Abstract
Embryonic stem (ES) cells fluctuate between self-renewal and the threshold of differentiation. Signalling via the fibroblast growth factor (Fgf)/Erk pathway is required to progress from this dynamic state and promote mouse ES cell differentiation. Retinoic acid also induces differentiation in many cellular contexts, but its mechanism of action in relation to Fgf/Erk signalling in ES cells is poorly understood. Here, we show for the first time that endogenous retinoid signalling is required for the timely acquisition of somatic cell fate in mouse ES cells and that exposure to retinoic acid advances differentiation by a dual mechanism: first increasing, but in the long-term decreasing, Fgf signalling. Rapid retinoid induction of Fgf8 and downstream Erk activity on day 1 in differentiation conditions may serve to ensure loss of self-renewal. However, more gradual repression of Fgf4 by retinoic acid is accompanied by an overall reduction in Erk activity on day 2, and the acquisition of neural and non-neural fates is now advanced by inhibition of Fgf signalling. So, although blocking Fgf/Erk activity is known to promote ES cell self-renewal, once cells have experienced a period of such signals, subsequent inhibition of Fgf signalling has the opposite effect and drives differentiation. We further show in the embryo that retinoid repression of Fgf signalling promotes neural differentiation onset in an analogous step in the extending embryonic body axis and so identify attenuation of Fgf signalling by retinoic acid as a conserved fundamental mechanism driving differentiation towards somatic cell fates.
Keywords: Fgf, Chick embryo, Differentiation, Embryonic stem cells, Mouse, Retinoic acid
INTRODUCTION
Differentiating mouse embryonic stem (ES) cells first downregulate pluripotency genes and transit through a primitive ectoderm-like state before adopting somatic cell fates. Blocking fibroblast growth factor (Fgf) signalling via the Erk1/2 MAP kinase (Mapk) pathway promotes self-renewal in mouse ES (mES) cells (Burdon et al., 1999; Ying et al., 2008) (reviewed by Silva and Smith, 2008), whereas exposure to such signalling is required for differentiation into neural and non-neural cell fates (Kunath et al., 2007; Stavridis et al., 2007). A period of Fgf/Erk signalling thus appears to be a first step away from the self-renewal cell state in this context. It is also evident, at least for neural differentiation, that such signals are only briefly required because, after 24 hours, blocking Erk signalling no longer inhibits the later onset of the neural progenitor marker Sox1 (Stavridis et al., 2007). This suggests that a particular Fgf/Erk signalling dynamic could underlie differentiation progression.
Another signalling molecule, retinoic acid (RA), also promotes differentiation in a range of tissues and cell lines, as well as in ES cells. Depending on culture conditions, retinoid signalling can promote ES cell differentiation into endoderm, adipocytes, fibroblast-like cells or neural tissue (reviewed by Soprano et al., 2007). In particular, neural fate is routinely elicited from ES cell aggregates (embryoid bodies) cultured in differentiation conditions for 4 days and then treated with RA (e.g. Aubert et al., 2002; Bain et al., 1995). The inclusion of a long period in an aggregated form in this protocol prior to exposure to RA suggests that, in this cellular context, RA acts after loss of self-renewal to promote differentiation. However, there is also evidence that RA has an earlier action via repression of the pluripotency gene Oct4 in ES cells (Gu et al., 2005) and that it might work directly via a retinoic acid response element (RARE) in the Oct4 promoter, as has been reported in P19 EC cells (Barnea and Bergman, 2000; Okazawa et al., 1991; Pikarsky et al., 1994). This may be one way in which RA influences Fgf signalling during differentiation, as Oct4 together with Sox2 promotes Fgf4 expression in ES cells (Yuan et al., 1995). Fgf4 is the principal source of Erk signalling in differentiating mES cells, as indicated by reduced dP-Erk levels and the poor differentiation of Fgf4−/− ES cells (Kunath et al., 2007), and so RA could limit an initial period of high Fgf/Erk activity by repressing Oct4.
The regulatory relationship between retinoid and Fgf signalling pathways differs depending on the cellular context. In the mouse and chick embryonic axis, RA promotes differentiation by inhibiting expression of Fgf8 as cells leave the tailbud (reviewed by Wilson et al., 2009); a step that may be analogous to RA-mediated downregulation of Fgf4 in ES cells. Furthermore, RA and Fgf pathways are mutually inhibitory in the embryonic axis (Diez del Corral et al., 2003), and elevated Fgf or reduced RA signalling is characteristic of many cancerous cell states (reviewed by Diez del Corral and Storey, 2004). These studies therefore suggest that RA attenuation of Fgf signalling is a fundamental signalling mechanism controlling cellular differentiation. In other contexts, however, RA can promote Fgf8 transcription, for example, in neurula-stage frog embryos (Moreno and Kintner, 2004). There is also evidence that RA receptors (RARs) can bind Fgf8 upstream elements (Brondani et al., 2002; Zhao et al., 2009), indicating that RA might directly regulate Fgf8. In addition, RA can activate the Erk pathway by so-called non-genomic mechanisms, which do not involve a transcriptional response: in PC12 and bronchial epithelial cells Erk activation does not require RARs (Aggarwal et al., 2006; Canon et al., 2004), but in neuroblastoma cells Erk activation involves direct binding and activation of PI 3-kinase subunits by liganded RAR (Masia et al., 2007), and RA can activate Erk in an RAR-dependent manner within 10 minutes in neurons (Chen and Napoli, 2008).
Here, we investigate the mechanisms by which retinoid signalling promotes mouse ES cell differentiation, using monolayer neural differentiation as an example. We demonstrate a requirement for endogenous RA for progression from a primitive ectoderm-like state towards the neural progenitor cell fate. We further show that exogenous RA requires an intact Fgf/Erk signalling pathway to drive ES cell differentiation. Indeed, RA treatment initially stimulates Erk activity and we find that this does not involve known non-genomic mechanisms, but is mediated at least in part by rapid Fgf8 induction. However, RA exposure also gradually represses Fgf4 and we reveal that, once cells have experienced a period of endogenous Fgf/Erk signalling and have acquired a primitive ectoderm-like state, RA treatment inhibits Erk activity. We show that inhibition of Fgfr signalling, rather than promoting self-renewal as it does in ES cells, now mimics the ability of RA to promote neural, or in the presence of Bmp4, non-neural differentiation, and that an analogous regulatory step initiates neural differentiation in the embryonic body axis.
MATERIALS AND METHODS
ES cell culture
Cells were grown, maintained and differentiated as described previously (Stavridis et al., 2007). 46C ES cells (expressing Sox1-GFP) were kindly provided by Austin Smith (University of Cambridge, UK) and Rex1-GFP/Oct4::CFP cells were generously provided by Hitoshi Niwa, Riken CDB (Toyooka et al., 2008). All-trans RA (Sigma) was used at 5 nM unless stated otherwise and Bmp4 (R&D Systems) at 10 ng/ml. PD173074 (Mohammadi et al., 1998; Mohammadi et al., 1997) (a kind gift of Pfizer) was added at 0.25 μM. RAR and RXR antagonists LG100815 and LG101208 (Sockanathan and Jessell, 1998) were a kind gift of Ligand Pharmaceuticals and were used at 0.5 μM. Cell viability was assessed following all inhibitor treatments by the proportion of non-viable cells staining with To-Pro3 in flow cytometry experiments. The Fgf8-blocking antibody and isotype control were supplied by R&D Systems (MAB323 and MAB002, respectively).
Immunoblotting
Immunoblotting was performed as described previously (Stavridis et al., 2007). All results shown are representative of three or more experiments, unless stated otherwise. Antibodies used were: anti-Crabp1 (Affinity Bioreagents, #MA3-813); anti-α-tubulin (Abcam, #ab7291); anti-phospho-Erk1/2 (Thr202/Tyr204; #9101), anti-Histone-H3 (#9717) and anti-total-Erk1/2 (#9102), all from Cell Signaling Technology. Secondary antibodies for fluorescence immunoblotting were 610132121 (Rockland) and A21109 (Invitrogen). Membranes were scanned on a LiCor scanner and analysed with Odyssey software.
