Abstract
Studies of cell transplantation therapeutics in animal models of traumatic spinal cord injury (SCI) are often hampered by partial or complete rejection of the graft by the host. Pharmacological immunosuppression is rarely sufficient to prevent rejection. Further, the immunological niche created by both the host immune response and immunosuppressant drugs could hypothetically influence the proliferation, differentiation, and fate of transplanted progenitor/stem cells. To avoid these confounds, we have previously used the constitutively immunodeficient non-obese diabetic severe combined immunodeficient (NOD-SCID) mouse as a model for transplantation studies following SCI. In the current study, we compare behavioral and histological recovery in NOD-SCID, C57BL/6, and BUB/BnJ mice of both sexes to better facilitate interpretation of data from studies using NOD-SCID mice. Of the strains examined, NOD-SCID mice exhibited the greatest locomotor recovery in the open field; no sex differences were detected in locomotor recovery in any of the strains. Stereologic estimation of the number of infiltrated neutrophils showed more cells in C57BL/6 mice than NOD-SCID mice, with BUB/BnJ mice having an intermediate number. The volume of macrophages/microglia did not differ between strains or sexes, though more rostral-caudal spreading was observed in C57BL/6 and BUB/BnJ than NOD-SCID mice. No significant differences were detected in lesion volume. Taken together these findings demonstrate that relative to other strains, NOD-SCID mice have both similar primary lesion volume and cellular inflammatory parameters after SCI, and support the applicability of the model for neurotransplantation studies.
Key words: animal studies, immunohistochemistry, inflammation, locomotor function, traumatic spinal cord injury
Introduction
Traumatic spinal cord injury (SCI) currently affects over 1,250,000 Americans. Animal models of SCI have been used to investigate a variety of therapeutic interventions, including treatments that reduce inflammation (Gris et al., 2004; Popovich et al., 1999; Stirling et al., 2004), promote regeneration (Freund et al., 2007; Low et al., 2008; Nikulina et al., 2004), or replace cells (Cummings et al., 2005; Hofstetter et al., 2002; Karimi-Abdolrezaee et al., 2006). While treatments including cellular replacement, particularly with stem cells, have become a hot topic, these studies often face the confound of cell rejection by the host.
To avoid rejection, many researchers turn to immunosuppressants including cyclosporine A (CsA) and tacrolimus (FK506). Despite the use of these drugs, engraftment rates rarely approach 100%, either in terms of the number of transplanted animals exhibiting cell survival at sacrifice, or the number of surviving cells relative to the initial number transplanted (Marsala et al., 2004; Parr et al., 2007; Sheth et al., 2008). These data suggest that immunosuppression is insufficient in the majority of studies. Additionally, the immune response has been implicated in affecting differentiation of the transplanted cells (Guo et al., 2007; Ideguchi et al., 2008; Monje et al., 2003). Further, both FK506 (Bavetta et al., 1999; Kaymaz et al., 2005; Madsen et al., 1998; Nottingham et al., 2002) and CsA (Diaz-Ruiz et al., 1999; Lopez-Vales et al., 2005; McEwen et al., 2007; Palladini et al., 1996; Ravikumar et al., 2007) have been shown to aid recovery from SCI, though the neuroprotective effect of CsA is debated (Rabchevsky et al., 2001). If pharmacological immunosuppression itself has an influence on recovery, interpretation of data involving transplants into immunosuppressed animals will be difficult.
To avoid the potential confounds of immunosuppression, we have used non-obese diabetic severe combined immunodeficient (NOD-SCID) mice as a model for transplantation studies (Cummings et al., 2005), as their immune system is constitutively deficient. Xenotransplantation studies in pharmacologically immunosuppressed animal models of central nervous system injury have rarely reported the percentage of transplanted animals exhibiting engraftment at the termination of a study. These studies have also shown poor cell survival, ranging from 1–37% of the initial transplant number in studies in which stereological quantification of this metric was performed (Karimi-Abdolrezaee et al., 2006; Marsala et al., 2004). In contrast, we have shown that 100% of NOD-SCID mice receiving xenografts of human neural stem cells exhibit engraftment at the time of sacrifice (Cummings et al., 2005). Additionally, we and others have reported quantification of transplanted cell survival ranging from 193–375% of the initial transplant number in constitutively immunodeficient rodent models (Cummings et al., 2005; Yan et al., 2007), suggesting an environment conducive to stem cell proliferation.
NOD-SCID mice have compromised innate immunity, as they have no complement hemolytic activity which is attributable to a lack of C5 (Shultz et al., 1995). Additionally, they are defective in adaptive immunity, lacking both mature B and T lymphocytes (Greiner et al., 1998). B cells and complement deposition are critical to antibody-mediated rejection (Baldwin et al., 1999; Serinsoz et al., 2005; Swijnenburg et al., 2008), and T cells are needed for cell-mediated rejection of grafts (Nozaki et al., 2008; Porrett et al., 2008; Swijnenburg et al., 2008). While they lack complement, other aspects of innate immunity, such as neutrophils and macrophages, remain intact in NOD-SCID mice. Neutrophils, which are generally first responders, are capable of mediating rejection responses when classical rejection pathways are blocked (El-Sawy et al., 2005; Miura et al., 2003; Surquin et al., 2005). Macrophages play a larger role in graft rejection, both directly and through T-cell activation (Armstrong et al., 2001; Cadili and Kneteman, 2008; Horne et al., 2008). Following SCI, these cells contribute to endogenous damage and repair processes through a diverse set of mechanisms, including promoting wound healing and axon regeneration, producing reactive oxygen and nitrogen species, removing cellular and myelin debris, and releasing cytokines (Carlson et al., 1998; Donnelly and Popovich, 2008; Popovich et al., 2002; Stirling et al., 2009).
In rodent studies of SCI, the strain of rat or mouse used affects factors such as inflammatory response and lesion volume, both of which may ultimately affect functional recovery (Kigerl et al., 2006; Popovich et al., 1997). The goal of the current study is to compare histopathological and behavioral outcomes of NOD-SCID mice with other laboratory strains. C57BL/6 mice are commonly used in SCI studies, though they have been reported to have higher amounts of cellular inflammation following injury than other common strains (Kigerl et al., 2006). As such, we have included another strain in our comparisons, BUB/BnJ, which may provide a more clinically relevant comparison, as BUB/BnJ mice exhibit the most human-like complement activation of all mouse strains (Galvan et al., 2008; Ong and Mattes, 1989), though there has been little investigation into other aspects of their innate immune response. These comparisons will aid in interpretation of data involving cellular transplants into SCI NOD-SCID mice.
Methods
Animals and injury procedures
All experiments were conducted in accordance with Institutional Animal Care and Use Committee guidelines. Average weight at the time of injury (±standard error) was as follows: C57BL/6 males 26.2 ± 0.4 g, C57BL/6 females 20.5 ± 0.3 g, NOD-SCID males 27.9 ± 0.8 g, NOD-SCID females 21.8 ± 0.5 g, BUB/BnJ males 28.5 ± 0.7 g, and BUB/BnJ females 23.5 ± 0.6 g. After anesthesia with Avertin (0.05% tribromoethane), the spinal cord was exposed by laminectomy of the T9 vertebral process, and a moderate contusion injury was administered (60 kilodynes) using the Infinite Horizon (IH) impactor (PSI Inc., Lexington, KY). After SCI, 0.9% sodium chloride solution (1 mL/day SC) was administered for the first 4 days post-injury (dpi) as additional fluid support. Four doses of buprenorphine were given as an analgesic spaced 12 hours apart, beginning immediately after surgery. All mice were maintained on thrice-daily manual bladder expression during the first 4 dpi, after which female bladder expression was reduced to twice daily. Enrofloxacin (2.5 mg/kg SC daily) was administered 2 days before surgery and then maintained for the entire study period. In an effort to avoid urolithiasis in male mice (described below), all animals were maintained on acidified drinking water (pH 6.5) after arrival at our facility and for the duration of the study.
