Abstract
Personality traits related to emotion processing are, at least in part, heritable and genetically determined. Dopamine D2 receptor signaling is involved in modulation of emotional behavior and activity of associated brain regions such as the amygdala and the prefrontal cortex. An intronic single nucleotide polymorphism within the D2 receptor gene (DRD2) (rs1076560, guanine > thymine or G > T) shifts splicing of the two protein isoforms (D2 short, mainly presynaptic, and D2 long) and has been associated with modulation of memory performance and brain activity. Here, our aim was to investigate the association of DRD2 rs1076560 genotype with personality traits of emotional stability and with brain physiology during processing of emotionally relevant stimuli. DRD2 genotype and Big Five Questionnaire scores were evaluated in 134 healthy subjects demonstrating that GG subjects have reduced “emotion control” compared with GT subjects. Functional magnetic resonance imaging in a sample of 24 individuals indicated greater amygdala activity during implicit processing and greater dorsolateral prefrontal cortex (DLPFC) response during explicit processing of facial emotional stimuli in GG subjects compared with GT. Other results also demonstrate an interaction between DRD2 genotype and facial emotional expression on functional connectivity of both amygdala and dorsolateral prefrontal regions with overlapping medial prefrontal areas. Moreover, rs1076560 genotype is associated with differential relationships between amygdala/DLPFC functional connectivity and emotion control scores. These results suggest that genetically determined D2 signaling may explain part of personality traits related to emotion processing and individual variability in specific brain responses to emotionally relevant inputs.
Introduction
Dopamine signaling is involved in emotion processing and control of emotional behavior (LeDoux, 1998; Salgado-Pineda et al., 2005). For example, dopaminergic psychostimulants affect emotional behavior (Hall et al., 1988). Consistently, during conditioned fear paradigms in animals, dopamine modulates activity in amygdala and in prefrontal cortex (Rosenkranz and Grace, 2001; Pezze and Feldon, 2004), key brain regions for emotion perception and control (Dolan, 2002; Ochsner and Gross, 2005). Moreover, genetic variation in cathecol-O-methyltransferase (COMT) (Val158Met), critically involved in dopamine catabolism, affects emotional behavior, related brain activity, and their relationship in both animals and humans (Gogos et al., 1998; Woo et al., 2002; Enoch et al., 2003; Smolka et al., 2005; Stein et al., 2005; Drabant et al., 2006).
Dopamine D2 receptors have been implicated in emotion processing (Pezze and Feldon, 2004). Administration of D2 agonists or antagonists in animal models modulates emotional responses, including emotional reactivity (Gendreau et al., 1998) and learning (Greba et al., 2001). However, little is known about possible associations between the D2 gene (DRD2) with emotional behavior (Drew et al., 2007).
D2 receptors exist in two alternatively spliced isoforms, the D2 long (D2L) located primarily postsynaptically and D2 short (D2S) functioning as presynaptic autoreceptor (Usiello et al., 2000). Studies in mice have suggested involvement of the D2L isoform in emotional responses. In particular, D2L-deficient mice have reduced explorative behavior and increased latency to escape from harmful situations (Hranilovic et al., 2008), suggesting that they are more “anxious.”
Expression of D2 isoforms is associated with a known genetic variant. Recently, we have characterized a novel functional single nucleotide polymorphism (SNP) within the DRD2 gene at intron 6, rs1076560 (G → T substitution). GG genotype is associated with relatively greater expression of D2S mRNA in prefrontal cortex and in striatum as well as with more efficient cognitive processing relative to GT genotype (Zhang et al., 2007).
Different models of human personality have been proposed identifying several traits or dimensions. Regardless of the model, personality traits are in part heritable (Jang et al., 1998), especially in terms of emotion control and stability (Jang et al., 1996; Lesch, 2005). In the present study in healthy humans, we investigated the association of DRD2 rs1076560 genotype with personality traits as measured with the Big Five Questionnaire (BFQ) (Caprara et al., 2003). Given the known relationship between dopamine signaling and control of emotional behavior, we hypothesized specific effects of DRD2 rs1076560 variants on the emotional stability dimension of the BFQ. Moreover, we explored with functional magnetic resonance imaging (fMRI) the potential association of DRD2 rs1076560 genotype with physiologic brain responses during emotional perceptual processing and explicit emotional evaluation. These emotional processes prominently engage the amygdala and the prefrontal cortex, respectively (Gusnard et al., 2001; Phillips et al., 2003; Ochsner et al., 2004a,b). Based on data in animal models (Hranilovic et al., 2008) and on previous knowledge of dopamine projections and D2 receptor distribution, we hypothesized differential activity and functional connectivity of these brain regions during emotion processing based on the DRD2 rs1076560 polymorphism.
Materials and Methods
Subjects.
One hundred thirty-four healthy subjects (84 females; mean ± SD, 26.4 ± 6.7 years) participated. Inclusion criteria were absence of any psychiatric disorder, as evaluated with the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders IV, of any significant neurological or medical condition revealed by clinical and magnetic resonance imaging evaluation, of history of head trauma with loss of consciousness, and of pharmacological treatment or drug abuse in the past year. The Wechsler Adult Intelligence Scale–Revised was used to evaluate intelligent quotient (IQ) (mean ± SD, 111.1 ± 13.9) and the Edinburgh Inventory (Oldfield, 1971) to measure handedness (mean ± SD, 0.7 ± 0.4).
To examine the association of genotypes with brain activity during emotion processing in groups matched for a series of variables, including sample size, we selected a subsample of 24 subjects (12 GG, six males; mean ± SD age of 24.7 ± 4.0 years, IQ of 115.7 ± 13.7, handedness 0.6 ± 0.37; 12 GT, six males, mean ± SD age of 24.0 + 4.7 years, IQ of 109.0 ± 16.7, handedness 0.5 ± 0.6) (p > 0.35 for all variables), who underwent fMRI during emotional perceptual processing and explicit emotional evaluation of faces with different facial expressions.
The present experimental protocol was approved by the local institutional review board. After complete description of the study to the subjects, written informed consent was obtained.
Genotyping.
Subjects were genotyped for DRD2 rs1076560. This SNP was analyzed with allele-specific PCR primers as described previously (Papp et al., 2003; Zhang et al., 2007; Bertolino et al., 2009a,b). One hundred six subjects were GG and 28 GT. This distribution displayed Hardy–Weinberg equilibrium (χ2 = 0.001; p = 0.99). None of the subjects was homozygous for the T allele, consistent with previous studies (Zhang et al., 2007).
