Abstract
Compromised memory functioning is one of the commonly reported cognitive sequelae seen following mild traumatic brain injury (mTBI). Diffusion tensor imaging (DTI) has been shown to be sufficiently sensitive at detecting early microstructural pathological alterations after mTBI. Given its location and shape, the cingulate, which is comprised of the cingulate gyrus (gray matter) and cingulum bundles (white matter), is selectively vulnerable to mTBI. In this study we examined the integrity of cingulum bundles using DTI, and the relationship between cingulum bundles and memory functioning. Twelve adolescents with mTBI and 11 demographically-matched healthy controls were studied. All participants with mTBI had a Glasgow Coma Scale score of 15, and were without intracranial findings on CT scan. Brain scans were performed on average 2.92 days post-injury, and all participants were administered the Verbal Selective Reminding Test (VSRT), an episodic verbal learning and memory task. Participants with mTBI had a significantly lower apparent diffusion coefficient (ADC) bilaterally than controls (p < 0.001). Despite the marginal significance of the group difference in fractional anisotropy (FA), the effect size between groups was moderate (d = 0.66). Cognitively, healthy controls performed better than the TBI group on immediate and delayed recall; however, the difference did not reach statistical significance. In the mTBI group, FA of the left cingulum bundle was significantly correlated with 30-min delayed recall (r = −0.56, p = 0.05). A marginally significant correlation was found between ADC of the left cingulum bundle and the total words of immediate recall (r = 0.59, p = 0.07). No significant correlation was found between DTI metrics and memory functioning for the control group. These preliminary findings indicate that cingulate injury likely contributes to the cognitive sequelae seen during the early phase post-mTBI.
Key words: diffusion tensor imaging, learning and memory, magnetic resonance imaging, pediatric brain injury, traumatic brain injury
Introduction
Memory or attentional impairment and slowed processing of information are the most frequent cognitive sequelae seen during the first 1–3 months after mild traumatic brain injury (mTBI) (Belanger et al., 2007; Kurca et al., 2006; Malojcic et al., 2008). Advances in magnetic resonance imaging (MRI), especially diffusion tensor imaging (DTI), have shown that DTI metrics, including fractional anisotropy (FA) and apparent diffusion coefficient (ADC), are sufficiently sensitive to detect early pathological alterations associated with injury (Alexander et al., 2007; Arfanakis et al., 2002; Nakayama et al., 2006; Xu et al., 2007) during the first 2 weeks after mTBI (Lo et al., 2009; Topal et al., 2008). Typically, higher FA and lower ADC are associated with preserved white matter tract integrity, and decreased FA and increased ADC in chronic TBI have been attributed to white matter injury and degeneration. However, in more acute stages of mTBI, a reversal of this pattern has been demonstrated, where increased FA and decreased ADC may be indicative of transient cytotoxic edema (Bazarian et al., 2007; Chu et al., in press; Wilde et al., 2008).
In moderate-to-severe TBI, the cingulate, which is comprised of both the gray matter of the cingulate gyrus (CG), as well as the underlying white matter tract of the cingulum bundle (CB), may be especially vulnerable to injury because of its location adjacent to the falx cerebri (Gean, 1994), including secondary effects from frontotemporal damage that disrupt afferent and efferent cingulate connections (Bigler, 2007). Furthermore, because of its long coursing nature, the CB is susceptible to traumatic axonal injury, and this injury is detectable by DTI in studies involving mild-to-severe TBI at a chronic phase (Bendlin et al., 2008; Kraus et al., 2007; Rutgers et al., 2008). Given the potential role that the CB plays in the emergence of cognitive and neuropsychiatric sequelae following mTBI (Stein and McAllister, 2009), we hypothesized that acute post-injury DTI measures of FA and ADC would be sensitive in detecting pathological changes within the CB that would relate to cognitive status in patients with mTBI.