Animal numbers and exclusion criteria
Male and female C57BL/6 (n = 59), BUB/BnJ (n = 35), and NOD-SCID (n = 55) mice 9–10 weeks old (Jackson Laboratory, Bar Harbor, ME) were used for the experiments. Animals with uneven spinal bruising and those exhibiting complications post-surgery, specifically urolithiasis, were excluded from the study to avoid interference with the immune response and behavioral assessment. A 28-dpi survival period was planned in the initial experiments; however, the onset of urolithiasis in a large percentage of the male animals (>50%) during this survival period made this time period impractical; however, many animals did not exhibit the onset of urolithiasis until more than 2 weeks post-injury. Several changes to our animal care procedures were instituted to limit urolithiasis, including giving the animals slightly acidic drinking water (pH 6.5), performing bladder expression three times daily, and injecting NaCl (0.5 mL SC) twice daily. Two cohorts of animals were tested independently for behavioral recovery for 15 dpi, and the data were pooled for analysis. Animals were randomly selected from the replication experiment to be used for histological analysis. A complete listing of the number of animals used in each portion of the study is provided in Table 1.
Table 1.
Numbers of Animals Used in the Experiments
| |
C57BL/6 |
BUB/BnJ |
NOD-SCID |
|||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |
| Pilot:a 28 days | ||||||
| 0 dpi | 9 | 9 | 8 | 7 | 10 | 7 |
| 15 dpi | 4 | 9 | 4 | 7 | 4 | 7 |
| 28 dpi | 0 | 9 | 0 | 7 | 0 | 5 |
| Cohort 1:b 15 days | ||||||
| 0 dpi | 13 | 12 | 2 | 7 | 12 | 12 |
| 15 dpi | 9 | 12 | 2 | 7 | 12 | 12 |
| Cohort 2:c 15 days | ||||||
| 0 dpi | 7 | 9 | 6 | 5 | 6 | 8 |
| 15 dpi | 7 | 9 | 6 | 5 | 6 | 8 |
| Totala,b,c | 29 | 30 | 16 | 19 | 28 | 27 |
| BMSb,c | 16 | 21 | 8 | 12 | 18 | 20 |
| Histologyc | 6 | 6 | 5 | 5 | 6 | 7 |
BMS, Basso mouse scale; dpi, days post-injury.
Groups used for analysis.
Behavioral assessment
The Basso mouse scale (BMS) open-field locomotor test (range 0–9) was used to assess functional recovery for a period of 15 dpi. Mice were tested using this scale before injury, and at 1, 7, and 15 dpi. Observations were performed by two trained observers at 4-min intervals (Basso et al., 2006).
Histological assessment
At 15 dpi, the mice were anesthetized with pentobarbital and transcardially perfused with phosphate-buffered saline (PBS), followed by 4% paraformaldehyde in PBS. The T6–T12 segment of the spinal cord was dissected by the dorsal root to provide an anatomically defined region for unbiased stereology, post-fixed in 4% paraformaldehyde/PBS for 4–8 h, transferred to 20% sucrose/4% paraformaldehyde/PBS overnight at 4°C, frozen in isopentane at −65°C, and stored at −80°C. Serial 20-μm parasagittal microtome sections were collected in PBS with sodium azide (0.02%) for free-floating immunohistochemical staining and stored at 4°C. In order to evaluate the lesion size and the immunological response, a panel of monoclonal antibodies was used as follows: rabbit anti-mouse fibronectin for lesion volume (1:500; Sigma, St. Louis, MO); rat anti-mouse 7/4 for neutrophils (1:50; Serotec, Raleigh, NC); and rat anti-mouse F4/80 for macrophages (1:50; Serotec). Donkey anti-rabbit and donkey anti-rat secondary antibodies were used to amplify immunostaining (1:500; Jackson ImmunoResearch, West Grove, PA).
In addition to spinal tissue, spleens were harvested from naïve animals and fixed in formalin. Spleens were sectioned on a sliding microtome and immunostained for T cells with rat anti-mouse CD3 (1:3000; Serotec), and B-cells with hamster anti-mouse CD79b (1:3000; Serotec). Donkey anti-rat IgG (1:500; Jackson ImmunoResearch), and goat anti-hamster IgG (1:500; Jackson ImmunoResearch) were used as secondary antibodies for T- and B-cell labeling, respectively.
Immunohistochemistry was conducted as previously described (Galvan et al., 2008) on every sixth section through the entire T6–T12 spinal cord segment. Briefly, the sections were washed for 5 min in 0.1 M Tris-buffered saline (pH 7.4), incubated for 20 min with 3% hydrogen peroxide and 10% methanol in Tris buffer, and washed for 15 min in Tris-A (Tris + 0.1% Triton X-100). After 30 min of blocking in Tris-B (Tris-A plus 2% bovine serum albumin [BSA] and 15 μL/mL of serum from the host in which the secondary antibody was raised), the sections were incubated with primary antibody overnight at 4°C on a rotator table, and then washed twice in Tris-A for 5 min each, in Tris-B for 30 min, and incubated for 1 h in secondary antibody. After washing twice with Tris-A for 5 min, followed by an additional 15 min in Tris-A, and 15 min in Tris-B (without serum), the sections were incubated in ABC (Vector Laboratories, Burlingame, CA) for 1 h at room temperature. After three washes in Tris of 5 min each, the sections were reacted for 4 min in diaminobenzidine (DAB; Vector Laboratories), mounted on slides, and dehydrated with serial dilutions of ethanol (50%, 70%, 95%, and 100%). Then they were cleared using 100% butanol, immersed twice for 5-min each in Histoclear (American MasterTech, Lodi, CA), and cover-slipped.
Quantification of cell number and section area
The total number of neutrophils was estimated using unbiased stereological 1/6 sampling of parasagittal sections of the spinal cord (as above). The sections were viewed with a 4 × objective to outline the area occupied by neutrophils, and a 60 × objective was used for cell counting. The grid sampling parameters were: area per unit [A(U)] = 1658 μm2, area per point [A(p)] = 414.5 μm2, with a resulting coefficient of error (CE) for analysis = 0.03. The investigators were blinded to experimental group for all stages of quantification.
The volume occupied by macrophages/microglia and their rostral-caudal spread were quantified by unbiased stereology using the Cavalieri sampling method on an Olympus C.A.S.T. grid system (Olympus, Ballerupt, Denmark) by tracing the area of dense immunoreactivity in the parasagittal sections using a 1/6 sampling scheme, and reconstructing the volume by applying the Cavalieri equation: V = T(A1 + A2 + A3 …), where T is the distance between sections, and A is the area of macrophage/microglial staining in each section (Engesser-Cesar et al., 2005).
Fibronectin staining was used to measure lesion volume (Nishi et al., 2007), and was quantified by unbiased stereology using the Cavalieri sampling method on an Olympus C.A.S.T. grid system. Fibronectin volume was determined by tracing the contours around the region of dense straining in serial sections (1/6). Volume was then reconstructed in the same manner as discussed above for macrophages/microglia.
Statistical analysis
Data were analyzed for statistical significance with Prism 5.0 (GraphPad Software, La Jolla, CA). Locomotor recovery over time was compared between male and female mice within each strain using repeated-measures analysis of variance (ANOVA). For all three strains, no sex differences were detected within a given strain (C57BL/6 p = 0.30, BUB/BnJ p = 0.12, and NOD-SCID p = 0.99). Therefore we pooled the sexes in each strain to increase power. Then we used repeated-measures ANOVA to compare locomotor recovery after injury between each sex-pooled strain, followed by Bonferroni post-hoc testing. The significance of the differences in neutrophil, macrophage/microglia, and fibronectin quantification (cell counts, volume, and spreading) were first analyzed with two-way ANOVA. As with the behavioral data, we did not detect any sex effects, and we thus collapsed the two sexes within a strain into a single group to increase power. We then analyzed the collapsed groups for statistical significance with one-way ANOVA, followed by Bonferroni post-hoc testing. Differences were considered significant at p < 0.05. All data are represented as mean ± SEM.
Results
Strain, but not sex, affects locomotor recovery after SCI
Locomotor performance was assessed using the BMS open-field locomotor task prior to injury, and then at 1, 7, and 15 dpi. Pilot studies extending to 28 dpi showed that behavioral assessment at 15 dpi was consistent with the emergence of a plateau for locomotor recovery. Male survival was low in the pilot study due to the high incidence of uroliathisis, and thus these males were excluded from plateau analysis. Female survival in the 28-day cohort was 100%. Within-strain comparison of BMS scores of females at 14 and 28 dpi by paired t-testing did not reveal any differences in locomotor performance, suggesting a behavioral plateau (C57BL/6 [n = 9] at 14 dpi = 4.1 ± 0.2, at 28 dpi = 4.4 ± 0.3, p = 0.06; BUB/BnJ [n = 7] at 14 dpi = 5.0 ± 0.4, at 28 dpi = 5.1 ± 0.3, p = 0.46; NOD-SCID [n = 5] at 14 dpi = 6.5 ± 0.3, at 28 dpi = 6.9 ± 0.5, p = 0.53). As such, the experiments were limited to 15 dpi, with analysis of males and females restricted to this single time point to allow direct comparison between sexes. Additionally, assessment of the innate immune response at 15 dpi was within the published peaks for both neutrophil and macrophage response for C57BL/6 mice (Kigerl et al., 2006).
