Introduction
The articles in this Special Topic cover a range of issues concerning long-distance projecting cortical GABAergic neurons, in the context of interneuron diversity. As several authors report, these neurons are attracting renewed attention spurred by new techniques and markers which show great potential for deciphering their role in cortical organization and microcircuitry. Other authors have emphasized developmental origins of particular subpopulations and their roles in early cortical circuitry. Notable recurring themes are species-specific features and probable implications for normal and pathological cortical functioning. A corollary theme, evident in many of these articles, concerns nomenclature. Several terms are almost interchangeably used, but nevertheless distinct; that is: subplate, layer 7, layer VIB, pioneer and interstitial neuron (see comments to follow Clancy et al., below, among others). In this article the main conclusions, and some of what the host editors (Kathleen Rockland and Javier DeFelipe) consider the most interesting remarks, have been extracted from each of the individual articles. These commentaries are not necessarily directly derived from the original work of the authors, and may be the result of the collective work of several different laboratories. This is followed by a section dedicated to more general comments and a discussion of the issues raised. The authors who have participated in this article are listed in alphabetical order.
Remarks and Main Conclusions
Cortical GABAergic neurons can be parceled into a number of subgroups based on variations in morphology, birthplace, mature locations, colocalized peptides, and electrophysiological parameters. Despite such diversity, conventional models of cortical function include GABAergic neurons as participators only in local connectivity, such that the designation of “interneuron” is often used interchangeably with GABAergic to describe cortical neurons that play an inhibitory role. However, cortical GABAergic categories recently were extended to include a subset of phylogenetically conserved neurons that project axons across long distances – the newly identified long-range interneurons, perhaps more precisely called cortical GABAergic projection neurons.
One intriguing aspect of the latest subgroup is that the majority of the long-range GABAergic projections extend from neurons located in cortical layer I, cortical white matter, and the subgriseal region of the cortex (subjacent to cortical layer VI). This prompted the suggestion that the GABAergic projection neurons might be a subset of the little-studied cells that persist in the adult brain from the developmental subplate.
Early in development, the cells of the future layer I, as well as the future subplate/white matter neurons, are merged as the preplate before neurons of the developing cortical plate split the preplate into a superficial region (later called layer 1) and a subgriseal region (later called the subplate). The exact percentage of subplate cells that survive into adulthood is somewhat difficult to identify, but it is well-documented that some survive an early wave of cell death to remain in the mature white matter in human and non-human primate cortex and in carnivores.
GABAergic neurons account for 15–25% of all the neurons in the region depicted as the persisting subplate, similar to their percentage in the cortex overall.
Similar to the sometimes confusing GABAergic nomenclature recently addressed by the Pettilla committee, surviving subplate cells have been assigned a variety of different names in mature cortex, including border neurons, white matter neurons, subgriseal neurons, layer VII, layer VIb, deep layer VI, upper subplate neurons, and the deep cortical band. In this study we will use the term “persisting subplate neurons” for those resilient cells that remain from the developmental subplate in and above the white matter across maturation, where they continue to participate in cortical function, and apparently include the GABAergic subset that sends projections for long distances.
Both GABAergic and subplate populations include numerous and diverse morphological subsets that are different from the more prevalent cortical pyramidal neurons (although each population may include cells with pyramidal morphology), and both populations contain a subset whose projections may travel long distances, sometimes crossing areal boundaries, as well as a subset that focuses projections on cortical layer I. Both populations are similarly heterogeneous in their electrophysiological properties and in the numerous signaling chemicals they sequester. Moreover, subsets may share a common non-cortical birthplace in the ganglionic eminences, raising the possibility that some may descend from similar sets of precursors. Supporting this notion, both populations use somewhat similar molecular modes of migration, different from the mechanism used by pyramidal cells.
Although the contribution of the GABAergic interneurons to cortical function is undisputed, and the critical role of the subplate in cortical development is well-accepted, conventional models of mature cognitive function do not yet incorporate contributions of either the projection GABAergic or the persisting subplate neurons. When numbers are reduced compared to other neural populations, there may be a tendency to simply dismiss those that persist as “sparse,” “remnants,” or “relics”. Unfortunately, such terminology implies a fairly unessential function, and it seems important to avoid such categorization until additional information on their function is available.
The mathematical principles underlying small-world networks suggest that sparse connectivity is a plausible design underlying important cognitive function. Long-range inhibition, even from relatively sparse connections, can be a potent network component. In small-world networks, clusters of cells link to their nearest neighbors, while some connect to distant clusters. This pattern can serve as the basis for a surprisingly strong communication network, especially when it is amplified by local input, as is likely the case for both the long-range GABAergic and the persisting subplate populations.
In a series of previous studies, mathematical models have been used to successfully identify both similarities and relative differences in the timing of neural “events” when comparing primate and nonprimate development. For the purpose of this review, “neural events” are defined as milestones pertaining to neural development such as the post conception (PC) date when neurons destined for the various cortical layers are generated. Mathematical approaches are valid because despite species differences, including differences in the duration of development, the size of most brain regions scales similarly across species. Central to this meta analysis, the timing of events that occur in most neural regions is remarkably conserved.
The most pragmatic application of statistical modeling is that neural events empirically derived in one species can be compared and successfully applied to another. Therefore, given the potentially important contributions of the long distance GABAergic and persisting subplate populations, we reasoned any additional information about these two populations, including comparative cross-species data, is likely to be useful. At this time, no empirical developmental data are yet available specifically for the cortical GABAergic projection neurons. However developmental data are available for both general populations that include them, the GABAergic and subplate populations. We assembled a database of GABAergic and subplate developmental events (e.g. the PC day subplate neurogenesis begins, the day GABAergic cells are first found in the subplate). We then applied cross-species statistical modeling, and tested if species similarities and differences might be indicated by statistical analysis of the developmental sequences.
Similarities between the two rodent populations (rat and mouse) are striking, permitting us to identify developmental dates for GABAergic and subplate neural events in rats that were previously identified only in mice, as well as the timing in mouse development for events previously identified in rats. Primate comparative data are also compelling, although slight variability in statistical error measurement indicates differences in primate GABAergic and subplate events when compared to rodents. Although human extrapolations are challenging because fewer empirical data points are available, and because human data display more variability, we also produce estimates of dates for GABAergic and subplate neural events that have not yet been, or cannot be, determined empirically in humans.
The pragmatic value of the cross species translations – in direct savings of time and resources when intervals for studies might be narrowed – is a compelling reason to add this type of analysis to the growing array of modern neuroanatomical tools. In some case, it might eliminate the necessity of repeating a study already accomplished in one species (as our data suggest is possible when translating from mice to rats, or rats to mice), or at the least contribute to a narrowing of time intervals (as our data suggest is possible for humans). And although correlations between data available in the empirical literature and data produced by the model were already significant, we expect future predictions will become more accurate as additional data points are added to our database.
On the other hand, we certainly do not suggest that there are no differences in the brain development of diverse mammalian species. Mathematical modeling can successfully adjust for some rodent/primate differences, such that comparisons and predictions are not overly distorted by differences in brain sizes, or what might seem to be a relatively prolonged time window for neurogenesis in primates when compared to rats. Yet there are questions that analysis of our database does not yet permit us to address, such as the possibility of an effect on differences in cell cycle mechanisms, or the effect of variability in the location of GABAergic proliferation when comparing rodents to humans.
However, it is clear that the timing of neurogenesis alone is an important factor in development, given the evidence that timing might predict such properties as laminar position, electrophysiology responses, and neuronal morphology, including projection patterns. As datapoints are added to our database, we hope to test if a neuron's role is more closely related to the location of its birthplace, or the timing of its birth date. One additional limitation we should point out arises because early postnatal days in rodent brain development correspond to in utero timing in humans. The result is that the effects of a perinatal wave of synaptogenesis, the onslaught of experience surrounding birth, and the mother/offspring interaction are not included, as we have not yet identified data points associated with these events that fit into statistical models.
General Comments and Discussion
Espinosa and fairén
Comment on point 2.
(a) It would be of interest to confirm by birth-dating the developmental association of GABA projection neurons with the preplate and its derivatives, marginal zone and subplate.
(b) What is the proportion of GABA projecting neurons among the total GABA neurons persisting from the subplate? Re: comments by DeFelipe and Rockland to Higo et al. (below).
Rockland
Comment on point 3.
If, as seems increasingly possible, subpopulations can be reliably identified by distinctive markers, a next step can be rigorous quantification across species, areas, and developmental epochs. In this regard, the relatively small size of the long-distance projecting GABAergic population in particular is a further advantage.
Clancy
Reply to Rockland point 3.
This seems a wonderful next step (or rather several wonderful next steps)!
Kanold
The identification of subpopulations is the necessary next step. But the comparison of different species here is almost even more important as well.
Rockland
Comment on point 5.
re Nomenclature, see DeFelipe comment, following Kanold article.
Clancy
Reply to Rockland point 5.
There would be value in establishing consistent terminology for persisting subplate cells. Those that remain from development in the white matter fibers have had some nomenclature stability after Cajal named them white matter interstitial cells in 1911 (although often abbreviated as interstitial neurons, or white matter neurons); prior to this they also had been called subcortical cells, extracortical (non plate) cells, and outlying cells (Okhotin and Kalinichenko, 2003). Assigning a distinct appropriate name for the persisting subplate cells that remain in a more superficial position has proved more difficult. As noted, they are referred to as layer VII (Reep, 2000), the deep cortical band (Kristt, 1979), upper subplate neurons (Marin-Padilla and Marin-Padilla, 1982), deep layer VI (McDonald and Burkhalter, 1993), layer VIb (Gomez-Pinilla and Cotman, 1992), subgriseal neurons (Clancy and Cauller, 1999), and border neurons (Hogan and Berman, 1992).
Espinosa and fairén
Comment on points 5–8.
re Nomenclature. Although the group of “persisting subplate neurons” includes both glutamatergic and long-range GABAergic neurons, we would suggest some problems of definition in point 8: “…both the long-range GABAergic and the persisting subplate populations”.
Clancy
Reply to Espinosa and Fairén.
In addition to the nomenclature problems, it seems persisting subplate cells are sometimes dismissed as simply a remnant population, likely because many die across development, or because they were once considered a rodent specialization (Reep and Goodwin, 1988). Yet because so many theories of human cognition are based on studies accomplished in rodents, more information on this subset of cells would be valuable even if they were specific to rodents. However, the “rodent specialization” assignment has evolved (Reep, 2000), and it seems unlikely that persisting subplate cells are mere remnants in adult cortex, such that their connectivity serves no definable purpose in mature cognitive function. For a subset of neural cells to persist as a remnant population would be an expensive metabolic strategy, so it is more likely that even if they began as remnants, they would eventually have been incorporated into some functional role. (It may also be worth noting that large numbers of cells in the spinal cord also die prior to maturity, but those that remain are not considered vestigial.)
Kanold
Reply to Clancy.
As far as “rodent specialization” goes, it would be very useful if one could compare the subtypes of subplate neurons (surviving or not), their associated circuits, and survival between rodents and at least one carnivore species (ferret, cat, etc).
I agree that remaining subplate neurons probably play some functional role, although it could be a very different role from their role in early development.
