Abstract
Purpose
To conduct a pilot study to determine the safety, feasibility, and engraftment of haploidentical natural killer (NK) cell infusions after an immunosuppressive regimen in children with acute myeloid leukemia (AML).
Patients and Methods
Ten patients (0.7 to 21 years old) who had completed chemotherapy and were in first complete remission of AML were enrolled on the Pilot Study of Haploidentical Natural Killer Cell Transplantation for Acute Myeloid Leukemia (NKAML) study. They received cyclophosphamide (60 mg/kg on day −7) and fludarabine (25 mg/m2/d on days −6 through −2), followed by killer immunoglobulin-like receptor–human leukocyte antigen (KIR-HLA) mismatched NK cells (median, 29 × 106/kg NK cells) and six doses of interleukin-2 (1 million U/m2). NK cell chimerism, phenotyping, and functional assays were performed on days 2, 7, 14, 21, and 28 after transplantation.
Results
All patients had transient engraftment for a median of 10 days (range, 2 to 189 days) and a significant expansion of KIR-mismatched NK cells (median, 5,800/mL of blood on day 14). Nonhematologic toxicity was limited, with no graft-versus-host disease. Median length of hospitalization was 2 days. With a median follow-up time of 964 days (range, 569 to 1,162 days), all patients remain in remission. The 2-year event-free survival estimate was 100% (95% CI, 63.1% to 100%).
Conclusion
Low-dose immunosuppression followed by donor-recipient inhibitory KIR-HLA mismatched NK cells is well tolerated by patients and results in successful engraftment. We propose to further investigate the efficacy of KIR-mismatched NK cells in a phase II trial as consolidation therapy to decrease relapse without increasing mortality in children with AML.
INTRODUCTION
Despite intensification of therapy and the use of new chemotherapeutic agents, one third to one half of children with acute myeloid leukemia (AML) continue to relapse.1,2 The Pediatric Oncology Group3 and Children's Cancer Group2 have reported event-free survival (EFS) estimates of 36% and 42%, respectively, on their recent trials. Contemporary European trials, including AML-BFM98,1 MRC 10,4,5 and NOPHO-AML93,6 have yielded similar EFS estimates (47% to 48%). Because the most common cause of treatment failure in these trials was early relapse, it is important to develop effective remission consolidation therapies. In a recent analysis of outcomes of 1,373 children with AML treated on cooperative group trials to evaluate the role of allogeneic hematopoietic stem-cell transplantation (HSCT), Horan et al7 found that 30% of favorable-risk and 54% of intermediate-risk patients assigned to chemotherapy relapsed, whereas 21% of favorable-risk and 26% of intermediate-risk patients assigned to HSCT relapsed.7 However, in the HSCT group, 16% of patients in each risk group died of treatment-related mortality compared with 7% to 9% in the chemotherapy groups. Clearly, there is an urgent need to develop novel approaches to decrease the risk of relapse without increasing the mortality associated with HSCT.
Natural killer (NK) cell transplantation is an ideal candidate for therapy for children with AML in remission, because it has the potential to provide antileukemic effects without causing graft-versus-host disease (GVHD) or organ toxicity. In humans, NK cells are regulated, in part, by inhibitory killer immunoglobulin-like receptors (KIRs) that recognize specific human leukocyte antigen (HLA) class I alleles.8 In HLA-nonidentical transplantation, NK cells will not be inhibited if the recipient lacks the appropriate HLA ligands to engage KIRs on donor NK cells. Animal studies have demonstrated that NK cell–mediated graft-versus-leukemia effects can occur in the absence of GVHD.9 In patients undergoing HSCT, donor NK cells may exert potent antileukemic effects if the patient's leukemia cells lack the class I epitope for the donor's inhibitory KIRs (ie, receptor-ligand mismatch), resulting in decreased risk of relapse.9–11 In addition, we have demonstrated the importance of direct assessment of the donor KIR repertoire, rather than donor KIR ligands, to predict donor-versus-leukemia effects.9,10
Miller et al12 have reported the transfer and expansion of haploidentical NK cells in the non-HSCT setting. However, the intensive conditioning regimen and high doses of interleukin-2 (IL-2) used in patients in their study resulted in significant hematologic and nonhematologic toxicities as well as prolonged hospitalization. To assess the safety and feasibility of low-dose immunosuppression followed by the infusion of purified haploidentical NK cells in children with AML, we initiated Pilot Study of Haploidentical Natural Killer Cell Transplantation for Acute Myeloid Leukemia (NKAML), a pilot study of haploidentical natural killer cell transplantation for acute myeloid leukemia. We administered a low-intensity regimen to patients to minimize toxicity while still allowing engraftment of haploidentical NK cells. To decrease the risks of GVHD and B-cell lymphoproliferative disease, we used a clinical-scale isolation method previously developed by us to obtain highly purified NK cells that have minimal contamination with T or B cells.10,13 Our results suggest that mild conditioning followed by donor-recipient inhibitory KIR-HLA mismatched NK cells is well tolerated by patients, results in successful engraftment, and is a promising novel therapy for reducing the risk of relapse in patients with AML.