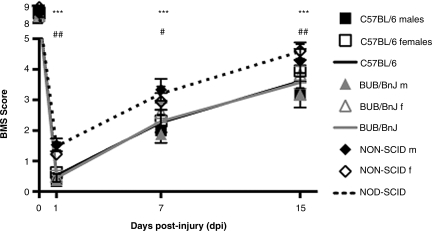
By 15 dpi, NOD-SCID males and females recovered to BMS scores of 4.3 ± 0.4 and 4.6 ± 0.3 15 dpi, respectively. In contrast, male and female BUB/BnJ exhibited less recovery, achieving BMS scores of 3.2 ± 0.2 and 3.8 ± 0.3, respectively. C57BL/6 mice performed similarly to BUB/BnJ mice, with males and females scoring 3.2 ± 0.4 and 4.0 ± 0.4 15 dpi, respectively (Fig. 1). Repeated-measures ANOVA was performed comparing the effect of sex within each strain over time. For all three strains, sex was not a factor (C57BL/6: p = 0.30; BUB/BnJ: p = 0.12; and NOD-SCID: p = 0.99). As sex was not a factor, the groups were collapsed within each strain to increase statistical power.
FIG. 1.
Non-obese diabetic severe combined immunodeficient (NOD-SCID) mice exhibited improved open-field locomotor recovery after contusion spinal cord injury (SCI), while C57BL/6 and BUB/BnJ mice did not. Two-way ANOVA comparison of BMS locomotor performance (sex and time) was performed for each strain. In each case, there were no sex differences in locomotor recovery. Thus the groups were collapsed within strains to increase power. Repeated-measures ANOVA of the collapsed groups showed significant main effects of strain (p < 0.0001), time (p < 0.0001), and strain × time interaction. Bonferroni post-hoc tests of the collapsed groups showed that NOD-SCID mice outperformed both C57BL/6 (***p < 0.001) and BUB/BnJ mice at all post-injury time points (#p < 0.05, ##p < 0.01). C57BL/6 and BUB/BnJ mice did not differ from each other at any time point (ANOVA, analysis of variance; BMS, Basso mouse scale).
By 15 dpi, NOD-SCID mice of both sexes averaged a BMS score of 4.6 ± 0.2, higher than both the C57BL/6 (3.6 ± 0.3) and the BUB/BnJ mice (3.6 ± 0.2). Repeated-measures ANOVA of the collapsed groups detected a significant main effect of strain [F (2,264) = 11.01, p = 0.0001], time [F (2,264) = 1151, p < 0.0001), and the strain × time interaction [F (6,264) = 2.583, p = 0.019). Bonferroni post-hoc testing showed that NOD-SCID mice had significantly improved locomotor recovery compared to both C57BL/6 and BUB/BnJ mice at all post-injury time points (NOD-SCID versus C57BL/6: 1, 7, and 15 dpi [p < 0.01]; NOD-SCID versus BUB/BnJ: 1 and 15 dpi [p < 0.01], 7 dpi [p < 0.05]). No significant differences were detected between C57BL/6 and BUB/BnJ mice.
Strain, but not sex, affects neutrophil infiltration into the injured spinal cord
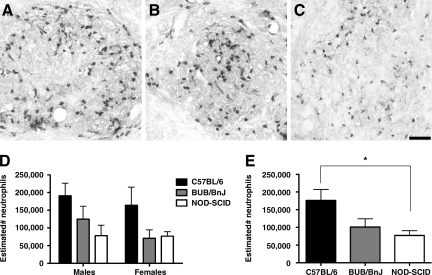
Unbiased stereology was used to estimate the number of neutrophils present in the injured spinal cord using dorsal roots T6 and T12 as anatomic landmarks (Fig. 2A–C). NOD-SCID males and females had similar numbers of neutrophils, with estimated totals of 78,150 ± 29,539 and 76,767 ± 12,713, respectively. BUB/BnJ females had similar PMN estimates to NOD-SCID mice, with 70,500 ± 24,278, though BUB/BnJ males had 125,160 ± 36,115. As expected, the estimates of neutrophil infiltration into C57BL/6 injured spinal cords were the highest, with males and females estimated to have 190,650 ± 35,936 and 164,186 ± 51,285 cells present, respectively. Two-way ANOVA showed a significant main effect of strain [F (2,31) = 5.133, p = 0.0119], but not sex [F (1,31) = 0.9240, p = 0.3439] or strain × sex interaction [F (2,31) = 0.2779, p = 0.7593; Fig. 2D]. As sex was not a factor, the groups were collapsed to increase power. One-way ANOVA revealed a significant difference between groups [F (2,34) = 5.328, p = 0.0097; Fig. 2E]. Bonferroni post-hoc tests showed a significant difference between NOD-SCID and C57BL/6 mice (p < 0.05), but no other comparisons were significant.
FIG. 2.
Neutrophil cell number in the injured spinal cord at 15 days post-SCI. Injured spinal cords from (A) C57BL/6, (B) BUB/BnJ, and (C) NOD-SCID mice were immunostained and quantified stereologically to estimate the (D) total number of infiltrated neutrophils. Two-way ANOVA detected strain differences, but no sex or strain × sex interaction differences. Thus the groups were collapsed within strains to increase power. (E) One-way ANOVA of the collapsed groups revealed a significant strain effect, with post-hoc tests confirming that there were more neutrophils in C57BL/6 mice than in NOD-SCID mice (ANOVA = p < 0.05, *Bonferroni post-hoc test = p < 0.05), though neither strain differed significantly from BUB/BnJ mice. Representative sections from female mice of each strain are illustrated, focusing on high-power comparison at the injury epicenter (scale bar = 100 μm; ANOVA, analysis of variance; NOD-SCID, non-obese diabetic severe combined immunodeficient; SCI, spinal cord injury).
Strain, but not sex, affects macrophage/microglia distribution in the injured spinal cord
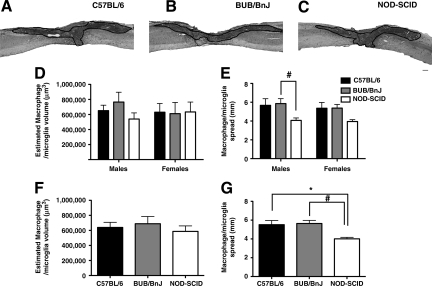
Unlike neutrophils, individual cell counts of F4/80-stained macrophages/microglia are not possible in the injured spinal cord, due to their “foamy” phenotype and dense packing, which prevents identification of individual nuclei (Fig. 3A–C). Therefore, instead of quantifying the number of cells present, we estimated the total volume they occupied using the Cavalieri sampling method of unbiased stereology. NOD-SCID male mice had a total F4/80-positive macrophage/microglia volume estimated to be 539,488 μm3 ± 82,069, while females had a total volume of 634,094 μm3 ± 131,370. Similarly, we estimated the total volume of macrophages/microglia to be 651,284 μm3 ± 72,548 in C57BL/6 males, and 630,071 μm3 ±116,350 in females. Female BUB/BnJ mice also had a similar macrophage/microglia volume (611,303 μm3 ± 148,562), while males had the highest volume, estimated to be 765,742 μm3 ±130,198. Two-way ANOVA did not reveal any differences in the volume occupied by macrophages/microglia by strain [F (2,23) = 0.3352, p = 0.6083], sex [F (1,23) = 0.0755, p =0.7859), or strain × sex interaction [F (2,23) = 0.5080, p = 0.6083; Fig. 3D]. As sex was not a factor, the groups were collapsed to increase power, but one-way ANOVA of the collapsed groups still failed to discriminate any differences between strains [F (2,26) = 0.3612, p = 0.7003; Fig. 3F].
FIG. 3.
