Abstract
Purpose
Plasma osteopontin (OPN) levels in advanced non–small-cell lung cancer (NSCLC) correlate with therapeutic response and survival, but the utility of plasma OPN for diagnosis and monitoring of early-stage NSCLC has not been investigated. We hypothesize that plasma OPN levels are elevated in early-stage NSCLC and decrease with resection.
Patients and Methods
Presurgery plasma OPN levels (in ng/mL) were measured by enzyme-linked immunosorbent assay (ELISA) in a discovery set of 60 patients with early-stage NSCLC and were compared with data from 56 cancer-free smokers. Presurgery OPN was validated in an independent cohort of 96 patients with resectable NSCLC. The presurgery levels in the latter cohort were compared with matched postsurgery levels. Perioperative OPN levels were correlated with demographics, tumor characteristics, and perioperative events. OPN was monitored during follow-up.
Results
Discovery set presurgery NSCLC OPN (271 ± 31 ng/mL) was higher than smokers (40 ± 2 ng/mL; P = .001). Presurgery OPN was similar in the NSCLC validation cohort (324 ng/mL ± 20 ng/mL; P = .134). Postsurgery OPN (256 ng/mL ± 21 ng/mL) measured at mean of 9.8 weeks (range, 2 to 46 weeks) was lower than presurgery OPN (P = .005). Time from surgery significantly impacted postsurgery OPN: OPN ≤ 6 weeks postsurgery (303 n/mL ± 26 ng/mL) was higher than OPN greater than 6 weeks postsurgery (177 ng/mL ± 29 ng/mL; P = .003). Multivariate analysis noted correlations between albumin and creatinine to presurgery OPN and use of thoracotomy to postsurgery OPN. Recurrence rate was 5% at 29 weeks mean follow-up. OPN at recurrence was elevated from postsurgery nadir.
Conclusion
Plasma OPN levels are elevated in early-stage NSCLC. They are reduced after resection and appear to increase with recurrence. Plasma OPN may have utility as a biomarker in early-stage NSCLC.
INTRODUCTION
Lung cancer is the world's leading cause of cancer death, and cure rates are less than 15%.1 Although a strong connection exists between tobacco use and the development of non–small-cell lung cancer (NSCLC), and although high-risk populations have been defined for the purpose of early detection, most patients have locally advanced or metastatic disease at the time of diagnosis. There is currently no screening modality with widely accepted efficacy. Furthermore, resection alone is the standard of care for stage I NSCLC, but 27% to 55% of patients will develop recurrence,2–4 which indicates a subgroup within this population that would benefit from additional therapies. In other solid tumors, biomarkers exist which aid in diagnosis, reliably define response to therapy, and serve as a marker for recurrence. The most clinically relevant of these include prostate-specific antigen (PSA) in prostate cancer, carcinoembryonic antigen (CEA) in colon cancer,5 and cancer antigen 125 (CA-125) in ovarian cancer. No such marker exists for NSCLC. Hypermethylation of promoters, mutations in K-ras and p53, and protein biomarkers such as CEA, cytokeratin 19 fragment (CYFRA 21-1), plasma kallikrein B1, and neuron-specific enolase6 have all been investigated, but they currently lack clinical utility.
Osteopontin (OPN) is a multifunctional glycophosphoprotein originally described as a secreted protein from malignant epithelial cells.7 It is identified in a remarkable range of normal and pathologic contexts,8 including the mediation of immune cell recruitment, wound healing, and tissue remodeling.9,10 The diverse biologic functions of OPN relate to cell adhesion, migration, and invasion and are mediated by integrin receptor binding and activation of its two highly preserved central binding domains.11
The importance of OPN in carcinogenesis and tumor dissemination is highlighted by gene transfer experiments, in which transfection of OPN increases the malignant phenotype12 and transfection with antisense oligonucleotides against OPN decreases malignant potential.13,14 OPN plays an important but poorly understood role in NSCLC pathogenesis. It is strongly overexpressed by immunohistochemistry in NSCLC tumors compared with normal lung tissue.15 Elevated plasma OPN levels in early-stage NSCLC are associated with increased hypoxic tumor conditions and an increased risk of recurrence.16 In advanced disease, elevated circulating OPN levels correlate with decreased response to therapy and poor prognosis.17,18
The utility of OPN as a biomarker has been investigated in other solid tumors. In malignant pleural mesothelioma, serum OPN levels are significantly higher than in asbestos-exposed patients without cancer.19 Cut point analysis has demonstrated that OPN values greater and less than 250 ng/mL are strongly associated with survival in mesothelioma, which characterizes OPN as a clinical biomarker with prognostic significance.20 Although elevations in circulating OPN are not unique to NSCLC, OPN appears to play a critical role in NSCLC carcinogenesis, and plasma levels have the potential to serve as an important biomarker in early-stage disease. The utility of plasma OPN levels to differentiate patients with early-stage NSCLC from smokers and high-risk populations, or as a marker of response to therapy, has not been previously investigated. We hypothesize that plasma OPN levels are elevated in patients with early-stage NSCLC compared with high-risk patients without cancer, and that resection results in a measurable reduction of the biomarker.