PATIENTS AND METHODS
Patients (0.7 to 21 years old) diagnosed with AML that was in complete remission and who had adequate organ function were eligible to be enrolled on the NKAML trial. Patients with Down syndrome, juvenile myelomonocytic leukemia, or acute promyelocytic leukemia were not eligible. After obtaining informed consent from parents and assent from patients, 10 patients who completed four or five courses of chemotherapy on the AML02 trial14 and remained in first complete remission were enrolled. Because patients with poor-risk AML received HSCT on the AML02 trial, only favorable- and intermediate-risk patients were enrolled on the NKAML trial. HLA typing was determined for each patient and surface expression of KIRs was determined for both parents, as previously described.9,15 The conditioning regimen for patients comprised 60 mg/kg cyclophosphamide intravenously on day −7 and 25 mg/m2/d fludarabine intravenously from day −6 to day 2. On alternate days, 1 million U/m2 of IL-2 was administered subcutaneously for six doses starting on day −1 to activate and expand circulating donor NK cells.
The donor underwent apheresis on day −1 and the product was purified on day 0 for CD3–CD56+ cells in our institutional Human Applications Laboratory by a two-step procedure using magnetic activated cell sorting.13 First, the CliniMACS system (Miltenyi Biotec, Woburn, MA) was used to deplete T cells from the mononuclear cell product obtained by leukapheresis by using anti-CD3 antibody-coated beads. Second, the CD3-depleted product was enriched for CD56+ cells by incubation with anti-CD56 antibody-coated beads. NK cell number and purity were determined by flow cytometry as previously described.13 Because our goal was to test the natural cytotoxicity of donor NK cells, the purified product was infused on day 0 immediately after cell enrichment without in vitro exposure to IL-2 or other cytokines.
NK cell chimerism, phenotyping, and functional assays were performed on days 2, 7, 14, 21, and 28 after transplantation, as previously described.9,10,16 Chimerism studies of immunologically sorted NK cells were performed by standard variable number of tandem repeats techniques,17 and NK cell phenotyping was determined by direct measurement of surface expression of KIRs by flow cytometry.9 In addition, the cytotoxicity of peripheral blood NK cells was measured in vitro by europium release assays.9 Bone marrow minimal residual disease (MRD) was determined by flow cytometry at enrollment and at 1, 2, and 4 months after the NK infusion.18
EFS was defined as the time elapsed from enrollment on the AML02 trial to relapse, the development of a secondary malignancy, or death, with those living and event-free censored at the time of last follow-up. EFS was computed by the Kaplan-Meier method and CIs were determined by binomial distribution because no events were observed. The binomial interval is based on the number of patients at risk. The NKAML trial was approved by the St. Jude institutional review board and was covered by the Investigational Device Exemption application 11533.
RESULTS
Patients had a median age of 2.5 years (range, 8 months to 21 years) and a median leukocyte count of 62 × 109/L (range, 4 × 109/L to 487 × 109/L) at diagnosis (Table 1). Of the patients, four had CBFβ-MYH11–positive leukemia and six had intermediate-risk features (RBM15-MKL1 in two patients and MLL-ENL, MLL-AF9, +21, and normal karyotype in one case each). All patients were MRD-negative at the time of enrollment on this study. Patients tolerated all doses of cyclophosphamide, fludarabine, and IL-2 and showed acceptable nonhematologic toxicity: one patient had swelling and erythema at the IL-2 injection site, one had a viral respiratory infection, and one had to be hospitalized for 2 days for febrile neutropenia. Median length of hospitalization was 2 days (range, 0 to 3 days) and median time to neutrophil recovery (absolute neutrophil count > 0.5 × 109/L) was 12 days (range, 9 to 56 days). All patients had neutrophil and platelet recovery by day +21, except for one patient (unique patient number [UPN] 8), who did not achieve neutrophil recovery until day +56 and platelet recovery until day +127.
