Abstract
Purpose
Event-free survival following all-trans-retinoic acid (ATRA) –based therapy for acute promyelocytic leukemia (APL) averages 70% at 5 years. While arsenic trioxide (ATO) can induce remissions in 95% of relapsed patients, few studies have addressed the integration of ATO into the primary management of APL. This study examines the efficacy of a single cycle of ATO-based consolidation therapy in a treatment regimen designed to decrease exposure to other cytotoxic agents.
Patients and Methods
After induction with ATRA and daunorubicin (DRN), untreated patients with APL received 3 days of cytarabine and DRN followed by 30 doses of ATO beginning on day 8. Molecular remitters received 2 years of risk-based maintenance therapy.
Results
Forty-one of 45 patients receiving induction therapy achieved remission; four patients died (one before treatment was initiated). Thirty-seven patients received consolidation and maintenance; of these one patient relapsed (CNS) and one died in remission during maintenance therapy (hepatic sickle cell crisis). With a median follow-up of 2.7 years, estimated disease-free survival was 90%; overall survival for all patients was 88%. Despite a total anthracycline dose of only 360 mg/m2, cardiac ejection fraction decreased by ≥ 20% in 20% of patients.
Conclusion
These data, combined with other recent studies using ATO in the primary management of APL, demonstrate the important role that ATO can play in the primary management of this curable disease. Future studies should continue to focus on reducing the toxicity of treatment without increasing the relapse rate.
INTRODUCTION
The incorporation of all-trans-retinoic acid (ATRA) in the primary management of patients with acute promyelocytic leukemia (APL) revolutionized the management of this disease, increasing 5-year overall survival rates from approximately 30% to 70%.1 Current concepts in APL include the use of ATRA in induction, usually concomitant with chemotherapy, significant anthracycline administration, and maintenance therapy.1–4 At least two successful treatment regimens have included anthracyclines as the only cytotoxic agents used in the context of ATRA-based therapy.5,6
Despite these remarkable advances in disease management, most major series published to date had treatment failure rates of 30%4,7,8; the Programa de Estudio y Tratamiento de las Hemopatías Malignas (PETHEMA) trial reported event-free survival of 85%.5,9 One percent to 7% of successfully treated patients develop treatment-induced myelodysplastic syndromes (MDS).10–13 Anthracycline exposure puts this highly curable population at risk for cardiac toxicity.
The high response rate to arsenic trioxide (ATO) monotherapy in relapsed patients identifies it as one of the most active drugs in this disease.14–16 With a favorable safety profile, incorporation of ATO into the first-line treatment of patients with APL holds the potential for simplifying and reducing the toxicity of therapy. We undertook a phase II study testing the ability of ATO to substitute for at least one cycle of cytotoxic chemotherapy in the treatment of APL. The goal was to determine whether the addition of ATO to first-line APL chemotherapy could reduce the total amount of chemotherapy administered to patients with APL without affecting event-free survival.
PATIENTS AND METHODS
Patients
Previously untreated patients (ages, 5 to 75 years) with morphologic and flow cytometric documentation of APL were eligible, regardless of morphologic subtype, performance status, or comorbid disease. No patients were excluded on the basis of left ventricular ejection fraction or QT interval. Consenting patients began treatment with ATRA immediately, pending confirmation of the PML-RARα gene by cytogenetic, fluorescence in situ hybridization (FISH), and/or molecular studies. Patients found to have cytogenetic abnormalities that did not produce the PML-RARα gene rearrangement, or normal cytogenetics in absence of PML-RARα gene rearrangement, were removed from study and treated for non-APL AML. All patients gave written informed consent as approved by the institutional review boards of the individual participating institution according to the Department of Health and Human Services guidelines. The clinical trial registration number is NCT00276601.
Treatment
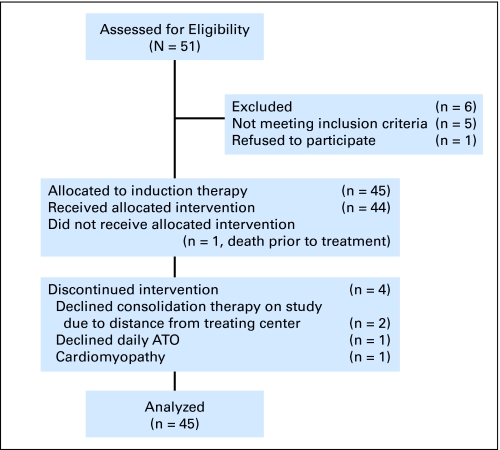
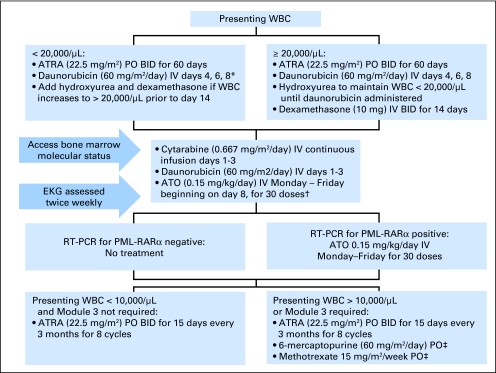
The CONSORT diagram is outlined in Figure 1 and treatment schema is shown in Figure 2.