Immunocytochemistry
Immunocytochemistry was performed as described in Kunath et al. (Kunath et al., 2007). Antibodies used were as follows: anti-Oct4 Santa Cruz (#sc-8628); LE61 supernatant (against keratins 8/18), provided by Birgit Lane (University of Dundee, UK), used neat on methanol-fixed samples; anti-Phospho-Erk (Cell Signaling Technologies, #4370); anti-Nanog (Abcam, ab21603); anti-Pax6 (Developmental Studies Hybridoma Bank, University of Iowa, USA).
Flow cytometry
Flow cytometry was performed as described previously (Stavridis et al., 2007). Results shown are from a representative of three or more experiments performed in triplicate unless stated otherwise.
QRT-PCR
Quantitative RT-PCR was performed as described previously (Stavridis et al., 2007). Samples were run and analysed on a Mastercycler Realplex2 (Eppendorf) using the Pfaffl method for quantification. Primer sequences and annealing temperatures are given in Table S1 in the supplementary material.
Embryo manipulations
Chick embryos at Hamburger and Hamilton stages HH9-HH10 were grafted with AGX beads soaked in carrier DMSO, RA (9-cis or All-trans RA, 0.5 μM), or Fgfr inhibitors SU5402 (4 mM) or PD173074 (4 mM), or with heparin-coated beads soaked in PBS, Fgf4 (100 ng/ml) or Fgf8b (200 ng/ml) (see Storey et al., 1998). Following incubation at 38°C for desired periods, embryos were processed for in situ hybridisation for chick Sox1 (kind gift of Hisato Kondoh, Osaka University, Japan) or chick Sox3, using standard procedures. Retinoid-deficient quail embryos were a gift of Emily Gale and Malcolm Maden (King's College London, UK), and normal and retinoid-deficient quail embryos were fixed and processed together (see Diez del Corral et al., 2003).
Statistics
Statistical analysis was performed using Student's t-test at P<0.05, unless stated otherwise.
RESULTS
Endogenous retinoid activity promotes ES cell neural differentiation
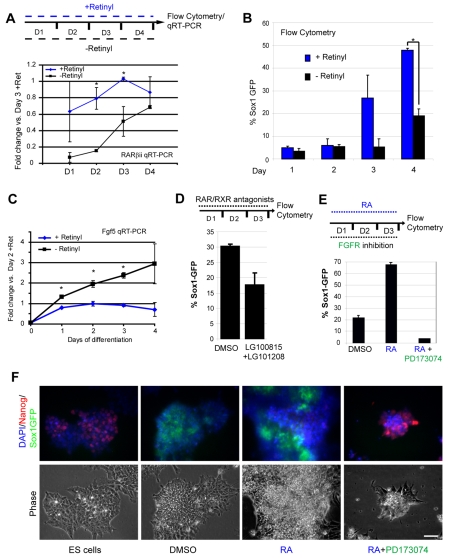
To investigate how retinoid signalling promotes ES cell differentiation, an ES cell line with GFP expression driven by the promoter of the neural progenitor marker gene Sox1 (Aubert et al., 2003; Ying et al., 2003) was used to provide cell quantification by flow cytometry. The requirement for endogenous retinoid production was first tested by plating ES cells in monolayer differentiation conditions [lacking serum and leukaemia inhibitory factor (Lif) in N2B27 medium] but using B27 supplement deficient in retinyl, a precursor of RA (− retinyl conditions). Reduction of retinoid signalling in retinyl-deficient conditions was assessed by analysis of the RA-responsive gene Rarb, detected by quantitative reverse-transcription PCR (qRT-PCR; Fig. 1A). Retinoid synthesis commences in ES cells on withdrawal of Lif (Lane et al., 1999) and Rarb can be detected from the first 24 hours of differentiation (with some variability) in ‘+ retinyl’ conditions; however, a consistent 10-fold increase in Rarb transcripts compared with in ‘− retinyl’ conditions is detected by day 2. This indicates that culture in retinyl-deficient media leads to attenuation of retinoid signalling in ES cells in differentiation conditions (at least for the first three days after plating). Importantly, the percentage of Sox1-GFP-positive cells was significantly lower in retinyl-deficient conditions (Fig. 1B). In these conditions, cells downregulate genes characteristic of the inner cell mass (ICM; Nanog and Rex1) (Chambers et al., 2003; Mitsui et al., 2003; Pelton et al., 2002), continue to express the pluripotency gene Oct4 (Nichols et al., 1998) (see Fig. S1 in the supplementary material), but accumulate in an Fgf5-positive state characteristic of primitive ectoderm (Haub and Goldfarb, 1991; Hebert et al., 1991), as indicated by the significant increase of Fgf5 transcripts over days 1-4 (Fig. 1C). Reduction of retinoid signalling by a different mechanism, using RAR (LG100815) and RXR (LG101208) antagonists to block RA signal transduction, also led to inhibition of the onset of Sox1-GFP expression (Fig. 1D). These experiments indicate that reduction of endogenous RA activity compromises progression from a primitive ectoderm-like state towards the neural progenitor cell fate and suggest that RA signals are normally required for acquisition of somatic cell fates.
Fig. 1.
Retinoid signalling is required for neural differentiation of mES cells and depends on Fgf signalling. (A) Rarb transcripts rise during 4 days of differentiation in standard N2B27 media (+Retinyl), but are significantly lower in cells in N2B27 lacking retinyl (−Retinyl); data from two independent experiments (*P<0.05). (B) Emergence of Sox1-GFP expressing neural progenitors is impaired without retinyl, as analysed by flow cytometry. Results are average values ±s.e.m. from two independent experiments (*P<0.05). (C) Fgf5 transcripts are increased in cells in −Retinyl conditions. Results are averages ±s.e.m. from a representative of two experiments performed in triplicate (*P<0.05). (D) Differentiation over 3 days is impaired in the presence of 0.5 mM of each of LG100815 and LG101208 (RAR and RXR inhibitors, respectively), as indicated by expression of Sox1-GFP. Results are average of two experiments performed in triplicate +s.e.m. (E) All-trans RA (5 nM) promotes neural differentiation in monolayer conditions, but this effect is blocked by Fgfr inhibitor PD173074 (250 nM). Results are averages from a representative experiment performed in triplicate ±s.e.m. (F) Photomicrographs of experiment as in E. In the presence of PD173074, RA fails to induce differentiation and Nanog levels remain high. Scale bar: 50 μm.
Retinoid signalling drives neural differentiation, but does not obviate the requirement for Fgf/Erk signalling
To examine the ability of retinoid signalling to promote neural differentiation, all-trans RA (5 nM) was added to ES cells at plating in differentiation conditions. At day 3, flow cytometry revealed a clear increase in the number of cells plated in RA that were Sox1-GFP positive (Fig. 1E). A similar result was also obtained with an independently isolated wild-type cell line (Collins et al., 2003), which exhibited upregulation of the neural progenitor marker nestin in response to RA (data not shown). This demonstrates that retinoid signalling can promote the acquisition of neural progenitor status in ES cells cultured in monolayer differentiation conditions.
As a period of Fgf/Erk signalling is required for ES cell differentiation (Kunath et al., 2007; Stavridis et al., 2007), we next tested whether the ability of RA to drive Sox1 expression is dependent on Fgf/Erk signalling. Cells were plated in differentiation conditions and cultured for 3 days either in the high affinity Fgf receptor inhibitor PD173074 together with RA, or with RA alone. The presence of PD173074 prevented RA induction of Sox1 expression (Fig. 1E,F) and cells remained in a self-renewing state, as indicated by persisting expression of the pluripotency gene Nanog (Fig. 1F). These findings indicate that retinoid signalling cannot substitute for Fgf/Erk signalling in order to initiate the normal differentiation process. This might indicate that a prior period of such signalling is required before RA can act, but it also raises the possibility that RA promotes differentiation by stimulating the Fgf signalling pathway.