Table 1.
Demographic, Hematologic, and Engraftment Features of Patients Enrolled Onto the NKAML Study
| UPN | At Diagnosis |
Recipient |
Donor KIR Mismatch* | Donor Ligand Mismatch† | Peak NK Cell Chimerism |
NK Cell Graft |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | Sex | WBC (109/L) | Karyotype | FAB | HLA-Bw | HLA-C | Day | % Donor | NK Cells (106/kg) | T Cells (106/kg) | B Cells (106/kg) | |||
| 1 | 0.9 | F | 20 | 46,XX | M5 | 4/6 | Asn80/Lys80 | None | None | 7 | 7 | 38.7 | ND | 0.106 |
| 2 | 3 | F | 14 | t(1;22) | M7 | 6/6 | Asn80/Asn80 | 2DL1, 3DL1 | None | 7 | 2 | 27.2 | ND | 1.700 |
| 3 | 0.2 | F | 41 | t(1;22) | M7 | 4/6 | Asn80/Asn80 | 2DL1 | Lys80 | 28 | 30 | 31.1 | ND | 0.652 |
| 4 | 2 | M | 145 | inv(16) | M4Eo | 4/4 | Asn80/Asn80 | 2DL1 | None | 2 | 6 | 37.3 | ND | 0.148 |
| 5 | 0.7 | M | 488 | t(9;11) | M5 | 6/6 | Lys80/Lys80 | 2DL2/3, 3DL1 | Bw4, Asn80 | 7 | 3 | 80.9 | ND | 0.135 |
| 6 | 11 | F | 32 | t(16;16) | M4Eo | 6/6 | Asn80/Asn80 | 2DL1, 3DL1 | Bw4 | 14 | 8 | 5.2 | ND | 0.007 |
| 7 | 21 | M | 110 | +21 | M1 | 4/6 | Asn80/Asn80 | 2DL1 | None | 7 | 1 | 7.3 | ND | 0.004 |
| 8 | 17 | F | 137 | t(16;16) | M4Eo | 4/4 | Lys80/Lys80 | 2DL2/3 | Asn80 | 28 | 29 | 13.3 | 0.001 | ND |
| 9 | 8 | M | 84 | t(16;16) | M4Eo | 6/6 | Asn80/Lys80 | 3DL1 | None | 7 | 15 | 47.7 | ND | 0.087 |
| 10 | 2 | M | 4 | t(11;19) | M5 | 6/6 | Asn80/Asn80 | 2DL1, 3DL1 | Lys80 | 7 | 2 | 13.4 | ND | 0.082 |
Abbreviations: NKAML, Pilot Study of Haploidentical Natural Killer Cell Transplantation for Acute Myeloid Leukemia; UPN, unique patient number; FAB, French-American-British; HLA, human leukocyte antigen; KIR, killer immunoglobulin-like receptor; NK, natural killer; ND, not determinable (ie, less than the detection threshold of 0.001).
Receptor-ligand model.
Ligand-ligand model.
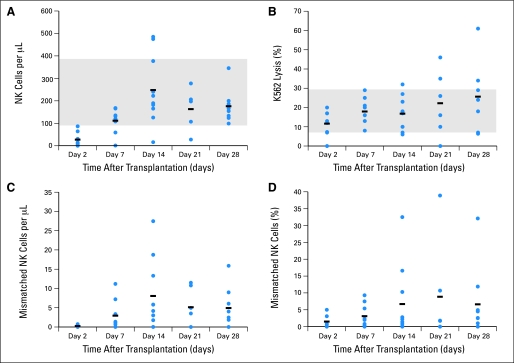
All donors underwent apheresis without complications. Patients received a median of 29 × 106/kg NK cells (range, 5 × 106/kg to 81 × 106/kg), with minimal B-cell contamination of products (median, 0.097 × 106/kg; Table 1). T cells were detected in the product given to one patient, but at a level that was more than 10-fold lower (1 × 103/kg) than the minimum number of T cells capable of causing GVHD.19 There were no adverse reactions during NK cell infusions and no GVHD. NK cell number in the blood normalized in nine of 10 patients by day 14 (Fig 1A), and the natural cytotoxicity against the K562 leukemia cell line was within the normal range in all patients by day 7 (Fig 1B).
