Abstract
Aims
To investigate the association between the presence and characteristics of uterine leiomyomata (UL) and self-reported stress urinary incontinence (SUI).
Methods
The study included 836 premenopausal participants (474 African American and 362 Caucasian) in the National Institute of Environmental Health Sciences (NIEHS) Uterine Fibroid Study. UL were characterized at baseline with ultrasound screening, and SUI was assessed at follow-up (after 4 years, on average). Linear risk models were used to estimate adjusted prevalence differences (aPD) and 95% confidence intervals (CI), controlling for age, ethnicity, body mass index (BMI), and number of deliveries.
Results
Compared with women without UL, SUI prevalence was higher among women with any UL (aPD = 7.4%, 95% CI 0.4-14.3) and women with UL 2–4 cm (aPD = 9.6%, 95% CI 1.3-17.9). Marginally significant results were found for the presence of UL ≥4 cm and anterior UL ≥2 cm.
Conclusions
The observed 7% increase in prevalence of this common condition for women with UL is of clinical importance. Further research is needed before concluding that treatment for larger UL might enhance SUI treatment in some women.
Introduction
Urinary incontinence is one of the most common types of lower urinary tract dysfunction, affecting about a third of adult women in the United States.1 The total cost of urinary incontinence in the United States was estimated to be $19.5 billion (in 2000 dollars).2 Stress urinary incontinence (SUI), the complaint of involuntary leakage of urine on effort or exertion or on sneezing or coughing,3 is the most common type of urinary incontinence in women4 and can have a substantial negative impact on quality of life.5 Established risk factors for self-reported SUI symptoms include older age,6 Caucasian vs. African American ethnicity,7,8 high body mass index (BMI),7,9 and parity,10 especially vaginal deliveries.11,12
Uterine enlargement due to the presence of uterine leiomyomata (UL), in particular large UL in the anterior lower uterus, has been hypothesized to cause SUI by compressing the urinary bladder.13 In particular, laughing, coughing, or sneezing may displace UL against the bladder and cause involuntary loss of urine (i.e., SUI). There is a case report of a pedunculated UL causing acute SUI.14 Although it has been hypothesized that UL may be associated with SUI,13,15,16 to our knowledge, only one population-based observational study has directly tested this hypothesis, and it found no association between self-reported UL and prevalent SUI.17 Therefore, the objective of this study was to investigate the association between SUI and UL, considering not only the presence of UL but also their characteristics. We examined this relationship in 474 African American and 362 Caucasian premenopausal women who participated in the first follow-up interview of the National Institute of Environmental Health Science's (NIEHS) Uterine Fibroid Study (UFS).
Materials and Methods
The NIEHS UFS was designed to measure the prevalence of UL, identify risk factors for UL, identify biological changes in tumor tissue compared with normal uterine muscle, and describe women's experience of symptoms associated with UL and how they change over time among African American and Caucasian women, aged 35–49. The study was approved by Human Subject Review Boards at the NIEHS and George Washington University. Enrollment occurred between 1996 and 1999, at which time participants were screened for UL by ultrasound and asked to provide baseline data by telephone interview and mail questionnaire. Premenopausal participants were followed up in 2001–2002 using a computer-assisted telephone interview. Details of the study have been published.18
Study participants
The UFS enrolled women aged 35–49 years selected randomly from a computerized list of health plan members at a Washington, DC, site. In addition to age and health plan membership requirements, eligible women had to be able to complete the UFS study interview in English. Of the 2102 women screened for enrollment, 316 (15%) were ineligible, primarily because they did not obtain care at the Washington, DC, health plan site. Of the 1786 eligible women, 335 (19%) refused to participate, and 21 (1%) were lost to contact prior to data collection. In total, 1430 women, constituting 80% of the eligible women, participated in the baseline phase of the UFS.
