Abstract
Purpose
In the absence of high-level evidence or clinical guidelines supporting any given active treatment approach over another for localized prostate cancer, clinician and patient preferences may lead to substantial variation in treatment use.
Methods
Data were analyzed from 36 clinical sites that contributed data to the Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) registry. Distribution of primary treatment use was measured over time. Prostate cancer risk was assessed using the D'Amico risk groups and the Cancer of the Prostate Risk Assessment (CAPRA) score. Descriptive analyses were performed, and a hierarchical model was constructed that controlled for year of diagnosis, cancer risk variables, and other patient factors to estimate the proportion of variation in primary treatment selection explicable by practice site.
Results
Among 11,892 men analyzed, 6.8% elected surveillance, 49.9% prostatectomy, 11.6% external-beam radiation, 13.3% brachytherapy, 4.0% cryoablation, and 14.4% androgen deprivation monotherapy. Prostate cancer risk drives treatment selection, but the data suggest both overtreatment of low-risk disease and undertreatment of high-risk disease. The former trend appears to be improving over time, while the latter is worsening. Treatment varies with age, comorbidity, and socioeconomic status. However, treatment patterns vary markedly across clinical sites, and this variation is not explained by case-mix variability or known patient factors. Practice site explains a proportion of this variation ranging from 13% for androgen deprivation monotherapy to 74% for cryoablation.
Conclusion
Substantial variation exists in management of localized prostate cancer that is not explained by measurable factors. A critical need exists for high-quality comparative effectiveness research in localized prostate cancer to help guide treatment decision making.
INTRODUCTION
An estimated 192,280 men will be diagnosed with prostate cancer this year and will face a complex decision with respect to timing of and type of initial management.1 Prostate cancer is second only to lung cancer as a cause of cancer mortality among men in the United States,1 but the natural history of the disease is variable and is frequently indolent, even in the setting of expectant management.2 The choice of any available treatment, moreover, is associated with risks of adverse effects and substantial impacts on health-related quality of life.3
No contemporary clinical trials randomly assigning men among any of the major active treatment modalities—radical prostatectomy, interstitial or external-beam radiation therapy, or androgen deprivation therapy—have been reported. Indeed, a recent systematic review commissioned by the Agency for Healthcare Research and Quality found that insufficient evidence exists to conclude that there is greater benefit for any given treatment approach over another.4 The recently updated clinical practice guideline published by the American Urological Association (AUA) endorses alternatives for localized disease, including active surveillance, prostatectomy, and radiation therapy (interstitial or external-beam), but again was unable to draw conclusions regarding the relative benefits of these alternatives.5
In the absence of high-quality evidence regarding the comparative effectiveness of these alternatives, clinician and patient preferences and values play a significant role in determining treatment approach6; along with various financial, legal, and other incentives, these preferences tend to lead to unwarranted variation in approaches to care across health care regions or localities.7 Indeed, previous studies have identified substantial local and regional variation in the use of radical prostatectomy and androgen deprivation therapy, but they were based on older data and were unable to adjust adequately for prostate cancer risk characteristics.8,9 We analyzed a large national registry of men with localized prostate cancer to determine trends over time in treatment of cancers at varying levels of progression risk, and to characterize and quantify variation in primary treatment at the level of the clinical practice site.
METHODS
Data were reviewed from the Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE), a national disease registry of men with biopsy-proven prostate adenocarcinoma. CaPSURE participants were accrued from 40 urology practices across the United States, of which three were academic and the remainder were community-based. Participating urologists consecutively recruited newly diagnosed men and reported initial and follow-up clinical data, including staging tests and treatments. Patients were treated per their clinicians' usual practices and were followed until time of death or withdrawal from the study. All patients provided written, informed consent under local and central institutional review board supervision. Details regarding CaPSURE's methodology have been reported previously.10,11
As of July 2008, there were 13,805 men enrolled in CaPSURE. Of these, 534 had advanced (clinical stage > T3aN0M0) disease at time of diagnosis and were excluded, as were 421 diagnosed before 1990. A total of 44 patients were treated at one of four practice sites contributing fewer than 30 patients each to the data set and were excluded. Finally, 914 men managed with a primary treatment other than radical prostatectomy, external-beam radiation therapy, brachytherapy, cryoablation, watchful waiting/active surveillance (CaPSURE does not distinguish these two terms), or primary androgen deprivation therapy, or whose primary treatment was unknown, were excluded. Thus, the analytic data set comprised 11,892 men managed at 36 practice sites.