To partially control for occult stratification within other genetic variants known to be associated with limbic brain activity, we also genotyped all subjects for COMT Val158Met (rs4680), 5-HTTLPR polymorphism (rs4795541) within the serotonin transporter gene (SLC6A4), BDNF Val66Met (rs6265), and monoamine oxidase A (MAO-A) u variable number of tandem repeat (uVNTR) (Bertolino et al., 2005; Smolka et al., 2005; Meyer-Lindenberg et al., 2006; Montag et al., 2008). COMT rs4680 genotype was determined as a restriction fragment length polymorphism after PCR amplification and digestion with NlaIII, as described previously (Bertolino et al., 2004). BDNF rs6265 genotype was determined by direct sequencing. Briefly, the rs6265 region was amplified using forward 5′-GGTGCAGCTGGAGTTTATCAC-3′ and reverse 5′-GGTCTCG-TAGAAGTATTGCTTC-3′ primers. PCR was performed in 25 μl of reaction volume containing 100 ng of genomic DNA, 2.5 μl of reaction buffer (100 mm Tris, pH 8.3, and 500 mm KCl), 2 mm MgCl2, 100 μm dNTP, 2 pmol of each primers, and 1 U of Ampli Taq Gold Polymerase (Applied Biosystems) with the following cycling profile: 12 min initial denaturation at 94°C; 35 cycles of denaturation at 94°C for 45 s, annealing at 58°C for 45 s, extension at 72°C for 1 min, followed by 7 min of final extension step at 72°C. PCR products were sequenced in both directions using BigDye Terminator chemistry and run on ABI Prism 3100 DNA Sequencer (Applied Biosystems).
Size polymorphisms of the MAO-A gene uVNTR polymorphism were screened by PCR and capillary electrophoresis. The uVNTR region was amplified using primers described previously (Sjöberg et al., 2008), the forward primer labeled with 6-FAM dye for visualization. PCR was performed on 30–50 ng of DNA, in buffer (100 mm Tris, pH 8.0, 500 mm KCl, and 3.0 mm MgCl2), 200 μm dNTP, 2 pmol of each primer, and 1 U of Taq polymerase (Applied Biosystems). Cycling conditions were as follows: initial denaturation at 95°C for 2 min, followed by 30 cycles of denaturation at 94°C for 30 s, annealing at 63°C for 30 s, and extension at 72°C for 45 s, with a 15 min final extension at 72°C. The samples were run on ABI3730XL DNA Analyzer (Applied Biosystems). Results were analyzed using GeneMapper version 3.5 (Applied Biosystems).
To determine the 5HTTLPR (rs4795541) polymorphism, we used a touchdown PCR protocol and capillary electrophoresis. Primer sequences have been described by Gelernter et al. (1997), the forward primer labeled with 6-FAM dye for visualization. PCR was performed on 30–50 ng of DNA in 5 μl of 5× Colorless GoTaq Reaction Buffer (Promega), 0.24 mm MgCl2, 150 μm dNTP, 10 pmol of each primer, and 1 U of GoTaq DNA Polymerase (Promega). Cycling conditions were as follows: initial denaturation at 94°C for 2 min; five cycles of denaturation at 94°C for 1 min, annealing at 66°C for 30 s, and extension at 72°C for 1 min; 20 cycles of denaturation at 94°C for 1 min, annealing at 64°C for 30 s, and extension at 72°C for 1 min; 10 cycles of denaturation at 94°C for 1 min, annealing at 62°C for 30 s, and extension at 72°C for 1 min with a 10 min final extension at 72°C. The samples were run on ABI3730XL DNA Analyzer (Applied Biosystems). Results were analyzed using GeneMapper version 3.5 (Applied Biosystems).
BFQ.
All subjects completed the BFQ (Caprara et al., 2003), measuring the Big Five Factors Model (McCrae and Costa, 1987). This questionnaire includes five dimensions (energy, friendliness, conscientiousness, emotional stability, and openness), which are organized into two facets each (energy: dynamism and dominance; friendliness: cooperativeness and politeness; conscientiousness: scrupulousness and perseverance; emotional stability: emotion control and impulse control; openness: openness to culture and openness to experience). In particular, the dimension “emotional stability” refers to aspects of “negative affectivity” (Caprara et al., 2003). In more detail, within this dimension, the facet “emotion control” is defined as the capacity to cope adequately with one's own anxiety and emotionality, whereas the “impulse control” facet refers to the capability of controlling irritation, discontent, and anger (Caprara et al., 2003).
fMRI experimental paradigm.
The event-related fMRI paradigm consisted of two runs: each run presented angry, fearful, happy, and neutral facial expressions from a validated set of facial pictures (NimStim, http://www.macbrain.org/resources.htm) (Tottenham et al., 2009). The order of stimuli was randomly distributed. However, the same stimuli were presented in both runs with the same order. During one run (emotional perceptual processing: implicit processing), subjects identified the gender of each face. In the other run (explicit emotional evaluation: explicit processing), they had to decide whether they would like to “approach” or “avoid” the face. From stimulus appearance, 2 s were allowed for behavioral responses. The presentation of the two runs was counterbalanced across subjects.
Each stimulus was presented for 500 ms, with the interstimulus interval randomly jittered between 2 and 7 s. The total number of stimuli was 144: 30 angry, 39 fearful, 37 happy, and 38 neutral faces. Duration of each run was 6 min 8 s. A fixation crosshair was presented during the interstimulus interval.
fMRI data acquisition.
fMRI was performed on a GE Signa 3T scanner with a gradient echo-planar imaging sequence (repetition time, 2000 ms; echo time, 28 ms; 26 interleaved slices, thickness of 4 mm, gap of 1 mm; voxel size, 4 × 4 × 5 mm; scan repetitions, 180; flip angle, 90°; field of view, 24 cm; matrix, 64 × 64). The first four scans were discarded to allow for signal saturation. All stimuli were presented via a back-projection system. A fiber optic response box was used to measure subject preference (and reaction time) for each stimulus:- left button for approach/male response and right button for avoid/female response.
Postscanning.
After the fMRI scanning session, we called back the subjects (17 of 24, 10 GG and 7 GT; the others were not available) to assess the emotional impact of each facial expression shown during the task. Subjects were asked to view again all pictures previously presented on a computer screen and to rate, in a forced-choice way, the emotional impact elicited by facial expressions in the pictures. Responses were given on the basis of a 5-point Likert scale (no impact, low impact, some impact, high impact, and maximum impact). This scale was then converted in numerical values (respectively, 1, 2, 3, 4, and 5) to allow correlation analysis with significant blood oxygen level-dependent (BOLD) responses.
Data analysis.