Premenopausal participants were eligible for follow-up. Among the 1430 baseline participants, 1142 were premenopausal African American or Caucasian women, and of these, 911 participated in the first follow-up of the NIEHS UFS. Of the 231 women who did not complete the follow-up, 107 (46%) refused to participate, 121 (52%) were unable to be contacted, and 3 (1%) were deceased. Compared with the 911 women who participated in the follow-up, the 231 women who did not complete the follow-up were more likely to have UL, to be African American, to be younger, and to have a higher BMI on average. Women were excluded from the current study if they were missing data on SUI at follow-up (n = 4) or UL status at baseline (n = 57). In addition, women were excluded if they were pregnant during the 12 months before follow-up, the time interval for which they reported SUI (n = 14). The final population included 836 premenopausal women, 474 African American and 362 Caucasian. Compared with the 836 women included in the study, the 75 women excluded from the study were similar with respect to race and were younger and had a lower BMI on average.
Determination of UL status
UL status was based on ultrasound evidence of fibroids. For 76% of the women, UL was identified or confirmed based on an ultrasound screening examination conducted for the study at the primary care site at baseline. The other women, who either had UL documented in a sonogram within the previous 5 years or were negative for UL based on a sonogram within the previous 2 years, were classified according to their previous sonogram.
Study ultrasound examinations included both transabdominal and transvaginal examinations to facilitate identification of UL in the upper uterus. Both examinations were performed by sonographers certified by the American Registry of Diagnostic Medical Sonographers, under the supervision of a radiologist with fellowship training in sonography. A single radiologist reviewed all questionable sonograms. Sonographers filled out a study-specific data collection form that included data on uterine size, uterine shape, the presence of diffuse heterogeneous echo pattern, number of focal UL, the size and location of the two largest UL (if at least 2 cm in diameter), and the size of the three largest submucosal UL.
UL status was classified according to (1) UL presence (no, yes), (2) UL size (no UL; ≥2 cm, small UL; 2–4 cm, medium UL; ≥4 cm, large UL), (3) the presence of an anterior UL ≥4 cm (large) in a nonretroverted uterus (no UL, yes, other UL), or (4) the presence of an anterior UL ≥2 cm (medium/large) in a nonretroverted uterus (no UL, yes, other UL). In addition, each woman's uterine size was classified as small (≤91.4 cm3, the lowest third of the overall uterine volume distribution), medium (>91.4 cm3 but ≤148.9 cm3, the middle third), large (>148.9 cm3 but ≤213.0 cm3, the 67th to 83rd percentile), and very large (>213.0 cm3, or above the 83rd percentile). Uterine volume was calculated using the prolate ellipsoid formula (0.52 × length × width × anterior/posterior diameter).
The “no UL” reference category included women who had neither focal UL nor a diffuse heterogeneous echo pattern present. The latter may result from multiple small focal UL or from larger UL that lack the usual distinct histological demarcation from the surrounding myometrium. We considered the diffuse heterogeneous echo pattern to be an indicator of UL because, like focal UL, this pattern was associated with uterine enlargement18 and excess bleeding.19 For the 89 women who had a diffuse heterogeneous echo pattern and no detectable focal UL, we assigned a UL size based on their uterine volume (calculated based on a regression model predicting size of largest fibroid from uterine size for all participants with both measurements). Sensitivity analyses were conducted with and without this group of women.
SUI determination
Data collected at the follow-up (2001–2002) during a computer-assisted telephone interview included self-reported SUI, which was classified as positive if women reported that they ever had urine leak when they coughed or sneezed during the 12-month interval before their follow-up reference date. The reference date for women who had UL treated by myomectomy, hystereoscopic resection, uterine artery embolization, or hysterectomy was the date of the procedure or the date of their last menstrual period, whichever came first. The reference date for women who did not undergo any of the procedures listed was the date of their follow-up interview if they had a recent menstruation or the date of their last period if they did not report a period in the last 2 months.