PATIENTS AND METHODS
We undertook a retrospective, nested-cohort study to evaluate presurgery and postsurgery plasma OPN levels in early-stage NSCLC. We compared OPN levels between presurgery patients with early-stage NSCLC and smokers without cancer, and we looked for differences between presurgery and postsurgery levels to monitor the impact of surgery on the biomarker. Because of the ubiquitous nature of OPN, we correlated perioperative OPN levels with patient demographics, tumor characteristics, and perioperative events.
Study Population
We studied three independent populations: a discovery cohort of 60 patients with early-stage NSCLC before resection and a control cohort of 56 current or former smokers with ≥ 40 pack-year smoking history without lung cancer and enrolled on a lung cancer screening trial; both groups were from Wayne State University (WSU) and were treated between 2002 and 2005. The third group was an independent validation cohort of 96 patients with early-stage NSCLC, diagnosed and treated at the New York University (NYU) School of Medicine from January 2006 to April 2009. The study was approved by the internal review board of each participating institution under an Early Detection Research Network (EDRN) –sponsored project for the detection of biomarkers in thoracic malignancies. All patients provided written informed consent for collection of blood, tissue, deidentified demographics, and clinical follow-up information. Plasma samples were collected in 10-mL EDTA tubes, were separated by centrifugation at 3,000 × g for 10 minutes, and were stored at −80°C until time of analysis.
The NYU cohort was selected by evaluating all patients with a pathologic diagnosis of NSCLC who underwent complete resection without the use of neoadjuvant chemotherapy or radiation between January 2006 and April 2009 that had matched presurgery and postsurgery plasma samples in the NYU-EDRN tissue repository. Six patients were excluded, two for second malignancies and four because initial postsurgery plasma specimens were collected greater than 52 weeks from surgery. We analyzed demographic and comorbid information, including age, sex, albumin, creatinine, history of hypertension, chronic steroid use, coronary artery disease, peripheral vascular disease, diabetes mellitus, tobacco consumption, and American Society of Anesthesiologist (ASA) class. A Glasgow Prognostic Score (ie, measure of immune reactivity in malignancy, which correlates with survival in colon cancer21) was calculated for each patient on the basis of C-reactive protein (CRP) (from the same plasma samples used to evaluate presurgery OPN levels) and was measured by Human C-Reactive Protein/CRP Quantikine ELISA kit (R&D Systems, Minneapolis, MN) and albumin from routine presurgery laboratory values. Tumor characteristics assessed included the pathologic TNM staging (according to the sixth and seventh AJCC staging system), size, lymphovascular invasion, histology, and tumor grade. Perioperative factors analyzed included the extent of resection, whether the operation was performed open or by video-assisted thoracoscopy (VATS) approach, and complications. Patients were observed serially for recurrence, and plasma OPN measurements were obtained during follow-up evaluation either at NYU visits or by primary care physicians and local oncologists.
OPN ELISA
The Human OPN ELISA Kit (ImmunoBiological Laboratories, Gunma, Japan) was used to determine plasma OPN levels as per manufacturer instruction and are reported in ng/mL. All specimens were tested blinded and in duplicate.
Statistical Analysis
The data analysis was generated by using SPSS version 13 (SPSS, Chicago, IL). The t test was used to compare mean OPN levels between the discovery cohort and the smoking and validation cohorts, and paired t test compared matched presurgery to postsurgery OPN levels. Independent t test and Spearman's correlation was used to assess correlations between plasma OPN levels and patient demographics, tumor characteristics, and perioperative events. All OPN levels are reported as mean ± SE.
RESULTS
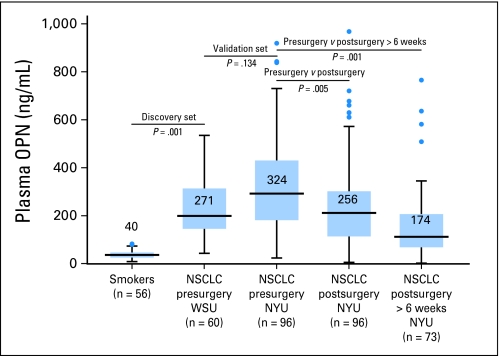
Mean plasma OPN in the discovery set of 60 patients with NSCLC from WSU (271 ng/mL ± 31 ng/mL) was significantly elevated compared with that of the cohort of smokers (40 ng/mL ± 2 ng/mL; P = .001). The presurgery OPN level was similar in the validation cohort of 96 patients with NSCLC from NYU before resection (324 ng/mL ± 20 ng/mL; P = .134). Matched postsurgery plasma OPN levels (256 ± 21 ng/mL) from the NYU cohort were significantly lower than presurgery group (P = .005). Postsurgery OPN was measured at a mean of 9.8 weeks (range, 1 to 46 weeks; Fig 2).
Fig 2.