Methods
Some results from this sample of participants (i.e., DTI metrics) have been reported in part by Wilde and associates (2008). Twelve (six males, six females) adolescents diagnosed with concussion or mTBI by a trauma physician were recruited for the study. All participants with mTBI had a Glasgow Coma Scale (GCS) score of 15 on arrival in the emergency department of Texas Children's Hospital (TCH), and had only brief loss of consciousness (<10 min). Although at least two participants had a period of memory disturbance, these data were not available for all participants. No participants had intracranial findings on computed tomography (CT) or demonstrated post-traumatic amnesia as assessed by the Galveston Orientation and Amnesia Test (Levin et al., 1979) at the time of clinical testing or scanning. However, 91% of the participants with mTBI reported post-concussive symptoms at the time of assessment. The mechanism of injury included motor vehicle crashes, sports, falls, and assaults. The mTBI group had a mean age of 15.3 years (±1.2 years; range 14–17 years), with 9.3 years of education (±1.7 years; range 7–12 years). The control group was comprised of 11 (six males, five females) healthy adolescents and young adults from the local community. The mean age and years of education for the control group were 15.8 (±1.8 years; range 14–19 years) and 10.0 (±1.8 years; range 8–12 years) respectively, which did not significantly differ from the mTBI group. All participants in both groups were right-hand dominant, and no participant had a history of previous brain injury, neurologic or psychiatric condition, or diagnosis of learning disability. DTI data were acquired with a Philips 3T Achieva scanner (Philips, Cleveland, OH) at TCH. Participants with mTBI were scanned on average 2.92 days post-injury (range 1–6 days). All participants were also administrated the six-trial version of the Verbal Selective Reminding Test (VSRT) (Larrabee et al., 2000). This is an episodic verbal learning and memory test in which participants are asked to repeatedly recall a list of words. Unlike other verbal list learning measures in which participants are provided the entire list after each trial, the administration of the VSRT requires examiners to give only words that were omitted during the previous trial. For the current analyses, the total number of words on immediate recall across all trials, and the number of words for the 30-min delayed recall were assessed. Informed consent was obtained from each study participant prior to participation in the study. Examiners performing neuropsychological assessments were blinded to the DTI results, and individuals performing the DTI analyses were blinded to the neuropsychological test results.
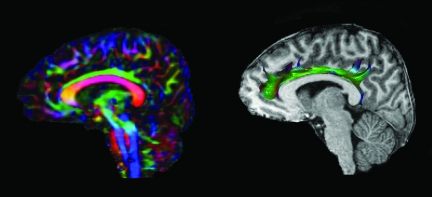
For this study, transverse multi-slice spin echo, single shot, echoplanar imaging sequences were applied (63,180.0 msec TR, 51 msec TE, 2.0-mm slices, 0-mm gap). Diffusivities were measured along 30 directions using an electrostatic gradient model (Jones et al., 1999) (number of b-value = 2, low b-value = 0, and high b-value = 1000 sec/mm2). To improve the robustness of the data, two acquisitions were employed and averaged using Philips diffusion affine registration program (Netsch, 2001). Each acquisition comprised of 70 slices took approximately 5 min. Prior to computing FA maps with the Philips fiber tracking 4.1V3 Beta 2 software, shear and eddy current distortion and head motion artifact were corrected using the Philips PRIDE-registration tool (Netsch, 2001). In all cases the data quality was considered excellent (Fig. 1). The bilateral CB were analyzed in the parasagittal plane individually using the automated Philips three-dimensional (3-D) fiber-tracking program, which examined fiber tracks passing through the regions of interest (ROI). Using the automatic 3-D ROI algorithm function, two to three seed points were placed linearly along the CB in the right parasagittal plane, usually within areas that appeared brightest on the FA color map. The resulting typical left CB is illustrated in Figure 1. The mean FA and ADC were automatically calculated by the software. The algorithm for fiber tracking is based upon the fiber assignment by continuous tracking method (Mori et al., 1999). If the FA in the voxels was less than 0.2, or if the angle between adjacent voxels was >7 degrees, fiber tracking was automatically terminated. To ensure intra- and inter-rater reliability, each ROI was separately analyzed twice by two independent raters, with excellent intra- and inter-rater reliability (range 0.975–1.000) being achieved.
FIG. 1.
Group Mean, Standard Deviation, and Effect Size for DTI Metrics in the Cingulum Bundles and Memory Task Performance
Statistical analysis
Student's t-tests were used to evaluate group differences for FA and ADC of the CB and memory performance; as this was an exploratory pilot study, correction for multiple comparisons was not necessary. To evaluate the relation between DTI indices for the CB and memory functioning, Spearman's rho correlation was utilized.