Data analysis
Potential confounders were selected by reviewing the literature on UL and SUI. They were age at the reference date (continuous or categorized as <40, 40–44, 45–49, ≥50), ethnicity (African American, Caucasian), BMI at baseline (kg/m2, categorized as <25, 25–29, 30–34, ≥35), and the number of deliveries prior to the reference date (categorical, 0, 1, 2, ≥3). A composite variable involving the number and the type of deliveries (none, only cesarean section deliveries, 1 vaginal delivery, 2 vaginal deliveries, ≥3 vaginal deliveries) was also evaluated as a potential confounder but was not used because results were comparable with those adjusted for the number of deliveries. A history of vaginal deliveries variable (no deliveries or only cesarean section deliveries, 1 or more vaginal deliveries) was also created to be evaluated as a potential effect modifier.
Univariate analyses were performed to describe distributions of exposures and outcomes and to assess missing data. We used linear risk models to estimate prevalence differences (PD) and corresponding 95% confidence intervals (CI),20 with separate linear risk models used to evaluate the association between each of the five UL variables of interest and self-reported SUI. We estimated crude and adjusted PD (aPD) and 95% CIs, which are reported as percent prevalence differences. As it was decided a priori to include all the potential confounders in the final models, the adjusted estimates were based on models that included age at reference (continuous), ethnicity, BMI at baseline, and number of deliveries. All model estimates were evaluated to confirm that predicted prevalences were between 0 and 1.
In secondary analyses, we explored the roles of ethnicity, parity, history of vaginal deliveries, and BMI as potential effect modifiers. Ethnicity (African American or Caucasian) was evaluated based on reported evidence consistent with ethnic differences in the association between UL and urinary incontinence.17 We evaluated effect modification by parity (nulliparous vs. parous) because pregnancy may increase susceptibility to SUI because of its effects on the pelvic floor. History of vaginal deliveries may act as an effect modifier if the presence of UL accelerates the development of SUI initiated by vaginal deliveries. BMI was investigated as a potential effect modifier, as the effect of a large UL may be different for obese and nonobese women. The evaluations of effect modification (specifically, risk difference modification) by ethnicity, parity, history of vaginal deliveries, and BMI were performed using likelihood ratio tests for corresponding interactions (α = 0.10). All analyses were performed using the SAS software package (Cary, NC).
Results
Over half of study participants were African American, and a third were nulliparous. Most women were aged 40–49 on the reference date (mean ± SD, 45.8 ± 3.9 years), and 60% were overweight or obese (mean BMI ± SD, 28.6 ± 7.6 km/m2). The average time from the ultrasound examination to the reference date was about 4 years (mean ± SD, 3.9 ± 2.0 years), and the average time from the reference date to the follow-up was 0.66 years (mean ± SD, 0.66 ± 1.3 years). The reference date preceded the follow-up date for 269 (32%) women: for 75 (9%) women because of treatment for UL and for 194 (23%) women because of reaching the menopause.
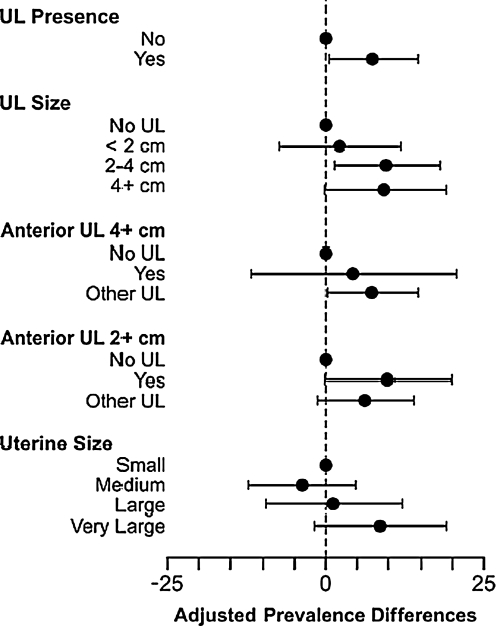
Overall, 414 of 836 (49.5%) study participants reported SUI during the reference period. African American women reported less SUI than Caucasian women (43% vs. 58%), and there was a general tendency for the prevalence of SUI to increase with age. Caucasian ethnicity, BMI at baseline, and number of deliveries were positively associated with SUI (Table 1). Sixty-four percent of participants had at least one UL (534 of 836), and the prevalence of SUI was 51% among women with UL compared with 46% among women without UL. The crude and adjusted prevalence differences and their 95% CIs for SUI in association with UL were PD = 5.0, 95% CI −2.1-12.0, and aPD = 7.4 95%, CI 0.4-14.3, respectively (Table 2 and Fig. 1).