Sociodemographic variables, including age, race, geographic region, insurance type, education, and household income, were recorded and compared across primary treatments, and comorbidity was assessed as previously described.12 Significance of differences across treatments was assessed with the χ2 test for categorical variables and the Mantel-Haenszel χ2 test for ordinal and categorized continuous variables. Prostate cancer risk was summarized using the D'Amico classification, which stratifies men to low, intermediate, and high risk based on the prostate-specific antigen (PSA), Gleason score, and clinical T stage as endorsed by the AUA guideline5; and the University of California, San Francisco (UCSF) Cancer of the Prostate Risk Assessment (CAPRA) score, which is a 0 to 10 multivariable score with improved accuracy compared with the risk groups, derived from the same variables with the addition of age and percent of positive biopsy cores. The CAPRA score has also been validated as a grouped score yielding three strata, with scores of 0 to 2, 3 to 5, and 6 to 10 indicating low, intermediate, and high risk, respectively.13
Treatment distributions were examined for patients at each individual CAPRA score level, and trends over time were examined for men in each risk stratum as defined by grouped CAPRA scores. These trends were tested for statistical significance with the Mantel-Haenszel χ2 test. Treatment distribution was plotted for each individual practice site, with sites sorted by case-mix in terms of prostate cancer risk as measured by the mean CAPRA score for all patients managed at each site. To estimate the proportion of treatment variation attributable to the practice site, a random effects hierarchical logit model was tested for each primary treatment modality, with adjustment for year of diagnosis, age, race/ethnicity, income, education, geographic region, Charlson score, PSA, Gleason score, clinical T stage, and percent of positive biopsy cores. The intraclass correlation coefficient (ICC) for clinical site was assessed to measure the proportion of treatment variation attributable to practice site. These analyses were repeated on the subset of patients who had low-risk features under the AUA guidelines (PSA < 10 ng/mL, Gleason score ≤ 6, and clinical stage ≤ T2a).5 The ICC was also calculated for use of neoadjuvant androgen deprivation therapy among men undergoing external-beam radiation therapy, and the frequency of neoadjuvant therapy among external-beam radiation patients was compared with the frequency of primary androgen deprivation therapy use across sites by calculation of Pearson's correlation coefficient (r). All statistical tests were two-sided, and all analyses were performed using STATA version 10.1 (STATA, College Station, TX).
RESULTS
Overall, of the 11,892 men in the analytic data set, 810 (6.8%) elected watchful waiting/active surveillance, 5,931 (49.9%) radical prostatectomy, 1,382 (11.6%) external-beam radiation therapy, 1,583 (13.3%) brachytherapy, 474 (4.0%) cryoablation, and 1,712 (14.4%) primary androgen deprivation monotherapy.
Table 1 presents the sociodemographic and clinical characteristics of men in each primary treatment group. In general terms, men undergoing prostatectomy were younger than those receiving other treatments, healthier in terms of comorbidity, more likely to have private insurance, and more likely to have high socioeconomic status. Brachytherapy and cryoablation patients were somewhat older and had lower socioeconomic status and higher comorbidity, followed by external-beam radiation therapy patients, and finally primary androgen deprivation and watchful waiting patients. All differences across treatment modalities were statistically significant (P < .001).