Results and Discussion
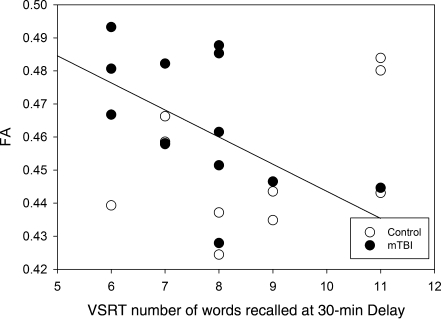
The results are summarized in Table 1. DTI findings demonstrated that in comparison to the healthy control group, the mTBI group had significantly lower ADC in both the right and left CB (all p < 0.0001). While a medium effect size (d = 0.66) for differences in CB FA was present between the groups, the difference was only marginally significant given the sample size. With regard to cognitive functioning, the mTBI group performed below the healthy controls on VSRT total words of immediate recall and delayed recall; however, the effect size for between-group differences was small to medium (d = 0.41 and 0.53), and the group difference did not reach statistical significance. It would be premature to draw conclusions regarding the clinical significance of these differences given the current sample size. It should be noted that the relations between DTI indices of CB and memory performance differed based on laterality. In the mTBI group, FA of the left CB significantly correlated with 30-min delayed recall (r = −0.56, p = 0.05; Fig. 2), where increased FA was related to poorer memory performance. A marginally significant correlation was found between ADC of the left CB and the total words of immediate recall (r = 0.59, p = 0.07), with reduced ADC associated with compromised memory functioning. No significant correlation was found between the DTI metrics and the VSRT variables in the control group, although several trends between FA of the right CB and immediate recall (r = 0.54, p = 0.09), ADC of the right CB and delayed recall (r = −0.55, p = 0.08), and ADC of the left CB and delayed recall (r = −0.52, p = 0.10) were found. As expected, the direction of the relations between DTI metrics and memory task performance was reversed in the control group such that higher FA and lower ADC were associated with better performance. Table 1 lists all means, standard deviations, p values, and effect sizes.
Table 1.
Group Mean, Standard Deviation, and Effect Size for DTI Metrics in the Cingulum Bundles and Memory Task Performance
| Variables | mTBI mean (SD) | Control mean (SD) | p Value | Cohen's d | |
|---|---|---|---|---|---|
| FA | Right | 0.44 (0.02) | 0.42 (0.03) | 0.06 | −0.66 |
| Left | 0.47 (0.02) | 0.45 (0.02) | 0.06 | −0.66 | |
| ADC | Right | 0.73 (0.02) | 0.76 (0.01) | <0.0001 | 1.25 |
| Left | 0.73 (0.02) | 0.75 (0.01) | <0.0001 | 1.21 | |
| VSRT total number of words during learning trials | 50.08 (6.95) | 53.00 (7.27) | 0.17 | 0.41 | |
| VSRT 30-min delayed recall | 7.67 (1.43) | 8.55 (1.81) | 0.11 | 0.53 | |
SD, standard deviation; FA, fractional anisotropy; ADC, apparent diffusion coefficient; VSRT, Verbal Selective Reminding Test; mTBI mild traumatic brain injury; DTI, diffusion tensor imaging.
For Cohen's d, 0.2–0.49 = small, 05–0.79 = medium, and >0.80 = large effect size. These were calculated based on values rounded to two decimal places.
All comparisons were performed with one-sided t-tests given the directional hypotheses.
FIG. 2.
Group Mean, Standard Deviation, and Effect Size for DTI Metrics in the Cingulum Bundles and Memory Task Performance
This study was conducted within 6 days of sustaining mTBI, with a mean time post-injury of just under 3 days. We acknowledge the heterogeneity in the time post-injury interval; however, 10 of the 12 participants were scanned within 4 days post-injury, and 8 of the 12 were scanned within 3 days. In the acute stage of brain injury, reduced ADC with increased FA may reflect transient cytotoxic edema (Wilde et al., 2008), and could be considered pathological. In contrast, DTI findings in the chronic phase of mild TBI have revealed decreased FA and increased ADC, presumably reflecting axonal injury. Experimental animal models have also shown a variety of inflammatory reactions within the cingulate after TBI, even when focal injury was distal to the CG (Dikranian et al., 2008; Kelley et al., 2007; Sargin et al., 2009), and may be apparent in the cingulate within hours to days following mild injury (Dikranian et al., 2008). In the human, the long-coursing white matter structures forming the cingulum bundles are especially vulnerable to deformation injury because of their proximity to the falx cerebri (Bigler, 2007; Gean, 1994). The current findings suggest that perturbation within CBs was present during the first week following mTBI, an effect possibly related to an early localized inflammatory reaction. Furthermore, this disruption in the left CB was related to 30-min delayed recall on a memory task. Some degree of hemispheric lateralization of CG function has been documented, wherein functional neuroimaging studies have demonstrated that left CG activation is more involved with language-based tasks (Christensen et al., 2008; Zago et al., 2008). As such, since the VSRT is a language-based episodic memory task in which the delayed component places greater demand on retention abilities, the current findings show that increased FA was associated with worse retention in the mTBI group. The effect sizes for both FA and ADC were medium to large, suggesting that even a mild injury may produce an inflammatory reaction sufficient to impact DTI metrics, and these changes are related to altered memory performance. This relation (between DTI metrics and common cognitive sequelae following mTBI) is consistent with findings of previous researchers (Kraus et al., 2007; Niogi et al., 2008), although the direction of the FA and ADC changes are in the opposite direction in this acute time frame.