Table 1.
Study Population Characteristics at Baseline and Stress Urinary Incontinence at Follow-up among 836 Premenopausal Women, NIEHS Uterine Fibroid Study
| |
Total |
SUI |
Crude |
|||
|---|---|---|---|---|---|---|
| Characteristic | n | % | n | % | PD (%) | 95% CI |
| Age at reference date | ||||||
| <40 | 58 | 6.9 | 25 | 43.1 | 0 | |
| 40–44 | 294 | 35.2 | 136 | 46.3 | 3.2 | −10.9-16.7 |
| 45–49 | 339 | 40.6 | 172 | 50.7 | 7.6 | −6.3-21.0 |
| ≥50 | 145 | 17.3 | 81 | 55.9 | 12.8 | −2.4-27.5 |
| Ethnicity | ||||||
| African American | 474 | 56.7 | 205 | 43.3 | 0 | |
| Caucasian | 362 | 43.3 | 209 | 57.7 | 14.5 | 7.7-21.2 |
| BMI at baseline (kg/m2) | ||||||
| <25 | 337 | 40.3 | 145 | 43.0 | 0 | |
| 25–29 | 221 | 26.4 | 117 | 52.9 | 9.9 | 1.5-18.3 |
| 30–34 | 126 | 15.1 | 64 | 50.8 | 7.8 | −2.4-17.9 |
| ≥35 | 152 | 18.2 | 88 | 57.9 | 14.9 | 5.3-24.2 |
| Number of deliveries | ||||||
| 0 | 304 | 36.4 | 136 | 44.7 | 0 | |
| 1 | 147 | 17.6 | 74 | 50.3 | 5.6 | −4.2-15.4 |
| 2 | 254 | 30.4 | 136 | 53.5 | 8.8 | 0.5-17.1 |
| ≥3 | 131 | 15.7 | 68 | 51.9 | 7.2 | −3.1-17.3 |
SUI, stress urinary incontinence; PD, prevalence differences; CI, confidence interval.
Table 2.
Estimated Prevalences and Crude and Adjusteda Percent Prevalence Differences for Stress Urinary Incontinence among 836 Premenopausal Women, NIEHS Uterine Fibroid Study
| |
Total |
SUI |
Crude |
Adjusted |
||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | n | % | n | % | PD (%) | 95% CI | PD (%) | 95% CI |
| UL presence | ||||||||
| No | 302 | 36.1 | 140 | 46.4 | 0 | 0 | ||
| Yes | 534 | 63.9 | 274 | 51.3 | 5.0 | −2.1-12.0 | 7.4 | 0.4-14.3 |
| UL size | ||||||||
| No UL | 302 | 36.1 | 140 | 46.4 | 0 | 0 | ||
| <2 cm | 143 | 17.1 | 68 | 47.6 | 1.2 | −8.7-11.1 | 2.1 | −7.5-11.7 |
| 2–4 cm | 237 | 28.4 | 126 | 53.2 | 6.8 | −1.7-15.2 | 9.6 | 1.3-17.9 |
| ≥4 cm | 154 | 18.4 | 80 | 52.0 | 5.6 | −4.1-15.2 | 9.3 | −0.4-18.9 |
| Anterior ULb ≥4 cm | ||||||||
| No UL | 302 | 37.4 | 140 | 46.4 | 0 | 0 | ||
| Yes | 35 | 4.3 | 17 | 48.6 | 2.2 | −14.8-19.4 | 4.3 | −11.9-20.6 |
| Other UL | 470 | 58.2 | 241 | 51.3 | 4.9 | −2.3-12.1 | 7.4 | 0.2-14.4 |
| Missing | 29 | |||||||
| Anterior ULb ≥2 cm | ||||||||
| No UL | 302 | 37.4 | 140 | 46.4 | 0 | 0 | ||
| Yes | 125 | 15.5 | 66 | 52.8 | 6.4 | −4.0-16.8 | 9.8 | −0.3-19.7 |
| Other UL | 380 | 47.1 | 192 | 50.5 | 4.2 | −3.4-11.7 | 6.2 | −1.3-13.7 |
| Missing | 29 | |||||||
| Uterine sizec | ||||||||
| Small | 258 | 32.9 | 120 | 46.5 | 0 | 0 | ||