Table 1.
Sociodemographic and Clinical Characteristics of Men by Primary Treatment Group
| Characteristic | WW/AS |
RP |
Brachytherapy |
EBRT |
Cryotherapy |
PADT |
Total |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | No. | % | |
| Age, years | ||||||||||||||
| < 50 | 4 | 1.3 | 276 | 88.2 | 15 | 4.8 | 6 | 1.9 | 1 | 0.3 | 11 | 3.5 | 313 | 2.6 |
| 50-65 | 130 | 2.5 | 3,813 | 73.6 | 520 | 10.0 | 288 | 5.6 | 146 | 2.8 | 285 | 5.5 | 5,182 | 43.6 |
| 65-75 | 345 | 7.4 | 1,796 | 38.6 | 801 | 17.2 | 751 | 16.1 | 273 | 5.9 | 686 | 14.7 | 4,652 | 39.1 |
| > 75 | 331 | 19.0 | 46 | 2.6 | 247 | 14.2 | 337 | 19.3 | 54 | 3.1 | 730 | 41.8 | 1,745 | 14.7 |
| Race | ||||||||||||||
| White | 710 | 6.9 | 5,191 | 50.7 | 1,394 | 13.6 | 1,142 | 11.1 | 436 | 4.3 | 1,373 | 13.4 | 10,246 | 86.2 |
| African American | 76 | 6.2 | 548 | 44.5 | 118 | 9.6 | 202 | 16.4 | 27 | 2.2 | 260 | 21.1 | 1,231 | 10.4 |
| Latino | 9 | 4.7 | 79 | 41.1 | 53 | 27.6 | 18 | 9.4 | 6 | 3.1 | 27 | 14.1 | 192 | 1.6 |
| Asian | 8 | 8.6 | 36 | 38.7 | 11 | 11.8 | 11 | 11.8 | 1 | 1.1 | 26 | 28.0 | 93 | 0.8 |
| Native American | 0 | 0.0 | 19 | 76.0 | 0 | 0.0 | 1 | 4.0 | 2 | 8.0 | 3 | 12.0 | 25 | 0.2 |
| Mixed | 2 | 4.4 | 27 | 60.0 | 6 | 13.3 | 2 | 4.4 | 2 | 4.4 | 6 | 13.3 | 45 | 0.4 |
| Unknown | 5 | 8.3 | 31 | 51.7 | 1 | 1.7 | 6 | 10.0 | 0 | 0.0 | 17 | 28.3 | 60 | 0.5 |
| Region | ||||||||||||||
| West | 227 | 13.1 | 829 | 47.8 | 202 | 11.7 | 130 | 7.5 | 66 | 3.8 | 279 | 16.1 | 1,733 | 14.6 |
| East | 303 | 5.7 | 2,785 | 51.9 | 631 | 11.8 | 772 | 14.4 | 304 | 5.7 | 566 | 10.6 | 5,361 | 45.1 |
| Midwest | 160 | 6.3 | 1,450 | 57.2 | 296 | 11.7 | 227 | 9.0 | 47 | 1.9 | 356 | 14.0 | 2,536 | 21.3 |
| South | 120 | 5.3 | 867 | 38.3 | 454 | 20.1 | 253 | 11.2 | 57 | 2.5 | 511 | 22.6 | 2,262 | 19.0 |
| Insurance | ||||||||||||||
| Private | 140 | 2.8 | 3,565 | 70.4 | 592 | 11.7 | 255 | 5.0 | 165 | 3.3 | 347 | 6.9 | 5,064 | 42.6 |
| Medicare | 157 | 9.2 | 501 | 29.2 | 255 | 14.9 | 333 | 19.4 | 106 | 6.2 | 361 | 21.1 | 1,713 | 14.4 |
| Medicare + supplemental insurance | 414 | 9.8 | 1,501 | 35.7 | 650 | 15.5 | 650 | 15.5 | 189 | 4.5 | 802 | 19.1 | 4,206 | 35.4 |
| Veterans affairs | 58 | 17.7 | 135 | 41.3 | 52 | 15.9 | 27 | 8.3 | 0 | 0.0 | 55 | 16.8 | 327 | 2.7 |
| Other/unknown | 41 | 7.0 | 229 | 39.3 | 34 | 5.8 | 117 | 20.1 | 14 | 2.4 | 147 | 25.3 | 582 | 4.9 |
| Education | ||||||||||||||
| No high school | 38 | 7.4 | 166 | 32.1 | 79 | 15.3 | 85 | 16.4 | 20 | 3.9 | 129 | 25.0 | 517 | 4.3 |
| Some high school | 98 | 10.6 | 345 | 37.2 | 138 | 14.9 | 123 | 13.3 | 42 | 4.5 | 182 | 19.6 | 928 | 7.8 |
| High school graduate | 169 | 7.2 | 1,130 | 48.0 | 311 | 13.2 | 293 | 12.4 | 125 | 5.3 | 327 | 13.9 | 2,355 | 19.8 |
| Some college | 126 | 7.1 | 936 | 52.8 | 221 | 12.5 | 177 | 10.0 | 77 | 4.3 | 235 | 13.3 | 1,772 | 14.9 |
| College graduate | 83 | 5.0 | 993 | 59.9 | 167 | 10.1 | 186 | 11.2 | 73 | 4.4 | 155 | 9.4 | 1,657 | 13.9 |
| Graduate school | 93 | 5.2 | 1,084 | 60.2 | 225 | 12.5 | 186 | 10.3 | 42 | 2.3 | 170 | 9.4 | 1,800 | 15.1 |
| Missing | 203 | 7.1 | 1,277 | 44.6 | 442 | 15.4 | 332 | 11.6 | 95 | 3.3 | 514 | 18.0 | 2,863 | 24.1 |
| Annual income, $ | ||||||||||||||
| Zero | 1 | 2.7 | 10 | 27.0 | 6 | 16.2 | 5 | 13.5 | 2 | 5.4 | 13 | 35.1 | 37 | 0.3 |
| < 5,000 | 5 | 6.0 | 17 | 20.2 | 9 | 10.7 | 17 | 20.2 | 4 | 4.8 | 32 | 38.1 | 84 | 0.7 |
| 5,000-10,000 | 19 | 6.8 | 89 | 31.9 | 31 | 11.1 | 39 | 14.0 | 11 | 3.9 | 90 | 32.3 | 279 | 2.3 |
| 10,000-20,000 | 98 | 10.9 | 269 | 29.8 | 156 | 17.3 | 146 | 16.2 | 48 | 5.3 | 185 | 20.5 | 902 | 7.6 |