The small sample size did not permit an investigation of regional differences within the CB, but given that effects were observed within the entire CB, it is anticipated that regional analyses will likely yield additional important relationships with functional changes during the acute phase of mTBI. For example, affective processing, pain, language, memory, and executive functioning all likely engage different regions of the cingulate (i.e., the anterior, body, and posterior regions), and may also show hemispheric differences (Behrens et al., 2009; Ebert and Ebmeier, 1996; Rushworth and Behrens, 2008). The investigation of these relations in acute brain injury would require a much larger sample size of patients with mTBI. Each of the potential behavioral and cognitive changes would need to be assessed with several neurobehavioral and/or neurocognitive measures, and therefore require a more complex study design. Nonetheless, these preliminary findings indicate that cingulate injury contributes to the cognitive sequelae seen during the early phase of mTBI.
Another limitation of the current investigation is that it examined relations between DTI and memory during the first week post-injury, and it is unknown if these relations persist into more chronic phases of mild TBI. Our data do not indicate whether CB DTI findings may help in differentiating and predicting those patients with mTBI who may experience persisting deficits versus those with good, rapid recovery. For example, Chen and colleagues (2008), in an fMRI investigation in athletes who sustained mTBI, found differences in activation in the anterior cingulate and medial frontal areas between those athletes whose sequelae resolved quickly and those with persistent symptoms. With atrophy of the cingulate reported after moderate-to-severe TBI (Chen et al., 2008; Gale et al., 2005; Levine et al., 2008; Yount et al., 2002), it is likely that injury to CB fiber tracts also occurs with milder forms of brain injury, and that further DTI investigations of these structures in mTBI are warranted.
Acknowledgments
This research was supported by National Institutes of Health/National Institute of Neurological Disorders and Stroke grant no. P01 NS056202. We would also like to acknowledge the generous support by Mission Connect of the TIRR Foundation (The Institute for Rehabilitation and Research).
Author Disclosure Statement
No competing financial interests exist.
References
- Alexander A.L. Lee J.E. Lazar M. Field A.S. Diffusion tensor imaging of the brain. J. Am. Soc. Exp. NeuroTherapeutics. 2007;4:316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arfanakis K. Haughton V.M. Carew J.D. Rogers B.P. Dempsey R.J. Meyerand M.E. Diffusion tensor MR imaging in diffuse axonal injury. Am. J. Neuroradiol. 2002;23:794–802. [PMC free article] [PubMed] [Google Scholar]
- Bazarian J.J. Zhong J. Blyth B. Zhu T. Kavcic V. Peterson D. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: a pilot study. J. Neurotrauma. 2007;24:1447–1459. doi: 10.1089/neu.2007.0241. [DOI] [PubMed] [Google Scholar]
- Behrens T.E. Hunt L.T. Rushworth M.F. The computation of social behavior. Science. 2009;324:1160–1164. doi: 10.1126/science.1169694. [DOI] [PubMed] [Google Scholar]
- Belanger H.G. Vanderploeg R.D. Curtiss G. Warden D.L. Recent neuroimaging techniques in mild traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 2007;19:5–20. doi: 10.1176/jnp.2007.19.1.5. [DOI] [PubMed] [Google Scholar]
