Abstract
Purpose
Recovery of lymphocyte populations after lymphocyte depletion is implicated in therapeutic immune pathways in animal models and in patients with cancer. We sought to evaluate the effects of chemotherapy-induced lymphodepletion followed by granulocyte-macrophage colony-stimulating factor (GM-CSF) and high-dose interleukin-2 (IL-2) therapy on clinical response and the recovery of lymphocyte subcompartments in patients with metastatic melanoma.
Patients and Methods
This was a two-stage phase II trial design. Patients with measurable metastatic melanoma were treated with intravenous cyclophosphamide (60 mg/kg, days 1 and 2) and fludarabine (25 mg/m2, day 3 through 7) followed by two 5-day courses of intravenous high-dose bolus IL-2 (600,000 U/kg; days 8 through 12 and 21 through 25). GM-CSF (250 μg/m2/d beginning day 8) was given until granulocyte recovery. Lymphocyte recovery profiles were determined by flow cytometric phenotyping at regular intervals, and clinical outcome was assessed by Response Evaluation Criteria in Solid Tumors (RECIST).
Results
The trial was stopped at the end of stage 1 with four of 18 objective responses noted. Twelve patients had detailed lymphocyte subcompartments evaluated. After lymphodepletion, we observed an induction of regulatory cells (CD4+ T regulatory cells; CD8+ T suppressor cells) and of T memory cells (CD8+ T central memory cells; T effector memory RA+ cells). Expansion of circulating melanoma-specific CD8+ cells was observed in one of four HLA-A2-positive patients.
Conclusion
Chemotherapy-induced lymphodepletion modulates the homeostatic repopulation of the lymphocyte compartment and influences recovering lymphocyte subpopulations. Clinical activity seems similar to standard high-dose aldesleukin alone.
INTRODUCTION
Metastatic stage IV melanoma remains a highly lethal disease.1 Immunotherapy with high-dose aldesleukin (HD interleukin-2 [IL-2]) can result in durable remissions, but only in a small percentage of patients.2–6 IL-2 eradication of tumor is thought to be mediated by enhancing T-cell function and increasing T-cell numbers.
Lymphopoiesis is partially driven by lymphopenia and homeostatic proliferation.7–9 During homeostatic recovery, even in the absence of antigen stimulus, lymphocyte subpopulations shift, favoring antigen-experienced memory phenotype and enhanced effector cell function. Prior animal studies demonstrated that sublethal irradiation of mice induces lymphocyte homeostatic proliferation, causing complete regression of established tumors.10,11 More recent animal models demonstrated that mice lymphodepleted by sublethal irradiation can reconstitute their tumor-specific effector cells from memory cells that mediate clinical reduction of MCA-205 pulmonary metastases.12 Lymphocyte depletion with anti-CD4 and anti-CD8 antibody was followed by lymphocyte homeostatic recovery, which was able to mediate allograft rejection when adoptively transferred to wild-type mice.13 CD4+Treg cells can suppress CD4+ and CD8+ T-cell proliferation. In murine models, lymphodepletion seemed to preferentially eliminate suppressive Treg lymphocytes.14 These laboratory models provide a strong rationale for therapeutic lymphodepletion in humans.15
We hypothesized that lymphodepleting chemotherapy provides a permissive environment for homeostatic regeneration of tumor-directed cytotoxic T lymphocytes. Regenerating populations of lymphocytes would be further influenced by HD IL-2 and granulocyte-macrophage colony-stimulating factor (GM-CSF) with resulting clinical benefit. We studied the clinical outcomes and systematic recovery of the mononuclear cell compartments after lymphodepletion, HD IL-2, and GM-CSF therapy in patients with metastatic melanoma.
PATIENTS AND METHODS
Inclusion/Exclusion Criteria
Patients were required to have histologically confirmed melanoma with measurable disease, a life expectancy ≥ 12 weeks, Karnofsky performance status ≥ 60%, no prior therapy within 4 weeks before entry (6 weeks for nitrosoureas), and adequate end-organ function (cardiac ejection fraction ≥ 50%, an forced expiratory volume in 1 second ≥ 2.0 L or ≥ 75% of predicted for height and age, and diffusing capacity of lung for carbon monoxide ≥ 60% predicted). Patients with brain metastases were excluded, as were lactating or pregnant women. Patients were excluded if they had been treated previously with IL-2; had second invasive malignancies fewer than 5 years before entry; had significant comorbid disease, such as autoimmune illnesses, uncontrolled diabetes mellitus, or active infection; or had positive serology for HIV, hepatitis B, or hepatitis C. All patients were required to sign an institutional review board–approved informed consent. Patients were treated at Dartmouth Hitchcock Medical Center, Loyola University, Beth Israel Deaconess Medical Center, and the City of Hope.
