Abstract
Methamphetamine (MA) use is associated with activation of microglia and, at high doses, can induce neurotoxicity. Given the changes in the neuroinflammatory environment associated with MA, we investigated whether MA sensitization, a model of stimulant psychosis and an indicator of drug addiction, would interfere with the thermoregulatory and neuroinflammatory response to a subsequent peripheral immune stimulus. C57BL6/J mice were given either 1mg/kg MA or saline i.p. once a day for 5 days to produce behavioral sensitization. Seventy-two hours following the last MA injection, 100μg/kg LPS or saline was co-administered with 1mg/kg MA or saline and blood and brains were collected. Here we report that while co-administration of LPS and MA did not affect the LPS-induced increase in central cytokine mRNA, mice sensitized to MA showed an attenuated central response to LPS. Interestingly, the peripheral response to LPS was not affected by MA sensitization. Plasma cytokines increased similarly in all groups after LPS. Further, c-Fos expression in the nucleus of the solitary tract did not differ between groups, suggesting that the periphery-to-brain immune signal is intact in MA-sensitized mice and that the deficit lies in the central cytokine compartment. We also show that MA sensitization decreased LPS- or acute MA-induced microglial Iba1 expression compared to non-sensitized mice. Taken together, these data show that MA sensitization interferes with the normal central immune response, preventing the CNS from efficiently responding to signals from the peripheral immune system.
Keywords: methamphetamine, neuroinflammation, fever, cytokine, mice, microglia
Introduction
Methamphetamine (MA) is one of the most commonly abused drugs in the U.S. Approximately 10.4 million Americans 12 years of age or older have used MA for non-medical reasons at least once in their lifetimes (NSDUH, 2005). MA is a functional dopamine agonist (Chang et al., 2004) and its effects may last for hours, adding to its potent addictive qualities. Repeated administration of MA in animals produces a phenotype that is characterized by behavioral sensitization to the locomotor enhancing effects of the drug and has been extensively used as a model of stimulant psychosis (Ujike, 2002) and as an indicator of drug addiction (Robinson and Berridge, 1993). This behavioral sensitization is long-lasting and can be reinstated after chronic administration has ceased. It represents a convergence of complex, long-term molecular changes and alterations in gene expression in the addicted brain (Nestler and Malenka, 2004).
There are several reports demonstrating that chronic pre-treatment with low-dose MA, a schedule that can produce sensitization, attenuates the neurotoxic effects of a subsequent, high-dose MA challenge (Cadet et al., 2009; Danaceau et al., 2007; Graham et al., 2008; Johnson-Davis et al., 2003; Stephans and Yamamoto, 1996), however little is known concerning the neuroinflammatory consequences of MA use and subsequent peripheral immune stimulation. In addition to its effects on the monoaminergic system, MA use is associated with cognitive deficits (Dellu et al., 2000) and long-term MA users show prominent microglial activation in certain brain regions that are sometimes apparent almost 2 years after MA use had ceased (Sekine et al., 2008). MA-induced microglial activation has also been shown in animals given neurotoxic doses of MA (Fantegrossi et al., 2008; Hozumi et al., 2008; Thomas and Kuhn, 2005) and there is evidence that both high and low doses of MA can induce inflammatory cytokines such as tumor necrosis factor α (TNFα) and interleukin-1β (IL-1β) in the brains of rodents (Flora et al., 2003; Halladay et al., 2003; Nakajima et al., 2004; Numachi et al., 2007). Acute and chronic MA administration have been shown to reduce the numbers of leukocytes as well as the NK cell activity of splenic lymphocytes (Saito et al., 2008). Chronic MA administration reduced Con A-induced T-cell proliferation as well as IL-2 and IFNγ production in mouse splenocytes (Yu et al., 2002). MA has also been shown to decrease IL-1 production by splenocytes in mice (In et al., 2005) and reduced IFNγ and IL-10 while increasing IL-4, MCP-1 and TNFα in plasma (Hozumi et al., 2008). Taken together, these data suggest that MA alters the production of both inflammatory and anti-inflammatory cytokines in the periphery while inducing a heightened inflammatory environment in the brain. This MA-induced increase in brain inflammation has the potential to influence the body's response to a subsequent immune stimulus. Indeed, Flora et al. (2003) reported that co-administration of MA and the HIV-1 protein tat induced a synergistic increase in TNFα and IL-1β in the CNS of mice.
Infection in the periphery results in the production of inflammatory cytokines which, through neural and humoral communication pathways, induce glial cells in discrete brain regions to produce the same inflammatory cytokines (Maier et al., 1998; Rivest, 2003). It is this central production of inflammatory cytokines that is responsible for inducing sickness behavior including fever, anorexia, as well as reduced locomotor and social behaviors. Excessive production of inflammatory cytokines has been shown to produce severe behavioral deficits and promote neurotoxicity (Block and Hong, 2005). Thus, circumstances that augment inflammatory cytokine production by microglial cells, such as MA use, are likely to lead to pronounced and prolonged behavioral deficits that are counterproductive to wellness. Because MA abuse may activate or sensitize microglial cells, we hypothesized that MA would exacerbate the neuroinflammatory response to a secondary stimulus, namely lipopolysaccharide (LPS). Here we report that mice sensitized to MA show a blunted febrile response that is paralleled by an attenuation of inflammatory cytokine mRNA expression in the brain when the immune system was peripherally activated by LPS.
Materials and Methods
Animals and Surgery
Adult (3-5 mo) male C57BL/6 mice from The Jackson Laboratories were used. Mice were housed in polypropylene cages and maintained at 23°C under a diurnal 12h light-dark cycle (lights on at 0700) with ad libitum access to water and rodent chow. To allow for continuous monitoring of body temperature (Tb) and locomotor activity, mice were anesthetized with an intraperitoneal (i.p.) injection of ketamine and xylazine (100 and 10mg, respectively) and implanted with a biotelemetry device (E-mitter, Mini Mitter, Bend, OR). Briefly, a midline abdominal incision was made 1cm below the diaphragm and the E-mitter was positioned in the abdominal cavity along the sagittal plane. The muscle wall was sutured with chromic gut and the skin sutured with silk surgical thread. Following surgery, mice were individually housed and each cage was placed on a receiver board (model ER-4000 Receiver, Mini Mitter). Data was collected every 5 min utilizing the Vital View data acquisition system (Mini Mitter Co., Bend, OR). Tb and locomotor activity were monitored from the time of implantation until the end of the experiment. Mice were allowed at least 7 days to recover from surgery before any experimental procedures took place. All procedures were in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the University of Illinois Institutional Animal Care and Use Committee.
Drugs
MA [(+)-Methamphetamine hydrochloride (Sigma, Cat # M-8750)] was dissolved in sterile saline and injected i.p. at a volume of 0.1ml/g body weight, equivalent to a dose of 1mg MA·HCl/kg body weight. Escherichia coli LPS (serotype 0127:B8, Sigma) was dissolved in sterile saline and injected i.p. at a volume of 0.01 ml/g body weight, or 100μg/kg body weight, a dose that reliably causes fever in mice. Control mice received an equivalent volume of saline.
