Abstract
Purpose
Therapeutic prostate-specific antigen (PSA) –targeted poxviral vaccines for prostate cancer have been well tolerated. PROSTVAC-VF treatment was evaluated for safety and for prolongation of progression-free survival (PFS) and overall survival (OS) in a randomized, controlled, and blinded phase II study.
Patients and Methods
In total, 125 patients were randomly assigned in a multicenter trial of vaccination series. Eligible patients had minimally symptomatic castration-resistant metastatic prostate cancer (mCRPC). PROSTVAC-VF comprises two recombinant viral vectors, each encoding transgenes for PSA, and three immune costimulatory molecules (B7.1, ICAM-1, and LFA-3). Vaccinia-based vector was used for priming followed by six planned fowlpox-based vector boosts. Patients were allocated (2:1) to PROSTVAC-VF plus granulocyte-macrophage colony-stimulating factor or to control empty vectors plus saline injections.
Results
Eighty-two patients received PROSTVAC-VF and 40 received control vectors. Patient characteristics were similar in both groups. The primary end point was PFS, which was similar in the two groups (P = .6). However, at 3 years post study, PROSTVAC-VF patients had a better OS with 25 (30%) of 82 alive versus 7 (17%) of 40 controls, longer median survival by 8.5 months (25.1 v 16.6 months for controls), an estimated hazard ratio of 0.56 (95% CI, 0.37 to 0.85), and stratified log-rank P = .0061.
Conclusion
PROSTVAC-VF immunotherapy was well tolerated and associated with a 44% reduction in the death rate and an 8.5-month improvement in median OS in men with mCRPC. These provocative data provide preliminary evidence of clinically meaningful benefit but need to be confirmed in a larger phase III study.
INTRODUCTION
Initial clinical studies with prostate-specific antigen (PSA) recombinant vaccinia vectors (rV-PSA) demonstrated safety and immunogenicity.1–3 It was determined that neutralizing antibody responses limited the ability for continued rV-PSA treatment, and a heterologous prime boost strategy was used with rFowlpox-PSA (rF-PSA) as a boosting agent. A small, randomized phase II study conducted by the Eastern Cooperative Oncology Group (ECOG) evaluated the effects of various sequences of vaccination and indicated that rV-PSA followed by rF-PSA boosts was associated with a longer PSA progression-free survival (PFS).4
Subsequent preclinical work has shown that the addition of an expanded repertoire of immune stimulatory molecules to the poxviral vectors results in augmented immune activation and induction of T cells with higher avidity for antigen.5,6 A number of costimulatory molecules have now been identified, but three well-characterized costimulatory molecules were found to be synergistic when added to the poxviral system. This triad, which includes B7.1 (CD80), ICAM-1 (CD54), and LFA-3 (CD58), is designated TRICOM and has been added to both the vaccinia priming vector and the fowlpox boosting vector. With PSA as the encoded antigen, this configuration constitutes PROSTVAC-VF, vaccinia-PSA-TRICOM, and fowlpox-PSA-TRICOM.
PROSTVAC-VF has been tested clinically in two phase I studies,7,8 and one single-arm phase II study. Both phase I studies demonstrated safety of the vectors, and the National Cancer Institute (NCI) study also evaluated biodistribution kinetics.8 The NCI has also recently completed a phase II study in 32 patients in whom immune and regulatory T-cell responses were evaluated.9
The objectives of this study were to evaluate PROSTVAC-VF in a randomized, controlled, and blinded manner and to assess the effects of treatment on PFS and overall survival (OS). The randomized phase II trial reported here was originated under industrial sponsorship with a collaborative research and development agreement (CRADA) with the NCI. The initial industrial sponsor, Therion Biologics, managed the trial through treatment, primary end point evaluation, and 1 year of OS follow-up.10,11 Subsequently BN ImmunoTherapeutics and the NCI established a new CRADA. BN ImmunoTherapeutics implemented long-term follow-up for vital status, and these follow-up data are the focus of this report. The original scientific leadership (P.W.K., T.J.S., D.L.P., J.L.G., and J.S.) and clinical contract research organization were involved throughout the program.
PATIENTS AND METHODS
Patient Eligibility
Men older than 18 years of age with a history of prior smallpox immunization were eligible. Patients had histologically confirmed adenocarcinoma of the prostate with radiologic evidence of metastasis by bone scan or computed tomography (CT) scan and were refractory to androgen deprivation therapy (ADT) with evidence of PSA progression by Prostate-Specific Antigen Working Group criteria.12 Other eligibility criteria included an ECOG performance status of 0 or 1 and a Gleason score ≤ 7 from the original biopsy. In addition, adequate hematologic, hepatic, and renal function were required.