Treatment
Table 1 outlines treatment schema with cyclophosphamide, sodium 2-mercaptoethanesulfonate, fludarabine, IL-2, and recombinant human GM-CSF. GM-CSF was given beginning on day 8 and continuing until absolute granulocyte count exceeded 5,000 cells/μL. Patients were hospitalized until their absolute neutrophil count reached 500 cells/μL and platelets were more than 20,000/μL. Patients received full supportive care, including transfusions of blood for hemoglobin less than 8.0g/dL, platelet transfusion for counts less than 5,000 cells/μL (< 10,000 if febrile), prophylactic antibiotics, and antiemetics when appropriate. Use of corticosteroids was avoided except in the case of life-threatening toxicity. All patients who received at least one dose of cyclophosphamide (day 1 of therapy) were considered evaluable for clinical response. All patients who received lymphodepleting chemotherapy and had two time points available for assessment of immune parameters were considered evaluable for correlative end points.
Table 1.
Treatment Schema
| Drug | Day 1 | Day 2 | Days 3-7 | Days 8-12 | Days 22-26 |
|---|---|---|---|---|---|
| Cyclophosphamide* | X | X | |||
| Fludarabine† | X | ||||
| Interleukin-2‡ | X | X | |||
| GM-CSF§ | X | ||||
| Mesna | X | X |
Abbreviation: GM-CSF, granulocyte-macrophage colony-stimulating factor.
Cyclophosphamide (60 mg/kg/d; Baxter, Deerfield, IL) intravenously (IV) for 2 days with sodium 2- mercaptoethanesulfonate (Mesna; Sicor, Irvine, CA) at 20% of cyclophosphamide dose IV 15 minutes before and 40% of the cyclophosphamide dose orally at 2 and 6 hours after the initiation of chemotherapy.
Fludarabine IV (25 mg/M2/day)–five daily doses from Day 3.
Interleukin-2 (aldesleukin) IV (600,000 U/kg; Chiron, Emeryville, CA): two 5-day courses on days 8 and 22. Interleukin-2 was given over 15 minutes every 8 hours. Goal is 14 doses/5-day course.
GM-CSF (250 μg/m2; Berlex, Montville, NJ) was given subcutaneously daily from day 8 until absolute granulocyte count exceeds 5,000 cells/mL for 2 consecutive days.
Treatment Evaluation
Tumor evaluation included physical examination and axial computed tomography scans before treatment, 4 weeks after completion of HD IL-2, and between 8 and 12 weeks after completion of treatment. Stable and responding patients were evaluated every 3 months with a history, physical examination, and computed tomography scans. Evaluation for brain metastases was done when clinically indicated. National Cancer Institute's Response Evaluation Criteria in Solid Tumors (RECIST) was used to determine clinical response. Responding patients were eligible for a second cycle of high-dose bolus IL-2 given without lymphodepleting chemotherapy or GM-CSF.
Correlative Immune Measurements
Lymphocyte subpopulations were evaluated before treatment and at planned time points during and post treatment. T-cell phenotype and T-cell receptor ß variable (vß) chain expression on CD3+ cells was assessed on peripheral blood using multicolor flow cytometry. In addition, the expansion of CD8+ melanoma-specific T cells of HLA-A2-positive patients was investigated.
Isolation and Purification of Human Cells
Twenty to 50 mL of whole blood was collected using Vacutainer sodium heparin blood collection tubes (BD, Franklin Lakes, NJ). Peripheral-blood mononuclear cells (PBMCs) were isolated via density gradient centrifugation using Ficoll-Paque Plus (Amersham Biosciences, Buckinghamshire, England). Phenotyping of patients' cells was performed on freshly isolated PBMCs. For confirmation of normal distribution of vβ T-cell receptors and other T-cell subsets, peripheral blood was obtained from healthy volunteers using a separate institutional review board–approved protocol. PBMCs for healthy donors were frozen in autologous serum plus 10% dimethyl sulfoxide (Sigma-Aldrich, St Louis, MO) and were stored at −140°C until use.
Patients underwent leukapheresis at two time points, pretreatment and post-treatment (day 78 to 85). Mononuclear cell fraction was obtained from the pheresis product using Ficoll-Hypaque centrifugal separation, and monocytes were further fractionated by cold aggregation, washed and frozen in 90% autologous serum plus 10% dimethyl sulfoxide, and stored at −140°C until use. Lymphocytes from this isolation were used for enumeration of CD8+ melanoma-specific T cells.