Box plots depicting plasma osteopontin (OPN) levels in discovery set of smokers and presurgery patients with early-stage non–small-cell lung cancer (NSCLC), and in a validation cohort of patients with NSCLC who were matched at presurgery and postsurgery times. Blue boxes represent 25th-75th quartile and whiskers represent two standard deviations from the mean, outliers are demonstrated with blue dots. OPN is significantly higher in patients with NSCLC than in smokers and is similar in the validation cohort. Postsurgery OPN was significantly decreased from pre-op levels, especially greater than 6 weeks from resection. NYU, New York University; WSU, Wayne State University.
Demographics from the 96 patients in the NYU cohort are in Table 1. The cohort is highly representative of a surgical series. The majority of patients with early-stage NSCLC and limited medical comorbidities were as follows: mean age was 68.5 years, 59% were women, 76% had stage I NSCLC, and 84% were current or former smokers. Patients were generally healthy and had a median albumin of 4.3 g/dL; median Glasgow Prognostic Score, 0.28; and median ASA class, 2.7. No patients received presurgery chemotherapy or radiotherapy. The majority of tumors were resected by VATS (n = 65) and lobectomy or greater (n = 62). All patients underwent mediastinal lymph node sampling or dissection. Operative morbidity was low (15%); eight patients developed atrial arrhythmia, six had prolonged air leak, and one developed acute renal insufficiency that resolved with fluid administration. The 30-day mortality was 0%, but all patients had to have been discharged and returned for postsurgery evaluation to be included in the cohort. Resected specimens averaged 2.2 cm in greatest dimension (range, 0.5 to 6.4 cm). 84% of tumors were adenocarcinoma. Recurrence rate was 5% at a median follow-up of 29 weeks (range, 2 to 120 weeks).
Table 1.
Patient Demographics and Clinical Characteristics of NYU Validation Cohort and Comparison by Time of Initial Postsurgery OPN Measurement
| Variable | NYU Validation Cohort (n = 96) | Postsurgery OPN Measured at ≤ 6 Weeks (n = 60) | Postsurgery OPN Measured at > 6 Weeks (n = 36) | P (< 6 v > 6 weeks) |
|---|---|---|---|---|
| Presurgery OPN | ||||
| Median | 324 | 322 | 327 | .924 |
| Range | 25-918 | 25-842 | 80-918 | |
| Postsurgery OPN | ||||
| Median | 256 | 304 | 177 | .003 |
| Range | 7-1051 | 7-967 | 25-1051 | |
| Age | ||||
| Median | 68.5 | 67.6 | 69.9 | .874 |
| Range | 40-88 | 41-84 | 40-88 | |
| Sex | ||||
| Female | 57 | 36 | 21 | .750 |
| Male | 39 | 24 | 15 | |
| Tobacco use | ||||
| Never | 15 | 11 | 4 | .680 |
| Former | 69 | 40 | 29 | |
| Current | 12 | 9 | 3 | |
| Pack years | ||||
| Median | 38 | 38 | 38 | .983 |
| Range | 0-160 | 0-120 | 0-160 | |
| COPD | ||||
| Yes | 20 | 16 | 4 | .070 |
| No | 76 | 44 | 32 | |
| Diabetes mellitus | ||||
| Yes | 9 | 5 | 4 | .675 |
| No | 87 | 55 | 32 | |
| Albumin | ||||
| Median | 4.3 | 4.3 | 4.3 | .364 |
| Range | 2.9-5.0 | 3.6-5.0 | 2.9-4.9 | |
| Creatinine | ||||
| Median | 0.9 | 1.0 | 0.9 | .559 |
| Range | 0.3-6.0 | 0.3-6.0 | 0.5-1.9 | |
| Histology | ||||
| Adeno | 81 | 49 | 32 | .223 |
| Squamous | 14 | 10 | 4 | |
| NOS | 1 | 1 | 0 | |
| Lymphovascular invasion | ||||
| Present | 68 | 15 | 8 | .770 |
| Absent | 23 | 42 | 26 | |
| NR | 5 | 3 | 2 | |
| p stage | ||||
| IA | 57 | 35 | 22 | .987 |
| IB | 16 | 9 | 7 | |
| IIA | 9 | 5 | 4 | |
| IIB | 5 | 4 | 1 | |
| IIIA | 6 | 4 | 2 | |
| IV | 1 | 1 | 0 | |
| Type of resection | ||||
| Wedge | 20 | 12 | 8 | .784 |
| Segmentectomy | 14 | 12 | 2 | |
| Lobectomy | 58 | 33 | 25 | |
| Bilobectomy | 3 | 2 | 1 | |
| Pneumonectomy | 1 | 1 | 0 | |
| Mode of resection | ||||
| VATS | 65 | 41 | 24 | .867 |
| Open | 31 | 19 | 12 |
Abbreviations: NYU, New York University; OPN, osteopontin; COPD, chronic obstructive pulmonary disease; adeno, adenocarcinoma; squamous, squamous cell carcinoma; NOS, not otherwise specified; NR, not recorded; p stage, pathologic stage by the 7th edition TNM staging criteria; VATS, video-assisted thoracic surgery; open, open thoracotomy.
