Abstract
We proposed that pharmacological manipulation of mesenchymal stem cells (MSCs) with diazoxide enhanced their survival and regenerative potential via NFκB regulation. MSCs preconditioned (PCMSCs) with diazoxide and later subjected to oxidant stress with 100 μmol/L H2O2 either immediately or after 24 h exhibited higher survival (p < 0.01 vs nonpreconditioned MSCs; Non-PCMSCs) with concomitantly increased phosphorylation of PI3K, Akt, GSK3β (cytoplasmic), and NF-κB (p65) (nuclear). Akt kinase activity was determined as a function of GSK3β activity. Pretreatment of PCMSCs with Wortmannin (Wt), NEMO-binding domain (NBD), or NF-κB (p50) siRNA abolished NF-κB (p65) activity. Preconditioning increased NF-κB-dependent elevation of secretable growth factors associated with their paracrine effects. Inhibition of PI3K activity with Wt reduced PCMSCs viability at both early and 24 h time-points. However, inhibition of NF-κB reduced viability of PCMSCs only at 24 h time-point. For in vivo studies, DMEM without cells (group-1) or containing 1 × 106 male Non-PCMSCs (group-2), PCMSCs (group-3), PCMSCs pretreated with Wortmannin (group-4) or NF-κB decoy (group-5) were transplanted in a female rat model of acute myocardial infarction. Group-3 showed highest cell survival and growth factor expression, increased angiomyogenesis, and functional improvement. We conclude that activation of NF-κB by preconditioning promoted PCMSCs survival and angiomyogenic potential in the infarcted heart. Antioxid. Redox Signal. 12, 693–702.
Introduction
Preconditioning of stem cells is an emerging strategy to curtail the massive death of cells after transplantation (13). Different strategies have been used to precondition the cells before transplantation (3). Despite encouraging results, the underlying mechanism of preconditioning-induced cytoprotection in stem cells remains contentious. Using skeletal myoblasts as a cellular model, we have previously shown that preconditioning of the cells with diazoxide (DZ) significantly enhanced their post-transplantation survival (13). DZ, a known activator of ATP sensitive potassium ion channels, is a preconditioning mimetic. Treatment with DZ causes local relaxation in smooth muscle by increasing membrane permeability to potassium ions and switching off voltage-gated calcium ion channels that inhibits the generation of an action potential (22). In this study however, we did not administer DZ to the animals. Rather, DZ was used to precondition MSCs to improve their survival post engraftment. Little is known regarding the mechanistic involvement of NF-κB in cytoprotective effects of DZ in general and in the preconditioned stem cells in particular. The present study elucidated the underlying mechanism of preconditioning-induced cytoprotection in mesenchymal stem cells with activation of nuclear factor-κB (NF-κB), besides determining the versatility of cellular preconditioning approach.
NF-κB is a family of inducible transcription factors that are assembled through dimerization of five subunits: RelA (p65), c-Rel, RelB, NF-κB1 (p50), and NF-κB2 (p52) (6). In a native unstimulated MSC, most NF-κB dimers are present in the cytoplasm in a bound state with the specific inhibitors of NF-κBs (IκBs). Cell stimulation however, activates the IκB kinase (IKK) complex that is composed of two catalytic subunits (IKK-α and IKK-β) and a regulatory subunit (IKK-γ/NEMO) (16). The activated IKK phosphorylates NF-κB-bound IκB proteins and targets them for polyubiquitination and rapid degradation. The freed NF-κB dimers thus translocate to the nucleus where they coordinate the transcriptional activation of their target genes (15, 24). NF-κB has been extensively studied in relation to immune reactions and cancer (6). Deletion of NF-κB (p65) subunit or expression of an inhibitor of NF-κB (IκB) inhibits the expression of several critical anti-apoptotic proteins including the specific inhibitor of caspase-8, caspase inhibitors cIAP1 and cIAP2, and Bcl-xL (7). The pro-survival role of NF-κB has also been attributed to the production of cytokines such as IL-6 which is encoded by an NF-κB target gene (1). Although extensively studied in neoplastic and immune cells, none of these studies have looked into the role of NF-κB in MSCs as a pro-survival factor.
Our results demonstrated that preconditioning of stem cells activated PI3K and Akt with a clear evidence of concomitant canonical activation of NF-κB. Besides, there is convincing evidence for the release of various pro-survival factors from preconditioned MSCs (PCMSCs) in an NF-κB dependent fashion. Although there was substantial evidence for phosphorylation and nuclear translocation of NF-κB (p65) in PCMSCs immediately after preconditioning, the cytoprotective effects of these molecular transitions were not evident during the first 4 h after preconditioning (early phase). However, blockade of NF-κB reduced the survival of PCMSCs assessed 24 h after preconditioning. Taken together, activated NF-κB has pro-survival activity in PCMSCs and regulated the expression of an array of growth factors in PCMSCs post-engraftment which acted in an autocrine or paracrine fashion for repair of the infarcted myocardium.
Materials and Methods
All experiments involving animals were approved by the Institutional Animal Care and Use Committee of University of Cincinnati.
Cell isolation
Bone marrow-derived MSCs were isolated from young female Fisher-344 rats, as described in Supplemental Methods (see online supplemental material at www.liebertonline.com/ars). The cells were cultured for no more than 3–5 passages before use in vitro, as well as in vivo studies.