T-Cell Subset and vß Analysis: Antibodies
Phenotypic analysis was performed using the following monoclonal antibodies purchased from Beckman Coulter (Fullerton, CA): anti-CD3, -CD45, and -CD62L conjugated to phycoerythrin-cyanine 5 (PECy5); anti-CD3, -CD4, -CD8, -CD14, -CD20, and anti-vβ1, 2, 5.1, 16, 21.3, and 22 conjugated to fluorescein isothiocyanate (FITC); anti-CD25, -CD28, -CD56, and anti-vβ7, 9, 12, 20, 18, and 23 conjugated to phycoerythrin (PE); anti-CD14 conjugated to allophycocyanin; and the respective isotype controls. Antibodies, human immunoglobulin G block (Sigma-Aldrich) and PBMCs were added to a 96-well plate and then incubated at 4°C for 1 hour on an Orbitron rotator (SPI, West Chester, PA). After incubation, cells were washed then fixed with 1% paraformaldehyde.
Tetramer Analysis: Antibodies
The antibody panel which was combined with HLA-A2 melanoma-specific tetramers was purchased from Beckman Coulter. CD3 (IM2635), CD4 (IM0448), and CD8 (Clone T8, No. 6607102) antibodies were combined with either negative control tetramer (product No. T01044) or one of three melanoma-specific tetramers: Mart-1 (T01008), gp100 (T01012), or tyrosinase (T01019; all tetramers from Beckman Coulter Immunomics). Tetramers were labeled with phycoerythrin (PE). Frozen lymphocytes were thawed, washed, then combined with tetramer and antibodies and incubated for 30 minutes. Cells were then washed and fixed in 1% paraformaldehyde.
Flow Cytometry and Data Analysis
For T-cell subsets, cells were acquired on a FACSCalibur flow cytometer (BD; 488 nm excitation; detection at 530 nm [FITC], 585 nm [PE], more than 670 nm [PECy5], and 635 nm excitation for detection at 661 nm [allophycocyanin]). Four-color analysis was used to assess the different subsets of the repopulating lymphocytes. Cells were gated by their forward and side scatter characteristics to include lymphocytes and monocytes. For some analyses, monocytes were excluded by gating out CD14 bright cells.
For tetramer analysis, cells were acquired on a FACSCanto flow cytometer (BD) with six-color capability (488 nm excitation, detection at 530 nm [FITC], 585 nm [PE], more than 670 nm [PECy5], and 780 nm [PECy7]). Approximately 400,000 CD3+ events were collected. Cells were gated as described above. CD3+ cells were further analyzed for CD8 versus tetramer to quantitate the percentage of CD8+/tetramer+ T cells.
Statistical Analysis
The trial was designed as a two-stage phase II trial. It was planned to study 18 evaluable patients in the first stage, and if more than four responses were observed in the first stage, an additional 15 would be recruited. The study was terminated after the first stage. For 12 of the 18 patients, flow cytometry and vβ end points were measured longitudinally. These repeated measures are summarized using means and standard deviations with transformations to achieve Gaussian distributions. Means are compared between time points using both paired t tests and mixed effects (ie, random intercept) models. A “normal range” of change within an individual for the vβ subsets was derived using changes from baseline for six time points (days 0, 8, 11, 15, 18, and 29) measured for four healthy donors.
RESULTS
Patient Characteristics
Eighteen patients (15 men and three women) of 20 considered were treated; demographic data are summarized in Table 2 (one patient declined therapy, one patient did not meet eligibility criteria). Accrual began in February 2004 and followed until December 2008. The mean age of our patients was 52 years (range, 30 to 71 years; M1a, n = 5; M1b, n = 8; M1c, n = 5). Ten of 18 patients received adjuvant interferon-containing therapies before entry onto this protocol. Two patients received prior radiation therapy. No patient received prior cytotoxic therapy. All patients were evaluable for tumor response and toxicity. Seventeen of 18 patients received 14 of 14 doses of IL-2 in week 1, with an average total number of IL-2 doses of 25 of 28.
Table 2.