Luhmann
Comment on point 8.
It has been recently demonstrated in hippocampal slices from immature rodents that a subpopulation of GABAergic interneurons with long axonal arborizations play a central role in synchronizing spontaneous network activity (“hub” neurons) (Bonifazi et al., 2009). These cells were preferentially located in the stratum oriens and lucidum at the two borders with the pyramidal cell layer. Two different morphological types of hub neurons were identified following intracellular labelling and reconstruction: (i) cells displaying a long axon spanning regions with sparse collaterals, and (ii) basketlike neurons with a dense but more local arborization pattern. Subplate cells may serve as hub neurons in the developing cerebral cortex (Voigt et al., 2001; Hanganu et al., 2009).
Remarks and Main Conclusions
GABAergic neurons with axons projecting in the ipsilateral hemisphere seem to have similar chemical features and often exhibit somatostatin (SS)-immunoreactivity (IR) (91%), neuropeptide Y (NPY)-IR (82%), and neuronal nitric oxide synthase (nNOS)-IR (71%). Considering these observations, the fact that most nNOS-positive neurons are a subpopulation of SS- and NPY-IR neurons, and nNOS-, NPY-, and SS-triple-positive cells are less than 0.5% of GABAergic neurons, it was speculated that the nNOS-positive GABAergic projection neurons compose a very small subpopulation in the neocortical GABAergic neurons.
Nicotinamide adenine dinucleotide phosphate diaphorase (NADPH-d)-positive somata in the white matter originate thick NADPH-d-positive fibers in the white matter of the cat neocortex. These emanate with other non-labeled arcuate fibers, without originating any thick branches, and without bending toward the gray matter of the neocortex. These features may indicate that the total length of NADPH-d-positive fibers in the white matter is long enough to be called as projection fibers. We regarded GABAergic neuron extending an axon longer than 1.5 mm from the soma as the GABAergic projection neuron in the mouse.
NADPH-d reaction labeled axon fibers of nNOS-positive GABAergic neurons in cats and mice, while GFP-immunohistochemistry in GAD67-Cre/GFP Cre-reporter mouse labeled both projection fibers originating in nNOS-positive GABAergic neurons and those in the other subtypes of GABAergic neurons. However, the distribution pattern of the labeled axon fragments was similar in both preparations. NADPH-d reactive axons and GFP-IR axons were found to be very sparse in the corpus callosum, sparse in the anterior commissure, and more numerous in the fimbria. Labeled fibers in the fimbria will include afferent- and efferent-fibers from and to the subcortical nuclei, in addition to the commissural fibers through the ventral hippocampal commissure. Excluding subcortical afferent and efferent fibers, we speculate that the GABAergic commissural fibers reciprocally interconnect archi- and paleo-cortex.
One of the important results elucidated in this study is that a large number of GABAergic neurons are also involved in the cortico-fugal, cortico-cortical, and callosal projections. Each subtype of GABAergic projection neuron may contribute to information processing in the neocortex in different ways.
General Comments and Discussion
DeFelipe
Comment on point 1.
Are parvalbumin expressing neurons not part of the subpopulation of GABA projecting cells?
Rockland
Reply to DeFelipe.
Tomioka et al. (2005) (mouse) and Tomioka and Rockland (2007) (primate) also reported an absence of PV+ neurons. However, see Jinno, below, about PV+ GABAergic projection neurons in the hippocampus.
Tamamaki
Reply to DeFelipe.
While parvalbumin expressing GABAergic neurons in the septum project axons to the neocortex, we did not find any of those in the neocortex project axons cortico-cortically. Less than 0.5% of GABAergic neurons (nNOS, NYP, and somatostatin-expressing GABAergic neurons) seemed to project axons cortico-cortically. However, we also agree that there will be other subtypes of GABAergic neurons projecting cortico-cortically in the neocortex. They would be generated in embryonic stage and maintained in adult rodent, feline, primate, and maybe, human brain. They may be found in very specific areas such as boundary between area 17 and 18. Although they may be very weak in GAD67 promoter activity and we could not describe them well in GAD67-GFP and GAD67-Cre/GFP-reporter mice, we would like to continue our observation of GABAergic projection neurons in the GAD67-GFP and GAD67-Cre knock-in mice.
DeFelipe
In Figures 2A,B of Higo et al. (2009), GFP-positive axons in the fimbria and anterior commissure of the GAD67-Cre/GFP Cre-reporter mouse brain have numerous varicosities. I am wondering if they establish synapses. Is there any information regarding this issue?
DeFelipe
Comment on points 1 and 4.
There is an apparent discrepancy between the claim of Higo and colleagues that a large number of GABAergic neurons are involved in the cortico-fugal, cortico-cortical and callosal projections (point 4), since it was estimated that the proportion of GABAergic neurons that project over long distances represent less than 0.5% (point 1). Thus, I am wondering whether 0.5% is an underestimation.
Rockland
Continuing DeFelipe.
Peters et al. (1990) demonstrated transcallosally projecting non-pyramidal neurons in the supragranular cortical layers of the area 17/18 border region in the cat. These were estimated as 10–32% of the total non-pyramidal cell population.
DeFelipe
Is the proportion of GABA projecting neurons similar in mouse, rat, cat and monkeys? Are these neurons more common in juvenile than in adult animals?
Espinosa and fairén
It seems that not all projection GABAergic neurons are associated to subplate or marginal zones? In neocortex there are exceptions (see Rockland comment on A. Peters). GABA projection neurons in the hippocampus, however, may be situated in every layer including for instance the stratum radiatum (see Jinno paper, this issue) and might not be related to the hippocampal preplate. Nevertheless, in general, hippocampal GABA neurons are among the earliest generated (Soriano et al., 1986, 1989).
Remarks and Main Conclusions
Using anatomical, molecular and electrophysiological approaches, several types of GABAergic projection neurons have been shown to exist in the hippocampus. The target areas of these cells are the subiculum and other retrohippocampal areas, the medial septum and the contralateral dentate gyrus. The long range GABAergic projection system of the hippocampus may serve to coordinate precisely the multiple activity patterns of widespread cortical cell assemblies in different brain states and among multiple functionally related areas.
The most studied remote target of the hippocampal GABAergic projection neurons is the medial septum. Using intraseptal injection of horseradish peroxidase as the retrograde tracer, it was found that a subset of GABAergic neurons of the hippocampus innervate the medial septum (H-MS cells) in the rat brain. The H-MS cells were found in all regions of the hippocampus, but they were distributed in a layer specific manner: in the CA1 region, they were mainly located in the stratum oriens; in the CA3 region, they were scattered throughout all the layers; and in the dentate gyrus (DG), they were exclusively located in the hilar area. The morphological characteristics of H-MS cells were examined using fixed slice preparations of the rat hippocampus, and the following cells were identified: stellate cells in the hilus, horizontal basket cells in the stratum oriens of CA1 and CA3, stellate cells in the stratum radiatum of CA3 and pyramid-like cells in the stratum radiatum of CA1. The main postsynaptic targets of H-MS cells in the medial septum were parvalbumin (PV)- expressing GABAergic neurons and, to a lesser extent, cholinergic neurons.
Several studies, employing different methods, have demonstrated conflicting results with regard to the local targets of H-MS cells. In juvenile rats, the local axons of CA1 H-MS cells recorded in vitro were reported to innervate predominantly hippocampal GABAergic neurons. By contrast, the main local targets of in vivo recorded or retrogradely labeled GABAergic cells projecting to the medial septum and subiculum were pyramidal neurons in the CA1 area of the adult rats. Although it is difficult to explain the discrepancy, differences in ages of animals and labeling methods might be related to the inconsistent results in local postsynaptic targets of H-MS cells.
The hippocampus also receives GABAergic inputs from the medial septum, and thus the medial septum and the hippocampus are connected reciprocally. The two major components of the septo-hippocampal projection are cholinergic and GABAergic neurons. The cholinergic projection terminates on all types of hippocampal cells, whereas septal GABAergic neurons specifically innervate hippocampal GABAergic neurons. Recently, Takács and coworkers demonstrated direct reciprocity by using combined retrograde and anterograde tracing; that is, H-MS cells are the postsynaptic targets of GABAergic septo-hippocampal axons. On the other hand, GABAergic terminals of H-MS cells in the medial septum were shown to innervate septo-hippocampal neurons retrogradely labeled from the ventral hippocampus. This reciprocal loop between the hippocampus and the medial septum via GABAergic neurons is considered to play a critical role for generating the rhythmic activity and synchronization.
Recent studies have demonstrated the existence of hippocampal GABAergic cells projecting to the subiculum (H-Sub cells). Using retrograde labeling, the distributions of H-Sub cells were estimated in the rat brain. Differently from the H-MS cells, the retrogradely labeled H-Sub cells were mainly found in the CA1 region, and only a few cells were detected in the CA3 region and the dentate hilus. In the CA1 region, H-Sub cells were scattered throughout all the layers. The majority of H-Sub cells in the stratum oriens were large-sized horizontal cells, while those in the strata radiatum and lacunosum-moleculare were small to medium-sized bipolar and multipolar cells. The postsynaptic targets of long-range axons in the subiculum were assumed to be pyramidal neurons, although the ratios were not determined due to technical limitations. The major local targets of seven H-Sub cells were the dendritic shafts of pyramidal neurons. But, one H-Sub cell expressing enkephalin (ENK) innervated dendritic shafts of GABAergic and pyramidal neurons. The targets of ENK-expressing H-Sub cells were associated with the location of axonal arbors, i.e., interneurons were the main targets in the alveus, both interneurons and pyramidal cell dendrites were innervated in the other layers, and interneurons were the exclusive targets in the subiculum.
It should be noted that four in vivo recorded cells projected from the CA1 stratum oriens to both the subiculum and the medial septum. Thus, there might be three groups of projection neurons in the CA1 region: those sending axons exclusively to the medial septum, those innervating both the medial septum and the subicular area, and those exclusively sending axons to the subicular/retrosplenial cortex. Although the cells exclusively projecting to the medial septum have not yet been identified, their existence has been suggested by the numerical data showing that the numbers of retrogradely labeled cells after injection into the medial septum are much larger than those after injection into the subiculum.
A few GABAergic neurons in the CA1 region are identified by injections of retrograde tracers into the retrosplenial cortex. The majority (about 65%) of hippocampal GABAergic neurons projecting to the granular retrosplenial cortex (H-Rsp cells) were detected at the border between strata radiatum and lacunosum-moleculare of the CA1 region, and a smaller population was located in the stratum radiatum. Many fewer cells (<10%) were found in the stratum oriens or stratum pyramidale of the CA1 region. In the CA3 and DG, virtually no cells were retrogradely labeled after the injection of tracer into the granular retrosplenial cortex.
A small number of GABAergic neurons in the hilus of the DG have axonal projections to the contralateral DG through the hippocampal commissure. Although the targets of these neurons are not strictly extrahippocampal, hilar GABAergic neurons with commissural projection should logically be included as long-range GABAergic projection neurons. The postsynaptic targets of GABAergic commissural projections are thought to be dendrites of granule cells. It has not been clearly proven whether the hilar cells innervating the medial septum simultaneously send commissural axons to the contralateral DG.