| Medium | 260 | 33.2 | 120 | 46.2 | 0.4 | −8.9-8.2 | −3.8 | −12.3-4.7 |
| Large | 132 | 16.8 | 66 | 50.0 | 3.5 | −7.0-13.9 | 1.1 | −9.7-11.8 |
| Very large | 134 | 17.1 | 76 | 56.7 | 10.2 | −0.2-20.4 | 8.7 | −1.9-19.0 |
| Missing | 52 | |||||||
Estimated using linear risk models, adjusted for age at reference date, ethnicity, BMI, and number of deliveries.
Anterior UL, UL located in the anterior wall of a nonretroverted uterus.
Small (≤91.4 cm3), medium (>91.4 cm3 and ≤148.9 cm3), large (>148.9 cm3 and ≤213.0 cm3), and very large (>213.0 cm3).
UL, uterine leiomyomata.
FIG. 1.
Study Population Characteristics at Baseline and Stress Urinary Incontinence at Follow-up among 836 Premenopausal Women, NIEHS Uterine Fibroid Study
The prevalence of SUI was similar for women with no UL and for those with UL <2cm but was statistically significantly increased among women with UL 2–4 cm (aPD = 9.6, 95% CI 1.3-17.9) and marginally significantly increased among women with UL ≥4 cm (aPD = 9.3, 95% CI −0.4-18.9) (Table 2 and Fig. 1). Contrary to expectations, the association between UL and SUI was not stronger for large (≥4 cm) anterior UL in a nonretroverted uterus (aPD = 4.3, 95% CI −11.9-20.6) than for other UL (aPD = 7.4, 95% CI 0.2-14.4); however, estimates were imprecise because of the small number of observations in the former group. The association between the presence of anterior UL ≥2 cm in a nonretroverted uterus and SUI (aPD = 9.8, 95% CI −0.3-19.7) was marginally significant and somewhat larger than the association estimated for other UL and SUI (aPD = 6.2, 95% CI −1.3-13.7).
After adjustment for the potential confounders, the prevalence of SUI among women with a very large uterine volume (>213 cm3) was increased relative to women with a small uterine volume (≤91.4 cm3) (aPD = 8.7, 95% CI −1.9-19.0), although the result was not statistically significant.
There was no evidence that the associations between UL or uterine size and SUI differed by race, parity, history of vaginal deliveries, or BMI (all p values for interactions from linear risk models >0.10). For example, race-specific associations between UL presence and SUI were aPD = 8.5, 95% CI −1.0-18.1 for African Americans and aPD = 6.3, 95% CI −3.8-16.3 for Caucasians (p value for test for interaction between ethnicity and UL presence 0.75). As the study did not have enough statistical power to detect effect modification, these results should be considered exploratory and interpreted with caution.