Macrophage/microglia spreading and volume in the injured spinal cord at 15 days post-SCI. Spinal cords from (A) C57BL/6, (B) BUB/BnJ, and (C) NOD-SCID mice were immunostained for macrophages/microglia. Note the predominant localization of immunopositive cells at the injury epicenter and in the dorsal columns, and for neutrophils this pattern was observed both rostral and caudal to the injury site. Unbiased stereological quantification was used to determine (D) volume occupied and (E) rostral-caudal spreading of macrophages/microglia. No differences were detected in cell volume. Two-way ANOVA of spreading revealed a significant strain, but not sex, effect (two-way ANOVA = p < 0.05; *Bonferroni post-hoc test = p < 0.05). Thus the groups were collapsed within strains to increase power. (F) No differences in macrophage/microglia volume were detected between the collapsed groups. (G) Bonferroni post-hoc tests following one-way ANOVA of the collapsed groups confirmed that NOD-SCID mice have less rostral-caudal spreading of macrophages/microglia than both C57BL/6 and BUB/BnJ mice (one-way ANOVA = p < 0.05; *#Bonferroni post-hoc test = p < 0.05). Representative sections of rostral-caudal spreading from female mice of each strain are illustrated (scale bar = 250 μm; ANOVA, analysis of variance; NOD-SCID, non-obese diabetic severe combined immunodeficient; SCI, spinal cord injury).
While we did not detect differences in the total spinal volume of macrophages/microglia, we also measured the rostral-caudal spread of the cells as a secondary measure of their distribution. We observed the least amount of spreading in NOD-SCID males (4.068 mm ± 0.263) and females (3.949 mm ±0.204), compared to C57BL/6 males (5.668 mm ± 0.709) and females (5.367 mm ± 0.613), or BUB/BnJ males (5.854 mm ±0.530) and females (5.378 mm ± 0.388). Two-way ANOVA of rostral-caudal spreading of macrophages/microglia showed a significant main effect of strain [F (2,27) = 6.688, p = 0.0044], but not sex [F (1,27) = 0.5243, p = 0.4752] or strain × sex interaction [F (2,27) = 0.05935, p = 0.9425; Fig. 3E]. Bonferroni post-hoc tests detected a significant difference between male NOD-SCID and BUB/BnJ mice (p < 0.05), but not in any other comparisons. As sex was not a factor in the two-way ANOVA, the groups were collapsed to increase power. One-way ANOVA of the collapsed groups yielded a significant difference between strains [F(2,30) = 7.406, p = 0.0024; Fig. 3G]. Bonferroni post-hoc tests confirmed that NOD-SCID mice had less macrophage spreading than C57BL/6 (p < 0.05) and BUB/BnJ mice (p < 0.05), but no differences were detected between C57BL/6 and BUB/BnJ mice.
Adaptive immunity in mouse strains
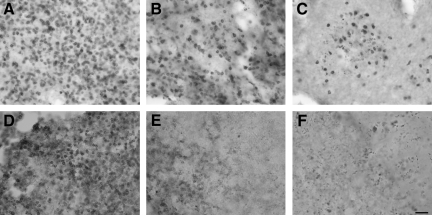
NOD-SCID mice have previously been characterized to have a reduced number of T and B lymphocytes compared to other strains, as well as having strong defects in lymphocyte function (Greiner et al., 1998). We confirmed the reduction of these lymphocytes in the spleen, as well as their presence in C57BL/6 and BUB/BnJ mice, by immunostaining sections from naïve animals (Fig. 4A–F).
FIG. 4.
T and B lymphocytes in spleen tissue from uninjured mice. Spleens from uninjured (A and D) C57BL/6, (B and E) BUB/BnJ, and (C and F) NOD-SCID mice were immunostained for (A–C) T lymphocytes (CD3), and (D–F) B lymphocytes (CD79b). For both cell types, the most cells were observed in C57BL/6 mice, followed by BUB/BnJ mice, with the fewest cells observed in NOD-SCID mice (NOD-SCID, non-obese diabetic severe combined immunodeficient).
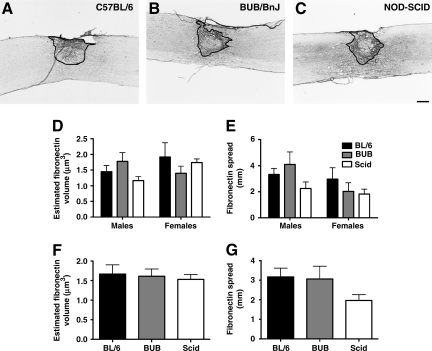
Neither strain nor sex affects SCI lesion volume
Immunohistochemical staining of fibronectin was performed to determine lesion volume following SCI (Fig. 5A–C). Unbiased stereology estimated that male NOD-SCID mice had a total estimated fibronectin volume of 1.163 μm3 ± 0.132 and females had 1.741 μm3 ± 0.114. Male C57BL/6 mice had a total estimated fibronectin volume of 1.449 μm3 ± 0.200 and females 1.915 μm3 ± 0.458. Estimates were similar in BUB/BnJ mice, with males having 1.778 μm3 ± 0.278 and females 1.399 μm3 ± 0.227 fibronectin. Two-way ANOVA did not detect any differences in fibronectin volume by strain [F (2,27) =0.3751, p = 0.6908], sex [F (1,27) = 0.9502, p = 0.3383), or strain × sex interaction [F (2,27) = 1.612, p = 0.2181; Fig. 5D], or in rostral-caudal fibronectin spreading by strain [F (2,29) = 1.775, p = 0.1874], sex [F (1,29) = 3.138, p = 0.0870], or strain × sex interaction [F (2,29) = 1.034, p = 0.3683; Fig. 5E]. As sex was not a significant factor, the groups were collapsed to increase power. One-way ANOVA did not detect any significant differences between the collapsed groups in fibronectin volume [F (2,30) = 0.1254, p = 0.8826; Fig. 5F], or spreading [F (2,30) = 2.092, p = 0.1400; Fig. 5G].
FIG. 5.
Fibronectin immunostaining, spreading, and volume in the injured spinal cord at 15 days post-SCI. Spinal cords were removed from (A) C57BL/6, (B) BUB/BnJ, and (C) NOD-SCID mice. Unbiased stereology was used to quantify (D) volume occupied by fibronectin, and (E) the extent of fibronectin's rostral-caudal spread. No significant differences were detected between groups, so the groups were collapsed within strains to increase power. No differences in (F) fibronectin volume or (G) spreading were detected between the collapsed groups. Representative sections from female mice of each strain are illustrated (scale bar = 250 μm; NOD-SCID, non-obese diabetic severe combined immunodeficient; SCI, spinal cord injury).
Discussion
In this study, we compared behavioral and histological outcomes in male and female NOD-SCID, C57BL/6, and BUB/BnJ mice. For all end-points, we did not detect any sex effects, and thus we collapsed the groups within each strain to increase statistical power. Our data show that NOD-SCID mice scored higher on an open-field locomotor task than C57BL/6 or BUB/BnJ mice. After sacrifice, we used unbiased stereology to quantify neutrophils and macrophages, the two most prevalent immune cells to infiltrate the spinal cord after injury. We detected an overall strain effect on neutrophil infiltration, and post-hoc tests revealed that C57BL/6 mice have greater neutrophil infiltration than NOD-SCID mice, though neither strain differed significantly from BUB/BnJ mice. Strain did not affect the volume of the spinal cord occupied by macrophages/microglia, although it did affect the rostral-caudal spreading of these cells, with less spreading observed in pooled NOD-SCIDs than pooled C57BL/6 and BUB/BnJ mice, with no differences observed between the latter two strains. Finally, we did not detect any differences in lesion volume or spreading between strains. These findings illustrate that the microenvironment of the injured spinal cord is relatively similar in C57BL/6, BUB/BnJ, and NOD-SCID mice.
Despite slight strain differences in cellular inflammation, we did not detect any differences in lesion volume or spreading between the groups. Unlike the cavitation that occurs after rat and most human SCI, mice form a fibronectin matrix at the lesion's epicenter days after injury that persists for at least 1 year (Camand et al., 2004; Chen et al., 2005). Lesion volume, in this case determined by fibronectin deposition, is a general indicator of the overall pathology of SCI, and we and others have previously correlated it with both locomotor recovery and spared white matter following graded injuries (Ma et al., 2001; Nishi et al., 2007). Our inability to detect any differences in lesion volume suggests that the gross pathology of SCI does not differ greatly between the strains examined here.