Preconditioning of MSCs and survival under oxidant stress
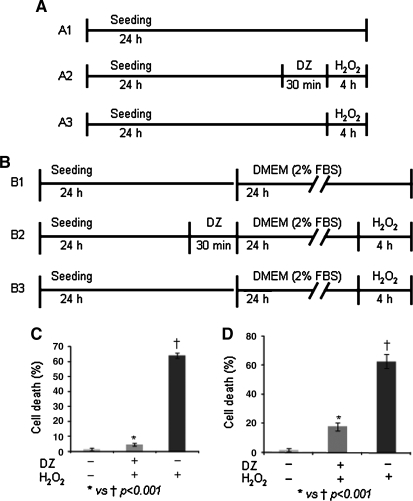
The pro-survival effects of preconditioning were assessed over a period of 24 h. The pro-survival effects observed immediately or later after preconditioning were referred as early phase and late phase effects, respectively. The experimental design for early and late phase effects is given in Fig. 1A and B, respectively. Briefly, 1 × 105 MSCs per 35 mm dish were seeded for 24 h to achieve a confluence of 80%–85%. The dishes were randomly divided into two sets, one for early phase effects (A1–A3) and the other for late phase effects (B1–B3). One dish in each set was preconditioned (PCMSCs) with 200 μmol/L DZ for 30 min. The other two dishes in each set were left untreated (Non-PCMSCs). The cell viability in PCMSCs and Non-PCMSCs during the early phase of preconditioning was assessed by treating dish A2 and A3 with 100 μmol/L H2O2 in glucose and serum-free DMEM for 4 h. The dish A1 served as an untreated control. To assess the cell viability in PCMSCs and Non-PCMSCs during the late phase after preconditioning, the dishes B1–B3 underwent the same treatment as described for A1–A3. However, at the end of 30 min preconditioning in B2, the medium in all three dishes (B1–B3) was replaced with low serum (2%) supplemented DMEM for 24 h. At the end of 24 h reperfusion, the PCMSCs (B2) and one dish of Non-PCMSCs (B3) were subjected to an oxidant stress of 100 μmol/L H2O2 in glucose and serum-free DMEM for 4 h. The dish B1 served as untreated control. Conditioned medium from each Petri dish was collected after 4 h of H2O2 treatment. Cell viability was determined by LDH measurement in the conditioned medium using CytoTox-ONE™ Homogeneous Membrane Integrity Assay (Promega, San Luis, CA). One dish of Non-PCMSCs treated with lysis solution (provided with the kit) achieved equivalent to 100% cell damage and was used as a positive control to calculate the percentage of cell damage in different groups. In addition to the LDH assay, cell viability was also determined by TUNEL immunostaining (Supplemental Methods, see online supplemental material at www.liebertonline.com/ars). The pro-survival effects of preconditioning during early phase and late phase were also studied in the presence of different inhibitors. The experimental protocol is detailed in Supplemental Methods and is shown in Supplemental Fig. 2A and B (see online supplemental material at www.liebertonline.com/ars) and the concentrations of inhibitors used in this study are described earlier (13).
FIG. 1.
Preconditioning of MSCs potentiated their viability under OGD. The experimental design for determination of (A) early and (B) late phase effects of DZ preconditioning. Cell survival assessed during (C) early phase and (D) late phase of preconditioning in PCMSCs and Non–PCMSCs.
Western blot analysis
Cell lysate was fractionated into cytoplasmic and nuclear fraction from different groups followed by Western blotting, as described in the Supplemental Methods (see online supplemental material at www.liebertonline.com/ars).
Quantitative assessment of Akt and NF-κB
The quantitative assessment of Akt was done by using Akt kinase assay (Cell Signaling, Danvers, MA). The activation of NF-κB was determined by using an ELISA-based kit from Active Motif (Carlsbad, CA) as described in the Supplemental Methods (see online supplemental material at www.liebertonline.com/ars).
Gene silencing studies
NF-κB acts either as homodimers of p50/p50 or heterodimers of p50/p65 subunits (6). Gene silencing studies were performed using NF-κB (p50) specific siRNA to block translocation of homodimers and heterodimers into the nucleus. The NF-κB (p50) specific siRNA and scrambled siRNA (control siRNA) were purchased from Qiagen (Valencia, CA) (Supplemental Methods, see online supplemental material at www.liebertonline.com/ars).
Real-time PCR
Real time PCR studies were performed using our published protocol (13). The primer sequence for different primers used is given in Supplemental Table 1 (see online supplemental material at www.liebertonline.com/ars).
In vivo studies
Experimental rat model of acute myocardial infarction and cell transplantation
All experimental procedures were performed in accordance with the standard human care guidelines of the “Guide for the Care and Use of Laboratory Animals” and Institutional Animal Care and Use of Committee of University of Cincinnati, which conforms to National Institutes of Health guidelines.
For cell transplantation studies, the cells were labeled with PKH26 cell tracker dye to study the fate of the transplanted cells (13). An experimental rat model of acute myocardial infarction in young female Fisher-344 rats was developed by permanent ligation of the coronary artery, as described earlier (13). Immediately after the coronary artery ligation, the animals were grouped to receive 60 μl DMEM without cells (group-1) or containing 1 × 106 male Non-PCMSCs (group-2), PCMSCs (group-3), PCMSCs pretreated with Wt (group-4), or PCMSCs pretreated with NF-κB decoy (group-5). The cells were injected intramyocardially under direct vision at multiple sites in and around the infarction area. For molecular analysis, the animals were euthanized on day-7 after their respective treatment, and the left ventricle was cut (n = 3 per group) and processed for isolation of gDNA and RNA for sry-gene analysis and growth factor expression analysis, respectively. Functional and histological studies were performed on the animals from groups 1–3 (n = 6 per group) after 6 weeks of their respective treatment, following which the animals were euthanized for collection of the heart tissue for histological studies. Histological studies were performed on paraffin-embedded or cryopreserved heart tissue samples, as described in the Supplemental Methods (see online supplemental material at www.liebertonline.com/ars).