Patient Demographics
| Patient | Sex | Age (years) | Disease Sites | M Stage | PS | Prior Treatment | Interleukin-2 |
||
|---|---|---|---|---|---|---|---|---|---|
| Cycle 1a | Cycle 1b | Total Doses | |||||||
| 001 | Male | 46 | Lymph node | M1a | 90 | LND | 14/14 | 8/14 | 22 |
| 002 | Male | 48 | Lung | M1b | 90 | WLE, SLND, tyrosinase DNA vaccine, PEG-IFN, temodar | 14/14 | 14/14 | 28 |
| 003 | Male | 63 | Soft tissue, lung | M1b | 90 | Lung resection | 14/14 | 11/14 | 25 |
| 004 | Male | 50 | Soft tissue, lung | M1b | 80 | WLE, LND | 14/14 | 8/14 | 22 |
| 005 | Male | 60 | Soft tissue | M1a | 80 | LND, RT, HD IFN | 14/14 | 13/14 | 27 |
| 006 | Male | 59 | Lung | M1b | 90 | WLE, SLND HD IFN | 14/14 | 14/14 | 28 |
| 007 | Male | 56 | Soft tissue | M1a | 80 | WLE, SLND, RT, HD IFN, surgery | 14/14 | 14/14 | 28 |
| 008 | Male | 54 | Liver, adrenal, lymph node, bone | M1c | 100 | Biopsy of axillary lymph node | 14/14 | 9/14 | 23 |
| 009 | Male | 71 | Lungs | M1b | 90 | WLE, SLND, surgery | 14/14 | 11/14 | 25 |
| 010 | Male | 43 | Lymph node, lungs, right axillary LN | M1b | 100 | LND | 14/14 | 14/14 | 28 |
| 011 | Male | 53 | RL back primary, R axillary LN, liver | M1c | 80 | WLE, LND, HD IFN | 14/14 | 10/14 | 24 |
| 012 | Female | 27 | Lymph node | M1a | 100 | WLE, SLN, adjuvant IFN, recurrence with L axillary LAN, pulmonary nodule | 13/14 | 9/14 | 22 |
| 013 | Male | 41 | Lung | M1b | 100 | LAN, Adjuvant IFN | 14/14 | 13/14 | 27 |
| 014 | Female | 54 | Paramediastinum, RLQ sc | M1a | 100 | WLE | 14/14 | 9/14 | 25 |
| 015 | Male | 58 | Rectum | M1c | 90 | WLE, HD IFN, R inguinal LN recurrence, LND | 14/14 | 10/14 | 24 |
| 016 | Male | 30 | Soft tissue, retroperitoneal mass, pulmonary nodules | M1c | 100 | WLE, inguinal LAN | 14/14 | 6/14 | 20 |
| 017 | Female | 65 | Right shin | M1a | 80 | WLE, adjuvant IFN, sargramostim therapy, surgery, temozolomide with progression of disease | 14/14 | 9/14 | 23 |
| 018 | Male | 53 | Lung | M1B | 100 | WLE, SLN, R axillary LAN, HD IFN, RU lobectomy and mediastinal LND | 14/14 | 14/14 | 24 |
Abbreviations: PS, performance status; LND, lymph node dissection; WLE, wide local excision; SLND, sentinel lymph node dissection; PEG IFN, pegylated interferon; IFN, interferon alfa; RT, radiation therapy; HD, high dose; L, left; LAN, lymph adenoathy; R, right; LN, lymph node; RLQ, right lower quadrant; sc, subcutaneous; SLN, sentinel lymph node; RU, right upper.
Toxicity
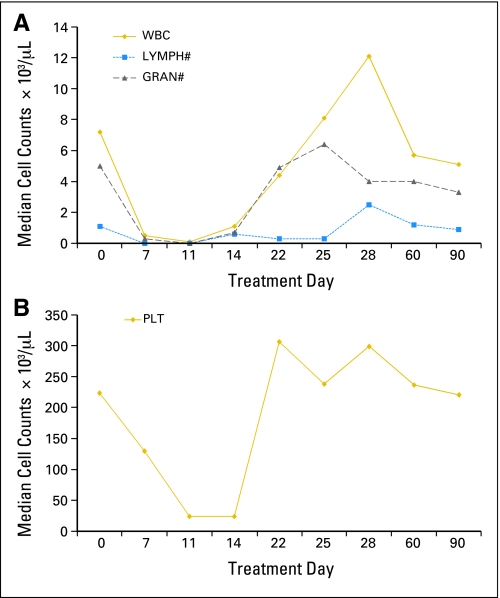
This lymphodepletion regimen in combination with high-dose IL-2 and GM-CSF was tolerated; there were no treatment-related deaths. All patients exhibited transient and reversible symptoms of IL-2–induced capillary leak syndrome, including edema, hypotension, creatinine elevation, hypoalbuminemia, electrolyte alteration, or decreased urine output. Hematologic toxicity was transient, with eight patients requiring packed RBC transfusion and four patients requiring platelet support (Fig 1). Platelet count was less than 50,000/μL for a median of 5.5 days (range, 0 to 7 days). All patients experienced neutropenia beginning on day 5 or 6, lasting a median of 8 days (range, 5 to 11 days). Ten of 18 patients experienced neutropenic fever, and three developed intravenous line infections. All patients experienced lymphopenia but recovered between day 14 and day 28.
Fig 1.
Peripheral-blood cell count recovery in 12 patients. (A) Leukocyte recovery. (B) Peripheral-blood platelet count recovery. LYMPH, lymphocytes; GRAN, granulocytes; PLT, platelets.