Several species differences have been reported in the functional organization of the hippocampus. Most notably, glutamatergic hilar mossy cells showed neurochemical discrepancies between mice and rats. The calcium-binding protein calretinin is expressed in mossy cells in the mouse ventral hilus, but not in the rat hilus. On the contrary, calcitonin gene-related peptide is localized in the rat mossy cells, but not in the mouse mossy cells. However, interestingly enough, the morphofunctional similarities in hippocampal GABAergic neurons have been repeatedly reported in mice and rats. The numerical densities of chemically defined subpopulations of GABAergic neurons in the mouse hippocampus were comparable to those in the rat hippocampus. Taken together, it is possible to hypothesize that the chemical characteristics of GABAergic projection neurons in the rat and mouse hippocampus are rather similar to each other.
Somatostatin (SOM) is one of the key molecules of H-MS cells. The vast majority of the H-MS cells express SOM in the mouse hippocampus. In contrast, one half of SOM-positive neuron in the CA3 region and DG, and one fourth in the CA1 region projected to the medial septum. Similar results were obtained from the rat hippocampus. In the CA1 region, one half of H-Sub cells were SOM-positive, while no H-Sub cells were immunoreactive for SOM in the strata radiatum and lacunosum-moleculare. In the CA3 strata radiatum/lacunosum-moleculare and the dentate hilus, SOM was detected in less than half and one-fourths of H-Sub cells, respectively. In the rat CA1 region, SOM was not detected in H-Rsp cells. On the other hand, the vast majority of SOM-positive cells project to the contralateral hippocampus via the commissural pathway in the rat dentate hilus.
In the Ammon's horn, less than half of H-MS cells were positive for neuropeptide Y (NPY), whereas virtually all H-MS cells in the DG contained NPY. The expression ratios of NPY in H-Sub cells were generally similar to those in H-MS cells.
Another key molecule of H-MS cells is calbindin D28K (CB). It has been reported that the majority of H-MS cells are CB-positive in the rat hippocampus. However, immunofluorescent multiple labeling showed that less than half of retrogradely identified H-MS cells were CB-positive in the mouse hippocampus. Approximately half of H-MS cells in the Ammon's horn expressed CB, while none of them were positive for CB in the DG. Similar results were shown in the rat hippocampus. Expression ratios of CB in retrogradely labeled H-Sub cells were generally lower than those in H-MS cells.
Parvalbumin (PV) is expressed in a small population of H-MS cells and H-Sub cells in the rat hippocampus. On the other hand, no H-Rsp cells contained PV. In the DG, the majority of PV-positive neurons commissurally project to the contralateral hippocampus.
It is well known that calretinin (CR) is expressed in one of the populations of GABAergic neurons called interneuron-specific (IS) cells, i.e., those exclusively innervating other GABAergic neurons. In addition, CR is also expressed in approximately one-fourths of H-MS cells of the rat hippocampus. But, neither H-Sub cells nor H-Rsp cells were positive for CR. In the DG, one third of CR-positive GABAergic neurons projected commissurally.
Muscarinic Acetylcholine (ACh) receptor type 2 (M2R) is commonly expressed in a considerable population (approximately 40%) of H-MS cells and H-Sub cells in the rat hippocampus. In contrast, a smaller subset (15%) of H-Rsp cells was positive for M2R.
In the Ammon's horn, the majority of H-MS cells expressed mGluR1α (69% in the CA1, and 84% in the CA3 region), whereas only 15% of H-MS were mGluR1α-positive in the DG. H-Sub cells also expressed mGluR1α, but the expression ratios were lower (40% in the CA1 region) than those of H-MS cells.
In the CA1 area, enkephalin (ENK) has been detected in a population of IS cells. In addition, a recent retrograde labeling study showed that ENK was expressed in approximately 10% of H-Sub cells. The ENK-positive H-Sub cells were located in the stratum radiatum. All tested ENK-positive H-Sub cells were co-labeled for the orphan nuclear receptor chicken ovalbumin upstream promoter-transcription factor II, but none for CR.
Interestingly, different types of cells innervating specific extrahippocampal targets fire with distinct spike timing during network oscillation. The diversity of GABAergic projection cell classes in the hippocampus may result from the need to coordinate precisely the multiple activities of distributed neural circuits in different brain states and among multiple functionally related brain areas.
There is a growing body of evidence suggesting that the spatially and temporally organized synaptic inhibition mediated by specific subclasses of GABAergic neurons is impaired in mental illness. Along these lines, it has also been postulated that cognitive and affective impairments in psychiatric disorders may be related to a failure to integrate the activity of widely spread neural circuits. Interestingly, recent imaging data suggest that functional connectivity between remote regions is impaired in individuals with mental illness, such as schizophrenia. Because GABAergic neurons are considered to play a critical role in the long-range fast synchronization of neural activities across brain regions, these findings suggest that defects of long-range GABAergic projection system might be associated with the neurobiology of psychiatric disorders.
General Comments and Discussion
DeFelipe
Comment on point 12.
Regarding the expression of CB in H-MS, there seems to be some discrepancy between the studies of Tóth and Freund (1992), Jinno and Kosaka (2002) and Jinno et al. (2007) (the majority versus half or less than half). Thus, it is not clear to me whether CB is also a key molecule in H-MS cells as the authors claim.
Jinno
Reply to DeFelipe point 12.
At present, it is difficult explain the reason of the discrepancy between the studies of Tóth and Freund (1992), Jinno and Kosaka (2002) and Jinno et al. (2007). It might be attributed to specificity differences in antibodies against CB. Even so, the expression ratios of CB in GABAergic projection cells are generally high, and I believe CB is one of the key molecules of projection neurons.
Rockland
Comment on PV+ subpopulation.
As I comment above (Higo et al.), the occurrence of PV-positive GABAergic projecting neurons in the hippocampal regions seems to be a specialization not reported so far for cortico-cortical GABAergic projections. However, Jinno and Kosaka (2004) describe PV-positive GABAergic corticostrial neurons in mouse. These were demonstrated from both retrosplenial and somatosensory cortices, and estimated to comprise 5% and 3%, respectively, of the total inhibitory neuron population.
DeFelipe
Comment on point 14.
CR is expressed in IS cells and while approximately one quarter of H-MS cells are also CR-positive, the vast majority of the H-MS cells express SOM (point 10). Thus, it follows that a subpopulation of H-MS represent IS cells that express both CR and SOM. Is this correct?
Jinno
Reply to DeFelipe point 14.
Yes. A subpopulation of H-MS cells represent IS cells, and they are considered to express both CR and SOM (please see Gulyas et al., 2003).
Remarks and Main Conclusions
Subplate neurons are among the earliest generated neurons in the cerebral cortex of mammals and are located in the developing white matter of all cortical regions. In humans subplate neurons comprise up to 50% of the cortical neurons in the second trimester and are present in the first few years of life (depending on cortical area). In rodents some subplate neurons can remain into adulthood forming layer 6b. Subplate neurons thus comprise additional cortical circuits that are only present during cortical development, and these circuits appear to play a major role in development and early cortical function, but are only beginning to be characterized.
Subplate neurons receive glutamatergic input from the thalamus before these thalamic axons grow to their targets in layer 4. Subplate axons mainly project to cortical layer 4, thus there is a time period when subplate neurons are in a key position to relay thalamic input to layer 4. After thalamic axons grow into layer 4, thalamocortical synapses and GABAergic circuits in layer 4 undergo refinement and maturation and over this time are particularly influenced by sensory experience (defining the “critical period”). During this time subplate neurons are still present, receive direct thalamic input, and project to layer 4. The majority of subplate neurons are gradually eliminated postnatally by programmed cell death and remaining neurons are retained as interstitial neurons.
Subplate neurons can be selectively ablated in early development by excitotoxic kainic acid injections. Ablation of subplate neurons before thalamic axons invade layer 4 causes these axons to bypass the ablated area and grow into layer 4 at areas that contain subplate neurons. Thus subplate neurons seem to provide a guidance role in targeting thalamic axons to layer 4. Since subplate neurons project radially to layer 4 they might provide a scaffold that enables thalamic axons, which travel tangentially below their eventual target layer, to find their targets.
After thalamic axons grow into layer 4 they make synaptic connections with layer 4 neurons and build up these connections over time to adult strength. The strengthening of the thalamocortical synapses from an initially weak state occurs while there is already strong input from subplate neurons and possibly intracortical connections. Recent experiments indicate that subplate neurons play a major role in the developmental strengthening of thalamocortical projections. Subplate ablation after thalamic afferents have grown into layer 4 but before these afferents have made a strong synapse with layer 4 neurons prevents the strengthening of thalamocortical connections. In addition, the frequency – but not amplitude – of spontaneous excitatory synaptic events in layer 4 cells is increased, which is consistent with a lack of functional refinement of cortical connections. Together these data indicated that without subplate neurons, there is a failure of appropriate synapses to strengthen and others to weaken, and the visual cortex becomes functionally decoupled from its thalamic inputs.
The maturation of intracortical inhibition is central to normal cortical function. In addition GABAergic activity is thought to be involved in the maturation of glutamatergic circuits. Despite this importance of inhibition, the cells and circuits that control inhibitory development are unknown. Key processes of inhibitory maturation occur postsynaptically by changes in the subunit composition of the GABAA receptor and the intracellular Cl−-concentration (which affects the ion flow through the GABAA receptor). The Cl−-reversal potential (ECl) controls if GABAA-ergic activity is depolarizing or hyperpolarizing. ECl is mediated by Cl− transporters such as KCC2 and NKCC1 that control Cl− levels in the cytosol. KCC2 levels are low (ECl high) in early development, thus GABA can be depolarizing. Depending on the amount of depolarization, depolarizing GABA can be excitatory or have a shunting inhibitory influence. Over development, KCC2 levels increase (decreasing ECl), rendering GABA inhibitory. The strengthening of both excitatory and inhibitory circuits while maintaining “appropriate” activity levels might be achieved by wiring up GABAergic circuits first and then utilizing depolarizing GABA to aid in maturing glutamatergic connections.
The maturation of inhibition depends on normal sensory experience. Sensory deprivations (i.e. dark rearing, deafness, whisker trimming) prevent inhibitory maturation and high expression levels of BNDF, which is involved in inhibitory maturation. Because subplate neurons form a crucial relay of sensory information, and because subplate neurons provide excitation to developing circuits, subplate neurons are in a key position to regulate the maturation of cortical GABAergic inhibition. In particular since subplate neurons are driven by thalamic afferents, strong synaptic inputs between subplate neurons and cortical neurons might amplify the action of sensory inputs.
Removal of subplate neurons at early ages, when inhibition is immature, prevents both the developmental increase in KCC2 expression and the expression of a mature complement of GABAA receptor subunits. Consistent with these molecular abnormalities, electrophysiological recordings showed that GABAergic circuits remain depolarizing. How then is KCC2 regulated, and how are subplate neurons involved? Recent experiments have led to the hypothesis that KCC2 expression can be regulated by GABAergic depolarization, while others report no influence of GABAergic signaling on KCC2 expression. However, there are other sources for depolarization. Blocking glutamatergic signaling during early ages in vivo is sufficient to prevent the developmental increase in KCC2. Thus early glutamatergic activity might be required for GABAergic maturation in layer 4.