Fig 1.
CONSORT flow chart of patient disposition. ATO, arsenic trioxide.
Fig 2.
Treatment schema. *Daunorubicin initiated earlier in case of acute promyelocytic leukemia differentiation syndrome or leukostatis. †Arsenic trioxide (ATO) was administered on an outpatient basis and was withheld only for symptomatic QT prolongation. ‡6-Mercaptopurine and methotrexate adjusted for hepatic transaminases > 300, absolute neutrophil count < 1,500/μL, or platelets < 75,000/μL. ATRA, all-trans-retinoic acid; PO, orally; IV, intravenously; EKG, electrocardiogram; RT-PCR, reverse-transcriptase polymerase chain reaction.
Induction (module 1).
ATRA/daunorubicin induction therapy was modified from the Gruppo Italiano Malattie Ematologiche dell'Adulto (GIMEMA) and PETHEMA trials5 as previously published (Fig 2).17 Patients whose presenting WBC was higher than 20,000/μL received hydroxyurea to maintain a WBC lower than 20,000/μL until daunorubicin was administered. Such patients also received prophylactic dexamethasone 10 mg every 12 hours for 14 days to prevent differentiation syndrome. Hydroxyurea and dexamethasone were also added for patients presenting with lower WBC if the count climbed to 20,000/μL before day 14. Patients with evidence of APL differentiation syndrome, as determined by individual investigators, received dexamethasone 10 mg every 12 hours for at least 14 days. ATRA administration was suspended in such patients until pulmonary symptoms resolved. Remission was assessed between days 60 and 67.
Consolidation therapy (modules 2 and 3).
All patients proceeded to module 2 regardless of achievement of CR in module 1, beginning on day 60 to 67 after initiation of module 1. Patients received cytarabine 0.667 gm/m2/d (2 gm/m2 total dose over 3 days), on days 1 to 3 by continuous intravenous infusion, and daunorubicin 60 mg/m2/d by intravenous push on day 1 through 3. ATO 0.15 mg/kg/d was given intravenously beginning on day 8, Monday through Friday, for a total of 30 doses, either as an inpatient or outpatient. After June 2007, ATO was supplied by the Cephalon Corporation; before that date, ATO was purchased through commercial suppliers.
Before initiation of module 2, routine echocardiograms were obtained on all patients. The daunorubicin dose was reduced to 30 mg/m2/d if left ventricular ejection fraction was lower than 45%. In patients whose ejection fraction was reduced to lower than 35%, institution of module 2 was delayed for 1 month and a cardiology consultation was obtained for consideration of endomyocardial biopsy. If biopsy documented anthracycline-induced cardiomyopathy, daunorubicin was discontinued. If the biopsy did not reveal anthracycline damage or was not performed, module 2 was delayed for an additional 28 days; in such cases, ATRA was reinstituted. If the ejection fraction improved to ≥ 40%, module 2 was begun. If the ejection fraction remained lower than 40%, module 2 was initiated at the discretion of the investigator, with daunorubicin being administered only after risk-benefit discussions with the patient.
An ECG (EKG) was obtained twice weekly during ATO administration. Asymptomatic patients with QT prolongation could continue to receive ATO with ongoing monitoring and electrolyte replacement despite the abnormal QT interval.
After module 2, response assessment was performed. An additional ATO-containing module (module 3), planned for patients not in molecular remission, was not required in any patient.
Maintenance therapy.
Risk-stratified maintenance therapy was administered next, with therapies and doses based on published studies.4 All patients received ATRA 22.5 mg/m2 twice daily for 15 days every 3 calendar months for eight cycles after documentation of recovery of blood counts per AML International Working Group remission criteria18 and molecular remission (negative qualitative reverse-transcriptase polymerase chain reaction [RT-PCR]; see below). Patients whose WBC on presentation was higher than 10,000/μL also received oral methotrexate 15 mg/m2/week and mercaptopurine. The planned mercaptopurine dose was decreased from 90 mg/m2/d to 60 mg/m2/d during the study based on a high rate of liver function test abnormalities. ATRA was not dose-modified during maintenance therapy. Methotrexate and mercaptopurine were modified for liver function test abnormalities and cytopenias (Fig 2).
Surveillance by Cytogenetic and Molecular Monitoring
Patients in remission were observed using standard cytogenetic analysis and both qualitative RT-PCR for bone marrow PML-RARα and quantitative real-time RT-PCR from peripheral blood (Table 1). Metaphase cytogenetic analysis and FISH for PML-RARα on bone marrow (BM) aspirates were performed in local laboratories by standard methods. Nonquantitative (qualitative), double-nested RT-PCR (QL-PCR) was performed according to the BIOMED-1 Concerted Action protocol.18 Real-time quantitative RT-PCR (RQ-PCR) was performed as previously used and described in a North American intergroup phase III APL clinical trial study (E2491).19 Details of the PCR techniques are in the Appendix (online only).20,21 Patients in remission were monitored over a 2-year period for minimal residual disease (MRD) in the peripheral blood (PB) by RQ-PCR monthly and for MRD in the BM or/and PB by QL-PCR every 3 months (Table 1). Before receiving funding for molecular monitoring, the study required only quarterly analysis of BM by QL-PCR, performed at local or commercial laboratories. Ten patients were enrolled on this initial protocol version (J0340).
