Abstract
Objective
To evaluate cervical cancer screening practices and barriers to screening in a sample of lesbians.
Methods
Cross-sectional survey data were collected from 225 self-identified lesbians who completed an online questionnaire.
Results
Of the respondents, 71% reported receiving a Pap screening test in the past 24 months (routine screeners), and 29% reported receiving a Pap screening test >24 months ago or never (nonroutine screeners). Routine screeners were more likely to be older (p < 0.01), white (p = 0.04), and college graduates (p < 0.01) than nonroutine screeners. Nonroutine screeners were more likely to delay seeking healthcare because of fear of discrimination (p < 0.01) and were less likely than routine screeners to disclose orientation to their primary care physician (p < 0.01). After adjusting for age, race, and education, nonroutine screeners perceived fewer benefits from (p < 0.01) and more barriers (p < 0.01) to Pap screening tests and were less knowledgeable about screening guidelines (p < 0.01) than routine screeners, but there was no difference in perceived susceptibility (p = 0.68), perceived seriousness (p = 0.68), or risk factor knowledge (p = 0.35) of cervical cancer.
Conclusions
Many lesbians do not screen for cervical cancer at recommended rates. Nonroutine screeners perceive fewer benefits, more barriers, and more discrimination and are less knowledgeable about screening guidelines than routine screeners.
Introduction
Cervical cancer is one of the most common reproductive cancers among women in the United States and is the second most common cancer among women worldwide.1–4 The National Cancer Institute (NCI) estimates that in 2009 roughly 11,070 cases of invasive cervical cancer will be diagnosed in the United States, and approximately 3870 women will die from cervical cancer. Of the women born in the United States today, 1 in 145 (0.69%) will be diagnosed with cervical cancer in her lifetime.1
Cervical cancer incidence and mortality rates overall have decreased significantly during the last 50 years as a result of widespread cervical cancer screening with the Papanicolaou (Pap) test.2 The Pap test is the most important and effective screening tool used to detect cervical abnormalities that can be treated to prevent development of invasive cervical cancer. Despite the efficacy of the Pap test, subgroups of women continue to be at elevated risk of cervical cancer as a result of underuse of screening services and lack of knowledge about risk factors for cervical cancer and appropriate methods of prevention. Lesbians are one such subgroup of women.3 Studies of lesbians indicate that negative experiences with the healthcare system and misinformation about disease risk and the preventive healthcare needs of lesbians contribute to underuse of medical services in general1,4–6 and routine cervical cancer screening in particular.7,8 Unpublished preliminary data obtained by the first author suggest that as many as 50% of lesbians do not get Pap screens at the recommended intervals and approximately 10% have never had a Pap screen (J.K. Tracy, unpublished observations). Failure to be screened is of great importance for this subgroup of women because inadequate screening may lead to diagnosis at a more advanced disease, which in turn may be related to greater overall morbidity and mortality.
Cervical cancer screening
Cervical cancer screening guidelines have been put forth by the American College of Obstetricians and Gynecologists (ACOG),9 the American Cancer Society (ACS),10 and the U.S. Preventive Services Task Force (USPSTF).11 Although there is slight variation in the recommendations of each agency, there is general agreement that screening should (1) begin no later than age 21 or within 3 years of the onset of sexual activity, (2) continue to at least age 65, and (3) occur at regular intervals, such as every 1–2 years up to age 30 and at least every 2–3 years thereafter if there is no history of cervical abnormality. The objective of cervical cancer screening is to prevent the occurrence of cervical cancer by detecting and treating premalignant lesions before invasive disease develops. Efforts to increase cervical cancer screening rates among women in general have been very successful. Data gathered in 2006 indicate that 84% of U.S. women ≥18 years report Pap smears within the last 3 years.12 This is in contrast to the available data for lesbians. Research to date indicates that lesbians are less likely than heterosexual women to receive regular Pap screening for cervical cancer. The majority of surveys report that only 44%–56% of lesbians have regular Pap smears5,6,8,13–15 (J.K. Tracy, unpublished observations).
Risk factors for cervical cancer
In contrast to many cancers, cervical cancer has a more clearly defined etiology, available course of treatment, and effective means of prevention. Risk factors associated with cervical cancer include persistent infection with certain oncogenic strains of human papillomavirus (HPV), young age of initiation of sexual activity, multiple past or present sexual partners (of either sex), history of a sexually transmitted infection (STI), smoking, poor diet, long-term use of oral contraceptives, low socioeconomic status (SES), family history of cervical cancer, and history of abnormal Pap smears.16 Infection with oncogenic strains of HPV is the biggest risk factor for cervical cancer, with standard estimates of the attributable risk fraction (proportion of disease that is related to HPV) ranging from 90%–98%.17 In the United States, exposure to strains of HPV is nearly ubiquitous for women who are sexually active. The cumulative lifetime prevalence of infection with HPV among samples of presumably heterosexual women has been estimated at upwards of 70%–80%.18,19 Population-based prevalence rates of HPV infection among lesbians do not exist; however, there are data to suggest that HPV can be sexually transmitted between two women. Studies by Marrazzo et al.20–24 note that 13%–30% of women who report having sex with women (WSW) tested positive for HPV infection. This contradicts the common misconception that lesbians have a minimal risk of contracting HPV. Marrazzo et al. conclude that at the very least, current recommendations for cervical cancer screening for lesbians (or WSW) should not differ from those for heterosexual women. This is particularly important given the finding by Grindel et al.25 that 80% of lesbian respondents from their nationwide survey reported having had an abnormal Pap smear.