ANOVA and χ2 test were used to compare demographics, distribution of the other genetic polymorphisms within DRD2 rs1076560 groups, and behavioral measures. Fisher's least significant difference test was used for post hoc comparisons. Statistical Parametric Mapping 5 (SPM5, http://www.fil.ion.ucl.ac.uk/spm) was used for imaging analysis. Images for each subject were realigned, spatially normalized into the Montreal Neurological Institute template (12-parameter affine model), and spatially smoothed (10 mm Gaussian filter). After realignment, datasets were also screened for high quality (scan stability) as demonstrated by small motion correction (<2 mm translation, <1.5° rotation). fMRI responses were modeled using a canonical hemodynamic response function and temporally filtered using a high-pass filter of 128 Hz to minimize scanner drift. For both runs, vectors were created for angry, fearful, happy, and neutral faces. Residual movement was modeled as regressor of no interest. Predetermined condition effects at each voxel were created using a t statistic, producing a statistical image for BOLD responses to brain processing of stimuli representative of each condition, i.e., angry, fearful, happy, and neutral faces versus fixation crosshairs during both runs. ANOVA was then used at the group level to investigate the main effect of task, of facial expression, of genotype, and their interactions. These analyses were constrained by a mask obtained by combining group activation maps associated with processing of each facial expression during each task. Therefore, voxels significant for either task or emotion were included in the final mask. To reduce the probability of type II errors, the statistical threshold used for these masks was set at p < 0.05, uncorrected.
Psychophysiological interaction (PPI) analysis was also performed to explore the association of DRD2 rs1076560 genotype with brain functional connectivity during the emotion task. With this purpose, we used as seeds clusters in which significant task × genotype interactions were found. In particular, we investigated functional connectivity of the left amygdala during implicit processing and of left dorsolateral prefrontal cortex (DLPFC) during explicit processing. The first eigenvariate of individual raw activation time courses was extracted by using singular value decomposition method from a volume of interest (VOI) centered on the subject-specific peak cluster within the seed regions. These time courses were then mean centered, high-pass filtered, and deconvolved. A general linear model was computed using three regressors: a physiological regressor (the time course response in the VOI), a psychological regressor (the presentation of emotional faces), and a psychophysiological interaction term, calculated as the cross-product of the previous two terms. The individual psychophysiological interaction contrasts for each seed were entered into separate ANOVAs to investigate the main effect of genotype, of emotion, and their interaction.
For fMRI analyses, a statistical threshold of p < 0.001, minimum cluster size (k) = 6, followed by familywise error small volume correction at p < 0.05 within the task associated clusters of activity in amygdala and DLPFC, as well as within the clusters of functional connectivity in medial prefrontal cortex.
Finally, BOLD responses and PPI values were extracted from significant clusters using MarsBar (http://marsbar.sourceforge.net/) and entered in Spearman's correlations to investigate the potential relationship between emotional behavior and brain responses in DRD2 rs1076560 genotype groups. In particular, we hypothesized a potential relationship between brain regions differentially activated by the two genotype groups during explicit emotion processing and postscanning scores relative to explicit evaluation of the emotional impact of the facial stimuli of the fMRI task. Therefore, Spearman's analysis was performed between BOLD responses in the DLPFC region showing a genotype × task interaction (see Results) and scores of emotional impact. Using Spearman's correlations, we also examined potential relationships between areas with differential activity and functional connectivity between the two genotype groups and emotional behavior. Given the exploratory nature of these latter correlations, results were corrected for the number of statistical tests performed.
Results
BFQ
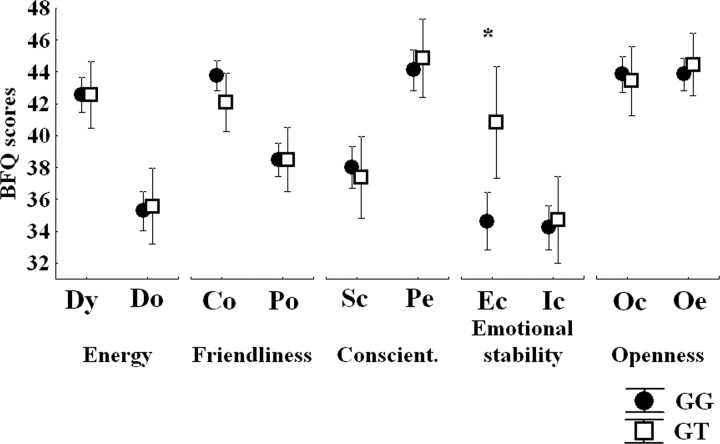
The two DRD2 rs1076560 genotype groups were well matched in terms of age, gender, IQ, handedness (all p > 0.25), and distribution of the other examined genotypes [COMT Val158Met (rs4680): df = 2, χ2 = 1.43, p > 0.4; 5HTTLPR rs4795541: df = 2, χ2 = 3.4, p > 0.1; BDNF Val66Met rs6265: df = 2, χ2 = 4.5, p > 0.1; MAO-A uVNTR: df = 2, χ2 = 3.2, p > 0.1]. A repeated-measures ANOVA, with DRD2 rs1076560 genotype as the independent factor together with the five dimensions and the 10 facets of the Big Five Questionnaire as repeated-measures dependent factors, showed no effect of genotype (F(1,132) = 0.78, p = 0.37), an interaction between genotype and dimension (F(4,528) = 2.40, p = 0.048), as well as an interaction between genotype, dimension, and facet (F(4,528) = 3.70, p = 0.005). This last interaction would also survive Bonferroni's correction for the number of facets (p = 0.05). Post hoc analysis with Fisher's test revealed that DRD2 rs1076560 genotype was associated with scores on the emotion control facet within the emotion stability dimension only (p = 0.002) such that GG subjects had significantly reduced scores on this specific facet (Fig. 1). No other significant association of DRD2 rs1076560 genotype with other BFQ facets was found (all p > 0.4) (Fig. 1).
Figure 1.
Plots showing the association of DRD2 rs1076560 with BFQ scores. Only the emotion control facet was significantly different between the two genotype groups. *p < 0.05. For statistics, see Results. Dy, Dynamism; DO, dominance; Co, cooperativeness; Po, politeness; Sc, scrupulousness; Pe, perseverance; Ec, emotion control; Ic, impulse control; Oc, openness to culture; Oe, openness to experience; Conscient, conscientiousness.