Exclusions included patients with visceral metastasis, cancer-related pain requiring narcotics, prior chemotherapy, current immunosuppressive therapy, or history of immunodeficiency. Patients with a history of eczema or exfoliative skin disorder, or a prior allergic reaction to smallpox (vaccinia) vaccine, eggs or egg products, or prior granulocyte-macrophage colony-stimulating factor (GM-CSF) were excluded. Patients needed to be able to avoid high-risk individuals for 3 weeks (ie, children younger than age 1 year, pregnant or lactating women, those with extensive eczema, or immunodeficient individuals).
ADT (eg, bicalutamide, nilutamide, or flutamide) needed to be withdrawn > 6 weeks before registration. Patients without orchiectomy continued on ADT with a luteinizing hormone releasing hormone agonist throughout the trial. All patients signed institutional review board (IRB)–approved informed consent forms before undergoing screening procedures. Central (Western) IRB and 18 individual IRBs approved the trial and consent forms. The National Institutes of Health Recombinant DNA Advisory Committee approved of the trial and biosafety procedures.
Study Design and Treatment
This double-blinded study involved 43 centers in the United States. Patients were to be randomly assigned to one of two arms designated as PROSTVAC arm and control arm. At the time of random assignment, patients were to be classified regarding whether they were using bisphosphonates. The basis for this stratification was the potential for bisphosphonate use to have an impact on progression rates. A centrally administered block randomization method was used to assign patients within center and stratification classification to the arms in a 2:1 ratio (PROSTVAC:control).
Study agent was to be administered on days 1, 14, 28, 56, 84, 112, and 140. The PROSTVAC arm patients were to receive priming immunization with rV-PSA-TRICOM (2 × 108 pfu) with subsequent boosts using rF-PSA-TRICOM (1 × 109 pfu), with recombinant GM-CSF (Leukine) used as an adjuvant for all vaccinations. PROSTVAC patients received GM-CSF at 100 μg subcutaneously on the day of each vaccination and for three consecutive days thereafter, all near the vaccination site (within 5 mm). The control arm patients were to receive priming immunization with empty vector vaccinia (2 × 108 pfu) and subsequent boosts with empty vector fowlpox (1 × 109 pfu), all with placebo saline injections in place of GM-CSF. All study agent administrations were given subcutaneously.
Vaccine Preparation
Vaccines consisted of PROSTVAC-V (rV-PSA-TRICOM), and PROSTVAC-F (rF-PSA-TRICOM), empty vector vaccinia (TBC-Wyeth), and empty vector fowlpox (TBC-FPV, Poxvac-TC). All four vaccines were manufactured using the same process at Therion Biologics (Cambridge, MA).7,8
Statistical Considerations
The planned primary end point was PFS defined as identification of two or more new sites of bone metastasis on the bone scan compared with the baseline scan, or an increase in the sum of measurable target lymph node metastasis on CT scan by > 20% according to Response Evaluation Criteria in Solid Tumors (RECIST) criteria compared with baseline. Patients who developed clinical signs or symptoms of progression but who did not meet the radiologic criteria were also considered to have progressed at the discretion of the investigator. Bone scans and CT scans were centrally reviewed, and the primary analysis was based on the centrally reviewed radiology. The analyses of time to progression or death and OS were preplanned.
The planned trial size requirement of 80 PROSTVAC arm patients and 40 control arm patients was computed on the basis of the proportion of patients who remained alive and progression free at day 168; other specifications included a type I error probability of two-sided 0.05, power of 80%, consequential effect size of 26-point difference in the proportion alive without progression at 6 months (12% for the control arm and 38% for the PROSTVAC arm), 2:1 randomization ratio, and use of χ2 test not corrected for continuity.
In this article, time-to-event end points were analyzed using the stratified log-rank test and stratified proportional hazard regression (for estimation of hazard ratios). Stratification is by the bisphosphonate use randomization factor. The CIs reported are 95%.
Effect modifier analyses were used to assess for lack of homogeneity of the arm effect across the levels of putatively influential factors.13 Each factor was analyzed separately in dichotomous form, with continuous factors dichotomized at the median, thereby defining the patient subgroups. The effect modifier analysis assessed the arm by factor interaction in a statistical model that also included the arm and factor main effects, with the interaction term P value referred to a two-sided 0.1 as evidence of effect modification. The main effect only model was estimated when the interaction model failed to provide evidence of effect modification and provided assessment of the persistence of the arm effect when there was adjustment for the factor.