One point that is often overlooked as a risk factor for cervical cancer is failure to obtain routine screening. After infection with oncogenic strains of HPV, lack of screening is the most common attributable risk factor in the development of invasive cervical cancer.26,27 Lesbians may be at elevated risk for cervical cancer as a result of lack of adherence to screening guidelines. Lesbians may have added risk because of behavioral risk factors for cervical cancer that are higher in prevalence among lesbians than in the general population of women. This includes a higher prevalence of obesity and smoking among lesbians.28 Consequently, it could be argued that even greater efforts should be directed toward encouraging cervical cancer screening in the lesbian population because of their lower Pap screen rates, comparable risk of HPV infection, and high prevalence of smoking and obesity.
The Health Belief Model (HBM) is an individual-level health behavior change model that has been used widely to evaluate factors associated with cancer screening (e.g., mammography,29–31 skin,32 prostate,33 cervical34). The HBM posits that health behavior and health behavior change are related to individual perceptions. According to the HBM, individuals take steps to prevent, screen for, or control health conditions if they believe themselves susceptible to the condition, if they believe the consequences of the condition are potentially serious, if they believe the recommended changes in health behaviors will reduce their susceptibility to the condition or lessen the severity of the condition, and if they believe the perceived benefits of taking action to prevent, screen, or control the health condition outweigh any perceived barriers.35 Across studies that have evaluated HBM-based interventions, perceived barriers have been the single most powerful predictor of positive health behavior change; therefore, major emphasis was placed on identifying perceived barriers to cervical cancer screening in lesbians.
The purpose of this survey was to evaluate cervical cancer screening practices of a sample of lesbians and to identify factors associated with cervical cancer screening in this group of women. Specifically, the study aimed to identify differences between routine screeners and nonroutine screeners on dimensions from the HBM, focusing on perceived barriers and benefits of cervical cancer screening as well as perceived susceptibility and seriousness of cervical cancer.
Materials and Methods
Requests for participation in an online survey were advertised for 1 month in Baltimore gay newspapers and once in the Lesbian Connection, which is a nationally distributed bimonthly magazine for lesbians with an estimated readership of 50,000. Advertisements in Baltimore gay newspapers ran for 4 weeks beginning in September 2005. To broaden the demographic characteristics of the sample, a second advertisement was placed in the March/April 2006 issue of the Lesbian Connection. This publication has a suggested subscription price, but it is provided free of charge to readers who cannot afford the subscription fee.
A woman was eligible to particicpate if she was 18–70 years old, self-identified as lesbian, had an intact cervix (i.e., not have had hysterectomy with removal of the cervix), and could read English. Women > age 70 were not included because current cervical cancer screening guidelines do not recommend screening past the age of 70. Women with previous hysterectomies with removal of the cervix were excluded because they are no longer at risk of developing cervical cancer. The call for participation advertisement provided a brief description of the study and the Internet address where the survey could be completed. The study was approved by the Institutional Review Board of the University of Maryland School of Medicine.
Measures
All study measures were collected via an interactive Internet-based survey designed by the research team using SurveyMonkeyTM (Portland, OR). Although the survey was constructed as one instrument, it included questions from the instruments described here.
Sociodemographic data
A modified version of the personal information questionnaire used by Jordan and Deluty36 and Tracy and Junginger37 was used to gather sociodemographic and health insurance coverage data as well as data pertaininig to respondents' sexual orientation and relationship status. Sexual orientation was determined based on respondents' self-identification as lesbian on a forced choice: How would you describe your sexual orientation? Response options included lesbian, bisexual, heterosexual. Although “lesbian” has been operationalized by others as being behaviorally based (i.e., having only or mostly sexual relations with and attractions to other women), we were interested in the self-identity of lesbian and the potential role that social stigma associated with lesbian self-identity plays in the decision to participate in cervical cancer screening.
Pap test screening and cervical cancer history
Standardized questions were used to ascertain the frequency of Pap screening,12 history of abnormal Pap screen results, and history of cervical cancer. Data pertaining to frequency of Pap screening was used to categorize respondents into our two screening groups of interest: routine screeners and nonroutine screeners.
Knowledge of cervical cancer risk factors
Knowledge of risk factors for cervical cancer was measured using 11 true/false questions from the Harvard Disease Risk Index Cervical Cancer Fact Sheet and Hislop et al.38 Knowledge of cervical cancer screening guidelines was determined using 4 questions from the ACS.10 Knowledge of screening guidelines questions included: (1) Women should be screened for cervical cancer every 3 years [true]. (2) It's unnecessary to be screened for cervical cancer if a women is abstinent [false]. (3) Women should begin screening for cervical cancer when they become sexually active [false]. (4) Women under the age of 30 years old should be screened for cervical cancer annually [true]. The knowledge score was created by summing the total number of correct responses to the 15 knowledge questions.
Health Belief Model Scales
Perceived susceptibility, seriousness, barriers, and benefits to cervical cancer screening were assessed using a modified version of Champion's Health Belief Model Scale (CHBMS).29–31 The CHBMS was designed to assess compliance with breast cancer screening and breast self-examination. The CHBMS remained unchanged except for modifications required for it to reference cervical cancer instead of breast cancer. The CHBMS includes scales for perceived susceptibility, seriousness, barriers, benefits, and general concern for overall health. Items are rated on a 5-point Likert-type scale (1 = strongly disagree, 5 = strongly agree). Cronbach's alpha internal consistency coefficients for the scales of the CHBMS range from 0.80 to 0.88 for the original instrument.
Perceived healthcare discrimination
Perceived healthcare discrimination was assessed using items from the Multi-site AIDS Cohort Study.39 Questions included (1) Have you ever felt you were discriminated against in any of the following healthcare settings (hospital, doctor or other healthcare provider's office, public health department or clinic, community health center, other)? (2) Has fear of being discriminated against because of your sexual orientation ever caused you to delay obtaining healthcare? (3) Has a doctor or other healthcare professional ever refused to treat you because you are a lesbian?