RA initially stimulates Fgf/Erk signalling
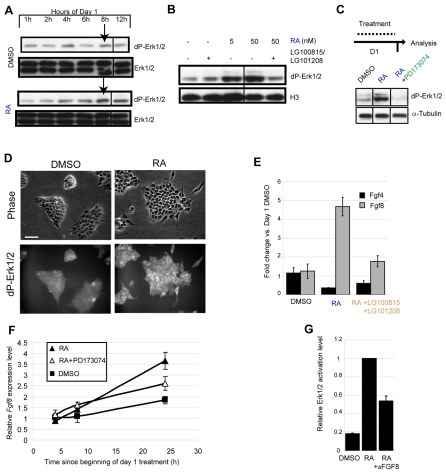
To investigate the impact of RA on the Fgf pathway, potential downstream Erk activity (measured by the phosphorylation status of Thr202/Tyr204) was assessed at 2-hour intervals in ES cells placed in monolayer differentiation conditions following exposure to RA or a DMSO-only control. Importantly, exposure to RA but not DMSO elicited an increase in Erk activity in these day 1 cells by 8 hours (Fig. 2A). This is unlikely to be generated by non-genomic mechanisms that are independent of retinoic acid receptors (Aggarwal et al., 2006; Canon et al., 2004), as Erk phosphorylation was blocked in the presence of RAR/RXR antagonists (Fig. 2B). Non-genomic actions of RA that do depend on RAR are either too fast acting to account for increased Erk activity at 8 hours (Chen and Napoli, 2008) or they rely on PI 3-kinase signalling (Masia et al., 2007), inhibition of which in the presence of RA does not reduce Erk signalling (see Fig. S2 in the supplementary material). These data suggest that RA does not stimulate Erk activity in this ES cell context by known non-genomic mechanisms and we therefore next set out to confirm that the ability of RA to promote an increase in Erk activity on day 1 is dependent on Fgfr signalling.
Fig. 2.
Initial exposure to RA induces Fgf8/Erk signalling during ES cell differentiation. (A) On day 1 of differentiation RA addition causes Erk activation by 8 hours, western blots represent two independent experiments, arrows indicate elevated dP-Erk in RA but not DMSO conditions. (B) Treatment with 0.5 mM of each of LG100815 and LG101208 blocks the ability of 50 nM RA to induce an increase in dP-Erk levels by 8 hours on day 1 of differentiation. This experiment was performed twice with the same result. (C) PD173074 blocks RA induction of dP-Erk by 24 hours on day 1 of differentiation. This experiment was performed twice with the same result. α-Tubulin was used as a loading control. (D) RA-treatment on day 1 increases dP-Erk in differentiating cells. Scale bar: 50 μm. (E) On day 1, RA reduces Fgf4 but increases Fgf8, and these effects are both inhibited by RAR/RXR antagonists. Results are weighted means ±s.e.m. from two independent experiments performed in triplicate. (F) Fgf8 transcripts rise in response to RA during day 1 even when differentiation is blocked by PD173074, representative of two experiments, ±s.e.m. (G) The ability of RA to induce dP-Erk1/2 is attenuated with 2.5 μg/ml anti-Fgf8 blocking antibody for 24 hours. dP-Erk was quantified by fluorescence immunoblotting. This experiment was carried out twice; result shown is the mean from a representative experiment performed in triplicate ±s.e.m.
At the 24-hour time point, RA still elicited robust high-level Erk activity in comparison with control DMSO-treated cells (Fig. 2C), and cell-by-cell analysis revealed a largely ubiquitous increase in Erk signalling across the day 1 cell population in response to RA (Fig. 2D). However, exposure to RA and an Fgfr inhibitor blocked this increase in Erk activity (Fig. 2C). This indicates that RA promotes Erk phosphorylation via Fgfrs and confirms that Fgfrs are responsible for Erk1/2 activity during early ES cell differentiation (Stavridis et al., 2007). To elucidate this signalling mechanism, the effects of RA on Fgf4 and Fgf8 expression were examined. RA treatment of differentiating ES cells on day 1 led to a reduction in Fgf4, but this was accompanied by a large increase in Fgf8, as measured by qRT-PCR, and both of these actions were found to be RAR/RXR dependent (Fig. 2E). Detailed analysis of Fgf8 transcription by qRT-PCR further shows progressive upregulation from ~8 hours in response to RA, consistent with increasing Erk activity within this timeframe (Fig. 2F). Furthermore, RA can induce Fgf8 expression even when differentiation is blocked with PD173074 (Fig. 2F), indicating that Fgf8 induction is not simply a downstream consequence of differentiation. Importantly, the RA-induced increase in Erk activity on day 1 is attenuated in the presence of an Fgf8-blocking antibody (but not by a non-specific isotype control, data not shown; Fig. 2G), indicating that Fgf8 contributes to this RA action. Overall, these data suggest that RA stimulates Erk activity in day 1 ES cells, at least in part, via a mechanism that involves RAR/RXR-mediated increase of Fgf8. This initial increase in Erk signalling in response to RA may help to ensure loss of ES cell self-renewal as Fgf4 levels begin to decline.
Following a period of endogenous Fgf activity, RA promotes differentiation by attenuating Fgf signalling
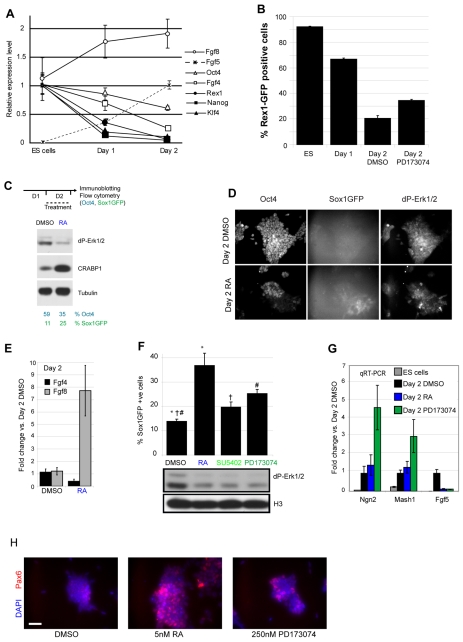
Retinoid signalling can repress Oct4 expression, and Oct4 in conjunction with Sox2 is required to maintain expression of Fgf4 in ES cells (Gu et al., 2005; Yuan et al., 1995). Because of the indirect way in which RA acts to repress Fgf4, this consequence of RA signalling may take longer than does the induction of Fgf8. Consistent with this, qRT-PCR analysis of changes in the endogenous transcripts of these ligands reveals that Fgf8 levels rise on day 1 prior to the major downregulation of Fgf4 on day 2 (Fig. 3A). To understand better the changes taking place over these first two days of differentiation, we next compared transcript levels of key pluripotency and differentiation genes across this period. At the end of day 1, cells have experienced endogenous Fgf/Erk signalling, Rarb (see Fig. 1A), Fgf8 and Fgf5 levels are beginning to rise, and expression of the pluripotency genes Nanog and Klf4, and also of Rex1, decline (Fig. 3A). By contrast, levels of Oct4 and Fgf4 show little change with respect to ES cell levels (indeed flow cytometry indicates that 99% of cells are Oct4 positive at this time point, data not shown). During day 2, however, Oct4 and Fgf4 levels begin to decline (Fig. 3A). At this time, Fgf5 is expressed at its highest levels (Fig. 1C), indicative of the acquisition of a primitive ectoderm-like state. Cell quantification analysis using GFP reporter lines indicates that there is a small increase in Sox1-expressing cells from <2% on day 1 to ~10% at the end of day 2 (Fig. 1B, and see below) (see also Lowell et al., 2006; Ying et al., 2003), and that Rex1-expressing cells (Toyooka et al., 2008) decline from 67% GFP-positive cells on day 1 to 19% by day 2 (Fig. 3B). Given the low level of Rex1 transcripts at these time points, this indicates some perdurance of the GFP protein, but also confirms that by day 2 the vast majority of cells are Rex1 negative. So, whereas at the end of day 1 31% of cells are likely to be primitive ectoderm-like cells (lacking Rex1 and Sox1 expression), by day 2 this proportion has increased to 71%. Although this is therefore a heterogeneous cell population, which includes some ES cells and possibly some Epi-stem-like cells [that retain Nanog, but that lack other ICM genes, e.g. Rex1 and Klf4 (Brons et al., 2007)], the prevailing transcriptional profile on day 2 is of a Nanog-, Rex1- and Klf4-negative, Oct4-, Fgf4-, Fgf8- and Fgf5-positive primitive ectoderm-like cell.