The results were not substantially different when the 89 women who had a diffuse heterogeneous echo pattern and no detectable focal UL were excluded. For example, after this exclusion, the adjusted PD and the 95% CI for the association between UL with SUI was aPD = 7.9, 95% CI 0.5-15.1. It is important to note that exclusion of the 125 women who had their reference date preceding the follow-up by 2 years or more did not substantially alter the results. For example, after their exclusion, the adjusted PD and the 95% CI for the association between UL with SUI were aPD = 7.6, 95% CI 0.2-15.1.
Discussion and Conclusions
Although several articles have hypothesized that UL may influence SUI,15,16 to our knowledge, this is only the second epidemiological study to systematically evaluate the association between UL and self-reported SUI. In addition to the presence or absence of any UL, we evaluated the size of the largest UL, UL location, and overall uterine volume because these characteristics may affect SUI by increasing pressure exerted on the bladder.
Almost half of the study population reported experiencing symptoms of SUI during the reference interval, but the prevalence of SUI was about 7% higher among women with UL compared with women without UL after adjusting for age, ethnicity, BMI, and number of deliveries. Relative to women without UL, the prevalence of SUI was about 9% higher among women with UL that were ≥2 cm and among women with a very large uterine volume. However, large anterior UL, which were hypothesized to be most likely to exert pressure on the bladder, were not more strongly associated with SUI than other UL; our estimates were imprecise, however, because few women had such UL. Although fibroids were not as strongly related to SUI as the established risk factors parity and high BMI (aPDs of 13%–23%, data not shown), a 7%–9% increase in prevalence is very important for this common condition.
To our knowledge, the association between the presence of UL and SUI has been investigated in only one other population-based epidemiological study. This community-based study included 3302 women aged 42–52 years enrolled in the Study of Women's Health Across the Nation (SWAN) cohort, a multiethnic longitudinal study of the natural history of menopausal transition.17 The presence of UL was based on self-reported diagnosis, and the presence of both stress and urge urinary incontinence (at least monthly during the previous year) was assessed at baseline and the first five follow-up visits. No association was found between self-reported UL and prevalent SUI. The study found an association with prevalent incontinence (stress, urge, or mixed combined) and an association with prevalent urge incontinence (aOR = 1.53, 95% CI 1.08-2.27), especially among the African American women (aOR = 1.95, 95% CI 1.07-3.54). No association was found between self-reported UL and incident incontinence, either overall or by specific subtype. It should be noted that another study also reported no association between UL (as the primary indication for hysterectomy) and self-reported SUI,21 but this was a highly selected sample, limited to women having hysterectomies, and only an unadjusted OR was reported.
There are also several studies that have evaluated the association between hysterectomy and SUI symptoms. In a prospective study, SUI prevalence dropped from 36% before surgery to 19% 6 months after surgery.22 Two randomized clinical trials also reported reductions in SUI after hysterectomy.23,24 In the first clinical trial, the reduction in SUI was associated with removal of uteri without UL as well as uteri with UL, whereas in the second study, the SUI reduction was attributed to the removal of uteri with large UL. We found no literature on the effect of myomectomy or uterine artery embolization on SUI, studies that would address more directly the efficacy of removal of UL on the treatment of SUI.
Strengths of our study include its sample size (474 African American and 362 Caucasian women) and the sampling method used to randomly select a sample of health plan participants. Another strength is that the UL status was based on ultrasound screening, therefore allowing women with both symptomatic and asymptomatic UL to be accurately classified with regard to UL status. The ultrasound data also allowed us to characterize the size and location of UL, characteristics not available from self-reported data. It should be noted, however, that analyses of UL by location were limited by the small number of women with large anterior UL.