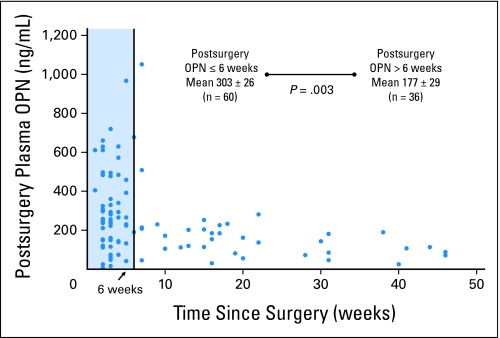
The time from surgery significantly impacted the postsurgery OPN level. When the initial postsurgery OPN measurement was made ≤ 6 weeks from surgery, the mean OPN level was 303 ng/mL ± 26 ng/mL (n = 60). This was significantly higher than when the initial postsurgery OPN measurement was greater than 6 weeks from surgery (mean, 177 ng/mL ± 29 ng/mL; n = 36; P = .003; Fig 3.) There were no significant differences between the groups who had an initial postsurgery OPN evaluation before or after 6 weeks from surgery with regard to presurgery OPN level, patient demographics, tumor characteristics, or perioperative events, as demonstrated in Table 1. The difference in plasma OPN on the basis of time from resection likely is due to the important role of OPN in wound healing. Therefore, we limited additional analysis to the first postsurgery OPN measurements made greater than 6 weeks from resection, whether that was the initial postsurgery or a subsequent postsurgery sample (Fig 1). The mean time for that sample from resection was 28 weeks.
Fig 3.
Demonstration of the effect of time from resection on the postsurgery osteopontin (OPN); each blue dot represents the initial postsurgery OPN measurement plotted against time from surgery. There is significant variability of the marker before 6 weeks (blue box), which likely was due to the OPN role in wound healing. After 6 weeks, most levels were less than 200 ng/mL.
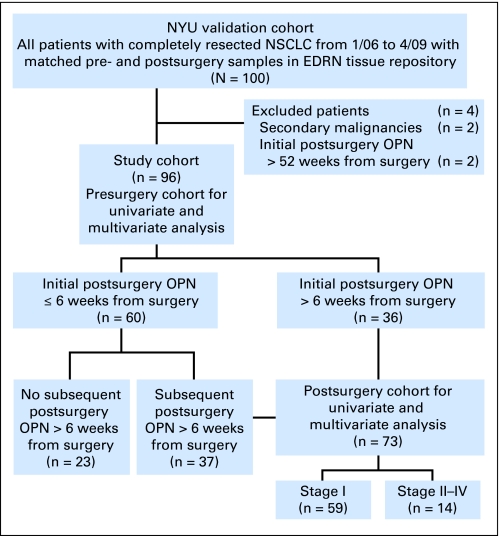
Fig 1.
RecommendCOPY COPY Marker Prognostic Studies (REMARK) diagram describes flow of the New York University (NYU) validation cohort through the study, including number of patients included in each stage of analysis.22 NSCLC, non–small-cell lung cancer; EDRN, Early Detection Research Network; OPN, osteopontin.
In analysis of patients with stage I NSCLC with postsurgery OPN measured more than 6 weeks from resection (n = 59), a significant decrease in OPN levels was appreciated as a result of surgery, from a mean presurgery of 323 ng/mL ± 26 ng/mL to a mean postsurgery level of 170 ng/mL ± 23 ng/mL (P < .001); this indicated sensitivity of the biomarker for stage I NSCLC.
Univariate analysis demonstrated a significant correlation between presurgery OPN levels and albumin, creatinine, hypertension, and coronary artery disease. When those factors with a P value less than .10 on univariate analysis were placed in multivariate analysis, significant correlations were noted between presurgery OPN and albumin and creatinine (Table 2). The postsurgery OPN level correlated with albumin, use of open thoracotomy, and presurgery OPN on univariate analysis. When those factors with a P value less than .10 were placed in multivariate analysis, a significant correlation was noted between postsurgery OPN level and the use of an open thoracotomy (Table 2).
Table 2.