In the fMRI sample, a t test to specifically evaluate the emotion control facet of the BFQ demonstrated a statistically significant effect of DRD2 rs1076560 genotype on this variable (df = 22, t = −3.6, p = 0.001), which was in the same direction as in the larger sample (GG < GT).
fMRI
Behavioral data
No statistically significant effects were present on accuracy data (all p > 0.4). Reaction time data indicated a main effect of task (F(1,22) = 20.9, p = 0.0001, explicit slower than implicit), of facial expression (F(3,66) = 6.1, p = 0.001, faster responses to happy faces than to all other expressions, all p < 0.05), and an interaction between task and facial expression (F(3,66) = 7.5, p = 0.0002, slower responses to angry, fearful, and neutral relative to happy facial expressions during explicit processing, all p < 0.03). Of note, there was a main effect of DRD2 genotype (F(1,22) = 4.5, p = 0.04) and an interaction between genotype and task (F(1,22) = 8.1, p = 0.01, slower responses for GG subjects only during explicit processing, p < 0.01) (Fig. 2A). No other significant effects were found (all p > 0.6).
Figure 2.
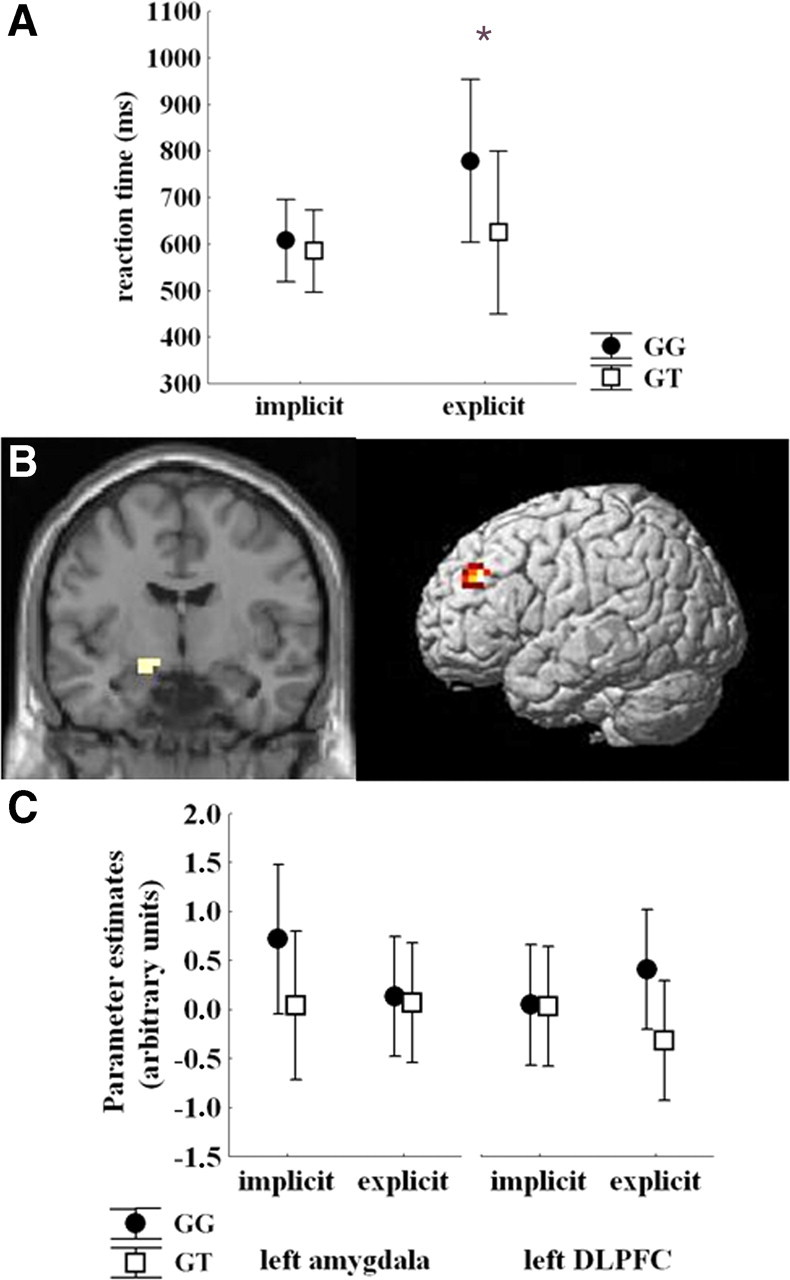
A, Plot showing reaction time data during implicit and explicit processing of faces stimuli. For statistics, see Results. *p < 0.05. B, Coronal section and rendered image showing brain regions in which a DRD2 rs1076560 genotype × task interaction was present, i.e., left amygdala and left lateral prefrontal cortex. C, For illustrative purposes, parameter estimates extracted from clusters shown in A displaying BOLD response in left amygdala and left lateral prefrontal cortex during the implicit and the explicit part of the task as a function of DRD2 rs1076560 genotype.
Effect of facial expression on approach/avoid judgments during the explicit processing run of the fMRI task
Repeated-measures ANOVA indicated an effect of facial expression on percentage of facial stimuli that received an avoid response (F(3,69) = 84.2, p < 0.0001). Fisher's post hoc analysis indicated no statistical difference between the number of fearful and angry faces that subjects judged they would avoid (mean avoided fearful faces, 77.1%; mean avoided angry faces, 83.2%; p > 0.2). These facial expressions received higher rates of avoid response. Furthermore, subjects judged they would avoid a significantly lower number of neutral relative to fearful and angry faces (mean avoided neutral faces, 40.0%; both p < 0.0001). Finally, happy expressions received lower percentage of avoid responses, with statically significant differences relative to all other facial expressions (mean avoided happy faces, 13.0%; all p < 0.0001).
Genotype and facial expression effects on postscanning emotional impact scores
Repeated-measures ANOVA on the emotional impact scores showed no effect of genotype (F(1,15) = 0.48, p = 0.5), an effect of facial expression (F(3,45) = 27.51, p < 0.0001), and no interaction between these variables (F(3,45) = 2.14, p = 0.1). Fisher's post hoc analysis revealed greater emotional impact for all emotionally charged relative to neutral expressions (p < 0.0001) but no significant difference between the different emotionally charged facial expressions (all p > 0.6).
Imaging data
BOLD response
ANOVA demonstrated a main effect of task in medial and lateral prefrontal regions, as well as in left amygdala: a main effect of facial expression in left amygdala (x, y, z: −26, −4, −19; k = 19; Z = 3.84) and left lateral prefrontal cortex [Brodmann area 45 (BA45); x, y, z: −60, 26, 11; k = 39; Z = 3.75], and a main effect of genotype in medial prefrontal cortex, left lateral prefrontal regions, and left amygdala (Table 1). An interaction between genotype and task was present in left amygdala and left DLPFC (Table 1, Fig. 2B). Specific post hoc contrasts revealed that GG subjects had greater left amygdala activity during implicit processing (Z = 4.87) and greater prefrontal response during explicit processing (Z = 6.06) in the clusters showing a gene × task interaction. Inspection of BOLD signal extracted from these amygdala and dorsolateral prefrontal clusters was consistent with the SPM data (Fig. 2C). No other statistically significant interactions were found.