Table 1.
Monitoring and Surveillance Schema
| Test by Year | Frequency |
|||
|---|---|---|---|---|
| Monthly | Every 2 Months | Every 3 Months | Every 4 Months | |
| 1-2 | ||||
| PB Q-PCR*† | X | |||
| PB qual PCR†‡ | X | |||
| BM Q-PCR† | X | |||
| BM qual PCR† | X | |||
| 3 | ||||
| PB Q-PCR | X | |||
| PB qual PCR | X | |||
| BM Q-PCR | X | |||
| BM qual PCR | X | |||
| 4-5 | ||||
| PB Q-PCR | X | |||
Abbreviations: PB, peripheral blood; Q-PCR, quantitative polymerase chain reaction; BM, bone marrow; qual, qualitative; GAPDH, glyceraldehyde-3'-phosphate dehydrogenase; NQ, normalized quotient; RT-PCR, reverse-transcriptase PCR.
Real-time PCR standardized to GAPDH copy number, and reported as NQ value: PML-RARα/GAPDH. If peripheral blood NQ > 10−6: obtain BM. If BM NQ > 10−6, but qual RT-PCR negative, resume monitoring. If BM qual RT-PCR positive but cytogenetics negative, repeat after 1 month. Patients were removed from the study if qual RT-PCR was persistently positive or cytogenetics were positive.
Samples run in triplicate.
Qual RT-PCR standardized to detect 1/104 (BIOMED-1 Concerted Action protocol).
Statistical Considerations
The primary goal of this protocol was to establish preliminary evidence that the proposed, potentially less toxic regimen, would lead to a comparable disease-free survival rate to those of current ATRA/chemotherapy-containing regimens (80%). A 3-year time point was selected because it represented a stable plateau on the disease-free survival curve in our previous trial and in published series.2,4,5 Initial accrual was planned for a sample size of 50 patients receiving consolidation therapy, which would result in a precision estimate of ± 0.14 for the proportion of patients who were disease-free. An independent data safety monitoring board convened every 6 months to examine the safety of ongoing accrual if the lower limit of the 95% CI of 1 year disease-free survival included 80%.
Accrual to the initial version of the protocol (J0340) began in January 2004; three institutions participated in the initial protocol (Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD, The Greenebaum Cancer Center, University of Maryland School of Medicine, Baltimore, MD, and Blood and Marrow Transplant Group of Georgia, Atlanta, GA). The final protocol (J0442) opened to accrual in November 2004. Accrual was suspended at most centers from November 2005 through June 2006 due to acquisition of ATO by Cephalon Corporation and contract negotiations for Cephalon to provide ATO. Accrual was terminated in December 2007 after 37 patients received consolidation therapy to ensure that funding would suffice for the required surveillance during remission, after determination that accrual of 10 additional patients would not greatly increase the precision of outcome estimation (precision ± 0.16 with 37 patients v ± 0.14 for 50 patients [standard error of mean]).
Survival analysis was performed according to the Kaplan-Meier method of using MedCalc software (Mariakerke, Belgium). All patients are included in intent-to-treat (ITT) analyses.
RESULTS
Patients
Fifty-one patients were enrolled: median age 50 years (range, 19 to 70); prognostic score (Sanz5): low (36%), intermediate (29%), high (32%). Five patients were found to lack PML-RARα and were removed from protocol; one revoked consent and was not treated on study. Thus, 45 patients with confirmed APL received induction therapy on protocol (Fig 2). Four patients died during induction, one before therapy could be administered. Two patients declined consolidation at the treating center due to travel distance and received identical therapy administered by local physicians. A third patient developed cardiomyopathy after induction and declined further intensive therapy, proceeding directly to maintenance therapy. A fourth patient declined daily ATO, receiving instead ATO three times weekly. Thus, 37 patients received consolidation (module 2). No patient expired during module 2. One patient declined further study participation due to prolonged QT interval during ATO consolidation (module 2).
Outcomes
All but three patients received 60 days of ATRA during module 1 (three patients in fact received 61 days). The median days of ATRA administration was 60 (range, 51 to 61). All 41 patients achieved a clinical remission during module 1, including 34 of 40 molecular remissions by QL-PCR. The 36 of 37 patients who completed module 2 achieved molecular remission and proceeded to planned maintenance therapy. One patient with a sickle cell variant (hemoglobin SC disease) developed hepatic failure attributed to intrahepatic sickle cell crisis while receiving 6-mercaptopurine and methotrexate and died while in remission.