-
9.
There are three sources of glutamatergic inputs to cortical layer 4: intracortical, thalamus and subplate neurons. Subplate removal by itself prevents inhibitory development despite the presence of intracortical and thalamic input. Thus, together these data suggest that glutamatergic excitation from subplate neurons is needed for inhibitory maturation. Such a role of subplate neurons in inhibitory maturation would require that subplate neurons depolarize layer 4 neurons, which can be achieved either by exciting GABAergic neurons and increasing early depolarizing GABAergic activity or by exciting the targets of GABAergic neurons directly. Thus by providing feed-forward excitation to the developing cortical circuits subplate neurons can regulate both the maturation of glutamatergic thalamocortical and GABAergic intracortical synapses. By controlling cortical inhibition, subplate neurons might also play a role in regulating cortical activity levels after GABAergic circuits have matured. Since subplate ablation prevents inhibitory maturation, maybe by directly activating GABAergic circuits, subplate activation is likely able to dampen cortical activity levels. Thus even temporary depression of subplate activity could lead to cortical hyperexcitability, which might underlie pathophysiological conditions.
-
10.
Lesioning subplate at a time when thalamocortical axons are present in layer 4, but before these projections have refined into a mature pattern, revealed a role for subplate neurons in thalamocortical patterning. The organizational pattern observed in the visual cortex is that of the ocular dominance columns (ODCs). These columns are formed by the segregation of thalamic afferents innervated by either eye into alternating bands of left eye or right eye dominance. In cat ODCs form during the postnatal period from an initially non-segregated state. Analysis of the ODCs following ablation of subplate neurons in V1 shows that subplate ablation prevents the formation of ODCs. This deficit in thalamocortical patterning is present even though both thalamic axons and their target neurons are present in layer 4. Thus subplate neurons are necessary for the patterned organization of the cerebral cortex.
-
11.
The function of inhibitory circuits is crucial for critical period plasticity in the visual cortex. Sensory manipulations that alter inhibition also result in impaired synaptic plasticity mechanisms that underlie critical period plasticity. Thus, there is a co-regulation of inhibition and critical period plasticity. Because subplate neurons play a crucial role in maturation of inhibition, it is likely that they also mediate cortical plasticity during the critical period.
-
12.
The disappearance of subplate neurons might ensure that certain processes, such as critical period plasticity, occur only once. While critical period plasticity might be distinct from adult plasticity by its extent and transience, the underlying mechanisms might be similar to processes underlying adult learning via attention based mechanisms. This attentional modulation of cortical circuits develops postnatally, thus subplate neurons could provide a circuit that enables large-scale cortical plasticity mechanisms before attention is functioning.
-
13.
Subplate activity can influence layer 4 synapses and enable thalamocortical strengthening to occur. In particular, subplate input to layer 4 can strongly depolarize layer 4 cells. Since subplate neurons are driven by thalamic activity, this subplate mediated depolarization of layer 4 cells occurs at the same time as direct thalamocortical input to layer 4 and may lead to a strengthening of thalamocortical synapses by associative long-term potentiation. The strength of the subplate to layer 4 connection is evidenced by the evoked disynaptic sinks in layer 4 during white matter stimulation. Therefore, subplate neurons can act somewhat like a “teacher” entraining layer 4 neurons to respond to appropriate thalamic inputs.
-
14.
Given the central role of subplate neurons in the maturation of cortical circuits, damage to subplate neurons at any point during development could lead to neurological diseases. Subplate neurons are particularly prone to injury (especially hypoxic-ischemic injuries) during development and are especially vulnerable at time points when injuries are associated with many neurodevelopmental disorders (second trimester). The enhanced vulnerability of subplate neurons may be due to their early maturation and therefore higher metabolic requirements. This vulnerability of subplate neurons might be more pronounced in infants born prematurely that are at a higher risk for neurodevelopmental disorders disorders as a large period of development occur ex utero. In animals, neonatal hypoxia damages subplate neurons and prevents normal critical period plasticity, supporting the idea that such injuries damage circuits needed for the development of normal tuning and plasticity. In humans, such hypoxic-ischemic injuries, especially in the second trimester are associated with various neurodevelopmental disorders such as cerebral palsy and epilepsy.
-
15.
Subplate ablation in animals is followed by a period of seizures indicating hyperactivity. These seizures develop a couple days after the time of ablation and thus are likely reflecting adjustments of the cortical network. The origin of these seizures is unclear. Seizures could be generating by depolarizing GABAergic activity (Kanold and Shatz, 2006) or alternatively be generated by glutamatergic activity that is not balanced appropriately by inhibitory GABAergic circuits. The different possible origins of seizures after subplate lesion are of clinical relevance. GABAA agonists can be used to treat seizures if they decrease firing probability. However, a GABAergic origin of seizures would also indicate that GABAA agonists would increase seizures instead of preventing them.
-
16.
Many neurodevelopmental disorders are characterized by abnormal neuronal activity, hyperexcitability, and learning impairments due to impaired inhibition, suggesting that altered inhibitory development underlies these disorders. Thus, a common outcome for early injuries and deprivations is that both alter inhibitory development, which in turn might alter critical period plasticity and normal development.
-
17.
In addition to subplate lesions, the activity of subplate neurons can be altered by neuromodulators such as GABA, acetylcholine and glycine. The subplate is also innervated by serotonergic fibers and subplate neurons selectively express progestin receptor. Thus, maternal or neonatal exposure to drugs (ranging from nicotine to sedatives and antidepressants) or hormones might alter subplate activity and thereby potentially disrupt cortical development. Therefore monitoring the status of subplate neurons in human infants is of high clinical relevance.
-
18.
Subplate neurons are present in the cerebral cortex of all mammals. Subplate neurons are more prominent in species with increased radial and tangential cortical connectivity such as cat, monkey and human, suggesting that subplate neurons might be needed for the establishment of more complex processing capabilities. The disappearance of subplate neurons over development suggests that their role is purely developmental. As discussed above, subplate neurons enable the functional maturation of cortical circuits. However, other areas in the brain (such as subcortical areas) seem mature without neurons equivalent to subplate neurons. Thus one can speculate that the role of subplate neurons might have to do with unique properties of the cerebral cortex. One hallmark of the cerebral cortex is complex interconnectivity and its ability to adjust its connectivity during early development in response to altered patterns of spontaneous and sensory inputs. This capability for rewiring of the cerebral cortex is greatly diminished after the critical period. Thus removing these enabling (or “teacher”) circuits is one way to ensure that plasticity occurs only once and only during early development and might allow the development of higher cognitive processes at later stages of cortical processing at later ages.
General Comments and Discussion
DeFelipe
Comment on points 1 and 18.
There is apparently a discrepancy between Kanold's article and that of Clancy and colleagues. Kanold states that subplate neurons contribute to cortical circuits that are only present during cortical development. However, as stated in the Remarks and main conclusions of the article by Clancy et al., and that of Luhmann et al., it is well-documented that some subplate cells persist in the white matter of the mature human and non-human cerebral cortex.
Espinosa and fairén
Comment on points 1 and 18.
Indeed, white matter neurons (carnivores and primates) and layer VIb neurons (rodents) are neither a small nor a residual population. Moreover, during development, some GABA neurons in the subplate are migratory neurons on their way to the cortical plate.
Clancy
Continuing DeFelipe, Clancy, Espinosa and Fairén.
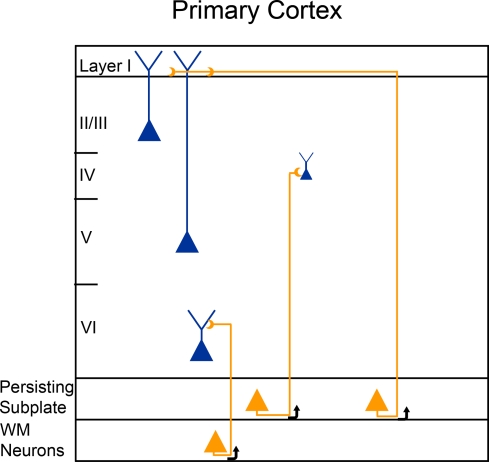
Connections from the persisting subplate cells in adult brains extend throughout the white matter and to all cortical layers (see Figure), including a stable projection that focuses specifically on cortical layer 1 (Clancy and Cauller, 1999; Clancy et al., 2001), evidence they retain (or reform) the earliest cortical developmental circuit. In layer 1 they converge with connections from non-specific thalamic and “top down” cortical projections, as well as callosal, brain stem nuclei, claustrum, zona incerta and nucleus basalis on the dendrites of cells in layers II/III and V. As a consequence, they may factor into one of the few neural correlates of primate conscious perception able to be isolated in a laboratory setting, the behaviorally relevant N1, an EEG deflection recorded following evoked sensory stimuli. Changes in the N1 component of a ERP accurately predict sensory perception in primates, a response that is initiated in cortical layer I (Cauller and Kulics, 1991; Cauller et al., 1998). Thus these connections, first formed in the developing subplate, are implicated as components of an interactive strategy for mature cognitive processing, within which sensory information from the environment may be primed, guided and interpreted (Koch and Davis, 1994; Cauller, 1995).
Figure.
Proposed persisting subplate/white matter connectivity circuits in mature mammalian cortex (Clancy and Cauller, 1999; Clancy et al., 2001; Friedlander and Torres-Reveron, 2009).
Kanold
Continuing DeFelipe, Clancy, Espinosa and Fairén.
While there are surviving subplate neurons in many species, the fractions of subplate neurons surviving is different between species, especially considering that there are more subplate neurons in higher species. A careful comparative accounting has to be done to see what is the percentage of subplate neurons and more importantly, which subplate neurons are eliminated/survive in different species. One key issue is the use of proper markers to identify subplate neurons at different ages that also do not label migrating neurons. For example, we can see and patch many subplate neurons at early ages that do not stain well with common markers (Zhao et al. 2009). Birth- dating might give the best estimate.
In addition, given the functional changes in the subplate (see Zhao et al., 2009) the role of the subplate in development can diminish (or change) simply based on the maturation of intrinsic membrane characteristics of subplate neurons and the maturation of the thalamocortical synapse.
As for subplate projections (figure above), functionally we find in young rodents excitatory subplate input to layer 4 and not layer 2/3 (Zhao et al., 2009) consistent with earlier work in cat by Friauf and Shatz (1991). Thus while subplate axons are present in other layers, their functional role might be limited. In addition the projection patterns might be different at different stages ofof subplate neurons might be changing over development.
Remarks and Main Conclusions
The subplate forms a transient layer in the developing cerebral cortex and consists of migratory and postmigratory neurons, dendrites, axons, growth cones, synapses and glial cells. The subplate is located between the intermediate zone and the cortical plate, which during further development differentiates into the neocortical layers II to VI.
Kostovic and Molliver were the first who identified the subplate as a distinct layer in the embryonic human cerebral cortex. Subsequently Rakic described this layer in the monkey neocortex. A subplate can be identified in all mammals although its relative thickness, developmental profile and persistence in adulthood vary among species. Anatomical data indicate an evolutionary difference in the ontogenetic fate of the subplate. In the rat and other rodents many subplate cells survive into adulthood forming layer VIb or VII.