Fig 1.
Natural killer (NK) cell engraftment and cytotoxicity. (A) Number of NK cells per microliter of blood. (B) Cytotoxicity against K562 cells with a target:effector cell ratio of 1:2. (C) Absolute number of killer immunoglobulin-like receptor (KIR)–mismatched NK cells. (D) Percentage of KIR-mismatched NK cells on days 2, 7, 14, 21, or 28. Gray areas in (A) and (B) represent the normal range established by analyzing donor blood before transplantation.
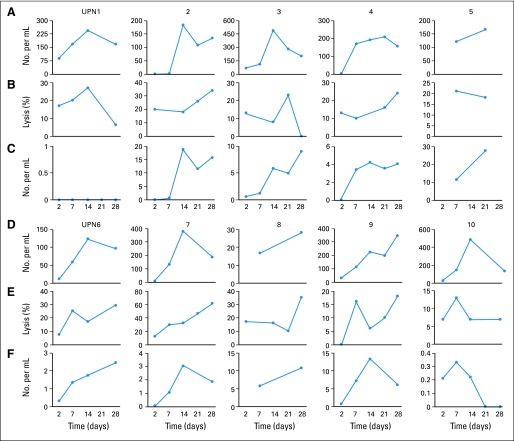
All patients had transient NK cell engraftment (median, 10 days; range, 2 to 189 days), with a median peak NK cell chimerism of 7% donor (range, 1% to 30%; Table 1). Three patients continued to have detectable donor NK cells at week 4 (range, 7% to 30%). Expansion of KIR-mismatched NK cells was observed in all nine patients with KIR-mismatched donors (Fig 1C and 1D), with a median peak expansion at day 14 (range, days 7 to 28; Fig 2C). The median number of KIR-mismatched NK cells was only 210/mL (range, 0 to 740/mL) of blood on day 2 and went up to 5,800/mL (range, 220 to 27,400/mL) on day 14. Patterns of engraftment for individual patients are shown in Figure 2.
Fig 2.
Patterns of natural killer (NK) cell engraftment. (A and D) Absolute number of NK cells per milliliter of blood for each patient. (B and E) Cytotoxicity against K562 cells with a target:effector cell ratio of 1:2. (C and F) Absolute number of killer immunoglobulin-like receptor (KIR)–mismatched NK cells for each patient. Unique patient number [UPN] 1 did not have a KIR-mismatched donor; donors for all other patients were KIR mismatched (Table 1).
One patient (UPN 8) had prolonged NK engraftment (2% donor NK cells at day +189) but no nonhematologic toxicity. This patient had delayed neutrophil and platelet recovery, as well as lymphopenia, with an absolute lymphocyte count less than 0.5 × 109/L until day +189. At day +261, this patient had no detectable donor NK cells and had complete hematopoietic recovery.
All patients remained MRD-negative at 1, 2, and 4 months after the NK infusion. With a median follow-up time of 964 days (range, 569 to 1,162 days), all patients are in remission (2-year EFS, 100%; 95% binomial CI, 63.1% to 100%).
DISCUSSION
The purpose of the NKAML pilot trial was to determine the feasibility and safety of immunosuppression with cyclophosphamide and fludarabine, followed by the infusion of donor-recipient inhibitory KIR-HLA–mismatched NK cells, in patients who had completed chemotherapy for AML. A study by Miller et al12 demonstrated that NK cells can be given to adults with relapsed AML, but administration of the conditioning regimen (two 60-mg/kg doses of cyclophosphamide) and the cytokine regimen (10 million units of IL-2 given three times per week) was associated with pancytopenia, fever, pleural effusions, hypoxemia, and constitutional symptoms, as well as prolonged hospitalization. Also in the Miller et al study,12 the number of T cells (2 × 105/kg) in NK cell products was potentially high enough to cause GVHD, and the number of B cells was high enough to cause fatal lymphoproliferative disease in one patient. In contrast, patients in our study were given a milder conditioning regimen and a lower IL-2 dose, which were well tolerated in the outpatient setting. Purified NK cell products were given directly to patients without prior in vitro exposure to cytokines, had minimal contamination by B or T cells, and were not associated with infusion-related toxicities. Hematologic toxicity was limited to delayed neutrophil and platelet recovery in one patient, suggesting that haploidentical, KIR-incompatible NK cells may not attack recipient hematopoietic progenitors. Most important, even with this safe, well-tolerated regimen, all patients showed evidence of engraftment, with expansion of donor KIR-mismatched NK cells 1 to 4 weeks after infusion. All patients remained in complete remission after a follow-up of approximately 32 months.