Recovery of Lymphocyte Subcompartments
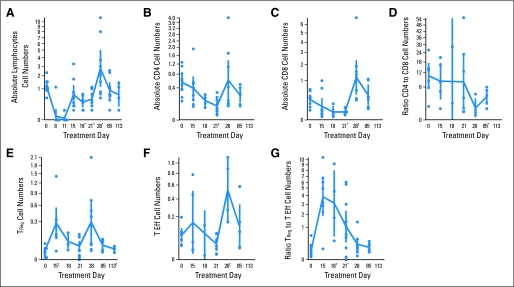
Lymphocyte recovery profiles are shown in Figure 2. The CD4+ lymphocyte numbers reached nadir at day 21 and were significantly different from baseline (.0001 < P ≤ .001). Measurements taken before the second 5-day course of IL-2 showed suppression of CD4+ lymphocytes. However, after the second course of IL-2, CD4+ lymphocytes rebounded to baseline. CD8+ lymphocyte numbers also reached nadir at day 21 and were significantly different from baseline (.001 < P ≤ .01). The mean CD8+ lymphocyte numbers rebounded above baseline on day 28 (.0001 < P ≤ .001). The mean percentage of natural killer cells on days 18 and 21 was significantly higher than on day 15 (P = .04 and .002, respectively). There was no significant difference between baseline levels and levels at days 85 and 113, but only data from three patients were available at these time points. The CD4/CD8 ratio reached nadir late and stayed low (Fig 2; .001 < P ≤ .01).
Fig 2.
Lymphocyte recovery: x-axis represents day of treatment from day 0. Days 8 and 11 were excluded because of insufficient cells to do subset phenotyping for most patients. y-axis represents cell numbers for each graph. The solid blue circles in each graph represent the number of cells for each patient, and the solid blue vertical lines represent the 95% CIs. The symbols represent statistically significant difference from baseline value: (*) .001 < P ≤ .01; (†) .0001 < P ≤ .001; (‡) P ≤ .0001. The panels represent absolute lymphocytes, absolute CD4+ cells, absolute CD8+ cells, CD4/CD8 ratio, number of TReg cells, T Eff cells; number of T effector memory CD8+/CD45RO+/CCR7+ cells, and TReg/CD8 T Eff cells ratio.
Regulatory (CD4+ Treg and CD8+ Tsup) and Effector (CD8+ Tem) Cells
We investigated CD4+ Treg cell numbers, defined as CD4+/CD25high/CD62L+. Although the phenotype that best identifies T-regulatory cells has evolved, at the time of this study, lymphocytes that expressed CD4, CD25bright/high, and CD62L on their cell surface were defined as T-regulatory cells. We first established that Treg cell levels in the peripheral blood remain relatively constant under normal conditions. In four healthy volunteers evaluated at six time points, Treg cell levels were fairly consistent, ranging from 0.07% to 1.34% (mean 0.5% ± 0.35%) of total lymphocytes. In our study patients, pretreatment peripheral-blood Treg cells ranged from 1.2% to 3.5% of all lymphocytes (mean 2.1% ± 0.8%). Treg cells were the earliest subpopulation of lymphocytes to recover from lymphodepletion and spiked above baseline levels at day 15 (.0001 < P ≤ .001). Measurements taken after the second 5-day course of IL-2 revealed another large spike in the number of Treg cells on day 28, returning to baseline by 2 months (P ≤ .0001; Fig 2).
Tsup cells (CD8+/CD28−) were also evaluated. The baseline percentage of Tsup cells in the lymphocyte population for the 11 study patients ranged from 0.1 to 23.1 (mean of 7.9). Tsup cell number remained lower than baseline through days 15 to 21.
CD8+/CD45RO+/CCR7+ Tem were seen to remain constant over time, and had a transient spike on day 28 (.0001 < P ≤ .001), returning to baseline at day 85 (Fig 2). The ratio of Treg to Tem spiked significantly at day 15 and remained significantly above baseline until day 28 (P ≤ .0001; Fig 2).
Oligoclonal Recovery of T Cells
To assess the pattern of oligoclonal expansion of CD3+ T lymphocytes, we determined the expression of 13 specific T-cell receptor vß chains in the 12 study patients throughout their treatment. In addition, we assessed the vß chain expression on T cells from four healthy volunteers at six intervals over the span of 1 month, demonstrating that in a resting state, the pattern of vß expression remains fairly constant within an individual. Using these data, we established a “normal range” of intradonor variation. In study patients, we compared day 28 with baseline and observed changes greater than the normal range in at least one vβ subset in 11 of 12 patients. Changes in at least one Vß subset less than the normal range were also observed in nine patients. There was no consistent pattern in vß expression among patients, but observed changes in proportions of individual vß subsets supports the interpretation of oligoclonal expansion of T cells consistent with the hypothesis of homeostatic recovery of T cells.