| 20,000-30,000 | 111 | 8.5 | 532 | 40.8 | 196 | 15.0 | 194 | 14.9 | 67 | 5.1 | 205 | 15.7 | 1,305 | 11.0 |
| 30,000-50,000 | 134 | 6.8 | 1,000 | 50.4 | 264 | 13.3 | 231 | 11.6 | 101 | 5.1 | 254 | 12.8 | 1,984 | 16.7 |
| 50,000-75,000 | 64 | 4.4 | 904 | 62.5 | 156 | 10.8 | 121 | 8.4 | 55 | 3.8 | 147 | 10.2 | 1,447 | 12.2 |
| > 75,000 | 80 | 4.1 | 1,396 | 71.0 | 188 | 9.6 | 147 | 7.5 | 45 | 2.3 | 110 | 5.6 | 1,966 | 16.5 |
| Unknown | 298 | 7.7 | 1,714 | 44.1 | 577 | 14.8 | 482 | 12.4 | 141 | 3.6 | 676 | 17.4 | 3,888 | 32.7 |
| Comorbidities | ||||||||||||||
| 0 | 72 | 4.4 | 1,059 | 65.5 | 145 | 9.0 | 147 | 9.1 | 54 | 3.3 | 141 | 8.7 | 1,618 | 13.6 |
| 1 | 117 | 4.8 | 1,453 | 59.3 | 283 | 11.5 | 254 | 10.4 | 114 | 4.6 | 231 | 9.4 | 2,452 | 20.6 |
| 2 | 154 | 6.6 | 1,158 | 49.7 | 317 | 13.6 | 285 | 12.2 | 102 | 4.4 | 312 | 13.4 | 2,328 | 19.6 |
| 3 | 137 | 8.9 | 645 | 41.8 | 225 | 14.6 | 188 | 12.2 | 72 | 4.7 | 275 | 17.8 | 1,542 | 13.0 |
| > 3 | 137 | 11.2 | 399 | 32.6 | 179 | 14.6 | 204 | 16.7 | 47 | 3.8 | 258 | 21.1 | 1,224 | 10.3 |
| Unknown | 193 | 7.1 | 1,217 | 44.6 | 434 | 15.9 | 304 | 11.1 | 85 | 3.1 | 495 | 18.1 | 2,728 | 22.9 |
| Risk group | ||||||||||||||
| Low | 397 | 9.2 | 2,449 | 56.8 | 691 | 16.0 | 315 | 7.3 | 132 | 3.1 | 330 | 7.6 | 4,314 | 36.3 |
| Intermediate | 209 | 4.8 | 2,283 | 52.9 | 581 | 13.5 | 530 | 12.3 | 194 | 4.5 | 515 | 11.9 | 4,312 | 36.3 |
| High | 58 | 3.2 | 576 | 32.2 | 135 | 7.5 | 324 | 18.1 | 109 | 6.1 | 588 | 32.8 | 1,790 | 15.1 |
| Unknown | 146 | 9.9 | 623 | 42.2 | 176 | 11.9 | 213 | 14.4 | 39 | 2.6 | 279 | 18.9 | 1,476 | 12.4 |
| CAPRA score | ||||||||||||||
| 0-2 | 358 | 7.4 | 2,845 | 58.9 | 810 | 16.8 | 315 | 6.5 | 153 | 3.2 | 352 | 7.3 | 4,833 | 40.6 |
| 3-5 | 166 | 4.8 | 1,720 | 49.7 | 453 | 13.1 | 465 | 13.4 | 167 | 4.8 | 487 | 14.1 | 3,458 | 29.1 |
| 6-10 | 33 | 2.6 | 338 | 26.6 | 109 | 8.6 | 272 | 21.4 | 74 | 5.8 | 445 | 35.0 | 1,271 | 10.7 |
| Unknown | 253 | 10.9 | 1,028 | 44.1 | 211 | 9.1 | 330 | 14.2 | 80 | 3.4 | 428 | 18.4 | 2,330 | 19.6 |
| Total | 810 | 5,931 | 1,583 | 1,382 | 474 | 1,712 | 11,892 | |||||||
Abbreviations: WW/AS, watchful waiting/active surveillance; RP, radical prostatectomy; EBRT, external-beam radiation therapy; PADT, primary androgen deprivation therapy; CAPRA, Cancer of the Prostate Risk Assessment.
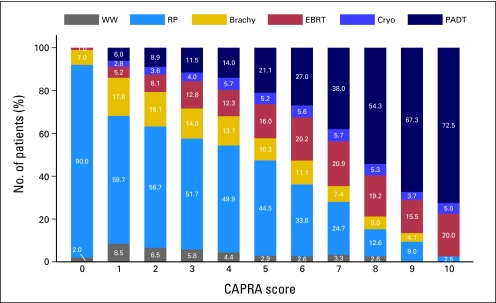
Prostate cancer risk was a strong driver of treatment selection, as summarized in Table 1 and Figure 1. With increasing risk as measured by the CAPRA score, proportions of prostatectomy, brachytherapy, and watchful waiting decreased consistently, while the proportion of primary androgen deprivation monotherapy increased substantially. The particularly high proportion of prostatectomy patients in the CAPRA 0 stratum reflects the fact that these patients are by definition relatively young (age < 50 years) as well as low risk in other respects. Use of external-beam radiation and cryoablation rose with risk through the low-risk group (CAPRA 0-2) and was relatively consistent across the intermediate-risk (CAPRA 3 to 5) and high-risk (CAPRA 6-10) groups (P < .001).
Fig 1.
Treatment patterns by risk level. Treatment distribution at each level of risk as measured by the Cancer of the Prostate Risk Assessment (CAPRA) score is illustrated. Treatment trends by risk group were statistically significant by Mantel-Haenszel χ2 test, P < .001. WW, watchful waiting/active surveillance; RP, radical prostatectomy; Brachy, brachytherapy; EBRT, external-beam radiation therapy; Cryo, cryoablation; PADT, primary androgen deprivation therapy.