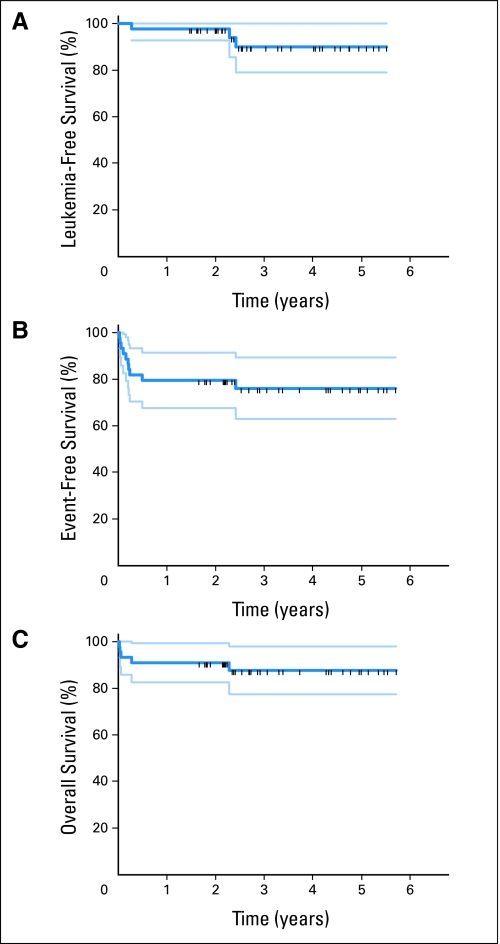
As of August 1, 2009, median follow-up of patients was 2.7 years (range, 1.5 to 5.5 years). Twenty-three patients have completed maintenance therapy; twelve patients continue to receive maintenance. A single patient has relapsed. This patient developed leptomeningeal leukemia 2.4 years into remission. No patient has developed a secondary myelodysplastic syndrome or acute myeloid leukemia to date. Figure 3A displays leukemia-free survival of patients achieving remission in the ITT population; patients who did not complete consolidation on protocol have been observed and are included in the analysis. Actuarial disease-free survival at 3 years was 88.7% ± 6%. Figure 3B demonstrates event-free survival; protocol exit for any reason is considered an event in this ITT analysis. Actuarial event-free survival was 76% ± 7%. Figure 3C depicts overall survival for the ITT population. Three-year actuarial overall survival was 88% ± 5%. Table 2 compares survival statistics from this study and other recent studies of APL.9,22–24
Fig 3.
Survival analyses (intent-to-treat population). (A) Leukemia-free survival. Long-term follow-up of patients who exited protocol prematurely is included. All relapses and deaths are considered events in this analysis. (B) Event-free survival. Premature protocol exit is considered an event. (C) Overall survival of all patients.
Table 2.
Comparison of Outcomes in Recent APL Trials
| Series | No. | Median Age (years) | Risk Group (Sanz) |
Follow-Up (years) | Overall Survival |
Disease-Free Survival |
Event-Free Survival* |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | Intermediate | High | % | SEM | % | SEM | % | SEM | ||||
| Current | 45 | 50 | 36 | 29 | 32 | 2.7 | 88 | 5 | 90 | 6 | 76 | 7 |
| C9710 ATO arm24 | 243 | NR | 28 | 45 | 24 | 2.4 | 86† | . | 77 | |||
| PETHEMA LPA999 | 410 | 37 | 18 | 57 | 25 | 5.6 | 93.7 | 86 | ||||
| APL2000 Ara-C arm9 | 178 | 43 | 24 | 30 | 46 | 5.2 | 90.5 | 85.6 | ||||
| Shanghai23 | 85 | 5.8 | 91.7 | 3 | 94.8‡ | 2.5 | 89.2 | 3.4 | ||||
| M. D. Anderson22 | 82 | 47 | 68 | 32§ | 1.9 | 84.1 | 90.6‖ | 82.9 | ||||
Abbreviations: APL, acute promyelocytic leukemia; SEM, standard error of mean; ATO, arsenic trioxide; PETHEMA, Programa de Estudio y Tratamiento de las Hemopatías Malignas; NR, not reported.
Premature protocol exit in the present study is considered an event.
From 2007 American Society of Clinical Oncology presentation online: http://media.asco.org/player/default.aspx?LectureID = 1327&conferenceFolder = vm2007&SessionFolder = 08,001&slideonly = yes&TrackID = N929&LectureTitle = Effect%20of%20consolidation%20with%20arsenic%20trioxide%20(As2O3)%20on%20event-free%20survival%20(EFS)%20and%20overall%20survival%20(OS)%20among%20patients%20with%20newly%20diagnosed%20acute%20promyelocytic% 20leukemia%20(APL):%20North%20American%20Intergroup%20Protocol%20C9710.&Key = vm_47_201_407_1327&SpeakerName = Presenter:%20Bayard%20L% 20Powell,%20MD&mediaURL = /media&ServerName = media.asco.org&max = 26&ext = jpg&useASX = false&playtype = &playtype = &playtype =.
Reported as relapse-free survival; no deaths were reported in remission.
Sanz risk score was not used in this study; rather, high risk was defined as presentation with a WBC of ≥ 10,000/μL. Actuarial survival figures are ± SEM.
Calculated from data in manuscript.
Pretreatment and postinduction echocardiograms were obtained in 29 patients. Ejection fraction decreased by a mean of 11.1% ± 3.6% (P < .005). Thirteen patients (45%) had a decrement in ejection fraction of at least 10% after induction. In six patients (21%), ejection fraction fell by more than 20% (range, 23% to 67%). The lowest ejection fraction recorded before module 2 was 20% in a 71-year-old woman with no prior cardiac history and pretreatment ejection fraction of 60%. Endomyocardial biopsy on two patients was diagnostic for anthracycline-associated cardiomyopathy. Twenty-two patients had repeat echocardiograms after module 2. Comparing ejection fraction after module 2 to baseline, the average decrement was 12.8% ± 4.6% (P = .01). Six patients had a decrement from baseline of 20% or greater (27%; range, 24% to 54%).