RESULTS
Characteristics of the Patients
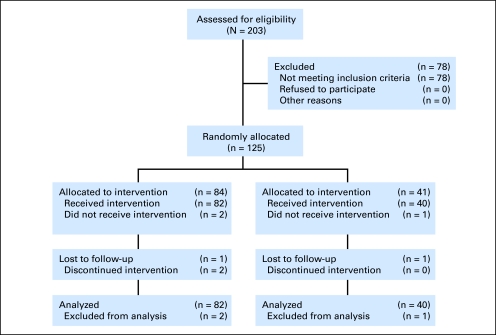
Between November 2003 and July 2005, 125 patients were enrolled at 43 study centers. Eighty-four patients were randomly assigned to the PROSTVAC arm, and 41 patients were randomly assigned to the control arm (Fig 1; CONSORT flow diagram). Three patients did not initiate study intervention and were excluded from analyses of this double-blind study. Two of the three excluded patients were randomly assigned to the PROSTVAC arm; one committed suicide and the other developed grade 3 hematuria. The excluded control arm patient was found to have a liver lesion on the screening CT scan. The 122 remaining patients, 82 in the PROSTVAC arm and 40 in the control arm, constitute the primary analysis set. Table 1 presents a between-arm comparison of the main baseline patient characteristics and shows that the arms were reasonably balanced, allowing for the 2:1 randomization and small trial size. The most notable difference is age: the PROSTVAC arm mean age is 72.6 years compared with 76.8 years for the control arm, but age is not a significant prognostic factor in prostate cancer.14 None of the other baseline clinical parameters were different. All patients had a Gleason score of ≤ 7 and were without visceral disease. The differences in the four laboratory components of the Halabi prognostic nomogram—PSA, lactate dehydrogenase, alkaline phosphatase, and hemoglobin—favor the PROSTVAC arm, though it should be noted that these four laboratory tests are highly correlated with each other (with all correlations having P < .05 both with and without logarithmic transformation; data not shown).
Fig 1.
Disposition of patients: CONSORT diagram.
Table 1.
Between-Arm Comparison of Baseline Patient Characteristics
| Characteristic | PROSTVAC-VF (n = 82) |
Placebo (n = 40) |
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| Age, years | ||||
| Median | 71.5 | 79 | ||
| Mean | 72 | 76 | ||
| IQR | 67-79 | 72-83 | ||
| Range | 52-94 | 55-90 | ||
| Race | ||||
| Caucasian | 71 | 86.6 | 33 | 82.5 |
| African American | 10 | 12.2 | 4 | 10 |
| Hispanic | 1 | 1.2 | 1 | 2.5 |
| Other | 0 | 2 | 5 | |
| Bisphosphonate use | ||||
| Yes | 35 | 42.7 | 16 | 40 |
| No | 47 | 57.3 | 24 | 60 |
| Gleason score | ||||
| 2-4 | 3 | 3.7 | 3 | 7.5 |
| 5 | 6 | 7.3 | 3 | 7.5 |
| 6 | 20 | 24.4 | 8 | 20 |
| 7 | 53 | 64.6 | 26 | 65 |
| ECOG performance status | ||||
| 0 | 56 | 68.3 | 27 | 67.5 |
| 1 | 26 | 31.7 | 13 | 32.5 |
| PSA | ||||
| Median | 36 | 45 | ||
| Mean | 131 | 183 | ||
| IQR | 17-107 | 20-85 | ||
| Range | 3-2,524 | 5-2,729 | ||
| Lactate dehydrogenase | ||||
| Median | 194 | 205 | ||
| Mean | 217 | 229 | ||
| IQR | 163-220 | 185-240 | ||
| Range | 124-1,380 | 107-480 | ||
| Alkaline phosphatase | ||||
| Median | 100 | 115 | ||
| Mean | 137 | 168 | ||
| IQR | 80-142 | 93-206 | ||
| Range | 52-828 | 67-555 | ||
| Hemoglobin | ||||
| Median | 13.0 | 12.65 | ||
| Mean | 12.97 | 12.77 | ||
| IQR | 12-13.8 | 11.5-13.9 | ||
| Range | 9.8-15.9 | 9.2-15.9 | ||
| Halabi-predicted survival, estimated No. of months | ||||
| Median | 22.5 | 20.4 | ||
| Mean | 21.4 | 20.4 | ||
| IQR | 18-24.5 | 16.2-24.5 | ||
| Range | 6.6-32.4 | 10.8-32.4 | ||
| Bone scan | ||||
| Median | 6 | 7 | ||
| Mean | 6.2 | 7.1 | ||
| IQR | 2-10 | 3-11 | ||
| Range | 0-20 | 1-19 | ||
| 0 | 8 | 9.8 | 0 | 0 |
| 1-4 | 31 | 37.8 | 15 | 37.5 |
| 5-9 | 17 | 20.7 | 11 | 27.5 |
| > 10 | 26 | 31.7 | 14 | 35 |
| Disease location | ||||
| Lymph node only | 8 | 9.8 | 0 | |
| Bone only | 37 | 45 | 21 | 52 |
| Bone and lymph node | 37 | 45 | 19 | 48 |
Abbreviations: PROSTVAC-VF, a vaccine containing two recombinant viral vectors (vaccinia and fowlpox) and three immune costimulatory molecules (B7.1, ICAM-1, and LFA3); IQR, interquartile range; ECOG, Eastern Cooperative Oncology Group; PSA, prostate-specific antigen.