Experimental Design
Behavioral sensitization
Behavioral sensitization is characterized by an enhanced locomotor response to a drug after chronic administration has ceased. To induce this, mice were subjected to a schedule adapted from (Itzhak, 1997; Maeda et al., 2006). Mice received 0 or 1mg/kg MA i.p. once per day for 5d. Seventy-two hours after the last administration, mice were challenged with 0 or 1mg/kg MA to test for sensitization.
MA sensitization and LPS
To investigate if MA administration affects the inflammatory response to peripheral immune activation, 72h after the last MA injection, non-sensitized and sensitized mice received i.p. injections of either MA (1mg/kg) and LPS (100μg/kg), MA and saline, saline and LPS, or saline and saline and Tb and locomotor activity were monitored for the next 24h. Mice were then killed by CO2 asphyxiation and blood and brains were collected for cytokine measurement. To measure cytokines at a time point when mice were actively sick, a separate group of mice not implanted with E-mitters was subjected to the same experimental protocol and killed at 4h post-LPS.
Cytokine mRNA measurement by quantitative real-time PCR
Total hypothalamic RNA was isolated using the Arcturus PicoPure™ RNA isolation kit as described by the manufacturer. DNase treatment was performed on a PicoPure column with a Qiagen RNase-free DNase set (Qiagen, Valenica, CA). RNA from the striatum and hippocampus was isolated using the Tri Reagent protocol (Sigma, St. Louis, MO). A QunatiTect Reverse Transcription Kit (Qiagen, Valencia, CA) was used for cDNA synthesis with integrated removal of genomic DNA contamination according to the manufacturer's protocol and previously described (Krzyszton et al., 2008). Quantitative real time PCR was performed using the Applied Biosystems (Foster, CA) Assay-on Demand Gene Expression protocol as previously described (Krzyszton et al.). In brief, cDNA was amplified by PCR where a target cDNA (IL-6, Mm00446190_m1; IL-1β, Mm00434228_m1; TNFα, Mm00443258_m1; MHC II antigen E, Mm00439221_m1; and IL-10, Mm00439616_m1) and a reference cDNA (glucose-3 phosphate dehydrogenase, Mm99999915_g1) were amplified simultaneously using an oligonucleotide probe with a 5′ fluorescent reporter dye (6-FAM) and a 3′ quencher dye (NFQ). PCR reactions were performed in triplicate under the following conditions: 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. Fluorescence was determined on an ABI PRISM 7900HT-sequence detection system (Perkin Elmer, Forest City, CA). Data were analyzed using the comparative threshold cycle (Ct) method, and results are expressed as fold difference.
Plasma Cytokines
Plasma samples were assayed for IL-1β, TNFα, IL-6, and IL-10 using a multiplex bead-based immunoassay kit combined with a Cytokine Reagent kit as described by the manufacturer (Bio-Rad, Hercules, CA). The multiplex assay was sensitive to <3 pg/ml for IL-1β, TNFα, IL-10, and IL-6. The inter-assay and intra-assay coefficients of variation were <8%.
Immunohistochemical staining and quantification
A separate group of mice was subjected to the MA sensitization regimen and then given either 100μg/kg of LPS or saline, or 1mg/kg MA or saline. Four hours after i.p. injection, mice were killed by CO2 asphyxiation and transcardially perfused with heparinized saline followed by 4% paraformaldehyde, and brains were removed. Brains were blocked in 3 parts (rostral, mid and caudal), postfixed over 2d in 4% paraformaldehyde, and then paraffin embedded. Coronal sections (4μm) were cut on a microtome and every 10th section was stained for Iba1 (ionized calcium binding adapter molecule 1; Wako Chemicals USA, Richmond, VA) at the level of the striatum (Bregma -0.82 mm) and at the hippocampus (Bregma -2.18 mm), or for c-Fos [c-Fos (4) sc-52; Santa Cruz Biotechnology, Santa Cruz, CA] at the level of the NTS (Bregma -7.48 mm). Sections were dewaxed and rehydrated through xylene and alcohols and were incubated in citrate buffer, pH 6, and microwaved for 10 min. Endogenous peroxidase was eliminated by incubating sections in 3% H2O2/methanol for 15 min. For microglia Iba1 staining, sections were washed in PBS and blocked with 5% normal goat serum in PBS before overnight incubation at 4°C with the primary antibody at 1:1600 dilution in 5% blocking serum. For c-Fos, sections were blocked with 20% normal goat serum before overnight incubation at 4°C with the primary antibody at 1:800 dilution in 5% blocking serum. For both antibodies, the sections were washed, and then incubated with biotinylated goat anti-rabbit antibody (Vector Laboratories, Burlingame, CA) for 1h. Staining was visualized using the ABC method and 3,3′-diaminobenzidine or nickel-enhanced 3,3′-diaminobenzidine as chromagen. Sections stained for Iba1 were counterstained with hemotoxylin. Isotype-matched IgG was used as negative control. Immunostaining was visualized using an Optronix Microfire camera (model S99808, Goleta, CA) attached to a Zeiss Axio Imager A.1 microscope (Gottingen, Germany). The numbers of Iba1-positive and c-Fos-positive cells were counted manually by two blind observers. The number of positive cells was determined in both hemispheres of a given section using a total of 4 sections per mouse and expressed as number of cells per 100 μm2.
Data Collection and Analysis
Tb and locomotor activity data were collected every 5 min and averaged over 30 minutes. Change in Tb and locomotor activity following injection was calculated as the 30-min average at each time point minus the 30-min average at time 0 (the time of injection). Data from the sensitization regimen were subjected to repeated-measures ANOVA in which Day was a within-subjects measure (i.e., repeated measure), and MA (MA or Saline) was a between subjects measure. Tb and locomotor activity data from LPS experiments were subjected to repeated-measures ANOVA in which Time was a within-subjects measure and Sensitization (Sensitized or Non-sensitized), MA (MA or Saline) and LPS (LPS or Saline) were between-subjects measures. Central cytokine mRNA levels and peripheral cytokines were analyzed using three-way ANOVA (Sensitization × MA × LPS). When ANOVAs revealed a significant effect of main factors or main factor interactions, differences in treatment group means were tested using Fisher's least-significant differences. All data are presented as means ± SEM.
Results
Behavioral sensitization
Repeated administration of MA in animals produces a phenotype that is characterized by behavioral sensitization to the locomotor enhancing effects of the drug and represents a convergence of complex, long-term molecular changes and alterations in gene expression in the addicted brain. Figures 1A and 1B show that repeated administration of MA caused a decrease in both body weight [F(1,37) = 14.24, p<0.001] and food intake [F(1,37) = 8.067, p<0.05] over the course of 5 days. Figure 1C shows the effect of 5 days of MA administration on locomotor activity. Here, there was a MA × Day interaction [F (5,125) = 4.655, p<0.0001], where 1mg/kg MA significantly increased locomotor activity compared to saline, and each subsequent MA injection resulted in greater activity than the injection the previous day. To test if sensitization had occurred, 1mg/kg MA was re-administered 72h later (i.e. day 8). This dose of MA produced enhanced locomotor activity compared to day 1 [F (1,25) = 9.028, p<0.01], signifying that MA sensitization had occurred.
Fig. 1. Behavioral sensitization to methamphetamine.
