #4 | 2006 | National; 100% cases | 164 | 7 | 4.3 | 9 | 5.5 |
| Pakistan* | Javaid et al, 200862 | NA | Convenience, 29 centers throughout the country | 1.8 | 11.3 | |||
| Palau | Report #4 | 2006 | Estimated | 1 (0,2) | 5.4 (1.7,16.5) | |||
| Panama | Report #4 | 2006 | Estimated | 47 (16,188) | 2.7 (0.9,11.0) | |||
| Papua New Guinea | Report #4 | 2006 | Estimated | 915 (285,3560) | 5.3 (1.7,20.1) | |||
| Paraguay | Report #4 | 2001 | National; proportionate cluster | 266 | 7 | 2.4 | 27 | 10.2 |
| Peru | Report #4 | 2006 | National; proportionate cluster | 2169 | 180 | 8.3 | 390 | 18 |
| Philippines | Report #4 | 2004 | National; proportionate cluster | 1094 | 66 | 6 | 180 | 16.5 |
| Poland | Report #4 | 2004 | National; 100% diagnostic units | 3239 | 51 | 1.6 | 194 | 6 |
| Puerto Rico | Report #4 | 2005 | National; 100% cases | 94 | 0 | 0 | 3 | 3.2 |
| Portugal | Report #4 | 2005 | National | 1579 | 28 | 1.8 | 210 | 13.3 |
| Qatar | Report #4 | 2006 | National; 100% cases | 278 | 3 | 1.1 | 25 | 9 |
| Romania | Report #4 | 2004 | National; 100% diagnostic units | 1251 | 67 | 5.4 | 181 | 14.5 |
| Russian Federation | Report #4 | 2006 | Estimated | 36037 (28992,50258) | 19.4 (17.1,24.6) | |||
| Ivanovo Oblast | Report #3 | 2002 | 100% cases | 505 | 133 | 26.3 | 147 | 29.1 |
| Orel Oblast | Report #4 | 2006 | 100% cases | 347 | 33 | 9.5 | 68 | 19.6 |
| Mary El Oblast* | Report #4 | 2006 | 100% cases | 304 | 38 | 12.5 | 53 | 17.4 |
| Tomsk Oblast* | Report #4 | 2005 | 100% cases | 515 | 77 | 15 | 105 | 20.3 |
| Rwanda | Report #4 | 2005 | National; 100% diagnostic units | 701 | 32 | 4.6 | 50 | 7.2 |
| Samoa | Report #4 | 2006 | Estimated | 2 (1,8) | 5.2 (1.8,18.6) | |||
| Saudi Arabia | Report #4 | 2006 | Estimated | 375 (124,1540) | 3.4 (1.1,13.6) | |||
| Senegal | Report #4 | 2006 | National; proportionate cluster | 279 | 12 | 4.3 | 26 | 9.3 |
| Serbia | Report #4 | 2005 | National; 100% cases | 1233 | 9 | 0.7 | 38 | 3.1 |
| Sierra Leone | Report #2 | 1997 | Nearly countrywide; random cluster | 130 | 4 | 3.1 | 33 | 25.4 |
| Singapore | Report #4 | 2005 | National; 100% cases | 1000 | 3 | 0.3 | 66 | 6.6 |
| Slovakia | Report #4 | 2005 | National; 100% cases | 311 | 8 | 2.6 | 21 | 6.7 |
| Slovenia | Report #4 | 2005 | National; 100% cases | 245 | 1 | 0.4 | 13 | 5.3 |
| Solomon Islands | Report #4 | 2004 | National; random cluster | 84 | 0 | 0 | 0 | 0 |
| Somalia | Report #4 | 2006 | Estimated | 412 (113,2229) | 2.1 (0.6,11.3) | |||
| South Africa | Report #3 | 2002 | National; proportionate cluster | 5708 | 175 | 3.1 | 388 | 6.8 |
| Spain | Report #4 | 2006 | Estimated | 48 (8,102) | 0.3 (0.1,0.7) | |||
| Galicia | Report #4 | 2005 | 100% cases | 634 | 2 | 0.3 | 44 | 7 |
| Aragon | Report #4 | 2005 | 100% cases | 226 | 4 | 0.8 | 14 | 6.2 |
| Barcelona | Report #4 | 2005 | 100% cases | 538 | 4 | 0.7 | 49 | 9.2 |
| Sri Lanka | Report #4 | 2006 | National; 100% cases | 624 | 1 | 0.2 | 10 | 1.6 |
| Sudan | Sharaf-Eldin et al, 200263 | 1998–1999 | Convenience; two TB clinics in Khartoum State | 4 | 33.3 | |||
| Swaziland | Report #1 | 1995 | National; proportionate cluster | 378 | 7 | 1.9 | 41 | 10.8 |
| Sweden | Report #4 | 2005 | National; 100% cases | 442 | 4 | 0.9 | 52 | 11.8 |
| Switzerland | Report #4 | 2005 | National; 100% cases | 457 | 5 | 1.1 | 19 | 4.2 |
| Syria | Report #4 | 2006 | Estimated | 287 (90,1195) | 4.4 (1.4,17.8) | |||
| Taiwan | Yu et al, 200864 | 2005 | National; 100% cases | 4 | 14.1 | |||
| Tajikistan | Report #4 | 2006 | Estimated | 3204 (1072,8916) | 20.0 (6.8,53.9) | |||
| Tanzania | Report #4 | 2007 | National; proportionate cluster | 418 | 4 | 1 | 27 | 6.4 |
| Thailand | Report #4 | 2006 | National; proportionate cluster | 1344 | 86 | 6.4 | 192 | 14.3 |
| Togo | Report #4 | 2006 | Estimated | 667 (190,3449) | 2.5 (0.7,12.6) | |||
| Tunisia | Report #4 | 2006 | Estimated | 84 (22,413) | 3.3 (0.9,15.7) | |||
| Turkey | Report #4 | 2006 | Estimated | 889 (284,3320) | 3.3 (1.1,12.3) | |||
| Turkmenistan | Report #3 | 2002 | Subnational; Dashoguz Velavat (Aral Sea Region); 100% diagnostic units | 203 | 22 | 10.8 | 71 | 35 |
| Uganda | Report #2 | 1997 | Subnational; 3 of 9 NTLP zones representing 50% of national population; proportionate cluster | 419 | 4 | 1 | 93 | 22.2 |
| Ukraine | Report #4 | 2006 | Donetsk; 100% diagnostic units | 1497 | 379 | 25.3 | 367 | 24.5 |
| United Arab Emirates | Report #4 | 2006 | Estimated | 27 (9,104) | 3.8 (1.3,14.2) | |||
| United Kingdom | Report #4 | 2005 | National; 100% cases | 4800 | 39 | 0.8 | 302 | 6.3 |
| United States | Report #4 | 2005 | National; 100% cases | 10584 | 124 | 1.2 | 1132 | 10.7 |
| Uruguay | Report #4 | 2005 | National | 368 | 2 | 0.5 | 8 | 2.2 |
| Uzbekistan | Report #4 | 2005 | Tashkent; 100% diagnostic units | 292 | 83 | 28.4 | 97 | 33.2 |
| Vanuatu | Report #4 | 2006 | National; random cluster | 29 | 0 | 0 | 1 | 3.4 |
| Venezuela | Report #3 | 1999 | National; proportionate cluster | 873 | 18 | 2.1 | 72 | 8.2 |
| Vietnam | Report #4 | 2006 | National; proportionate cluster | 826 | 84 | 4.6 | 242 | 29.3 |
| Yemen | Report #4 | 2004 | National; 100% diagnostic units | 563 | 21 | 3.7 | 39 | 7 |
| Zambia | Report #3 | 2000 | National; proportionate cluster | 489 | 9 | 1.8 | 48 | 9.9 |
| Zimbabwe | Report #1 | 1995 | Nearly countrywide; all diagnostic centers | 712 | 16 | 2.2 | 11 | 1.6 |
Reported among new TB cases only.
All reports are from the WHO/IUATLD Global Project on Anti-tuberculosis Drug Resistance Surveillance and can be accessed online at http://www.who.int/tb/publications/en/index.html.
Source population and sampling strategy are specified for those countries or regions for which sampling was conducted. In the absence of reported data, estimates were obtained with logistic regression modeling, as described in the 4th WHO/IUATLD report.
The absence of the number tested indicates that the number and proportion of MDR-TB cases are estimates.
Estimated burden and 95% confidence intervals are drawn from the 4th WHO/IUATLD report.
No information, either reported or estimated, was available for the following countries: Barbados, Grenada, Laos, Liberia, Liechtenstein, Mauritania, Monaco, Sao Tome e Principe, Suriname, Trinidad, and Tobago.
An overall lack of current data defines the situation in much of Africa. Of the 22 African countries ever evaluated, only six were surveyed in the last 5 years. In 2006, 66,711 (95% CI: 55,607 to 137,264) cases of MDR-TB were estimated to have occurred (2.2% of TB patients) on the continent. Reports of 3.9% MDR-TB among new cases in Rwanda, 18% among previously treated cases in Senegal, and widespread MDR-TB and XDR-TB in southern Africa66–69 suggest that the true burden of drug resistance in this region may be underestimated. In particular, more data are needed to refine estimates of MDR-TB in high-HIV incidence settings in Africa where an estimated 58,296 MDR-TB cases occurred; the upper confidence limit was more than two times that figure (118,506).
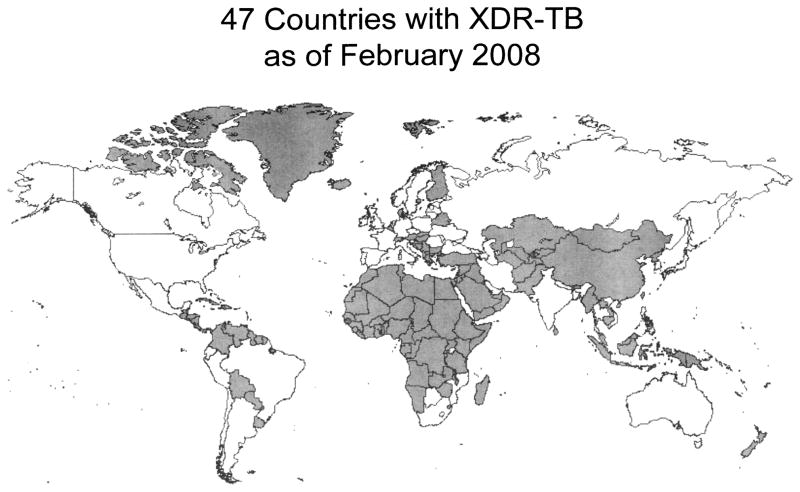
Even more uncertain is our current knowledge of the global burden of XDR-TB. The 2008 report summarized available data on XDR-TB, which has been documented in at least 46 countries (Fig. 2). Surveillance data revealed XDR-TB among 1.9% (95% CI: 1.1,3.1) of MDR-TB patients in the United States and 23.7% (95% CI: 18.5,29.5) in Estonia. Survey results ranged from 0% in Rwanda and Tanzania to 15.0% (95% CI: 3.2, 37.9) in Donetsk Oblast, Ukraine. Knowledge of the true extent of the XDR-TB problem is hampered for several reasons, in addition to those already mentioned. First, surveillance data are available from a very small number of patients in only a few countries, and only those with extensive drug susceptibility testing (DST) capacity. Second, the denominator in nearly all sites is confirmed MDR-TB, so the true burden of XDR-TB in the population remains unknown. Lastly, the short duration of surveys may yield biased results, particularly in light of oft-reported seasonal variation in TB notification.70–76
Figure 2.