Progression
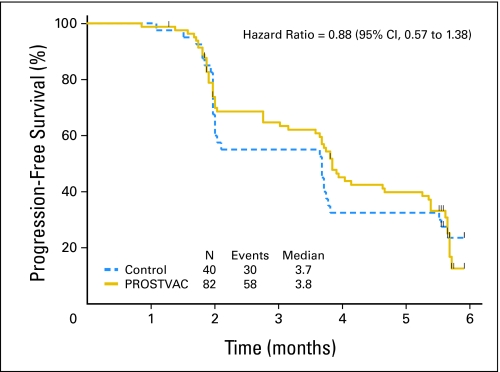
The assessment for PFS was scheduled at months 2, 4, and 6. All patients had at least one progression assessment, and three patients did not have central radiologic review. Two patients died before 180 days with progressive disease but without having a date of progression recorded. One patient in the control arm died at 62 days and one patient in the PROSTVAC arm died at 142 days. At 180 days, 10 (25%) of 40 patients in the control arm had not experienced progression or death compared with 19 (23%) of 82 patients in the PROSTVAC arm. The Kaplan-Meier estimates of the PFS distributions are shown in Figure 2. The hazard ratio estimated from stratified proportional hazard regression is 0.884 (95% CI, 0.568 to 1.375), and the stratified log-rank P value for PFS is .60.
Fig 2.
Primary end point is progression-free survival. Kaplan-Meier estimator for PROSTVAC (a vaccine containing two recombinant viral vectors [vaccinia and fowlpox] and three immune costimulatory molecules [B7.1, ICAM-1, and LFA3]) arm is shown as a solid gold line and the estimator for the control arm is a dashed blue line. The small vertical tic marks show the censoring times. The estimated median progression-free survival is 3.8 months in the PROSTVAC arm and 3.7 months in the control arm.
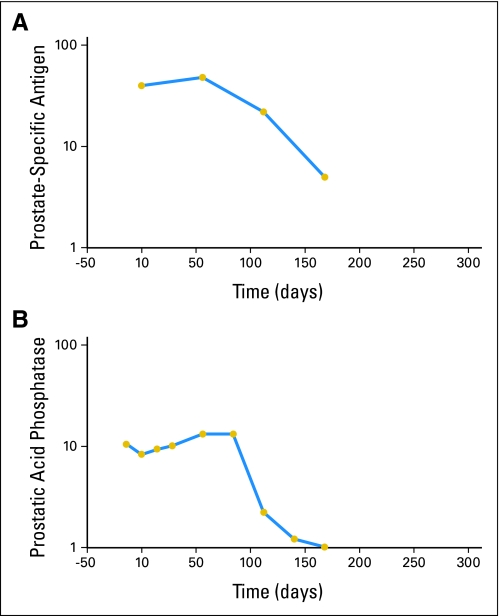
PSA responses were infrequent. One PROSTVAC-treated patient had a PSA decline of > 80%. Interestingly, this patient also experienced a > 80% drop in blood prostatic acid phosphatase levels over a similar time course. These results are shown in Figure 3. No patient with measurable lymph node enlargement had a complete or partial response per RECIST criteria. The mean number of vaccinations was 5.4 for PROSTVAC patients and 5.3 for controls. After progression, patients were unblinded, and those in the control arm were offered cross-over treatment with PROSTVAC-VF. Approximately half, 19 of the 40 patients in the control arm, crossed over to PROSTVAC-VF treatment. They received a mean number of 3.4 further vaccinations.
Fig 3.
Serum tumor marker response. Semi-log graphs of (A) prostate-specific antigen levels from 40 to 5 ng/mL and of (B) prostatic acid phosphatase levels from 10 to 1 ng/mL over time in a specific PROSTVAC-treated patient (PROSTVAC is a vaccine containing two recombinant viral vectors [vaccinia and fowlpox] and three immune costimulatory molecules [B7.1, ICAM-1, and LFA3]). Gold circles represent time points for the samples. The responses occurred over 3 to 6 months (end of study). The patient survived 929 days.
Overall Survival
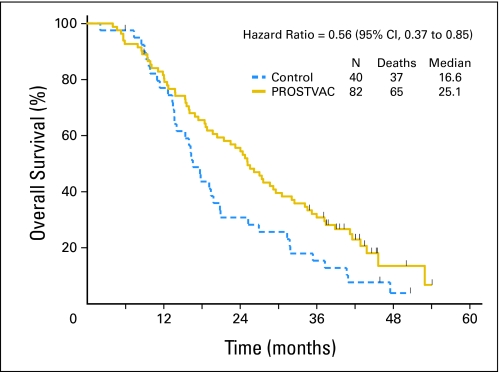
Patient data were collected between May 2008 and November 2008. Two patients (one in each arm) were lost to follow-up: one PROSTVAC patient at 8.9 months and one control patient at 6 months follow-up. Figure 4 shows graphs of the Kaplan-Meier estimator of the survival distributions. The estimated median survivals for the PROSTVAC arm and control arm are 25.1 and 16.6 months, respectively. At 3 years post treatment, 25 of 82 patients in the PROSTVAC arm are not known to have died compared with 7 of 40 patients in the control arm (30.5% v 17.5%). At this analysis, 17 (20.7%) of 82 patients in the PROSTVAC arm are not known to have died with a median follow-up of 41.3 months (range, 8.9 to 54.1 months), compared with three (7.5%) of 40 patients in the control arm with follow-up times of 6.0, 45.9, and 50.7 months.