Echocardiography
Cardiac function was assessed by echocardiography on day 7 and 6 weeks after their respective treatment as described earlier (13).
Statistics
All the data were described as mean ± SEM. To analyze the data statistically, we performed Student's t-test and 1-way ANOVA with post-hoc analysis and considered a value of p < 0.05 as statistically significant.
Results
Preconditioning of MSCs potentiated their survival under OGD
PCMSCs showed better survival than Non-PCMSCs over a period of 24 h. The percentage of cell death assessed during the early phase of preconditioning was significantly less in the PCMSCs (4.7 ± 1.8%) as compared to Non-PCMSCs (64.3 ± 4.7%; p < 0.001) (Fig. 1C). The survival of PCMSCs was decreased as a function of time as indicated by higher cell death (18 ± 3%) during late phase of preconditioning (Fig.1D). However, the degree of cell death in PCMSCs during late phase remained significantly lower (18 ± 3%) as compared to the corresponding Non-PCMSCs (66 ± 3.9%; p < 0.001) (Fig. 1D). These results clearly showed that cytoprotective effects of preconditioning lasted at least 24 h. The number of TUNEL-positive cells was significantly less in the PCMSCs (24 ± 7.6% and 34 ± 10.3%) as compared with the Non-PCMSCs (64.3 ± 4.7% and 75 ± 9.4%) during early and late phases, respectively (Supplemental Fig. 1A and B).
Preconditioning of MSCs induced canonical activation of NF-κB
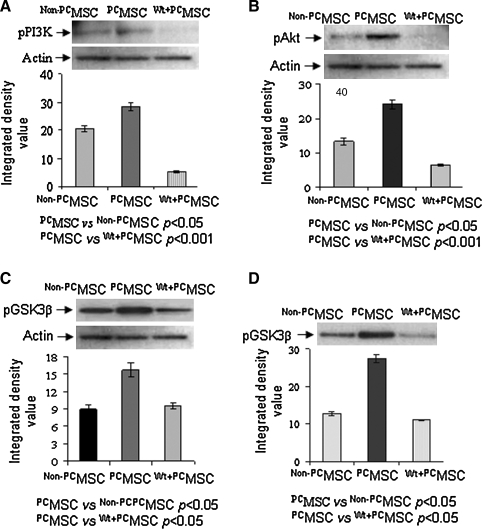
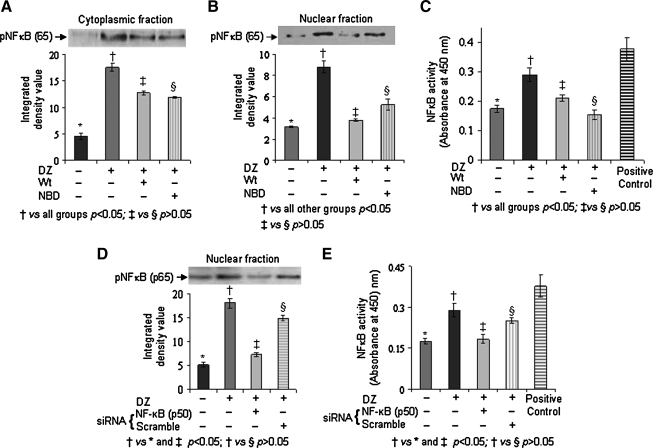
In order to delineate the pro-survival pathway in PCMSCs, we isolated the cell lysate from different treatment groups for Western blot studies which showed increased phosphorylation of PI3K (Fig. 2A), Akt (Fig. 2B), and GSK3β (Fig. 2C) in PCMSCs as compared with Non-PCMSCs. Activity of Akt determined by an Akt-kinase assay using GSK3β as substrate was also significantly higher in the PCMSCs as compared to Non-PCMSCs (Fig. 2D). Pretreatment of PCMSCs with Wt significantly abrogated these molecular events. These data indicate that phosphorylation of Akt and GSK3β in PCMSCs was mediated via activation of PI3K. Next we examined the activity of NF-κB in different treatment groups of the cells. Nuclear and cytoplasmic fraction of the cell lysate from each treatment group was isolated after 30 min of preconditioning. The pNF-κB (p65) subunit (phosphorylated NF-κB) was used as a surrogate marker for NF-κB activity. The amount of pNF-κB (p65) was significantly increased in cytoplasmic and nuclear protein fractions of PCMSCs as compared to Non-PCMSCs (Fig. 3A and B) which was significantly reduced in PCMSCs in the presence of Wt and NBD (IKKα/IKKß Inhibitor). TransAM NF-κB (p65) assay performed to detect and quantify the activity of NF-κB in the nuclear fraction was consistent with Western blot data (Fig. 3C). The reduction of pNF-κB (p65) in PCMSCs in the presence of NBD confirmed that this activation occurred via IKKα/IKKß. This is the first evidence for canonical activation of NF-κB in PCMSCs. Gene silencing studies also supported the activation of NF-κB in PCMSCs. Immunoblotting performed on nuclear fraction from PCMSCs transfected with NF-κB (p50) siRNA showed abrogation of pNF-κB (p65) as compared to PCMSCs transfected with scramble siRNA (Fig. 3D). The data from TransAM assay performed on the same nuclear lysate was in accordance with Western blotting results (Fig. 3E).
FIG. 2.