Global distribution of XDRTB, reported through February 2008. Adapted from reference 2.
Nevertheless, existing data warrant intensive, rapid action. Given the heterogeneity of observed and estimated burden, a standardized approach is unlikely to be appropriate. However, certain principles of MDR-TB management apply across disparate settings and are essential to scale-up efforts.
PROGRAMMATIC MANAGEMENT OF MDR-TB
Laboratory Networks
Prominent among the challenges of scaling up MDR-TB treatment programs has been the process of building laboratory capacity. Although DOTS requires only smear microscopy, MDR-TB treatment demands culture and DST capacity for the following: individual regimen design, regional surveillance to guide standardized regimen composition, and treatment monitoring. These services are unlikely to be available at point of care in the immediate future. Implementation, therefore, requires a laboratory network to efficiently transmit samples and results between laboratories and clinical settings.
At present, many developing countries are unable to diagnose TB with certainty, much less MDR-TB or XDR-TB77–80; this is especially true in the presence of HIV coinfection.81 Countries in the process of implementing MDR-TB treatment programs must therefore include a plan to enhance laboratory infrastructure. Adequate, local DST and culture capacity may lag behind treatment capacity but should not hamper swift “roll-out” of treatment services. Instead, “bridging” infrastructure can draw from a variety of resources, such as: established, quality-assured reference laboratories abroad; laboratory capacity built through translational and operational research82; and laboratory support from local private commercial or academic laboratories.
An MDR-TB laboratory network comprises not only participating local, regional, and national laboratories, but also clinical providers and policy makers who work with laboratory directors to establish and adhere to a rational plan for DST and culture capacity consistent with National TB Program policy.82 Simple, rapid, and inexpensive DST methods, currently becoming available, should be implemented whenever possible.83–85 However, the benefits of rapid DST will be limited if delays in specimen transport, test result communication, and treatment initiation are not simultaneously addressed.86,87
Programmatic Approaches to MDR-TB Treatment
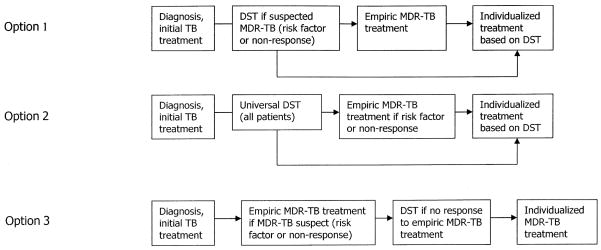
There is a broad spectrum of programmatic approaches to MDR-TB management, with variability in the following elements: (1) when to screen and treat, (2) whether to use empirical therapy prior to laboratory confirmation of MDR-TB, (3) how much to individualize the regimen, (4) how to monitor treatment response, and (5) where to deliver care. Below, we recommend approaches for each of these elements, gleaned from management experiences in many sites.88–92
Delayed initiation of appropriate treatment in suspected MDR-TB cases is associated with excess morbidity.93 Because drug resistance is often suspected before DST is performed, a proactive approach to identifying drug resistant cases optimizes the chances of timely initiation of therapy. All patients with prior TB treatment or delayed or unfavorable response to first-line treatment should be evaluated for drug resistance. Close contacts of cases with active MDR-TB—for instance, those exposed in prisons, hospitals, and households—should be screened for active disease and if confirmed, screened for MDR-TB. Moreover, in light of the poor prognosis among patients coinfected with HIV, prompt referral for diagnosis and treatment of MDR-TB in this population is critical. Additional indications for empirical MDR-TB treatment and/or DST should be guided by data on local or regional factors associated with drug resistance. Universal DST should be the ultimate goal. Figure 3 provides several examples of programmatic approaches, without being exhaustive. A common element among the examples is adherence to the principle of initiating empirical therapy when there is high clinical suspicion of MDR-TB in individual patients. The importance of timely and adequate empirical MDR therapy cannot be overemphasized. Improved outcomes have been demonstrated when patients with MDR-TB receive prompt therapy with multiple drugs that the patient has not received before.94,95 The use of inappropriate therapy (e.g., a re-treatment regimen containing only first-line drugs or regimens containing an inadequate number of second-line drugs) while awaiting susceptibility results may fail to result in clinical improvement. Moreover, drug pressure may lead to amplification of drug resistance, rendering the DST data unreliable when they are finally available. Once effective, empirical therapy has been initiated, the regimen can be optimized if susceptibility results become available.
Figure 3.
Possible strategies for screening and referral to treatment for DR-TB.
All MDR-TB treatment follows several basic principles. MDR-TB regimens are constructed using the most active drugs available. Agents used to treat MDR-TB are described in Table 2. A regimen typically consists of at least four to five drugs to which the infecting strain is likely susceptible, including a parenteral, a fluoroquinolone, and any first-line drugs to which the infecting strain is likely susceptible. Likely susceptibility is determined by several criteria, including extent of prior exposure: a patient-isolate is considered likely susceptible if the patient has not previously received the drug for more than 1 month. If the patient is a close contact of someone with MDR-TB and DST results on the new patient are not available, the contact can be presumed to have MDR-TB, although the specific resistance pattern may vary.96,97 Drugs to which in vitro resistance is laboratory confirmed—or to which the patient has a documented allergy—should also not be prescribed. If individual DST results are not available or are incomplete, regional resistance data are considered; for instance, in regions where streptomycin resistance is endemic, the regimen should use an alternative parenteral agent.
Table 2.
Chemotherapeutic Agents Used in the Treatment of Tuberculosis
| Drug Name | Description | Administration (Adult Doses) | Side Effects |
|---|---|---|---|
| FIRST-LINE DRUGS | |||
| Isoniazid (INH)1 | Nicotinic acid hydrazide. Bactericidal. Inhibits mycolic acid synthesis most effectively in dividing cells. Hepatically metabolized. | Regular dose: 300 mg or 5 mg/kg PO once a day | Incidence of adverse reactions: 5.4%. |
| High dose: 900 mg or 15 mg/kg PO 2x/wk (for strains resistant to low-dose INH) | Common: hepatitis (10–20% have elevated transaminases; INH discontinuation indicated in symptomatic hepatitis; increased risk with alcohol ingestion), peripheral neuropathy (dose-related; increased risk with malnutrition, alcoholism, diabetes, concurrent use of aminoglycosides or Prothio/Ethio) | ||
| Administer with pyridoxine 150–300 mg once a day | Less common: fever, GI upset, gynecomastia, rash (2%) | ||
| Rare: agranulocytosis, anemia, encephalopathy, eosinophilia, hypersensitivity, memory impairment, optic neuritis, positive antinuclear antibody, psychosis, seizure, thrombocytopenia, vasculitis | |||
| Drug interactions: increases phenytoin levels | |||
| Rifampin (Rifampicin, RIF) | Bactericidal. Produced by Streptomyces spp. Inhibits protein synthesis by blocking mRNA transcription and synthesis. Hepatically metabolized. | 600 mg or 10 mg/kg PO once a day | Incidence adverse reactions: <4% |
| Common: orange-colored bodily secretions; transient transaminitis | |||
| Less common: GI upset (1.5%), hepatitis | |||
| Rare: cholestatic jaundice, drowsiness, fatigue, fever (0.5%), gynecomastia, headache, pruritis, rash (0.8%), renal insufficiency, thrombocytopenia (especially in conjunction with EMB), urticaria | |||
| Drug interactions: decreased reliability of oral contraceptives, protease inhibitor (PI) levels decreased by RIF, decreased activity of drugs metabolized by P450 system (e.g., CPX, corticosteroids, dapsone, diazepam, digitoxin, fluconazole, haloperidol, methadone, oral hypoglycemics, phenytoin, quinidine, theophylline, warfarin) | |||
| Pyrazinamide (PZA) | Nicotinamide derivative. Bactericidal. Mechanism unknown. Effective in acid milieu (e.g., cavitary disease, intracellular organisms). Hepatically metabolized, renally excreted. | 25–35 mg/kg PO once a day | Common: arthropathy, hepatotoxicity, hyperuricemia |
| Less common: GI upset, impaired diabetic control, rash | |||
| Rare: dysuria, fever, hypersensitivity reactions, malaise | |||
| Drug interactions: none reported | |||
| Ethambutol (EMB) | Bacteriostatic at conventional dosing (15 mg/kg). Inhibits lipid and cell wall metabolism. Renally excreted. | 15–25 mg/kg PO once a day | Incidence adverse reactions: <2% |
| Adjust for renal insufficiency | Less common: arthralgia, GI upset, headache, malaise | ||
| Rare: disorientation, dizziness, fever (0.3%), hallucination, peripheral neuropathy, pleuritis, rash (0.5%), retrobulbar neuritis (0.8%, dose-related and reversible, increased risk with renal insufficiency) | |||
| Drug interactions: none reported | |||
| PARENTERAL AGENTS | |||
| Aminoglycosides | Bactericidal. Inhibits protein synthesis through disruption of ribosomal function. Less effective in acid, intracellular environment. Renally excreted | Amikacin: 1 g or 15 mg/kg IM/IV once a day | Incidence adverse reactions: 8.2% |
| Amikacin (AMK) | SM least nephrotoxic. AMK has been shown to be highly mycobactericidal compared with other aminoglycosides in vitro. Cross-resistance rare between SM and other aminoglycosides. Frequent cross-resistance between KM and AMK | Kanamycin: 1 g IM/IV once a day | Common: pain at injection site |
| Kanamycin (KM) | Streptomycin: 1 g or 15 m/kg IM once a day | Less common: cochlear otoxocity (hearing loss, dose-related to cumulative and peak concentrations, increased risk with renal insufficiency, may be irreversible), facial paresthesia, nephrotoxicity (dose-related to cumulative and peak concentrations, increased risk with renal insufficiency, may be irreversible), peripheral neuropathy, rash, vestibular toxicity (nausea, vomiting, and vertigo) | |
| Streptomycin (SM) | Adjust for renal insufficiency | Rare: anaphylaxis, hemolytic anemia, neuromuscular blockade, pancytopenia | |
| Every other day or biweekly dosing not recommended | Drug interactions: otoxocity potentiated by certain diuretics | ||
| Polypeptide Capreomycin (CM) | Polypeptide isolated from Streptomyces capreolus. Renally excreted. Varying degrees of cross-resistance reported between KM and CM; no cross-resistance reported between SM and CM; frequent cross-resistance between viomycin and CM. | 1 g IM once a day | Common: pain at injection site |