Despite the similarity in lesion volume, NOD-SCID mice exhibited improved locomotor recovery relative to C57BL/6 and BUB/BnJ mice. Interestingly, NOD-SCID mice had higher BMS scores as early as 1 dpi. In the present study, the three strains were equivalent in macrophage volume after SCI, and the BUB/BnJ mice were similar to NOD-SCID mice in numbers of infiltrating neutrophils. Therefore these parameters alone cannot account for the improved recovery seen in the NOD-SCID mice relative to the other strains, suggesting that factors other than the magnitude of the infiltrating immune cells must play a role. For example, there could be alterations in the chemokine/cytokine profiles of these cells across strains. Additionally, there may be strain-dependent neuroprotective effects, including variations in cellular vulnerability to excitotoxicity (Schauwecker and Steward, 1997).
While this study is among the first to use NOD-SCID mice in spinal cord injury research, it is not the first to demonstrate that genetic diversity between mouse strains influences functional and histological outcome. Strain differences in recovery have been reported after both spinal contusion and transection (Basso et al., 2006; Lapointe et al., 2006). Differential recovery from SCI may be based on several factors. Neuroanatomical differences between strains have been reported in the brain, but have not been investigated in the spinal cord (Chen et al., 2006; Maeda et al., 1998). The organization of descending tracts and/or specific topographic disruption of those tracts may account for the differences seen in functional recovery. Sensorimotor processing also differs between strains (Paylor and Crawley, 1997), potentially affecting gait parameters. Finally, neuroinflammation varies between strains (Kigerl et al., 2006); the lack of functional T and B cells in NOD-SCID mice may be responsible for their improved recovery, as these cells can lead to the development of autoimmunity, exacerbating the pathology of SCI (Ankeny et al., 2006; Gonzalez et al., 2003; Jones et al., 2004). We did not quantify T and B cell numbers in the injured spinal cords, but this information would likely have contributed little to the interpretation of this study. It is likely that we would detect elevated lymphocyte numbers in C57BL/6 compared to BUB/BnJ mice, as C57BL/6 mice have previously been reported to have higher T-cell counts than other common strains (Kigerl et al., 2006). We would still be unable to determine the role of adaptive immunity in functional recovery, as these two strains exhibited identical patterns of locomotor recovery.
Inflammation can have broad effects on recovery from SCI (Bethea, 2000; Trivedi et al., 2006), and thus it was critical to confirm its presence in the NOD-SCID mouse to validate this strain as an appropriate model for studying SCI. While NOD-SCID mice lack adaptive immunity, here we show that they have limited innate immunity, with similar amounts of neutrophil infiltration into the injured spinal cord as BUB/BnJ mice, but not C57BL/6 mice, which have previously been reported to have more neutrophils than other common strains (Kigerl et al., 2006). Additionally, NOD-SCID mice have an equal volume of macrophages/microglia as the other two strains, but less rostral-caudal spreading than either. Both neutrophils and macrophages/microglia secrete many cytokines into the microenvironment (Mantovani et al., 2004; Pineau and Lacroix, 2007; Stoll et al., 2002). It is critical that these signaling molecules are present in models of cellular transplantation, as cytokines drive migration and fate determination in several cell types, including embryonic (Alberti et al., 2008; Ideguchi et al., 2008), hematopoietic (Dentelli and Brizzi, 2008; Pitchford et al., 2009), and neural stem cells (Nakashima and Taga, 2002; Wang et al., 2007).
Another important mediator of inflammation are the complement proteins, which play a role in immune cell recruitment, opsonization, and cell lysis. Male C57BL/6 and BUB/BnJ mice have been shown to have similar serum titers of both C3 and C5, and though the strains do not differ in their ability to opsonize bacteria with C3b, BUB/BnJ mice have greater complement-mediated hemolytic activity (Osmers et al., 2006). We have shown that male BUB/BnJ mice have equal C3, but elevated C5 activity, in C3H50 and C5H50 replacement assays. Further, we have replicated the finding of increased complement-mediated hemolysis in BUB/BnJ mice (Galvan et al., 2008). Taken together, these data suggest that complement activity does not necessarily correlate with protein expression levels between strains. One possible explanation for this phenomenon is that point mutations may reduce protein activity, as has been demonstrated in human populations (Abe et al., 2009; Arnold et al., 2009; Lopez-Lera et al., 2009; Wu et al., 2009).
While most SCI researchers use female animals to avoid the complications associated with the more complex male urinary anatomy, occasionally males are the more suitable model. For instance, investigation of the complement cascade generally uses male animals, as they have significantly higher titers of complement proteins than females (Galvan et al., 2008; Ong and Mattes, 1989). Another area of research requiring male animals is sexual function (Nout et al., 2005), which has been identified as a top priority for the human SCI population (Anderson, 2004). As there is a demonstrated need for experimentation on male SCI mice, we included sex comparisons in our data set; however, we did not detect significant differences between sexes in any of our end-points within strains.
The lack of a sex effect in our data was not completely unexpected, as debate about sex effects following central nervous system trauma continues in both laboratory and clinical studies. There is a report that female C57BL/6 mice have vastly improved locomotor recovery after compression SCI compared to males (Farooque et al., 2006), while in another study researchers found no sex differences after traumatic brain injury (Hall et al., 2005). Clinical data are equally mixed in other studies, with some showing greater recovery in females (Groswasser et al., 1998), some in males (Gunnarsson and Fehlings, 2003), mixed results depending on injury level (Sipski et al., 2004), and no sex effects at all (Greenwald et al., 2001). It has also been noted that sex differences exist in non-neurological end-points such as deep venous thrombosis or reactive depression (Furlan et al., 2005), effects not likely to be detected in short-term rodent studies.
Findings of the current study demonstrate that the NOD-SCID mouse exhibits slightly improved locomotor recovery and similar histopathological recovery to other mouse strains. It is critical that researchers choose an appropriate model to address a specific question, as this may necessitate the selection of alternative genetic backgrounds or transgenic strains. Further, until autologous cell transplantation techniques are universally adopted, there will be a need for studies of pharmacologically-immunosuppressed animals. Nonetheless, the absence of a functional adaptive immune system makes the NOD-SCID mouse an ideal model for proof-of-principle to test the potential of transplanted cells without the confounding elements of rejection or the effects of the immunosuppressants themselves.
Acknowledgments
We thank Rebecca Nishi and the Christopher and Dana Reeve Foundation SCI core for surgical assistance, Chelsea Pagan and Lena Nguyen for general technical assistance, Desirée Salazar for editorial suggestions, and Andrea Tenner for technical advice. This work was supported by National Institutes of Health grants R01NS043428-05 and R01NS049885-05 to A.J.A., and the Christopher and Dana Reeve Foundation.
Author Disclosure Statement
No competing financial interests exist.