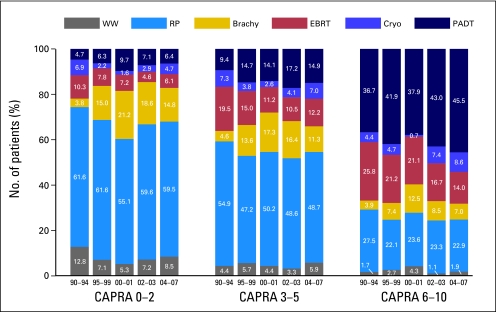
Treatment trends over time were discordant across risk groups, as presented in Figure 2. For low-risk patients, use of brachytherapy and primary androgen deprivation rose through the 1990s at the expense of prostatectomy and watchful waiting, while in the current decade, these trends appear to be reversing. Among intermediate-risk patients, treatment patterns have been relatively stable since the mid-1990s; for high-risk patients, use of prostatectomy has been relatively stable while use of primary androgen deprivation monotherapy has been rising, apparently at the expense of external-beam radiation therapy in particular.
Fig 2.
Treatment trends over time by risk group. Changes over time in primary treatment patterns across all practice sites, by risk groups defined by categorized Cancer of the Prostate Risk Assessment (CAPRA) scores. Trends over time for each risk group were statistically significant by Mantel-Haenszel χ2 test, P < .001. WW, watchful waiting; RP, radical prostatectomy; Brachy, brachytherapy; EBRT, external-beam radiation therapy; Cryo, cryoablation; PADT, primary androgen deprivation therapy.
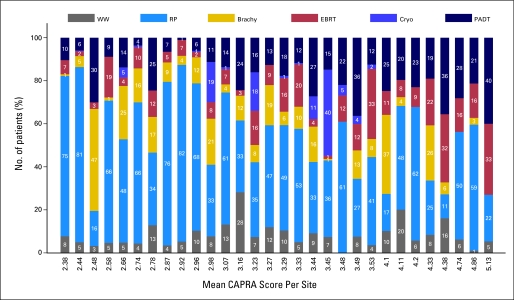
Figure 3 illustrates the substantial variation that exists across practice sites in terms of primary treatment selection for localized prostate cancer. Use of watchful waiting/active surveillance ranged from 0% to 28% across sites, use of prostatectomy from 11% to 82%, use of brachytherapy from 0% to 47%, use of external-beam radiation therapy from 1% to 33%, use of cryoablation from 0% to 40%, and use of primary androgen deprivation monotherapy from 1% to 40%. The sites in Figure 3 are sorted according to case-mix as assessed by the mean CAPRA score. There is no discernable pattern from left to right (as mean CAPRA score doubles from 2.38 to 5.13) suggesting that the variation in treatment selection across sites was not substantially explained by disease characteristics.
Fig 3.
Treatment variation among practice sites. Primary treatment distribution from 1990 to 2006 at each clinical site for patients with localized prostate cancer. Sites had at least 30 patients. Clinical sites are arranged in order of increasing average disease risk as assessed by the mean Cancer of the Prostate Risk Assessment (CAPRA) score for that site (listed on x-axis). WW, watchful waiting; RP, radical prostatectomy; Brachy, brachytherapy; EBRT, external-beam radiation therapy; Cryo, cryoablation; PADT, primary androgen deprivation therapy.
The results of the multivariable analysis are presented in Table 2. Proportion of variation attributable to practice site ranged from 13% for primary androgen deprivation to 74% for cryoablation; among D'Amico low-risk patients only, this proportion ranged from 21% for watchful waiting to 81% for cryoablation. For neoadjuvant androgen deprivation therapy with external-beam radiation, 14% of variation was attributable to practice site. Use of neoadjuvant androgen deprivation therapy correlated modestly with use of primary androgen deprivation at each site (r = 0.48; P < .001).
Table 2.
Treatment Variation Attributable to Practice Site
| Treatment | ρ (all patients) | 95% CI | ρ (low-risk patients only) | 95% CI |
|---|---|---|---|---|
| WW/AS | 0.17 | 0.10 to 0.28 | 0.21 | 0.11 to 0.37 |
| RP | 0.30 | 0.20 to 0.42 | 0.29 | 0.18 to 0.42 |
| EBRT | 0.20 | 0.12 to 0.31 | 0.22 | 0.12 to 0.37 |
| Brachytherapy | 0.36 | 0.23 to 0.52 | 0.32 | 0.19 to 0.49 |
| Cryotherapy | 0.74 | 0.56 to 0.87 | 0.81 | 0.63 to 0.91 |
| PADT | 0.13 | 0.07 to 0.21 | 0.23 | 0.12 to 0.39 |
| NADT | 0.14 | 0.08 to 0.23 |
NOTE. Proportion of variation (ρ) attributable to practice site is presented with 95% CIs for each primary treatment type, and for NADT given with EBRT.
Abbreviations: WW/AS, watchful waiting/active surveillance; RP, radical prostatectomy; EBRT, external-beam radiation therapy; PADT, primary androgen deprivation therapy; NADT, neoadjuvant androgen deprivation therapy.
DISCUSSION
In a large, prospectively accrued cohort of men with prostate cancer, disease risk as measured by the CAPRA score does appear to be a major driver of treatment (Fig 1), though the patterns observed are of some concern for both overtreatment of low-risk disease—identified in prior analyses of CaPSURE and other data sources14,15—and undertreatment of high-risk localized disease.16 More men with low-risk disease should be candidates for active surveillance, whereas most with high-risk but clinically localized disease should be offered a chance at cure with multimodal therapy, including either radiation or surgery as the primary local modality.5 Interestingly, trends over time (Fig 2) suggest that overtreatment of low-risk disease may be ameliorating slightly, whereas potential undertreatment of high-risk disease appears to be a growing concern.