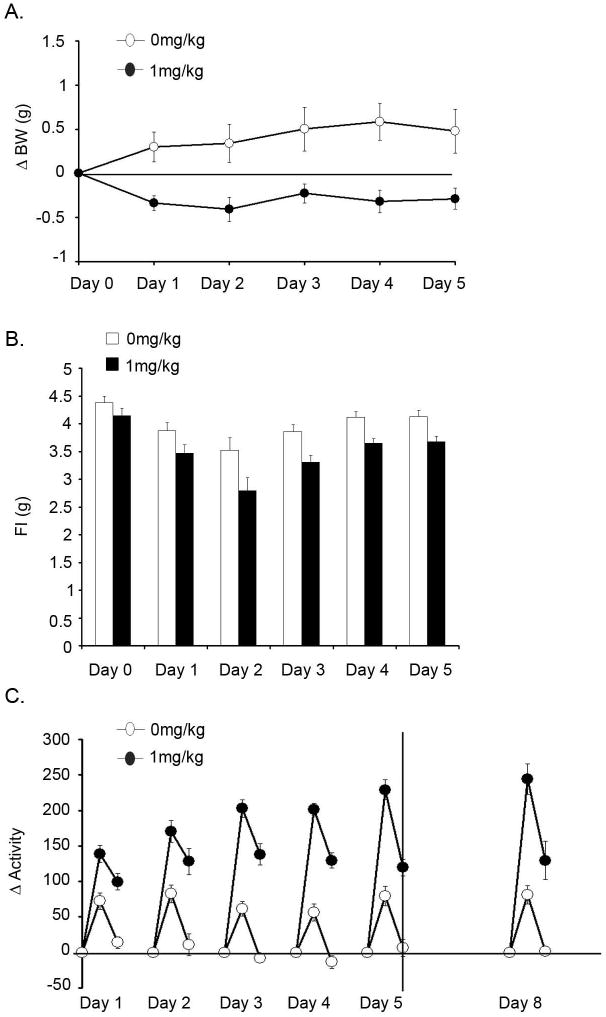
Effect of 5 days of MA administration on body weight (A), food intake (B) and locomotor activity (C) over 5 days of MA administration followed by behavioral sensitization test 72h later. Open bars or symbols represent saline controls and closed symbols represent MA administration (n=10-12 per group). Activity data is presented as 30 minute averages from time of injection to 1h post-injection.
Methamphetamine sensitization attenuates the febrile but not locomotor response to LPS
We next investigated if MA administration would interfere with the response to a peripheral immune stimulus (LPS, 100μg/kg). Non-sensitized and sensitized mice received i.p. injections of either MA (1mg/kg) and LPS (100μg/kg), MA and saline, saline and LPS, or saline and saline and body weight, Tb and locomotor activity were monitored over the next 24h. Figure 2A shows the effects of LPS on body weight in mice sensitized to MA. There was a Sensitization × LPS interaction whereby mice sensitized to MA lost less body weight than did non-sensitized mice [F (1,48) = 4.175, p<0.05]. Initially we thought that this interaction might be due to the loss in BW occurring during MA sensitization however, the starting BW between the two groups (Non-sensitized vs Sensitized) was not appreciably different (27.6±0.28 and 27.45±0.24, respectively).
Fig. 2. Locomotor activity and body temperature response to LPS in mice sensitized to methamphetamine.
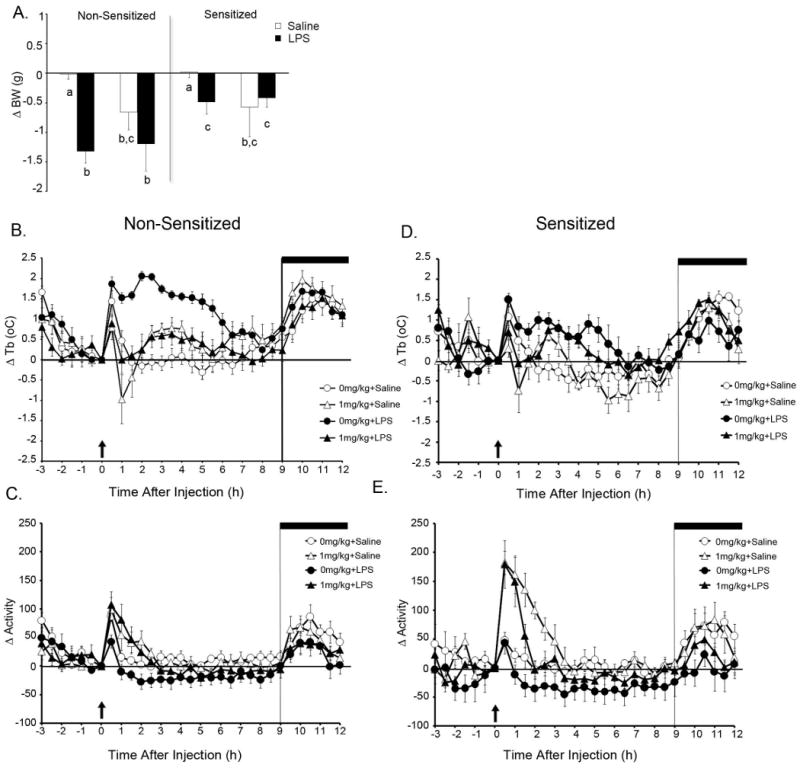
Change in body weight (A) and Tb and locomotor activity in non-sensitized (B and C) and sensitized (D and E) mice after LPS and/or MA. Mice were subjected to a 5d regimen of 0 or 1mg/kg MA per day and then injected with LPS and/or MA 72h later (n=5-10 per group). Open circles represent saline administration, open triangles respresent MA administration, closed circles represent LPS administration and closed triangles represent mice co-administered MA and LPS. Arrow = injection, dark bar = lights off. For Tb there were significant main effects of Sensitization and LPS as well as a significant Sensitization × LPS interaction.
Figures 2B-E show changes in Tb and locomotor behavior after LPS and/or MA in mice sensitized to MA. For the sake of clarity, data from non-sensitized and sensitized animals are shown in separate panels [Fig 2B-C and 2D-E, respectively]. For Tb, there were main effects of Sensitization [F (1,48) = 11.061, p<0.01] and LPS [F (1,48) = 16.688, p<0.001]. MA administration, regardless of sensitization, induced a decrease in Tb 1h after administration, followed by a rise in Tb that peaked 2-3h post-injection. As evidenced by the large error bars, this hypothermic response was fairly varied, with 5 out of 10 mice given MA showing a drop in Tb ranging from -0.5 to -1.5°C. There was also a MA × LPS interaction [F (1,48) = 5.942, p<0.02] where mice given MA, regardless of sensitization, had significantly lower fevers than did mice receiving LPS alone. This difference in the LPS-induced rise in Tb was not due to a disparity in baseline Tb between sensitized (36.2 ± 0.1°C) and non-sensitized (36.0 ± 0.1°C) mice.
As expected, for locomotor activity there was a main effect of MA [F (1,48) = 8.804, p<0.01] where mice given MA showed increased activity compared to those given saline. There was also a main effect of LPS [F (1,48) = 9.004, p<0.01] where LPS decreased locomotor activity regardless of MA administration.