Intensive Molecular Surveillance
Of 36 patients who completed consolidation (module 2), 27 followed an intensive (monthly) PB molecular monitoring schedule for 2 years (Table 1). With all patients having completed at least 1 year and 12 patients having completed longer than 2 years of postconsolidation monitoring, the normalized quotient (NQ) value exceeded the increased relapse-risk value of 10−6 in only three patients. In two patients this was a transient, single point elevation (NQ = 1.08 × 10−6 and 1.63 × 10−6) and was not associated with a positive QL-PCR assay in either BM or PB or with any clinical consequence. These single point RQ-PCR elevations were not likely due to false-positive assays, however, since the succeeding one or two monthly PB samples had detectable PML-RARα at a lower level (NQ = 5.87 × 10−6 to 8.07 × 10−7), while the vast majority of RQ-PCR assay points in these and other patients were completely negative. In one patient, the PB NQ value exceeded the 10−6 risk cutoff value 22 months after the completion of consolidation, and this was succeeded by CNS relapse 6 months later. Beginning 3 months after consolidation, the NQ values in this patient became intermittently detectable in the more than 10−6 to less than 10−6 range in both PB and BM, and at 9 months postconsolidation an unconfirmed QL-PCR BM assay was positive. Thereafter, the QL-PCR assay was persistently negative in BM specimens, while the QL-PCR assays became consistently positive in PB. At CNS relapse there was no cytological evidence of APL relapse in the BM.
DISCUSSION
Combination ATRA and anthracycline-based chemotherapy (ABCT) is the currently recognized standard for the induction and consolidation therapy of newly diagnosed APL,23 with induction death rate of approximately 10% but cumulative incidence of relapse below 20%. The majority of patients with low-risk disease who achieve long-term remission and apparent cure may in fact be overtreated, potentially associated with a risk of treatment-related death during early disease remission as well as longer-term risks of secondary MDS/AML and late-developing anthracycline-related cardiomyopathy.23,24
We formulated the current protocol with the following features: a reduced overall dose of anthracycline used in only two treatment cycles, the first as a single cytotoxic agent in combination with ATRA according to the standard Italian-Spanish format5; a single course of consolidation therapy that, in addition to anthracycline, included a 3-day infusion of moderate-dose cytarabine followed by a single 30-day course of ATO; and differential treatment of high-risk patients by including methotrexate and mercaptopurine in addition to ATRA in 2 years of maintenance therapy (Fig 2; Tables 2 and 3). As detailed in the Results, among 37 patients who completed consolidation there has been only one relapse (2.7% incidence), confined to the CNS, and one death in remission, which did not involve the disease process (sickle cell crisis). Notably, with 2.7 years median follow-up none of 12 high-risk patients has relapsed, although the CNS relapse case had a rising WBC count at 9,200/uL before beginning therapy and was treated with high-risk, methotrexate and mercaptopurine–containing maintenance therapy per investigator's clinical judgment.
Table 3.
Comparison of Drug Exposure in APL Trials
| Series | No. | Anthracycline Total Dose (daunorubicin equivalent/m2)* | Ara-C Total Dose (g/m2) | ATRA Total Dose (days of 45 mg/m2/d)† | ATO Total Dose (mg/kg) | No. of Cycles Including Parenteral Chemotherapy |
|---|---|---|---|---|---|---|
| Current | 45 | 360 | 2 | 60 | 4.5 | 2 |
| C9710 ATO arm24 | 243 | 500 | 1.4 | Until CR + 14 d during consolidation | 7.5 | 5 |
| PETHEMA LPA 999 | 410 | 525-625‡ | 0 | Until CR + 45 d during consolidation | 0 | 4 |
| APL20009 | 178 | 495 | 10.8-22.4‡ | Until CR | 0 | 3 |
| Shanghai23 | 85 | 405§ | 17.7-26.7§ | Until CR‖ | 28.3§ | 4 |
| M. D. Anderson22 | 82 | 0¶ | 0 | 128¶ | 14.7 | 1 |
Abbreviations: APL, acute promyelocytic leukemia; ATRA, all-trans-retinoic acid; ATO, arsenic trioxide; PETHEMA, Programa de Estudio y Tratamiento de las Hemopatías Malignas; CR, complete response.
Daunorubicin = 1; mitoxantrone = 2.5; idarubicin = 5.
Does not include maintenance.
Selected based on Sanz risk score.
An undisclosed number received idarubicin during induction; such patients received an additional 90 mg/m2 anthracycline equivalent; such patients also received an additional 0.7 g/m2 of cytarabine. Calculation of total dose of ATO based on reported median time to CR documentation of 27 days.
ATRA at 25 mg/m2/day.
High-risk patients received a dose of gemtuzumab ozogamycin during induction. Total ATRA dose calculation based on reported median time to remission attainment of 30 days.