Aside from these observed trends over time and risk, this analysis confirms wide variation in practice patterns across clinical sites (Fig 3). Indeed, a substantial proportion of variation in primary treatment selection for localized prostate cancer is attributable to practice site. The degrees of variation observed were similar when the analysis was restricted to men with low-risk disease only. These findings suggest that factors other than cancer risk and patient clinical and sociodemographic factors influence treatment decision making. In fact, treatment of localized prostate cancer is a model of what has been termed “preference-sensitive” health care, in which patient or clinician preferences, beliefs, or values drive decision making in the absence of strong scientific evidence.7
Indeed, clinicians and patients making decisions regarding localized prostate cancer do so in the setting of a relative dearth of high-quality data comparing outcomes following the various available treatments.4 Only one randomized trial of substantial size and quality has been reported,17 finding a survival benefit for prostatectomy over watchful waiting; however, the generalizability of the findings to contemporary patients, and to subgroups of various ages and levels of risk, has been the subject of ongoing debate. The Prostate Cancer Intervention Versus Observation Trial (PIVOT) study, also randomly assigning patients to surgery versus watchful waiting, completed accrual; results are expected during 2010.18
However, no trials that randomly assign patients among active treatment modalities have yet been completed. The Surgical Prostatectomy Versus Interstitial Radiation Intervention Trial (SPIRIT), intended to compare surgery with brachytherapy, closed because of poor accrual.19 The Prostate Testing for Cancer and Treatment (ProtecT) study successfully randomized men in the United Kingdom to a three-arm trial comparing prostatectomy, radiation, and surveillance, though results will not be available until the middle of the next decade.20
Previous analyses have documented substantial local variation for specific prostate cancer treatments. Using Medicare data from the mid-1990s, for example, the Dartmouth Atlas of Health Care project analyzed the 10 most commonly performed surgical procedures in the United States, including radical prostatectomy. Findings show that among the 10 procedures, prostatectomy was characterized by the greatest local variation: more than 12-fold greater than the procedure (hip fracture repair) with the least variation and more than eight-fold greater than colectomy for colon cancer. The absolute rates of prostatectomy, adjusted for prevalence of prostate cancer, varied by a factor of nearly 10, from 0.5 to 4.7 per 1,000 Medicare enrollees.8
Another recently published study focused on the use of androgen deprivation therapy, based on 1990s data from the Surveillance, Epidemiology, and End Results (SEER)–Medicare linked database. The investigators performed separate analyses for evidence-based androgen deprivation therapy—that is, therapy given together with radiation therapy for high-risk disease (stage T3 or stage T2/poorly differentiated)—and uncertain-benefit therapy, including all other uses. For the evidence-based setting, they found, based on ICC calculations, that disease characteristics accounted for 6.6% of variation, other patient characteristics explained 7.3%, and the treating urologist accounted for 25.4% of variation. For the uncertain benefit setting, the corresponding proportions were 5.3%, 5.0%, and 22.7%. From the early to the late part of the decade, the proportion of variation attributable to the urologist appeared to be increasing.9
The major limitation of these studies, aside from the somewhat older data used for analysis, was the lack of adequate cancer risk assessment available in the data sets analyzed: Medicare has no data on risk factors, and the 1990s-era SEER-Medicare data had only stage and differentiation but not PSA levels or Gleason scores. In addition, analyses based on Medicare data by definition are restricted to patients older than age 65 years. The present analysis of treatment variation comprises updated data, including all of the major treatment approaches for localized prostate cancer, and incorporates a robust, well-validated risk assessment score for case-mix adjustment.
The proportions of variation attributable to clinical site for androgen deprivation—13% and 14% for primary and neoadjuvant therapy, respectively—are slightly lower than those observed in the SEER-Medicare analysis,9 perhaps reflecting better control for patient and tumor factors in CaPSURE. What is striking, however, is the fact that site-specific variation was higher for every other treatment modality than for androgen ablation therapy (Table 2). Use of prostatectomy varied from 11% to 82%, nearly as great a range as the 10-fold variation observed in the Dartmouth Atlas of Health Care project.8
Explanations for the observed variation are speculative, but presumably reflect variable physician training, experience, and personal outcomes; payor mix, reimbursement patterns, and other financial incentives; the local medicolegal environment; uneven penetrance of novel technologies; impact of local culture on patient beliefs and preferences; and many other factors. An aggressive local or personal philosophy with respect to prostate cancer screening may also correspond to an increased tendency toward treatment, even controlling for varying stage at presentation. A similar observation has been made, for example, in correlating rates of coronary angiography with angioplasty and/or bypass surgery.8 The protracted time course of prostate cancer, the proliferation of treatment options, and the nature of the potential complications of treatment all likely contribute additionally to the problem in the case of prostate cancer.