Preconditioning induced expression of pro-survival proteins. Immunoblots from the protein samples collected from the cells harvested 4 h after their respective treatment. Immunodetection with respective antibody showed significantly increased phosphorylation of (A) PI3K, (B) Akt, and (C) GSK3β in Non-PCMSCs, PCMSCs, and PCMSCs treated with Wt. The densitometry was done by using β-actin as internal control. (D) Immunoblot showing phospho-GSK3β from Akt kinase assay which used GSK3β as substrate.
FIG. 3.
Preconditioning of MSCs induced canonical activation of NF-κB. Immunoblots from the protein samples collected from the cells harvested 4 h after their respective treatment. Immunodetection with respective antibodies showed phospho-NF-κB (p65) in (A) cytoplasmic and (B) nuclear protein fraction in Non-PCMSCs, PCMSCs, PCMSCs treated with Wt and PCMSCs treated with NBD. (C) TransAM assay showing activity of phospho-NF-κB (p65) in the nuclear fraction in the same samples. (D) Immunoblot showing phospho-NF-κB (p65) in the nuclear protein fraction from Non-PCMSCs, PCMSCs, and PCMSCs transfected with NF-κB (p50) siRNA and PCMSCs transfected with scrambled siRNA. (E) TransAM assay showing activity of NF-κB (p65) in the nuclear fraction from different groups used in gene silencing studies. The densitometry (IDV) in all the blots was determined by using β-actin (Octamer-1 for nuclear fraction) as internal control.
Canonical activation of NF-κB results in sustained survival of MSCs
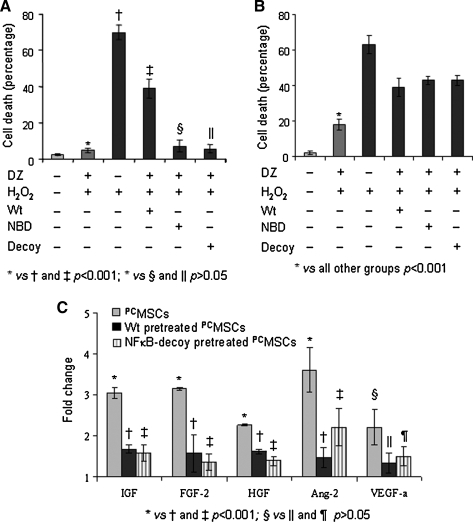
We speculated that early effects of preconditioning were dependent on Akt activation but independent of NF-κB activation. Therefore, in order to determine the functional significance of NF-κB activation in PCMSCs, cell survival studies were performed during early phase and late phase preconditioning in the presence of different inhibitors. Pharmacological blockers (Wt and NBD) and nonpharmacological blockers (NF-κB decoy) were used to elucidate the survival signaling. The experimental design is shown in Supplemental Fig. 2A and B. Our results showed that presence of Wt during preconditioning resulted in higher cell death during early phase (39 ± 6.2%; Fig. 4A) and late phase (46 ± 2.8%; Fig. 4B) of preconditioning as compared to the corresponding PCMSCs without Wt pretreatment (4.8 ± 1.3% and 18 ± 3%, respectively). Although the presence of NBD during preconditioning significantly blocked NF-κB (p65) activation (Fig. 3A–C), nevertheless it failed to abolish the cytoprotective effects of preconditioning during the early phase (Fig. 4A). The cell death in this group was insignificantly different from PCMSCs (p > 0.05). The transfection of NF-κB decoy also failed to reduce PCMSCs survival during the early phase of preconditioning (Fig. 4A). The survival studies with NBD and NF-κB decoy showed that activation of NF-κB has a minimal role in cytoprotection during the early phase after preconditioning. Interestingly, both NBD treatment and NF-κB decoy significantly reduced the survival of PCMSCs during the late phase of preconditioning (43 ± 3.2 and 44 ± 4.5, respectively), thus signifying the predominant role of NF-κB activation during the late phase (Fig. 4B).
FIG. 4.
Canonical activation of NF-κB resulted in survival of MSCs. Cell viability during (A) early phase of preconditioning and (B) late phase of preconditioning in different groups in the presence of inhibitors. (C) Real time PCR showing expression of prosurvival and pro-angiogenic factors in PCMSCs as compared to Wt-treated and NF-κB decoy-treated PCMSCs. The fold change was calculated by using Non-PCMSCs as normalizing control.
Activation of NF-κB induced expression of pro-survival and angiogenic factors
The significant contribution of NF-κB activation during the late phase preconditioning prompted us to look for the expression and association of different pro-survival molecules associated with NF-κB activation in PCMSCs. Real-time PCR showed higher expression of HGF, IGF, FGF-2, and Ang-2 in PCMSCs (Fig. 4C) which was abrogated in the presence of Wt and NF- κB decoy (p < 0.05). These data indicated that preconditioning-induced activation of NF-κB led to higher expression of pro-survival factors that were presumably responsible for enhanced survival of PCMSCs during the late phase.