Although based on small numbers and requiring confirmation, the results of this trial compare very favorably to those based on current state-of-the-art, risk-adapted ATRA/ABCT regimens, while using considerably reduced amounts of anthracycline (daunorubicin or calculated equivalent), 360 mg/m2 versus 495 to 625 mg/m2 (Tables 2 and 3). These results resemble those recently reported for two other ATO-containing clinical trials (Tables 2 and 3).22,23 The M. D. Anderson trial which used either no cytotoxic chemotherapy or a single dose of gemtuzumab ozogamycin as the only cytotoxic agent (Table 2)22 led to seven (8.5%) of 82 induction deaths, and three of 75 relapses (median follow-up, 1.9 years). Four other patients died in remission (three due to second, nonhematologic malignancies; one cause unknown). The Shanghai group administered combined ATRA/ATO remission induction followed by three courses of consolidation chemotherapy including 405 mg/m2 total dose of daunorubicin (Tables 2 and 3).23 Five (5.9%) of 85 patients died during induction therapy, four (5.0%) of 80 relapsed (all in the CNS) and no deaths occurred in remission (median follow-up 5.8 years).
Our trial results also apparently resemble those of the ATO-containing arm of the North American Intergroup phase III trial C9710 in which two 25-day cycles of ATO consolidation therapy were sandwiched between ATRA/ABCT induction therapy and two courses of consolidation therapy (Tables 2 and 3).24 With a median follow-up of 3 years (reported in abstract format), the early death rate was 8% and EFS was 77% on the ATO-containing treatment arm.
The systemic monitoring of ejection fraction before and after induction therapy in this study showed that this parameter decreased by 20% or more in 21% of patients after only 180 mg/m2 of anthracycline. This raises the question of whether ATRA may sensitize the myocardium to anthracycline toxicity, or whether initial studies of anthracycline toxicities performed in solid tumor patients are applicable to patients with AML.
The aggregate data from the four ATO-based clinical trials indicate that first-line ATO therapy markedly decreases the incidence of relapse, particularly medullary relapse, in de novo APL. The data also indicate that high DFS/RFS rates can be achieved with much lower doses of anthracycline than now being used in current ATRA/ABCT regimens, regardless of pretreatment risk assessment. Together, these studies strongly support the routine incorporation of ATO into the first-line therapy of de novo APL. Future clinical trials based on combined ATRA-ATO therapy can be safely designed to include markedly reduced amounts of cytotoxic agents, potentially avoiding their recognized short-term and not fully understood long-term effects in the relatively young patient population affected by APL. The question of routine CNS prophylaxis should be addressed in future trials. However, despite great strides in minimizing relapse, the greatest barrier to curing APL remains death during induction. A concerted cooperative group effort to study supportive care strategies during induction, particularly of high-risk patients, could bring overall survival rates even closer to 100% in this curable malignancy.
Acknowledgment
We thank our data safety and monitoring board: Steven Piantadosi, MD, chair (Cedars Sinai Cancer Center, Los Angeles, CA); Richard Jones, MD (Johns Hopkins, Baltimore, MD); Jerry Spivak, MD (Johns Hopkins); and Wendy Stock, MD (University of Chicago, Chicago, IL).
Appendix
Surveillance by cytogenetic and molecular monitoring.
Patients in remission were observed using standard cytogenetic analysis and both qualitative RT-PCR for bone marrow PML-RARα and quantitative real-time RT-PCR from peripheral blood (Table 1). Metaphase cytogenetic analysis and FISH for PML-RARα on bone marrow (BM) aspirates were performed in local laboratories by standard methods. Nonquantitative (qualitative), double-nested RT-PCR (QL-PCR) was performed according to the BIOMED-1 Concerted Action protocol (van Dongen JJ, Macintyre EA, Gabert JA, et al: Leukemia 13:1901-1928, 1999). Real-time quantitative RT-PCR (RQ-PCR) was performed as previously used and described in a North American intergroup phase III APL clinical trial study (E2491) (Gallagher RE, Yeap BY, Bi W, et al: Blood 101:2521-2528, 2003). Patients in remission were monitored over a 2-year period for MRD in the peripheral blood (PB) by RQ-PCR monthly and for MRD in the BM or/and PB by QL-PCR every 3 months (Table 1). The detection sensitivities of these two assays are approximately 1 in 10−4 and 10−5, respectively. An effective detection sensitivity of 10−4 was demonstrated in all molecular monitoring assays by results obtained in parallel with an appropriate external dilution control for either the long (L) form or short (S) form of PML-RARα. Monitoring of patients with the variable (V) form of PML-RARα was performed using a commercial kit (Ipsogen Inc, Windsor, CT) or, in cases in which the upstream Ipsogen primer was determined by sequence analysis to be beyond the 5′ anchor site, by QL-PCR alone using a 5′-proximal PML exon 6 primer (Gallagher RE, Li YP, Rao S, et al: Blood 86:1540-1547, 1995). In samples with a detectable level of PML-RARα by RQ-PCR (defined as ≥ 2 of the triplicate assays with a cycle threshold, CT, < 40), the quantitative level of PML-RARα (transcript copy number) was calculated relative to the copy number of the housekeeping gene glyceraldehyde-3′-phosphate dehydrogenase (GAPDH) in the same sample (each determined by reference to dilution standard curves), and expressed as the normalized quotient (NQ; ie, NQ = PML-RARα copy number/GAPDH copy number; Gallagher RE, Yeap BY, Bi W, et al: Blood 101:2521-2528, 2003).