Certainly the absence of high-quality comparative effectiveness data, along with controversy regarding interpretation of the data that do exist, creates a fertile substrate in which variation would be expected to thrive. Even where high-quality evidence exists, however, incorporation into clinical practice may be variable. Randomized trials supporting the use of androgen deprivation together with radiation therapy, for example, were reported in the late 1990s, and those finding no benefit for androgen deprivation given before prostatectomy were reported in the early 2000s.21 Within a few years, the use of androgen deprivation therapy with external-beam radiation for high-risk patients rose to 85%, whereas use of such therapy before prostatectomy appeared to be rising to more than 10% by the mid- to late-2000s.16,21
Caveats to this analysis must be noted, the most important of which is that although the practices in CaPSURE represent a range of practice sizes and geographic locations, they were not chosen at random and do not represent a statistically valid sample of the US population. We have previously compared the CaPSURE population to the SEER population of prostate cancer patients and found that the median age among patients is similar, though white men are relatively over-represented in CaPSURE compared with the general population.13 Previous studies have found that race and ethnicity have little impact on degrees of variation in health care.22 Men in CaPSURE also have slightly higher socioeconomic status on average than the overall population.11 It is reassuring that the findings with respect to variation in use of prostatectomy and androgen deprivation therapy are generally consistent with the previous analyses from Medicare and SEER-Medicare; certainly there is no reason to suspect that the current analysis underestimates the true extent of variation at the population level.
Data in CaPSURE are submitted only by patients and urologists; thus any treatments by other clinicians that are not reported by patients either to their urologists or in their resource utilization questionnaires may be missed. Extant quality assurance mechanisms, including medical records review of all hospital admissions, is expected to minimize this problem. Despite these cautionary notes, we believe our data provide the best available description of case-mix adjusted national practice patterns.
Examination of treatment patterns in a large, national disease registry confirms substantial practice-level variation in management of localized prostate cancer that cannot be explained by disease case-mix variability. A growing body of evidence suggests that improved decision support may not only improve decision quality23 and reduce decisional regret24 but may also be a means to reduce unwarranted variation in health care.7 Incorporation of such decision support into clinical practice, while challenging, should be a priority. Even more important is the need for better data on outcomes of prostate cancer treatment. The Institute of Medicine recently included treatment for localized prostate cancer among the 25 most important topics for future comparative effectiveness research.25 Only through such research, based on both prospective clinical trials and retrospective review of high-quality, clinically rich data sources, will clinicians and patient be able to assess more accurately the relative merits, risks, and costs of treatment alternatives and, by extension, to reduce variation in their selections among these alternatives.
Footnotes
Support for CaPSURE is provided in part by Abbott Labs, Abbott Park, IL; by the Department of Urology, University of California, San Francisco; and by Special Program of Research Excellence (SPORE) Grant No. P50CA89520 from the National Institutes of Health/National Cancer Institute, University of California, San Francisco.
Presented at the 45th Annual Meeting of the American Society of Clinical Oncology, May 29-June 2, 2009, Orlando, FL.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: None Stock Ownership: None Honoraria: Matthew R. Cooperberg, Abbott Laboratories, Takeda Pharmaceutical; Jeanette M. Broering, Takeda Pharmaceutical; Peter R. Carroll, Takeda Pharmaceutical Research Funding: None Expert Testimony: None Other Remuneration: Jeanette M. Broering, Takeda Pharmaceutical
AUTHOR CONTRIBUTIONS
Conception and design: Matthew R. Cooperberg, Peter R. Carroll
Financial support: Peter R. Carroll
Administrative support: Jeanette M. Broering, Peter R. Carroll
Provision of study materials or patients: Jeanette M. Broering
Collection and assembly of data: Jeanette M. Broering
Data analysis and interpretation: Matthew R. Cooperberg
Manuscript writing: Matthew R. Cooperberg
Final approval of manuscript: Matthew R. Cooperberg, Jeanette M. Broering, Peter R. Carroll
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Lu-Yao GL, Albertsen PC, Moore DF, et al. Outcomes of localized prostate cancer following conservative management. JAMA. 2009;302:1202–1209. doi: 10.1001/jama.2009.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wei JT, Dunn RL, Sandler HM, et al. Comprehensive comparison of health-related quality of life after contemporary therapies for localized prostate cancer. J Clin Oncol. 2002;20:557–566. doi: 10.1200/JCO.2002.20.2.557. [DOI] [PubMed] [Google Scholar]
- 4.Wilt TJ, MacDonald R, Rutks I, et al. Systematic review: Comparative effectiveness and harms of treatments for clinically localized prostate cancer. Ann Intern Med. 2008;148:435–448. doi: 10.7326/0003-4819-148-6-200803180-00209. [DOI] [PubMed] [Google Scholar]