| Adjust for renal insufficiency | Less common: otoxocity and nephrotoxicity (dose-both to cumulative and peak concentrations, increased risk with renal insufficiency) | ||
| Every other day or biweekly dosing not recommended | Rarely: electrolyte abnormalities, eosinophilia, hypersensitivity, neuromuscular blockade | ||
| Drug interactions: Enhanced risk of neuromuscular blockade with ether anesthesia | |||
| FLUOROQUINOLONES | |||
| Ciprofloxacin (CPX) | Likely bactericidal. DNA-gyrase inhibitor. Not FDA-approved for use during pregnancy (associated with arthropathies in studies with immature animals). Renally excreted. Levofloxacin active moiety and thus possibly the drug of choice. Cross-resistance among first-generation fluoroquinolones thought to be near complete. | Ciprofloxacin: 750 mg PO twice a day | Well-tolerated, well-absorbed |
| Levofloxacin (LFX) | Sparfloxacin: 200 mg PO twice a day | Less common: diarrhea, dizziness, GI upset, headache, insomnia, photosensitivity (8% occurrence with SPX), rash, vaginitis | |
| Ofloxacin (OFX) | Ofloxacin 400 mg PO twice a day | Rare: arthralgia, interstitial nephritis, palpitations, psychosis, seizure, transaminitis (CNS effects seen almost exclusively in elderly) | |
| Sparfloxacin (SPX) | Levofloxacin 500 mg PO once a day | Drug interactions: CPX, OFX prolong half-life of theophylline with increased risk of toxicity; CaSO4 or FeSO4 and antacids with Al, Mg may inhibit GI absorption of fluoroquinolones; altered phenytoin levels (increased and decreased); exacerbated hypoglycemic effect of glyburide; increased coumadin levels reported with CPX, OFX; probenacid increases CPX, OFX levels; use of SPX contraindicated in persons receiving any drug that prolongs the Q-T interval | |
| Moxifloxacin (MFX) | Adjust doses for creatinine clearance <50 mL/min | ||
| Gatifloxacin (GFX) | |||
| OTHER SECOND-LINE DRUGS | |||
| Cycloserine (CS) | Alanine analogue. Bacteriostatic. Interferes with cell-wall proteoglycan synthesis. Renally excreted. Recommended for TB of CNS given ready penetration into CNS. | 750–1000 mg PO once a day | Common: neurological and psychiatric disturbances including headaches, irritability, tremors |
| Administer with pyridoxine 150–300 mg once a day | Less common: hypersensitivity, psychosis, peripheral neuropathy, seizures (increased risk of CNS effects with concurrent use of ethanol, INH, Prothio/Ethio, or other centrally acting medications). Neurologic adverse effects may be lessened by pyridoxine coadministration. | ||
| Increase gradually to maximum dose. | Drug interactions: phenytoin | ||
| Ethionamide (Ethio) | Derivative of isonicotinic acid. Bacteriostatic. Partial cross-resistance with thiacetazone. Hepatically metabolized, renally excreted. Efficacy profiles similar; prothionamide may cause fewer side effects. | Ethionamide: 750–1000 mg PO once a day | Common: GI upset (nausea, vomiting, abdominal pain, loss of appetite), metallic taste, hypothyroidism (especially when taken with PAS) |
| Prothionamide (Prothio) | Prothionamide: 500–1000 mg PO once a day | Less common: arthralgia, dermatitis, gynecomastia, hepatitis, impotence, peripheral neuropathy, photosensitivity | |
| Increase gradually to maximum dose | Rarely: optic neuritis, psychosis, seizure (Increased risk of CNS effects with concurrent use of ethanol, INH, CS, or other centrally acting medications) | ||
| Administer with pyridoxine 150–300 mg once a day | Drug interactions: transiently increased INH levels | ||
| Para-aminosalicylic acid (PAS) | Bacteriostatic. Hepatic acetylation, renally excreted. | 4 g PO three times a day | Incidence adverse reactions: 10% |
| Delayed-release granules should be administered with acidic food or drink | Common: GI upset (nausea, vomiting, diarrhea), hypersensitivity (5–10%), hypothyroidism (especially when taken with ethionamide) | ||
| Less common: hepatitis, electrolyte abnormalities | |||
| Drug interactions: decreased INH acetylation, decreased RIF absorption in nongranular preparation, decreased B12 uptake | |||
| Rifabutin (RFB) | Bactericidal. Rifamycin spiropiperidyl derivative. Cross-resistance with rifampin >70%. | Rifabutin: 150–300 mg PO once a day | Similar or lesser side effect profile and drug interactions compared with RIF, including reduced activity of drugs metabolized by P450 system |
| Rifapentine (RFP) | Rifapentine: 600 mg PO 2x/wk | Drug interactions: RFB interacts less with PI levels than does RIF; RFB and RFP decrease protease inhibitor levels; RFB levels increased by PIs | |
| Thiacetazone (THZ) | Weakly bactericidal. Inhibition of mycolic acid synthesis. | 150 mg PO three times a day | Common: GI upset (nausea, vomiting), hypersensitivity |
| Rare: cutaneous reactions (including Stevens-Johnson syndrome, increased risk in HIV-infected patients), jaundice, reversible bone-marrow suppression | |||
| Drug interactions: may potentiate ototoxicity of aminoglycosides | |||
| THIRD-LINE DRUGS | |||
| Amoxicillin-clavulanate | Beta-lactam antibiotic with a β-lactamase inhibitor. Bactericidal effect demonstrated in vitro. | 500 mg PO three times a day | Common: GI upset |
| Administer with food. | Less common: hypersensitivity | ||
| Drug interactions: none reported | |||
| Clarithromycin | Semisynthetic erythromycin derivative. Demonstrated efficacy against Mycobacterium avium complex; in vitro bactericidal effect on susceptible strains of Mycobacterium tuberculosis. | 500 mg PO twice a day | Well tolerated |
| Less common: GI side effects (abdominal pain, diarrhea, metallic taste) | |||
| Rare: ototoxicity | |||
| Drug interactions: increased theophylline and carbamazepine levels; use of terfenadine is contraindicated | |||
| Clofazimine | Substituted iminophenazine bright-red dye. Bacteriostatic. Transcription inhibition by binding guanine residues of mycobacterial DNA. | 200–300 mg PO once a day | Common: discoloration of skin and eyes, GI upset |
| Initiate dose at 300 mg; decrease dose to 200 mg when skin bronzes. | Less common: photosensitivity, malabsorption, severe abdominal distress due to crystal deposition | ||
| Drug interactions: none reported | |||
CNS, central nervous system; FDA, Food and Drug Administration; GI, gastrointestinal.
In addition to the foundation of four to five drugs, reinforcement with additional drugs (e.g., drugs to which resistance is possible given prior exposure but not confirmed) may be considered in cases with advanced disease and/or confirmed high-grade drug resistance. Drugs that possess in vitro efficacy without proven in vivo effect may also be included for reinforcement. None of these drugs, however, should be considered part of the foundation. Other drugs—including new compounds in clinical development—have been suggested for use against MDR-TB, but additional safety and efficacy testing is still required.98–112
Much emphasis has been placed on whether regimens are tailored to each patient’s drug susceptibility data and treatment history or standardized according to population resistance data. In reality, hybrid approaches, which combine available population and individual data, are most often applied. Irrespective of the degree of individualization, the principles of regimen design remain the same and depend on prudent attention to treatment history and timely, representative resistance data.52
Bacteriologic, clinical, and radiographic parameters all aid in monitoring treatment response. Because less active drugs may merely suppress bacillary growth without sterilization, current guidelines recommend monthly sputum smear and culture, at least prior to bacteriologic conversion. Culture conversion occurs at an average of 60 days into MDR-TB therapy and is associated with favorable treatment outcome.113 In cases of delayed culture conversion (i.e., positive after 4 months of treatment) and/or lack of clinical and/or radiographic improvement, patients should be evaluated for surgery and reinforcement of their MDR-TB regimen. Adherence to treatment should be reassessed and repeat DST considered.
There is considerable debate around the optimal frequency of bacteriologic monitoring after initial conversion. Some have argued that the risk of reconversion, as well as its associated consequences, is high enough to warrant monthly follow-up with smear and culture. Others cite programmatic and laboratory burden and suggest less frequent monitoring, especially with culture. Current guidelines recommend at least quarterly monitoring, after conversion, with both smear microscopy and culture.114 A parenteral agent should be provided for 6 months after achieving sputum-culture conversion. Oral MDR-TB treatment is recommended for at least 18 months after culture conversion.
Treatment support and directly observed therapy (DOT) of antimycobacterial agents are especially important for MDR-TB patients. Prolonged treatment with frequent adverse reactions presents adherence challenges for even the most motivated patients. Adverse events associated with MDR-TB medications, summarized in Table 2, should be managed aggressively to minimize risk of treatment default.115,116 Therapy is best supervised by individuals trained in the use of second-line drugs. Experience notes, however, that these can include specially trained lay personnel.117,118
Both inpatient119–121 and ambulatory117,122,123 models of MDR-TB care have been successful.124 Inpatient management allows close management of adverse events and facilitates treatment adherence. Ambulatory care permits increased flexibility, eliminates the bottleneck of inpatient treatment initiation, and decreases the risk of nosocomial transmission of drug resistant strains to other patients and health workers. With DOT and intensive monitoring, ambulatory care may be comparable (or superior) to inpatient modes of care.
There are several advantages to community-based MDR-TB treatment. First, scheduling and treatment locale can be flexible without compromising supervision or regularity. This permits treatment completion, usually of two doses a day for 18 to 24 months, without jeopardizing patients’ family and work obligations. Second, community-based DOT should minimize MDR-TB transmission in health centers. Third, community health workers provide additional support, including emotional and educational support; monitoring of and referral for medical and/or psychosocial problems that may threaten treatment completion; and screening of household contacts for TB. Finally, task shifting to community health workers represents an important potential contribution to health systems strengthening beyond MDR-TB treatment.