Methamphetamine sensitization attenuates the neuroinflammatory response to LPS
To examine the neuroinflammatory response to LPS in MA sensitized mice, non-sensitized and sensitized mice were killed and blood and brains were collected at 4h post LPS and/or MA administration. We were particularly interested in the hypothalamus and hippocampus because they are intimately involved in the sickness response, and the striatum because it is sensitive to the effects of MA. As expected, LPS administration increased IL-1β, IL-6 and TNFα in all 3 brain regions. Figure 3 shows the fold change in IL-1β, IL-6, TNFα and IL-10 mRNA in 3 brain regions 4h after LPS. In all 3 brain regions, co-administration of MA and LPS did not affect the LPS-induced increase in inflammatory cytokines in non-sensitized mice. Indeed, with the exception of the hypothalamus, MA administration alone did not induce any increase in cytokine mRNA 4h after injection. However, sensitization significantly influenced the LPS-induced increase in cytokine mRNA. For IL-1β mRNA (Fig 3A, E, and H), there was a Sensitization × LPS interaction whereby MA sensitization attenuated the LPS-induced increase in the hypothalamus [F (1,35) = 12.161, p<0.01], hippocampus [F (1,35) = 10.846, p<0.01] and striatum [F (1,35) = 12.856 p<0.01]. This Sensitization × LPS interaction was also observed for IL-6 mRNA in the hypothalamus [F (1,35) = 15.710, p<0.0001], hippocampus [F (1,35) = 10.270, p<0.01] and striatum [F (1,35) = 4.121, p=0.05], and for TNFα mRNA in the hypothalamus [F (1,35) = 7.560, p<0.01] and striatum [F (1,35) 14.269, p<0.0001].
Fig. 3. Cytokine mRNA expression 4h after LPS and/or MA in mice sensitized to methamphetamine.
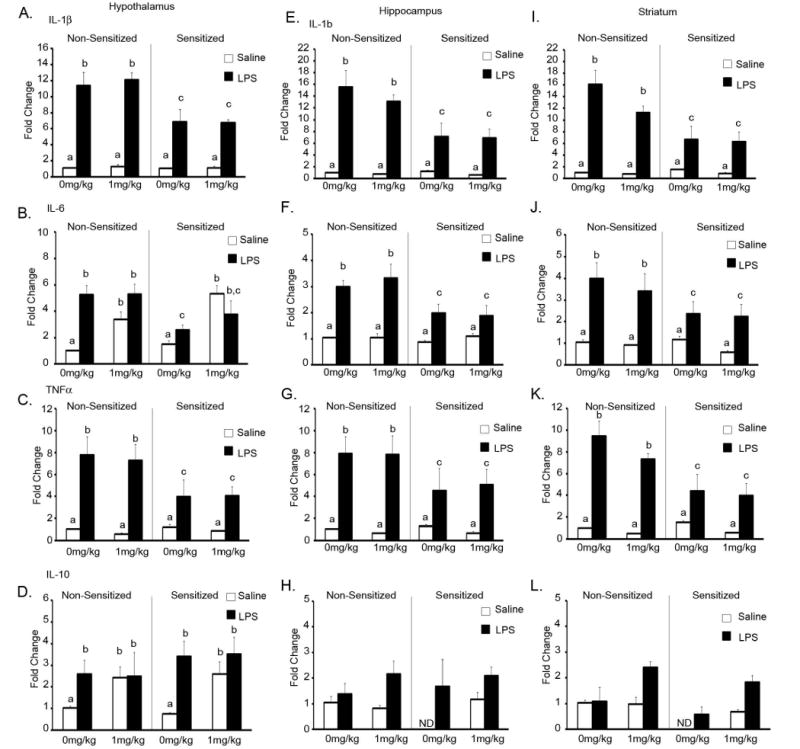
Cytokine mRNA expression in mice sensitized to MA 4h after LPS administration in the hypothalamus (A-D), hippocampus (E-H), and striatum (I-L). Non-sensitized and sensitized mice were given LPS and/or MA and brains were collected 4h post-LPS (n=5-6 per group). Means with different letters are at least p<0.05 different from each other. ND=not detectable.
In the hypothalamus MA increased IL-6 in both non-sensitized and sensitized animals (p<0.0001). There was also a MA × LPS interaction whereby MA alone increased IL-6 mRNA to the same extent as LPS alone or LPS + MA combined [F (1,35) = 9.650, p<0.01]. IL-10 mRNA was either undetectable or detected at very low levels in the hippocampus and striatum 4h after injection in sensitized mice given saline. It was however, detected in all other groups, suggesting that sensitization decreased IL-10 mRNA levels to below the detection level of the assay. In the hypothalamus LPS induced an increase in IL-10 mRNA regardless of MA sensitization (Fig 3A, p<0.04). There was also a MA × LPS interaction [F (1,33) = 7.507, p<0.01] whereby MA administration, regardless of sensitization, increased IL-10 mRNA to the same extent as LPS alone or LPS + MA combined.
Methamphetamine sensitization does not influence the plasma cytokine response to LPS
To investigate the peripheral cytokine response to LPS in MA sensitized mice, blood was collected 4h post LPS and/or MA. Table 1 shows values for cytokine levels 4h after LPS. MA alone increased plasma levels of IL-1β [F (1,34) = 11.346, p<0.01] in both sensitized and non-sensitized mice. For this cytokine, there was also a MA × LPS interaction [F (1,34) = 4.925, p<0.04] whereby MA increased plasma levels of IL-1β to the same extent as LPS + MA or LPS alone. However, unlike the inflammatory response in the brain, sensitization had no effect on magnitude of the LPS-induced increase in any of the cytokine levels. At 4h post LPS, IL-1β [F (1,34) = 5.289, p<0.03], IL-6 [F (1,35) = 27.207, p<0.001] and IL-10 [F (1,35) = 4.829, p<0.03] were all increased compared to saline controls. These data suggest that while MA alone increases IL-1β 4h after administration, the peripheral cytokine response to LPS is unaffected by MA sensitization.
Table 1.
Plasma cytokines 4h after LPS in mice sensitized to MA.
| Non-Sensitized | Sensitized | |||||||
|---|---|---|---|---|---|---|---|---|
| 0mg/kg MA | 1mg/kg MA | 0mg/kg MA | 1mg/kg MA | |||||
| Saline | LPS | Saline | LPS | Saline | LPS | Saline | LPS | |
| IL-1β | 63.86±31.57 | 196.34±27.81** | 226.59±25.8** | 156.21±34.26** | 77.10±20.51 | 164.34±26.77* | 176.10±47.51** | 260.41±42.61* |
| IL-6 | 34.32±12.34 | 168.05±30.15** | 11.86±1.64 | 81.54±36.59** | 10.40±2.97 | 120.417±35.37* | 9.318±3 | 95.8±63.37* |
| TNFα | 277.92±123.93 | 580.34±247.4 | 272.54±12.8 | 211.52±29.51 | 190.32±71.12 | 554.74±242.71 | 234.03±65.14 | 337.93±11.53 |
| IL-10 | 119.99±32.08 | 193.58±35.33** | 87.29±14.28 | 73.94±30.84** | 70.05±11.78 | 197.71±55.03** | 88.34±27.53 | 129.12±28.11** |
Mice were given saline or MA (1mg/kg) i.p. for 5 days to induce behavioral sensitization. Seventy-two hours after the last MA injection, mice were given either saline or LPS (100μg/kg), along with MA (1mg/kg) or saline i.p. and blood was collected at 4 hrs post-injection. LPS increased plasma cytokines at regardless of sensitization.
p<0.05,
p<0.001, different from saline controls.