Fig. 3.
Inhibition of Fgf signalling mimics retinoid induction of neural differentiation. (A) Gene expression profile of key genes during monolayer differentiation relative to expression in undifferentiated ES cells (for Fgf5 relative to expression at day 2). Transcript levels in ES cells and at day 1 are significantly different for all genes (P<0.05), except Oct4, Fgf4 and Fgf8, comparison of levels in ES cells and at day 2 for Oct4 and Fgf4 are P<0.05 and 0.06, respectively. Data are means ±s.e.m. from a representative experiment performed in triplicate. (B) Rex1-GFP cells in N2B27, analysed by flow cytometry. PD173074 treatment during day 2 increases Rex1-GFP+ cells compared to control (DMSO), suggesting a reversion of some cells to the pluripotent state. Results are means of two experiments performed in triplicate +s.e.m. All pairs of treatments are significantly different (P<0.05). (C) RA treatment for 24 hours during day 2 causes a decrease in dP-Erk and induction of Crabp1 [an RA-responsive gene (Lane et al., 2008)] by western blotting, but a decrease in Oct4-positive cells and an increase in Sox1-positive cells as analysed by flow cytometry. Results are representative of three experiments performed in triplicate ±s.e.m. (D) Cells labelled for Oct4, dP-Erk1/2 and Sox1-GFP, following day 2 treatment. RA treatment reduced dP-Erk and Oct4 levels, but increased the number of Sox1-GFP-positive cells. (E) At the end of day 2, RA causes a further decrease in Fgf4 and increase in Fgf8, data from two independent experiments performed in triplicate. (F) On day 2, treatment with RA or Fgfr inhibitors (PD173074 or SU5402) reduces levels of dP-Erk and increases the number of Sox1-GFP-positive cells. Symbols indicate statistically significant differences between treatments at P<0.05 (paired t-test, n=5 independent experiments performed in triplicate). (G) Fgfr inhibition or RA treatment on day 2 causes a decrease in Fgf5 transcripts and an increase neural progenitor markers Ngn2 and Mash1 (P<0.05), except for RA-induction of proneural genes, which was only statistically significant on exposure to 50 nM RA (Ngn2, P<0.05). Results are averages of three experiments performed in triplicate ±s.e.m. (H) Following day 2 treatment, cells were labelled with an antibody against Pax6. Both RA and PD173074 cause an increase in the number of Pax6-positive cells, with RA having a stronger effect. Result is representative of five randomly selected fields. Scale bars: 50 μm.
To investigate the mechanism by which RA promotes differentiation after day 1, we exposed day 1 cells to RA for 24 hours and assessed the impact on Oct4 and Sox1 expression and on Erk activity. The number of Oct4 cells decreased and the number of Sox1 cells increased in response to RA (Fig. 3C). Strikingly, in contrast to exposure to RA from plating, RA now elicited a clear decrease in Erk phosphorylation by the end of day 2 (Fig. 3C). This reduction in Erk activity and increased differentiation progression, as indicated by flow cytometry analysis of Sox1-GFP, were also detected following RA treatment for 24 hours on day 3 or day 4 (see Fig. S3 in the supplementary material). These changes were further analysed on a cell-by-cell basis to determine the extent of the cell population experiencing changes in Erk activity. This revealed widespread and largely uniform Erk activity in the day 2 cell population, consistent with paracrine Fgf signalling, and that exposure to RA for 24 hours now resulted in a reduction of Oct4 and phospho-Erk1/2 levels and an increase in the number of Sox1-positive cells (Fig. 3D). In addition, RA treatment on day 2 led to further reduction in Fgf4 levels, while Fgf8 levels still increased (Fig. 2E, Fig. 3E). These findings are consistent with Fgf4 being the principal stimulator of Erk signalling in differentiating ES cells (Kunath et al., 2007), and support the hypothesis that the initial rapid increase in Fgf8 when Fgf4 is still high leads to the net dP-Erk increase on day 1, and that the slower, indirect inhibition of Fgf4 underlies the Erk decrease on day 2. Several alternative mechanisms by which RA might elicit a decrease in Erk activity were also investigated: (1) the induction of Dusp genes encoding Erk phosphatases (Mason et al., 1996; Moreno and Kintner, 2004); (2) induction of the Fgfr/Erk antagonist Sprouty2 (Minowada et al., 1999); and (3) the repression of Fgfrs (McDonald and Heath, 1994; Mummery et al., 1990). However, RA did not induce these transcriptional changes in this context (see Fig. S4A-C in the supplementary material). Overall, these data suggest that a consequence of RA driven differentiation on day 2 is the attenuation of Fgf/Erk signalling due to downregulation of Fgf4.
Inhibition of Fgfr signalling also promotes differentiation on day 2
If RA promotes differentiation on day 2 via its ability to inhibit Fgf4, simply blocking Fgfr signalling should also drive this process. Comparison of the effects of RA and the Fgfr inhibitors SU5402 or PD173074 on day 2 shows that Fgfr inhibition can mimic the ability of RA to increase the emergence of Sox1-GFP-positive cells (Fig. 3F). As these small molecule inhibitors also block related Vegf receptors (Mohammadi et al., 1998), cells were exposed to KRN633, a Vegfr-specific inhibitor that does not block Fgfr signalling (Nakamura et al., 2004). This had no effect on Sox1 expression, supporting specific Fgfr inhibition by SU5402 and PD173074 in this context (see Fig. S5 in the supplementary material). PD173074 generated a ~2-fold increase in Sox1-positive cells (Fig. 3G). A reduction in Fgf5 transcription in response to RA or Fgfr inhibition was also observed, together with increases in the expression of further neural progenitor markers, such as Ngn2, Mash1 and Pax6 (Fig. 3G,H).
To define better the cell population differentiating in response to Fgfr inhibition, we further analysed the levels of Rex1-GFP expression following exposure to PD173074. This revealed that although the expression of neural progenitor markers increased (Fig. 3E), a subset of cells (~13%) now also had increased Rex1 levels compared with the DMSO control (Fig. 3B). This ‘reversion’ identifies those Rex1-negative cells that are still able to return to the ES cell state, and indicates that at the end of day 2 (when 80% of cells are Rex1 negative) 67% of cells have now embarked on differentiation.
The induction of Sox1 by PD173074 was not, interestingly, mimicked by the Mek inhibitor PD184352, indicating that attenuation of Erk signalling alone is insufficient to promote differentiation (data not shown) and implicating further Fgfr downstream consequences in this process. Importantly, these changes in gene expression in response to RA on day 2 were not accompanied by any alteration in cell cycle phase distribution (see Fig. S6 in the supplementary material). This suggests that reduction of Fgf5 and increased acquisition of neural progenitor status is unlikely to be due to preferential expansion of a sub-population of cells. These findings show that RA-mediated neural differentiation of day 2 primitive ectoderm-like cells can be mimicked by inhibition of Fgf signalling, which is consistent with a mechanism involving RA repression of Fgf4.
RA or Fgfr inhibition drive non-neural differentiation in the presence of Bmp4
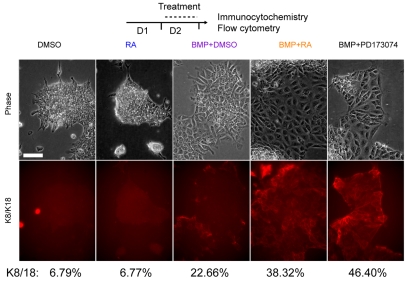
To address whether RA and Fgfr inhibition serve as generic differentiation agents or specifically promote the acquisition of a neural cell fate, the same monolayer differentiation protocol was used, but cells were instead exposed to bone morphogenetic protein 4 (Bmp4), which strongly suppresses neural fate and causes cells to adopt an epithelial morphology resembling surface ectoderm (Kunath et al., 2007; Ying et al., 2003). The early epithelial markers keratin 8 and 18 (K8/18) begin to be expressed after two days of differentiation at very low levels (Fig. 4A). Addition of RA alone during day 2 did not increase K8/18, but the presence of Bmp4 promoted K8/18 expression and exposure to RA increased this effect (Fig. 4A). Moreover, inhibition of Fgfr signalling in the presence of Bmp4 mimicked the effects of RA addition on day 2 and increased K8/18 expression. These findings therefore indicate that RA attenuation of Fgf signalling is not a neural specific step, but a generic mechanism that can promote differentiation in multiple cellular contexts.