The study has also several limitations. Because the ultrasound was performed on average about 4 years before the assessment of SUI symptoms and the development of new UL and the growth of existing UL during the period are not accounted for, our results may have underestimated the true effect of UL presence and characteristics. Limitations regarding the assessment of urinary incontinence symptoms included the definition used, which asked only about urine leakage when the women coughed or sneezed, without any assessment of severity or the presence of urge urinary incontinence. Another important study limitation was the retrospective ascertainment of SUI during the 12-month interval prior to the reference date, given that 21% of the women had their reference date preceding the follow-up by ≥1 year and 15% of them had their reference date ≥2 years before the follow-up. It is important to note, however, that excluding the latter group caused only minor changes to the results. Regarding the severity, assuming a causal relationship, the effect of UL would have been stronger if the outcome had been restricted to more severe SUI symptoms. In addition, although UL status was determined at baseline and SUI at follow-up, the ages of onset of UL and SUI were unknown. It is possible that the observed association between UL and SUI was a consequence of shared causal factors.
In summary, we found an increase in SUI prevalence of about 7% associated with the presence of UL, with slightly larger increases in prevalence associated with larger UL. These findings must be confirmed by other studies using a more rigorous assessment of SUI before concluding that the effective treatment of SUI may include treatment for large UL in some cases.
Acknowledgments
Dr. Michael Hill supervised the ultrasound screening examinations, and Dr. Joel Schectman administered the clinical aspects of the NIEHS UFS. Ms. Glenn Heartwell managed the study. Drs. Shannon Laughlin and Anne Marie Jukic reviewed an earlier draft of the manuscript. This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences.
Disclosure Statement
The authors have no conflicts of interest to report.
References
- 1.DuBeau CE. Urinary incontinence management: New questions from old assumptions. J Am Geriatr Soc. 2001;49:829–830. doi: 10.1046/j.1532-5415.2001.49163.x. [DOI] [PubMed] [Google Scholar]
- 2.Hu TW. Wagner TH. Bentkover JD. Leblanc K. Zhou SZ. Hunt T. Costs of urinary incontinence and overactive bladder in the United States: A comparative study. Urology. 2004;63:461–465. doi: 10.1016/j.urology.2003.10.037. [DOI] [PubMed] [Google Scholar]
- 3.Abrams P. Cardozo L. Fall M, et al. The standardization of terminology of lower urinary tract function: Report from the Standardization Sub-committee of the International Continence Society. Am J Obstet Gynecol. 2002;187:116–126. doi: 10.1067/mob.2002.125704. [DOI] [PubMed] [Google Scholar]
- 4.Hampel C. Wienhold D. Benken N. Eggersmann C. Thuroff JW. Prevalence and natural history of female incontinence. Eur Urol. 1997;32(Suppl 2):3–12. [PubMed] [Google Scholar]
- 5.Contreras-Ortiz O. Stress urinary incontinence in the gynecological practice. Int J Gynaecol Obstet. 2004;86(Suppl 1):S6–S16. doi: 10.1016/j.ijgo.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Rohr G. Støvring H. Christensen K. Gaist D. Nybo H. Kragstrup J. Characteristics of middle-aged and elderly women with urinary incontinence. Scand J Prim Health Care. 2005;23:203–208. doi: 10.1080/02813430500362803. [DOI] [PubMed] [Google Scholar]
- 7.Jackson RA. Vittinghoff E. Kanaya AM, et al. Urinary incontinence in elderly women: Findings from the Health, Aging, and Body Composition Study. Obstet Gynecol. 2004;104:301–307. doi: 10.1097/01.AOG.0000133482.20685.d1. [DOI] [PubMed] [Google Scholar]