Table 1.
Local maxima of brain activity and functional connectivity showing significant DRD2 rs1076650 effects during implicit and explicit processing of emotional stimuli as elicited by the task used in this study
| Brain region | BA | MNI coordinates |
k | Z | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Activity | ||||||
| Main effect of genotype | ||||||
| Left amygdala | −15 | −7 | −22 | 10 | 5.09 | |
| Left inferior frontal gyrus | 51 | −60 | 26 | 11 | 51 | 4.89 |
| Left superior frontal gyrus | 6 | −11 | 15 | 68 | 6 | 3.62 |
| Main effect of task | ||||||
| Left inferior frontal gyrus | 46 | −49 | 41 | 15 | 42 | 4.94 |
| Left superior frontal gyrus | 6 | −4 | 19 | 53 | 19 | 3.81 |
| Left amygdala | −15 | −7 | −11 | 10 | 3.35 | |
| Interaction: genotype × task | ||||||
| Left amygdala | −15 | −7 | −11 | 6 | 3.22 | |
| Left superior frontal gyrus | 9 | −38 | 45 | 34 | 19 | 4.15 |
| PPI | ||||||
| Explicit processing: seed in left BA9 | ||||||
| Interaction: genotype × emotion | ||||||
| Left superior frontal gyrus | 8 | −15 | 41 | 45 | 6 | 3.42 |
| Implicit processing: seed in left amygdala | ||||||
| Interaction: genotype × emotion | ||||||
| Left superior frontal gyrus | 8 | −15 | 38 | 49 | 12 | 3.35 |
MNI, Montreal Neurological Institute.
We also examined potential relationships between activity during the explicit condition in the DLPFC region showing a task × genotype interaction and the postscanning ratings of emotional impact. Spearman's correlation analysis indicated in GG subjects a positive correlation between mean scores of emotional impact and DLPFC activity (ρ = 0.7, p = 0.01). This relationship was not evident in GT subjects (ρ = −0.03, p = 0.9).
PPI
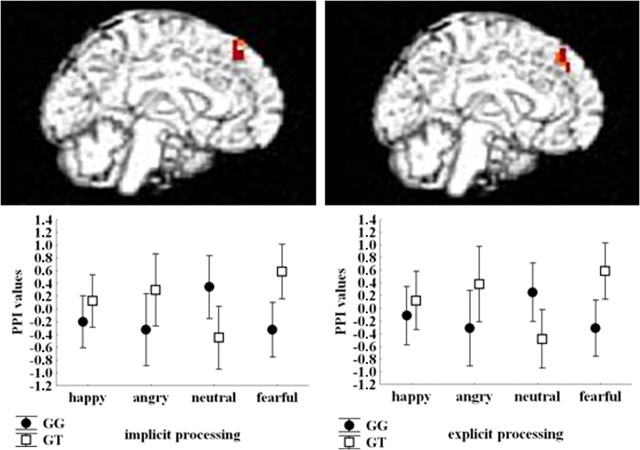
PPI analyses indicated no main effect of genotype on left amygdala and left DLPFC functional connectivity during implicit and explicit emotional processing, respectively. However, an interaction between genotype and facial expression was found for PPI between both seeds and clusters in medial prefrontal cortex (Table 1, Fig. 3). PPI measures extracted from both these clusters indicated positive values for emotionally charged facial expressions (angry, fearful, and happy) and negative for neutral faces in GT subjects, whereas the opposite pattern was evident in GG individuals (Fig. 3).
Figure 3.
Rendered images of sagittal sections of the brain showing medial prefrontal clusters in which significant interaction between DRD2 rs1076560 and facial expressions was present on functional connectivity with left amygdala during implicit processing (left) and with left DLPFC during explicit processing (right). Mean ± SE. PPI values are shown in the plots.
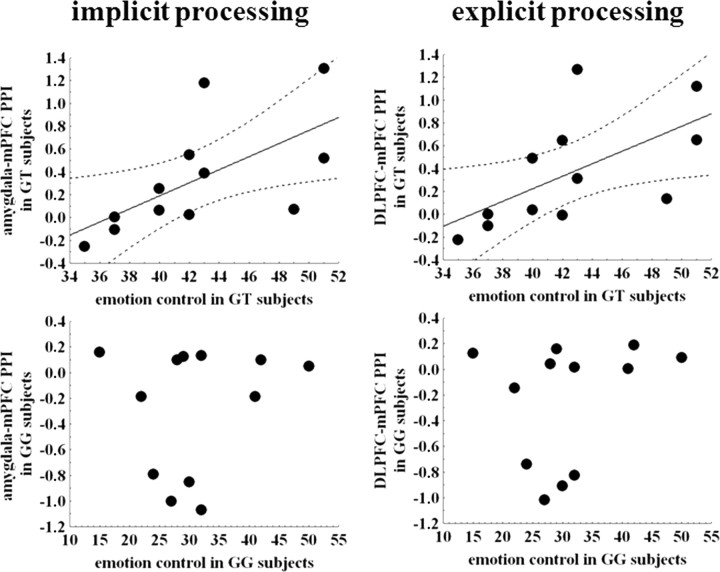
Spearman's test in GT individuals revealed positive correlations of emotion control scores with mean PPI values between medial prefrontal regions and both left amygdala during implicit processing and left DLPFC during explicit processing of emotionally charged facial expressions (left amygdala with medial prefrontal cortex: ρ = 0.81, p = 0.001, after correction for the number of correlations, p = 0.008; left DLPFC with medial prefrontal cortex: ρ = 0.77, p = 0.004, after correction for the number of correlations, p = 0.03). Conversely, no such relationship was evident in GG subjects (left amygdala–medial prefrontal cortex: ρ = −0.05, p = 0.87; left DLPFC–medial prefrontal cortex: ρ = −0.24, p = 0.46) (Fig. 4).
Figure 4.
Scatter plots showing relationships between mean PPI values of emotionally charged faces (angry, fearful, and happy) extracted from clusters displayed in Figure 3 during implicit (x, y, z: −15, 38, 49) (left scatter plots) and explicit (x, y, z: −15, 41, 45) (right scatter plots) processing and emotion control scores as measured with the BFQ. In GT individuals, positive relationships are present during both implicit and explicit processing, whereas in GG subjects, no such relationships have been found. For statistics, see Results. mPFC, Medial prefrontal cortex.
