Abstract
Chronic kidney disease affects 1 out of 9 Americans and leads to kidney failure and death. Recent scientific evidence showed increased activation of the disintegrin and metalloenzyme ADAM-17 during development of the disease and it was suggested that threonine phosphorylation of ADAM-17 may be an important regulator of the enzyme activity. The goal of our study was to investigate whether profibrotic serotonin (5-HT) induces phosphorylation of ADAM-17 and whether phosphorylation of ADAM-17 increases its enzymatic activity. We used kidney mesangial cells as our model system and found that 5-HT treatment (1 μM for 10 min) induced a significant 3-fold increase in ADAM-17 phosphorylation. PD98059 (1 μM) an inhibitor of extracellular signal regulated kinase (ERK) activation completely inhibited ADAM-17 phosphorylation. In co-immunoprecipitation analysis we observed increased (2.7-fold) binding of activated ERK to ADAM-17 after 10 min of 5-HT stimulation. In ADAM-17 activity assay using a fluorogenic ADAM-17 peptide substrate MCA-PLAQAV(Dpa) we found that 5-HT treatment induced a 2.3-fold activation of ADAM-17 and that pre-treatment with 1 μM of PD98059 significantly attenuated 5-HT-induced ADAM-17 activity (to 1.5-fold over control). We conclude that during profibrotic stimulus ERK phosphorylates ADAM-17 on threonines in kidney cells and that phosphorylation of ADAM-17 induces concomitant increase in the enzyme activity. Our data suggest that inhibiting ADAM-17 phosphorylation can have potential therapeutic significance to slow down kidney fibrosis.
INTRODUCTION
Chronic kidney disease is a growing health problem in the United States and word wide. Inhibitors of the renin-angiotensin-aldosterone system were successfully used to slow down diseases progression in the past years1. Recent scientific data indicate that activation of the epidermal growth factor receptor (EGFR) has an important role in promoting kidney fibrosis by inducing matrix deposition of mesangial cells in the glomeruli and promoting cell proliferation. Pro-fibrotic molecules like angiotensin-II and serotonin (5-HT) which are known activators of G protein-coupled receptors were shown to transactivate the EGFR in in vivo and in vitro models of kidney fibrosis2,3. ADAM-17, a member of the “a disintegrin and metalloenzyme” family4, was identified as the main mediator of this transactivation process. ADAM-17 cleaves (sheds) cell surface bound EGF-like growth factors which in turn activate the EGFR5.
Phosphorylation of proteins is widely recognized as an important regulator of their activity. Phosphorylation of ADAM-17 cytoplasmic tail on serine (through growth factor stimulation) or threonine (through PKC activation) was shown by ERK6. Further, phosphoinositide-dependent kinase 1 (PDK1) was implicated to induce serine and threonine phosphorylation of ADAM-17 during GPCR-EGFR crosstalk in cancer cells7. Despite these excessive studies on ADAM-17 phosphorylation we still do not have evidence whether phosphorylation of the enzyme results in increased enzymatic activity.
The objective of this summer research program was to test whether 5-HT induces ADAM-17 phosphorylation in mesangial cells and whether increased phosphorylation of the enzyme increases ADAM-17 enzymatic activity. We also aimed to identify the kinase responsible for ADAM-17 phosphorylation in this model of kidney fibrosis.
MATERIALS AND METHODS
Cell Culture
Primary rat mesangial cells were obtained from cortical sections of kidneys from 100–150-g Sprague-Dawley rats by collagenase treatment and a standard sieving technique3. The kidneys were harvested in accordance with protocols approved by the Institutional Animal Care and Use Committees of the Medical University of South Carolina and the Ralph H. Johnson Veterans Affairs Medical Center. Cells were cultured in RPMI 1640 medium supplemented with 20% fetal bovine serum and antibiotics (100 units/ml penicillin and 100μg/ml streptomycin) at 37 °C. Cells were subcultured weekly and used between passages 6 and 14.
Immunoprecipitation and Western blotting
RMES cells were plated in 10 cm dishes, serum starved for 48 h, and treated with 10 μM kinase inhibitor (PD98059-ERK inhibitor, EMD Biosciences) or negative control for 1 hr then stimulated with 1 μM serotonin for 10 min. Cells were cellular proteins were immunoprecipitated using ADAM-17 antibody (QED) or phospho-ERK antibody (Cell Signaling) employing standard immunoprecipiation protocol3. Precipitates were analyzed by anti-ADAM-17 antibody (QED), anti-phospho-ERK antibody (Cell Signaling) or anti-phospho-Threonine antibody (Zymed) in Western blotting. Immunoreactive protein bands were visualized using enhanced chemiluminescence and recorded on Kodak BioMax XR film.
