Abstract
Sec1/Munc18 (SM) proteins and SNAREs form part of the core intracellular membrane fusion machinery, but it is unclear how they cooperate in membrane fusion. The synaptic vesicle SNARE synaptobrevin and the plasma membrane SNAREs syntaxin-1 and SNAP-25 assemble into a tight SNARE complex that includes a four-helix bundle formed by their SNARE motifs and is key for fusion. The neuronal SM protein Munc18-1 binds to syntaxin-1 and to the SNARE complex through interactions with the syntaxin-1 N-terminal region that are critical for neurotransmitter release. It has been proposed that Munc18-1 also binds to synaptobrevin and to the SNARE four-helix bundle, and that such interactions might be crucial for membrane fusion, but definitive, direct evidence for these interactions has not been described. Using diverse biophysical approaches, we now demonstrate that Munc18-1 indeed binds to synaptobrevin and to the SNARE four-helix bundle. Both interactions have similar affinities (in the low micromolar range) and appear to involve the same cavity of Munc18-1 that binds to syntaxin-1. Correspondingly, the N-terminal region of syntaxin-1 competes with the SNARE four-helix bundle and synaptobrevin for Munc18-1 binding. Importantly, the Munc18-1 binding site on synaptobrevin is located at the C-terminus of its SNARE motif, suggesting that this interaction places Munc18-1 right at the site where fusion occurs. These results suggest a model whereby neurotransmitter release involves a sequence of three different types of Munc18-1/SNARE interactions, and whereby Munc18-1 plays a direct, active role in membrane fusion in cooperation with the SNAREs.
Intracellular membrane traffic is governed by a conserved machinery formed by members of several protein families (1;2). Particularly important among these components are Sec1/Munc18 (SM) proteins and soluble N-ethylmaleimide sensitive factor attachment protein receptors (SNAREs), which are believed to form the core of the fusion machinery (3–5). The primary function of the SNAREs became clear from studies of the synaptic vesicle protein synaptobrevin and the plasma membrane proteins syntaxin-1 and SNAP-25, the SNAREs that control neurotransmitter release. These studies showed that SNAREs form tight ‘SNARE complexes’ through sequences called SNARE motifs (6). These motifs form a four-helix bundle (7;8) that brings the two membranes into close proximity (9), which is key for membrane fusion (1–5). The role of SM proteins is still highly unclear despite intense research.
Early studies showed that the neuronal SM protein Munc18-1 is essential for neurotransmitter release (10) and binds tightly to syntaxin-1 (11). In addition to a SNARE motif that precedes a C-terminal transmembrane (TM) sequence, syntaxin-1 contains an N-terminal region that includes a three-helix bundle domain called the Habc domain (12). Isolated syntaxin-1 forms a closed conformation that involves packing of the SNARE motif against the Habc domain and is incompatible with the SNARE complex, but is critical for Munc18-1 binding (13). The Munc18-1/syntaxin-1 interaction involves a characteristic cavity formed in Munc18-1 due to its arch-shaped architecture (14), and gates entry of syntaxin-1 into SNARE complexes (15). However, studies of other SM proteins suggested that this interaction is not general, as the SM protein from the yeast plasma membrane, Sec1p, binds to assembled SNARE complexes rather than to the syntaxin homologue Sso1p (16), and the yeast vacuolar syntaxin Vam3p lacks a closed conformation (17). Moreover, SM proteins from other membrane compartments were found to bind to an N-terminal sequence of their cognate syntaxins (18–21).