Procedure
Participants anonymously completed the standardized survey online, which included questions related to demographics, cervical cancer screening behaviors, perceived healthcare discrimination, disclosure of sexual orientation, knowledge of cervical cancer risk factors and screening guidelines, and a modified version of CHBMS.29,30 The survey software prevented a woman from completing the survey more than once.
A total of 255 women responded to the survey over a period of 12 months; 239 of these women self-identified as lesbian, and the remaining 14 women (10 bisexuals and 4 heterosexuals) were excluded, as they did not meet the inclusion criteria for the study. Following the previously described recommendations for screening from the ACS, ACOG, and the USPSTF, respondents were divided into two groups: routine screeners and nonroutine screeners. Women reporting completion of a Pap test in the 24 months prior to the survey were classified as routine screeners. Women not reporting completion of a Pap test in the 24 months prior to the survey were classified as nonroutine screeners. The cutoff point of 24 months was selected to represent a moderately conservative interpretation of cervical cancer screening guidelines provided by the aforementioned sources (recommendations vary from being as frequent as annually to the less frequent 2–3 years, provided the individual has never had an abnormal Pap test result). Fourteen women did not provide any information about when (if ever) they had received a Pap test and were excluded from analyses, resulting in a final analytic sample size of 225 women.
Statistical analyses
Data were examined for normality and completeness. Univariate analyses were performed to evaluate group differences between routine screeners and nonroutine screeners on sociodemographic variables; group differences were assessed using Student's t tests (continuous variables) and chi-square/Fisher's exact tests (categorical variables). Multivariate linear regression was used to determine the independent association between cervical cancer screening behavior (e.g., routine screener vs. nonroutine screener) and the following dependent variables: cervical cancer screening knowledge, perceived susceptibility, barriers and benefits, controlling for confounders identified by univariate analyses. All statistical analyses were done using Stata 10 (Stata Corp., College Station, TX). Statistical significance was inferred at p ≤ 0.05.
Results
Participant characteristics
Respondents were 225 self-identified lesbians between the ages of 18 and 68 years (mean 41, SD 12). Table 1 provides the sociodemographic and screening characteristics of the analytic sample. The women were predominantly college graduates (76%), white (88%), and employed full-time (71%). Additionally, the majority of the respondents were in a committed relationship with one woman (57%). Respondents lived in 41 of the 50 states, with relatively equal representation across the four major census regions. That the proportion of respondents from the southern region was somewhat higher than that of the other three regions reflects the fact that initial recruitment focused on the Baltimore metro area; recruitment was later broadened through advertising to achieve a more representative sample. The majority of respondents had some form of health insurance (38% private/commercial, 34% HMO/managed care). It is noteworthy that 28% of respondents had no health insurance; this is nearly twice the rate of being uninsured in the general population.
Table 1.
Sociodemographic and Screening Characteristics of Survey Sample
| Characteristic | Routine screeners n = 161 | Nonroutine screeners n = 64 | Total n = 225 | p valuea |
|---|---|---|---|---|
| Age (years, mean ± SD) | 43 ± 11 | 37 ± 12 | 41 ± 12 | <0.01 |
| White (%) | 90 | 81 | 87 | 0.04 |
| Black | 6 | 6 | 6 | |
| Asian | 0 | 3 | 1 | |
| Native American | 0 | 2 | <1 | |
| Other | 4 | 8 | 5 | |
| College graduate (%) | 82 | 61 | 76 | <0.01 |
| Employment status (%) | 0.19 | |||
| Full-time | 74 | 63 | 71 | |
| Part-time | 11 | 13 | 12 | |
| Unemployed | 8 | 8 | 8 | |
| Student | 7 | 16 | 10 | |
| Relationship status (%) | 0.10 | |||
| Single | 22 | 36 | 26 | |
| Casually dating 1 woman | 3 | 6 | 4 | |
| Casually dating >1 woman | 3 | 5 | 4 | |
| Seriously dating 1 woman | 11 | 6 | 9 | |
| Committed to 1 woman | 61 | 47 | 57 | |
| States represented (n) | 34 | 41 | – | |
| Census regions (%) | ||||
| West | 21 | 8 | 17 | 0.06 |
| Midwest | 22 | 18 | 21 | |
| South | 38 | 53 | 42 | |
| Northeast | 17 | 15 | 16 | |
| Not reported | 2 | 6 | 4 | |
| Source of payment for medical care (%) | 0.09 | |||
| Private/commercial | 40 | 36 | 38 | |
| HMO/managed | 38 | 21 | 34 | |
| Medical assistance | 2 | 2 | 2 | |
| Self–pay | 9 | 21 | 12 | |
| Other | 12 | 19 | 14 | |
| Most recent Pap test (%) | – | |||
| <12 months | 80 | – | 57 | |
| 12–24 months | 20 | – | 14 | |
| >24–36 months | – | 31 | 9 | |
| >36 months | – | 47 | 13 | |
| Never | – | 22 | 6 |
p values from t test, chi–square test, or Fisher's exact test, as appropriate.
With respect to cervical cancer screening behavior, 57% had received a Pap screening test in the previous 12 months, and an additional 14% had received a Pap screening test in the previous 13–24 months. These 161 women constituted the routine screeners. Nine percent had received a Pap screening test in the previous 25–36 months, 13% had received one more than 36 months ago, and 6% had never received one. These 64 women constituted the nonroutine screeners. Univariate analysis indicated that routine screeners were older (p < 0.01), more likely to be white (p = 0.04), and more likely to have graduated from college (p < 0.01). Routine screeners and nonroutine screeners did not differ in employment, relationship, or insurance status; further, both screening groups had respondents from a wide range of geographic locations.