- Bendlin B.B. Ries M.L. Lazar M. Alexander A.L. Dempsey R.J. Rowley H.A. Sherman J.E. Johnson S.C. Longitudinal changes in patients with traumatic brain injury assessed with diffusion-tensor and volumetric imaging. NeuroImage. 2008;42:503–514. doi: 10.1016/j.neuroimage.2008.04.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigler E.D. Anterior and middle cranial fossa in traumatic brain injury: relevant neuroanatomy and neuropathology in the study of neuropsychological outcome. Neuropsychology. 2007;21:515–531. doi: 10.1037/0894-4105.21.5.515. [DOI] [PubMed] [Google Scholar]
- Chen J.K. Johnston K.M. Petrides M. Ptito A. Neural substrates of symptoms of depression following concussion in male athletes with persisting postconcussion symptoms. Arch. Gen. Psychiatry. 2008;65:81–90. doi: 10.1001/archgenpsychiatry.2007.8. [DOI] [PubMed] [Google Scholar]
- Christensen T.A. Antonucci S.M. Lockwood J.L. Kittleson M. Palante E. Cortical and subcortical contributions to the attentive processing of speech. Neuroreport. 2008;19:1101–1105. doi: 10.1097/WNR.0b013e3283060a9d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu Z. Wilde E.A. Hunter J.V. McCauley S.R. Bigler E.D. Troyanskaya M. Yallampalli R. Chia J.M. Levin H.S. Voxel-based analysis of diffusion tensor imaging in mild traumatic brain injury in adolescents. Am. J. Neuroradiol. 2009 doi: 10.3174/ajnr.A1806. Dec 3 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikranian K. Cohen R. MacDonald C. Pan Y. Brakefield D. Bayly P. Parsadanian A. Mild traumatic brain injury to the infant mouse causes robust white matter axonal degeneration which precedes apoptotic death of cortical and thalamic neurons. Exp. Neurol. 2008;211:551–560. doi: 10.1016/j.expneurol.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert D. Ebmeier K.P. The role of the cingulate gyrus in depression: from functional anatomy to neurochemistry. Biol. Psychiatry. 1996;39:1044–1050. doi: 10.1016/0006-3223(95)00320-7. [DOI] [PubMed] [Google Scholar]
- Gale S.D. Baxter L. Roundy N. Johnson S.C. Traumatic brain injury and grey matter concentration: a preliminary voxel based morphometry study. J. Neurol. Neurosurg. Psychiatry. 2005;76:984–988. doi: 10.1136/jnnp.2004.036210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gean A.D. Imaging of Head Trauma. New York. Raven Press; 1994. [Google Scholar]
- Jones D.K. Horsfield M.A. Simmons A. Optimal strategies for measuring diffusion in anisotropic systems by magnetic resonance imaging. Magn. Reson. Med. 1999;42:515–525. [PubMed] [Google Scholar]
- Kelley B.J. Lifshitz J. Povlishock J.T. Neuroinflammatory responses after experimental diffuse traumatic brain injury. J. Neuropathol. Exper. Neurol. 2007;66:989–1001. doi: 10.1097/NEN.0b013e3181588245. [DOI] [PubMed] [Google Scholar]
- Kraus M.F. Susmaras T. Caughlin B.P. Walker C.J. Sweeney J.A. Little D.M. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain. 2007;130:2608–2519. doi: 10.1093/brain/awm216. [DOI] [PubMed] [Google Scholar]
- Kurca E. Sivak S. Kucera P. Impaired cognitive functions in mild traumatic brain injury patients with normal and pathological magnetic resonance imaging. Neuroradiology. 2006;48:661–669. doi: 10.1007/s00234-006-0109-9. [DOI] [PubMed] [Google Scholar]
- Larrabee G.J. Trahan D.E. Levin H.S. Normative data for a six-trial administration of the Verbal Selective Reminding Test. Clin. Neuropsychologist. 2000;14:110–118. doi: 10.1076/1385-4046(200002)14:1;1-8;FT110. [DOI] [PubMed] [Google Scholar]
- Levine B. Kovacevic N. Nica E.I. Cheung G. Gao F. Schwartz M.L. Black S.E. The Toronto traumatic brain injury study: injury severity and quantified MRI. Neurology. 2008;70:771–778. doi: 10.1212/01.wnl.0000304108.32283.aa. [DOI] [PubMed] [Google Scholar]
- Levin H.S. O'Donnell V.M. Grossman R.G. The Galveston Orientation and Amnesia Test. A practical scale to assess cognition after head injury. J. Nerv. Mental Dis. 1979;167:675–684. doi: 10.1097/00005053-197911000-00004. [DOI] [PubMed] [Google Scholar]