Prominent species differences also exist in the relative thickness of the subplate, which increased during evolution. In humans the subplate develops to approximately six times the thickness of the cortical plate around 29 weeks of gestation (Mrzljak et al., 1990), whereas in rodents it remains a relatively thin layer during development (Uylings et al., 1990).
A substantial proportion of subplate cells are not born in the ventricular neuroepithelium, but instead originate in the medial ganglionic eminence and follow a tangential migratory route to their positions in the developing cortex.
Subplate cells represent a rather heterogeneous neuronal population according to their morphology, neurotransmitter identity and connectivity.
Subplate cells play important roles in the structural and functional organization of the cerebral cortex and in early necortical plasticity. Axons arising from subplate neurons pioneer the cortico-fugal pathway and have been proposed to form a cellular scaffold for guiding thalamocortical axons. Subplate neurons receive a transient synaptic input from “waiting” thalamic axons and early deletion of subplate neurons in kitten visual cortex prevents the segregation of thalamocortical axons within layer IV and the formation of ocular dominance columns. Furthermore, subplate cells regulate the maturation of GABAergic synaptic transmission and establish the balance between excitation and inhibition in the developing neocortical network.
Subplate neurons reveal a large variety of morphologies. Inverted pyramidal-like and horizontal cells as well as polymorphic neurons with different shapes and spiny or smooth dendrites have been classified as subplate neurons. Due to their earlier generation and more mature developmental stage, subplate neurons show a relatively extensive dendritic tree when compared to the more immature pyramidal neurons of the cortical plate. Descending dendrites from subplate neurons may invade the underlying intermediate zone and ascending dendrites may extend into the cortical plate. This morphological heterogeneity is accompanied by a large variation in immunoreactivity. Subplate neurons reveal markers for GABA or glutamate and may co-express various peptides. The morphological and neurochemical heterogeneity also explains, why various attempts failed to identify a specific marker for subplate neurons. Recently Hoerder-Suabedissen and coworkers succeeded in identifying a number of novel markers for murine subplate cells, which may soon allow the definition of different subpopulations of subplate neurons.
Subplate cells participate in local and long-distance axonal connections indicating that these neurons may function as local circuit as well as projection neurons. They show a dense axonal arborization within the subplate, but also project to the marginal zone/layer I and to the cortical plate, where they form axonal collaterals within layer IV.
In ferrets and cats, the majority of the subplate neurons projecting into the cortical plate reside in the upper half of the subplate and provide a glutamatergic synaptic input to the developing cortical plate, including the layer IV neurons. Long-distance axons from subplate neurons invade the thalamus during early stages of corticogenesis and form an axonal scaffold for the establishment of cortical efferent and afferent projections. Beside these local and long-distance projections arising from glutamatergic subplate neurons, GABAergic subplate cells also project to both neighbouring and more distant neocortical regions and form a cortico-cortical synaptic network. Since GABA may act as an excitatory neurotransmitter during early cortical development, the postsynaptic action of GABAergic subplate neurons may be also depolarizing.
Ultrastructural studies of subplate cells in various species have demonstrated symmetrical as well as asymmetrical synapses with relatively mature properties, indicating that subplate neurons receive GABAergic as well as glutamatergic synaptic inputs. As suggested by Kostovic and Rakic, glutamatergic inputs onto subplate neurons may arise from the thalamus and other neocortical areas, whereas GABAergic synaptic inputs may originate from GABAergic interneurons in the subplate. Thalamocortical synaptic contacts with spines and shafts of subplate neuron dendrites have been demonstrated in the neonatal ferret and a dense network of cortico-cortical fibers have been reported in the subplate of the embryonic mouse.
N-Methyl-d-aspartate (NMDA), α-amino-3-hydroxy 5 methylisoxazole-4-propionic acid (AMPA) and kainate receptors and the essential subunits for their receptor function have been demonstrated in the subplate of various species, suggesting the presence of functional glutamatergic synapses in subplate neurons. The expression of benzodiazepine binding sites, GABAA receptors and GABAA receptor subunits in the subplate indicate that functional GABAergic synaptic inputs should also be present in subplate neurons. These morphological, ultrastructural and immunohistochemical data are complemented by functional studies on the properties of the subplate and single subplate cells in different mammalian species.
In contrast to the heterogeneity in morphological and chemical appearance, electrophysiological recordings from single subplate neurons in rodents demonstrate rather homogeneous functional properties. Subplate neurons exhibit relatively uniform passive membrane properties.
In comparison to other neurons in the immature cerebral cortex, subplate cells also reveal the most mature properties in action potential characteristics and in the biophysical properties of voltage-dependent sodium and calcium currents. These observations have been made in developing rodent as well as in human cerebral cortex, indicating that these relatively mature functional properties enable subplate cells to transmit afferent neuronal activity faithfully to the developing cortical plate.
Intracellular labeling of single subplate cells with fluorescent dyes or biocytin revealed an extensive neuronal network of dye-coupled neurons in the subplate and cortical plate. In newborn rats, on average about nine neurons are dye-coupled to a single subplate cell and these gap junction coupled networks are often organized in a columnar manner.
Subplate cells are strongly coupled via electrical synapses and form a functional columnar syncytium with neurons located in the cortical plate. It is tempting to speculate that this early columnar organization results from the radial, column-like neuronal migration of newly-generated neurons into the developing neocortex, which is also controlled by gap junctional coupling.
Electrophysiological and optical imaging recordings further support the hypothesis that subplate neurons are well integrated in the developing cerebral cortex. In vitro intracellular recordings and current-source density analyses in late embryonic and early postnatal kitten visual cortex demonstrated that subplate neurons receive functional excitatory synaptic inputs from axons that course in the developing white matter. Subplate cells in newborn rat somatosensory cortical slices reveal a substantial amount of spontaneous postsynaptic currents (sPSCs) with different kinetics and pharmacological profile demonstrating that subplate neurons receive functional synaptic inputs mediated by AMPA, NMDA and GABAA receptors.
Beside a glutamatergic thalamocortical input with relatively mature functional properties (short delay, fast kinetics, reliable responses), subplate cells in newborn rodents receive additional intracortical synaptic inputs from various presynaptic sources. Local glutamatergic synaptic inputs arise from pyramidal neurons in the cortical plate and from glutamatergic subplate cells, which both activate postsynaptic AMPA/kainite and NMDA receptors. This intra-subplate glutamatergic input differs from the thalamocortical input. Anatomical studies in rodent and human immature cerebral cortex indicate that glutamatergic synaptic inputs most likely also arise from other neocortical sources via cortico-cortical connections, but the functional properties of these long-distance synaptic inputs onto subplate cells are currently unknown.
Subplate cells in rodents receive a GABAergic synaptic input from neighbouring GABAergic neurons located in the subplate and probably also in the cortical plate. However, as in other immature brain structures, this GABAergic input most likely has a pure excitatory postsynaptic effect.
Subplate neurons are not only well integrated in the developing cortical circuit, but during certain developmental periods they also receive a very selective input from neuromodulatory brain structures. Both in primates as well as in rodents the subplate is specifically innervated by cholinergic fibers arising from the basal forebrain and from monoaminergic inputs. The activation of postsynaptic nicotinic acetylcholine receptors elicits in subplate cells a marked depolarization which is largely mediated by the activation of α4/β2 receptors. The responsiveness to activation of muscarinic acetylcholine receptors (mAChR) is more complex and reveals a remarkable oscillatory discharge mode of subplate cells.
Gap junctional coupling, depolarizing GABA actions and a tonic non-synaptic GABA release contribute to the generation and maintenance of the cholinergic network oscillations.
Electrophysiological and calcium imaging recordings in acute neocortical slices from newborn rodents as well as calcium imaging data obtained in neocortical cell cultures indicate that GABAergic subplate cells play a central role in generating synchronous neuronal network activity. Voigt and coworkers estimated that a minimal number of two GABAergic subplate neurons per square millimeter are required for the occurrence of synchronous network activity. The unique structural and functional properties enable subplate cells to receive, synchronize and amplify the afferent and intrinsic synaptic inputs.
A large proportion of newly-generated GABAergic interneurons arising from the medial ganglionic eminence and pyramidal neurons from the ventricular zone must migrate through the subplate on their way to the developing cortical plate. Therefore the subplate may also have a profound influence on the migration pattern. GABAergic and glutamatergic subplate neurons partly release their neurotransmitter in a paracrine, non-synaptic manner, thereby regulating the neuronal migration pattern. Neurotransmitter release in the subplate may rise substantially during oscillatory network activity, thereby activating low affinity or extrasynaptic receptors causing alterations in neuronal migration.
On the basis of the currently available data we suggest the following model: During early cortical development, in most mammals before birth, subplate cells with relatively mature structural properties (elaborated dendritic tree, complex axonal projections, mature symmetrical and asymmetrical synapses) receive a functional glutamatergic synaptic input from specific thalamic nuclei and a selective input from neuromodulatory systems (e.g. cholinergic inputs from the basal forebrain). In rodents, subplate neurons are densely interconnected via electrical and chemical synapses and upon activation of muscarinic (and probably also other metabotropic) receptors discharge in repetitive ∼20-Hz bursts. GABAergic subplate neurons releasing GABA in a synaptic and tonic non-synaptic manner facilitate the generation of oscillatory network activity. Intra-subplate connections arising from glutamatergic subplate cells may boost the subplate activity in the 10–40-Hz frequency range. Subplate cells are dye-coupled to cortical plate neurons in a columnar manner and faithfully transmit synchronized 10–20-Hz network oscillations generated and amplified in the subplate to the cortical plate and marginal zone.
Elimination or interruption of the subplate prevents the generation of the subplate-driven synchronized activity patterns and disturbs the maturation of the columnar architecture. Under normal conditions and with further development, subplate cells disappear by apoptosis or transform into white matter interstitial cells or layer VIb (layer VII) neurons. In the normal mature cerebral cortex, subplate neurons have lost their capabilities to receive and amplify incoming neuronal activity and to generate synchronized network oscillations. However, as already suggested by Jones, disturbances in the pattern of programmed cell death in the subplate may cause a failure to establish normal patterns of connections in the overlying cerebral cortex, leading to long-term neurological deficits such as schizophrenia. Abnormal placement of (surviving subplate?) neurons in the white matter and atypical circuits have been observed in the prefrontal cortex of schizophrenic patients. It has been further suggested that subplate-like neurons may persist in cortical dysplasia and contribute to the manifestation of pharmacoresistant epilepsy in adults. Interestingly in the resectioned human tissue GABA application induced depolarizing postsynaptic responses and spontaneous GABAergic synaptic potentials even elicited action potentials.
General Comments and Discussion
DeFelipe
Comment on point 24.
It seems that most, if not all, interstitial neurons of the adult neocortical white matter are derived from the population of subplate neurons (e.g., Chun and Shatz, 1989). According to Luhmann and colleagues, these neurons have lost their capacity to receive and amplify incoming neuronal activity, and to generate synchronized network oscillations. This implies that during the transformation of subplate neurons to interstitial cells, the latter do not receive afferent inputs? Are there other anatomical, molecular and electrophysiological differences between interstitial neurons and subplate neurons? Are there any clues about what may be the function of interstitial cells?