Methamphetamine sensitization does not influence LPS-induced c-Fos immunoreactivity in the NTS
Peripheral inflammatory stimuli like LPS and cytokines activate vagal afferent pathways and induce neuronal responses that stimulate the production of cytokines within the brain. Neurons in the NTS are particularly responsive to peripheral LPS. Given that mice sensitized to MA showed an attenuated central response to peripheral LPS but no difference in peripheral cytokine production, we determined whether MA sensitization affected activation of the immune-responsive NTS by staining c-Fos, a widely used marker for functional activation. Because MA administration alone had no effect on the LPS-induced increase in cytokine mRNA, and the overwhelming significant finding for cytokine mRNA was that of sensitization, MA was not co-administered in this experiment. Figure 4 shows c-Fos staining in the NTS 4h after LPS in mice sensitized to MA. LPS reliably activated Fos regardless of methamphetamine sensitization [F (1,16) = 13.525, p<0.01]. These data indicate that MA sensitization does not interfere with traditional immune-to-brain communication pathways when the peripheral innate immune system is stimulated and suggest that the attenuated response to LPS in MA sensitized mice is mediated at a central, not peripheral, level.
Fig. 4. c-Fos immunoreactivity in the NTS 4h after LPS in mice sensitized to methamphetamine.
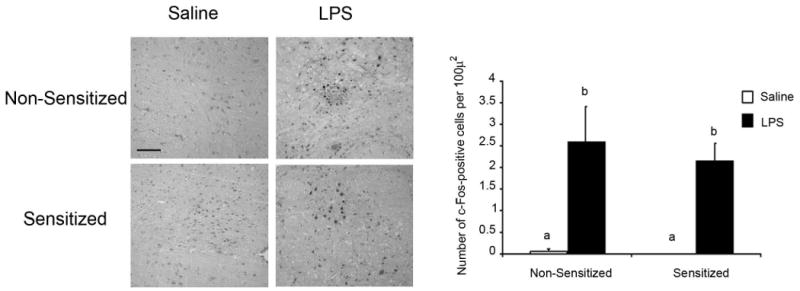
Immunohistochemical staining of c-Fos in the NTS of mice sensitized to MA prior to LPS administration at 20× magnification. Mice were subjected to a 5d regimen of 0 or 1mg/kg MA per day and then injected with either LPS or saline 72h later and brains were collected 4h after LPS administration. Pictures are representative photomicrocraphs of the NTS of a sensitized and non-sensitized mouse given LPS or saline. Scale bar = 100μm.
Methamphetamine sensitization alters microglial expression of Iba1
MA administration, at doses high enough to be considered toxic, reliably activates microglia. The dosing schedule used here was not expected to significantly activate microglia. However, given that the attenuated response to LPS in MA sensitized mice appears to be mediated centrally, we investigated if MA sensitization altered the microglial response to LPS. Ionized calcium-binding adapter molecule 1 (Iba1) is specifically expressed in microglia and expression is particularly enhanced under pathological conditions (Ito et al., 1998; Ito et al., 2001; Qin et al., 2007; Sandhir et al., 2008). Figures 5A and 5B show Iba1 staining in the dentate gyrus of the hippocampus and the striatum 4h after LPS administration. In the dentate gyrus there were main effects for both Sensitization [F (1,15) = 6.360, p<0.03] and LPS [F (1,15) = 8.729, p<0.01]. In the striatum there was a Sensitization × LPS interaction [F (1,14) = 4.718, p<0.05]. In both regions, expression of Iba1 4h after LPS appeared to be diminished in mice sensitized to MA compared to non-sensitized mice.
Fig. 5. Iba1 expression 4h after LPS in mice sensitized to methamphetamine.
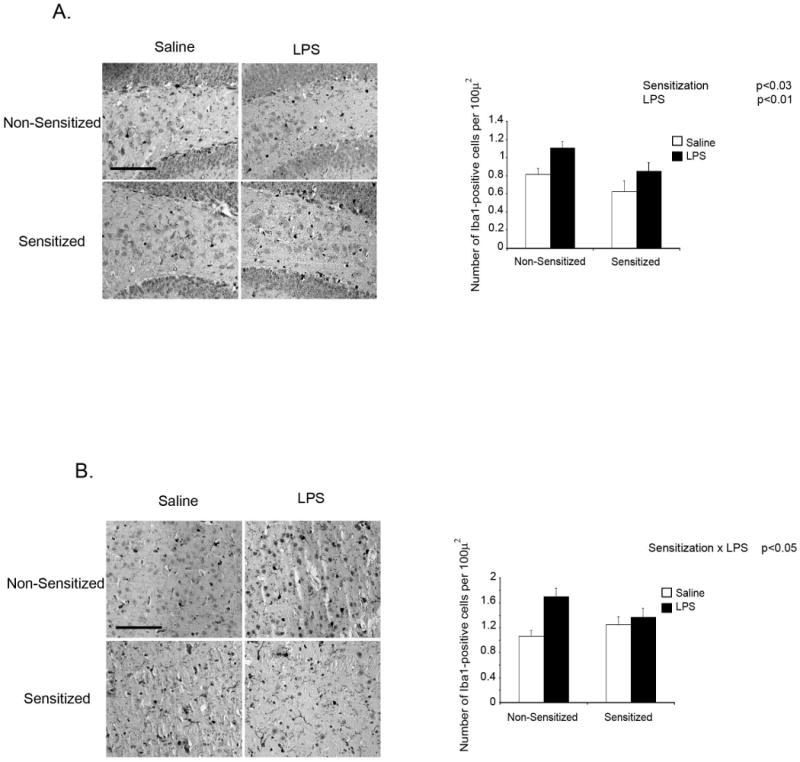
Immunohistochemical staining of Iba1 in the dentate gyrus of the hippocampus (A) and the striatum (B) of mice sensitized to MA prior to LPS administration at 40× magnification. Mice were subjected to a 5d regimen of 0 or 1mg/kg MA per day and then given either LPS or saline 72h later. Brains were collected 4h after LPS administration. Scale bar = 100 μm.
While high doses of MA have been shown to activate microglia, it has also been shown that repeated administration of a lower dose of MA, one that induces only minimal microglial activation, can induce tolerance whereby a subsequent, high-dose MA challenge will not activate microglia above control levels (Thomas and Kuhn, 2005). It is not known whether the MA schedule used here could influence the microglia response to a low-, rather than high-dose MA challenge. We therefore measured Iba1 expression in non-sensitized and sensitized mice challenged with 1mg/kg MA 72h after the last MA administration. Fig 6 shows Iba1 expression 4h after MA in non-sensitized and sensitized mice. There was a Sensitization × MA interaction in both the hippocampus [F (1,15) = 14.930, p<0.01] and striatum [F (1,15) = 9.331, p<0.01] whereby non-sensitized mice showed increased Iba1 expression 4h after an acute injection of MA compared to sensitized mice. A similar interaction was observed in the hippocampus for MHCII mRNA [F (1,16) = 19.057, p<0.001] (data not shown). Taken together, these data suggest that sensitization diminished the microglial response to a subsequent, low-dose MA challenge.