References
- Abe K. Endo Y. Nakazawa N. Kanno K. Okubo M. Hoshino T. Fujita T. Unique phenotypes of C1s deficiency and abnormality caused by two compound heterozygosities in a Japanese family. J. Immunol. 2009;182:1681–1688. doi: 10.4049/jimmunol.182.3.1681. [DOI] [PubMed] [Google Scholar]
- Alberti K. Davey R.E. Onishi K. George S. Salchert K. Seib F.P. Bornhauser M. Pompe T. Nagy A. Werner C. Zandstra P.W. Functional immobilization of signaling proteins enables control of stem cell fate. Nat. Methods. 2008;5:645–650. doi: 10.1038/nmeth.1222. [DOI] [PubMed] [Google Scholar]
- Anderson K.D. Targeting recovery: priorities of the spinal cord-injured population. J. Neurotrauma. 2004;21:1371–1383. doi: 10.1089/neu.2004.21.1371. [DOI] [PubMed] [Google Scholar]
- Ankeny D.P. Lucin K.M. Sanders V.M. McGaughy V.M. Popovich P.G. Spinal cord injury triggers systemic autoimmunity: evidence for chronic B lymphocyte activation and lupus-like autoantibody synthesis. J. Neurochem. 2006;99:1073–1087. doi: 10.1111/j.1471-4159.2006.04147.x. [DOI] [PubMed] [Google Scholar]
- Armstrong R.J. Harrower T.P. Hurelbrink C.B. McLaughin M. Ratcliffe E.L. Tyers P. Richards A. Dunnett S.B. Rosser A.E. Barker R.A. Porcine neural xenografts in the immunocompetent rat: immune response following grafting of expanded neural precursor cells. Neuroscience. 2001;106:201–216. doi: 10.1016/s0306-4522(01)00273-1. [DOI] [PubMed] [Google Scholar]
- Arnold D.F. Roberts A.G. Thomas A. Ferry B. Morgan B.P. Chapel H. A novel mutation in a patient with a deficiency of the eighth component of complement associated with recurrent meningococcal meningitis. J. Clin. 2009;Immunol.29:691–695. doi: 10.1007/s10875-009-9295-7. [DOI] [PubMed] [Google Scholar]
- Baldwin W.M., 3rd. Samaniego-Picota M. Kasper E.K. Clark A.M. Czader M. Rohde C. Zachary A.A. Sanfilippo F. Hruban R.H. Complement deposition in early cardiac transplant biopsies is associated with ischemic injury and subsequent rejection episodes. Transplantation. 1999;68:894–900. doi: 10.1097/00007890-199909270-00024. [DOI] [PubMed] [Google Scholar]
- Basso D.M. Fisher L.C. Anderson A.J. Jakeman L.B. McTigue D.M. Popovich P.G. Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J. Neurotrauma. 2006;23:635–659. doi: 10.1089/neu.2006.23.635. [DOI] [PubMed] [Google Scholar]
- Bavetta S. Hamlyn P.J. Burnstock G. Lieberman A.R. Anderson P.N. The effects of FK506 on dorsal column axons following spinal cord injury in adult rats: neuroprotection and local regeneration. Exp. Neurol. 1999;158:382–393. doi: 10.1006/exnr.1999.7119. [DOI] [PubMed] [Google Scholar]
- Bethea J.R. Spinal cord injury-induced inflammation: a dual-edged sword. Prog. Brain Res. 2000;128:33–42. doi: 10.1016/S0079-6123(00)28005-9. [DOI] [PubMed] [Google Scholar]
- Cadili A. Kneteman N. The role of macrophages in xenograft rejection. Transplant Proc. 2008;40:3289–3293. doi: 10.1016/j.transproceed.2008.08.125. [DOI] [PubMed] [Google Scholar]
- Camand E. Morel M.P. Faissner A. Sotelo C. Dusart I. Long-term changes in the molecular composition of the glial scar and progressive increase of serotoninergic fibre sprouting after hemisection of the mouse spinal cord. Eur. J. Neurosci. 2004;20:1161–1176. doi: 10.1111/j.1460-9568.2004.03558.x. [DOI] [PubMed] [Google Scholar]
- Carlson S.L. Parrish M.E. Springer J.E. Doty K. Dossett L. Acute inflammatory response in spinal cord following impact injury. Exp. Neurol. 1998;151:77–88. doi: 10.1006/exnr.1998.6785. [DOI] [PubMed] [Google Scholar]
- Chen J. Leong S.Y. Schachner M. Differential expression of cell fate determinants in neurons and glial cells of adult mouse spinal cord after compression injury. Eur. J. Neurosci. 2005;22:1895–1906. doi: 10.1111/j.1460-9568.2005.04348.x. [DOI] [PubMed] [Google Scholar]
- Chen X.J. Kovacevic N. Lobaugh N.J. Sled J.G. Henkelman R.M. Henderson J.T. Neuroanatomical differences between mouse strains as shown by high-resolution 3D MRI. Neuroimage. 2006;29:99–105. doi: 10.1016/j.neuroimage.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Cummings B.J. Uchida N. Tamaki S.J. Salazar D.L. Hooshmand M. Summers R. Gage F.H. Anderson A.J. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc. Natl. Acad. Sci. U.S.A. 2005;102:14069–14074. doi: 10.1073/pnas.0507063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dentelli P. Brizzi M.F. Inflammatory microenvironment promotes hemopoietic-derived angiogenic cell expansion and arterial specification. Scientific World J. 2008;8:1111–1115. doi: 10.1100/tsw.2008.142. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Diaz-Ruiz A. Rios C. Duarte I. Correa D. Guizar-Sahagun G. Grijalva I. Ibarra A. Cyclosporin-A inhibits lipid peroxidation after spinal cord injury in rats. Neurosci. Lett. 1999;266:61–64. doi: 10.1016/s0304-3940(99)00255-4. [DOI] [PubMed] [Google Scholar]
- Donnelly D.J. Popovich P.G. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp. Neurol. 2008;209:378–388. doi: 10.1016/j.expneurol.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sawy T. Belperio J.A. Strieter R.M. Remick D.G. Fairchild R.L. Inhibition of polymorphonuclear leukocyte-mediated graft damage synergizes with short-term costimulatory blockade to prevent cardiac allograft rejection. Circulation. 2005;112:320–331. doi: 10.1161/CIRCULATIONAHA.104.516708. [DOI] [PubMed] [Google Scholar]
- Engesser-Cesar C. Anderson A.J. Basso D.M. Edgerton V.R. Cotman C.W. Voluntary wheel running improves recovery from a moderate spinal cord injury. J. Neurotrauma. 2005;22:157–171. doi: 10.1089/neu.2005.22.157. [DOI] [PubMed] [Google Scholar]
- Farooque M. Suo Z. Arnold P.M. Wulser M.J. Chou C.T. Vancura R.W. Fowler S. Festoff B.W. Gender-related differences in recovery of locomotor function after spinal cord injury in mice. Spinal Cord. 2006;44:182–187. doi: 10.1038/sj.sc.3101816. [DOI] [PubMed] [Google Scholar]
- Freund P. Wannier T. Schmidlin E. Bloch J. Mir A. Schwab M.E. Rouiller E.M. Anti-Nogo-A antibody treatment enhances sprouting of corticospinal axons rostral to a unilateral cervical spinal cord lesion in adult macaque monkey. J. Comp. Neurol. 2007;502:644–659. doi: 10.1002/cne.21321. [DOI] [PubMed] [Google Scholar]
- Furlan J.C. Krassioukov A.V. Fehlings M.G. The effects of gender on clinical and neurological outcomes after acute cervical spinal cord injury. J. Neurotrauma. 2005;22:368–381. doi: 10.1089/neu.2005.22.368. [DOI] [PubMed] [Google Scholar]
- Galvan M.D. Luchetti S. Burgos A.M. Nguyen H.X. Hooshmand M.J. Hamers F.P. Anderson A.J. Deficiency in complement C1q improves histological and functional locomotor outcome after spinal cord injury. J. Neurosci. 2008;28:13876–13888. doi: 10.1523/JNEUROSCI.2823-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R. Glaser J. Liu M.T. Lane T.E. Keirstead H.S. Reducing inflammation decreases secondary degeneration and functional deficit after spinal cord injury. Exp. Neurol. 2003;184:456–463. doi: 10.1016/s0014-4886(03)00257-7. [DOI] [PubMed] [Google Scholar]
- Greenwald B.D. Seel R.T. Cifu D.X. Shah A.N. Gender-related differences in acute rehabilitation lengths of stay, charges, and functional outcomes for a matched sample with spinal cord injury: a multicenter investigation. Arch. Phys. Med. Rehabil. 2001;82:1181–1187. doi: 10.1053/apmr.2001.24891. [DOI] [PubMed] [Google Scholar]
- Greiner D.L. Hesselton R.A. Shultz L.D. SCID mouse models of human stem cell engraftment. Stem Cells. 1998;16:166–177. doi: 10.1002/stem.160166. [DOI] [PubMed] [Google Scholar]