Infection Control
Infection control in health establishments, households, and laboratories is an essential component of MDR-TB management. TB infection control measures can be planned at three levels: administrative, environmental, and personal, as described by the Centers for Disease Control and Prevention (CDC). First, and most often overlooked, are practical administrative measures to separate patients according to reduce risk of (and from) transmission of TB, including MDR-TB. In resource-poor settings, it can be difficult to screen and identify TB and/or MDR-TB patients in overcrowded waiting rooms with poor ventilation and minimal staff. However, simple strategies can be employed, such as (1) separating coughing patients from others in emergency and waiting rooms; (2) separating HIV-positive individuals in emergency wards and consultation services; (3) designating different times or spaces for consultations and DOT for pansusceptible versus MDR-TB patients. Whenever possible, inpatient contact between HIV-positive patients and patients with confirmed or suspected MDR-TB should be minimized. Similarly, HIV-positive health care workers may be protected by minimizing their exposure to smear- and culture-positive MDR-TB cases.
Infection control in patient homes and communities has been largely neglected, resulting in an absence of metrics of transmission risk. Nevertheless, adapting from hospital and laboratory infection control research, several practices may reduce transmission and stigma. First, there should be adequate ventilation 125,126 and space. Households should be assessed for transmission risk, and, if necessary, renovations (such as windows, to allow cross ventilation; or construction of a separate room to allow the MDR-TB patient to sleep alone). These measures should be undertaken by TB programs or social services as part of public health efforts to reduce community transmission. Families should also be counseled on behavior modifications to reduce transmission, such as using outdoor spaces whenever possible and minimizing intimate contact (e.g., breastfeeding, sexual relations) while a family member is culture-positive. Culture-positive patients should also be discouraged from attending work or school. Conversely, myths should be dispelled (e.g., refusing to share eating utensils or refraining from close contact once the patient is culture-negative).
MEETING THE CHALLENGE
“Universal access to high-quality diagnosis and patient-centered treatment” is the first objective of the 2006 Stop TB Strategy.127 A companion document, the MDR-TB/XDR-TB Response Plan set as a target, treatment of 1.6 million MDR-TB patients by 2015.3 This number represents only 25 to 50% of estimated cases expected to occur before then.1 Even with unprecedented opportunities created through global advocacy and substantially increased funding for treatment of DR-TB, implementation remains daunting and the goal elusive. Limitations in available human resources, as well as in current diagnostic and treatment tools, give pause to scale-up efforts. The risks of accelerating scale-up with incomplete information and partial infrastructure, however, need to be weighed against the consequences of continued limited action. In this final section, we present some of the primary concerns that have been raised and propose pragmatic responses to this global crisis.
If MDR-TB Treatment Is Made Widely Available, Limited Second-Line Drugs Will Be Squandered through Emergence of Additional Resistance
MDR-TB and XDR-TB are humanmade epidemics that result from inadequate TB treatment, outdated policy, and transmission. Experiences in the former Soviet Union, parts of Asia, and Latin America reveal that historical poor TB control led to high levels of resistant TB.128–130 Motivated by a perceived need for a universal, cost-effective solution and preservation of costly, toxic, second-line drugs, the response was to impose rigid TB control,131 which relied on repeated courses of standardized treatment with first-line drugs132–134 and severely restricted the distribution of second-line drugs.135
This strategy has been extremely successful in the treatment of drug-susceptible TB. In settings of important, extant resistance, however, this approach has been markedly less successful.33–35,136 The 2004 GPADRS report suggests reasons for this failure: “present treatment practices create significant numbers of new resistant cases and amplify already present resistance,” singling out DOTS re-treatment regimens as problematic: “These results corroborate recently emerging evidence that standard re-treatment regimens containing first-line drugs for failures of standard treatment should be abandoned in some settings.”137
When second-line regimens are finally introduced in this context, they are often inadequate for the resistance profiles of circulating strains.52 In two examples, Peru and Korea, outcomes of standardized second-line treatment were poor.138,139 Evidence from Peru and South Africa—where a similar standardized regimen was used—also reveals that, not surprisingly, resistance was further aggravated.140,141 Moreover, this approach has yet to yield a detectable reduction in the overall burden of resistant disease in Peru or Korea.2 Stable and decreasing trends in drug resistance were documented, in contrast, only in places with TB control strategies that include universal DST at first TB diagnosis and treatment with second-line drugs when indicated by one or more of the following: DST, prior exposure, or contact history (Fig. 3, option 2). Examples include Hong Kong, the United States, Latvia, Estonia, and several western European countries.2
As with many infectious diseases, selection of resistant strains is inevitable in the presence of exposure to antimicrobial therapy. The rate at which they emerge, and the negative consequences of their emergence— morbidity, mortality, amplification of resistance, and transmission—can, however, be minimized through adherence to several principles. MDR-TB treatment with a consistent supply of quality-assured second-line drugs should be initiated early; advanced disease diminishes the chance of cure117,142 and increases opportunity for transmission. Suboptimal regimens containing second-line drugs should be avoided,52 and treatment adherence should be assured to impede resistance amplification. Measures must also be implemented to reduce transmission risk: these include hospitalizing only those patients who require it for medical reasons and improving administrative and environmental controls in hospitals, health centers, and communities. The GLC currently facilitates treatment consistent with these principles for a small number of TB programs that meet criteria for good TB control.
An alternative and dangerous reality, however, prevails: an estimated 71,000 patients are being treated outside GLC auspices in 2007–08; during that interval only 22,000 patients were even approved under the GLC mechanism, and many fewer treated. Outside the GLC, patients are often treated with drugs of unknown quality, with untested and unsupervised regimens. Patients are often forced to purchase their own medicines and can only do so sporadically. Consequently, although the GLC mechanism limits distribution and use of quality- assured, second-line drugs,143 non-quality-assured drugs circulate widely.
Broader, not more limited, distribution of quality-assured drugs will be essential to achievement of targets. Control over their distribution will have to be exercised at the local, rather than the international level. When administered to new patients, or patients having failed only one prior treatment, second-line regimens will require fewer toxic drugs and will achieve better outcomes.142 Nevertheless, regimens should be constructed to maximize probability of cure, not designed to “hold in reserve” some second-line drugs. The dangers of the alternatives are now well understood. And, with two new drugs already in clinical development for MDR-TB110,111—and a third showing promise in animal models101—treatment options will not always be so limited.
Global Drug Supply Is Too Limited to Permit Scale-Up
In addition to required policy changes described earlier, increased production is essential to permit wider distribution of quality-assured drugs. Current delays in delivery of orders through the GLC mechanism often exceed 6 months. Second-line drug manufacturers are stymied by inaccurate forecasting of drug needs. Lengthy lead time required to manufacture drugs, and, in some cases, shortages of raw materials, further aggravates the supply problem.† An additional obstacle to a growing drug supply is the perceived small market, represented by those initiating GLC-approved treatment.
Dramatic scale-up of second-line drug production is critical but will not occur without increased demand and/or new incentives: with more aggressive implementation of laboratory support and treatment programs, in the context of health-systems strengthening, patient numbers will increase. Supply could also be bolstered through increased prequalification of manufacturers and products through the WHO’s Essential Drugs Program. Only 17 anti-TB products have been prequalified— among them one second-line drug—whereas 147 antiretroviral drugs or combinations are on the prequalification list.144 Dozens of generic manufacturers are operating in high-burden MDR-TB countries, which have laws that preclude purchase of generic drugs made elsewhere. Enhanced access to prequalification could substantially increase the high-quality products available in and out of these settings. Lastly, novel incentives for drug discovery, development, and manufacturing must be explored.145,146
MDR-TB Cannot Be Managed without Local Laboratory Capacity
Although current protocols recommend baseline drug-susceptibility testing and frequent monitoring by sputum culture, treatment need not be delayed until these tests can be performed locally. Partnerships with reference laboratories, often with excess capacity and little TB, can be established to fill the gap while laboratory capacity is developed locally. This approach provides additional training opportunities for staff in laboratories in low TB-incidence settings. The Laboratory Strengthening Sub-Group of the DOTS Expansion Working Group of the Stop TB Partnership should facilitate these partnerships by developing a directory of laboratories interested in collaborating to ensure the successful shipment of samples and timely transmission of reliable results. These partnerships can further help to develop local laboratory capacity and facilitate external quality assurance. 82
Implementation of MDR-TB Treatment Should Follow Resistance Survey or Surveillance Data
Designing treatment strategies and forecasting drug demand are challenging without good data. Yet, available models and empirical evidence demonstrate the substantial risks associated with a “business as usual” approach33–35,140,141 while awaiting better data. Even if DOTS coverage and cure targets are achieved, frequency of failure will likely rise as the proportion of patients with resistant disease increases relative to the number of all TB patients.147 Inadequately treated patients will transmit resistant strains and ultimately die. This cycle will be accelerated in settings with high HIV prevalence due to the increased incidence of TB.148
It is essential to develop a range of mechanisms that facilitate treatment with second-line drugs in patients with risk factors for MDR-TB, or for poor outcomes. If local epidemiological data are not available to identify these groups, selection criteria should at least include patients in whom prior treatment failed to result in a sustained cure, those not responding to first-line TB treatment (especially if they are HIV coinfected), and individuals with close exposure to MDR-TB patients (e.g., household contacts, health care workers, prisoners, etc.). Empirical regimens can be constructed based on treatment history and available DST results.
There Are Too Few Hospital Beds and Too Few Health Care Workers to Scale Up MDR-TB Treatment
Inpatient initiation of MDR-TB therapy is unlikely to be the optimal strategy in settings where limited numbers of hospital beds, doctors, and nurses as well as the prohibitive cost of inpatient care result in enrollment bottlenecks. Moreover, the current lack of infection control virtually guarantees transmission to other patients and health care workers.149,150 In some settings, forced hospitalization has raised serious human rights concerns,151,152 especially in light of poor treatment outcomes.149
Ambulatory, especially community-based, treatment can alleviate these problems.153 Task-shifting to community health workers—supervised by nurses and doctors—for the bulk of the patient contact also has significant benefits in light of the human resource and financial challenges confronting many health systems. Hospitalization should be reserved for patients for whom it is medically indicated. Infection control efforts should prioritize administrative and environmental measures.
In Light of Limited Resources for TB Control, Scaled-Up Treatment of MDR-TB Is Unrealistic
Building an MDR-TB program on an already fragile and burdened TB service is daunting. Management of adverse events, HIV-coinfected patients, and often-tenuous relationships with private providers represent significant challenges. Addressing MDR-TB, however, need not threaten the health system. Rather, it can infuse additional resources, including: access to new funding sources, training of new health care workers, and integration of services.154,155 Moreover, it can be used to raise the standard of care: while still treating ~25,000 new TB patients annually in Peru, the National Tuberculosis Program (NTP) now assures culture and DST to all patients failing first-line treatment. This is especially true when ambulatory systems of treatment support are developed and used to deliver integrated primary care services. Private providers, who have been successfully engaged in the implementation of DOTS with quality-assured drugs, can also be trained to manage resistant disease. In addition, the funding climate has changed profoundly since the myths surrounding MDR-TB in resource-poor settings were first exposed in 1998156: the recent influx of international funding (e.g., from the Global Fund to Fight AIDS, Tuberculosis and Malaria and the International Drug Purchase Facility, referred to as UNITAID) and outside expertise should counter the scarcity mentality. Although the challenge of building a program is enormous, resources are no longer the obstacle.