Fig. 4.
Retinoic acid or Fgfr inhibition promote BMP-induced non-neural differentiation. During day 2 of differentiation in the presence of Bmp4, RA or Fgfr inhibitor treatment causes an increase in the proportion of keratin 8/18-positive cells, as indicated by immunocytochemistry and flow cytometry. Results shown are representative of two independent experiments. Scale bar: 25 μm.
The Fgf/RA signalling switch initiates neural differentiation in the embryonic body axis
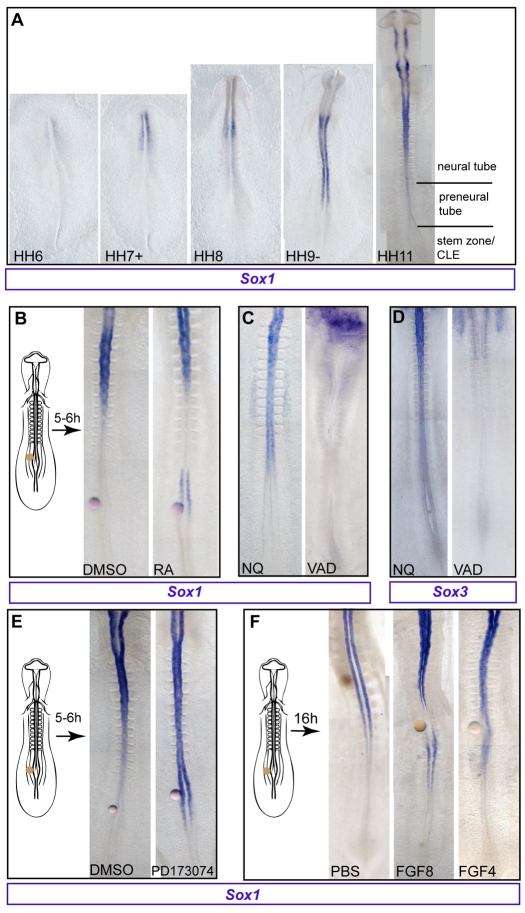
An analogous differentiation step has been identified in the extending embryonic body axis, where the temporal sequence of differentiation events is finely spatially separated as cells leave the epiblast cell population in the region of the primitive streak. Here, RA inhibition of Fgf signalling promotes differentiation as cells exit the caudal lateral epiblast (CLE)/tailbud, a region of high Fgf/Erk signalling (Diez del Corral et al., 2003; Lunn et al., 2007; Molotkova et al., 2005; Ribes et al., 2009). To examine whether onset of Sox1 transcription is similarly regulated in this embryonic context, the Sox1 mRNA expression pattern was examined in early chick embryos, which are readily amenable to local experimental manipulation of signalling pathways. Importantly, at the 10-somite stage Sox1 is specifically absent from the stem zone (the caudal lateral epiblast found adjacent to the node/primitive streak), appearing first in the more rostral preneural tube (Fig. 5A, HH9-HH11).
Fig. 5.
Sox1 onset in the embryonic axis is regulated by Fgf/retinoid signalling. (A) Sox1 mRNA is first detected in head-fold-stage chick embryos (HH stage 6) and appears progressively in a rostral to caudal direction, but is absent from the stem zone/caudal lateral epiblast (CLE). (B) Control DMSO or retinoic acid (RA) beads were grafted as indicated. All embryos were subject to in situ hybridisation for Sox1 except where stated otherwise. (C) Sox1 expression in normal quail (NQ) embryos and its reduction in vitamin A deficient (VAD) quails. (D) Sox3 expression in normal quails and its reduction in VAD embryos. (E) Beads soaked in DMSO (control) or the Fgfr inhibitor PD173074 were grafted as indicated. Sox1 expression is extended caudally in embryos grafted with PD173074-soaked beads. (F) Control (PBS), Fgf8 or Fgf4 presenting beads were grafted as indicated. Sox1 expression is locally inhibited when beads soaked in Fgf8 or Fgf4 are grafted near the preneural tube.
To test whether precocious exposure to RA is able to promote Sox1 expression, beads delivering RA were grafted caudal to the normal onset level, adjacent to the preneural tube. This elicited local ectopic or caudal extension of Sox1 expression after 5-6 hours (8/12 cases), compared with control DMSO vehicle only (2/7 embryos with Sox1 near bead) (Fig. 5B). Conversely, Sox1 expression extends caudally in the neural tube, at least to the most recently formed somite in normal quail embryos at HH10-HH11 (6/7 cases), but is lacking in the neural tube of retinoid (vitamin A)-deficient (VAD) quail embryos (8/11 cases; Fig. 5C). Such embryos also lack Sox3 transcripts (5/5 cases), whereas Sox3 is strongly expressed in normal quails in this region (3/3 cases; Fig. 5D).
As one of the key actions of retinoid signalling in this region of the axis is attenuation of Fgf signalling, we next investigated whether premature loss of signalling via this pathway could mimic the ability of retinoid signalling to promote Sox1. Beads delivering the Fgf receptor inhibitors SU5402 or PD173074 were grafted adjacent to the preneural tube and embryos were cultured for 5-6 hours. As with RA, Sox1 expression was induced precociously or extended more caudally on loss of Fgfr signalling (6/6 SU5402, 0/2 DMSO-only control embryos; 10/12 PD173074, 1/7 DMSO-only control embryos; Fig. 5E). To determine whether prolonging exposure to Fgf signals blocks onset of Sox1 expression, beads presenting Fgf4 or Fgf8 were grafted adjacent to the stem zone/preneural tube and embryos cultured for 16 hours (Fig. 5F). Local onset of Sox1 expression was inhibited by both Fgf4 (6/7 cases) and Fgf8 (5/7), whereas PBS beads had no effect (n=6, n=3 respectively; Fig. 5F). These findings indicate that onset of Sox1 expression in the embryo is regulated, as in ES cells, by retinoid-mediated attenuation of Fgf signalling, and suggest that this regulatory step is a conserved differentiation mechanism.
DISCUSSION
This study reveals that endogenous retinoid signalling is required for timely acquisition of somatic cell fate during ES cell differentiation and that exposure to RA promotes differentiation in this context by creating a distinct Fgf signalling signature. This involves an initial increase in Fgf/Erk signalling followed by a decrease, and our findings suggest that this is achieved by the rapid induction of Fgf8 and a more gradual repression of Fgf4. These actions could explain why RA is such a potent differentiation agent; increased Erk signalling might ensure loss of ES cell self-renewal, whereas the subsequent inhibition of Fgf signalling induces the loss of primitive ectoderm markers and the onset of expression of somatic cell-specific genes. Importantly, RA or Fgfr inhibition in primitive ectoderm-like cells increases the expression of neural or non-neural genes indicating that this second step is a generic differentiation mechanism, which we demonstrate is conserved in the developing embryonic axis.
Retinoid signalling advances acquisition of somatic cell fates
We demonstrate here using defined monolayer culture conditions that endogenous retinoid signalling is required for progression towards neural differentiation, as indicated by Sox1-GFP expression. We show that levels of endogenous Rarb and Fgf8 transcripts rise during the first two days in differentiation conditions. This is consistent with induction of Fgf8 in response to exogenous RA, which we show is RAR/RXR dependent, and leads to an increase in Erk activity. Recent work has shown that undifferentiated ES cell cultures constitute a mixture of cells with gene expression profiles characteristic of ICM or primitive ectoderm, and that these states are reversible in vitro (Chambers et al., 2007; Hayashi et al., 2008; Kalmar et al., 2009; Silva and Smith, 2008; Toyooka et al., 2008). Our findings show that RA requires Fgfr signalling in order to promote neural differentiation and this may indicate that cells need to experience a period of such signalling before RA can act. Induction of Fgf8 and increased Erk activity elicited by exogenous RA might also help to resolve this bistable ES cell state in favour of differentiation. Interestingly, we have found previously that ES cells cultured in the presence of a Mek inhibitor upregulate pluripotency genes, but can still progress as far as Fgf5 expression, indicative of the primitive ectoderm (Stavridis et al., 2007). These observations therefore suggest that high Erk signalling is required to resolve fluctuation between ICM and primitive ectoderm cell states. Endogenous retinoid signalling could contribute to this step by promoting Fgf8. However, we find that cells lacking retinoid signalling accumulate in an Fgf5-positive state characteristic of primitive ectoderm cells and do not require high-level retinoid activity in order to downregulate ICM genes. This suggests that the essential action of endogenous retinoid signalling during differentiation is to promote progression from a primitive ectoderm-like cell state towards somatic cell fates.