Fig. 6. Iba1 expression 4h after methamphetamine administration in mice sensitized to methamphetamine.
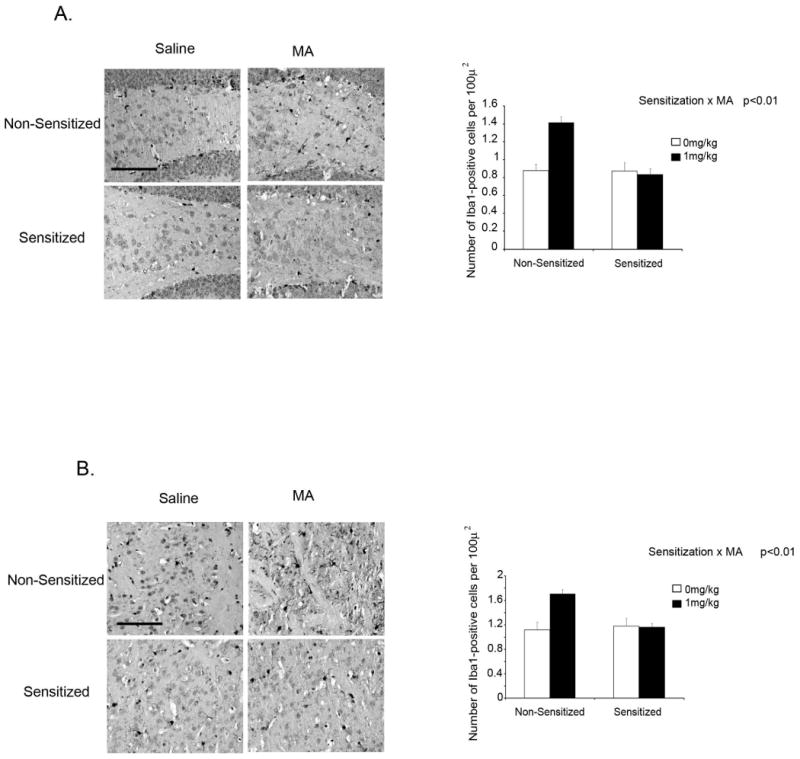
Immunohistochemical staining of Iba1 in the dentate gyrus of the hippocampus (A) and the striatum (B) of mice sensitized to MA prior to MA administration at 40× magnification. Mice were subjected to a 5d regimen of 0 or 1mg/kg MA per day and then given either 1mg/kg MA or saline 72h later. Brains were collected 4h after MA administration. Scale bar = 100 μm.
Discussion
MA is a popular drug of abuse that has several central effects and prolonged, or heavy usage can activate the primary inflammatory cells of the brain; microglia. Because MA has been shown to heighten the inflammatory environment of the brain and has also been shown to have a synergistic effect when administered concomitantly with an immune stimulus such as the HIV-1 Tat protein (Flora et al., 2003), we hypothesized that MA administration would exacerbate LPS-induced sickness behavior as well as the neuroinflammatory cytokine response. However, here we demonstrate that, while co-administration of MA and LPS did not affect the LPS-induce increase in neuroinflammation in mice not sensitized to MA, mice sensitized to MA lose less weight, generate lower fevers, and show attenuated expression of central inflammatory cytokine mRNA in response to LPS than do mice not sensitized to MA. In short, MA sensitization appears to attenuate the sickness response to LPS.
As noted, sensitized mice given LPS showed a blunted fever that was paralleled by an attenuated central cytokine response seen 4h post injection. However, non-sensitized mice given MA also showed a blunted Tb response to LPS, despite the increase in cytokine mRNA. Indeed, the Tb response to co-administration of MA and LPS was similar in both non-sensitized and sensitized mice. It is difficult to interpret the Tb response to co-administration of MA and LPS since MA administration alone results in such dramatic Tb and locomotor responses. It is likely that the initial response to MA significantly masked the effects of LPS on the fever response. Here we show that MA administration alone, while typically associated with hyperthermia, induced hypothermia 1h post-administration before a resultant increase in Tb 2-3h after administration. While this was somewhat surprising it is not unprecedented, especially in rodents pre-exposed to MA (Myles et al., 2008; Myles and Sabol, 2008). Possibly, this response to MA in both sensitized and non-sensitized mice interfered with the thermoregulatory processes of generating a fever. Despite this difference in the thermoregulatory response to MA+LPS, the overwhelming significant finding throughout is that of MA sensitization. Co-administration of MA and LPS did not affect the LPS-induced increase in central cytokine mRNA in non-sensitized mice however, sensitized mice showed a significant attenuation in LPS-induced cytokines, regardless of MA co-administration.
Peripheral immune stimulation activates microglia in the brain. This is accomplished by the actions of peripheral cytokines at the level of the BBB and/or activation of projections of the vagus nerve to the NTS. In the current study there was no difference in LPS-induced plasma cytokines with regard to MA sensitization, indicating that the peripheral cytokine response to LPS was not influenced by MA. Given that these high levels of circulating inflammatory cytokines were not sufficient to induce cytokine mRNA expression in the brains of MA sensitized mice to the same extent as seen in mice not sensitized to MA, it is possible that MA sensitization is somehow influencing the communication between periphery and brain, whereby the signal reaching the brain in the MA sensitized mice is diminished, resulting in the attenuated fever and cytokine response to LPS. We therefore explored the possibility that MA sensitization interfered with the vagal afferent pathway that is responsive to peripheral LPS. Neurons in the NTS receive input from vagal afferents and project to brain areas mediating sickness behavior (Rutecki, 1990) and several studies show that subdiaphragmatic vagotomy attenuates sickness behavior in response to low doses of LPS as well as peripheral administration of IL-1β (Gaykema et al., 2000; Hansen and Krueger, 1997; Romanovsky et al., 1997). Therefore, if MA sensitization blunted the vagal afferent pathway, it might explain why MA sensitized mice showed attenuated fever as well as diminished central cytokine mRNA expression after LPS. However, we show here that c-Fos immunoreactivity in the NTS was increased similarly in both MA sensitized and non-sensitized mice 4h after LPS administration, suggesting that the periphery-to-brain immune signal is intact and that the deficit lies in the central cytokine compartment.
There are several reports suggesting that pre-treatment with varying schedules of MA attenuates the neurotoxic effects of a subsequent, high-dose MA challenge. Most of these studies focus on the damage to monoaminergic systems in response to a high-dose MA challenge. For instance, non-toxic chronic MA administration protected against the toxic effects to the dopaminergic (Cadet et al., 2009; Danaceau et al., 2007; Graham et al., 2008) and serotonergic (Danaceau et al., 2007; Johnson-Davis et al., 2003; Stephans and Yamamoto, 1996) systems associated with a subsequent, high-dose MA challenge. This phenomenon has also been reported in microglia (Thomas and Kuhn, 2005). While high doses of MA have been shown to activate microglia, it has also been shown that repeated administration of a lower dose of MA, one that induces only minimal microglial activation, can induce tolerance whereby a subsequent MA administration will not activate microglia above control levels (Thomas and Kuhn, 2005). If such was the case in terms of an alternate immune challenge, the microglia of mice pre-exposed to MA may produce lesser amounts of inflammatory molecules upon subsequent immune stimulation. We therefore measured expression of Iba1, a molecule specifically expressed in microglia, to see if MA sensitization altered the microglial response to LPS and, in a separate experiment, if MA sensitization altered the microglial response to a subsequent, low-dose MA challenge. The fact that there were no differences in Iba1 expression between non-sensitized and sensitized mice suggests that any effects of the low-dose sensitization schedule on microglia is gone by 72h. Overall, MA sensitization appeared to diminish expression of Iba1 4h after LPS compared to non-sensitized mice. Further, MA alone increased Iba1 expression 4h after administration, but only in animals not exposed to the MA sensitization regimen. Taken together, these data suggest that sensitization diminished the microglial response to a subsequent, low-dose MA challenge as well as a subsequent LPS challenge, which may be indicative of lower levels of microglial activation, resulting in attenuated cytokine gene expression in the brain. This may also help explain why the LPS-induced cytokine response in non-sensitized and sensitized mice co-administered MA and LPS is so different.