This apparently confusing picture was partially clarified by increasing evidence showing that SM proteins generally bind to SNARE complexes and that the syntaxin N-terminal sequences often contribute to binding [(16;22–27); but see (28)]. These findings suggest that formation of SM protein/SNARE complex assemblies might underlie the key functional importance of SM proteins, as predicted in a model whereby the core fusion machinery is formed by SM-protein/SNARE complex assemblies rather than SNARE complexes alone (23;29) (Suppl. Fig. 1). Indeed, Munc18-1 binding to the SNARE complex enhances SNARE-dependent lipid mixing in reconstitution assays (24;30) and is critical for neurotransmitter release (31), activities that depend on the interaction of Munc18-1 with the syntaxin-1 N-terminal sequence (24;31). Moreover, interactions of Munc18-1 with the syntaxin-1 Habc domain within the SNARE complex are also crucial for priming synaptic vesicles to a readily-releasable state (32). However, these interactions do not appear to be important for downstream events that lead to Ca2+-triggered release (32) and, mechanistically, it is unclear how binding of Munc18-1 to the syntaxin-1 N-terminal region can help inducing membrane fusion. One possibility is that Munc18-1 could assist in SNARE complex assembly after syntaxin-1 is opened (14;33), and evidence for this proposal was described recently (24). In this scenario, Munc18-1 does not play a direct role in fusion but acts as an accessory factor in SNARE complex assembly. However, it seems difficult to reconcile this model with the strict requirement of Munc18-1 and SM proteins in general for membrane fusion (34), and some evidence supported a role for Sec1p in yeast exocytosis after SNARE complex formation (35).
The model assigning a direct role for Munc18-1 in membrane fusion that we proposed (Suppl. Fig. 1) emerged from the realization that Munc18-1/SNARE complex assemblies might be much more efficient than SNARE complexes alone in exerting mechanical force on the membranes to induce fusion (29). Munc18-1 binding to the four-helix bundle formed by the SNARE motifs of syntaxin-1, SNAP-25 and synaptobrevin (below referred to as the SNARE four-helix bundle) is a central aspect of this model. Some data suggested that Munc18-1 indeed binds to the SNARE four-helix bundle (23;24), and the stimulation of SNARE-dependent lipid mixing caused by Munc18-1 in reconstitution assays appeared to depend on Munc18-1/synaptobrevin interactions (24). However, no direct binding of Munc18-1 to synaptobrevin could be detected in this study (24), and isothermal titration calorimetry (ITC) data indicated that Munc18-1 has similar affinity for the SNARE complex and the syntaxin-1 N-terminal region, suggesting that this region is solely responsible for SNARE complex binding to Munc18-1 (36). Hence, it is still unclear whether Munc18-1 indeed binds to synaptobrevin and to the SNARE four-helix bundle, despite the central importance that these interactions could have to couple the functions of Munc18-1 and the SNAREs in membrane fusion.
In this study, we have addressed these questions using a combination of biophysical approaches. We demonstrate that Munc18-1 binds directly to the SNARE motif of synaptobrevin and to the SNARE four-helix bundle with an affinity in the low micromolar range. Our data suggest that these interactions involve the same cavity of Munc18-1 involved in binding to the syntaxin-1 closed conformation, and map the Munc18-1 binding site on synaptobrevin to the very C-terminus of its SNARE motif. These results suggest that synaptobrevin binding places Munc18-1 right at the site of membrane fusion, and that Munc18-1 may indeed have a direct role in fusion in cooperation with the SNAREs.
EXPERIMENTAL PROCEDURES
Protein expression and purification
Vectors to express rat Munc18-1, and fragments corresponding to the cytoplasmic regions of rat syntaxin-1A (residues 2-253) or rat synaptobrevin-2 (1–96), to the syntaxin-1A N-terminal region (residues 1-180), or to the SNARE motifs of syntaxin-1A (residues 191-253), synaptobrevin-2 (29–93) and human SNAP-25B (11–82 and 141–203) as GST-fusion proteins were described previously (12;13;23;37). A vector to express squid Munc18-1 as a His-tagged protein was a kind gift from W. Weissenhorn (38). Cysteine mutations of Munc18-1 (K125C, K308C), syntaxin-1A(2-253) (D27C) and synaptobrevin(1-96) (S61C) were performed with QuickChange site-directed mutagenesis kit (Stratagene). All proteins were expressed and purified as described (12;13;23;37). SNARE complexes were assembled and purified as described (23;32;37).