Retinoid signalling promotes somatic cell fates by repressing Fgf signalling
We show that, once cells have experienced endogenous Fgf/Erk signalling and have progressed to a high Fgf5-positive state at the end of day 1, exposure to RA or inhibition of Fgfr signalling now advances their differentiation towards neural or, in the presence of Bmp4, non-neural fates. Importantly, as noted above, if Fgfr and Erk activity are inhibited in ES cells, this promotes self-renewal (Ying et al., 2008), not differentiation. These results therefore suggest that once ES cells have experienced sufficient Fgf/Erk signalling they lose the ability to return to the ES cell state in response to inhibition of such signals, as this action instead now propels their differentiation (Fig. 6). Although day 2 cells represent a heterogeneous cell population, it is clear that pluripotency genes characteristic of the ICM (Nanog, Klf4 and Rex1) are decreased, that only a minority of cells retain the ability to return to the pluripotent ES cell state (as indicated by increased Rex1 expression on Fgfr inhibition), and that the prevailing gene expression profile is indicative of a primitive ectoderm cell state. Furthermore, cell-by-cell analysis revealed extensive Erk activity in this cell population and the widespread loss of such signalling in the presence of RA. These findings therefore suggest that RA acts on the majority and not a sub-population of day 2 cells to promote differentiation. We show that exposure to RA represses Fgf4 expression, and we propose that this step is responsible for the overall reduction in Erk signalling on day 2 in response to RA. Fgf4−/− ES cells exhibit greatly reduced Erk activity, indicating that Fgf4 is the major endogenous source of Erk signalling in ES cells (Kunath et al., 2007). The repression of Oct4 by RA and the reliance of Fgf4 expression on Oct4 and Sox2 (Gu et al., 2005; Yuan et al., 1995) further indicate that RA acts indirectly to repress Fgf4. These findings therefore suggest that a ‘rise and fall’ Fgf/Erk signalling signature is elicited by exogenous RA via an initial rapid increase in Fgf8, when Fgf4 is still high, followed by a slower, indirect inhibition of Fgf4.
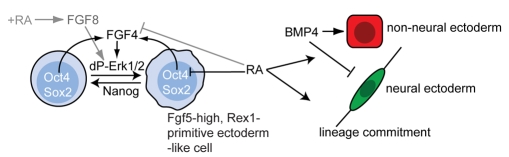
Fig. 6.
Initial steps in mouse ES cell differentiation. Summary of regulatory relationships between RA and Fgf pathways during ES cell differentiation, diagram adapted from Silva and Smith (Silva and Smith, 2008). Fgf/Erk signalling is required for loss of self-renewal and addition of RA stimulates Fgf8 transcription, which may ensure this step (indicated in grey). RA exposure also indirectly inhibits Fgf4 expression (indicated in grey). Cells lacking retinoid signalling accumulate in a Rex1-negative, Fgf5-positive state and exhibit greatly reduced neural differentiation, indicating that endogenous RA signalling normally promotes lineage commitment.
Neural fate can be induced by forced bHLH proneural gene expression in ES cells (Kanda et al., 2004) and our data show that blocking Fgfr signalling induces proneural genes (Mash1 and Ngn2) more efficiently than does treatment with RA over 24 hours (Fig. 3G). This contrasts with the tendency of RA to promote two further neural progenitor markers, Sox1 and Pax6, more effectively than loss of Fgfr signalling, suggesting that proneural gene onset may have a specific requirement for Fgf attenuation. This is consistent with RA taking longer to reduce Fgf signalling than direct inhibition of Fgfr, and with regulation of Ngn2 in the early mouse embryo, which involves both repression of Fgf signalling and regulation by RAREs in the Ngn2 promoter (Ribes et al., 2008). It is therefore possible that upregulation of proneural genes by direct action of RA and/or Fgfr inhibition is part of the mechanism that promotes neural fate in ES cells, but our data additionally show that RA or Fgfr inhibition also drive acquisition of a non-neural fate when Bmp4 is provided. This suggests that RA has two functions here, to promote differentiation beyond the primitive ectoderm cell state and to induce neural genes. During neural differentiation these two functions cannot be easily distinguished, but in the presence of Bmp4 the neural differentiation action is suppressed in favour of epithelial fates, revealing a generic effect of RA in driving primitive ectoderm differentiation. This step must involve downregulation of Fgf5, which we demonstrate is elicited by RA or Fgfr inhibition. Furthermore, as Fgfr inhibition promotes both neural and non-neural fates, this indicates that this generic differentiation action is mediated by RA repression of Fgfr signalling (Fig. 6).
An analogous signalling mechanism in the early embryo
In the mouse embryo, the earliest reported retinoid activity is sporadic detection of RARE-driven β-galactosidase activity in E3.5 ICM (Rossant et al., 1991), and this is followed by transient activity in the primitive streak, node ectoderm, epiblast and emerging paraxial mesoderm at E6.75 (Ribes et al., 2009; Rossant et al., 1991). Fgf8 is first expressed in primitive ectoderm of the pre-streak mouse embryo at E6.0 (Crossley and Martin, 1995). Fgf4 is first detected in the ICM of late blastocysts (E4.5), persists in early epiblast and is downregulated in this tissue, along with Fgf5, as the primitive steak forms (Haub and Goldfarb, 1991; Hebert et al., 1991; Niswander and Martin, 1992). The onset of Fgf8 expression in the primitive ectoderm as RA activity rises and downregulation of Fgf4 and Fgf5 in this tissue are thus consistent with the sequence of events we observe in differentiating ES cells. A short time later, at headfold stage, retinoid activity is excluded from the primitive streak and the adjacent caudal lateral epiblast (CLE; stem zone/caudal neural plate), but detected in the differentiating paraxial mesoderm and neural tube (Ribes et al., 2009). Conversely, Fgf4 and Fgf8 are now confined to the primitive streak, the newly formed paraxial mesoderm and the CLE (Crossley and Martin, 1995; Niswander and Martin, 1992). This separation of Fgf and retinoid signalling centres persists as the body axis is generated progressively from these caudal tissues, with cells experiencing Fgf and subsequently retinoid signalling. This spatial separation of the temporal events of differentiation provides a unique opportunity to locally manipulate signalling activity in the embryo. This approach has already revealed that retinoid signalling acts via repression of Fgf signalling to promote onset of neuronal differentiation and ventral neural tube patterning, as well as mesoderm differentiation (Diez del Corral et al., 2003; Molotkova et al., 2005; Morimoto et al., 2005; Ribes et al., 2009; Ribes et al., 2008). In the embryonic axis, however, RA now represses expression of Fgf8 (and also Fgf4; Isabel Olivera-Martinez and K.G.S., unpublished). This is likely to reflect the differences in cell state between pluripotent ES cells newly exposed to differentiation medium and cells in the CLE/tailbud, which lack pluripotency genes, already express Fgf8, have experienced high-level Erk signalling and are poised to commence somatic cell fate differentiation.
Despite the difference in Fgf8 regulation in these two contexts, we show here for the first time that exposure to RA or inhibition of Fgfr signalling promotes the onset of neural progenitor markers in the avian embryonic body axis, as well as in ES cells. RA or Fgfr inhibitors can locally accelerate Sox1 onset along the forming neural axis, whereas expression of Sox1 and Sox3 is depleted in retinoid-deficient embryos and following ectopic maintenance of Fgf. Although an initial analysis of mice lacking the retinoid synthesising enzyme Raldh2 suggested that Sox1 and Sox2 expression are unaffected by RA reduction (Molotkova et al., 2005), recent work indicates that onset of Sox2 is indeed defective in early Raldh2 mutant embryos (Ribes et al., 2009). It is also noteworthy that in the early mouse embryo (E6.5) neural differentiation, as indicated by expression of the anterior neural maker Hesx1, is increased when Fgf signalling is blocked in embryos lacking BMP signalling (Di-Gregorio et al., 2007), identifying Fgf signalling levels as crucial regulators of differentiation progression in this context as well.