Psychometric data
The CHBMS was originally designed to capture health beliefs related to breast cancer, not cervical cancer. The CHBMS was modified for the current study to refer to cervical cancer and Pap screening. To demonstrate preservation of the underlying psychometric properties of the CHBMS, internal consistency coefficients were computed. Internal consistency coefficients (Cronbach's alpha) for all CHBMS scales were comparable to or better than those reliability coefficients reported for the original instrument29,30; reliability coefficients were 0.90, 0.88, 0.79, and 0.81 for the susceptibility, seriousness, benefits, and barriers scales, respectively.
Univariate analyses
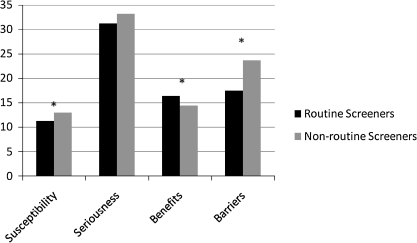
As shown in Figure 1, nonroutine screeners perceived themselves as more susceptible to cervical cancer (p < 0.01) but did not differ from routine screeners with respect to their perceived seriousness of cervical cancer (p = 0.11). Nonroutine screeners perceived fewer benefits (p < 0.01) and more barriers (p < 0.01) to screening than did routine screeners. Nonroutine screeners also perceived greater discrimination because of sexual orientation in a variety of healthcare settings, including hospitals (p = 0.01), public health clinics (p < 0.01), community-based health clinics (p < 0.01), and other healthcare settings (p = 0.03) (Table 2). In addition, nonroutine screeners were more likely than routine screeners to report that fear of discrimination caused them to delay seeking healthcare (p < 0.01) and were less likely to disclose sexual orientation to their primary care physician (p < 0.01) or gynecologist (p < 0.01). Nonroutine screeners were less knowledgeable about Pap screening guidelines (p = 0.01) but did not differ from routine screeners in their knowledge of cervical risk factors (p = 0.24) (Table 3).
Fig. 1.
Discrimination Due to Sexual Orientation and Disclosure of Sexual Orientation
Table 2.
Discrimination Due to Sexual Orientation and Disclosure of Sexual Orientation
| Routine screeners n = 161 | Nonroutine screeners n = 64 | Total n = 225 | p valuea | |
|---|---|---|---|---|
| Respondents reporting discrimination due to sexual orientation, by location (%) | ||||
| Hospital | 23 | 41 | 28 | 0.01 |
| Doctor's office | 48 | 38 | 41 | 0.16 |
| Public health clinic | 12 | 30 | 17 | <0.01 |
| Community-based clinic | 6 | 28 | 12 | <0.01 |
| Other healthcare setting | 13 | 26 | 17 | 0.03 |
| Fear of discrimination caused delay in seeking healthcare (%) | 20 | 39 | 25 | <0.01 |
| Disclosure of sexual orientation, by healthcare provider (mean ± SD)b | ||||
| Primary care physician | 5.4 ± 2.1 | 4.0 ± 2.3 | 5.0 ± 2.2 | <0.01 |
| Gynecologist | 5.6 ± 2.1 | 3.7 ± 2.2 | 5.1 ± 2.2 | <0.01 |
p values from t test, chi-square test, or Fisher's exact test, as appropriate.
Results are from a 7-point Likert scale using the following anchors: 1, person definitely does not know about your sexual orientation status; 2, person might know about your status, but it is never talked about; 3, person probably knows about your status, but it is never talked about; 4, person probably knows about your status, but it is rarely talked about; 5, person definitely knows about your status, but it is rarely talked about; 6, person definitely knows about your status, and it is sometimes talked about; 7, person definitely knows about your status, and it is openly talked about.
Table 3.
Knowledge of Cervical Cancer
| Routine screeners n = 161 | Nonroutine screeners n = 64 | Total n = 225 | p valuea | |
|---|---|---|---|---|
| Cervical cancer risk factorsb | 6.8 ± 2.1 | 6.4 ± 2.1 | 6.7 ± 2.1 | 0.24 |
| Cervical cancer screening guidelinesc | 2.8 ± 0.7 | 2.4 ± 0.7 | 2.7 ± 0.7 | <0.01 |
p values from chi-square test or Fisher's exact test, as appropriate.
Mean ± SD of number of correct answers to 11 true/false questions.
Mean ± SD of number of correct answers to 4 true/false questions.
Multiple regression analyses
Many of the differences between routine and nonroutine screeners persisted after controlling for age, race, and education. Nonroutine screeners were less knowledgeable about current cervical cancer screening guidelines (p = 0.001) and still perceived fewer benefits (p < 0.001) and more barriers (p < 0.001) to Pap screening examinations than routine screeners. However, there was no statistically significant difference in perceived susceptibility, perceived seriousness, or knowledge of risk factors for cervical cancer between nonroutine screeners and routine screeners after adjusting for age, race, and education (Table 4).
Table 4.
Multivariate Regressiona
| Characteristic | Odds ratio (95% CI) | p value |
|---|---|---|
| Knowledge of risk factors | 1.08 (0.92-1.26) | 0.319 |
| Knowledge of screening guidelines | 2.30 (1.43-3.69) | 0.001 |
| Perceived susceptibility | 0.98 (0.90-1.07) | 0.650 |
| Perceived seriousness | 0.99 (0.95-1.03) | 0.697 |
| Perceived benefits | 1.23 (1.10-1.36) | <0.001 |
| Perceived barriers | 0.83 (0.77-0.88) | <0.001 |
Multivariate logistic regression adjusted for age (continuous), education (college graduate and above vs. less than college graduate), and race (white vs. not white) with screening group (routine screener v. nonroutine screener).