Luhmann
Reply.
Torres-Reveron and Friedlander (Torres-Reveron and Friedlander, 2007) demonstrated in adolescent (P10–P20) rat visual cortex that surviving interstitial cells in the white matter and also subplate cells (layer VIb or VII) receive glutamatergic and GABAergic synaptic inputs. Studies in mature (>2-months rodents) cortex are lacking and it is unkown whether interstitial cells or subplate neurons in adolescent or adult cortex can synchronize network oscillations.
Interstitial cells and subplate neurons in adolescent rat cortex reveal similar passive and active electrophysiological properties (Torres-Reveron and Friedlander, 2007).
The exact function of interstitial cells is unknown, but it is tempting to speculate that surviving interstitial cells may play a pathophysiological role in subcortical or white matter heterotopia (Ackman et al., 2009; Croquelois et al., 2009; Meroni et al., 2009).
Rockland
Comment on points 8 and 9.
From this and other articles, information is emerging on the circuitry of long-distance projection neurons. A key point remains the identification of the principal postsynaptic targets. An interesting observation, emphasized in this article, is that GABAergic projecting neurons can have both local and long-distance targets.
Espinosa and fairén
Comment on points 4, 7, 10 and 8.
These cells coming from the medial ganglionic eminence are GABA neurons. Some of these cells in the subplate are just interneurons en route to the cortical plate (see point 22), and this should be taken into account for interpreting peptide expression in subplate neurons. Some GABA neurons may have neuronal interactions in subplate neuronal circuits, or may become persisting GABA neurons in the white matter or layer VIb.
Rockland
Comment on point 21.
It's fascinating that as little as two GABAergic subplate neurons per square millimeter can be effective for synchronous network activity.
Kanold
Continuing Rockland on point 21.
It is interesting that relatively small gap junction conductances can drive cortical oscillations. In addition it is very interesting that the morphological diversity in subplate neurons so far is not reflected in obvious physiological diversity.
Luhmann
Reply to Rockland point 21.
Yes and please see also my comment to point 8 of Clancy et al.
Remarks and Main Conclusions
Up to this date most of our knowledge regarding GABAergic neuron development is based on studies performed in rodents. However, differences in cortical GABAergic circuitry exist between rodents and primates. Although many types of cortical interneurons appear to be common to all species, some types in primates, like the double bouquet cells, or interneurons of cortical layer I, display more elaborate features. These types may represent evolutionary specializations. In addition, several studies suggest an increased proportion of cortical GABAergic neurons between rodents and primates. Such increases in the number and diversity of GABAergic neurons have been suggested to be closely related to the tremendous increased brain complexity that occurs during mammalian evolution.
Whereas there is a consensus for all species examined that principal glutamatergic neurons originate in the local ventricular (VZ) and subventricular zones (SVZ) of the dorsal telencephalon (pallium) and migrate along radial glia to their target cortical layer, this seems not to be the case for GABAergic neurons. In rodents, there are compelling evidences that cortical GABAergic neurons are not generated in these cortical (pallial) proliferative zones. They are produced in proliferative zones of the ventral (basal) telencephalon (subpallium), the ganglionic eminence (GE). From this region, newly born GABAergic neurons migrate tangentially into the cortex. Surprisingly, data obtained in the human and monkey cortex point out significant evolutionary changes with respect to the origin of cortical GABAergic neurons.
In rodents GABAergic neurons originate exclusively from the ganglionic eminence and migrate tangentially to the cortex. These migrating neurons follow several routes to reach their target regions. The major stream of tangentially migrating GABAergic neurons is present at the border of IZ and SVZ (lower part of IZ and upper part of SVZ). In addition, smaller streams of migrating cells are present in the subplate (SP) and the upper part of MZ.
There are compelling evidences that different subdomains within the GE generate different populations of cortical GABAergic neurons. The GE displays two major divisions: the medial and lateral GE. Recent findings demonstrated the existence of two additional components, the caudal and septal divisions. The vast majority of cortical GABAergic neurons are provided by the medial GE and to a lesser extent by the caudal GE. However, significant, although smaller numbers of cortical GABAergic neurons originate from the lateral GE, especially at later stages. Among the different subpopulations of GABAergic neurons, most of somatostatin-expressing neurons and all parvalbumin-containing cells derive from the medial GE whereas the majority of calretinin-containing cells and half of the NPY-expressing neurons arise from the caudal GE. Furthermore, somatostatin cells are primarily generated within the dorsal part of the medial GE whereas parvalbumin neurons are provided by the ventral part of the medial GE. Therefore, the GE is organized in molecularly different subdomains that produce different subpopulations of cortical GABAergic neurons.
In addition to spatial diversity in the origin of GABAergic neuron subpopulations, the generation of these different subgroups may occur at specific time windows. However, the precise spatio-temporal pattern of production, for the different GABAergic subtypes, has yet to be established.
In human and non-human primates, cortical GABAergic neurons are produced not only by the GE (as in rodents), but also massively by the proliferative zones of the dorsal telencephalon.
Both in human and macaque, neurogenesis of GABAergic neurons within the ventral and dorsal telencephalon occurs with distinct temporal profiles. Whereas at early stages of primate fetal development, GE is an exclusive site of origin for cortical GABAergic neurons, later on the dorsal telencephalic proliferative layers is the major source for these neurons.
The dorsal and ventral sites of neurogenesis produce different populations of cortical GABAergic neurons. A recent study from Fertuzinhos and coworkers investigate the proportion of different subpopulations of GABAergic neurons in brains of human fetuses or infants affected by holoprosencephaly. In holoprosencephaly, the numbers of cortical GABAergic neurons expressing nitric oxide synthase, neuropeptide Y, or somatostatin were significantly reduced in comparison to healthy infants. In contrast, calretinin-containing neurons were present in normal numbers as well as principal neurons. These findings show that, in human, nitric oxide synthase-, neuropeptide Y- and somatostatin-containing neurons originate from the GE whereas calretinin neurons are generated by the dorsal telencephalon.
The population of calretinin neurons, generated mainly in the dorsal telencephalon in human, corresponds to the population of GABAergic neurons whose proportion increases dramatically in primates and which displays primate-specific subtypes such as double bouquet cells. Altogether, these studies favor the hypothesis that dorsal production occurs principally as an answer to an increased evolutionary need for specific classes of cortical GABAergic neurons.
During tangential migration, prospective GABAergic neurons move parallel to the surface of the brain. They pass by a complex route between numerous growing axon bundles and cross regional boundaries. The length of their trajectory is greater than that of radially migrating neurons. Therefore, the mechanisms of cellular interactions enabling proper positioning and specification of GABAergic neurons are more complex than for radially migrating neurons. One can suggest that, dorsal production of cortical GABAergic neurons in primates might occur in order to facilitate migration routes through an expanding neocortex. An exclusive ventral telencephalic origin of cortical GABAergic neurons in primates would imply extremely long and complex migratory routes for such neurons. In keeping with this hypothesis, it might be expected that the percentage of GABAergic neurons produced dorsally will increase in larger brains in order to keep migratory routes shorter and simpler. However, the data obtained in ferret do not support this hypothesis. That is, the ferret brain displays a convoluted neocortex significantly larger compared to rodents, but shows limited cortical GABAergic neurons generated by the dorsal telencephalon such as described in mouse. Extensive dorsal production of GABAergic neurons in primates can be related to an increased need in number and/or specific types for GABAergic neurons in brains with more complex cortical circuitries.
The very massive dorsal telencephalic origin of cortical GABAergic neurons suggests distinct properties of dorsal telencephalic progenitors in primates compared to rodents. Interestingly, it was demonstrated that the proliferative behavior of cortical neuronal precursors during neurogenesis differs between rodents and primates. This leads to significant differences in morphology and function of their dorsal proliferative zones.
In the human embryo, in comparison to the rodent embryos, there is a dramatic increase in surface area and thickness of the VZ at the earliest stage of proliferation. In comparison to other species, the primate SVZ is larger and more complex. It displays a different type of cellular organization. There is a new, outer compartment within the SVZ, which displays a number of unique features, and exists much longer during development. So, it is not unreasonable to suggest that the increase in complexity, or even the specific cellular and laminar organization of dorsal proliferative zones in primates are significantly connected to the massive production of GABAergic neurons. However, no data to prove this hypothesis are currently available.
General Comments and Discussion
Rockland
It will be interesting to establish whether common spatial-temporal origins denote share functional characteristics.
Espinosa and fairén
The finding of interneuron precursors in the dorsal cortex by combination of Ki67 and GAD65 labeling is fascinating and significant.
Remarks and Main Conclusions
The preplate and its derivatives contain projection neurons, collectively named pioneer neurons, whose axonal arborizations establish the earliest cortico-fugal projection systems during cortical development. These neurons have traditionally been associated with the subplate. However, certain preplate neurons never associate with the subplate but instead with the marginal zone.
Proteins that are strongly expressed in pioneer neurons include the calcium-binding proteins calbindin (CB) and calretinin (CR) in rats. CB and CR co-label a single population of preplate pioneer neurons that end up in the marginal zone. In the preplate, another population is made up of CB+ cells interspersed among the CB+/CR+ cells. Most of these CB+-only cells end up in the subplate.
Surprisingly, neither of these two calcium-binding proteins labels early projecting neurons in mice. In mice, CB is a marker for most migrating GABA interneurons at the earliest stages of cortical histogenesis, including early migrating GAD67-GFP+ interneurons that arrived to the preplate before its partition. We found that in rats as well, CB and CR decorated putative migrating interneurons.
Thus, we searched for alternative immunohistochemical markers to try to identify the homologues in mice of the calcium-binding protein- expressing pioneer neurons of rats. We used antibodies to the neural cell adhesion and recognition molecules L1 and TAG-1/cntn2/axonin1, found previously to label pioneer cells in mice, in embryos of the two rodent species.
We have identified two subtypes of non-subplate pioneer neurons in rats: the cells of the first subtype co-express CB, CR and L1 and are situated in the upper part of the preplate before its partition. Cells of the second subtype are characterized by the expression of TAG-1 and are located slightly deeper than the previous population in the preplate. After the preplate partition, the neurons co-expressing CB, CR and L1 remain in the marginal zone whereas those expressing TAG-1 become localized in the upper cortical plate. In mice, we identified two subtypes of non-subplate pioneer neurons that express either L1 or cntn2 (TAG-1). We propose that these two subtypes of mouse cells are homologues of the two subtypes of non-subplate pioneer neurons of the rat. The anatomical distribution of these neuron populations is identical in rat and mouse.
The T-box transcription factor Tbr1 is expressed early after the differentiation of cortical progenitors, and is functionally important for corticogenesis. Tbr1 is expressed by Cajal-Retzius cells, subplate cells and glutamatergic neurons, but not by GABAergic cells. Since we characterized the CB+/CR+ cells of the rat preplate and marginal zone as projecting neurons, we wished to confirm that these cells co-expressed the T-box transcription factor Tbr1. This was indeed the case, and we additionally observed that Tbr1 was expressed in the mouse preplate by pioneer neurons both of the L1 and the TAG-1 subtypes.