Fluorescence Enzymatic Activity Measurement
Mesangial cells were seeded into 96-well plates. After serum depletion for 48 h, cells were pretreated with 10 μM PD98059 or vehicle control for 1 h at 37°C. Immediately after addition of 1 μM of 5-HT or vehicle control to the cells fluorogenic ADAM-17 substrate MCA-PLAQAV(Dpa) (R&D System) was added as described before3. Enzymatic activities as time resolved fluorescence were measured using SpectraMax5 instrument (Molecular Devices) and SoftMaxPro software.
Statistical Analysis
Values are presented as mean ± standard error of mean. Statistical difference between groups was determined by analysis of variance (ANOVA) using Graph Pad Prizm Software. P values< 0.05 were considered statistically significant.
RESULTS
PD98059 attenuates 5-HT- induced ADAM-17 phosphorylation
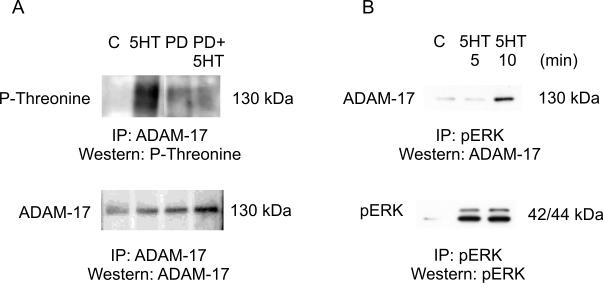
ADAM-17 immunoprecipitation and Western blotting which probed for phospho-threonine (an indicator of phosphorylated ADAM-17) showed that 5-HT treatment induced a 3-fold increase (p<0.01) in phospho-threonine band intensity compared to unstimulated control. Cells pre-treated with PD98059 to inhibit activation of ERK (PD + 5HT) showed significantly less band intensity compared to 5-HT alone. For phosphorylated ADAM-17 we expected a band at 125 kDa. The presence of a smear between 100-150 kDa indicates a mixture of phosphorylated ADAM-17 species, differing in degree of posttranslational modification. The blot was re-probed for total ADAM-17 as an indication of equal loading of samples. The total ADAM-17 signal in the lane corresponding to cells pre-treated with kinase inhibitor and treated with 5-HT (PD+ 5HT) appears to be stronger than the other bands, indicating more total protein for this sample. Despite having more protein, this sample still showed significantly less phosphorylated ADAM-17 than the 5-HT control. We also probed for phospho-tyrosine and phospho-serine after ADAM-17 immunoprecipitation, but we did not see any increase during 5-HT stimulation in our cells.
ADAM-17 co-precipitates with activated ERK
We showed previously that 5-HT treatment activates ERK in mesangial cells4. To show that activated ERK (pERK) directly interacts with ADAM-17 we performed pERK immunoprecipitation followed by ADAM-17 Western blotting. As shown in Figure 1B increased activation of ERK significantly increased its binding (up to 2.7-fold) to ADAM-17 (upper panel). We also investigated whether PDK1 kinase, which was shown to phosphorylate ADAM-17 in cancer cells, interacts with ADAM-17 in kidney cells. However, in our co-immunoprecipitation experiments we did not see binding of PDK1 to ADAM-17 (data not shown).
Figure 1.
ADAM-17 phosphorylation is ERK dependent. A) PD98059 (PD) inhibits 5-HT-induced ADAM-17 threonine phosphorylation (p-Threonine). B) Phosphorylated ERK interacts with ADAM-17 during 5-HT treatment in mesangial cells. IP: immunoprecipitation. Figure shows one representative experiment out of 3.
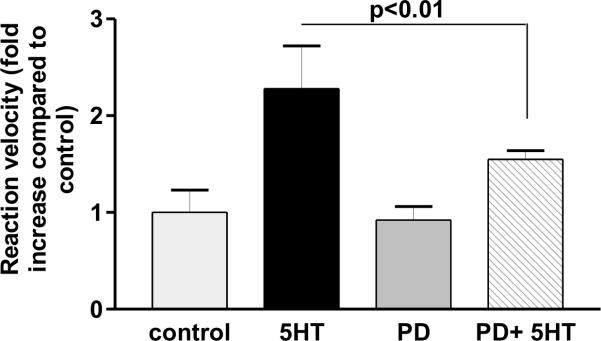
Serotonin-induced ADAM-17 activity is repressed by ERK inhibition
Next we wanted to see whether phosphorylation of ADAM-17 correlates with increased ADAM-17 activity. Indeed, 5-HT stimulation induced a significant (p<0.01) 2.3-fold increase in ADAM-17 activity. At the same time fluorescence enzymatic activity assay of cells pre-treated with PD98059 before 5-HT treatment showed significantly (p<0.01) decreased ADAM-17 activity when compared with 5-HT stimulation alone.