Univariate and Multivariate Analysis Conducted for Pre-Operative OPN and Post-Operative OPN
| Variable | Pre-Operative OPN (n = 96) |
Post-Operative OPN (N = 73) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Univariate |
Multivariate |
Univariate |
Multivariate |
|||||||
| RR | 95% CI | P | RR | P | RR | 95% CI | P | RR | P | |
| Age | 0.023 | −25 to 130 | .075 | 0.002 | .640 | 0.030 | −46 to 118 | .141 | ||
| Sex | 0.010 | −69 to 91 | .784 | 0.026 | −92 to 73 | .825 | ||||
| Smoker (ever v never) | 0.011 | −49 to 158 | .304 | 0.002 | −134 to 95 | .730 | ||||
| Albumin | 0.105 | 44 to 194 | .001* | 0.060 | .012* | 0.014 | 0.03 to 161 | .023* | 0.001 | .866 |
| Creatinine | 0.091 | 4 to 164 | .003* | 0.068 | .006* | 0.073 | −151 to 22 | .749 | ||
| HTN | 0.040 | 0.8-to 153 | .003* | 0.020* | .160 | 0.024 | −73 to 91 | .841 | ||
| CAD | 0.068 | 28 to 207 | .010* | 0.025* | .120 | 0.046 | −191 to 6.9 | .068 | 0.006 | .529 |
| VATS v open | 0.004 | −59 to 108 | .557 | 0.077 | 18 to 181 | .017* | 0.080 | .016* | ||
| p stage (IASLC) | 0.010 | .909 | 0.010 | .942 | ||||||
| LV invasion | 0.010 | −188 to 49 | .348 | 0.014 | −147 to 49 | .322 | ||||
| Presurgery OPN | NA | NA | NA | NA | NA | .067 | .001* | 0.059 | .066 | |
Abbreviations: OPN, osteopontin; RR, relative risk; HTN, hypertension; CAD, coronary artery disease; VATS, video-assisted thoracic surgery; open, open thoracotomy; p stage, pathologic stage by the 7th edition TNM staging criteria; IASLC, International Association for the Study of Lung Cancer 2009 Lung Cancer Staging System; LV, lymphovascular; NA, not applicable.
Significant.
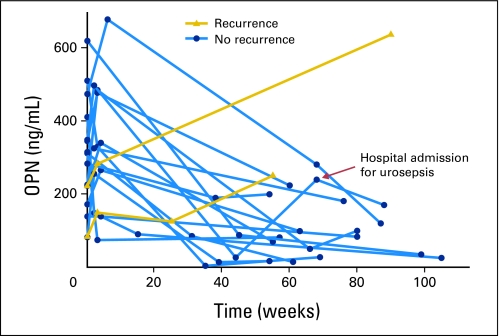
Serial OPN levels were monitored in the NYU cohort during follow-up, as patients were evaluated for recurrence. In the majority of patients, perioperative fluctuations in OPN subsided by 6 weeks after resection, after which time levels remained at or less than 200 ng/mL. At a median follow-up of 29 weeks, five patients had documented recurrent disease confirmed by biopsy. In three patients OPN levels were not available at the time of recurrence, but OPN levels were measured and noted to be increased from each patient's postsurgery nadir (Fig 4).
Fig 4.
Longitudinal trends in postsurgery plasma osteopontin (OPN); each dot or triangle represents an individual postsurgery OPN measurements plotted against time from surgery for all patients with follow-up greater than 52 weeks. In patients without recurrence, perioperative fluctuations subside by 6 weeks, and levels remained less than 200 ng/mL. Patients with recurrence had an accompanying increase in plasma OPN from their postsurgery nadirs.
All patients with plasma OPN measurements and clinical follow-up for greater than 52 weeks without evidence of disease recurrence (n = 14) had a significant reduction in plasma OPN from presurgery mean 298 ng/mL ± 41 ng/mL to a mean of 101 ± 23 postsurgery (P < .001). Of note, one patient was hospitalized for urosepsis at 70 weeks postsurgery. A corresponding elevation of plasma OPN was noted, but positron emission tomography/computed tomography suggested no evidence of disease recurrence. Subsequent plasma OPN at 90 weeks postsurgery demonstrated a reduction toward the patient's previously established postsurgery nadir (Fig 4). The fluctuation in plasma OPN was likely a result of a systemic inflammatory response with secretion of OPN by immune cell mediators associated with bacteremia.
DISCUSSION
Biomarkers ideally play a central role in the pathogenesis of the disease they identify, can be used to differentiate patients with and without the disease, carry prognostic significance, and serve as a monitoring tool for disease progression.23 NSCLC is the leading cause of cancer death in the United States and is a diagnostic marker used alone or in combination with CT imaging for screening high-risk populations and disease surveillance is essential to improving survival. Although OPN has numerous functions, and although plasma elevations are not exclusive to a diagnosis of NSCLC, we believe OPN has the potential to be an important biomarker in this setting.
OPN was first characterized in NSCLC and other solid tumors two decades ago, with demonstration of increased mRNA expression compared to normal tissues.24 OPN is overexpressed by immunohistochemistry in human NSCLC tumors compared with surrounding normal lung.15 Increased tumor OPN levels are associated with increased stage, lymph node involvement, and poor long-term survival.15,25,26
The prognostic value of circulating OPN has been investigated in a variety of human malignancies, including mesothelioma, ovarian, breast, pancreatic, and head and neck carcinomas, for which elevated OPN levels have poor prognostic significance in advanced disease.19,27–32 Characterization of plasma OPN levels in patients with NSCLC was first assessed by Hu,15 who demonstrated a median plasma OPN level of 319.1 ng/mL for patients with NSCLC of all stages, which was significantly higher than OPN levels in healthy volunteers (17.9 ng/mL) or patients with benign pulmonary disease (161.6 ng/mL).15 A comparison between patients with stage I and II NSCLC and those with stage III and IV showed a significant increase in plasma levels with more advanced disease. The considerable difference between plasma OPN levels in benign versus malignant disease introduced the potential diagnostic relevance of OPN as a biomarker for NSCLC.