Recovery of Melanoma-Specific Cytotoxic Lymphocytes
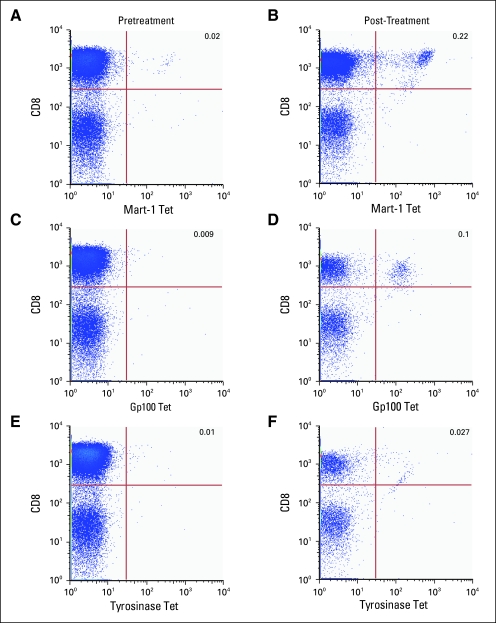
We evaluated the four HLA-A2-positive patients for the induction of detectable melanoma-specific CD8+ T cells after treatment. One patient had significant enhancement of an oligoclonal expansion of cells that were tetramer positive for some of the melanoma antigens tested (Mart-1, gp100; Fig 3). This patient did not demonstrate a clinical response to the treatment.
Fig 3.
Dot plots from flow cytometric analysis of tetramer (Tet) -positive (A, C, E) pretreatment and (B, D, F) post-treatment CD8 cells. One of four HLA-A2-positive patients had a treatment related shift in tetramer-positive cells. CD8+ cells are on the y-axis; tetramer-positive cells are on the x axis. (A, B) Mart 1; (C, D) gp100; (E, F) tyrosinase.
Clinical Response
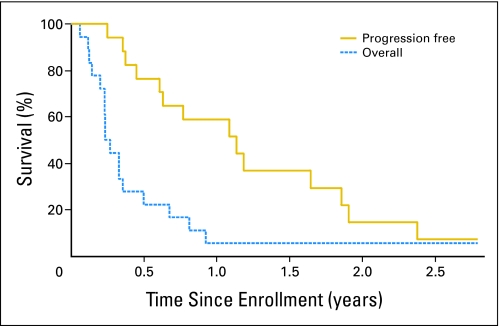
We observed one complete response and three partial responses by RECIST criteria. The complete responder has no evidence of disease for 54+ months. The range of duration of response in three partial responders was 1 to 11 months. Time to progression and overall survival of this group of patients are shown in Figure 4.
Fig 4.
Kaplan-Meier estimates for progression-free and overall survival. (Survival with 95% CI and progression-free survival with 95% CI.)
DISCUSSION
In this study, we confirmed the safety of lymphodepleting chemotherapy with HD IL-2 in a multicenter setting. Patients received a median of 25 of 28 aldesleukin doses compared with the historical data of 18 of 28 doses.16There were no treatment-related deaths, and all patients recovered hematopoietic function. The pattern of lymphocyte recovery after high-dose combination cyclophosphamide and fludarabine is not known, and thus we can only conclude that the effects we saw are attributed to treatment with cyclophosphamide, fludarabine, IL-2, and GM-CSF working in concert. We demonstrated rapid recovery of CD4+Treg and skewing of lymphocytes subtypes. The rapid recovery of CD4+Treg and the high Treg to Tem ratio in the first 3 weeks suggests a persistence of significant immunologic barriers. The observation of Treg cells repopulated before CD8+ effector cells provides an explanation for the failure to enhance the response rates over that anticipated with IL-2 alone. CD8+Tsup cells remained low through day 21, but spiked later in recovery. The CD4/CD8 ratio was significantly below baseline levels at day 28 and at day 85 (2 months after completion of therapy). This observation, combined with the increase in Treg cells, the high Treg/CD8+ ratio suggest a less than optimal in vivo environment for adoptive cellular therapy. Antony et al14 showed that removal of the Treg subset allows helper function of the remaining Thelper cells. They also showed that Treg cells suppressed adoptive immunotherapy of an established tumor. Kline et al17 showed that combined CD25 depletion and homeostatic proliferation support a potent antitumor response in animal studies. Although preliminary data using adoptive cellular therapy with lymphodepletion seem to have significant impact on metastatic melanoma, our data suggest that other strategies for lymphocyte depletion and blocking Treg cell early recovery may further improve the outcome.