Preconditioning enhanced MSCs survival after transplantation via activation of NF-κB
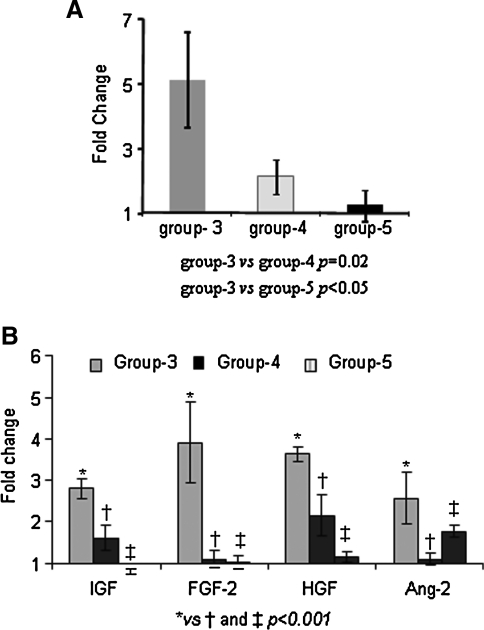
The survival of male MSCs was assessed by sry-gene detection in a female rat model of acute myocardial infarction on day 7 after transplantation. The cells transplanted in group-3 showed higher survival as compared with group-4 and group-5 (Fig. 5A). The fold change was calculated by using group-2 as control. Group-1 (DMEM injected) hearts did not show any amplification of sry-gene and served as negative controls. The rate of cell survival in various treatment groups was not determined. The left ventricular tissue used for sry-gene studies was also used for transcriptional profiling of pro-survival and pro-angiogenic factors. The results showed higher expression of HGF, IGF, FGF-2, and Ang-2 in group-3 as compared to group-4 and group-5 (Fig. 5B). These results supported our rationale that preconditioning which activated NF-κB in PCMSCs improved the survival of PCMSCs and led to higher expression of pro-survival factors in the ischemic myocardium.
FIG. 5.
Activation of NF-κB induced pro-survival factors and enhanced survival of MSCs post-transplantation. Real time PCR for (A) sry-gene and (B) growth factor expression in group-3, as compared to group-4 and group-5 using group-2 as normalizing control.
PCMSCs improved cardiac function and attenuated infarction size
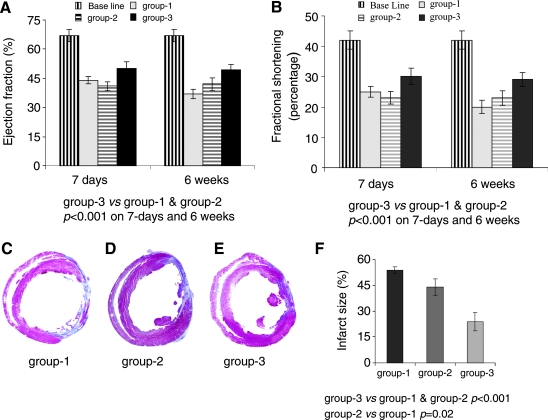
Baseline transthoracic echocardiography measurements for LV ejection fraction (LVEF) 67 ± 3.4% and LV fractional shortening (LVFS) 42 ± 2.3% were obtained in pre-surgical animals (n = 6) to serve as a baseline control (Fig. 6A and B). LVEF after 7 days of surgery was significantly reduced in group-1 (44 ± 1.9 %) and group-2 (41 ± 1.2%) as compared to group-3 (50 ± 5.7%). Unlike group-1 which showed a continued functional deterioration as indicated by LVEF (37.3 ± 3.3%) at 6 weeks, LVEF was preserved in group-2 (42 ± 3.7%) and group-3 (50 ± 5.7%) (Fig. 6A). End diastolic and systolic dimensions as indices of LV remodeling were also preserved in group-3 (Supplemental Fig. 3A–D). Masson trichrome-stained sections of the hearts harvested after 6 weeks of transplantation had reduced infarction size in group-2 and group-3 as compared with group-1 (Fig. 6C–E). The infarction size was significantly reduced in group-3 (24 ± 5.3%) as compared to group-2 (42 ± 4.8%; p < 0.02) and group-1 (50 ± 1.8%; p < 0.001) (Fig. 6F).
FIG. 6.
Transplantation of PCMSCs improved cardiac function and attenuated infarction size. (A) LVEF and (B) LVFS on day 7 post-infarction deteriorated significantly in all the treatment groups as compared with the baseline. However, further deterioration in heart function was observed in group-1 until 6 weeks of observation, as compared to non-PCMSCs transplanted group-2 and PCMSCs transplanted group-3 (p < 0.05). Group-3 showed significantly preserved LV contractile function indices as compared to group-2. (C–F) Quantitative assessment of infarction size in Masson's trichrome-stained histological tissue sections (C) group-1, (D) group-2, and (E) group-3 at 6 weeks after their respective treatment. (F) Comparison of the fibrotic area in the infarcted heart (n = 4/group) showed significantly attenuated infarct size in group-3 as compared with group-2 (p < 0.02) and group-1 (p < 0.001).
PCMSCs promoted myoangiogenesis
Using DMEM injected animal hearts (group-1) as a control (Supplemental Figs. 4A and B), extensive presence of PKH26 labeled donor cells was observed in the infarction and peri-infarction areas in group-2 and group-3 animal hearts both at day 7 and 6 weeks after cell engraftment (Supplemental Figs. 4C–F). Immunostaining for cardiac actin (green) was co-localized with PKH-26 labeled cells (red), thus revealing neomyogenesis after 7 days of cell transplantation (Supplemental Figs. 4C and E). Similarly, double fluorescent immunostaining for cardiac actin (green, Supplemental Fig. 4D; cyan, Supplemental Fig. 4F) and connexin-43 (purple, Supplemental Fig. 4D; yellow, Supplemental Fig. 4F) co-localized with PKH-26 labeled PCMSCs showed their myogenic differentiation and functional integration at 6 weeks after cell transplantation. Myogenic differentiation in group-2 however, was markedly less.