Discussion
Overall, rates of participation in cervical cancer screening were somewhat higher than previously reported8 (J.K. Tracy, unpublished observations). This is an encouraging finding and may reflect greater awareness of the need for screening among lesbians. Alternatively, higher levels of participation may be reflective of increasing childbearing among lesbians and the cue to be screened that occurs when one is interested in having children. Data from the current study indicate that, similar to the general population of women, minority and less educated lesbians are less likely to participate in screening at recommended intervals. In contrast to the general population, however, lesbians who adhere to screening guidelines are more likely to be older than those who do not adhere. One possible explanation for this difference is the necessity among the heterosexual female general population to obtain annual Pap screening examinations in order to obtain a prescription for oral contraception. Thus, younger heterosexual women likely have this cue to action to adhere to cervical cancer screening guidelines. As the heterosexual population ages and the need for birth control decreases, the cue to action follows suit, and screening rates decline. Although many lesbians report a previous history of sexual relations with men, without the need for birth control, it is possible that the lesbian population does not experience a cue to action for cervical cancer screening until they reach an age where mammography screening is also recommended, suggesting that among this population, cervical cancer screening rates may rise with age. Future studies should explore differences in cervical cancer screening behaviors of lesbians who choose to have children and those who do not have children to evaluate the extent to which participation in this type of gynecological care process affects cervical cancer screening behaviors. Further, efforts should be made in future studies to systematically evaluate the role of physician recommendation for participation in cervical cancer screening.
Although knowledge of risk factors for cervical cancer did not differ for nonroutine and routine screeners in our study, the screening groups did differ in their knowledge of recommended screening guidelines. Nonroutine screeners were less knowledgeable than routine screeners about current screening guidelines, even after adjusting for age, race, and education, indicating that screening behavior among lesbians is related to knowledge of screening guidelines but not knowledge of risk factors. This suggests that information campaigns promoting routine cervical cancer screening among lesbians will have more potent effects by addressing knowledge of screening guidelines instead of knowledge of risk factors.
The present study indicated that lesbians who were nonroutine screeners perceived fewer benefits and more barriers to screening compared with lesbians who were in the routine screener group. These trends were independent of the age, race, and education level of the respondents. Interestingly, nonroutine screeners also perceived themselves as more susceptible to cervical cancer, perhaps because of self-awareness of the risk of not adhering to recommended guidelines for prevention. Belief in the benefits of cervical cancer screening has also been consistently associated with regular screening in the general population.40
Although income data were not directly collected, there was no difference between screening groups with regard to employment or insurance status, potentially indicating a similar overall economic situation for each screening group. Thus, differences in screening are unlikely to be merely a reflection of SES and affordability of screening.
Our study of lesbians provides empirical evidence that lesbians who are nonroutine screeners are more likely than routine screeners to report discrimination because of sexual orientation in a variety of healthcare settings, with the notable exception of the primary care doctor's office. Nonroutine screeners were also less likely than routine screeners to disclose their sexual orientation to their physician or gynecologist or to discuss how sexual orientation may modify their health risks.
Despite significant improvement in Pap screening rates overall, some women do not participate in cervical cancer screening according to recommended guidelines. Sociodemographic and cultural factors associated with underuse of cervical cancer screening in the general population include age, race/ethnicity, educational background, and economic status. In contrast to young women, older women obtain Pap screening examinations at lower rates.41 Analysis of data from the National Health Interview Survey (NHIS) found that nearly one half of women aged 50–64 years did not obtain a Pap smear in the preceding 3 years.42 Cervical cancer screening rates for women from minority groups are consistently below the rates of nonminority women.43–45 Using data for self-reported Pap screening behavior from the Behavioral Risk Factor Surveillance System (BRFSS), Coughlin et al.46 found that women from minority racial/ethnic groups, lower education level, and lower income or the unemployed were less likely to participate in screening.
Women's knowledge and attitudes about screening also appear to be related to cervical cancer screening behavior in the general population. Many studies have shown a relationship between knowledge related to cervical cancer risk factors and screening guidelines and adherence to recommended screening guidelines. Knowledge of risk factors associated with cervical cancer tends to be higher among women who participate in regular cervical cancer screening. In secondary analysis of data collected as part of the Cancer Control Supplement of the NHIS, Pearlman et al.47 noted that knowledge of risk factors related to cervical cancer was an important barrier to women's participation in regular cervical cancer screening. Behbakht et al.48 analyzed differences between women with cervical cancer who were previously screened compared with women with cervical cancer who had not been screened and noted that women who had not been screened before their diagnosis lacked knowledge about their risk for cervical cancer. This stands in contrast to the finding in our study of lesbians that knowledge of cervical cancer risk factors was comparable between screening groups.
Reasons for not participating in routine cervical cancer screening have not been well studied in lesbians. A review of the scientific literature revealed a paucity of research related to any aspect of cervical cancer in lesbians.49 A single study has provided some evidence that lesbians perceive themselves to be less susceptible to cervical cancer than heterosexual women,50 although this finding has not been empirically confirmed. Other factors have been proposed as potential barriers to routine cervical cancer screening in lesbians. These include experiences of discrimination and homophobia in the healthcare system, lack of health insurance, and fewer cues to action, such as contraceptive needs, that might otherwise trigger routine gynecological care.28
Additional barriers to participation in routine cervical cancer screening may include lack of healthcare providers' knowledge of disease risk in this population, providers' failure to obtain a complete sexual history from lesbians, and lesbians' lack of willingness to disclose sexual orientation to care providers.5,6,51–53 Although these factors have been offered as possible explanations for lesbians' low rates of adherence to recommended cervical cancer screening guidelines, few studies have directly evaluated these hypothesized associations empirically in lesbians or care providers of lesbians.