There Is Too Little Expertise Available Globally to Support Scale Up
With GLC-approved projects functioning since 2000, there is growing global expertise in the management of MDR-TB. Consultants can be drawn from more experienced programs to support implementation and scale-up in settings with similar conditions. Regional centers of excellence in all elements of DR-TB management represent one possible approach to facilitating scale-up. Referral to the cadre of experts in management, scale-up, laboratory, and infection control, which is no longer limited to a handful of individuals from industrialized countries, should be facilitated by the MDR-TB Working Group of the STOP TB Partnership.
Treatment Recommendations Are Based on Expert Opinion, Rather than on Evidence from Randomized, Controlled Trials
Although no large-scale randomized, controlled trials of MDR-TB regimens have been implemented, a growing body of evidence from observational studies has been used to develop recommendations for MDR-TB management. 114 These recommendations do not advocate a single approach, rather they present a range of options, adaptable to local conditions. Questions about optimal drug combinations and duration persist.157 Nevertheless, programs following existing recommendations have achieved cure in up to 80% of patients.117,120,121,124 The dangers of waiting until these controversies have been resolved for broader implementation include ongoing transmission, morbidity, mortality, and generation of increasingly drug resistant strains.
CONCLUSION
We have reviewed existing data on the global burden of DR-TB, and argue that, despite gaps in knowledge, sufficient evidence exists to exhort global action. We have described a broad range of models, emphasizing the need to adapt these models based on local context. Finally, we confront some of the perceived obstacles that have resulted in exceptionally slow scale-up and urge innovation to permit achievement of targets.
Acknowledgments
We are grateful for the efforts of Eva Tomczyk in the production of this manuscript. Support for Dr. Mitnick was provided by the National Institutes of Allergy and Infectious Diseases career development award (5 K01 A1065836).
Footnotes
Results from the four reports of the Global Anti-Tuberculosis Drug Resistance Surveillance Project are adapted and summarized in Table 1. For countries for which no representative survey results have been published, data from the most recent published reports of convenience samples are included.
Personal communication, P. Zintl, chair, drug-procurement subgroup, June 2008.
References
- 1.Zignol M, Hosseini MS, Wright A, et al. Global incidence of multidrug-resistant tuberculosis. J Infect Dis. 2006;194:479–485. doi: 10.1086/505877. [DOI] [PubMed] [Google Scholar]
- 2.Anti-Tuberculosis Drug Resistance in the World: Fourth Global Report. Geneva: World Health Organization; 2008. WHO/IUATLD Global Project on Anti-Tuberculosis Drug Resistance Surveillance. [Google Scholar]
- 3.World Health Organization, STOP-TB Partnership. The Global MDR-TB & XDR-TB Response Plan 2007. WHO/HTM/TB/2007; 2007. p. 387. [Google Scholar]
- 4.Fauci AS: NIAID Tuberculosis Working Group. Multidrug-resistant and extensively drug-resistant tuberculosis: The National Institute of Allergy and Infectious Diseases Research Agenda and Recommendations for Priority Research. J Infect Dis. 2008;197:1493–1498. doi: 10.1086/587904. [DOI] [PubMed] [Google Scholar]
- 5.Matteelli A, Migliori GB, Cirillo D, Centis R, Girard E, Raviglion M. Multidrug-resistant and extensively drug-resistant Mycobacterium tuberculosis: epidemiology and control. Expert Rev Anti Infect Ther. 2007;5:857–871. doi: 10.1586/14787210.5.5.857. [DOI] [PubMed] [Google Scholar]
- 6.Sharma SK, Mohan A. Multidrug-resistant tuberculosis: a menace that threatens to destabilize tuberculosis control. Chest. 2006;130:261–272. doi: 10.1378/chest.130.1.261. [DOI] [PubMed] [Google Scholar]
- 7.Harries AD, Dye C. Tuberculosis. Ann Trop Med Parasitol. 2006;100:415–431. doi: 10.1179/136485906X91477. [DOI] [PubMed] [Google Scholar]
- 8.Wells CD, Cegielski JP, Nelson LJ, et al. HIV infection and multidrug-resistant tuberculosis: the perfect storm. J Infect Dis. 2007;196(Suppl 1):S86–S107. doi: 10.1086/518665. [DOI] [PubMed] [Google Scholar]
- 9.Yew WW, Leung CC. Management of multidrug-resistant tuberculosis: update 2007. Respirology. 2008;13:21–46. doi: 10.1111/j.1440-1843.2007.01180.x. [DOI] [PubMed] [Google Scholar]
- 10.Meya DB, McAdam KP. The TB pandemic: an old problem seeking new solutions. J Intern Med. 2007;261:309–329. doi: 10.1111/j.1365-2796.2007.01795.x. [DOI] [PubMed] [Google Scholar]
- 11.Ducati RG, Ruffino-Netto A, Basso LA, Santos DS. The resumption of consumption–a review on tuberculosis. Mem Inst Oswaldo Cruz. 2006;101:697–714. doi: 10.1590/s0074-02762006000700001. [DOI] [PubMed] [Google Scholar]
- 12.Faustini A, Hall AJ, Perucci CA. Risk factors for multidrug resistant tuberculosis in Europe: a systematic review. Thorax. 2006;61:158–163. doi: 10.1136/thx.2005.045963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saltini C. Chemotherapy and diagnosis of tuberculosis. Respir Med. 2006;100:2085–2097. doi: 10.1016/j.rmed.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 14.Johnson R, Streicher EM, Louw GE, Warren RM, van Helden PD, Victor TC. Drug resistance in Mycobacterium tuberculosis. Curr Issues Mol Biol. 2006;8:97–111. [PubMed] [Google Scholar]
- 15.Singh P, Mishra AK, Malonia SK, et al. The paradox of pyrazinamide: an update on the molecular mechanisms of pyrazinamide resistance in Mycobacteria. J Commun Dis. 2006;38:288–298. [PubMed] [Google Scholar]
- 16.Woodford N, Ellington MJ. The emergence of antibiotic resistance by mutation. Clin Microbiol Infect. 2007;13:5–18. doi: 10.1111/j.1469-0691.2006.01492.x. [DOI] [PubMed] [Google Scholar]
- 17.van Helden PD, Donald PR, Victor TC, et al. Antimicrobial resistance in tuberculosis: an international perspective. Expert Rev Anti Infect Ther. 2006;4:759–766. doi: 10.1586/14787210.4.5.759. [DOI] [PubMed] [Google Scholar]
- 18.Goldman RC, Plumley KV, Laughon BE. The evolution of extensively drug resistant tuberculosis (XDR-TB): history, status and issues for global control. Infect Disord Drug Targets. 2007;7:73–91. doi: 10.2174/187152607781001844. [DOI] [PubMed] [Google Scholar]
- 19.Shi R, Itagaki N, Sugawara I. Overview of anti-tuberculosis (TB) drugs and their resistance mechanisms. Mini Rev Med Chem. 2007;7:1177–1185. doi: 10.2174/138955707782331740. [DOI] [PubMed] [Google Scholar]
- 20.Friedland G. Tuberculosis, drug resistance, and HIV/AIDS: a triple threat. Curr Infect Dis Rep. 2007;9:252–261. doi: 10.1007/s11908-007-0039-7. [DOI] [PubMed] [Google Scholar]
- 21.Chakrabarti B, Davies PD. Key issues in multidrug-resistant tuberculosis. Future Microbiol. 2007;2:51–61. doi: 10.2217/17460913.2.1.51. [DOI] [PubMed] [Google Scholar]
- 22.David HL. Probability distribution of drug-resistant mutants in unselected populations of Mycobacterium tuberculosis. Appl Microbiol. 1970;20:810–814. doi: 10.1128/am.20.5.810-814.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anti-tuberculosis Drug Resistance in the World. Geneva: World Health Organization; 1997. WHO/IUATLD Global Project on Anti-Tuberculosis Drug Resistance Surveillance 1994–1997. [Google Scholar]
- 24.Alangaden GJ, Manavathu EK, Vakulenko SB, Zvonok NM, Lerner SA. Characterization of fluoroquinolone-resistant mutant strains of Mycobacterium tuberculosis selected in the laboratory and isolated from patients. Antimicrob Agents Chemother. 1995;39:1700–1703. doi: 10.1128/aac.39.8.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crofton J, Mitchison D. Streptomycin resistance in pulmonary tuberculosis. BMJ. 1948;2:1009–1015. doi: 10.1136/bmj.2.4588.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Medical Research Council. Streptomycin treatment of pulmonary tuberculosis: a Medical Research Council Investigation. BMJ. 1948;4582:769–782. [PMC free article] [PubMed] [Google Scholar]
- 27.Medical Research Council. Prevention of streptomycin resistance by combined chemotherapy. BMJ. 1952;1:1157–1162. [PMC free article] [PubMed] [Google Scholar]
- 28.Mitchison DA. The diagnosis and therapy of tuberculosis during the past 100 years. Am J Respir Crit Care Med. 2005;171:699–706. doi: 10.1164/rccm.200411-1603OE. [DOI] [PubMed] [Google Scholar]
- 29.American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America. Treatment of Tuberculosis. Am J Respir Crit Care Med. 2003;167:603–662. doi: 10.1164/rccm.167.4.603. [DOI] [PubMed] [Google Scholar]
- 30.An expanded DOTS framework for effective tuberculosis control. Int J Tuberc Lung Dis. 2002;6:378–388. [PubMed] [Google Scholar]
- 31.Aziz MA, Wright A, Laszlo A, et al. Epidemiology of antituberculosis drug resistance (the Global Project on Anti-tuberculosis Drug Resistance Surveillance): an updated analysis. Lancet. 2006;368:2142–2154. doi: 10.1016/S0140-6736(06)69863-2. [DOI] [PubMed] [Google Scholar]