An extensive angiogenic response was observed in group-3 as compared with the group-2. Immunostaining for vWF-VIII was performed to elucidate the angiogenic response after 6-weeks of transplantation (Supplemental Fig. 5). The blood vessel density was significantly higher in the peri-infarct and infarct areas in group-3 as compared with group-1 and group-2. Blood vessel density per surface area (0.155 mm2) at 400 × magnification in the infarct and peri-infarct regions was 35.5 ± 8.6 and 58 ± 9.8, respectively, in group-3 as compared with group-2 (14.2 ± 1.4; p < 0.001; 21.7 ± 3.6; p < 0.001).
Discussion
Preconditioning of stem cells before transplantation is a novel albeit less studied concept in heart cell therapy. The previous studies have used different preconditioning strategies (2, 13, 18). The present study provides new insight into the mechanistic involvement of NF-κB as a pro-survival transcription factor that also stimulated the expression of multiple growth factors. The salient findings of our study include a) preconditioning enhanced survival of MSCs against oxidant stress; b) the cytoprotective effects during the early phase after preconditioning were predominantly due to increased phosphorylation of PI3K; and Akt; c) the cytoprotective effects after preconditioning were NF-κB dependent; and d) preconditioning significantly enhanced the survival and growth factor expression of transplanted MSCs in NF-κB -dependent manner with resultant attenuated infarct size and improved cardiac function after 6 weeks.
The massive death of transplanted cells remains a major challenge in heart cell therapy (17). The underlying cause of unusually high and accelerated cell death is multifactorial. Besides other mechanisms, the dynamics of the early phase cell death implicates local immune and inflammatory responses, loss of trophic factors and local tissue ischemia as the prime factors (3). Hence, a strategy aimed at modifying the cytokine-rich harsh milieu of the ischemic myocardium to become friendlier may significantly enhance the stem cell survival. Alternatively, donor cells can be modified to enhance their resistance to ischemic stress. Our approach of pharmacological preconditioning of stem cells prior to engraftment is not only limited to their better survival through paracrine effects, it also improves their therapeutic effectiveness by promoting their myogenic differentiation and integration with the host myocytes. The immediate protection in the early phase of preconditioning was mediated by activation of diverse signaling pathways and allowed the cells ample time to acclimatize in the harsh microenvironment of the infarcted heart. The cytoprotective effects in the late phase of preconditioning was the result of upregulation of various transcription factors and synthesis of various growth factor proteins. Furthermore, such manipulation also promotes their paracrine activity to exert cytoprotective effects on the host cardiomyocytes and angiomyogenic activity in the infarcted heart.
NF-κB is critical for survival of PCMSCs
Activation of NFκB is an important molecular event in both pro-apoptotic and pro-survival signaling (8, 11). On account of its extensive involvement in diverse biological processes, the dysfunctioning NFκB has been implied in various cardiovascular pathologies (4). Experimental animal studies have also implicated NF-κB in the late phase of ischemic preconditioning of heart (14). Using a murine model of ischemia-reperfusion injury, we have already reported that activation of NF-κB was responsible for cardioprotection during the late phase of preconditioning (21). Our present study is an extrapolation of this observation to the stem cells and showed that preconditioning-induced canonical activation of NFκB is downstream of Akt phosphorylation in PCMSCs. Similar results have been recently reported by Zhang and colleagues who showed that activation of Toll-like receptors on MSCs using lipopolysaccharides was cytoprotective and involved phosphorylation of Akt (Ser 473) and NF-κB (Ser 536) (23).
In the process of NFκB activation, pAkt activates IκB kinase, thus increasing IκB phosphorylation and degradation (5). NF-κB dimer (i.e., p50/p65) then translocates to the nucleus to bind with its cognate DNA element and activates transcription. We observed that NBD significantly reduced p65/NFκB phosphorylation in the nuclear fraction, thus revealing canonical activation of p65/NFκB. The induction of nuclear translocation of NFκB and its activation by preconditioning was sensitive to Wt pretreatment that significantly abrogated PI3K/Akt activation along with abrogation of p65/NFκB activation and its nuclear translocation. Moreover, Wt pretreatment of PCMSCs reduced their viability in the early phase of preconditioning. Although activation and nuclear translocation of NFκB immediately after preconditioning of the cells were observed, inhibition with NF-κB decoy and NBD in PCMSCs did not affect their survival under subsequent oxidant stress. These results suggest that NF-κB activation and nuclear translocation has little significance in the early phase preconditioning-induced cell survival. On the other hand, the use of NBD or an NF-κB decoy in late phase preconditioning reaffirms that activation of NF-κB is not an epiphenomenon and NF-κB activation is critical for sustained cytoprotection of PCMSCs during the late phase preconditioning. The use of NBD to block the activity of regulatory subunit (IKK-γ/NEMO) of IKK complex in PCMSCs was supportive of canonical activation of NF-κB in these cells. Due to the nature of many kinases having multiple phosphorylation targets, kinase inhibitors may exhibit nonspecific “off-target” effects in addition to NF-κB blockade. Therefore, transcription factor decoys were preferred over kinase inhibitors for inhibition of NF-κB due to the increased specificity that can be achieved with the decoys.
Paracrine factors and cardioprotection
There is mounting evidence that stem cells orchestrate the repair process in the heart by secreting an array of angiogenic, anti-apoptotic, and anti-inflammatory cytokines that act in a paracrine manner (7, 13). The ability to release paracrine factors was accentuated in response to preconditioning with PCMSCs expressing copious levels of the secretable growth factors. The higher expression of HGF, IGF, FGF-2, and Ang-2 in PCMSCs occurred in Akt and NF-κB dependent fashion. There is a possibility that a part of the elevated growth factor expression was contributed by the resident or mobilized inflammatory cells homing into the infarcted myocardium as a part of the intrinsic repair process. Nevertheless, a comparison between the PCMSCs (group-3) transplanted heart tissues with those transplanted with Non-PCMSCs (group-) clearly support our conclusion that a major contribution of these growth factors was from the transplanted PCMSCs. Moreover, the significant reduction in the growth factor expression observed in groups-4 and -5 animal hearts that received PCMSCs pretreated with Wt and NF-kB decoy, respectively, further vindicate our inference that the observed elevated expression of growth factors in group-3 animal hearts was mainly contributed by the transplanted PCMSCs. In the light of the earlier study results that the use of individual growth factors is cardioprotective (7), we anticipated that delivery of PCMSCs capable of releasing a multitude of pro-survival factors would be more appealing than individual proteins.