Discussion
The results of the present study suggest that DRD2 rs1076560 genotype is associated with specific parameters of emotion processing. At the behavioral level, there was a specific effect within the emotion stability dimension of the BFQ. In particular, GG subjects had reduced emotion control scores relative to GT subjects. Furthermore, GG subjects were slower when explicitly processing facial stimuli. The fMRI data indicated that GG subjects had greater left amygdala activity during implicit processing and greater left DLPFC signal during explicit processing of facial expressions. Consistently, activity in DLPFC of GG subjects was correlated with ratings of the emotional impact of facial stimuli presented during the task. Furthermore, DRD2 rs1076560 genotype and facial expressions were differentially associated with functional connectivity of medial prefrontal areas with amygdala during implicit processing and with DLPFC during explicit processing. In particular, GG individuals had negative PPI while evaluating emotionally charged faces, whereas PPI was positive in GT subjects. Conversely, processing of neutral expressions was associated with the opposite pattern. Behavioral relevance of these data is further suggested by analysis of the relationship between amygdala/DLPFC–medial prefrontal cortex functional connectivity during processing of emotionally charged faces and emotion control scores, which indicated significant positive correlations in GT subjects only.
Studies in animal models have indicated that dopamine D2 receptor signaling is involved in emotional behavior (Gendreau et al., 1998; Guarraci et al., 2000; Greba et al., 2001) and in activity of amygdala and prefrontal cortex, brain regions centrally implicated in emotion processing (Levey et al., 1993; Rosenkranz and Grace, 2001; Seamans and Yang, 2004). Of note, these brain areas are functionally connected during management of emotional inputs (Pezze and Feldon, 2004; Stein et al., 2007). Our results suggesting association of emotional behavior and physiology during emotion processing with genetically determined D2 signaling are consistent with these data. In particular, in GG subjects, we found lower emotion control scores and lower speed of processing of emotionally relevant explicit stimuli, as well as greater amygdala and DLPFC activity, respectively, during implicit and explicit processing of emotionally relevant stimuli. These results may indicate that a ratio of D2 receptors favoring the presynaptic form (D2S) may predispose GG subjects to lower capacity of handling emotional inputs. In this sense, these data are consistent with previous findings in animals expressing only the D2S receptor, which has greater levels of behavioral anxiety in the form of lower level of exploration and increased latency to escape from harmful situations (Hranilovic et al., 2008). Conversely, lack of DRD2 rs1076560 genotype association with the impulse control scale within the emotional stability dimension of the BFQ is consistent with other studies indicating no effect of D2 signaling on impulsive behavior (Arnsten and Li, 2005; van Gaalen et al., 2006) and suggests a specific association of DRD2 rs1076560 genotype with emotion control.
The association of this DRD2 genotype with amygdala activity during implicit processing is consistent with the known role of this brain region in perception of emotionally relevant inputs (LeDoux, 1998; Hariri et al., 2000; Salgado-Pineda et al., 2005) and with data suggesting involvement of D2 receptors in amygdala responses (Pezze and Feldon, 2004). Furthermore, differential DLPFC response associated with this polymorphism during explicit processing is in line with several studies involving this brain region in emotional regulation (Phillips et al., 2003), as well as with the well established relevance of D2 signaling in activity of prefrontal neurons (Seamans and Yang, 2004).
Altogether, these results suggest that genetically determined D2 signaling is associated with task- and region-specific effects. In fact, the two DRD2 rs1076560 genotype groups have differential BOLD responses in amygdala and DLPFC during tasks in which these brain regions are more specifically involved, i.e., implicit emotion perception and explicit emotional evaluation, respectively. Furthermore, specificity of the present results for the emotional domain is also suggested by our previous data on association of brain activity with DRD2 rs1076560 genotype during cognition. In particular, differential activity between GG and GT subjects in striatum and DLPFC was found during working memory. However, the results of that study were in the opposite direction, with greater BOLD responses in GT subjects relative to GG individuals (Zhang et al., 2007). In this respect, the present data are consistent with those reported in other studies investigating the COMT Val158Met (rs4680) polymorphism revealing pleiotropic opposite effects in the cognitive and emotional domains (Blasi et al., 2005; Smolka et al., 2005).
The DRD2 rs1076560 polymorphism was also associated with opposite effects on functional connectivity between amygdala and DLPFC with medial prefrontal regions during implicit and explicit processing of emotionally and non-emotionally charged facial expressions. During both emotional processes investigated with our task, positive PPI values (i.e., a positive relationship between activity in amygdala and in DLPFC with that in medial prefrontal regions) were found in GT subjects while evaluating emotionally charged facial expressions, whereas they were negative (i.e., the opposite as before) in GG individuals. Of note, these medial prefrontal regions had overlapping spatial location during both implicit and explicit processing. Previous studies have associated dorsal regions of the medial prefrontal cortex with processing of emotional stimuli. For example, fMRI studies have indicated involvement of these medial brain areas in reappraisal of stimuli with negative valence (Ochsner et al., 2004b), attention to own emotional response to external stimuli (Gusnard et al., 2001), regulation of negative emotions (Ochsner et al., 2004a), and cognitive evaluation of emotional stimuli (Rubino et al., 2007). Therefore, the results of the present study suggest that functional connections between relevant nodes of the emotional network, such as the medial prefrontal cortex, the amygdala, and the DLPFC, during different emotional processes may be associated with genetically determined dopamine signaling. Furthermore, GT subjects had positive correlations between amygdala/DLPFC functional connections with medial prefrontal regions and emotion control scores, whereas no such relationship was present in GG individuals. These results are in line with other studies showing effects of genetically determined modulation of dopamine signaling on the relationship between physiology and behavior (Drabant et al., 2006). Moreover, they suggest that variations in dopamine signaling related with the DRD2 rs1076560 polymorphism may be implicated in changes of functional connections within the emotional network and in their impact on processing of emotionally salient stimuli.
Interestingly, the results of the present study vary with regard to their specificity for emotionally charged stimuli. For example, there was no interaction of BOLD effects with facial expression, which was instead associated with PPI effects. Differential effects of specific variables on either activity of discrete brain regions or brain functional coupling is not new in the fMRI literature (Williams et al., 2006; Meyer-Lindenberg, 2009; Prata et al., 2009). Some recent studies have also reported no effect of genotype on regional brain activation along with very robust effects on PPI (Esslinger et al., 2009). Another factor that may be associated with these differences is that perception of different facial expressions may involve greater functional integration of activity of different brain regions. Therefore, the physiological factors associated with such processing may more likely and more strongly affect measures of functional coupling in some brain regions.
A limitation of this study is that the present data do not allow us to clarify the relevance of the examined variant for the risk architecture of psychiatric disorders, because neither evidence for an association of these particular phenotypes with patient populations nor evidence for their heritability is provided. However, investigation of these phenotypes in patient populations was beyond the aim of this work.