- 32.Balabanova Y, Drobniewski F, Fedorin I, et al. The Directly Observed Therapy Short-Course (DOTS) strategy in Samara Oblast, Russian Federation. Respir Res. 2006;7:44. doi: 10.1186/1465-9921-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Espinal MA, Kim SJ, Suarez PG, et al. Standard short-course chemotherapy for drug-resistant tuberculosis: treatment outcomes in 6 countries. JAMA. 2000;283:2537–2545. doi: 10.1001/jama.283.19.2537. [DOI] [PubMed] [Google Scholar]
- 34.Coninx R, Mathieu C, Debacker M, et al. First-line tuberculosis therapy and drug-resistant Mycobacterium tuberculosis in prisons. Lancet. 1999;353:969–973. doi: 10.1016/s0140-6736(98)08341-x. [DOI] [PubMed] [Google Scholar]
- 35.Kimerling ME, Kluge H, Vezhnina N, et al. Inadequacy of the current WHO re-treatment regimen in a central Siberian prison: treatment failure and MDR-TB. Int J Tuberc Lung Dis. 1999;3:451–453. [PubMed] [Google Scholar]
- 36.Quy HT, Lan NT, Borgdorff MW, et al. Drug resistance among failure and relapse cases of tuberculosis: is the standard re-treatment regimen adequate? Int J Tuberc Lung Dis. 2003;7:631–636. [PubMed] [Google Scholar]
- 37.Migliori GB, Espinal M, Danilova ID, Punga VV, Grzemska M, Raviglione MC. Frequency of recurrence among MDR-TB cases ‘successfully’ treated with standardised short-course chemotherapy. Int J Tuberc Lung Dis. 2002;6:858–864. [PubMed] [Google Scholar]
- 38.Mak A, Thomas A, Del Granado M, Zaleskis R, Mouzafarova N, Menzies D. Influence of multidrug resistance on tuberculosis treatment outcomes with standardized regimens. Am J Respir Crit Care Med. 2008 May 29; doi: 10.1164/rccm.200802-240OC. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 39.Jeon CY, Hwang SH, Min JH, et al. Extensively drug-resistant tuberculosis in South Korea: risk factors and treatment outcomes among patients at a tertiary referral hospital. Clin Infect Dis. 2008;46:42–49. doi: 10.1086/524017. [DOI] [PubMed] [Google Scholar]
- 40.Kim HR, Hwang SS, Kim HJ, et al. Impact of extensive drug resistance on treatment outcomes in non-HIV-infected patients with multidrug-resistant tuberculosis. Clin Infect Dis. 2007;45:1290–1295. doi: 10.1086/522537. [DOI] [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention. Emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs—worldwide, 2000–2004. MMWR Morb Mortal Wkly Rep. 2006;55:301–305. [PubMed] [Google Scholar]
- 42.Migliori GB, Besozzi G, Girardi E, et al. Clinical and operational value of the extensively drug-resistant tuberculosis definition. Eur Respir J. 2007;30:623–626. doi: 10.1183/09031936.00077307. [DOI] [PubMed] [Google Scholar]
- 43.Pablos-Mendez A, Raviglione MC, Laszlo A, et al. Global surveillance for antituberculosis-drug resistance, 1994–1997. World Health Organization-International Union against Tuberculosis and Lung Disease Working Group on Anti-Tuberculosis Drug Resistance Surveillance. N Engl J Med. 1998;338:1641–1649. doi: 10.1056/NEJM199806043382301. [DOI] [PubMed] [Google Scholar]
- 44.Espinal MA, Laszlo A, Simonsen L, et al. Global trends in resistance to antituberculosis drugs. World Health Organization- International Union against Tuberculosis and Lung Disease Working Group on Anti-Tuberculosis Drug Resistance Surveillance. N Engl J Med. 2001;344:1294–1303. doi: 10.1056/NEJM200104263441706. [DOI] [PubMed] [Google Scholar]
- 45.Cohen T, Colijn C, Wright A, Zignol M, Pym A, Murray M. Challenges in estimating the total burden of drug-resistant tuberculosis. Am J Respir Crit Care Med. 2008;177:1302–1306. doi: 10.1164/rccm.200801-175PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choi JC, Lim SY, Suh GY, et al. Drug resistance rates of Mycobacterium tuberculosis at a private referral center in Korea. J Korean Med Sci. 2007;22:677–681. doi: 10.3346/jkms.2007.22.4.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lönnroth K, Lambregts K, Nhien DT, Quy HT, Diwan VK. Private pharmacies and tuberculosis control: a survey of case detection skills and reported anti-tuberculosis drug dispensing in private pharmacies in Ho Chi Minh City, Vietnam. Int J Tuberc Lung Dis. 2000;4:1052–1059. [PubMed] [Google Scholar]
- 48.Uplekar M, Pathania V, Raviglione M. Private practitioners and public health: weak links in tuberculosis control. Lancet. 2001;358:912–916. doi: 10.1016/S0140-6736(01)06076-7. [DOI] [PubMed] [Google Scholar]
- 49.Greaves F, Ouyang H, Pefole M, MacCarthy S, Cash RA. Compliance with DOTS diagnosis and treatment recommendations by private practitioners in Kerala, India. Int J Tuberc Lung Dis. 2007;11:110–112. [PubMed] [Google Scholar]
- 50.Harries AD, Maher D, Nunn P. An approach to the problems of diagnosing and treating adult smear- negative pulmonary tuberculosis in high-HIV-prevalence settings in sub-Saharan Africa. Bull World Health Organ. 1998;76:651–662. [PMC free article] [PubMed] [Google Scholar]
- 51.Storla DG, Yimer S, Bjune GA. A systematic review of delay in the diagnosis and treatment of tuberculosis. BMC Public Health. 2008;8:15. doi: 10.1186/1471-2458-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rich ML, Socci AR, Mitnick CD, et al. Representative drug susceptibility patterns for guiding design of retreatment regimens for MDR-TB. Int J Tuberc Lung Dis. 2006;10:290–296. [PubMed] [Google Scholar]
- 53.Van Deun A, Aung KJ, Chowdhury S, et al. Drug susceptibility of Mycobacterium tuberculosis in a rural area of Bangladesh and its relevance to the national treatment regimens. Int J Tuberc Lung Dis. 1999;3:143–148. [PubMed] [Google Scholar]
- 54.Sanders M, Van Deun A, Ntakirutimana D, et al. Rifampicin mono-resistant Mycobacterium tuberculosis in Bujumbura, Burundi: results of a drug resistance survey. Int J Tuberc Lung Dis. 2006;10:178–183. [PubMed] [Google Scholar]
- 55.Kelly PM, Lumb R, Pinto A, da Costa G, Sarmento J, Bastian I. Analysis of Mycobacterium tuberculosis isolates from treatment failure patients living in East Timor. Int J Tuberc Lung Dis. 2005;9:81–86. [PubMed] [Google Scholar]
- 56.Tudo G, Gonzalez J, Obama R, et al. Study of resistance to anti-tuberculosis drugs in five districts of Equatorial Guinea: rates, risk factors, genotyping of gene mutations and molecular epidemiology. Int J Tuberc Lung Dis. 2004;8:15–22. [PubMed] [Google Scholar]
- 57.Menner N, Gunther I, Orawa H, et al. High frequency of multidrug-resistant Mycobacterium tuberculosis isolates in Georgetown, Guyana. Trop Med Int Health. 2005;10:1215–1218. doi: 10.1111/j.1365-3156.2005.01521.x. [DOI] [PubMed] [Google Scholar]
- 58.Ferdinand S, Sola C, Verdol B, et al. Molecular characterization and drug resistance patterns of strains of Mycobacterium tuberculosis isolated from patients in an AIDS counseling center in Port-au-Prince, Haiti: a 1-year study. J Clin Microbiol. 2003;41:694–702. doi: 10.1128/JCM.41.2.694-702.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mokaddas E, Ahmad S, Samir I. Secular trends in susceptibility patterns of Mycobacterium tuberculosis isolates in Kuwait, 1996–2005. Int J Tuberc Lung Dis. 2008;12:319–325. [PubMed] [Google Scholar]
- 60.Salaniponi FM, Nyirenda TE, Kemp JR, Squire SB, Godfrey-Faussett P, Harries AD. Characteristics, management and outcome of patients with recurrent tuberculosis under routine programme conditions in Malawi. Int J Tuberc Lung Dis. 2003;7:948–952. [PubMed] [Google Scholar]
- 61.Kehinde AO, Obaseki FA, Ishola OC, Ibrahim KD. Multidrug resistance to Mycobacterium tuberculosis in a tertiary hospital. J Natl Med Assoc. 2007;99:1185–1189. [PMC free article] [PubMed] [Google Scholar]
- 62.Javaid A, Hasan R, Zafar A, et al. Prevalence of primary multidrug resistance to anti-tuberculosis drugs in Pakistan. Int J Tuberc Lung Dis. 2008;12:326–331. [PubMed] [Google Scholar]
- 63.Sharaf-Eldin GS, Saeed NS, Hamid ME, et al. Molecular analysis of clinical isolates of Mycobacterium tuberculosis collected from patients with persistent disease in the Khartoum region of Sudan. J Infect. 2002;44:244–251. doi: 10.1053/jinf.2001.0992. [DOI] [PubMed] [Google Scholar]
- 64.Yu MC, Wu MH, Jou R. Extensively drug-resistant tuberculosis, Taiwan. Emerg Infect Dis. 2008;14:849–850. doi: 10.3201/eid1405.071398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zager EM, McNerney R. Multidrug-resistant tuberculosis. BMC Infect Dis. 2008;8:10. doi: 10.1186/1471-2334-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kapp C. XDR tuberculosis spreads across South Africa. Lancet. 2007;369:729. doi: 10.1016/S0140-6736(07)60341-9. [DOI] [PubMed] [Google Scholar]
- 67.Jones KD, Hesketh T, Yudkin J. Extensively drug-resistant tuberculosis in sub-Saharan Africa: an emerging public-health concern. Trans R Soc Trop Med Hyg. 2008;102:219–224. doi: 10.1016/j.trstmh.2007.11.014. [DOI] [PubMed] [Google Scholar]
- 68.Alcorn K. [Accessed June 9, 2008];Two XDR TB cases reported in Botswana. http://www.aidsmap.com/en/news/1B5F0A58-9125-461F-9F37-36CC930B6E79.asp.
- 69.Sibeene P. [Accessed June 9, 2008];Namibia: No Drugs for Virulent TB Strain. http://allafrica.com/stories/200805271002.html.