Although it is probable that components of successful interventions among presumably heterosexual women are appropriate to use when targeting the lesbian population, the unique challenges faced by this group require a customized approach. The data presented here indicate several ways in which public health interventions may need to be modified to promote cervical cancer screening and improve adherence to cervical cancer screening guidelines among lesbians. Perceived benefits and barriers to screening should be targeted, as these predictors remained even after controlling for age, race, and education. Interventions targeted to lesbians should address the benefits of screening and offer strategies for overcoming barriers. In addition, our screening groups did not differ with respect to their knowledge of risk factors for cervical cancer; however, the groups did differ in their understanding of current guidelines for cervical cancer screening. Consequently, an information campaign directed to lesbians as the target population would be most effective in promoting screening if focused on providing information on screening guidelines rather than providing information about risk factors. Unique predictors of nonadherence to screening recommendations among lesbians were discrimination because of sexual orientation when interacting with healthcare systems and lack of disclosure of sexual orientation to a healthcare provider. Effective intervention strategies for this group of women should encourage positive and productive interactions with the healthcare community; such interventions may be most effective when targeted both to lesbians as the target population and to care providers. Future studies may advance these findings by exploring the extent to which healthcare provider attitudes contribute to cervical cancer screening practices of lesbians.
An issue that was beyond the scope of this report but that warrants further study in future investigations is that of the relation between sexual behaviors, including sexual histories with male and female partners and the perception of risk for acquiring HPV and cervical cancer. Incorporation of these types of questions into the evaluation of barriers to screening may reveal valuable information on the decision-making processes related to cervical cancer screening. Specifically, future studies that incorporate collection of sexual history data and risk perception may help disentangle the complex ways in which previous sexual behavior affects risk perception and subsequent screening behavior.
Limitations
This study presents important findings related to cervical cancer screening knowledge, attitudes, and beliefs among lesbians, but it is not without shortcomings. First, our survey relied on self-report for categorizing women as routine or nonroutine screeners. Although there is evidence in the extant literature that women tend to overestimate their adherence with cervical cancer screening,54 others55 have suggested that self-report offers a reasonable approximation of cervical cancer screening behavior. Future studies of this important issue should attempt to reduce this source of potential bias by validating self-report data with provider confirmation.
Although use of an Internet-based survey for this type of study represents an improvement over the methods used historically to recruit hidden populations in general and lesbian respondents, specifically (e.g., recruitment through gay bars, gay social organizations, other social gatherings of convenience), it is possible that the use of this method contributed to recruitment of a more select sample. A degree of comfort with technology was required in order to navigate the web survey. Lesbians who were less comfortable with computer and Internet use were less likely to be recruited for the study. The consequence of this is that generalizability of results may be limited; therefore, the findings reported herein may not be representative of all lesbian women.
Given the preliminary nature of this investigation, caution should be exercised when generalizing its results. Although it was our intention to recruit a geographically and demographically diverse sample, our participants were recruited via nonprobability sampling techniques, and the sample is somewhat select. We achieved a level of geographic and sociodemographic variability within the sample respondents; however, in general, our sample was highly educated (a large proportion had at least some college education) and had some form of insurance; the racial and ethnic diversity of respondents was also somewhat limited. It was noteworthy, however, that 28% of respondents had no health insurance; this is nearly twice the rate of being uninsured reported for the general population and suggests that a notable proportion of respondents were not of middle or upper SES. Findings from this study should be interpreted with caution, as they may not generalize to samples that are more varied with respect to sociodemographic characteristics.
Conclusions
Cervical cancer is a preventable disease that disproportionately affects underserved groups of women in the United States. Lesbians constitute one such group. Lesbians have a higher prevalence of several modifiable cervical cancer risk factors, such as smoking and obesity, compared with the general population of women. Lesbians are at risk of contracting HPV from a female partner but do not participate in Pap screening examinations at recommended rates. Lesbians who do not screen for cervical cancer at recommended rates were more likely to report experiences of discrimination in healthcare systems, less likely to disclose sexual orientation to a healthcare provider, and less likely to be knowledgeable of current cervical cancer screening guidelines than those who do screen at recommended rates. Additionally, lesbians who do not adhere to screening guidelines perceive more barriers and fewer benefits to screening than those who do adhere to screening guidelines, even after adjusting for age, race, and education. Public health interventions focused on increasing adherence to cervical cancer screening guidelines in lesbians should be designed accordingly.
Disclosure Statement
The authors have no conflicts of interest to report.