- Lo C. Shifteh K. Gold T. Bello J.A. Lipton M.L. Diffusion tensor imaging abnormalities in patients with mild traumatic brain injury and neurocognitive impairment. J. Comput. Assist. Tomogr. 2009;33:293–297. doi: 10.1097/RCT.0b013e31817579d1. [DOI] [PubMed] [Google Scholar]
- Malojcic B. Mubrin Z. Coric B. Susnic M. Spilich G.J. Consequences of mild traumatic brain injury on information processing assessed with attention and short-term memory tasks. J. Neurotrauma. 2008;25:30–37. doi: 10.1089/neu.2007.0384. [DOI] [PubMed] [Google Scholar]
- Mori S. Crain B.J. Chacko V.P. Vanzijl P.C. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann. Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Nakayama N. Okumura A. Shinoda J. Yasokawa Y.T. Yoshimura S.I. Iwama T. Evidence for white matter disruption in traumatic brain injury without macroscopic lesions. J. Neurol. Neurosurg. Psychiatry. 2006;77:850–855. doi: 10.1136/jnnp.2005.077875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netsch T. Paper presented at the International Conference on Computer Vision Towards Real-Time Multi-Modality 3-D Medical Image Registration. 2001 [Google Scholar]
- Niogi S.N. Mukherjee P. Ghajar J. Johnson C.E. Kolster R. Lee H. Suh M. Zimmerman R.D. Manley G.T. McCandliss B.D. Structural dissociation of attentional control and memory in adults with and without mild traumatic brain injury. Brain. 2008;131:3209–3221. doi: 10.1093/brain/awn247. [DOI] [PubMed] [Google Scholar]
- Rushworth M.F. Behrens T.E. Choice, uncertainty and value in prefrontal and cingualte cortex. Nature Neurosci. 2008;11:389–397. doi: 10.1038/nn2066. [DOI] [PubMed] [Google Scholar]
- Rutgers D.R. Toulgoat F. Cazejust J. Fillard P. Lasjaunias P. Ducreux D. White matter abnormalities in mild traumatic brain injury: a diffusion tensor imaging study. Am. J. Neuroradiol. 2008;29:514–519. doi: 10.3174/ajnr.A0856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargin D. Hassouna I. Sperling S. Siren A.L. Ehrenreich H. Uncoupling of neurodegeneration and gliosis in a murine model of juvenile cortical lesion. Glia. 2009;57:693–702. doi: 10.1002/glia.20797. [DOI] [PubMed] [Google Scholar]
- Stein M.B. McAllister T.W. Exploring the convergence of posttraumatic stress disorder and mild traumatic brain injury. Am. J. Psychiatry. 2009;166:768–776. doi: 10.1176/appi.ajp.2009.08101604. [DOI] [PubMed] [Google Scholar]
- Topal N.B. Hakyemez B. Erdogan C. Bulut M. Koksal O. Akkose S. Dogan S. Parlak M. Ozguc H. Korfali E. MR imaging in the detection of diffuse axonal injury with mild traumatic brain injury. Neurological Res. 2008;30:974–978. doi: 10.1179/016164108X323799. [DOI] [PubMed] [Google Scholar]
- Wilde E.A. McCauley S.R. Hunter J.V. Bigler E.D. Chu Z. Wang Z.J. Hanten G.R. Troyanskaya M. Yallampalli R. Li X. Chia J. Levin H.S. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology. 2008;70:948–955. doi: 10.1212/01.wnl.0000305961.68029.54. [DOI] [PubMed] [Google Scholar]
- Xu J. Rasmussen I. Lagopoulos J. Haberg A. Diffuse axonal injury in severe traumatic brain injury visualized using high-resolution diffusion tensor imaging. J. Neurotrauma. 2007;24:753–765. doi: 10.1089/neu.2006.0208. [DOI] [PubMed] [Google Scholar]
- Yount R. Raschke K.A. Biru M. Tate D.F. Miller M.J. Abildskov T. Gandhi P. Ryser D. Hopkins R.O. Bigler E.D. Traumatic brain injury and atrophy of the cingulate gyrus. J. Neuropsychiatry Clin. Neurosci. 2002;14:416–423. doi: 10.1176/jnp.14.4.416. [DOI] [PubMed] [Google Scholar]
- Zago L. Turbeline L.P. Andersson M.R. Vigneau F.M. Tzourio-Mazoyer N. How verbal and spatial manipulation networks contribute to calculation: an fMRI study. Neuropsychologia. 2008;46:2401–2014. doi: 10.1016/j.neuropsychologia.2008.03.001. [DOI] [PubMed] [Google Scholar]