Preconditioning of MSCs enhanced their differentiation potential
Preconditioning is also believed to enhance the differentiation potential of stem cells. The marked difference between PCMSCs and Non-PCMSCs in their myogenic differentiation post engraftment in the heart may be attributed to the inhibition of pGSK3β. Previous studies have shown that inhibition of GSK-3β downstream of pAkt is sufficient to duplicate the effects of IGF-1 on protein synthesis and muscle differentiation (19, 20). We have observed increased pGSK3 in PCMSCs after transplantation of PCMSCs and anticipate that this molecular event contributed to improved myogenesis in the PCMSCs transplanted animal hearts as compared with Non-PCMSCs transplanted hearts. Taken together, we speculate that activation of NF-κB is a primary event leading to the secretion of different paracrine factors that play an important role in the longevity of transplanted cells, thus increasing their chances of successful engraftment.
In conclusion, preconditioning is an important strategy to enhance survival of MSCs after transplantation. Moreover, activation of NF-κB during the late phase of preconditioning (24 h after preconditioning) is the primary event leading to upregulation of different angiogenic growth factors, pro-survival and anti-apoptotic genes which are responsible for sustained protection of MSCs after preconditioning. Ex-vivo MSC modification may be an important strategy to improve MSC mediated paracrine protection.
Study limitations
Despite portraying the significance of PI3/Akt signaling and the involvement of downstream NFκB in pharmacological preconditioning of stem cells, there are some study limitations. Most importantly, the effect of preconditioning was assessed only on two time points, 4 h and 24 h. Experiments at time points in between 4 h and 24 h will determine the significance of NF-κB phosphorylation as a pro-survival event in PCMSCs. An important feature of our study was the dependence of growth factor expression on NF-κB activation in PCMSCs. An extensive and in-depth analysis of the secretable growth factors other than angiogenic factors, which are upregulated in PCMSCs and are released in NFκB dependent manner, would be of greater interest.
Supplementary Material
Abbreviations Used
- DZ
diazoxide
- EF
ejection fraction
- FS
fractional shortening
- IDV
integrated densitometric value
- LV
left ventricle
- MSCs
mesenchymal stem cells
- NBD
NF-κB essential modulator (NEMO)-binding domain peptide
- NF-κB
nuclear factor- κB
- Non-PCMSCs
nonpreconditioned MSCs
- OGD
oxygen-glucose deprivation
- PCMSCs
preconditioned MSCs
- TUNEL
Terminal Deoxynucleotidyl Transferase dUTP nick end labeling (TUNEL)
- Wt
Wortmannin
Acknowledgments
We greatly acknowledge Dr. Keith L Jones and Mike Tranter from University of Cincinnati, Ohio for providing the NF-κB decoy. Financial support for this study were National Institutes of Health (NIH) Grants # R37-HL074272; HL-080686; HL-087246 to (MA) and HL-087288; HL-089535 (KhHH).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Becker C. Fantini MC. Schramm C. Lehr HA. Wirtz S. Nikolaev A. Burg J. Strand S. Kiesslich R. Huber S. Ito H. Nishimoto N. Yoshizaki K. Kishimoto T. Galle PR. Blessing M. Rose-John S. Neurath MF. TGF-β suppresses tumor progression in colon cancer by inhibition of IL-6 trans-signaling. Immunity. 2004;21:491–501. doi: 10.1016/j.immuni.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 2.Hahn JY. Hahn JY. Cho HJ. Kang HJ. Kim TS. Kim MH. Chung JH. Bae JW. Oh BH. Park YB. Kim HS. Pre-treatment of mesenchymal stem cells with a combination of growth factors enhances gap junction formation, cytoprotective effect on cardiomyocytes, and therapeutic efficacy for myocardial infarction. J Am Coll Cardiol. 2008;9:933–943. doi: 10.1016/j.jacc.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 3.Haider HKh. Ashraf M. Strategies to promote donor cell survival: combining preconditioning approach with stem cell transplantation. J Mol Cell Cardiol. 2008;45:554–566. doi: 10.1016/j.yjmcc.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall G. Hasday JD. Rogers TB. Regulating the regulator: NF-kappaB signaling in heart. J Mol Cell Cardiol. 2008;41:580–591. doi: 10.1016/j.yjmcc.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 5.Hoffmann A. Levchenko A. Scott ML. Baltimore D. The IkappaB-NF-kappaB signaling module: Temporal control and selective gene activation. Science. 2002;298:1241–1245. doi: 10.1126/science.1071914. [DOI] [PubMed] [Google Scholar]
- 6.Karin M. Nuclear factor-kappa B in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 7.Karin M. Lin A. NF-κB at the crossroads of life and death. Nature Immunol. 2002;3:221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 8.Kuhnel F. Zender L. Paul Y. Tietze MK. Trautwein C. Manns M. Kubicka S. NFkappaB mediates apoptosis through transcriptional activation of Fas (CD95) in adenoviral hepatitis. J Biol Chem. 2000;275:6421–6427. doi: 10.1074/jbc.275.9.6421. [DOI] [PubMed] [Google Scholar]