Fig 4.
Overall survival. Kaplan-Meier estimator for PROSTVAC (a vaccine containing two recombinant viral vectors [vaccinia and fowlpox] and three immune co-stimulatory molecules [B7.1, ICAM-1, and LFA3]) arm is shown as a solid gold line and estimator for the control arm is a dashed blue line. The small vertical tic marks show the censoring times. The estimated median overall survival is 25.1 months for the PROSTVAC arm and 16.6 months for the control arm.
For the primary analysis set, the hazard ratio estimated from stratified proportional hazard regression is 0.56 (95% CI, 0.37 to 0.85), and the log-rank P value is .0061. For the analysis of all randomly assigned patients (N = 125), the hazard ratio estimated from stratified proportional hazard regression is 0.58 (95% CI, 0.38 to 0.88), and the log-rank P value is .0095.
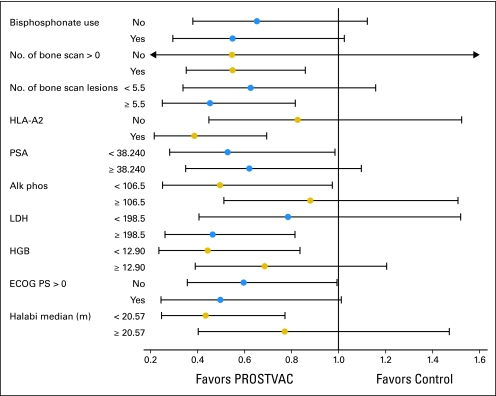
The robustness of the survival hazard ratio effect estimate of 0.56 was assessed through effect modification analysis of potential effect modifiers. Figure 5 is a forest plot showing the hazard ratio estimates (and 95% CIs) for the levels of the potential effect modifier analyzed. There is no evidence of effect modification for any of the potential effect modifiers analyzed using the criterion Pinteraction = .1; however, the trial size is small as can be seen from the widths of the CIs in the forest plot. The hazard ratio estimates in Figure 5 are consistent with the PROSTVAC effect, and no evidence of an alternative explanation for the PROSTVAC effect is evident.
Fig 5.
Effect modifier analysis. Forest plot for putatively prognostic factors. Continuous factors are analyzed by dichotomization at the common median. PROSTVAC, a vaccine containing two recombinant viral vectors (vaccinia and fowlpox) and three immune costimulatory molecules (B7.1, ICAM-1, and LFA3); HLA-A2, human leukocyte antigen A2; PSA, prostate-specific antigen; Alk phos, alkaline phosphatase; LDH, lactate dehydrogenase; HGB, hemoglobin; ECOG PS, Eastern Cooperative Oncology Group performance status.
Humoral Immune Responses
There were no detectable antibody responses to PSA. All titers remained less than 1:100. All patients had augmented antibody responses (approximately four- to eight-fold) to vaccinia vector (median final titer, 1:3,200), and all but one (of those who had more than two booster vaccinations) generated de novo antibody responses to fowlpox vector (median final titer, 1:12,800). There was no correlation of antivector antibody responses with OS.
Toxicity
Poxviral immunization therapy was well tolerated. Most adverse events (AEs) were injection site reactions with only a subset of patients experiencing associated systemic AEs such as fatigue, fevers, and nausea. Table 2 lists the most common AEs that were present in either treatment group at > 10%. Typical injection site reactions were mild, with only one clear G3 injection site event throughout the whole study: an injection site cellulitis. The AE profile of primary vaccinia immunization was equivalent to that induced by the fowlpox booster immunizations.
Table 2.
Common Adverse Events
| Adverse Event | PROSTVAC-VF (n = 82) |
Control (n = 40) |
||
|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | |
| Injection site reactions | ||||
| Erythema | 48 | 58.5 | 22 | 55.0 |
| Pain | 29 | 35.4 | 14 | 35.0 |
| Swelling | 23 | 28.0 | 5 | 12.5 |
| Pruritus | 17 | 20.7 | 4 | 10.0 |
| Induration | 10 | 12.2 | 6 | 15.0 |
| General disorders | ||||
| Fatigue | 35 | 42.7 | 8 | 20.0 |
| Pyrexia | 15 | 18.3 | 6 | 15.0 |
| Peripheral edema | 11 | 13.4 | 4 | 10.0 |
| Chills | 12 | 14.6 | 1 | 2.5 |
| GI disorders | ||||
| Constipation | 9 | 11.0 | 6 | 15.0 |
| Diarrhea | 7 | 8.5 | 6 | 15.0 |
| Nausea | 17 | 20.7 | 2 | 5.0 |
| Musculoskeletal and connective tissue disorders | ||||
| Arthralgia | 10 | 12.2 | 10 | 25.0 |
| Nervous system disorders | ||||
| Dizziness | 10 | 12.2 | 3 | 7.5 |
NOTE. At each level of patient summarization, a patient is counted only once if the patient reported one or more events. Adverse events are coded according to the Medical Dictionary for Regulatory Activities Version 6.0.