Finally, other pluripotent cells, including human ES and Epi-stem cells (EpiSCs) self-renew under Fgf signalling, and mouse EpiSCs cells are maintained by Fgf and activin. It has been argued that these cells are more finely poised to differentiate than mES cells, as indicated by lineage bias in hES cell lines and increased expression of early mesodermal and endodermal genes in EpiSCs (reviewed by Rossant, 2008). In this study, we show that RA or inhibition of Fgfrs can advance differentiation from a primitive ectoderm-like state, and it may be that a primary action of RA in these later pluripotent cell contexts is to repress Fgf signalling and thereby promote both loss of self-renewal and the rapid acquisition of somatic cell fates.
Supplementary Material
Acknowledgements
We are grateful to Tom Burdon, Ying Liu, Ruth Diez del Corral, Claudio Stern, Arno Müller and Janet Rossant for critical comments. This work was funded by an MRC grant (G0301013) to K.G.S. and a joint MRC/BBSRC funded Career Development Award in Stem Cell Research to M.P.S. (G113/18). Deposited in PMC for release after 6 months.
Footnotes
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material for this article is available at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.043117/-/DC1
References
- Aggarwal S., Kim S. W., Cheon K., Tabassam F. H., Yoon J. H., Koo J. S. (2006). Nonclassical action of retinoic acid on the activation of the cAMP response element-binding protein in normal human bronchial epithelial cells. Mol. Biol. Cell 17, 566-575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert J., Dunstan H., Chambers I., Smith A. (2002). Functional gene screening in embryonic stem cells implicates Wnt antagonism in neural differentiation. Nat. Biotechnol. 20, 1240-1245 [DOI] [PubMed] [Google Scholar]
- Aubert J., Stavridis M. P., Tweedie S., O'Reilly M., Vierlinger K., Li M., Ghazal P., Pratt T., Mason J. O., Roy D., et al. (2003). Screening for mammalian neural genes via fluorescence-activated cell sorter purification of neural precursors from Sox1-gfp knock-in mice. Proc. Natl. Acad. Sci. USA 100Suppl. 1, 11836-11841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain G., Kitchens D., Yao M., Huettner J. E., Gottlieb D. I. (1995). Embryonic stem cells express neuronal properties in vitro. Dev. Biol. 168, 342-357 [DOI] [PubMed] [Google Scholar]
- Barnea E., Bergman Y. (2000). Synergy of SF1 and RAR in activation of Oct-3/4 promoter. J. Biol. Chem. 275, 6608-6619 [DOI] [PubMed] [Google Scholar]
- Brondani V., Klimkait T., Egly J. M., Hamy F. (2002). Promoter of FGF8 reveals a unique regulation by unliganded RARalpha. J. Mol. Biol. 319, 715-728 [DOI] [PubMed] [Google Scholar]
- Brons I. G., Smithers L. E., Trotter M. W., Rugg-Gunn P., Sun B., Chuva de Sousa Lopes S. M., Howlett S. K., Clarkson A., Ahrlund-Richter L., Pedersen R. A., et al. (2007). Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 448, 191-195 [DOI] [PubMed] [Google Scholar]
- Burdon T., Stracey C., Chambers I., Nichols J., Smith A. (1999). Suppression of SHP-2 and ERK signalling promotes self-renewal of mouse embryonic stem cells. Dev. Biol. 210, 30-43 [DOI] [PubMed] [Google Scholar]
- Canon E., Cosgaya J. M., Scsucova S., Aranda A. (2004). Rapid effects of retinoic acid on CREB and ERK phosphorylation in neuronal cells. Mol. Biol. Cell 15, 5583-5592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers I., Colby D., Robertson M., Nichols J., Lee S., Tweedie S., Smith A. (2003). Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell 113, 643-655 [DOI] [PubMed] [Google Scholar]
- Chambers I., Silva J., Colby D., Nichols J., Nijmeijer B., Robertson M., Vrana J., Jones K., Grotewold L., Smith A. (2007). Nanog safeguards pluripotency and mediates germline development. Nature 450, 1230-1234 [DOI] [PubMed] [Google Scholar]
- Chen N., Napoli J. L. (2008). All-trans-retinoic acid stimulates translation and induces spine formation in hippocampal neurons through a membrane-associated RARalpha. FASEB J. 22, 236-245 [DOI] [PubMed] [Google Scholar]
- Collins B. J., Deak M., Arthur J. S., Armit L. J., Alessi D. R. (2003). In vivo role of the PIF-binding docking site of PDK1 defined by knock-in mutation. EMBO J. 22, 4202-4211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley P. H., Martin G. R. (1995). The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development 121, 439-451 [DOI] [PubMed] [Google Scholar]
- Di-Gregorio A., Sancho M., Stuckey D. W., Crompton L. A., Godwin J., Mishina Y., Rodriguez T. A. (2007). BMP signalling inhibits premature neural differentiation in the mouse embryo. Development 134, 3359-3369 [DOI] [PubMed] [Google Scholar]
- Diez del Corral R., Storey K. G. (2004). Opposing FGF and retinoid pathways: a signalling switch that controls differentiation and patterning onset in the extending vertebrate body axis. BioEssays 26, 857-869 [DOI] [PubMed] [Google Scholar]
- Diez del Corral R., Olivera-Martinez I., Goriely A., Gale E., Maden M., Storey K. (2003). Opposing FGF and retinoid pathways control ventral neural pattern, neuronal differentiation, and segmentation during body axis extension. Neuron 40, 65-79 [DOI] [PubMed] [Google Scholar]
- Gu P., LeMenuet D., Chung A. C., Mancini M., Wheeler D. A., Cooney A. J. (2005). Orphan nuclear receptor GCNF is required for the repression of pluripotency genes during retinoic acid-induced embryonic stem cell differentiation. Mol. Cell. Biol. 25, 8507-8519 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Haub O., Goldfarb M. (1991). Expression of the fibroblast growth factor-5 gene in the mouse embryo. Development 112, 397-406 [DOI] [PubMed] [Google Scholar]
- Hayashi K., Lopes S. M., Tang F., Surani M. A. (2008). Dynamic equilibrium and heterogeneity of mouse pluripotent stem cells with distinct functional and epigenetic states. Cell Stem Cell 3, 391-401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert J. M., Boyle M., Martin G. R. (1991). mRNA localization studies suggest that murine FGF-5 plays a role in gastrulation. Development 112, 407-415 [DOI] [PubMed] [Google Scholar]
- Kalmar T., Lim C., Hayward P., Munoz-Descalzo S., Nichols J., Garcia-Ojalvo J., Martinez Arias A. (2009). Regulated fluctuations in nanog expression mediate cell fate decisions in embryonic stem cells. PLoS Biol. 7, e1000149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda S., Tamada Y., Yoshidome A., Hayashi I., Nishiyama T. (2004). Over-expression of bHLH genes facilitate neural formation of mouse embryonic stem (ES) cells in vitro. Int. J. Dev. Neurosci. 22, 149-156 [DOI] [PubMed] [Google Scholar]
- Kunath T., Saba-El-Leil M. K., Almousailleakh M., Wray J., Meloche S., Smith A. (2007). FGF stimulation of the Erk1/2 signalling cascade triggers transition of pluripotent embryonic stem cells from self-renewal to lineage commitment. Development 134, 2895-2902 [DOI] [PubMed] [Google Scholar]
- Lane M. A., Xu J., Wilen E. W., Sylvester R., Derguini F., Gudas L. J. (2008). LIF removal increases CRABPI and CRABPII transcripts in embryonic stem cells cultured in retinol or 4-oxoretinol. Mol. Cell. Endocrinol. 280, 63-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell S., Benchoua A., Heavey B., Smith A. G. (2006). Notch promotes neural lineage entry by pluripotent embryonic stem cells. PLoS Biol. 4, e121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn J. S., Fishwick K. J., Halley P. A., Storey K. G. (2007). A spatial and temporal map of FGF/Erk1/2 activity and response repertoires in the early chick embryo. Dev. Biol. 302, 536-552 [DOI] [PubMed] [Google Scholar]