In a separate analysis by Chang et al33 of patients with advanced-stage NSCLC, circulating plasma OPN correlated with stage disease. In the series by Chang et al, histology also influenced plasma OPN levels; adenocarcinomas were associated with the higher plasma levels of OPN. Mack et al17 additionally characterized circulating plasma OPN in patients with advanced NSCLC from the SWOG S0003 study population, and this correlates plasma OPN with response to therapy as well as to progression-free and overall survival. This observation of favorable prognosis associated with lower OPN levels in late-stage NSCLC was recently validated in serum.18
In this study, plasma OPN levels from patients with early-stage NSCLC were significantly elevated compared with smokers without cancer and were similar in two independent cohorts of patients with early-stage NSCLC before resection. The baseline presurgery plasma OPN levels in both cohorts were similar to those seen by Hu et al,15 Mack et al,17 and Le et al.16 The mean presurgery OPN levels from the WSU discovery set is the lowest from any of those series and appears similar to the initial postsurgery level from the NYU cohort. (Fig 2) This is likely due to the influence of early postsurgery variability of the biomarker. When analysis is limited to those measurements made greater than 6 weeks from resection, the differences are more clear (Fig 2). Statistically, the NYU presurgery cohort was compared with the discovery WSU discovery cohort using a t test, but comparisons to the initial and greater than 6 weeks NYU postsurgery cohorts were made by using a paired t test because data points are matched, allowing for a more sensitive detection of difference.
We did not note differences in plasma OPN associated with stage, histology, or lymph node involvement. This is likely due to the uniform nature of our study population and small number of patients with nonadenocarcinoma histology or disease other than stage I. Operative resection resulted in a significant reduction in plasma OPN. Postsurgery OPN levels are most reliably measured after 6 weeks because of the important role of OPN in wound healing and tissue remodeling.8,10,34–36 This provides important information as to when reliable monitoring of postsurgery OPN levels is appropriate for future studies designed to evaluate the prognostic utility of perioperative plasma OPN. After 6 weeks, postsurgery OPN plasma levels were significantly decreased toward a mean greater than 200 ng/mL and remained at or less than that level in serial follow-up. Interestingly, OPN levels never appear to decrease back to the level of healthy volunteers or smokers without cancer, but at less than 52 weeks postsurgery OPN levels decrease to levels similar to those seen by Hu et al15 in patients with benign pulmonary disease (161 ng/mL).15 This is possibly because 30% of these patients had COPD or may indicate some level of pulmonary inflammation that persisted for an extended period after surgery.
In two patients with documented recurrence, plasma OPN was elevated to greater than the postsurgery nadir at the time of recurrence. This provides preliminary evidence of a relationship between plasma OPN levels and NSCLC recurrence that warrants additional investigation. Although the utility of biomarker elevation alone as a trigger to initiate therapy is of questionable value, its utility in helping to determining recurrence with other clinical indicators has significant merit.
Univariate and multivariate analysis demonstrated correlations between perioperative plasma OPN levels and albumin, creatinine, hypertension, and open thoracotomy. This is not surprising; OPN is a multifunctional protein, and the significance of elevated plasma OPN to an NSCLC diagnosis cannot be analyzed without taking other systemic sources into account. A larger study designed to address these variables is necessary to outline their precise impacts. The important connection between OPN and wound healing was highlighted in the multivariate analysis of postsurgery OPN. A correlation was noted between open thoracotomy and elevated plasma OPN levels, but only for postsurgery OPN measurements greater than 6 weeks from resection. In the immediate postsurgery period, OPN levels in both VATS and open thoracotomy groups were elevated; however, by 6 weeks, postsurgery OPN levels in the VATS population were lower than the thoracotomy population, likely because of the more complete wound healing.
In conclusion, plasma OPN levels in patients with early-stage NSCLC are significantly elevated compared to smokers without cancer. Surgical resection results in a significant decrease in the plasma biomarker, even in stage I disease. There are significant perioperative fluctuations in plasma OPN because of the important role of OPN in wound healing and its production by immune cell mediators. Perioperative fluctuations subside by 6 weeks, which represents a reliable time to begin to monitor the biomarker. In a limited population, we noted an increase in plasma OPN with tumor recurrence, which suggests the ability of the biomarker to monitor disease progression. Additional investigations into the prognostic significance of perioperative plasma OPN levels could provide a powerful tool for surveillance and treatment in NSCLC.
Footnotes
Supported by Early Detection Research Network Grant No. U01 CA111295-03 (H.I.P.); the Stephen Banner Lung Cancer Foundation; donations from Belluck and Fox LLP; and funding from Wayne State University (H.I.P).