- Gris D. Marsh D.R. Oatway M.A. Chen Y. Hamilton E.F. Dekaban G.A. Weaver L.C. Transient blockade of the CD11d/CD18 integrin reduces secondary damage after spinal cord injury, improving sensory, autonomic, and motor function. J. Neurosci. 2004;24:4043–4051. doi: 10.1523/JNEUROSCI.5343-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groswasser Z. Cohen M. Keren O. Female TBI patients recover better than males. Brain Inj. 1998;12:805–808. doi: 10.1080/026990598122197. [DOI] [PubMed] [Google Scholar]
- Gunnarsson T. Fehlings M.G. Acute neurosurgical management of traumatic brain injury and spinal cord injury. Curr. Opin. Neurol. 2003;16:717–723. doi: 10.1097/01.wco.0000102629.16692.ee. [DOI] [PubMed] [Google Scholar]
- Guo J. Zeng Y. Liang Y. Wang L. Su H. Wu W. Cyclosporine affects the proliferation and differentiation of neural stem cells in culture. Neuroreport. 2007;18:863–868. doi: 10.1097/WNR.0b013e32811d6d36. [DOI] [PubMed] [Google Scholar]
- Hall E.D. Gibson T.R. Pavel K.M. Lack of a gender difference in post-traumatic neurodegeneration in the mouse controlled cortical impact injury model. J. Neurotrauma. 2005;22:669–679. doi: 10.1089/neu.2005.22.669. [DOI] [PubMed] [Google Scholar]
- Hofstetter C.P. Schwarz E.J. Hess D. Widenfalk J. El Manira A. Prockop D.J. Olson L. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc. Natl. Acad. Sci. U.S.A. 2002;99:2199–2204. doi: 10.1073/pnas.042678299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne P.H. Zimmerer J.M. Fisher M.G. Lunsford K.E. Nadasdy G. Nadasdy T. van Rooijen N. Bumgardner GL. Critical role of effector macrophages in mediating CD4-dependent alloimmune injury of transplanted liver parenchymal cells. J. Immunol. 2008;181:1224–1231. doi: 10.4049/jimmunol.181.2.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ideguchi M. Shinoyama M. Gomi M. Hayashi H. Hashimoto N. Takahashi J. Immune or inflammatory response by the host brain suppresses neuronal differentiation of transplanted ES cell-derived neural precursor cells. J. Neurosci. Res. 2008;86:1936–1943. doi: 10.1002/jnr.21652. [DOI] [PubMed] [Google Scholar]
- Jones T.B. Ankeny D.P. Guan Z. McGaughy V. Fisher L.C. Basso D.M. Popovich P.G. Passive or active immunization with myelin basic protein impairs neurological function and exacerbates neuropathology after spinal cord injury in rats. J. Neurosci. 2004;24:3752–3761. doi: 10.1523/JNEUROSCI.0406-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi-Abdolrezaee S. Eftekharpour E. Wang J. Morshead C.M. Fehlings M.G. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J. Neurosci. 2006;26:3377–3389. doi: 10.1523/JNEUROSCI.4184-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaymaz M. Emmez H. Bukan N. Dursun A. Kurt G. Pasaoglu H. Pasaoglu A. Effectiveness of FK506 on lipid peroxidation in the spinal cord following experimental traumatic injury. Spinal Cord. 2005;43:22–26. doi: 10.1038/sj.sc.3101621. [DOI] [PubMed] [Google Scholar]
- Kigerl K.A. McGaughy V.M. Popovich P.G. Comparative analysis of lesion development and intraspinal inflammation in four strains of mice following spinal contusion injury. J. Comp. Neurol. 2006;494:578–594. doi: 10.1002/cne.20827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapointe N.P. Ung R.V. Bergeron M. Cote M. Guertin P.A. Strain-dependent recovery of spontaneous hindlimb movement in spinal cord transected mice (CD1, C57BL/6, BALB/c) Behav. Neurosci. 2006;120:826–834. doi: 10.1037/0735-7044.120.4.826. [DOI] [PubMed] [Google Scholar]
- Lopez-Lera A. Garrido S. de la Cruz R.M. Fontan G. Lopez-Trascasa M. Molecular characterization of three new mutations causing C5 deficiency in two non-related families. Mol. Immunol. 2009;46:2340–2347. doi: 10.1016/j.molimm.2009.03.026. [DOI] [PubMed] [Google Scholar]
- Lopez-Vales R. Garcia-Alias G. Fores J. Udina E. Gold B.G. Navarro X. Verdu E. FK 506 reduces tissue damage and prevents functional deficit after spinal cord injury in the rat. J. Neurosci. Res. 2005;81:827–836. doi: 10.1002/jnr.20605. [DOI] [PubMed] [Google Scholar]
- Low K. Culbertson M. Bradke F. Tessier-Lavigne M. Tuszynski M.H. Netrin-1 is a novel myelin-associated inhibitor to axon growth. J. Neurosci. 2008;28:1099–1108. doi: 10.1523/JNEUROSCI.4906-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M. Basso D.M. Walters P. Stokes B.T. Jakeman L.B. Behavioral and histological outcomes following graded spinal cord contusion injury in the C57Bl/6 mouse. Exp. Neurol. 2001;169:239–254. doi: 10.1006/exnr.2001.7679. [DOI] [PubMed] [Google Scholar]
- Madsen J.R. MacDonald P. Irwin N. Goldberg D.E. Yao G.L. Meiri K.F. Rimm I.J. Stieg P.E. Benowitz L.I. Tacrolimus (FK506) increases neuronal expression of GAP-43 and improves functional recovery after spinal cord injury in rats. Exp. Neurol. 1998;154:673–683. doi: 10.1006/exnr.1998.6974. [DOI] [PubMed] [Google Scholar]
- Maeda K. Hata R. Hossmann K.A. Differences in the cerebrovascular anatomy of C57black/6 and SV129 mice. Neuroreport. 1998;9:1317–1319. doi: 10.1097/00001756-199805110-00012. [DOI] [PubMed] [Google Scholar]
- Mantovani A. Sica A. Sozzani S. Allavena P. Vecchi A. Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Marsala M. Kakinohana O. Yaksh T.L. Tomori Z. Marsala S. Cizkova D. Spinal implantation of hNT neurons and neuronal precursors: graft survival and functional effects in rats with ischemic spastic paraplegia. Eur. J. Neurosci. 2004;20:2401–2414. doi: 10.1111/j.1460-9568.2004.03702.x. [DOI] [PubMed] [Google Scholar]
- McEwen M.L. Sullivan P.G. Springer J.E. Pretreatment with the cyclosporin derivative, NIM811, improves the function of synaptic mitochondria following spinal cord contusion in rats. J. Neurotrauma. 2007;24:613–624. doi: 10.1089/neu.2006.9969. [DOI] [PubMed] [Google Scholar]
- Miura M. El-Sawy T. Fairchild R.L. Neutrophils mediate parenchymal tissue necrosis and accelerate the rejection of complete major histocompatibility complex-disparate cardiac allografts in the absence of interferon-gamma. Am. J. Pathol. 2003;162:509–519. doi: 10.1016/s0002-9440(10)63845-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monje M.L. Toda H. Palmer T.D. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Nakashima K. Taga T. Mechanisms underlying cytokine-mediated cell-fate regulation in the nervous system. Mol. Neurobiol. 2002;25:233–244. doi: 10.1385/MN:25:3:233. [DOI] [PubMed] [Google Scholar]
- Nikulina E. Tidwell J.L. Dai H.N. Bregman B.S. Filbin M.T. The phosphodiesterase inhibitor rolipram delivered after a spinal cord lesion promotes axonal regeneration and functional recovery. Proc. Natl. Acad. Sci. U.S.A. 2004;101:8786–8790. doi: 10.1073/pnas.0402595101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi R.A. Liu H. Chu Y. Hamamura M. Su M.Y. Nalcioglu O. Anderson A.J. Behavioral, histological, and ex vivo magnetic resonance imaging assessment of graded contusion spinal cord injury in mice. J. Neurotrauma. 2007;24:674–689. doi: 10.1089/neu.2006.0204. [DOI] [PubMed] [Google Scholar]
- Nottingham S. Knapp P. Springer J. FK506 treatment inhibits caspase-3 activation and promotes oligodendroglial survival following traumatic spinal cord injury. Exp. Neurol. 2002;177:242–251. doi: 10.1006/exnr.2002.7975. [DOI] [PubMed] [Google Scholar]
- Nout Y.S. Schmidt M.H. Tovar C.A. Culp E. Beattie M.S. Bresnahan J.C. Telemetric monitoring of corpus spongiosum penis pressure in conscious rats for assessment of micturition and sexual function following spinal cord contusion injury. J. Neurotrauma. 2005;22:429–441. doi: 10.1089/neu.2005.22.429. [DOI] [PubMed] [Google Scholar]