- Masia S., Alvarez S., de Lera A. R., Barettino D. (2007). Rapid, nongenomic actions of retinoic acid on phosphatidylinositol-3-kinase signaling pathway mediated by the retinoic acid receptor. Mol. Endocrinol. 21, 2391-2402 [DOI] [PubMed] [Google Scholar]
- Mason C., Lake M., Nebreda A., Old R. (1996). A novel MAP kinase phosphatase is localised in the branchial arch region and tail tip of Xenopus embryos and is inducible by retinoic acid. Mech. Dev. 55, 133-144 [DOI] [PubMed] [Google Scholar]
- McDonald F. J., Heath J. K. (1994). Developmentally regulated expression of fibroblast growth factor receptor genes and splice variants by murine embryonic stem and embryonal carcinoma cells. Dev. Genet. 15, 148-154 [DOI] [PubMed] [Google Scholar]
- Minowada G., Jarvis L. A., Chi C. L., Neubuser A., Sun X., Hacohen N., Krasnow M. A., Martin G. R. (1999). Vertebrate Sprouty genes are induced by FGF signaling and can cause chondrodysplasia when overexpressed. Development 126, 4465-4475 [DOI] [PubMed] [Google Scholar]
- Mitsui K., Tokuzawa Y., Itoh H., Segawa K., Murakami M., Takahashi K., Maruyama M., Maeda M., Yamanaka S. (2003). The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell 113, 631-642 [DOI] [PubMed] [Google Scholar]
- Mohammadi M., McMahon G., Sun L., Tang C., Hirth P., Yeh B. K., Hubbard S. R., Schlessinger J. (1997). Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science 276, 955-960 [DOI] [PubMed] [Google Scholar]
- Mohammadi M., Froum S., Hamby J. M., Schroeder M. C., Panek R. L., Lu G. H., Eliseenkova A. V., Green D., Schlessinger J., Hubbard S. R. (1998). Crystal structure of an angiogenesis inhibitor bound to the FGF receptor tyrosine kinase domain. EMBO J. 17, 5896-5904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molotkova N., Molotkov A., Sirbu I. O., Duester G. (2005). Requirement of mesodermal retinoic acid generated by Raldh2 for posterior neural transformation. Mech. Dev. 122, 145-155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno T. A., Kintner C. (2004). Regulation of segmental patterning by retinoic acid signaling during Xenopus somitogenesis. Dev. Cell 6, 205-218 [DOI] [PubMed] [Google Scholar]
- Morimoto M., Takahashi Y., Endo M., Saga Y. (2005). The Mesp2 transcription factor establishes segmental borders by suppressing Notch activity. Nature 435, 354-359 [DOI] [PubMed] [Google Scholar]
- Mummery C. L., van den Eijnden-van Raaij A. J., Feijen A., Freund E., Hulskotte E., Schoorlemmer J., Kruijer W. (1990). Expression of growth factors during the differentiation of embryonic stem cells in monolayer. Dev. Biol. 142, 406-413 [DOI] [PubMed] [Google Scholar]
- Nakamura K., Yamamoto A., Kamishohara M., Takahashi K., Taguchi E., Miura T., Kubo K., Shibuya M., Isoe T. (2004). KRN633: A selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase that suppresses tumor angiogenesis and growth. Mol. Cancer Ther. 3, 1639-1649 [PubMed] [Google Scholar]
- Nichols J., Zevnik B., Anastassiadis K., Niwa H., Klewe-Nebenius D., Chambers I., Scholer H., Smith A. (1998). Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell 95, 379-391 [DOI] [PubMed] [Google Scholar]
- Niswander L., Martin G. R. (1992). Fgf-4 expression during gastrulation, myogenesis, limb and tooth development in the mouse. Development 114, 755-768 [DOI] [PubMed] [Google Scholar]
- Okazawa H., Okamoto K., Ishino F., Ishino-Kaneko T., Takeda S., Toyoda Y., Muramatsu M., Hamada H. (1991). The oct3 gene, a gene for an embryonic transcription factor, is controlled by a retinoic acid repressible enhancer. EMBO J. 10, 2997-3005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelton T. A., Sharma S., Schulz T. C., Rathjen J., Rathjen P. D. (2002). Transient pluripotent cell populations during primitive ectoderm formation: correlation of in vivo and in vitro pluripotent cell development. J. Cell Sci. 115, 329-339 [DOI] [PubMed] [Google Scholar]
- Pikarsky E., Sharir H., Ben-Shushan E., Bergman Y. (1994). Retinoic acid represses Oct-3/4 gene expression through several retinoic acid-responsive elements located in the promoter-enhancer region. Mol. Cell. Biol. 14, 1026-1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribes V., Stutzmann F., Bianchetti L., Guillemot F., Dolle P., Le Roux I. (2008). Combinatorial signalling controls Neurogenin2 expression at the onset of spinal neurogenesis. Dev. Biol. 321, 470-481 [DOI] [PubMed] [Google Scholar]
- Ribes V., Le Roux I., Rhinn M., Schuhbaur B., Dolle P. (2009). Early mouse caudal development relies on crosstalk between retinoic acid, Shh and Fgf signalling pathways. Development 136, 665-676 [DOI] [PubMed] [Google Scholar]
- Rossant J. (2008). Stem cells and early lineage development. Cell 132, 527-531 [DOI] [PubMed] [Google Scholar]
- Rossant J., Zirngibl R., Cado D., Shago M., Giguere V. (1991). Expression of a retinoic acid response element-hsplacZ transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes Dev. 5, 1333-1344 [DOI] [PubMed] [Google Scholar]
- Silva J., Smith A. (2008). Capturing pluripotency. Cell 132, 532-536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sockanathan S., Jessell T. M. (1998). Motor-neuron derived retinoid signalling specifies the subtype identity of spinal motor neurons. Cell 94, 503-514 [DOI] [PubMed] [Google Scholar]
- Soprano D. R., Teets B. W., Soprano K. J. (2007). Role of retinoic acid in the differentiation of embryonal carcinoma and embryonic stem cells. Vitam. Horm. 75, 69-95 [DOI] [PubMed] [Google Scholar]
- Stavridis M. P., Lunn J. S., Collins B. J., Storey K. G. (2007). A discrete period of FGF-induced Erk1/2 signalling is required for vertebrate neural specification. Development 134, 2889-2894 [DOI] [PubMed] [Google Scholar]
- Storey K. G., Goriely A., Sargent C. M., Brown J. M., Burns H. D., Abud H. M., Heath J. K. (1998). Early posterior neural tissue is induced by FGF in the chick embryo. Development 125, 473-484 [DOI] [PubMed] [Google Scholar]
- Toyooka Y., Shimosato D., Murakami K., Takahashi K., Niwa H. (2008). Identification and characterization of subpopulations in undifferentiated ES cell culture. Development 135, 909-918 [DOI] [PubMed] [Google Scholar]
- Wilson V., Olivera-Martinez I., Storey K. G. (2009). Stem cells, signals and vertebrate body axis extension. Development 136, 1591-1604 [DOI] [PubMed] [Google Scholar]
- Ying Q. L., Stavridis M., Griffiths D., Li M., Smith A. (2003). Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat. Biotechnol. 21, 183-186 [DOI] [PubMed] [Google Scholar]
- Ying Q. L., Wray J., Nichols J., Batlle-Morera L., Doble B., Woodgett J., Cohen P., Smith A. (2008). The ground state of embryonic stem cell self-renewal. Nature 453, 519-523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H., Corbi N., Basilico C., Dailey L. (1995). Developmental-specific activity of the FGF-4 enhancer requires the synergistic action of Sox2 and Oct-3. Genes Dev. 9, 2635-2645 [DOI] [PubMed] [Google Scholar]
- Zhao X., Sirbu I. O., Mic F. A., Molotkova N., Molotkov A., Kumar S., Duester G. (2009). Retinoic acid promotes limb induction through effects on body axis extension but is unnecessary for limb patterning. Curr. Biol. 19, 1050-1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.