Other studies of similar lymphodepleting chemotherapy used in conjunction with adoptive transfer of T cells showed persistent depression of absolute CD4+ cell counts from day 31 to 1 year after lymphodepletion.15 In our study, CD4+ lymphocyte number remained below baseline, but recovered by day 85. The affect of high-dose bolus IL-2 in vivo on Treg function was not addressed in our study. In vitro functional analyses of human regulatory CD4+ Treg cells has revealed that additional IL-2 can result in low-level proliferation of these cells and complete loss of regulation.18
The CD8+ Tsup cells, a subpopulation of the CD8+CD28− cells, were decreased in numbers after chemotherapy, but rebounded after the first dose of IL-2 therapy. They continued to increase after the second dose of IL-2 and remained above baseline up to 3 months after therapy. Tsup cells inhibit immune responses in an antigen-specific, major histocompatibility complex–restricted fashion.19 Thus the pattern of CD8+CD28− Tsup cell recovery may further influence the function of CD8+ cytolytic T-lymphocytes (CTLs).
Melanoma-specific CTL are known to exist de novo in patients with melanoma.20,21 Thus it is possible that under homeostatic repopulation, there will be a skewing of recovery of these CD8+ CTLs. T-cell receptors are heterodimeric cell-surface protein complexes made up of an α and β chain. The α chain has a variable (v) and a joining (j) segment, and the β chain contains a dominant (d), v, and j segment. The diversity required for T cells to recognize millions of antigen-derived peptides is created by recombination of these chains into unique receptor proteins. T cells undergoing clonal expansion can be detected by analyzing the vβ repertoires using flow cytometry. Our analysis of vβ chains in four healthy donors demonstrates only slight changes in expression over time within a donor and consistency from person to person. During recovery of patient cells from lymphodepletion, the majority of the T-cell expansions were temporary. Because of limitation of peripheral-blood cell availability, we were unable to determine antigen specificity of this expansion. Of four HLA-A2-positive patients, we observed an increase in one patient's melanoma-specific CD8 cells by tetramer staining. The data support the need to adoptively transfer melanoma-specific effector cells and to inhibit Treg pathways after depletion.
The approach of lymphodepletion is in its infancy. Although we have demonstrated the feasibility of a multicenter trial of chemotherapy lymphodepletion and HD IL-2, the clinical results failed to show improvement in clinical response relative to HD IL-2 alone.22 The lymphocyte recovery data support the need for improved strategies to address the balance of Treg, Tsup, and Thelp cell populations to further optimize adoptive cellular therapy.
Footnotes
Supported in part by National Cancer Institute Grant No. R21CA112761, Chiron, and Berlex.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00085423.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: David McDermott, Novartis (C); Michael B. Atkins, Novartis (C) Stock Ownership: None Honoraria: Nancy A. Crosby, Novartis/Chiron; Joseph I. Clark, Novartis Research Funding: Kenneth R. Meehan, Berlex Pharmaceuticals; Joseph I. Clark, Novartis; Marc S. Emstoff, Novartis Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Kenneth R. Meehan, Todd S. Crocenzi, Edward J. Usherwood, Mary Jo Turk, Cory Ahonen, Shinichiro Fuse, Jan L. Fisher, Randolph J. Noelle, Marc S. Ernstoff
Financial support: Marc S. Ernstoff
Administrative support: Marc S. Ernstoff
Provision of study materials or patients: Kenneth R. Meehan, Todd S. Crocenzi, David McDermott, Kim A. Margolin, Nancy A. Crosby, Michael B. Atkins, Marc S. Ernstoff
Collection and assembly of data: Krishna S. Gunturu, Nancy A. Crosby, Joseph I. Clark, Jan L. Fisher, Marc S. Ernstoff
Data analysis and interpretation: Krishna S. Gunturu, Kenneth R. Meehan, Todd A. Mackenzie, David McDermott, Edward J. Usherwood, Kim A. Margolin, Nancy A. Crosby, Michael B. Atkins, Mary Jo Turk, Cory Ahonen, Shinichiro Fuse, Joseph I. Clark, Jan L. Fisher, Randolph J. Noelle, Marc S. Ernstoff
Manuscript writing: Krishna S. Gunturu, Kenneth R. Meehan, Todd A. Mackenzie, Todd S. Crocenzi, David McDermott, Kim A. Margolin, Nancy A. Crosby, Michael B. Atkins, Mary Jo Turk, Cory Ahonen, Joseph I. Clark, Jan L. Fisher, Randolph J. Noelle, Marc S. Ernstoff