Presented at the 45th Annual Meeting of the American Society of Clinical Oncology, May 30-June 3, 2009, Orlando, FL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Justin D. Blasberg, Harvey I. Pass, Raja M. Flores, Jessica S. Donington
Financial support: Harvey I. Pass
Administrative support: Harvey I. Pass, Suzie Lee
Provision of study materials or patients: Harvey I. Pass, Jessica S. Donington
Collection and assembly of data: Justin D. Blasberg, Chandra M. Goparaju, Suzie Lee, Jessica S. Donington
Data analysis and interpretation: Justin D. Blasberg, Jessica S. Donington
Manuscript writing: Justin D. Blasberg, Jessica S. Donington
Final approval of manuscript: Harvey I. Pass, Chandra M. Goparaju, Raja M. Flores, Suzie Lee, Jessica S. Donington
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Nesbitt JC, Putnam JB, Jr, Walsh GL, et al. Survival in early-stage non–small-cell lung cancer. Annals Thor Surg. 1995;60:466–472. doi: 10.1016/0003-4975(95)00169-l. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman PC, Mauer AM, Vokes EE. Lung cancer. Lancet. 2000;35:479–485. doi: 10.1016/S0140-6736(00)82038-3. [DOI] [PubMed] [Google Scholar]
- 4.Rami-Porta R, Ball D, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for the revision of the T descriptors in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol. 2007;2:593–602. doi: 10.1097/JTO.0b013e31807a2f81. [DOI] [PubMed] [Google Scholar]
- 5.Chu DZ, Erickson CA, Russell MP, et al. Prognostic significance of carcinoembryonic antigen in colorectal carcinoma. Serum levels before and after resection and before recurrence. Arch Surg. 1991;126:314–316. doi: 10.1001/archsurg.1991.01410270054010. [DOI] [PubMed] [Google Scholar]
- 6.Sung HJ, Cho JY. Biomarkers for the lung cancer diagnosis and their advances in proteomics. BMB Rep. 2008;41:615–625. doi: 10.5483/bmbrep.2008.41.9.615. [DOI] [PubMed] [Google Scholar]
- 7.Senger DR, Wirth DF, Hynes RO. Transformed mammalian cells secrete specific proteins and phosphoproteins. Cell. 1979;16:885–893. doi: 10.1016/0092-8674(79)90103-x. [DOI] [PubMed] [Google Scholar]
- 8.O'Regan A. The role of osteopontin in lung disease. Cytokine Growth Factor Rev. 2003;14:479–488. doi: 10.1016/s1359-6101(03)00055-8. [DOI] [PubMed] [Google Scholar]
- 9.Ashkar S, Weber GF, Panoutsakopoulou V, et al. Eta-1 (osteopontin): An early component of type-1 (cell-mediated) immunity. Science. 2000;287:860–864. doi: 10.1126/science.287.5454.860. [DOI] [PubMed] [Google Scholar]
- 10.Liaw L, Birk DE, Ballas CB, et al. Altered wound healing in mice lacking a functional osteopontin gene. J Clin Invest. 1998;101(suppl 1):1468–1478. doi: 10.1172/JCI1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das R, Philip S, Mahabeleshwar GH, et al. Osteopontin: Its role in regulation of cell motility and nuclear factor kappa B-mediated urokinase type plasminogen activator expression. IUBMB Life. 2005;57:441–447. doi: 10.1080/15216540500159424. [DOI] [PubMed] [Google Scholar]
- 12.Denhardt DT, Guo X. Osteopontin: A protein with diverse functions. Faseb J. 1993;7:1475–1482. [PubMed] [Google Scholar]
- 13.Behrend EI, Craig AM, Wilson SM, et al. Reduced malignancy of ras-transformed NIH 3T3 cells expressing antisense osteopontin RNA. Cancer Res. 1994;54:832–837. [PubMed] [Google Scholar]
- 14.Gardner HA, Berse B, Senger DR. Specific reduction in osteopontin synthesis by antisense RNA inhibits the tumorigenicity of transformed Rat1 fibroblasts. Oncogene. 1994;9:2321–2326. [PubMed] [Google Scholar]
- 15.Hu Z, Lin D, Yuan J, et al. Overexpression of osteopontin is associated with more aggressive phenotypes in human non—small-cell lung cancer. Clin Cancer Res. 2005;11:4646–4652. doi: 10.1158/1078-0432.CCR-04-2013. [DOI] [PubMed] [Google Scholar]
- 16.Le QT, Chen E, Salim A, et al. An evaluation of tumor oxygenation and gene expression in patients with early stage non–small-cell lung cancers. Clin Cancer Res. 2006;12:1507–1514. doi: 10.1158/1078-0432.CCR-05-2049. [DOI] [PubMed] [Google Scholar]
- 17.Mack PC, Redman MW, Chansky K, et al. Lower osteopontin plasma levels are associated with superior outcomes in advanced non–small-cell lung cancer patients receiving platinum-based chemotherapy: SWOG Study S0003. J Clin Oncol. 2008;26:4771–4776. doi: 10.1200/JCO.2008.17.0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Isa S, Kawaguchi T, Teramukai S, et al. Serum osteopontin levels are highly prognostic for survival in advanced non–small-cell lung cancer: Results from JMTO LC 0004. J Thorac Oncol. 2009;4:1104–1110. doi: 10.1097/JTO.0b013e3181ae2844. [DOI] [PubMed] [Google Scholar]