- 70.Luquero FJ, Sanchez-Padilla E, Simon-Soria F, Eiros JM, Golub JE. Trend and seasonality of tuberculosis in Spain, 1996–2004. Int J Tuberc Lung Dis. 2008;12:221–224. [PubMed] [Google Scholar]
- 71.Akhtar S, Mohammad HG. Seasonality in pulmonary tuberculosis among migrant workers entering Kuwait. BMC Infect Dis. 2008;8:3. doi: 10.1186/1471-2334-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nagayama N, Ohmori M. Seasonality in various forms of tuberculosis. Int J Tuberc Lung Dis. 2006;10:1117–1122. [PubMed] [Google Scholar]
- 73.Leung CC, Yew WW, Chan TY, et al. Seasonal pattern of tuberculosis in Hong Kong. Int J Epidemiol. 2005;34:924–930. doi: 10.1093/ije/dyi080. [DOI] [PubMed] [Google Scholar]
- 74.Thorpe LE, Frieden TR, Laserson KF, Wells C, Khatri GR. Seasonality of tuberculosis in India: is it real and what does it tell us? Lancet. 2004;364:1613–1614. doi: 10.1016/S0140-6736(04)17316-9. [DOI] [PubMed] [Google Scholar]
- 75.Douglas AS, Strachan DP, Maxwell JD. Seasonality of tuberculosis: the reverse of other respiratory diseases in the UK. Thorax. 1996;51:944–946. doi: 10.1136/thx.51.9.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schaaf HS, Nel ED, Beyers N, Gie RP, Scott F, Donald PR. A decade of experience with Mycobacterium tuberculosis culture from children: a seasonal influence on incidence of childhood tuberculosis. Tuber Lung Dis. 1996;77:43–46. doi: 10.1016/s0962-8479(96)90074-x. [DOI] [PubMed] [Google Scholar]
- 77.Addressing the threat of tuberculosis caused by extensively drug-resistant Mycobacterium tuberculosis. Wkly Epidemiol Rec. 2006;81:386–390. [PubMed] [Google Scholar]
- 78.Dukes Hamilton C, Sterling TR, Blumberg HM, et al. Extensively drug-resistant tuberculosis: are we learning from history or repeating it? Clin Infect Dis. 2007;45:338–342. doi: 10.1086/519292. [DOI] [PubMed] [Google Scholar]
- 79.Fisher M. Diagnosis of MDR-TB: a developing world problem on a developed world budget. Expert Rev Mol Diagn. 2002;2:151–159. doi: 10.1586/14737159.2.2.151. [DOI] [PubMed] [Google Scholar]
- 80.Raviglione MC, Smith IM. XDR tuberculosis: implications for global public health. N Engl J Med. 2007;356:656–659. doi: 10.1056/NEJMp068273. [DOI] [PubMed] [Google Scholar]
- 81.Perkins MD, Cunningham J. Facing the crisis: improving the diagnosis of tuberculosis in the HIV era. J Infect Dis. 2007;196(Suppl 1):S15–S27. doi: 10.1086/518656. [DOI] [PubMed] [Google Scholar]
- 82.Shin SS, Yagui M, Ascencios L, et al. Scale-up of multidrug-resistant tuberculosis laboratory services, Peru. Emerg Infect Dis. 2008;14:701–708. doi: 10.3201/eid1405.070721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Martin A, Portaels F, Palomino JC. Colorimetric redoxindicator methods for the rapid detection of multidrug resistance in Mycobacterium tuberculosis: a systematic review and meta-analysis. J Antimicrob Chemother. 2007;59:175–183. doi: 10.1093/jac/dkl477. [DOI] [PubMed] [Google Scholar]
- 84.Palomino JC. Newer diagnostics for tuberculosis and multidrug resistant tuberculosis. Curr Opin Pulm Med. 2006;12:172–178. doi: 10.1097/01.mcp.0000219265.50310.9b. [DOI] [PubMed] [Google Scholar]
- 85.Palomino JC. Nonconventional and new methods in the diagnosis of tuberculosis: feasibility and applicability in the field. Eur Respir J. 2005;26:339–350. doi: 10.1183/09031936.05.00050305. [DOI] [PubMed] [Google Scholar]
- 86.Nic Fhogartaigh CJ, Vargas-Prada S, Huancare V, Lopez S, Rodriguez J, Moore DA. Physician-initiated courtesy MODS testing for TB and MDR-TB diagnosis and patient management. Int J Tuberc Lung Dis. 2008;12:555–560. [PubMed] [Google Scholar]
- 87.Yagui M, Perales MT, Asencios L, et al. Timely diagnosis of MDR-TB under program conditions: is rapid drug susceptibility testing sufficient? Int J Tuberc Lung Dis. 2006;10:838–843. [PMC free article] [PubMed] [Google Scholar]
- 88.Furin J. The clinical management of drug-resistant tuberculosis. Curr Opin Pulm Med. 2007;13:212–217. doi: 10.1097/MCP.0b013e3280f3c0b2. [DOI] [PubMed] [Google Scholar]
- 89.World Health Organization. Guidelines for the Management of Drug-Resistant Tuberculosis. Geneva, Switzerland: WHO; 2006. [Google Scholar]
- 90.Partners In Health. The PIH Guide to the Medical Management of Multidrug-Resistant Tuberculosis. Boston: Partners In Health; 2003. [Google Scholar]
- 91.Mukherjee JS, Rich ML, Socci AR, et al. Programmes and principles in treatment of multidrug-resistant tuberculosis. Lancet. 2004;363:474–481. doi: 10.1016/S0140-6736(04)15496-2. [DOI] [PubMed] [Google Scholar]
- 92.Iseman MD. Treatment of multidrug-resistant tuberculosis. N Engl J Med. 1993;329:784–791. doi: 10.1056/NEJM199309093291108. [DOI] [PubMed] [Google Scholar]
- 93.Telzak EE, Sepkowitz K, Alpert P, et al. Multidrug-resistant tuberculosis in patients without HIV infection. N Engl J Med. 1995;333:907–911. doi: 10.1056/NEJM199510053331404. [DOI] [PubMed] [Google Scholar]
- 94.Park MM, Davis AL, Schluger NW, Cohen H, Rom WN. Outcome of MDR-TB patients, 1983–1993. prolonged survival with appropriate therapy. Am J Respir Crit Care Med. 1996;153:317–324. doi: 10.1164/ajrccm.153.1.8542137. [DOI] [PubMed] [Google Scholar]
- 95.Turett GS, Telzak EE, Torian LV, et al. Improved outcomes for patients with multidrug-resistant tuberculosis. Clin Infect Dis. 1995;21:1238–1244. doi: 10.1093/clinids/21.5.1238. [DOI] [PubMed] [Google Scholar]
- 96.Schaaf HS, Shean K, Donald PR. Culture confirmed multidrug resistant tuberculosis: diagnostic delay, clinical features, and outcome. Arch Dis Child. 2003;88:1106–1111. doi: 10.1136/adc.88.12.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bayona J, Chavez-Pachas AM, Palacios E, Llaro K, Sapag R, Becerra MC. Contact investigations as a means of detection and timely treatment of persons with infectious multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2003;7(12 Suppl 3):S501–S509. [PubMed] [Google Scholar]
- 98.Brooks JV, Furney SK, Orme IM. Metronidazole therapy in mice infected with tuberculosis. Antimicrob Agents Chemother. 1999;43:1285–1288. doi: 10.1128/aac.43.5.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Paramasivan CN, Kubendiran G, Herbert D. Action of metronidazole in combination with isoniazid & rifampicin on persisting organisms in experimental murine tuberculosis. Indian J Med Res. 1998;108:115–119. [PubMed] [Google Scholar]
- 100.Watt B, Edwards JR, Rayner A, Grindey AJ, Harris G. In vitro activity of meropenem and imipenem against mycobacteria: development of a daily antibiotic dosing schedule. Tuber Lung Dis. 1992;73:134–136. doi: 10.1016/0962-8479(92)90145-A. [DOI] [PubMed] [Google Scholar]
- 101.Nuermberger E, Tyagi S, Tasneen R, et al. Powerful bactericidal and sterilizing activity of a regimen containing PA-824, moxifloxacin, and pyrazinamide in a murine model of tuberculosis. Antimicrob Agents Chemother. 2008;52:1522–1524. doi: 10.1128/AAC.00074-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rodriguez JC, Ruiz M, Lopez M, Royo G. In vitro activity of moxifloxacin, levofloxacin, gatifloxacin and linezolid against Mycobacterium tuberculosis. Int J Antimicrob Agents. 2002;20:464–467. doi: 10.1016/s0924-8579(02)00239-x. [DOI] [PubMed] [Google Scholar]
- 103.Park IN, Hong SB, Oh YM, et al. Efficacy and tolerability of daily-half dose linezolid in patients with intractable multidrug-resistant tuberculosis. J Antimicrob Chemother. 2006;58:701–704. doi: 10.1093/jac/dkl298. [DOI] [PubMed] [Google Scholar]
- 104.von der Lippe B, Sandven P, Brubakk O. Efficacy and safety of linezolid in multidrug resistant tuberculosis (MDR-TB): a report of ten cases. J Infect. 2006;52:92–96. doi: 10.1016/j.jinf.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 105.Fortun J, Martin-Davila P, Navas E, et al. Linezolid for the treatment of multidrug-resistant tuberculosis. J Antimicrob Chemother. 2005;56:180–185. doi: 10.1093/jac/dki148. [DOI] [PubMed] [Google Scholar]
- 106.Huang TS, Liu YC, Sy CL, Chen YS, Tu HZ, Chen BC. In vitro activities of linezolid against clinical isolates of Mycobacterium tuberculosis complex isolated in Taiwan over 10 years. Antimicrob Agents Chemother. 2008;52:2226–2227. doi: 10.1128/AAC.00414-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yew WW, Chau CH, Wen KH. Linezolid in the treatment of ‘difficult’ multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2008;12:345–346. [PubMed] [Google Scholar]
- 108.Barry PJ, O’Connor TM. Novel agents in the management of Mycobacterium tuberculosis disease. Curr Med Chem. 2007;14:2000–2008. doi: 10.2174/092986707781368496. [DOI] [PubMed] [Google Scholar]
- 109.Chambers HF, Turner J, Schecter GF, Kawamura M, Hopewell PC. Imipenem for treatment of tuberculosis in mice and humans. Antimicrob Agents Chemother. 2005;49:2816–2821. doi: 10.1128/AAC.49.7.2816-2821.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Matsumoto M, Hashizume H, Tomishige T, et al. OPC-67683, a nitro-dihydro-imidazooxazole derivative with promising action against tuberculosis in vitro and in mice. PLoS Med. 2006;3:e466. doi: 10.1371/journal.pmed.0030466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Andries K, Verhasselt P, Guillemont J, et al. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science. 2005;307:223–227. doi: 10.1126/science.1106753. [DOI] [PubMed] [Google Scholar]
- 112.Rustomjee R, Diacon AH, Allen J, et al. Early bactericidal activity and pharmacokinetics of the Diarylquinoline TMC 207 in pulmonary tuberculosis. Antimicrob Agents Chemother. 2008 May 27; doi: 10.1128/AAC.01204-07. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Holtz TH, Sternberg M, Kammerer S, et al. Time to sputum culture conversion in multidrug-resistant tuberculosis: predictors and relationship to treatment outcome. Ann Intern Med. 2006;144:650–659. doi: 10.7326/0003-4819-144-9-200605020-00008. [DOI] [PubMed] [Google Scholar]