In conclusion, all these data indicate that genetic variation in DRD2 modulating D2 presynaptic versus postsynaptic signaling are relevant to specific aspects of behavior and physiology during emotion processing. Additional studies are needed to elucidate the role of DRD2 rs1076560 in brain disorders with relevant emotional deficits, including schizophrenia, depression, and anxiety disorders.
Footnotes
Part of this work was supported by National Institutes of Health Grant DA022199 (W.S.). We are grateful to Dr. Ahmad Hariri and Dr. Venkata S. Mattay for their help with the design of the task. We are also grateful to Riccarda Lomuscio for her help in data acquisition and all people that participated in this study.
References
- Arnsten AF, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry. 2005;57:1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Caforio G, Blasi G, De Candia M, Latorre V, Petruzzella V, Altamura M, Nappi G, Papa S, Callicott JH, Mattay VS, Bellomo A, Scarabino T, Weinberger DR, Nardini M. Interaction of COMT Val108/158 Met genotype and olanzapine treatment on prefrontal cortical function in patients with schizophrenia. Am J Psychiatry. 2004;161:1798–1805. doi: 10.1176/ajp.161.10.1798. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Arciero G, Rubino V, Latorre V, De Candia M, Mazzola V, Blasi G, Caforio G, Hariri A, Kolachana B, Nardini M, Weinberger DR, Scarabino T. Variation of human amygdala response during threatening stimuli as a function of 5′HTTLPR genotype and personality style. Biol Psychiatry. 2005;57:1517–1525. doi: 10.1016/j.biopsych.2005.02.031. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Fazio L, Caforio G, Blasi G, Rampino A, Romano R, Di Giorgio A, Taurisano P, Papp A, Pinsonneault J, Wang D, Nardini M, Popolizio T, Sadee W. Functional variants of the dopamine receptor D2 gene modulate prefronto-striatal phenotypes in schizophrenia. Brain. 2009a;132:417–425. doi: 10.1093/brain/awn248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino A, Fazio L, Di Giorgio A, Blasi G, Romano R, Taurisano P, Caforio G, Sinibaldi L, Ursini G, Popolizio T, Tirotta E, Papp A, Dallapiccola B, Borrelli E, Sadee W. Genetically determined interaction between the dopamine transporter and the D2 receptor on prefronto-striatal activity and volume in humans. J Neurosci. 2009b;29:1224–1234. doi: 10.1523/JNEUROSCI.4858-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi G, Mattay VS, Bertolino A, Elvevåg B, Callicott JH, Das S, Kolachana BS, Egan MF, Goldberg TE, Weinberger DR. Effect of catechol-O-methyltransferase val158met genotype on attentional control. J Neurosci. 2005;25:5038–5045. doi: 10.1523/JNEUROSCI.0476-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprara G, Barbarelli C, Borgogni L, Perugini M. The “Big Five Questionnaire”: a new questionnaire to assess the five factor model. Pers Individ Diff. 2003;15:281–288. [Google Scholar]
- Dolan RJ. Emotion, cognition, and behavior. Science. 2002;298:1191–1194. doi: 10.1126/science.1076358. [DOI] [PubMed] [Google Scholar]
- Drabant EM, Hariri AR, Meyer-Lindenberg A, Munoz KE, Mattay VS, Kolachana BS, Egan MF, Weinberger DR. Catechol O-methyltransferase val158met genotype and neural mechanisms related to affective arousal and regulation. Arch Gen Psychiatry. 2006;63:1396–1406. doi: 10.1001/archpsyc.63.12.1396. [DOI] [PubMed] [Google Scholar]
- Drew MR, Simpson EH, Kellendonk C, Herzberg WG, Lipatova O, Fairhurst S, Kandel ER, Malapani C, Balsam PD. Transient overexpression of striatal D2 receptors impairs operant motivation and interval timing. J Neurosci. 2007;27:7731–7739. doi: 10.1523/JNEUROSCI.1736-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, Xu K, Ferro E, Harris CR, Goldman D. Genetic origins of anxiety in women: a role for a functional catechol-O-methyltransferase polymorphism. Psychiatr Genet. 2003;13:33–41. doi: 10.1097/00041444-200303000-00006. [DOI] [PubMed] [Google Scholar]
- Esslinger C, Walter H, Kirsch P, Erk S, Schnell K, Arnold C, Haddad L, Mier D, Opitz von Boberfeld C, Raab K, Witt SH, Rietschel M, Cichon S, Meyer-Lindenberg A. Neural mechanisms of a genome-wide supported psychosis variant. Science. 2009;324:605. doi: 10.1126/science.1167768. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Kranzler H, Cubells JF. Serotonin transporter protein (SLC6A4) allele and haplotype frequencies and linkage disequilibria in African- and European-American and Japanese populations and in alcohol-dependent subjects. Hum Genet. 1997;101:243–246. doi: 10.1007/s004390050624. [DOI] [PubMed] [Google Scholar]
- Gendreau PL, Petitto JM, Gariépy JL, Lewis MH. D2-like dopamine receptor mediation of social-emotional reactivity in a mouse model of anxiety: strain and experience effects. Neuropsychopharmacology. 1998;18:210–221. doi: 10.1016/S0893-133X(97)00131-0. [DOI] [PubMed] [Google Scholar]
- Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, Karayiorgou M. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behavior. Proc Natl Acad Sci U S A. 1998;95:9991–9996. doi: 10.1073/pnas.95.17.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greba Q, Gifkins A, Kokkinidis L. Inhibition of amygdaloid dopamine D2 receptors impairs emotional learning measured with fear-potentiated startle. Brain Res. 2001;899:218–226. doi: 10.1016/s0006-8993(01)02243-0. [DOI] [PubMed] [Google Scholar]
- Guarraci FA, Frohardt RJ, Falls WA, Kapp BS. The effects of intra-amygdaloid infusions of a D2 dopamine receptor antagonist on Pavlovian fear conditioning. Behav Neurosci. 2000;114:647–651. doi: 10.1037//0735-7044.114.3.647. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall RC, Popkin MK, Beresford TP, Hall AK. Amphetamine psychosis: clinical presentations and differential diagnosis. Psychiatr Med. 1988;6:73–79. [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11:43–48. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Hranilovic D, Bucan M, Wang Y. Emotional response in dopamine D2L receptor-deficient mice. Behav Brain Res. 2008;195:246–250. doi: 10.1016/j.bbr.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang KL, Livesley WJ, Vernon PA. Heritability of the big five personality dimensions and their facets: a twin study. J Pers. 1996;64:577–591. doi: 10.1111/j.1467-6494.1996.tb00522.x. [DOI] [PubMed] [Google Scholar]