- Nozaki T. Rosenblum J.M. Ishii D. Tanabe K. Fairchild R.L. CD4 T cell-mediated rejection of cardiac allografts in B cell-deficient mice. J. Immunol. 2008;181:5257–5263. doi: 10.4049/jimmunol.181.8.5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong G.L. Mattes M.J. Mouse strains with typical mammalian levels of complement activity. J. Immunol. Methods. 1989;125:147–158. doi: 10.1016/0022-1759(89)90088-4. [DOI] [PubMed] [Google Scholar]
- Osmers I. Szalai A.J. Tenner A.J. Barnum S.R. Complement in BuB/BnJ mice revisited: serum C3 levels and complement opsonic activity are not elevated. Mol. Immunol. 2006;43:1722–1725. doi: 10.1016/j.molimm.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Palladini G. Caronti B. Pozzessere G. Teichner A. Buttarelli F.R. Morselli E. Valle E. Venturini G. Fortuna A. Pontieri F.E. Treatment with cyclosporine A promotes axonal regeneration in rats submitted to transverse section of the spinal cord—II—Recovery of function. J. Hirnforsch. 1996;37:145–153. [PubMed] [Google Scholar]
- Parr A.M. Kulbatski I. Tator C.H. Transplantation of adult rat spinal cord stem/progenitor cells for spinal cord injury. J. Neurotrauma. 2007;24:835–845. doi: 10.1089/neu.2006.3771. [DOI] [PubMed] [Google Scholar]
- Paylor R. Crawley J.N. Inbred strain differences in prepulse inhibition of the mouse startle response. Psychopharmacology (Berl.) 1997;132:169–180. doi: 10.1007/s002130050333. [DOI] [PubMed] [Google Scholar]
- Pineau I. Lacroix S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: Multiphasic expression pattern and identification of the cell types involved. J. Comp. Neurol. 2007;500:267–285. doi: 10.1002/cne.21149. [DOI] [PubMed] [Google Scholar]
- Pitchford S.C. Furze R.C. Jones C.P. Wengner A.M. Rankin S.M. Differential mobilization of subsets of progenitor cells from the bone marrow. Cell Stem Cell. 2009;4:62–72. doi: 10.1016/j.stem.2008.10.017. [DOI] [PubMed] [Google Scholar]
- Popovich P.G. Guan Z. McGaughy V. Fisher L. Hickey W.F. Basso D.M. The neuropathological and behavioral consequences of intraspinal microglial/macrophage activation. J. Neuropathol. Exp. Neurol. 2002;61:623–633. doi: 10.1093/jnen/61.7.623. [DOI] [PubMed] [Google Scholar]
- Popovich P.G. Guan Z. Wei P. Huitinga I. van Rooijen N. Stokes B.T. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp. Neurol. 1999;158:351–365. doi: 10.1006/exnr.1999.7118. [DOI] [PubMed] [Google Scholar]
- Popovich P.G. Wei P. Stokes B.T. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J. Comp. Neurol. 1997;377:443–464. doi: 10.1002/(sici)1096-9861(19970120)377:3<443::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Porrett P.M. Lee M.Kt. Lian M.M. Wang J. Caton A.J. Deng S. Markmann J.F. Moore D.J. A direct comparison of rejection by CD8 and CD4 T cells in a transgenic model of allotransplantation. Arch. Immunol. Ther. Exp. (Warsz.) 2008;56:193–200. doi: 10.1007/s00005-008-0019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabchevsky A.G. Fugaccia I. Sullivan P.G. Scheff S.W. Cyclosporin A treatment following spinal cord injury to the rat: behavioral effects and stereological assessment of tissue sparing. J. Neurotrauma. 2001;18:513–522. doi: 10.1089/089771501300227314. [DOI] [PubMed] [Google Scholar]
- Ravikumar R. McEwen M.L. Springer J.E. Post-treatment with the cyclosporin derivative, NIM811, reduced indices of cell death and increased the volume of spared tissue in the acute period following spinal cord contusion. J. Neurotrauma. 2007;24:1618–1630. doi: 10.1089/neu.2007.0329. [DOI] [PubMed] [Google Scholar]
- Schauwecker P.E. Steward O. Genetic determinants of susceptibility to excitotoxic cell death: implications for gene targeting approaches. Proc. Natl. Acad. Sci. U.S.A. 1997;94:4103–4108. doi: 10.1073/pnas.94.8.4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serinsoz E. Bock O. Gwinner W. Schwarz A. Haller H. Kreipe H. Mengel M. Local complement C3 expression is upregulated in humoral and cellular rejection of renal allografts. Am. J. Transplant. 2005;5:1490–1494. doi: 10.1111/j.1600-6143.2005.00873.x. [DOI] [PubMed] [Google Scholar]
- Sheth R.N. Manzano G. Li X. Levi A.D. Transplantation of human bone marrow-derived stromal cells into the contused spinal cord of nude rats. J. Neurosurg. Spine. 2008;8:153–162. doi: 10.3171/SPI/2008/8/2/153. [DOI] [PubMed] [Google Scholar]
- Shultz L.D. Schweitzer P.A. Christianson S.W. Gott B. Schweitzer I.B. Tennent B. McKenna S. Mobraaten L. Rajan T.V. Greiner D.L. Leiter E.H. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J. Immunol. 1995;154:180–191. [PubMed] [Google Scholar]
- Sipski M.L. Jackson A.B. Gomez-Marin O. Estores I. Stein A. Effects of gender on neurologic and functional recovery after spinal cord injury. Arch. Phys. Med. Rehabil. 2004;85:1826–1836. doi: 10.1016/j.apmr.2004.04.031. [DOI] [PubMed] [Google Scholar]
- Stirling D.P. Khodarahmi K. Liu J. McPhail L.T. McBride C.B. Steeves J.D. Ramer M.S. Tetzlaff W. Minocycline treatment reduces delayed oligodendrocyte death, attenuates axonal dieback, and improves functional outcome after spinal cord injury. J. Neurosci. 2004;24:2182–2190. doi: 10.1523/JNEUROSCI.5275-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirling D.P. Liu S. Kubes P. Yong V.W. Depletion of Ly6G/Gr-1 leukocytes after spinal cord injury in mice alters wound healing and worsens neurological outcome. J. Neurosci. 2009;29:753–764. doi: 10.1523/JNEUROSCI.4918-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll G. Jander S. Schroeter M. Detrimental and beneficial effects of injury-induced inflammation and cytokine expression in the nervous system. Adv. Exp. Med. Biol. 2002;513:87–113. doi: 10.1007/978-1-4615-0123-7_3. [DOI] [PubMed] [Google Scholar]
- Surquin M. Le Moine A. Flamand V. Rombaut K. Demoor F.X. Salmon I. Goldman M. Abramowicz D. IL-4 deficiency prevents eosinophilic rejection and uncovers a role for neutrophils in the rejection of MHC class II disparate skin grafts. Transplantation. 2005;80:1485–1492. doi: 10.1097/01.tp.0000176486.01697.3f. [DOI] [PubMed] [Google Scholar]
- Swijnenburg R.J. Schrepfer S. Govaert J.A. Cao F. Ransohoff K. Sheikh A.Y. Haddad M. Connolly A.J. Davis M.M. Robbins R.C. Wu J.C. Immunosuppressive therapy mitigates immunological rejection of human embryonic stem cell xenografts. Proc. Natl. Acad. Sci. U.S.A. 2008;105:12991–12996. doi: 10.1073/pnas.0805802105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi A. Olivas A.D. Noble-Haeusslein L.J. Inflammation and spinal cord injury: Infiltrating leukocytes as determinants of injury and repair processes. Clin. Neurosci. Res. 2006;6:283–292. doi: 10.1016/j.cnr.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. Fu S. Wang Y. Yu P. Hu J. Gu W. Xu X.M. Lu P. Interleukin-1beta mediates proliferation and differentiation of multipotent neural precursor cells through the activation of SAPK/JNK pathway. Mol. Cell Neurosci. 2007;36:343–354. doi: 10.1016/j.mcn.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Wu Y.L. Hauptmann G. Viguier M. Yu C.Y. Molecular basis of complete complement C4 deficiency in two North-African families with systemic lupus erythematosus. Genes Immun. 2009;10:433–445. doi: 10.1038/gene.2009.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J. Xu L. Welsh A.M. Hatfield G. Hazel T. Johe K. Koliatsos V.E. Extensive neuronal differentiation of human neural stem cell grafts in adult rat spinal cord. PLoS Med. 2007;4:e39. doi: 10.1371/journal.pmed.0040039. [DOI] [PMC free article] [PubMed] [Google Scholar]