References
- 1.American Cancer Society. Cancer facts and figures 2009. Atlanta: ACS; 2009. [Google Scholar]
- 2.Schiffman MH. Brinton LA. Devesa SS. Fraumeni JF., Jr . In: Cancer epidemiology and prevention. Schottenfeld D, editor; Fraumeni JF Jr., editor. New York: Oxford University Press; 1996. pp. 1090–1116. [Google Scholar]
- 3.Matthews AK. Brandenburg DL. Johnson TP. Hughes TL. Correlates of underutilization of gynecological cancer screening among lesbian and heterosexual women. Prev Med. 2004;38:105–113. doi: 10.1016/j.ypmed.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 4.Harris Interactive. New national survey shows financial concerns and lack of adequate health insurance are top causes for delay by lesbians in obtaining health care. 2005. www.harrisinteractive.com/news/allnewsbydate.asp?NewsID = 899 www.harrisinteractive.com/news/allnewsbydate.asp?NewsID = 899
- 5.Diamant AL. Schuster MA. Lever J. Receipt of preventive health care services by lesbians. Am J Prev Med. 2000;19:141–148. doi: 10.1016/s0749-3797(00)00192-6. [DOI] [PubMed] [Google Scholar]
- 6.Diamant AL. Wold C. Spritzer K. Gelberg L. Health behaviors, health status, and access to and use of health care: A population-based study of lesbian, bisexual, and heterosexual women. Arch Fam Med. 2000;9:1043–1051. doi: 10.1001/archfami.9.10.1043. [DOI] [PubMed] [Google Scholar]
- 7.Rankow EJ. Lesbian health issues for the primary care provider. J Fam Pract. 1995;40:486–496. [PubMed] [Google Scholar]
- 8.Rankow EJ. Tessaro I. Cervical cancer risk and Papanicolaou screening in a sample of lesbian and bisexual women. J Fam Pract. 1998;47:139–143. [PubMed] [Google Scholar]
- 9.American College of Obstetricians and Gynecologists. Revised cervical cancer screening guidelines require re-education of women and physicians. 2008. www.acog.org/from_home/publications/press_releases/nr05-04-04-1.cfm www.acog.org/from_home/publications/press_releases/nr05-04-04-1.cfm
- 10.American Cancer Society. Risk factors for cervical cancer fact sheet. 2009. www.cancer.org www.cancer.org
- 11.U.S. Preventive Services Task Force. Screening for cervical cancer. www.ahrq.gov/clinic/uspstf/uspscerv.htm www.ahrq.gov/clinic/uspstf/uspscerv.htm
- 12.Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System. www.cdc.gov/brfss/ www.cdc.gov/brfss/
- 13.Bradford J. Ryan C. The national lesbian health care survey final report. Washington, DC: National Lesbian and Gay Health Foundation; 1987. [Google Scholar]
- 14.Johnson SR. Smith EM. Guenther SM. Comparison of gynecologic health care problems between lesbians and bisexual women. A survey of 2,345 women. J Reprod Med. 1987;32:805–811. [PubMed] [Google Scholar]
- 15.Roberts SJ. Sorensen L. Health-related behaviors and cancer screening of lesbians: Results from the Boston lesbian health project. Women Health. 1999;28:1–12. doi: 10.1300/J013v28n04_01. [DOI] [PubMed] [Google Scholar]
- 16.Solarz A. Lesbian health: Current assessment and directions for the future. Washington, DC: National Academies Press; 1999. [PubMed] [Google Scholar]
- 17.Munoz N. Bosch FX. de Sanjose S, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–527. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- 18.Koutsky LA. Galloway DA. Holmes KK. Epidemiology of genital human papillomavirus infection. Epidemiol Rev. 1988;10:122–163. doi: 10.1093/oxfordjournals.epirev.a036020. [DOI] [PubMed] [Google Scholar]
- 19.Koutsky L. Epidemiology of genital human papillomavirus infection. Am J Med. 1997;102:3–8. doi: 10.1016/s0002-9343(97)00177-0. [DOI] [PubMed] [Google Scholar]
- 20.Marrazzo JM. Koutsky LA. Stine KL, et al. Genital human papillomavirus infection in women who have sex with women. J Infect Dis. 1998;178:1604–1609. doi: 10.1086/314494. [DOI] [PubMed] [Google Scholar]
- 21.Marrazzo JM. Stine K. Koutsky LA. Genital human papillomavirus infection in women who have sex with women: A review. Am J Obstet Gynecol. 2000;183:770–774. doi: 10.1067/mob.2000.106681. [DOI] [PubMed] [Google Scholar]
- 22.Marrazzo JM. Koutsky LA. Kiviat NB. Kuypers JM. Stine K. Papanicolaou test screening and prevalence of genital human papillomavirus among women who have sex with women. Am J Public Health. 2001;91:947–952. doi: 10.2105/ajph.91.6.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marrazzo JM. Koutsky LA. Handsfield HH. Characteristics of female sexually transmitted disease clinic clients who report same-sex behaviour. Int J STD AIDS. 2001;12:41–46. [PubMed] [Google Scholar]
- 24.Marrazzo JM. Genital human papillomavirus infection in women who have sex with women: A concern for patients and providers. AIDS Patient Care STDS. 2000;14:447–451. doi: 10.1089/108729100416669. [DOI] [PubMed] [Google Scholar]
- 25.Grindel CG. McGehee LA. Patsdaughter CA. Roberts SJ. Cancer prevention and screening behaviors in lesbians. Women Health. 2006;44:15–39. doi: 10.1300/j013v44n02_02. [DOI] [PubMed] [Google Scholar]
- 26.Leyden WA. Manos MM. Geiger AM, et al. Cervical cancer in women with comprehensive health care access: Attributable factors in the screening process. J Natl Cancer Inst. 2005;97:675–683. doi: 10.1093/jnci/dji115. [DOI] [PubMed] [Google Scholar]
- 27.Kenter GG. Schoonderwald EM. Koelma IA. Arentz N. Hermans J. Fleuren GJ. The cytological screening history of 469 patients with squamous cell carcinoma of the cervix uteri: Does interval carcinoma exist? Acta Obstet Gynecol Scand. 1996;75:400–403. doi: 10.3109/00016349609033339. [DOI] [PubMed] [Google Scholar]
- 28.Cochran SD. Mays VM. Bowen D, et al. Cancer-related risk indicators and preventive screening behaviors among lesbians and bisexual women. Am J Public Health. 2001;91:591–597. doi: 10.2105/ajph.91.4.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Champion VL. Instrument refinement for breast cancer screening behaviors. Nurs Res. 1993;42:139–143. [PubMed] [Google Scholar]