Abbreviation: PROSTVAC-VF, a vaccine containing two recombinant viral vectors (vaccinia and fowlpox) and three immune costimulatory molecules (B7.1, ICAM-1, and LFA3).
Two PROSTVAC-treated patients discontinued therapy because of treatment-related AEs. One had recurrent lip edema after second and third vaccinations. The other patient developed multiple AEs and serious AEs associated with thrombotic thrombocytopenic purpura and myocardial infarction. The case was reported as possibly related to treatment. Thrombotic thrombocytopenic purpura has not been reported in association with vaccinia immunization.15
DISCUSSION
This randomized, controlled, and double-blinded phase II study was designed and powered for the short-term end point of PFS, and it failed to find an association between treatment arm and progression. However, a strong association between treatment arm and OS was observed. The magnitude of the effect (estimated hazard ratio of 0.56 and an observed difference in median survival of 8.5 months) suggests a clinically meaningful outcome. This study, with blinded control patients, strongly suggests that PROSTVAC-VF immunotherapy may produce an OS benefit. Thus PSA-targeted immunotherapy may offer a new complementary approach to treating prostate cancer.
In a search for alternative explanations for the differential survival outcome, analysis of known prognostic factors between arms was undertaken. There were no major differences between the groups that could explain the result. While the two treatment arms were reasonably well balanced considering the small size of the trial and the 2:1 randomization, there was a slight imbalance in favor of the PROSTVAC arm in mean and median laboratory values for PSA, hemoglobin, lactate dehydrogenase, and alkaline phosphatase. The magnitude of the differences is not likely to be clinically meaningful. Further, integration of these four highly correlated factors plus performance status in the Halabi nomogram revealed a 1-month mean and 2-month median difference in predicted survival (mean and median of 20.4 months for controls v mean of 21.4 months and median of 22.5 months for PROSTVAC; Table 1). The observed survival difference of 8.5 months far exceeds that predicted by the Halabi nomogram. Finally, the effect modifier analysis, including the Halabi score, failed to find evidence of effect modification and, in fact, found suggestions of effect for all subgroups (Fig 4). Thus, while there are some between-arm baseline differences, it does not seem plausible that these differences could be the explanation for the observed effect size for OS.
With the current PROSTVAC data, there are parallels with respect to other immunotherapy-based approaches to prostate cancer. Treatment of a similar group of metastatic prostate cancer patients with a good prognosis (median Halabi predicted survival of 21 months) with sipuleucel-T provided an improved median OS of 4.5 months (25.9 months for sipuleucel-T v 21.4 months for controls) and OS benefit (3-year OS of 33% v 11%), yet demonstrated only a trend toward delayed short-term disease progression.16 A larger phase III study with more than 500 patients has recently confirmed these results.17 These studies of immunotherapy in prostate cancer may represent an emerging theme of prolonged survival, without a demonstrable signal of tumor shrinkage or delay in short-term disease progression.
A potential limitation of this study of OS is the lack of treatment data after completion of the treatment phase of the trial. Imbalances due to chance may have occurred in treatments after progression. However, only docetaxel has been shown to affect survival in metastatic prostate cancer patients, and only by approximately 3 months.18 Thus, we think it unlikely that a potential imbalance in post-study chemotherapy treatment could explain the survival result.
The role of GM-CSF in the treatment effect is unclear. Murine data support its use as an adjuvant19; however, clinical data are less definitive, and only small numbers of patients have been evaluated in poxviral vaccine trials with and without GM-CSF.9,20,21 While there has been single-agent activity of GM-CSF in prostate cancer (mainly PSA response), those studies used high doses (250 μg/m2) for 14 days on and 14 days off and for multiple cycles, and effects on OS are not known.22
No detectable antibody titers to PSA were generated. This is consistent with prior observations with PSA-based poxviral vector vaccinations, where only 1 of 200 patients developed anti-PSA antibodies in previous clinical trials.1–4,7–9 Unfortunately, T-cell immune responses were not evaluated. However, in the recent phase II clinical study of PROSTVAC-VF in 32 patients conducted by the NCI, gamma-interferon ELISPOTs were analyzed.9 In that study, 13 of 28 evaluable patients had more than two-fold increases in PSA epitope-specific immune responses, and four of five high responders (more than a six-fold increase) survived > 40 months, while low or nonresponders had a median OS of 20 months.