Apparent differences in anatomy and tempo of deployment of early axonal projections of subplate vs. non-subplate neurons may result from technical factors; that is, the different advantages and pitfalls of the axonal tract-tracing methods utilized in different studies. Here, we decided to trace axonal projections by taking advantage of the chemical properties of the early neurons, as detected by immunohistochemistry. In fortunate cases, this approach produced selective staining of different neuronal populations together with high resolution anatomical detail of their projecting axons.
The different populations of non-subplate pioneer neurons differ in their axonal projections. Non-subplate pioneer neurons of the L1 subtype project to the ganglionic eminences and the anterior preoptic area, but avoid following the internal capsule that leads to the dorsal thalamus. Thus, we can conclude there is no projection of neurons of L1 subtype to the dorsal thalamus. The projections of the neurons of the TAG-1 subtype end up at the lateral parts of the ganglionic eminences. A corticothalamic TAG-1+ projection becomes apparent much later, in postnatal mice.
A major discrepancy among the various studies of the early cortico-fugal connections concerns whether or not subplate axons are considered as reaching the dorsal thalamus, or, according to more recent studies, these subplate axons (and axons deriving from the marginal zone) are considered as entering only the part of the internal capsule that traverses the ganglionic eminences. However, subplate axons seem not to project to the dorsal thalamus at the initial steps of corticogenesis. If this is so, the subcortical distributions of subplate axons are similar to that of the non-subplate axons described here.
A caveat: both adhesion molecules TAG-1/cntn2 and L1 are simultaneously expressed by different neurons of the marginal zone and the subplate. This makes it hard to distinguish which descending cortico-fugal projections originate from neurons located in each one of these two transitory cortical compartments, or from neurons residing in both places. Unfortunately, this seems to somewhat limit the usefulness of most neurochemical markers of the marginal zone and the subplate described so far.
The diversified group of early cortical neurons with their diversified axonal projections that we have considered here has not yet been identified in species other than rodents. However, further study is highly relevant to advancing our understanding of cerebral cortex development. Not only would this support the development of functional murine experimental models, but there is the promise of extending these observations to non-human primates and humans. To emphasize the importance of such a comparative approach, it may suffice to consider two examples; namely, the pioneer plate described in humans by Meyer et al. (2000) and the predecessor neurons described in the human preplate by Bystron et al. (2006).
We have described here a close anatomical relationship of pioneer L1+ and TAG-1+ axons with migrating GABA interneurons. Medial L1+ axons coincide with a massive stream of migrating interneurons during their exit from the ganglionic eminences to the pallium, along the intermediate zone of the cortex. Other less abundant interneurons migrate in a compartment located more laterally, where they acquire spatial relationships with L1+ and TAG-1+ axons. TAG-1+ axons also accumulate below the subplate, and are accompanied by migrating interneurons in this territory.
Our observations strongly suggest that L1+ and TAG1+ cortico-fugal axons could be a substrate for migration of interneurons along the cortical intermediate zone, even though a role for L1 in the migration of cortical interneurons has been excluded in in vitro perturbation experiments. The role of TAG-1 in interneuron migration is controversial, although this molecule is active in the control of tangential migration of other neuron cohorts.
General Comments and Discussion
Rockland
Comment on point 5.
Do you think that L1 and TAG-1 are actively involved in the spatial sorting of these subtypes; that is, neurons co-expressing CB, CR, and L1 in the marginal zone vs. a deeper location, in the upper cortical plate, for TAG-1 expressing neurons?
Espinosa and fairén
Reply.
This is an attractive idea, and of course something of that sort could be expected on the grounds of the exquisite specificity of the two markers for distinct projection neuron populations. Our work in progress seems to indicate that the absence of L1 in knockout mice embryos affects the distribution of early Tbr1+ neurons in the cortical anlage.
Espinosa and fairén
Comment on point 11.
We need novel data about molecular markers of pioneer cells or predecessor cells described in humans, in order to determine (1) if they are unique to the human brain or may have homologues in other mammals and (2), how the patterns of early cortico-fugal projections are shared in mammalian evolution.
Espinosa and fairén
Comment on point 13.
The observation that migrating GABAergic interneurons contact certain sets of cortico-fugal axons does not directly imply a function for either L1 or TAG-1 in the control of these migrations, because experimental proof is still lacking. It is the authors’ opinion, however, that the issue of axonophilic migration of certain sets of GABAergic interneurons merits further analyses. Interneurons migrating along the marginal zone do not seem to use any axonal system as scaffold.
Remarks and Main Conclusions
One of the most fundamental features of the brain is its plasticity, a constant interplay between neural structure, function and experience. The scope and complexity of neuroplasticity appear to increase during evolution in parallel with the enlargement of brain through encephalization, corticalization and gyrification. Thus, plasticity is likely the foundation of the so-called “higher brain functions” (e.g., learning, memory, decision making, or intelligence) that are mostly sophisticated in humans.
In general, the associative cortical areas together with the amygdala and hippocampal formation, which are greatly expanded during late mammalian evolution, are the anatomic niches for many high-level cognitive functions. Greater neuroplasticity is likely inherent with higher vulnerability to environmental and internal insults. In line with this notion, cellular deficits and structural changes in the associative cortical and limbic areas are associated with some neurological or neuropsychiatric disorders (e.g., mode disorders, schizophrenia, epilepsy, Alzheimer's disease) in the adolescent, adult and aged human populations.
Neuroplasticity is a complex process involving structural modulations at synaptic, neuronal and circuitry/pathway levels. Of particular interest, formation of new neurons in the adult brain has been recently recognized as a key substrate for neuroplasticity and cognition. For instance, adult neurogenesis in the forebrain subventricular and subgranular zones (SVZ, SGZ) appears to be essential for olfaction and hippocampus-dependent learning and memory in rodents.
Newly-generated neurons in the SVZ and SGZ express a set of immature neuronal markers including doublecortin (DCX+) and polysialylated neural cell adhesion molecule (PSA-NCAM). Concurrent with morphological development, these new neurons differentiate through a correlated process of downregulation of the above-mentioned immature markers and upregulation of common (e.g., neuron-specific nuclear protein, NeuN) or specific terminal neuronal markers, thus eventually become fully integrated into functional neuronal circuitries in the adult brain.
Besides the SVZ/SGZ, a novel population of DCX+ cells coexpressing other immature neuronal markers has been recently characterized in the cerebral cortex of guinea pigs, rabbits, cats and primates from young to mid-age adulthood. These DCX+ cells are more common in the associative relative to primary cortical areas, and appear to develop into interneuron subgroups in guinea pigs and cats. We show here in rhesus monkeys that DCX+ cells persist into advanced ages in the associative frontal and temporal lobe cortex and amygdala, even in very old animals with substantial cerebral amyloid pathology. In contrast, DCX+ cells are rare in the hippocampus by midage.
Recent studies by our group and others have gathered substantial information on cortical DCX+ cells or alike in various mammalian species, which now allows a comparison between these novel cortical cells and the conventional adult-born neuronal populations in SVZ and SGZ. Taking the newly-generated SGZ immature neuronal population as an example, we attempted to update relevant features of cortical and hippocampal DCX+ cells. Similar properties of these two populations include that both groups: (1) express the very same set of immature neuronal markers including DCX, PSA-NCAM, neuron-specific β-tubulin-III (TuJ1) and Hu; (2) exhibit a diverse variation in somal size and shape, nuclear appearance in bisbenzimide stain and complexity of neuritic processes, all suggesting cell growth and morphological maturation; (3) display a pattern of transient expression of immature neuronal markers that overlap partially with the emergence of mature neuronal markers but correlate with morphological development; (4) may arrange as tightly-apposed cell clusters and/or as migratory chains, suggestive of certain neuroblastlike behavior or property; (5) reduce in number with age in all studied mammals; (6) might be modulated by some antidepressants at least according to rodent studies.
The most confusing issue regarding cortical DCX+ cells is whether they are generated prenatally, postnatally or even throughout adult life. Several groups report a low rate of BrdU incorporation into DCX+ cells in the piriform or temporal lobe cortex in small laboratory rodents and primates. In contrast, others suggest that this population of cortical cells is produced prenatally. With this regard, the current finding of persistent occurrence of DCX+ cells in the cortex and amygdale virtually to the end of life in non-human primates is very puzzling.
The arrangement of cortical DCX+ cells as extended tangential migratory chains and expanded/distorted migratory apparatus in layers I/II in mid-age and aged primates is of interest. Cortical DCX+ cells in young adult cats and monkeys appear to mostly migrate from layer II to deeper layers. Thus, the increased tendency of tangential cell migration in older animals might implicate certain type of age-related alteration in the distribution of cortical DCX+ cells.
Previous studies suggest that the radial migration of newly-generated hippocampal neurons might be impaired in aged rodents, dogs and monkeys. Thus, DCX+ cells in aged dentate gyrus tend to retain at the SGZ with their long somal diameter parallel to the GCL. In contract, dentate DCX+ cells in younger animals migrate across the GCL during their morphological maturation. Thus, the extended tangential migration and distorted clusterization of DCX+ cells around layers I and II in aged monkeys could potentially reflect certain deficit of migration or dispersion/descending of these cells in the cortex.
Little is currently known about the functional implication for a life-long presence of putative immature and developing neurons in mammalian associative cerebral cortex and amygdala. Giving their potential GABAergic fate, it seems plausible that these novel cells might be involved in interneuron plasticity under physiological conditions, and perhaps interneuron dysfunction under certain disease conditions. Recent studies suggest that interneuron deficits might relate to the etiology of certain neuropsychiatric diseases. It appears that bipolar disorder, major depression and schizophrenia are associated with reduced GABAergic interneurons and/or altered inhibitory neurotransmission in the prefrontal cortex.
A role for impaired neurogenesis in cognitive decline during aging and in Alzheimer's disease has been lately proposed according to studies of adult-born neuronal populations in the hippocampus and SVZ. We observe diminished DCX+ subgranular cells in mid-age and aged monkeys, consistent with other reports that hippocampal neurogenesis is dramatically reduced around mid-age in most mammals. Our current study however reveals a remarkably intriguing fact that putative immature cortical neurons persist into very old age in non-human primates, in sharp contrast to a great loss of hippocampal neurogenesis well before the onset of senescence. Thus, there exists an extended time window for a potential involvement of cortical and amygdalar DCX+ cells in aging-related changes in neuronal plasticity and perhaps cognitive decline in non-human primates.
General Comments and Discussion
Rockland
Comment on point 5.
Can you make any finer demarcation of where in the “associative frontal and temporal lobe cortex” the DCX+ cells can be found? In particular, both lobes have neocortical and limboid components (respectively, dorsolateral frontal and lateral temporal vs. orbitofrontal and entorhinal areas). Any correlation or trend?
Yan
Reply.