- 8.Thom DH. van den Eeden SK. Ragins AI, et al. Differences in prevalence of urinary incontinence by race/ethnicity. J Urol. 2006;175:259–264. doi: 10.1016/S0022-5347(05)00039-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mishra GD. Hardy R. Cardozo L. Kuh D. Body weight through adult life and risk of urinary incontinence in middle-aged women: Results from a British prospective cohort. Int J Obes (Lond) 2008;32:1415–1422. doi: 10.1038/ijo.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rortveit G. Hannestad YS. Daltveit AK. Hunskaar S. Age- and type-dependent effects of parity on urinary incontinence: The Norwegian EPINCONT study. Obstet Gynecol. 2001;98:1004–1010. doi: 10.1016/s0029-7844(01)01566-6. [DOI] [PubMed] [Google Scholar]
- 11.Rortveit G. Daltveit AK. Hannestad YS. Hunskaar S. Norwegian EPINCONT Study. Urinary incontinence after vaginal delivery or cesarean section. N Engl J Med. 2003;348:900–907. doi: 10.1056/NEJMoa021788. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg RP. Abramov Y. Botros S, et al. Delivery mode is a major environmental determinant of stress urinary incontinence: Results of the Evanston-Northwestern Twin Sisters Study. Am J Obstet Gynecol. 2005;193:2149–2153. doi: 10.1016/j.ajog.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 13.Haney AF. Clinical decision making regarding leiomyomata: What we need in the next millenium. Environ Health Perspect. 2000;108(Suppl 5):835–839. doi: 10.1289/ehp.00108s5835. [DOI] [PubMed] [Google Scholar]
- 14.Isherwood PJ. Rane A. Pedunculated uterine leiomyoma causing acute urinary stress incontinence. J Obstet Gynaecol. 1999;19:440–441. doi: 10.1080/01443619964913. [DOI] [PubMed] [Google Scholar]
- 15.Stovall DW. Clinical symptomatology of uterine leiomyomas. Clin Obstet Gynecol. 2001;44:364–371. doi: 10.1097/00003081-200106000-00022. [DOI] [PubMed] [Google Scholar]
- 16.Nygaard IE. Heit M. Stress urinary incontinence. Obstet Gynecol. 2004;104:607–620. doi: 10.1097/01.AOG.0000137874.84862.94. [DOI] [PubMed] [Google Scholar]
- 17.Waetjen LE. Liao S. Johnson WO, et al. Factors associated with prevalent and incident urinary incontinence in a cohort of midlife women: A longitudinal analysis of data: Study of Women's Health Across the Nation. Am J Epidemiol. 2007;165:309–318. doi: 10.1093/aje/kwk018. [DOI] [PubMed] [Google Scholar]
- 18.Baird DD. Dunson DB. Hill MC. Cousins D. Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: Ultrasound evidence. Am J Obstet Gynecol. 2003;188:100–107. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- 19.Wegienka G. Baird DD. Hertz-Picciotto I, et al. Self-reported heavy bleeding associated with uterine leiomyomata. Obstet Gynecol. 2003;101:431–437. doi: 10.1016/s0029-7844(02)03121-6. [DOI] [PubMed] [Google Scholar]
- 20.Spiegelman D. Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 21.Handa VL. Harvey L. Fox HE. Kjerulff KH. Parity and route of delivery: Does cesarean delivery reduce bladder symptoms later in life? Am J Obstet Gynecol. 2004;191:463–469. doi: 10.1016/j.ajog.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 22.El-Toukhy TA. Hefni M. Davies A. Mahadevan S. The effect of different types of hysterectomy on urinary and sexual functions: A prospective study. J Obstet Gynaecol. 2004;24:420–425. doi: 10.1080/01443610410001685574. [DOI] [PubMed] [Google Scholar]
- 23.Thakar R. Ayers S. Clarkson P. Stanton S. Manyonda I. Outcomes after total versus subtotal abdominal hysterectomy. N Engl J Med. 2002;347:1318–1325. doi: 10.1056/NEJMoa013336. [DOI] [PubMed] [Google Scholar]
- 24.Gimbel H. Zobbe V. Andersen BJ, et al. Lower urinary tract symptoms after total and subtotal hysterectomy: Results of a randomized controlled trial. Int Urogynecol J Pelvic Floor Dysfunction. 2005;16:257–262. doi: 10.1007/s00192-005-1291-8. [DOI] [PubMed] [Google Scholar]