Fluorescence spectroscopy
Labeling with fluorescence probes was performed with samples of 50–100 μM single cysteine mutated proteins in 20mM HEPES(pH7.2), 100mM KCl, by incubating with a 10- to 20-fold excess of N-(2-aminoethyl) maleimide Bodipy FL, tetramethylrhodamine-5-iodoacetamide dihydroiodide, or Texas Red C5-bromoacetamide (Molecular Probes) at RT for 6h, and 10mM DTT was added to stop the reaction (39). Fluorescence emission spectra (480–680 nm; excitation at 465 nm) were acquired on a PTI fluorimeter using 50–100 nM samples of Bodipy-FL-labeled Munc18-1 dissolved in 20mM HEPES(pH7.2), 100mM KCl, and variable concentrations of SNARE complexes or fragments.
Chemical cross-linking
Rat munc18-1 (17 μM) and 1X-5X synaptobrevin((29-96)) were incubated with 5 mM 1-Ethyl-3-[3-dimethylaminopropyl]carbodiimide (EDC) or 1 mM Bis(sulfosuccinimidyl)suberate (BS3) for 1 hr at RT and quenched with 25 mM Tris, pH8.0. Samples of 18 μg of protein were loaded to SDS gels for PAGE and LC-MS-MS analysis.
Miscellaneous
1H-15N HSQC spectra were acquired on Varian INOVA600 spectrometers at 25 oC using samples dissolved in 20 mM HEPES (pH 7.2), 120 mM KCl. ITC experiments were performed using a VP-ITC system (MicroCal) at 20oC in 20 mM HEPES (pH 7.4), 100 mM KCl, with 20 μM squid Munc18-1 and successive injections of 0.4 mM synaptobrevin(49-96) or 0.5 mM synaptobrevin(77-96). The data were fitted using a single-site binding model with Origin for ITC v.5.0.
RESULTS
Munc18-1 binds to synaptobrevin and to the SNARE four-helix bundle
To directly test for diverse types of Munc18-1/SNARE interactions using FRET, we prepared two mutants of rat Munc18-1 where residue 125 or 308 was mutated to cysteine, and labeled them with BODIPY-FL as a donor fluorescence probe. Specific labeling of residue 125 or 308 was verified by trypsin digestion and mass spectrometry (MS), and was favored by the exposed nature of these side chains (the native cysteines of Munc18-1 are buried). We will refer to the labeled Munc18-1 proteins as Munc18-125-BP and Munc18-308-BP. We also introduced single cysteine mutations in residue 27 of the cytoplasmic region of syntaxin-1 (residues 2-253) where the only native cysteine (residue 145) was mutated to serine, and in residue 61 of the cytoplasmic region of synaptobrevin (residues 1-96). The mutants were labeled with Rhodamine or Texas Red as fluorescence acceptor probes [referred to as syntaxin-27-Rho or –TR, and synaptobrevin-61-Rho or -TR]. Representative experiments with one or the other probe are shown below, but the nature of the probe did not alter the results observed.
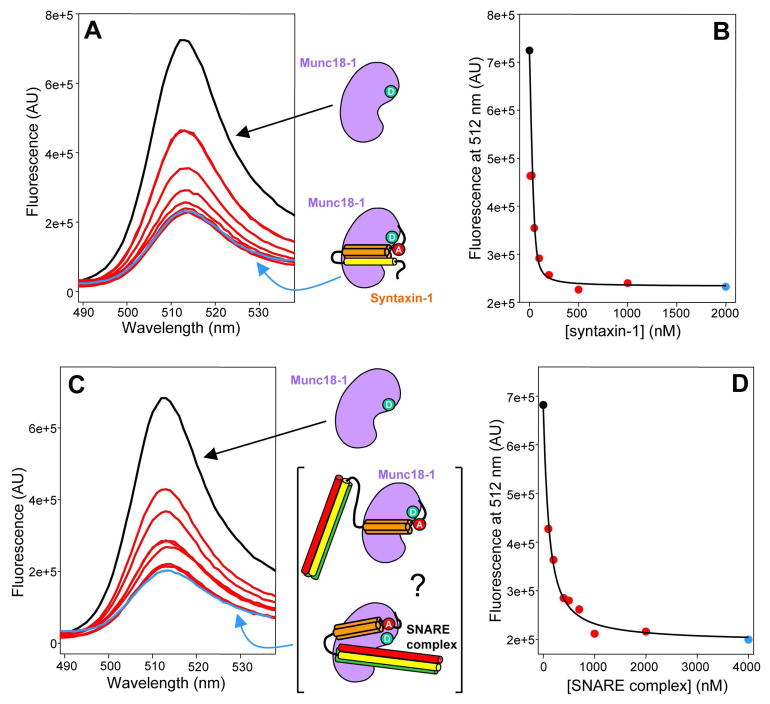