While it may appear that animals sensitized to MA respond better (i.e. get less sick) to an immune stimulus than their drug-free counterparts, this is not necessarily beneficial. LPS reliably induces cytokines in both the brain and the periphery. It is this central cytokine response that is essentially responsible for the initiation of sickness behavior, which is an integrated, adaptive and protective response to illness that is considered to be necessary for recovery and survival. Indeed, early fever research has shown that preventing the febrile response, which would also be expected to attenuate the cytokine response, is detrimental to survival (Kluger, 1991). Therefore, the attenuated febrile and neuroinflammatory response to LPS in MA sensitized mice may present a very serious problem; hampering the immune response of the CNS. MA use is associated with an increased prevalence of HIV infection, as well as hepatitis and fungal infection (Gonzales et al., 2006; Halkitis et al., 2001; Miller et al., 2009; Purcell et al., 2001). Therefore, the potential that MA use can hamper the immune response also presents a serious problem in the human population since, despite the negative symptoms of sickness, the inflammatory response to an invading pathogen is necessary for survival and recovery. Sickness behavior is a highly adaptive and beneficial response to illness and the severity of the symptoms is what directs individuals to seek medical help. A dampened response to infection could present a problem in that it could delay medical treatment and consequently prolong illness and recovery. It is therefore important to determine the underlying cause of the dampened central response to a subsequent immune stimulus in animals sensitized to MA.
Acknowledgments
This research was supported by the NIH DA024443. The authors declare no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005;76:77–98. doi: 10.1016/j.pneurobio.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Cadet JL, Krasnova IN, Ladenheim B, Cai NS, McCoy MT, Atianjoh FE. Methamphetamine preconditioning: differential protective effects on monoaminergic systems in the rat brain. Neurotox Res. 2009;15:252–259. doi: 10.1007/s12640-009-9026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang WP, Koelsch G, Wong S, Downs D, Da H, Weerasena V, Gordon B, Devasamudram T, Bilcer G, Ghosh AK, Tang J. In vivo inhibition of Abeta production by memapsin 2 (beta-secretase) inhibitors. J Neurochem. 2004;89:1409–1416. doi: 10.1111/j.1471-4159.2004.02452.x. [DOI] [PubMed] [Google Scholar]
- Danaceau JP, Deering CE, Day JE, Smeal SJ, Johnson-Davis KL, Fleckenstein AE, Wilkins DG. Persistence of tolerance to methamphetamine-induced monoamine deficits. Eur J Pharmacol. 2007;559:46–54. doi: 10.1016/j.ejphar.2006.11.045. [DOI] [PubMed] [Google Scholar]
- Dellu F, Contarino A, Simon H, Koob GF, Gold LH. Genetic differences in response to novelty and spatial memory using a two-trial recognition task in mice. Neurobiol Learn Mem. 2000;73:31–48. doi: 10.1006/nlme.1999.3919. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Ciullo JR, Wakabayashi KT, De La Garza R, 2nd, Traynor JR, Woods JH. A comparison of the physiological, behavioral, neurochemical and microglial effects of methamphetamine and 3,4-methylenedioxymethamphetamine in the mouse. Neuroscience. 2008;151:533–543. doi: 10.1016/j.neuroscience.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flora G, Lee YW, Nath A, Hennig B, Maragos W, Toborek M. Methamphetamine potentiates HIV-1 Tat protein-mediated activation of redox-sensitive pathways in discrete regions of the brain. Exp Neurol. 2003;179:60–70. doi: 10.1006/exnr.2002.8048. [DOI] [PubMed] [Google Scholar]
- Gaykema RP, Goehler LE, Hansen MK, Maier SF, Watkins LR. Subdiaphragmatic vagotomy blocks interleukin-1beta-induced fever but does not reduce IL-1beta levels in the circulation. Auton Neurosci. 2000;85:72–77. doi: 10.1016/s1566-0702(00)00222-8. [DOI] [PubMed] [Google Scholar]
- Gonzales R, Marinelli-Casey P, Shoptaw S, Ang A, Rawson RA. Hepatitis C virus infection among methamphetamine-dependent individuals in outpatient treatment. J Subst Abuse Treat. 2006;31:195–202. doi: 10.1016/j.jsat.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Graham DL, Noailles PA, Cadet JL. Differential neurochemical consequences of an escalating dose-binge regimen followed by single-day multiple-dose methamphetamine challenges. J Neurochem. 2008;105:1873–1885. doi: 10.1111/j.1471-4159.2008.05269.x. [DOI] [PubMed] [Google Scholar]
- Halkitis PN, Parsons JT, Stirratt MJ. A double epidemic: crystal methamphetamine drug use in relation to HIV transmission among gay men. J Homosex. 2001;41:17–35. doi: 10.1300/J082v41n02_02. [DOI] [PubMed] [Google Scholar]
- Halladay AK, Kusnecov A, Michna L, Kita T, Hara C, Wagner GC. Relationship between methamphetamine-induced dopamine release, hyperthermia, self-injurious behaviour and long term dopamine depletion in BALB/c and C57BL/6 mice. Pharmacol Toxicol. 2003;93:33–41. doi: 10.1034/j.1600-0773.2003.930105.x. [DOI] [PubMed] [Google Scholar]
- Hansen MK, Krueger JM. Subdiaphragmatic vagotomy blocks the sleep- and fever-promoting effects of interleukin-1beta. Am J Physiol. 1997;273:R1246–1253. doi: 10.1152/ajpregu.1997.273.4.R1246. [DOI] [PubMed] [Google Scholar]
- Hozumi H, Asanuma M, Miyazaki I, Fukuoka S, Kikkawa Y, Kimoto N, Kitamura Y, Sendo T, Kita T, Gomita Y. Protective effects of interferon-gamma against methamphetamine-induced neurotoxicity. Toxicol Lett. 2008;177:123–129. doi: 10.1016/j.toxlet.2008.01.005. [DOI] [PubMed] [Google Scholar]
- In SW, Son EW, Rhee DK, Pyo S. Methamphetamine administration produces immunomodulation in mice. J Toxicol Environ Health A. 2005;68:2133–2145. doi: 10.1080/15287390500177156. [DOI] [PubMed] [Google Scholar]
- Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Brain Res Mol Brain Res. 1998;57:1–9. doi: 10.1016/s0169-328x(98)00040-0. [DOI] [PubMed] [Google Scholar]