In summary, PROSTVAC immunotherapy in this randomized, controlled, and blinded study was associated with an improved OS. The estimated hazard ratio is 0.56 (95% CI, 0.37 to 0.85), and the observed difference in median survival of 8.5 months suggests significant impact. Nonetheless, while these data are statistically and potentially clinically meaningful, these remarkable findings are regarded as hypothesis generating. PROSTVAC immunotherapy is a promising approach, and a larger pivotal phase III trial is planned.
Acknowledgment
We gratefully acknowledge the work of the investigators, coordinators, and patients who participated in and contributed to this PROSTVAC trial.
Footnotes
See accompanying editorial on page 1085
Supported by the Intramural Research Program of the National Cancer Institute, Center for Cancer Research, National Institutes of Health. Additional support was provided by the Laboratory of Tumor Immunology and Biology, via the National Cancer Institute, which had a Collaborative Research and Development Agreement with Therion Biologics and currently has one with BN ImmunoTherapeutics.
Presented in part as an oral presentation at the 42nd Annual Meeting of the American Society of Clinical Oncology (ASCO), June 2-6, 2006, Atlanta, GA; the 45th Annual Meeting of ASCO, May 29-June 2, 2009, Orlando, FL; and the 15th Annual Meeting of the European Cancer Organization, September 20-24, 2009, Berlin, Germany.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00078585.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: Reiner Laus, BN ImmunoTherapeutics (C); Wayne R. Godfrey, BN ImmunoTherapeutics (C) Consultant or Advisory Role: Brent A. Blumenstein, BN ImmunoTherapeutics (C); Dennis L. Panicali, BN ImmunoTherapeutics (C) Stock Ownership: Reiner Laus, BN ImmunoTherapeutics; Wayne R. Godfrey, BN ImmunoTherapeutics Honoraria: None Research Funding: L. Michael Glode, Therion Biologics Expert Testimony: None Other Remuneration: Thomas J. Schuetz, Therion Biologics
AUTHOR CONTRIBUTIONS
Conception and design: Philip W. Kantoff, Thomas J. Schuetz, Michael Wyand, Kelledy Manson, Dennis L. Panicali, Jeffrey Schlom, William L. Dahut, Philip M. Arlen, James L. Gulley
Financial support: Reiner Laus
Provision of study materials or patients: Philip W. Kantoff, Thomas J. Schuetz, L. Michael Glode, David L. Bilhartz, Michael Wyand, Dennis L. Panicali
Collection and assembly of data: Philip W. Kantoff, Thomas J. Schuetz, Brent A. Blumenstein, David L. Bilhartz, Michael Wyand, Kelledy Manson, Wayne R. Godfrey
Data analysis and interpretation: Philip W. Kantoff, Thomas J. Schuetz, Brent A. Blumenstein, Michael Wyand, Dennis L. Panicali, Reiner Laus, Jeffrey Schlom, Philip M. Arlen, James L. Gulley, Wayne R. Godfrey
Manuscript writing: Philip W. Kantoff, Thomas J. Schuetz, Brent A. Blumenstein, L. Michael Glode, Kelledy Manson, Jeffrey Schlom, Philip M. Arlen, James L. Gulley, Wayne R. Godfrey
Final approval of manuscript: Philip W. Kantoff, Thomas J. Schuetz, Brent A. Blumenstein, L. Michael Glode, David L. Bilhartz, Michael Wyand, Dennis L. Panicali, Reiner Laus, Jeffrey Schlom, William L. Dahut, Philip M. Arlen, James L. Gulley, Wayne R. Godfrey
REFERENCES
- 1.Sanda MG, Smith DC, Linda GC, et al. Recombinant vaccinia-PSA (PROSTVAC) can induce a prostate-specific immune response in androgen-modulated human prostate cancer. Urology. 1999;53:260–266. doi: 10.1016/s0090-4295(98)00539-1. [DOI] [PubMed] [Google Scholar]
- 2.Eder JP, Kantoff PW, Roper K, et al. A phase I trial of a recombinant vaccinia virus expressing prostate-specific antigen in advanced prostate cancer. Clin Cancer Res. 2000;6:1632–1638. [PubMed] [Google Scholar]
- 3.Gulley J, Chen AP, Dahut W, et al. Phase I study of a vaccine using recombinant vaccinia virus expressing PSA (rV-PSA) in patients with metastatic androgen-independent prostate cancer. Prostate. 2002;53:109–117. doi: 10.1002/pros.10130. [DOI] [PubMed] [Google Scholar]
- 4.Kaufman HL, Wang W, Manola J, et al. Phase II randomized study of vaccine treatment of advanced prostate cancer (E7897): A trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2004;22:2122–2132. doi: 10.1200/JCO.2004.08.083. [DOI] [PubMed] [Google Scholar]
- 5.Hodge JW, Sabzevari H, Yafal AG, et al. A triad of costimulatory molecules synergize to amplify T-cell activation. Cancer Res. 1999;59:5800–5807. [PubMed] [Google Scholar]