Final approval of manuscript: Krishna S. Gunturu, Kenneth R. Meehan, Todd A. Mackenzie, Todd S. Crocenzi, David McDermott, Edward J. Usherwood, Kim A. Margolin, Nancy A. Crosby, Michael B. Atkins, Mary Jo Turk, Cory Ahonen, Shinichiro Fuse, Joseph I. Clark, Jan L. Fisher, Randolph J. Noelle, Marc S. Ernstoff
REFERENCES
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Ribas A, Camacho LH, Lopez-Berestein G, et al. Antitumor activity in melanoma and anti-self responses in a phase I trial with the anti-cytotoxic T lymphocyte-associated antigen 4 monoclonal antibody CP-675,206. J Clin Oncol. 2005;23:8968–8977. doi: 10.1200/JCO.2005.01.109. [DOI] [PubMed] [Google Scholar]
- 3.Hodi FS, Mihm MC, Soiffer RJ, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A. 2003;100:4712–4717. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phan GQ, Yang JC, Sherry RM, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2003;100:8372–8377. doi: 10.1073/pnas.1533209100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanderson K, Scotland R, Lee P, et al. Autoimmunity in a phase I trial of a fully human anti-cytotoxic T-lymphocyte antigen-4 monoclonal antibody with multiple melanoma peptides and Montanide ISA 51 for patients with resected stages III and IV melanoma. J Clin Oncol. 2005;23:741–750. doi: 10.1200/JCO.2005.01.128. [DOI] [PubMed] [Google Scholar]
- 6.Korman A, Yellin M, Keler T. Tumor immunotherapy: Preclinical and clinical activity of anti-CTLA4 antibodies. Curr Opin Investig Drugs. 2005;6:582–591. [PubMed] [Google Scholar]
- 7.Goldrath AW, Bogatzki LY, Bevan MJ. Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. J Exp Med. 2000;192:557–564. doi: 10.1084/jem.192.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murali-Krishna K, Ahmed R. Cutting edge: Naive T cells masquerading as memory cells. J Immunol. 2000;165:1733–1737. doi: 10.4049/jimmunol.165.4.1733. [DOI] [PubMed] [Google Scholar]
- 9.Surh CD, Sprent J. Regulation of mature T cell homeostasis. Semin Immunol. 2005;17:183–191. doi: 10.1016/j.smim.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Hellström KE, Hellstrom I, Kant JA, et al. Regression and inhibition of sarcoma growth by interference with a radiosensitive T-cell population. J Exp Med. 1978;148:799–804. doi: 10.1084/jem.148.3.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maine GN, Mule JJ. Making room for T cells. J Clin Invest. 2002;110:157–159. doi: 10.1172/JCI16166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang LX, Kjaergaard J, Cohen PA, et al. Memory T cells originate from adoptively transferred effector and reconstituting host cells after sequential lymphodepletion and adoptive immunotherapy. J Immunol. 2004;172:3462–3468. doi: 10.4049/jimmunol.172.6.3462. [DOI] [PubMed] [Google Scholar]
- 13.Wu Z, Bensinger SJ, Zhang J, et al. Homeostatic proliferation is a barrier to transplantation tolerance. Nat Med. 2004;10:87–92. doi: 10.1038/nm965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antony PA, Piccirillo CA, Akpinarli A, et al. CD8+ T cell immunity against a tumor/self -antigen is augmented by CD4+ T helper cells and hindered by naturally occurring T regulatory cells. J Immunol. 2005;174:2591–2601. doi: 10.4049/jimmunol.174.5.2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reference deleted.
- 16. Reference deleted.
- 17.Kline J, Brown IE, Zha YY, et al. homeostatic proliferation plus regulatory T-cell depletion promotes potent rejection of B16 melanoma. Clin Cancer Res. 2008;14:3156–3167. doi: 10.1158/1078-0432.CCR-07-4696. [DOI] [PubMed] [Google Scholar]
- 18.Baecher-Allan C, Wolf E, Hafler DA. Functional analysis of highly defined, FACS-isolated populations of human regulatory CD4+CD25+ T cells. Clin Immunol. 2005;115:10–18. doi: 10.1016/j.clim.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 19.Chang CC, Ciubotariu R, Manavalan JS, et al. Tolerization of dendritic cells by TS cells: The crucial role of inhibitory receptors ILT3 and ILT4. Nat Immunol. 2002;3:237–243. doi: 10.1038/ni760. [DOI] [PubMed] [Google Scholar]
- 20.Letsch A, Keilholz U, Schadendorf D, et al. High frequencies of circulating melanoma-reactive CD8+ T cells in patients with advanced melanoma. Int J Cancer. 2000;87:659–664. [PubMed] [Google Scholar]
- 21.Hu HM, Poehlein CH, Urba WJ, et al. Development of antitumor immune responses in reconstituted lymphopenic hosts. Cancer Res. 2002;62:3914–3919. [PubMed] [Google Scholar]
- 22.Atkins MB, Lotze MT, Dutcher JP, et al. High dose recombinant interleukin-2 therapy for patients with metastatic melanoma: Analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]