- Jang KL, McCrae RR, Angleitner A, Riemann R, Livesley WJ. Heritability of facet-level traits in a cross-cultural twin sample: support for a hierarchical model of personality. J Pers Soc Psychol. 1998;74:1556–1565. doi: 10.1037//0022-3514.74.6.1556. [DOI] [PubMed] [Google Scholar]
- LeDoux J. Fear and the brain: where have we been, and where are we going? Biol Psychiatry. 1998;44:1229–1238. doi: 10.1016/s0006-3223(98)00282-0. [DOI] [PubMed] [Google Scholar]
- Lesch KP. Alcohol dependence and gene x environment interaction in emotion regulation: Is serotonin the link? Eur J Pharmacol. 2005;526:113–124. doi: 10.1016/j.ejphar.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Levey AI, Hersch SM, Rye DB, Sunahara RK, Niznik HB, Kitt CA, Price DL, Maggio R, Brann MR, Ciliax BJ. Localization of D1 and D2 dopamine receptors in brain with subtype-specific antibodies. Proc Natl Acad Sci U S A. 1993;90:8861–8865. doi: 10.1073/pnas.90.19.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrae RR, Costa PT., Jr Validation of the five-factor model of personality across instruments and observers. J Pers Soc Psychol. 1987;52:81–90. doi: 10.1037//0022-3514.52.1.81. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A. Neural connectivity as an intermediate phenotype: brain networks under genetic control. Hum Brain Mapp. 2009;30:1938–1946. doi: 10.1002/hbm.20639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Buckholtz JW, Kolachana B, Hariri A, Pezawas L, Blasi G, Wabnitz A, Honea R, Verchinski B, Callicott JH, Egan M, Mattay V, Weinberger DR. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Natl Acad Sci U S A. 2006;103:6269–6274. doi: 10.1073/pnas.0511311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montag C, Reuter M, Newport B, Elger C, Weber B. The BDNF Val66Met polymorphism affects amygdala activity in response to emotional stimuli: evidence from a genetic imaging study. Neuroimage. 2008;42:1554–1559. doi: 10.1016/j.neuroimage.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD, Gross JJ. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004a;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover G, Mackey SC. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. J Cogn Neurosci. 2004b;16:1746–1772. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Papp AC, Pinsonneault JK, Cooke G, Sadée W. Single nucleotide polymorphism genotyping using allele-specific PCR and fluorescence melting curves. Biotechniques. 2003;34:1068–1072. doi: 10.2144/03345dd03. [DOI] [PubMed] [Google Scholar]
- Pezze MA, Feldon J. Mesolimbic dopaminergic pathways in fear conditioning. Prog Neurobiol. 2004;74:301–320. doi: 10.1016/j.pneurobio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biol Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Prata DP, Mechelli A, Fu CH, Picchioni M, Kane F, Kalidindi S, McDonald C, Howes O, Kravariti E, Demjaha A, Toulopoulou T, Diforti M, Murray RM, Collier DA, McGuire PK. Opposite effects of catechol-O-methyltransferase Val158Met on cortical function in healthy subjects and patients with schizophrenia. Biol Psychiatry. 2009;65:473–480. doi: 10.1016/j.biopsych.2008.09.027. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Dopamine attenuates prefrontal cortical suppression of sensory inputs to the basolateral amygdala of rats. J Neurosci. 2001;21:4090–4103. doi: 10.1523/JNEUROSCI.21-11-04090.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino V, Blasi G, Latorre V, Fazio L, d'Errico I, Mazzola V, Caforio G, Nardini M, Popolizio T, Hariri A, Arciero G, Bertolino A. Activity in medial prefrontal cortex during cognitive evaluation of threatening stimuli as a function of personality style. Brain Res Bull. 2007;74:250–257. doi: 10.1016/j.brainresbull.2007.06.019. [DOI] [PubMed] [Google Scholar]
- Salgado-Pineda P, Delaveau P, Blin O, Nieoullon A. Dopaminergic contribution to the regulation of emotional perception. Clin Neuropharmacol. 2005;28:228–237. doi: 10.1097/01.wnf.0000185824.57690.f0. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol. 2004;74:1–58. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Sjöberg RL, Ducci F, Barr CS, Newman TK, Dell'osso L, Virkkunen M, Goldman D. A non-additive interaction of a functional MAO-A VNTR and testosterone predicts antisocial behavior. Neuropsychopharmacology. 2008;33:425–430. doi: 10.1038/sj.npp.1301417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolka MN, Schumann G, Wrase J, Grüsser SM, Flor H, Mann K, Braus DF, Goldman D, Büchel C, Heinz A. Catechol-O-methyltransferase val158met genotype affects processing of emotional stimuli in the amygdala and prefrontal cortex. J Neurosci. 2005;25:836–842. doi: 10.1523/JNEUROSCI.1792-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, Meyer-Lindenberg A. A validated network of effective amygdala connectivity. Neuroimage. 2007;36:736–745. doi: 10.1016/j.neuroimage.2007.03.022. [DOI] [PubMed] [Google Scholar]
- Stein MB, Fallin MD, Schork NJ, Gelernter J. COMT polymorphisms and anxiety-related personality traits. Neuropsychopharmacology. 2005;30:2092–2102. doi: 10.1038/sj.npp.1300787. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson C. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usiello A, Baik JH, Rougé-Pont F, Picetti R, Dierich A, LeMeur M, Piazza PV, Borrelli E. Distinct functions of the two isoforms of dopamine D2 receptors. Nature. 2000;408:199–203. doi: 10.1038/35041572. [DOI] [PubMed] [Google Scholar]
- van Gaalen MM, van Koten R, Schoffelmeer AN, Vanderschuren LJ. Critical involvement of dopaminergic neurotransmission in impulsive decision making. Biol Psychiatry. 2006;60:66–73. doi: 10.1016/j.biopsych.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Williams LM, Das P, Liddell BJ, Kemp AH, Rennie CJ, Gordon E. Mode of functional connectivity in amygdala pathways dissociates level of awareness for signals of fear. J Neurosci. 2006;26:9264–9271. doi: 10.1523/JNEUROSCI.1016-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo JM, Yoon KS, Yu BH. Catechol O-methyltransferase genetic polymorphism in panic disorder. Am J Psychiatry. 2002;159:1785–1787. doi: 10.1176/appi.ajp.159.10.1785. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Bertolino A, Fazio L, Blasi G, Rampino A, Romano R, Lee ML, Xiao T, Papp A, Wang D, Sadée W. Polymorphisms in human dopamine D2 receptor gene affect gene expression, splicing, and neuronal activity during working memory. Proc Natl Acad Sci U S A. 2007;104:20552–20557. doi: 10.1073/pnas.0707106104. [DOI] [PMC free article] [PubMed] [Google Scholar]