- 9.Li Q. Guo Y. Tan W. Ou Q. Wu WJ. Sturza D. Dawn B. Hunt G. Cui C. Bolli R. Cardioprotection afforded by inducible nitric oxide synthase gene therapy is mediated by cyclooxygenase-2 via a nuclear factor-kappaB dependent pathway. Circulation. 2007;14:1577–1584. doi: 10.1161/CIRCULATIONAHA.107.689810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mirotsou M. Zhang Z. Deb A. Zhang L. Gnecchi M. Noiseux N. Mu H. Pachori A. Dzau V. Secreted frizzled related protein 2 (Sfrp2) is the key Akt-mesenchymal stem cell-released paracrine factor mediating myocardial survival and repair. Proc Natl Acad Sci USA. 2007;5:643–648. doi: 10.1073/pnas.0610024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Misra A. Haudek SB. Knuefermann P. Vallejo JG. Chen ZJ. Michael LH. Sivasubramanian N. Olson EN. Entman ML. Mann DL. Nuclear factor-kappaB protects the adult cardiac myocyte against ischemia-induced apoptosis in a murine model of acute myocardial infarction. Circulation. 2003;108:3075–3078. doi: 10.1161/01.CIR.0000108929.93074.0B. [DOI] [PubMed] [Google Scholar]
- 12.Murry CE. Jennings RB. Reimer KA. Preconditioning with ischemia: A delay of lethal cell injury in ischemic myocardium. Circulation. 1986;5:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 13.Niagara MI. Haider H. Jiang S. Ashraf M. Pharmacologically preconditioned skeletal myoblasts are resistant to oxidative stress and promote angiomyogenesis via release of paracrine factors in the infarcted heart. Circ Res. 2007;100:545–555. doi: 10.1161/01.RES.0000258460.41160.ef. [DOI] [PubMed] [Google Scholar]
- 14.Ockaili R. Emani VR. Okubo S. Brown M. Krottapalli K. Kukreja RC. Opening of mitochondrial KATP channel induces early and delayed cardioprotective effect: role of nitric oxide. Am J Physiol. 1999;277:25–34. doi: 10.1152/ajpheart.1999.277.6.H2425. [DOI] [PubMed] [Google Scholar]
- 15.Park JM. Greten FR. Wong A. Westrick RJ. Arthur JS. Otsu K. Hoffmann A. Montminy M. Karin M. Signaling pathways and genes that inhibit pathogen-induced macrophage apoptosis: CREB and NF-κB as key regulators. Immunity. 2005;23:319–329. doi: 10.1016/j.immuni.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Rothwarf DM. Karin M. The NF-κB activation pathway: A paradigm in information transfer from membrane to nucleus. Sci STKE. 1999;1999:RE1. doi: 10.1126/stke.1999.5.re1. [DOI] [PubMed] [Google Scholar]
- 17.Toma C. Pittenger MF. Cahill KS. Byrne BJ. Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;1:93–98. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 18.Urbanek K. Rota M. Cascapera S. Bearzi C. Nascimbene A. De Angelis A. Hosoda T. Chimenti S. Baker M. Limana F. Nurzynska D. Torella D. Rotatori F. Rastaldo R. Musso E. Quaini F. Leri A. Kajstura J. Anversa P. Cardiac stem cells possess growth factor-receptor systems that after activation regenerate the infarcted myocardium, improving ventricular function and long-term survival. Circ Res. 2005;7:663–673. doi: 10.1161/01.RES.0000183733.53101.11. [DOI] [PubMed] [Google Scholar]
- 19.van der Velden JL. Schols AM. Willems J. Kelders MC. Langen RC. Glycogen synthase kinase 3 suppresses myogenic differentiation through negative regulation of NFATc3. J Biol Chem. 2008;283:358–366. doi: 10.1074/jbc.M707812200. [DOI] [PubMed] [Google Scholar]
- 20.van der Velden JL. Langen RC. Kelders MC. Wouters EF. Janssen–Heininger YM. Schols AM. Inhibition of glycogen synthase kinase-3beta activity is sufficient to stimulate myogenic differentiation. Am J Physiol Cell Physiol. 2006;290:C453–462. doi: 10.1152/ajpcell.00068.2005. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y. Kudo M. Xu M. Ayub A. Ashraf M. Mitochondrial K (ATP) channel as an end effector of cardioprotection during late preconditioning: Triggering role of nitric oxide. J Mol Cell Cardiol. 2001;11:2037–2046. doi: 10.1006/jmcc.2001.1468. [DOI] [PubMed] [Google Scholar]
- 22.Wang Y. Takashi E. Xu M. Ayub A. Ashraf M. Downregulation of protein kinase C inhibits activation of mitochondrial K(ATP) channels by diazoxide. Circulation. 2001;104:85–90. doi: 10.1161/01.cir.104.1.85. [DOI] [PubMed] [Google Scholar]
- 23.Wang ZJ. Zhang FM. Wang LS. Yao YW. Zhao Q. Gao X. Lipopolysaccharides can protect mesenchymal stem cells (MSCs) from oxidative stress-induced apoptosis and enhance proliferation of MSCs via Toll-like receptor(TLR)-4 and PI3K/Akt. Cell Biol Int. 2009;33:665–674. doi: 10.1016/j.cellbi.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Werner SL. Barken D. Hoffmann A. Stimulus specificity of gene expression programs determined by temporal control of IKK activity. Science. 2005;309:1857–1861. doi: 10.1126/science.1113319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.