We did not correlate the pattern of DCX+ cells with Brodmann areas in part because there are age-related changes in area distribution. In addition, the distribution appeared to be more regional than area-specific. In general, we found that labeled cells are more numerous ventrally across the anterior-posterior cerebral dimension, which is particularly clear in young adults. In aged monkeys, DCX+ cells remain fairly numerous in temporal lobe areas, both the paleo- and neo-cortical areas. In addition, a small number of these cells also persist in the medial and ventral prefrontal cortex including the medial and lateral orbital gyri, as well as around the ventral portion of the occipital lobe cortex.
References
- Ackman J. B., Aniksztejn L., Crépel V., Becq H., Pellegrino C., Cardoso C., Ben-Ari Y., Represa A. (2009). Abnormal network activity in a targeted genetic model of human double cortex. J. Neurosci. 29, 313–327 10.1523/JNEUROSCI.4093-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifazi P., Goldin M., Picardo M. A., Jorquera I., Cattani A., Bianconi G., Represa A., Ben-Ari Y., Cossart R. (2009). GABAergic hub neurons orchestrate synchrony in developing hippocampal networks. Science 326, 1419–1424 10.1126/science.1175509 [DOI] [PubMed] [Google Scholar]
- Bystron I., Rakic P., Molnár Z., Blakemore C. (2006). The first neurons of the human cerebral cortex. Nat. Neurosci. 9, 880–886 10.1038/nn1726 [DOI] [PubMed] [Google Scholar]
- Cauller L. (1995). Layer I of primary sensory neocortex: where top-down converges upon bottom-up. Behav. Brain Res. 71, 163–170 10.1016/0166-4328(95)00032-1 [DOI] [PubMed] [Google Scholar]
- Cauller L. J., Clancy B., Connors B. W. (1998). Backward cortical projections to primary somatosensory cortex in rats extend long horizontal axons in layer I. J. Comp. Neurol. 390, 297–310 [DOI] [PubMed] [Google Scholar]
- Cauller L. J., Kulics A. T. (1991). The neural basis of the behaviorally relevant N1 component of the somatosensory-evoked potential in SI cortex of awake monkeys: evidence that backward cortical projections signal conscious touch sensation. Exp. Brain Res. 84, 607–619 10.1007/BF00230973 [DOI] [PubMed] [Google Scholar]
- Chun J. J., Shatz C. J. (1989). Interstitial cells of the adult neocortical white matter are the remnant of the early generated subplate neuron population. J. Comp. Neurol. 282, 555–569 10.1002/cne.902820407 [DOI] [PubMed] [Google Scholar]
- Clancy B., Cauller L. J. (1999). Widespread projections from subgriseal neurons (layer VII) to layer I in adult rat cortex. J. Comp. Neurol. 407, 275–286 [DOI] [PubMed] [Google Scholar]
- Clancy B., Silva-Filho M., Friedlander M. J. (2001). Structure and projections of white matter neurons in the postnatal rat visual cortex. J. Comp. Neurol. 434, 233–252 10.1002/cne.1174 [DOI] [PubMed] [Google Scholar]
- Croquelois A., Giuliani F., Savary C., Kielar M., Amiot C., Schenk F., Welker E. (2009). Characterization of the HeCo mutant mouse: a new model of subcortical band heterotopia associated with seizures and behavioral deficits. Cereb. Cortex 19, 563–575 10.1093/cercor/bhn106 [DOI] [PubMed] [Google Scholar]
- Friauf E., Shatz C. J. (1991). Changing patterns of synaptic input to subplate and cortical plate during development of visual cortex. J. Neurophysiol. 66, 2059–2071 [DOI] [PubMed] [Google Scholar]
- Friedlander M. J., Torres-Reveron J. (2009). The changing roles of neurons in the cortical subplate. Front. Neuroanat. 3:15 doi: 10.3389/neuro.05.015.2009. 10.3389/neuro.05.015.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Pinilla F., Cotman C. W. (1992). Transient lesion-induced increase of basic fibroblast growth factor and its receptor in layer VIb (subplate cells) of the adult rat cerebral cortex. Neuroscience 49, 771–780 10.1016/0306-4522(92)90355-6 [DOI] [PubMed] [Google Scholar]
- Gulyas A. I., Hajos N., Katona I., Freund T. F. (2003). Interneurons are the local targets of hippocampal inhibitory cells which project to the medial septum. Eur. J. Neurosci. 17, 1861–1872 10.1046/j.1460-9568.2003.02630.x [DOI] [PubMed] [Google Scholar]
- Hanganu I. L., Okabe A., Lessmann V., Luhmann H. J. (2009). Cellular mechanisms of subplate-driven and cholinergic input-dependent network activity in the neonatal rat somatosensory cortex. Cereb. Cortex 19, 89–105 10.1093/cercor/bhn061 [DOI] [PubMed] [Google Scholar]
- Hogan D., Berman N. E. (1992). The development of neuropeptide Y immunoreactive neurons in cat visual cortical areas. Brain Res. Dev. Brain Res. 67, 343–369 10.1016/0165-3806(92)90236-P [DOI] [PubMed] [Google Scholar]
- Jinno S., Klausberger T., Marton L. F., Dalezios Y., Roberts J. D., Fuentealba P., Bushong E. A., Henze D., Buzsaki G., Somogyi P. (2007). Neuronal diversity in GABAergic long-range projections from the hippocampus. J. Neurosci. 27, 8790–8804 10.1523/JNEUROSCI.1847-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinno S., Kosaka T. (2002). Immunocytochemical characterization of hipocamposeptal projecting GABAergic nonprincipal neurons in the mouse brain: a retrograde labeling study. Brain Res. 945, 219–231 10.1016/S0006-8993(02)02804-4 [DOI] [PubMed] [Google Scholar]
- Jinno S., Kosaka T. (2004). Parvalbumin is expressed in glutamatergic and GABAergic corticostriatal pathway in mice. J. Comp. Neurol. 477, 188–201 10.1002/cne.20246 [DOI] [PubMed] [Google Scholar]
- Kanold P. O., Shatz C. J. (2006). Subplate neurons regulate maturation of cortical inhibition and outcome of ocular dominance plasticity. Neuron 51, 627–638 10.1016/j.neuron.2006.07.008 [DOI] [PubMed] [Google Scholar]
- Koch C., Davis J. L. (1994). Large-scale neuronal theories of the brain. Cambridge, MA, MIT Press [Google Scholar]
- Kristt D. A. (1979). Development of neocortical circuitry: histochemical localization of acetylcholinesterase in relation to the cell layers of rat somatosensory cortex. J. Comp. Neurol. 186, 1–15 10.1002/cne.901860102 [DOI] [PubMed] [Google Scholar]
- Marin-Padilla M., Marin-Padilla T. M. (1982). Origin, prenatal development and structural organization of layer I of the human cerebral (motor) cortex. A Golgi study. Anat. Embryol. 164, 161–206 10.1007/BF00318504 [DOI] [PubMed] [Google Scholar]
- McDonald C. T., Burkhalter A. (1993). Organization of long-range inhibitory connections with rat visual cortex. J. Neurosci. 13, 768–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meroni A., Galli C., Bramerio M., Tassi L., Colombo N., Cossu M., Lo R. G., Garbelli R., Spreafico R. (2009). Nodular heterotopia: a neuropathological study of 24 patients undergoing surgery for drug-resistant epilepsy. Epilepsia 50, 116–124 10.1111/j.1528-1167.2008.01717.x [DOI] [PubMed] [Google Scholar]
- Meyer G., Schaaps J. P., Moreau L., Goffinet A. M. (2000). Embryonic and early fetal development of the human neocortex. J. Neurosci. 20, 1858–1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mrzljak L., Uylings H. B., Van Eden C. G., Judas M. (1990). Neuronal development in human prefrontal cortex in prenatal and postnatal stages. Prog. Brain Res. 85, 185–222 10.1016/S0079-6123(08)62681-3 [DOI] [PubMed] [Google Scholar]
- Okhotin V. E., Kalinichenko S. G. (2003). Subcortical white matter interstitial cells: their connections, neurochemical specialization, and role in the histogenesis of the cortex. Neurosci. Behav. Physiol. 33, 177–194 10.1023/A:1021778015886 [DOI] [PubMed] [Google Scholar]
- Peters A., Payne B. R., Josephson K. (1990). Transcallosal non-pyramidal cell projections from visual cortex in the cat. J. Comp. Neurol. 302, 124–142 10.1002/cne.903020110 [DOI] [PubMed] [Google Scholar]
- Reep R. L. (2000). Cortical layer VII and persistent subplate cells in mammalian brains. Brain Behav. Evol. 56, 212–234 10.1159/000047206 [DOI] [PubMed] [Google Scholar]
- Reep R. L., Goodwin G. S. (1988). Layer VII of rodent cerebral cortex. Neurosci. Lett. 90, 15–20 10.1016/0304-3940(88)90779-3 [DOI] [PubMed] [Google Scholar]
- Soriano E., Cobas A., Fairén A. (1986). Asynchronism in the neurogenesis of GABAergic and non-GABAergic neurons in the mouse hippocampus. Brain Res. 395, 88–92 10.1016/0165-3806(86)90134-3 [DOI] [PubMed] [Google Scholar]
- Soriano E., Cobas A., Fairén A. (1989). Neurogenesis of glutamic acid decarboxylase immunoreactive cells in the hippocampus of the mouse. I: Regio superior and regio inferior. J. Comp. Neurol. 281, 586–602 10.1002/cne.902810408 [DOI] [PubMed] [Google Scholar]
- Tomioka R., Okamoto K., Furuta T., Fujiyama F., Iwasato T., Yanagawa Y., Obata K., Kanako T., Tamamamki N. (2005). Demonstration of long-range GABAergic connections distributed throughout the mouse neocortex. Eur. J. Neurosci. 21, 1587–1600 10.1111/j.1460-9568.2005.03989.x [DOI] [PubMed] [Google Scholar]
- Tomioka R., Rockland K. S. (2007). Long-distance corticocortical GABAergic neurons in the adult monkey white and gray matter. J. Comp. Neurol. 505, 526–538 10.1002/cne.21504 [DOI] [PubMed] [Google Scholar]
- Torres-Reveron J., Friedlander M. J. (2007). Properties of persistent postnatal cortical subplate neurons. J. Neurosci. 27, 9962–9974 10.1523/JNEUROSCI.1536-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth K., Freund T. F. (1992). Calbindin D28k-containing nonpyramidal cells in the rat hippocampus: their immunoreactivity for GABA and projection to the medial septum. Neuroscience 49, 793–805 10.1016/0306-4522(92)90357-8 [DOI] [PubMed] [Google Scholar]
- Uylings H. B. M., Van Eden C. G., Parnavelas J. G., Kalsbeek A. (1990). The prenatal and postnatal development of rat cerebral cortex. In The Cerebral Cortex of the Rat, Kolb B., Tees R. C., eds (Cambridge, MIT Press: ), pp. 35–76 [Google Scholar]
- Voigt T., Opitz T., De Lima A. D. (2001). Synchronous oscillatory activity in immature cortical network is driven by GABAergic preplate neurons. J. Neurosci. 21, 8895–8905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C., Kao J. P., Kanold P. O. (2009). Functional excitatory microcircuits in neonatal cortex connect thalamus and layer 4. J. Neurosci. 29, 15479–15488 10.1523/JNEUROSCI.4471-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]