- 114.World Health Organization. Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis. Geneva: World Health Organization; 2006. [Google Scholar]
- 115.Nathanson E, Gupta R, Huamani P, et al. Adverse events in the treatment of multidrug-resistant tuberculosis: results from the DOTS-Plus initiative. Int J Tuberc Lung Dis. 2004;8:1382–1384. [PubMed] [Google Scholar]
- 116.Furin JJ, Mitnick CD, Shin SS, et al. Occurrence of serious adverse effects in patients receiving community-based therapy for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2001;5:648–655. [PubMed] [Google Scholar]
- 117.Mitnick C, Bayona J, Palacios E, et al. Community-based therapy for multidrug-resistant tuberculosis in Lima, Peru. N Engl J Med. 2003;348:119–128. doi: 10.1056/NEJMoa022928. [DOI] [PubMed] [Google Scholar]
- 118.Shin S, Furin J, Bayona J, Mate K, Kim JY, Farmer P. Community-based treatment of multidrug-resistant tuberculosis in Lima, Peru: 7 years of experience. Soc Sci Med. 2004;59:1529–1539. doi: 10.1016/j.socscimed.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 119.Riekstina V, Leimane V, Holtz TH, Leimans J, Wells CD. Treatment outcome cohort analysis in an integrated DOTS and DOTS-Plus TB program in Latvia. Int J Tuberc Lung Dis. 2007;11:585–587. [PubMed] [Google Scholar]
- 120.Shin SS, Pasechnikov AD, Gelmanova IY, et al. Treatment outcomes in an integrated civilian and prison MDR-TB treatment program in Russia. Int J Tuberc Lung Dis. 2006;10:402–408. [PubMed] [Google Scholar]
- 121.Tahaoglu K, Torun T, Sevim T, et al. The treatment of multidrug-resistant tuberculosis in Turkey. N Engl J Med. 2001;345:170–174. doi: 10.1056/NEJM200107193450303. [DOI] [PubMed] [Google Scholar]
- 122.Tupasi TE, Gupta R, Quelapio MI, et al. Feasibility and cost-effectiveness of treating multidrug-resistant tuberculosis: a cohort study in the Philippines. PLoS Med. 2006;3:e352. doi: 10.1371/journal.pmed.0030352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Van Deun A, Salim MA, Das AP, Bastian I, Portaels F. Results of a standardised regimen for multidrug-resistant tuberculosis in Bangladesh. Int J Tuberc Lung Dis. 2004;8:560–567. [PubMed] [Google Scholar]
- 124.Nathanson E, Lambregts-van Weezenbeek C, Rich ML, et al. Multidrug-resistant tuberculosis management in resource-limited settings. Emerg Infect Dis. 2006;12:1389–1397. doi: 10.3201/eid1209.051618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Menzies D, Fanning A, Yuan L, FitzGerald JM. Hospital ventilation and risk for tuberculous infection in Canadian health care workers. Canadian Collaborative Group in Nosocomial Transmission of TB. Ann Intern Med. 2000;133:779–789. doi: 10.7326/0003-4819-133-10-200011210-00010. [DOI] [PubMed] [Google Scholar]
- 126.Escombe AR, Oeser CC, Gilman RH, et al. Natural ventilation for the prevention of airborne contagion. PLoS Med. 2007;4:e68. doi: 10.1371/journal.pmed.0040068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Stop TB Partnership and World Health Organization. Global Plan to Stop TB 2006–2015. Geneva: World Health Organization; 2006. [Google Scholar]
- 128.Hopewell PC, Sanchez-Hernandez M, Baron RB, Ganter B. Operational evaluation of treatment for tuberculosis: results of a “standard” 12-month regimen in Peru. Am Rev Respir Dis. 1984;129:439–443. doi: 10.1164/arrd.1984.129.3.439. [DOI] [PubMed] [Google Scholar]
- 129.Hopewell PC, Ganter B, Baron RB, Sanchez-Hernandez M. Operational evaluation of treatment for tuberculosis: results of 8- and 12-month regimens in Peru. Am Rev Respir Dis. 1985;132:737–741. doi: 10.1164/arrd.1985.132.4.737. [DOI] [PubMed] [Google Scholar]
- 130.Manalo F, Tan F, Sbarbaro JA, Iseman MD. Community-based short-course treatment of pulmonary tuberculosis in a developing nation: initial report of an eight-month, largely intermittent regimen in a population with a high prevalence of drug resistance. Am Rev Respir Dis. 1990;142(6 Pt 1):1301–1305. doi: 10.1164/ajrccm/142.6_Pt_1.1301. [DOI] [PubMed] [Google Scholar]
- 131.World Health Organization. Framework for Effective Tuberculosis Control. Geneva: World Health Organization; 1994. WHO/TB/94.179. [Google Scholar]
- 132.Dye C, Williams BG, Espinal MA, Raviglione MC. Erasing the world’s slow stain: strategies to beat multidrug- resistant tuberculosis. Science. 2002;295:2042–2046. doi: 10.1126/science.1063814. [DOI] [PubMed] [Google Scholar]
- 133.Maher D, Chaulet P, Spinaci S, Harries A. Treatment of Tuberculosis: Guidelines for National Programmes. Geneva: World Health Organization; 1997. [Google Scholar]
- 134.Espinal MA, Dye C, Raviglione MC, Kochi A. Rational “DOTS Plus” for the control of MDR-TB. Int J Tuberc Lung Dis. 1999;3:561–563. [PubMed] [Google Scholar]
- 135.Crofton J, Chaulet P, Maher D. Guidelines on the Management of Drug-Resistant Tuberculosis. Geneva: World Health Organization; 1996. [Google Scholar]
- 136.Bonnet M, Sizaire V, Kebede Y, et al. Does one size fit all? Drug resistance and standard treatments: results of six tuberculosis programmes in former Soviet countries. Int J Tuberc Lung Dis. 2005;9:1147–1154. [PubMed] [Google Scholar]
- 137.Anti-tuberculosis Drug Resistance in the World: Third Global Report. Geneva: World Health Organization; 2004. WHO/IUATLD Global Project on Anti-Tuberculosis Drug Resistance Surveillance. [Google Scholar]
- 138.Suarez PG, Floyd K, Portocarrero J, et al. Feasibility and cost-effectiveness of standardised second-line drug treatment for chronic tuberculosis patients: a national cohort study in Peru. Lancet. 2002;359:1980–1989. doi: 10.1016/S0140-6736(02)08830-X. [DOI] [PubMed] [Google Scholar]
- 139.Park SK, Lee WC, Lee DH, Mitnick CD, Han L, Seung KJ. Self-administered, standardized regimens for multidrug-resistant tuberculosis in South Korea. Int J Tuberc Lung Dis. 2004;8:361–368. [PubMed] [Google Scholar]
- 140.Pillay M, Sturm AW. Evolution of the extensively drug-resistant F15/LAM4/KZN strain of Mycobacterium tuberculosis in KwaZulu-Natal, South Africa. Clin Infect Dis. 2007;45:1409–1414. doi: 10.1086/522987. [DOI] [PubMed] [Google Scholar]
- 141.Han LL, Sloutsky A, Canales R, et al. Acquisition of drug resistance in multidrug-resistant Mycobacterium tuberculosis during directly observed empiric retreatment with standardized regimens. Int J Tuberc Lung Dis. 2005;9:818–821. [PubMed] [Google Scholar]
- 142.Leimane V, Riekstina V, Holtz TH, et al. Clinical outcome of individualised treatment of multidrug-resistant tuberculosis in Latvia: a retrospective cohort study. Lancet. 2005;365:318–326. doi: 10.1016/S0140-6736(05)17786-1. [DOI] [PubMed] [Google Scholar]
- 143.McKinsey & Company McKinsey & Company. [Accessed June 9, 2008];Independent External Evaluation of the Stop TB Partnership: Final Report. http://www.stoptb.org/resource_center/documents.asp.
- 144.World Health Organization. [Accessed June 9, 2008];Prequalification Programme: A United Nations Programme Managed by WHO. http://healthtech.who.int/pq/
- 145.Pogge T. Montreal Statement on the Human Right to Essential Medicines. Camb Q Healthc Ethics. 2007;16:97–108. doi: 10.1017/s0963180107070107. [DOI] [PubMed] [Google Scholar]
- 146.The Sixty-first World Health Assembly. [Accessed June 9, 2008];Global Strategy and Plan of Action on Public Health, Innovation and Intellectual Property. http://www.who.int/gb/ebwha/pdf_files/A61/A61_R21-en.pdf.
- 147.Blower SM, Gerberding JL. Understanding, predicting and controlling the emergence of drug- resistant tuberculosis: a theoretical framework. J Mol Med. 1998;76:624–636. doi: 10.1007/s001090050260. [DOI] [PubMed] [Google Scholar]
- 148.Nelson LJ, Talbot EA, Mwasekaga MJ, et al. Antituberculosis drug resistance and anonymous HIV surveillance in tuberculosis patients in Botswana, 2002. Lancet. 2005;366:488–490. doi: 10.1016/S0140-6736(05)67062-6. [DOI] [PubMed] [Google Scholar]
- 149.Gandhi NR, Moll A, Sturm AW, et al. Extensively drug-resistant tuberculosis as a cause of death in patients coinfected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368:1575–1580. doi: 10.1016/S0140-6736(06)69573-1. [DOI] [PubMed] [Google Scholar]
- 150.Basu S, Andrews JR, Poolman EM, et al. Prevention of nosocomial transmission of extensively drug-resistant tuberculosis in rural South African district hospitals: an epidemiological modelling study. Lancet. 2007;370:1500–1507. doi: 10.1016/S0140-6736(07)61636-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Singh JA, Upshur R, Padayatchi N. XDR-TB in South Africa: no time for denial or complacency. PLoS Med. 2007;4:e50. doi: 10.1371/journal.pmed.0040050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.McGregor SS. [Accessed 2008, June 9];Africa Urged to Isolate “Killer” TB Patients. http://www.alertnet.org/thenews/newsdesk/L23916489.htm.
- 153.Goemaere E, Ford N, Berman D, McDermid C, Cohen R. XDR-TB in South Africa: detention is not the priority. PLoS Med. 2007;4:e162. doi: 10.1371/journal.pmed.0040162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Keshavjee S, Gelmanova I, Pasechnikov A, et al. Treating multi-drug resistant tuberculosis in Tomsk, Russia: developing programs that address the linkage between poverty and disease. Ann N Y Acad Sci. 2007 Oct 22; doi: 10.1196/annals.1425.009. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 155.Bonilla C, Bayona J. Building political commitment in Peru for TB control through expansion of the DOTS strategy. Bull World Health Organ. 2007;85:402. [Google Scholar]
- 156.Farmer P, Bayona J, Becerra M, et al. The dilemma of MDR-TB in the global era. Int J Tuberc Lung Dis. 1998;2:869–876. [PubMed] [Google Scholar]
- 157.Caminero JA. Treatment of multidrug-resistant tuberculosis: evidence and controversies. Int J Tuberc Lung Dis. 2006;10:829–837. [PubMed] [Google Scholar]