Although this was a pilot study to assess toxicity, our results indicate that transplantation of haploidentical NK cells is a promising consolidation therapy for patients with AML in remission. Additional studies are needed to confirm the safety of and establish the effectiveness of NK cell therapy. Although allogeneic HSCT is efficacious in patients with AML, it is associated with relapse rates of 21% to 26% and treatment-related mortality rates of approximately 16%.7 In addition, the morbidity, late effects, and financial costs associated with HSCT are substantial.20 In contrast, NK cell therapy appears to be safe and relatively inexpensive treatment that will likely have few late effects. Additionally, it is feasible to obtain large numbers of NK cells from readily available and willing family members. To determine whether NK cell therapy is a safe alternative to HSCT and reduces the risk of relapse in children with AML, we have initiated a phase II trial to evaluate the efficacy of KIR-mismatched NK cells as consolidation therapy in patients with intermediate-risk AML.
Acknowledgment
We thank Vani Shanker for expert editorial review, Vicky Turner for performing HLA typing, Xueyuan Cao for statistical analysis, and Dario Campana for critical review of the manuscript.
Footnotes
See accompanying editorial on page 909
Supported in part by Cancer Center Support (CORE) Grant No. P30 CA021765-30 from the National Institutes of Health, by a Center of Excellence grant from the State of Tennessee, and by the American Lebanese Syrian Associated Charities.
Presented, in part, at the 45th Annual Meeting of the American Society of Clinical Oncology, May 29-June 2, 2009.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00187096.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Jeffrey E. Rubnitz, Hiroto Inaba, Raul C. Ribeiro, Stanley Pounds, Ching-Hon Pui, Wing Leung
Provision of study materials or patients: Jeffrey E. Rubnitz, Hiroto Inaba, Raul C. Ribeiro, Barbara Rooney, Teresa Bell, Wing Leung
Collection and assembly of data: Jeffrey E. Rubnitz, Barbara Rooney, Teresa Bell, Wing Leung
Data analysis and interpretation: Jeffrey E. Rubnitz, Stanley Pounds, Barbara Rooney, Teresa Bell, Ching-Hon Pui, Wing Leung
Manuscript writing: Jeffrey E. Rubnitz, Hiroto Inaba, Raul C. Ribeiro, Stanley Pounds, Ching-Hon Pui, Wing Leung
Final approval of manuscript: Jeffrey E. Rubnitz, Hiroto Inaba, Raul C. Ribeiro, Stanley Pounds, Barbara Rooney, Teresa Bell, Ching-Hon Pui, Wing Leung
REFERENCES
- 1.Creutzig U, Zimmermann M, Lehrnbecher T, et al. Less toxicity by optimizing chemotherapy, but not by addition of granulocyte colony-stimulating factor in children and adolescents with acute myeloid leukemia: Results of AML-BFM 98. J Clin Oncol. 2006;24:4499–4506. doi: 10.1200/JCO.2006.06.5037. [DOI] [PubMed] [Google Scholar]
- 2.Lange BJ, Smith FO, Feusner J, et al. Outcomes in CCG-2961, a Children's Oncology Group phase 3 trial for untreated pediatric acute myeloid leukemia: A report from the Children's Oncology Group. Blood. 2008;111:1044–1053. doi: 10.1182/blood-2007-04-084293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becton D, Dahl GV, Ravindranath Y, et al. Randomized use of cyclosporin A (CsA) to modulate P-glycoprotein in children with AML in remission: Pediatric Oncology Group Study 9421. Blood. 2006;107:1315–1324. doi: 10.1182/blood-2004-08-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stevens RF, Hann IM, Wheatley K, et al. Marked improvements in outcome with chemotherapy alone in paediatric acute myeloid leukemia: Results of the United Kingdom Medical Research Council's 10th AML trial. MRC Childhood Leukaemia Working Party. Br J Haematol. 1998;101:130–140. doi: 10.1046/j.1365-2141.1998.00677.x. [DOI] [PubMed] [Google Scholar]