DISCUSSION
ADAM17 is a key enzyme mediating cross-talk between G-protein coupled receptors (like angiotensin receptor and serotonin receptor) and receptor tyrosine kinases (like EGFR, TNFR). Because it participates both in proliferative and inflammatory processes it gained substantial attention in recent years. Several inhibitors against ADAM-17 were developed and reached clinical phase II trials8. Because of the side effects these trials were terminated. In our study we aimed to understand how ADAM-17 is activated in kidney mesangial cells during GPCR/RTK crosstalk to provide future therapeutic target for the regulation of ADAM-17 activity. Using a chemical inhibitor targeting ERK activation we have identified ERK as the kinase phosphorylating ADAM-17 on threonine residues. Further, we showed increased co-precipitation of the kinase with ADAM-17 suggesting physical interaction of the molecules by 5-HT stimulation. We also provided evidence on the functional role of ADAM-17 threonine phosphorylation: using a fluorogenic substrate we showed that ERK inhibitor significantly attenuated ADAM-17 activity during 5-HT treatment. The results of IP/Western blots and the fluorescence activity assays together point to a positive correlation between the phosphorylation and increased activity of ADAM-17 under conditions of serotonin stimulation in mesangial cells. Our data also confirm the important role of mitogenic ERK in the activity regulation of ADAM-17 during pro-fibrotic stimulus and suggest that targeting ERK in the future could be a possible therapeutic target for treating chronic kidney fibrosis.
Figure 2.
ADAM-17 activity is ERK dependent. PD98059 (PD) inhibits 5-HT-induced ADAM-17 activation measured by cleavage of the quenched fluorogenic peptide substrate MCA-PLAQAV(Dpa). Figure shows data from 4 experiments performed in triplicates.
Sources of support
This work was supported by the NIH/NIDDK K01 DK070054 and the Paul Teschan Research Fund to MG, and the MUSC Graduate School Summer Health Professions Research Fellowship by NIH NIDDK to HLB (medical student) and MG (mentor).
Footnotes
Disclosure: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Song JH, Cha SH, Hong SB, et al. Dual blockade of the renin-angiotensin system with angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers in chronic kidney disease. J Hypertens Suppl. 2006;24:S101–6. doi: 10.1097/01.hjh.0000220414.99610.6b. [DOI] [PubMed] [Google Scholar]
- 2.Lautrette A, Li S, Alili R, et al. Angiotensin II and EGF receptor cross-talk in chronic kidney diseases: a new therapeutic approach. Nat Med. 2005;11:867–74. doi: 10.1038/nm1275. [DOI] [PubMed] [Google Scholar]
- 3.Gooz M, Gooz P, Luttrell LM, et al. 5-HT2A receptor induces ERK phosphorylation and proliferation through ADAM-17 tumor necrosis factor-alpha-converting enzyme (TACE) activation and heparin-bound epidermal growth factor-like growth factor (HB-EGF) shedding in mesangial cells. J Biol Chem. 2006;281:21004–12. doi: 10.1074/jbc.M512096200. [DOI] [PubMed] [Google Scholar]
- 4.Black RA, Rauch CT, Kozlosky CJ, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729–33. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 5.Primakoff P, Myles DG. The ADAM gene family: surface proteins with adhesion and protease activity. Trends Genet. 2000;16:83–87. doi: 10.1016/s0168-9525(99)01926-5. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Q, Thomas SM, Lui VW, et al. Phosphorylation of TNF-alpha converting enzyme by gastrin-releasing peptide induces amphiregulin release and EGF receptor activation. Proc Natl Acad Sci USA. 2006;103:6901–6. doi: 10.1073/pnas.0509719103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soond SM, Everson B, Riches DW, et al. ERK-mediated phosphorylation of Thr735 in TNFalpha-converting enzyme and its potential role in ADAM-17 protein trafficking. J Cell Sci. 2005;118:2371–80. doi: 10.1242/jcs.02357. [DOI] [PubMed] [Google Scholar]
- 8.DasGupta S, Murumkar PR, Giridhar R, et al. Current perspective of ADAM-17 inhibitors: a review. Bioorg Med Chem. 2009;17:444–59. doi: 10.1016/j.bmc.2008.11.067. [DOI] [PubMed] [Google Scholar]