- 30.Champion VL. Instrument development for Health Belief Model constructs. ANS Adv Nurs Sci. 1984;6:73–85. doi: 10.1097/00012272-198404000-00011. [DOI] [PubMed] [Google Scholar]
- 31.Champion VL. Skinner CS. Differences in perceptions of risk, benefits, and barriers by stage of mammography adoption. J Womens Health. 2003;12:277–286. doi: 10.1089/154099903321667618. [DOI] [PubMed] [Google Scholar]
- 32.Carmel S. Shani E. Rosenberg L. The role of age and an expanded health belief model in predicting skin cancer protective behavior. Health Educ Res. 1994;9:433–447. doi: 10.1093/her/9.4.433. [DOI] [PubMed] [Google Scholar]
- 33.Plowden KO. Using the Health Belief Model in understanding prostate cancer in African American men. ABNF J. 1999;10:4–8. [PubMed] [Google Scholar]
- 34.Gillam SJ. Understanding the uptake of cervical cancer screening: The contribution of the Health Belief Model. Br J Gen Pract. 1991;41:510–513. [PMC free article] [PubMed] [Google Scholar]
- 35.Janz N. Champion V. Strecher V. The Health Belief Model. In: Health behavior and health education: Theory, research, and practice. 3rd. San Francisco, CA: Jossey-Bass; 2002. [Google Scholar]
- 36.Jordan KM. Deluty RH. Coming out for lesbian women: Its relation to anxiety, positive affectivity, self-esteem, and social support. J Homosex. 1998;35:41–63. doi: 10.1300/J082v35n02_03. [DOI] [PubMed] [Google Scholar]
- 37.Tracy JK. Junginger J. Correlates of lesbian sexual functioning. J Womens Health. 2007;16:499–509. doi: 10.1089/jwh.2006.0308. [DOI] [PubMed] [Google Scholar]
- 38.Hislop TG. Deschamps M. Teh C, et al. Facilitators and barriers to cervical cancer screening among Chinese Canadian women. Can J Public Health. 2003;94:68–73. doi: 10.1007/BF03405056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kass NE. Faden RR. Fox R. Dudley J. Homosexual and bisexual men's perceptions of discrimination in health services. Am J Public Health. 1992;82:1277–1279. doi: 10.2105/ajph.82.9.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thomas VN. Saleem T. Abraham R. Barriers to effective uptake of cancer screening among black and minority ethnic groups. Int J Palliat Nurs. 2005;11(562):564–571. doi: 10.12968/ijpn.2005.11.11.20096. [DOI] [PubMed] [Google Scholar]
- 41.Simoes EJ. Newschaffer CJ. Hagdrup N, et al. Predictors of compliance with recommended cervical cancer screening schedule: A population-based study. J Community Health. 1999;24:115–130. doi: 10.1023/a:1018754307718. [DOI] [PubMed] [Google Scholar]
- 42.Marcus AC. Crane LA. A review of cervical cancer screening intervention research: Implications for public health programs and future research. Prev Med. 1998;27:13–31. doi: 10.1006/pmed.1997.0251. [DOI] [PubMed] [Google Scholar]
- 43.Garner EI. Cervical cancer: Disparities in screening, treatment, and survival. Cancer Epidemiol Biomarkers Prev. 2003;12:242s–247s. [PubMed] [Google Scholar]
- 44.Owusu GA. Eve SB. Cready CM, et al. Race and ethnic disparities in cervical cancer screening in a safety-net system. Matern Child Health J. 2005;9:285–295. doi: 10.1007/s10995-005-0004-8. [DOI] [PubMed] [Google Scholar]
- 45.Rodriguez MA. Ward LM. Perez-Stable EJ. Breast and cervical cancer screening: Impact of health insurance status, ethnicity, and nativity of latinas. Ann Fam Med. 2005;3:235–241. doi: 10.1370/afm.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coughlin SS. Uhler RJ. Hall HI. Briss PA. Nonadherence to breast and cervical cancer screening: What are the linkages to chronic disease risk? Prev Chronic Dis. 2004;1:A04. [PMC free article] [PubMed] [Google Scholar]
- 47.Pearlman DN. Clark MA. Rakowski W. Ehrich B. Screening for breast and cervical cancers: The importance of knowledge and perceived cancer survivability. Women Health. 1999;28:93–112. doi: 10.1300/J013v28n04_06. [DOI] [PubMed] [Google Scholar]
- 48.Behbakht K. Lynch A. Teal S. Degeest K. Massad S. Social and cultural barriers to Papanicolaou test screening in an urban population. Obstet Gynecol. 2004;104:1355–1361. doi: 10.1097/01.AOG.0000143881.53058.81. [DOI] [PubMed] [Google Scholar]
- 49.Brown JP. Tracy JK. Lesbians and cancer: An overlooked health disparity. Cancer Causes Control. 2008;19:1009–1020. doi: 10.1007/s10552-008-9176-z. [DOI] [PubMed] [Google Scholar]
- 50.Price JH. Easton AN. Telljohann SK. Wallace PB. Perceptions of cervical cancer and Pap smear screening behavior by women's sexual orientation. J Community Health. 1996;21:89–105. doi: 10.1007/BF01682301. [DOI] [PubMed] [Google Scholar]
- 51.O'Hanlan KA. Crum CP. Human papillomavirus-associated cervical intraepithelial neoplasia following lesbian sex. Obstet Gynecol. 1996;88:702–703. doi: 10.1016/0029-7844(96)00206-2. [DOI] [PubMed] [Google Scholar]
- 52.Roberts SJ. Health care recommendations for lesbian women. J Obstet Gynecol Neonat Nurs. 2006;35:583–591. doi: 10.1111/j.1552-6909.2006.00081.x. [DOI] [PubMed] [Google Scholar]
- 53.Mravcak SA. Primary care for lesbians and bisexual women. Am Fam Physician. 2006;74:279–286. [PubMed] [Google Scholar]
- 54.McPhee SJ. Nguyen TT. Shema SJ, et al. Validation of recall of breast and cervical cancer screening by women in an ethnically diverse population. Prev Med. 2002;35:463–473. doi: 10.1006/pmed.2002.1096. [DOI] [PubMed] [Google Scholar]
- 55.Caplan LS. McQueen DV. Qualters JR. Leff M. Garrett C. Calonge N. Validity of women's self-reports of cancer screening test utilization in a managed care population. Cancer Epidemiol Biomarkers Prev. 2003;12:1182–1187. [PubMed] [Google Scholar]