- 5.Gibson BE, Wheatley K, Hann IM, et al. Treatment strategy and long-term results in paediatric patients treated in consecutive UK AML trials. Leukemia. 2005;19:2130–2138. doi: 10.1038/sj.leu.2403924. [DOI] [PubMed] [Google Scholar]
- 6.Lie SO, Abrahamsson J, Clausen N, et al. Treatment stratification based on initial in vivo response in acute myeloid leukaemia in children without Down's syndrome: Results of NOPHO-AML trials. Br J Haematol. 2003;122:217–225. doi: 10.1046/j.1365-2141.2003.04418.x. [DOI] [PubMed] [Google Scholar]
- 7.Horan JT, Alonzo TA, Lyman GH, et al. Impact of disease risk on efficacy of matched related bone marrow transplantation for pediatric acute myeloid leukemia: The Children's Oncology Group. J Clin Oncol. 2008;26:5797–5801. doi: 10.1200/JCO.2007.13.5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leung W, Iyengar R, Turner V, et al. Determinants of antileukemia effects of allogeneic NK cells. J Immunol. 2004;172:644–650. doi: 10.4049/jimmunol.172.1.644. [DOI] [PubMed] [Google Scholar]
- 10.Leung W, Iyengar R, Triplett B, et al. Comparison of killer Ig-like receptor genotyping and phenotyping for selection of allogeneic blood stem cell donors. J Immunol. 2005;174:6540–6545. doi: 10.4049/jimmunol.174.10.6540. [DOI] [PubMed] [Google Scholar]
- 11.Hsu KC, Keever-Taylor CA, Wilton A, et al. Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood. 2005;105:4878–4884. doi: 10.1182/blood-2004-12-4825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller JS, Soignier Y, Panoskaltsis-Mortari A, et al. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 13.Iyengar R, Handgretinger R, Babarin-Dorner A, et al. Purification of human natural killer cells using a clinical-scale immunomagnetic method. Cytotherapy. 2003;5:479–484. doi: 10.1080/14653240310003558. [DOI] [PubMed] [Google Scholar]
- 14.Kurt B, Flynn P, Shenep JL, et al. Prophylactic antibiotics reduce morbidity due to septicemia during intensive treatment for pediatric acute myeloid leukemia. Cancer. 2008;113:376–382. doi: 10.1002/cncr.23563. [DOI] [PubMed] [Google Scholar]
- 15.Leung WH, Turner V, Richardson SL, et al. Effect of HLA class I or class II incompatibility in pediatric marrow transplantation from unrelated and related donors. Hum Immunol. 2001;62:399–407. doi: 10.1016/s0198-8859(01)00220-8. [DOI] [PubMed] [Google Scholar]
- 16.Leung W, Iyengar R, Leimig T, et al. Phenotype and function of human natural killer cells purified by using a clinical-scale immunomagnetic method. Cancer Immunol Immunother. 2005;54:389–394. doi: 10.1007/s00262-004-0609-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schichman SA, Suess P, Vertino AM, et al. Comparison of short tandem repeat and variable number tandem repeat genetic markers for quantitative determination of allogeneic bone marrow transplant engraftment. Bone Marrow Transplant. 2002;29:243–248. doi: 10.1038/sj.bmt.1703360. [DOI] [PubMed] [Google Scholar]
- 18.Coustan-Smith E, Ribeiro RC, Rubnitz JE, et al. Clinical significance of residual disease during treatment in childhood acute myeloid leukaemia. Br J Haematol. 2003;123:243–252. doi: 10.1046/j.1365-2141.2003.04610.x. [DOI] [PubMed] [Google Scholar]
- 19.Müller S, Schulz A, Reiss U, et al. Definition of a critical T cell threshold for prevention of GVHD after HLA non-identical PBPC transplantation in children. Bone Marrow Transplant. 1999;24:575–581. doi: 10.1038/sj.bmt.1701970. [DOI] [PubMed] [Google Scholar]
- 20.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]