Molecular relapse was defined as a positive QL-PCR assay confirmed in a second BM sample by QL-PCR. If during monthly PB monitoring by RQ-PCR, the NQ value exceeded 10−6, a value associated with a hazard ratio for clinical relapse of 7.6 in the E2491 trial (Gallagher RE, Yeap BY, Bi W, et al: Blood 101:2521-2528, 2003), a BM sample was immediately obtained and evaluated by QL-PCR for potential molecular relapse by the above criteria. If this QL-PCR assay was negative, protocol treatment continued with repeat BM analysis by QL-PCR in 1 month. Any positive cytogenetic study was a basis for declaring treatment failure.
Before receiving funding for molecular monitoring, the study required only quarterly analysis of BM by QL-PCR, performed at local or commercial laboratories. Ten patients were enrolled on this initial protocol version (J0340).
Footnotes
Supported by Grants No. R01 CA100904 and K24 CA111717; the first year of the trial was supported in part by Cell Therapeutics. S.D.G. was a Scholar in Clinical Research of the Leukemia and Lymphoma Society of America.
Presented in part at the 50th Annual Meeting of the American Society of Hematology, December 6-9, 2008, New Orleans, LA.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00276601.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Ivana Gojo, Cephalon (C) Stock Ownership: None Honoraria: Ivana Gojo, Cephalon Research Funding: Steven D. Gore, Cell Therapeutics Incorporated, Cephalon Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Steven D. Gore, Ivana Gojo, Lawrence Morris, Robert Arceci, Robert E. Gallagher
Administrative support: Tianna Dauses
Provision of study materials or patients: Steven D. Gore, Ivana Gojo, Mikkael A. Sekeres, Lawrence Morris, Marcel Devetten, Katarzyna Jamieson, Robert L. Redner, Robert Arceci
Collection and assembly of data: Steven D. Gore, Ibitayo Owoeye, Tianna Dauses, Esther Schachter-Tokarz, Robert E. Gallagher
Data analysis and interpretation: Steven D. Gore, Mikkael A. Sekeres, Lawrence Morris, Marcel Devetten, Katarzyna Jamieson, Robert L. Redner, Robert Arceci, Ibitayo Owoeye, Esther Schachter-Tokarz, Robert E. Gallagher
Manuscript writing: Steven D. Gore, Ivana Gojo, Mikkael A. Sekeres, Lawrence Morris, Marcel Devetten, Katarzyna Jamieson, Robert L. Redner, Robert Arceci, Robert E. Gallagher
Final approval of manuscript: Steven D. Gore, Ivana Gojo, Mikkael A. Sekeres, Lawrence Morris, Marcel Devetten, Katarzyna Jamieson, Robert L. Redner, Robert Arceci, Ibitayo Owoeye, Tianna Dauses, Esther Schachter-Tokarz, Robert E. Gallagher
REFERENCES
- 1.Tallman MS, Nabhan C, Feusner JH, et al. Acute promyelocytic leukemia: Evolving therapeutic strategies. Blood. 2002;99:759–767. doi: 10.1182/blood.v99.3.759. [DOI] [PubMed] [Google Scholar]
- 2.Tallman MS, Andersen JW, Schiffer CA, et al. All-trans retinoic acid in acute promyelocytic leukemia: Long-term outcome and prognostic factor analysis from the North American Intergroup protocol. Blood. 2002;100:4298–4302. doi: 10.1182/blood-2002-02-0632. [DOI] [PubMed] [Google Scholar]
- 3.Fenaux P, Le Deley MC, Castaigne S, et al. Effect of all transretinoic acid in newly diagnosed acute promyelocytic leukemia. Results of a multicenter randomized trial European APL 91 Group. Blood. 1993;82:3241–3249. [PubMed] [Google Scholar]
- 4.Fenaux P, Chastang C, Chevret S, et al. A randomized comparison of all transretinoic acid (ATRA) followed by chemotherapy and ATRA plus chemotherapy and the role of maintenance therapy in newly diagnosed acute promyelocytic leukemia: The European APL Group. Blood. 1999;94:1192–1200. [PubMed] [Google Scholar]
- 5.Sanz MA, Lo CF, Martin G, et al. Definition of relapse risk and role of nonanthracycline drugs for consolidation in patients with acute promyelocytic leukemia: A joint study of the PETHEMA and GIMEMA cooperative groups. Blood. 2000;96:1247–1253. [PubMed] [Google Scholar]
- 6.Estey E, Thall PF, Pierce S, et al. Treatment of newly diagnosed acute promyelocytic leukemia without cytarabine. J Clin Oncol. 1997;15:483–490. doi: 10.1200/JCO.1997.15.2.483. [DOI] [PubMed] [Google Scholar]
- 7.Tallman MS, Andersen JW, Schiffer CA, et al. All-trans-retinoic acid in acute promyelocytic leukemia. N Engl J Med. 1997;337:1021–1028. doi: 10.1056/NEJM199710093371501. [DOI] [PubMed] [Google Scholar]
- 8.Avvisati G, Lo CF, Diverio D, et al. AIDA (all-trans retinoic acid + idarubicin) in newly diagnosed acute promyelocytic leukemia: A Gruppo Italiano Malattie Ematologiche Maligne dell'Adulto (GIMEMA) pilot study. Blood. 1996;88:1390–1398. [PubMed] [Google Scholar]