- 6.Hodge JW, Greiner JW, Tsang KY, et al. Costimulatory molecules as adjuvants for immunotherapy. Front Biosci. 2006;11:788–803. doi: 10.2741/1837. [DOI] [PubMed] [Google Scholar]
- 7.DiPaola RS, Plante M, Kaufman H, et al. A phase I trial of pox PSA vaccines (PROSTVAC-VF) with B7-1, ICAM-1, and LFA-3 co-stimulatory molecules (TRICOM) in patients with prostate cancer. J Transl Med. 2006;4:1–5. doi: 10.1186/1479-5876-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arlen PM, Skarupa L, Pazdur M, et al. Clinical safety of a viral vector based prostate cancer vaccine strategy. J Urol. 2007;178:1515–1520. doi: 10.1016/j.juro.2007.05.117. [DOI] [PubMed] [Google Scholar]
- 9.Gulley JL, Arlen PM, Madan RA, et al. Immunologic and prognostic factors associated with overall survival employing a poxviral-based PSA vaccine in metastatic castrate-resistant prostate cancer. Cancer Immunol Immunother. doi: 10.1007/s00262-009-0782-8. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kantoff PW, Glode LM, Tannenbaum SI, et al. Randomized, double-blind, vector-controlled study of targeted immunotherapy in patients (pts) with hormone-refractory prostate cancer (HRPC) J Clin Oncol. 2006;24(suppl):100s. abstr 2501. [Google Scholar]
- 11.Schlom J, Arlen PM, Gulley JL. Cancer vaccines: Moving beyond current paradigms. Clin Cancer Res. 2007;13:3776–3782. doi: 10.1158/1078-0432.CCR-07-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bubley GJ, Carducci M, Dahut W, et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: Recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol. 1999;17:3461–3467. doi: 10.1200/JCO.1999.17.11.3461. [DOI] [PubMed] [Google Scholar]
- 13.Wang R, Lagakos SW, Ware JH, et al. Statistics in medicine: Reporting of subgroup analyses in clinical trials. N Engl J Med. 2007;357:2189–2194. doi: 10.1056/NEJMsr077003. [DOI] [PubMed] [Google Scholar]
- 14.Halabi S, Small EJ, Kantoff PW, et al. Prognostic model for predicting survival in men with hormone-refractory metastatic prostate cancer. J Clin Oncol. 2003;21:1232–1237. doi: 10.1200/JCO.2003.06.100. [DOI] [PubMed] [Google Scholar]
- 15.Poland GA, Grabenstein JD, Neff JM. The US smallpox vaccination program: A review of a large modern era smallpox vaccination implementation program. Vaccine. 2005;23:2078–2081. doi: 10.1016/j.vaccine.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Small EJ, Schellhammer PF, Higano CS, et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J Clin Oncol. 2006;24:3089–3094. doi: 10.1200/JCO.2005.04.5252. [DOI] [PubMed] [Google Scholar]
- 17.Schellhammer PF, Higano C, Berger ER, et al. A randomized, double-blind, placebo-controlled, multi-center, phase III trial of sipuleucel-T in men with metastatic, androgen independent prostatic adenocarcinoma (AIPC). Presented at the Annual Meeting of the American Urological Association; April 25-30, 2009; Chicago, IL. [Google Scholar]
- 18.Berthold DR, Pond GR, Soban F, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: Updated survival in the TAX 327 study. J Clin Oncol. 2008;26:242–245. doi: 10.1200/JCO.2007.12.4008. [DOI] [PubMed] [Google Scholar]
- 19.Aarts WM, Schlom J, Hodge JW. Vector-based vaccine/cytokine combination therapy to enhance induction of immune responses to a self-antigen and antitumor activity. Cancer Res. 2002;62:5770–5777. [PubMed] [Google Scholar]
- 20.von Mehren M, Arlen P, Gulley J, et al. The influence of granulocyte macrophage colony-stimulating factor and prior chemotherapy on the immunological response to a vaccine (ALVAC-CEA B7.1) in patients with metastatic carcinoma. Clin Cancer Res. 2001;7:1181–1191. [PubMed] [Google Scholar]
- 21.Marshall JL, Gulley JL, Arlen PM, et al. Phase I study of sequential vaccinations with fowlpox-CEA(6D)-TRICOM alone and sequentially with vaccinia-CEA(6D)-TRICOM, with and without granulocyte-macrophage colony-stimulating factor, in patients with carcinoembryonic antigen-expressing carcinomas. J Clin Oncol. 2005;23:720–731. doi: 10.1200/JCO.2005.10.206. [DOI] [PubMed] [Google Scholar]
- 22.Small EJ, Reese DM, Um B, et al. Therapy of advanced prostate cancer with granulocyte macrophage colony-stimulating factor. Clin Cancer Res. 1999;5:1738–1744. [PubMed] [Google Scholar]