- Ito D, Tanaka K, Suzuki S, Dembo T, Fukuuchi Y. Enhanced expression of Iba1, ionized calcium-binding adapter molecule 1, after transient focal cerebral ischemia in rat brain. Stroke. 2001;32:1208–1215. doi: 10.1161/01.str.32.5.1208. [DOI] [PubMed] [Google Scholar]
- Itzhak Y. Modulation of cocaine- and methamphetamine-induced behavioral sensitization by inhibition of brain nitric oxide synthase. J Pharmacol Exp Ther. 1997;282:521–527. [PubMed] [Google Scholar]
- Johnson-Davis KL, Fleckenstein AE, Wilkins DG. The role of hyperthermia and metabolism as mechanisms of tolerance to methamphetamine neurotoxicity. Eur J Pharmacol. 2003;482:151–154. doi: 10.1016/j.ejphar.2003.09.063. [DOI] [PubMed] [Google Scholar]
- Kluger M. Fever: role of pyrogens and cryogens. Physiology Reviews. 1991;71:93–127. doi: 10.1152/physrev.1991.71.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzyszton CP, Sparkman NL, Grant RW, Buchanan JB, Broussard SR, Woods J, Johnson RW. Exacerbated fatigue and motor deficits in interleukin-10-deficient mice after peripheral immune stimulation. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1109–1114. doi: 10.1152/ajpregu.90302.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, Kiguchi N, Fukazawa Y, Yamamoto A, Ozaki M, Kishioka S. Peroxisome Proliferator-Activated Receptor Gamma Activation Relieves Expression of Behavioral Sensitization to Methamphetamine in Mice. Neuropsychopharmacology. 2006 doi: 10.1038/sj.npp.1301213. [DOI] [PubMed] [Google Scholar]
- Maier SF, Goehler LE, Fleshner M, Watkins LR. The role of the vagus nerve in cytokine-to-brain communication. Ann N Y Acad Sci. 1998;840:289–300. doi: 10.1111/j.1749-6632.1998.tb09569.x. [DOI] [PubMed] [Google Scholar]
- Miller CL, Kerr T, Fischer B, Zhang R, Wood E. Methamphetamine injection independently predicts hepatitis C infection among street-involved youth in a Canadian setting. J Adolesc Health. 2009;44:302–304. doi: 10.1016/j.jadohealth.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myles BJ, Jarrett LA, Broom SL, Speaker HA, Sabol KE. The effects of methamphetamine on core body temperature in the rat--part 1: chronic treatment and ambient temperature. Psychopharmacology (Berl) 2008;198:301–311. doi: 10.1007/s00213-007-1061-z. [DOI] [PubMed] [Google Scholar]
- Myles BJ, Sabol KE. The effects of methamphetamine on core body temperature in the rat--part 2: an escalating regimen. Psychopharmacology (Berl) 2008;198:313–322. doi: 10.1007/s00213-007-1060-0. [DOI] [PubMed] [Google Scholar]
- Nakajima A, Yamada K, Nagai T, Uchiyama T, Miyamoto Y, Mamiya T, He J, Nitta A, Mizuno M, Tran MH, Seto A, Yoshimura M, Kitaichi K, Hasegawa T, Saito K, Yamada Y, Seishima M, Sekikawa K, Kim HC, Nabeshima T. Role of tumor necrosis factor-alpha in methamphetamine-induced drug dependence and neurotoxicity. J Neurosci. 2004;24:2212–2225. doi: 10.1523/JNEUROSCI.4847-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Malenka RC. The addicted brain. Sci Am. 2004;290:78–85. doi: 10.1038/scientificamerican0304-78. [DOI] [PubMed] [Google Scholar]
- Numachi Y, Ohara A, Yamashita M, Fukushima S, Kobayashi H, Hata H, Watanabe H, Hall FS, Lesch KP, Murphy DL, Uhl GR, Sora I. Methamphetamine-induced hyperthermia and lethal toxicity: role of the dopamine and serotonin transporters. Eur J Pharmacol. 2007;572:120–128. doi: 10.1016/j.ejphar.2007.06.022. [DOI] [PubMed] [Google Scholar]
- Purcell DW, Parsons JT, Halkitis PN, Mizuno Y, Woods WJ. Substance use and sexual transmission risk behavior of HIV-positive men who have sex with men. J Subst Abuse. 2001;13:185–200. doi: 10.1016/s0899-3289(01)00072-4. [DOI] [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivest S. Molecular insights on the cerebral innate immune system. Brain Behav Immun. 2003;17:13–19. doi: 10.1016/s0889-1591(02)00055-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Romanovsky AA, Simons CT, Szekely M, Kulchitsky VA. The vagus nerve in the thermoregulatory response to systemic inflammation. Am J Physiol. 1997;273:R407–413. doi: 10.1152/ajpregu.1997.273.1.R407. [DOI] [PubMed] [Google Scholar]
- Rutecki P. Anatomical, physiological, and theoretical basis for the antiepileptic effect of vagus nerve stimulation. Epilepsia. 1990;31 2:S1–6. doi: 10.1111/j.1528-1157.1990.tb05843.x. [DOI] [PubMed] [Google Scholar]
- Saito M, Terada M, Kawata T, Ito H, Shigematsu N, Kromkhun P, Yokosuka M, Saito TR. Effects of single or repeated administrations of methamphetamine on immune response in mice. Exp Anim. 2008;57:35–43. doi: 10.1538/expanim.57.35. [DOI] [PubMed] [Google Scholar]
- Sandhir R, Onyszchuk G, Berman NE. Exacerbated glial response in the aged mouse hippocampus following controlled cortical impact injury. Exp Neurol. 2008;213:372–380. doi: 10.1016/j.expneurol.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekine Y, Ouchi Y, Sugihara G, Takei N, Yoshikawa E, Nakamura K, Iwata Y, Tsuchiya KJ, Suda S, Suzuki K, Kawai M, Takebayashi K, Yamamoto S, Matsuzaki H, Ueki T, Mori N, Gold MS, Cadet JL. Methamphetamine causes microglial activation in the brains of human abusers. J Neurosci. 2008;28:5756–5761. doi: 10.1523/JNEUROSCI.1179-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephans S, Yamamoto B. Methamphetamines pretreatment and the vulnerability of the striatum to methamphetamine neurotoxicity. Neuroscience. 1996;72:593–600. doi: 10.1016/0306-4522(95)00587-0. [DOI] [PubMed] [Google Scholar]
- Thomas DM, Kuhn DM. Attenuated microglial activation mediates tolerance to the neurotoxic effects of methamphetamine. J Neurochem. 2005;92:790–797. doi: 10.1111/j.1471-4159.2004.02906.x. [DOI] [PubMed] [Google Scholar]
- Ujike H. Stimulant-induced psychosis and schizophrenia: the role of sensitization. Curr Psychiatry Rep. 2002;4:177–184. doi: 10.1007/s11920-002-0024-7. [DOI] [PubMed] [Google Scholar]
- Yu Q, Montes S, Larson DF, Watson RR. Effects of chronic methamphetamine exposure on heart function in uninfected and retrovirus-infected mice. Life Sci. 2002;71:953–965. doi: 10.1016/s0024-3205(02)01769-1. [DOI] [PubMed] [Google Scholar]