- 5.Thompson I, Thrasher JB, Aus G, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177:2106–2131. doi: 10.1016/j.juro.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Kramer KM, Bennett CL, Pickard AS, et al. Patient preferences in prostate cancer: A clinician's guide to understanding health utilities. Clin Prostate Cancer. 2005;4:15–23. doi: 10.3816/cgc.2005.n.007. [DOI] [PubMed] [Google Scholar]
- 7.O'Connor AM, Llewellyn-Thomas HA, Flood AB. Modifying unwarranted variations in health care: Shared decision making using patient decision aids. Health Aff (Millwood) 2004;(Suppl Web Exclusives):VAR63–VAR72. doi: 10.1377/hlthaff.var.63. [DOI] [PubMed] [Google Scholar]
- 8.Cooper MM, Birkmeyer JD, Bronner KK, et al. Chicago, IL: Health Forum; The Quality of Medical Care in the United States: A Report on the Medicare Program. The Center for the Evaluative Clinical Sciences, Dartmouth Medical School, 1999. [Google Scholar]
- 9.Shahinian VB, Kuo YF, Freeman JL, et al. Determinants of androgen deprivation therapy use for prostate cancer: Role of the urologist. J Natl Cancer Inst. 2006;98:839–845. doi: 10.1093/jnci/djj230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lubeck DP, Litwin MS, Henning JM, et al. The CaPSURE database: A methodology for clinical practice and research in prostate cancer—CaPSURE Research Panel. Cancer of the Prostate Strategic Urologic Research Endeavor. Urology. 1996;48:773–777. doi: 10.1016/s0090-4295(96)00226-9. [DOI] [PubMed] [Google Scholar]
- 11.Cooperberg MR, Broering JM, Litwin MS, et al. The contemporary management of prostate cancer in the United States: Lessons from the Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE), a national disease registry. J Urol. 2004;171:1393–1401. doi: 10.1097/01.ju.0000107247.81471.06. [DOI] [PubMed] [Google Scholar]
- 12.Marr PL, Elkin EP, Arredondo SA, et al. Comorbidity and primary treatment for localized prostate cancer: Data from CaPSURE. J Urol. 2006;175:1326–1331. doi: 10.1016/S0022-5347(05)00647-6. [DOI] [PubMed] [Google Scholar]
- 13.Cooperberg MR, Broering JM, Carroll PR. Risk assessment for prostate cancer metastasis and mortality at the time of diagnosis. J Natl Cancer Inst. 2009;101:878–887. doi: 10.1093/jnci/djp122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cooperberg MR, Lubeck DP, Meng MV, et al. The changing face of low-risk prostate cancer: Trends in clinical presentation and primary management. J Clin Oncol. 2004;22:2141–2149. doi: 10.1200/JCO.2004.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller DC, Gruber SB, Hollenbeck BK, et al. Incidence of initial local therapy among men with lower-risk prostate cancer in the United States. J Natl Cancer Inst. 2006;98:1134–1141. doi: 10.1093/jnci/djj308. [DOI] [PubMed] [Google Scholar]
- 16.Cooperberg MR, Cowan J, Broering JM, et al. High-risk prostate cancer in the United States, 1990-2007. World J Urol. 2008;26:211–218. doi: 10.1007/s00345-008-0250-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bill-Axelson A, Holmberg L, Filen F, et al. Radical prostatectomy versus watchful waiting in localized prostate cancer: The Scandinavian Prostate Cancer Group-4 randomized trial. J Natl Cancer Inst. 2008;100:1144–1154. doi: 10.1093/jnci/djn255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilt TJ, Brawer MK, Barry MJ, et al. The Prostate Cancer Intervention Versus Observation Trial:VA/NCI/AHRQ Cooperative Studies Program #407 (PIVOT): Design and baseline results of a randomized controlled trial comparing radical prostatectomy to watchful waiting for men with clinically localized prostate cancer. Contemp Clin Trials. 2009;30:81–87. doi: 10.1016/j.cct.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Wallace K, Fleshner N, Jewett M, et al. Impact of a multi-disciplinary patient education session on accrual to a difficult clinical trial: The Toronto experience with the surgical prostatectomy versus interstitial radiation intervention trial. J Clin Oncol. 2006;24:4158–4162. doi: 10.1200/JCO.2006.06.3875. [DOI] [PubMed] [Google Scholar]
- 20.Donovan JL, Lane JA, Peters TJ, et al. Development of a complex intervention improved randomization and informed consent in a randomized controlled trial. J Clin Epidemiol. 2009;62:29–36. doi: 10.1016/j.jclinepi.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Cooperberg MR, Grossfeld GD, Lubeck DP, et al. National practice patterns and time trends in androgen ablation for localized prostate cancer. J Natl Cancer Inst. 2003;95:981–989. doi: 10.1093/jnci/95.13.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baicker K, Chandra A, Skinner JS, et al. Who you are and where you live: How race and geography affect the treatment of Medicare beneficiaries. Health Aff (Millwood) 2004;(Suppl Web Exclusives):VAR33–VAR44. doi: 10.1377/hlthaff.var.33. [DOI] [PubMed] [Google Scholar]
- 23.Gwede CK, Pow-Sang J, Seigne J, et al. Treatment decision-making strategies and influences in patients with localized prostate carcinoma. Cancer. 2005;104:1381–1390. doi: 10.1002/cncr.21330. [DOI] [PubMed] [Google Scholar]
- 24.Davison BJ, So AI, Goldenberg SL. Quality of life, sexual function and decisional regret at 1 year after surgical treatment for localized prostate cancer. BJU Int. 2007;100:780–785. doi: 10.1111/j.1464-410X.2007.07043.x. [DOI] [PubMed] [Google Scholar]
- 25.Washington, DC: National Academies Press; 2009. Institute of Medicine Committee on Comparative Effectiveness Research Prioritization: Initial national priorities for comparative effectiveness research. [Google Scholar]