- 9.Ades L, Sanz MA, Chevret S, et al. Treatment of newly diagnosed acute promyelocytic leukemia (APL): A comparison of French-Belgian-Swiss and PETHEMA results. Blood. 2008;111:1078–1084. doi: 10.1182/blood-2007-07-099978. [DOI] [PubMed] [Google Scholar]
- 10.Latagliata R, Petti MC, Fenu S, et al. Therapy-related myelodysplastic syndrome-acute myelogenous leukemia in patients treated for acute promyelocytic leukemia: An emerging problem. Blood. 2002;99:822–824. doi: 10.1182/blood.v99.3.822. [DOI] [PubMed] [Google Scholar]
- 11.Todisco E, Testi AM, Avvisati G, et al. Therapy-related acute myelomonocytic leukemia following successful treatment for acute promyelocytic leukemia. Leukemia. 1995;9:1583–1585. [PubMed] [Google Scholar]
- 12.Garcia-Manero G, Kantarjian HM, Kornblau S, et al. Therapy-related myelodysplastic syndrome or acute myelogenous leukemia in patients with acute promyelocytic leukemia (APL). Leukemia. 2002;16:1888. doi: 10.1038/sj.leu.2402616. [DOI] [PubMed] [Google Scholar]
- 13.Lobe I, Rigal-Huguet F, Vekhoff A, et al. Myelodysplastic syndrome after acute promyelocytic leukemia: The European APL group experience. Leukemia. 2003;17:1600–1604. doi: 10.1038/sj.leu.2403034. [DOI] [PubMed] [Google Scholar]
- 14.Niu C, Yan H, Yu T, et al. Studies on treatment of acute promyelocytic leukemia with arsenic trioxide: Remission induction, follow-up, and molecular monitoring in 11 newly diagnosed and 47 relapsed acute promyelocytic leukemia patients. Blood. 1999;94:3315–3324. [PubMed] [Google Scholar]
- 15.Shen ZX, Chen GQ, Ni JH, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): II: Clinical efficacy and pharmacokinetics in relapsed patients. Blood. 1997;89:3354–3360. [PubMed] [Google Scholar]
- 16.Soignet SL, Frankel SR, Douer D, et al. United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. J Clin Oncol. 2001;19:3852–3860. doi: 10.1200/JCO.2001.19.18.3852. [DOI] [PubMed] [Google Scholar]
- 17.Gore SD, Smith BD, Gojo I, et al. Durable molecular remissions with a single cycle of timed sequential consolidation chemotherapy in acute promyelocytic leukemia. Am J Hematol. 2005;79:119–127. doi: 10.1002/ajh.20354. [DOI] [PubMed] [Google Scholar]
- 18.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for diagnosis, standardization of response criteria, treatment outcomes, and reporting standards for therapeutic trials in acute myeloid leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 19.van Dongen JJ, Macintyre EA, Gabert JA, et al. Standardized RT-PCR analysis of fusion gene transcripts from chromosome aberrations in acute leukemia for detection of minimal residual disease: Report of the BIOMED-1 Concerted Action: Investigation of minimal residual disease in acute leukemia. Leukemia. 1999;13:1901–1928. doi: 10.1038/sj.leu.2401592. [DOI] [PubMed] [Google Scholar]
- 20.Gallagher RE, Yeap BY, Bi W, et al. Quantitative real-time RT-PCR analysis of PML-RAR alpha mRNA in acute promyelocytic leukemia: Assessment of prognostic significance in adult patients from intergroup protocol 0129. Blood. 2003;101:2521–2528. doi: 10.1182/blood-2002-05-1357. [DOI] [PubMed] [Google Scholar]
- 21. Reference deleted.
- 22.Ravandi F, Estey E, Jones D, et al. Effective treatment of acute promyelocytic leukemia with all-trans-retinoic acid, arsenic trioxide, and gemtuzumab ozogamicin. J Clin Oncol. 2009;27:504–510. doi: 10.1200/JCO.2008.18.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu J, Liu YF, Wu CF, et al. Long-term efficacy and safety of all-trans retinoic acid/arsenic trioxide-based therapy in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci U S A. 2009;106:3342–3347. doi: 10.1073/pnas.0813280106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Powell BL, Moser B, Stock W, et al. Effect of consolidation with arsenic trioxide (As2O3) on event-free survival (EFS) and overall survival (OS) among patients with newly diagnosed acute promyelocytic leukemia (APL): North American Intergroup Protocol C9710. J Clin Oncol. 2007;25(suppl):1s. abstr 2. [Google Scholar]
- 25.Sanz MA, Grimwade D, Tallman MS, et al. Management of acute promyelocytic leukemia: Recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2009;113:1875–1891. doi: 10.1182/blood-2008-04-150250. [DOI] [PubMed] [Google Scholar]
- 26.Thomas X, Le QH, Fiere D. Anthracycline-related toxicity requiring cardiac transplantation in long-term disease-free survivors with acute promyelocytic leukemia. Ann Hematol. 2002;81:504–507. doi: 10.1007/s00277-002-0534-8. [DOI] [PubMed] [Google Scholar]