- 19.Pass HI, Lott D, Lonardo F, et al. Asbestos exposure, pleural mesothelioma, and serum osteopontin levels. N Engl J Med. 2005;353:1564–1573. doi: 10.1056/NEJMoa051185. [DOI] [PubMed] [Google Scholar]
- 20.Mazumdar M, Glassman JR. Categorizing a prognostic variable: Review of methods, code for easy implementation and applications to decision-making about cancer treatments. Stat Med. 2000;19:113–132. doi: 10.1002/(sici)1097-0258(20000115)19:1<113::aid-sim245>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 21.Brown DJ, Milroy R, Preston T, et al. The relationship between an inflammation-based prognostic score (Glasgow Prognostic Score) and changes in serum biochemical variables in patients with advanced lung and gastrointestinal cancer. J Clin Pathol. 2007;60:705–708. doi: 10.1136/jcp.2005.033217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McShane LM, Altman DG, Sauerbrei W, et al. REporting recommendations for tumor MARKer prognostic studies (REMARK) Nat Clin Pract. 2005;2:416–422. [PubMed] [Google Scholar]
- 23.McShane LM, Altman DG, Sauerbrei W, et al. Reporting recommendations for tumor marker prognostic studies. J Clin Oncol. 2005;23:9067–9072. doi: 10.1200/JCO.2004.01.0454. [DOI] [PubMed] [Google Scholar]
- 24.Brown LF, Papadopoulos-Sergiou A, Berse B, et al. Osteopontin expression and distribution in human carcinomas. Am J Pathol. 1994;145:610–623. [PMC free article] [PubMed] [Google Scholar]
- 25.Shijubo N, Uede T, Kon S, et al. Vascular endothelial growth factor and osteopontin in stage I lung adenocarcinoma. Am J Respir Crit Care Med. 1999;160:1269–1273. doi: 10.1164/ajrccm.160.4.9807094. [DOI] [PubMed] [Google Scholar]
- 26.Donati V, Boldrini L, Dell'Omodarme M, et al. Osteopontin expression and prognostic significance in non-small cell lung cancer. Clin Cancer Res. 2005;11:6459–6465. doi: 10.1158/1078-0432.CCR-05-0541. [DOI] [PubMed] [Google Scholar]
- 27.Zhang H, Ye QH, Ren N, et al. The prognostic significance of presurgery plasma levels of osteopontin in patients with hepatocellular carcinoma. J Cancer Res Clin Oncol. 2006;132:709–717. doi: 10.1007/s00432-006-0119-3. [DOI] [PubMed] [Google Scholar]
- 28.Cui BK, Zhang CQ, Zhang Y, et al. Osteopontin as a potential biomarker of metastasis and recurrence for hepatocellular carcinoma [in Chinese] Ai Zheng. 2006;25:876–879. [PubMed] [Google Scholar]
- 29.Kim JH, Skates SJ, Uede T, et al. Osteopontin as a potential diagnostic biomarker for ovarian cancer. JAMA. 2002;287:1671–1679. doi: 10.1001/jama.287.13.1671. [DOI] [PubMed] [Google Scholar]
- 30.Singhal H, Bautista DS, Tonkin KS, et al. Elevated plasma osteopontin in metastatic breast cancer associated with increased tumor burden and decreased survival. Clin Cancer Res. 1997;3:605–611. [PubMed] [Google Scholar]
- 31.Koopmann J, Fedarko NS, Jain A, et al. Evaluation of osteopontin as biomarker for pancreatic adenocarcinoma. Cancer Epidemiol Biomarkers Prev. 2004;13:487–491. [PubMed] [Google Scholar]
- 32.Le QT, Sutphin PD, Raychaudhuri S, et al. Identification of osteopontin as a prognostic plasma marker for head and neck squamous cell carcinomas. Clin Cancer Res. 2003;9:59–67. [PubMed] [Google Scholar]
- 33.Chang YS, Kim HJ, Chang J, Ahn CM, Kim SK, Kim SK. Elevated circulating level of osteopontin is associated with advanced disease state of non-small cell lung cancer. Lung Cancer. 2007;57:373–380. doi: 10.1016/j.lungcan.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Cho HJ, Kim HS. Osteopontin: A multifunctional protein at the crossroads of inflammation, atherosclerosis, and vascular calcification. Curr Atheroscler Rep. 2009;11:206–213. doi: 10.1007/s11883-009-0032-8. [DOI] [PubMed] [Google Scholar]
- 35.O'Regan AW, Chupp GL, Lowry JA, et al. Osteopontin is associated with T cells in sarcoid granulomas and has T cell adhesive and cytokine-like properties in vitro. J Immunol. 1999;162:1024–1031. [PubMed] [Google Scholar]
- 36.O'Regan AW, Hayden JM, Berman JS. Osteopontin augments CD3-mediated interferon-gamma and CD40 ligand expression by T cells, which results in IL-12 production from peripheral blood mononuclear cells. J Leukoc Biol. 2000;68:495–502. [PubMed] [Google Scholar]