To test the labeled proteins in a well-characterize interaction, we first tested Munc18-1/syntaxin-1 binding. We observed a high FRET efficiency (E > 70%) between syntaxin-27-Rho and Munc18-125-BP (Figure 1A), as expected from the proximity of residue 125 of Munc18-1 to residue 27 of syntaxin-1 in their complex (ca. 23 Å; ref. 14) and assuming a Forster radius of 50 Å. Titrations with variable syntaxin-27-Rho concentrations yielded saturable dose-response curves (e.g. Figures 1A,B) and an approximate dissociation constant (kD) of 5 nM, consistent with published data (31;32;36). Similar results were obtained with Munc18-308-BP (Suppl. Fig. 2). We also analyzed binding of SNARE complex containing syntaxin-27-TR or -Rho to Munc18-125-BP or Munc18-308-BP. We observed similar FRET efficiencies to those observed with isolated syntaxin-27-Rho or –TR, as expected if the Habc domain occupies a similar position upon binding of syntaxin-1 or the SNARE complex to Munc18-1 (31;32;36). Titrations with different SNARE complex concentrations yielded saturable binding curves with a kD of ca. 100 nM (Figures 1C,D, and data not shown), comparable to those determined previously by NMR spectroscopy (23;32). Hence, all these data show that the fluorescently labeled mutants exhibit analogous interactions to those between the WT proteins.
Figure 1.
Binding of Munc18-1 to closed syntaxin-1 and neuronal SNARE complex monitored by FRET. (A,C) Emission fluorescence spectra of 50 nM Munc18-125-BP and variable concentrations of syntaxin-27-Rho (A) or SNARE complex containing syntaxin-27-TR (C). The SNARE complex was formed with syntaxin-27-TR, and the SNARE motifs of syntaptobrevin and SNAP-25. The diagrams next to the spectra represent Munc18-1 (purple) with a donor fluorescence probe (green), syntaxin-1(2-253) (SNARE motif, yellow; Habc domain orange) labeled with an acceptor fluorescence probe (red), and SNARE complexes containing syntaxin-1(2-253), synaptobrevin (red) and SNAP-25 (green). In the bracket, two different types of Munc18-1/SNARE complex assemblies are represented where the SNARE four-helix bundle is interacting or not with Munc18-1. (B,D) Plots of the fluorescence emission intensity at 512 nm as function of syntaxin-27-Rho concentration (B) or SNARE complex concentration (D), derived from the spectra shown in (A) and (C), respectively. The data were fit to a standard single binding site model.
These data do not clarify whether the SNARE four-helix bundle binds to Munc18-1. To directly address this question, we studied binding of Munc18-1 to SNARE complexes containing synaptobrevin-61-Rho or –TR, and lacking the syntaxin-1 N-terminal region (i.e. using a syntaxin-1 fragment spanning residues 191-253). Importantly, these complexes exhibited substantial FRET with Munc18-308-BP and less efficient FRET with Munc18-125-BP (Figure 2A and data not shown). In titration experiments, we were unable to reach complete saturation because of limited availability of the fluorescently-labeled SNARE four-helix bundles, but the data could be fit well with a standard protein-ligand binding equation (Figure 2B), yielding estimated kD values on the order of 6 μM. Hence, these results demonstrate that Munc18-1 binds to the SNARE four-helix bundle with low micromolar affinity.
Figure 2.
Munc18-1 binds to synaptobrevin and the SNARE four-helix bundle. (A,C) Emission fluorescence spectra of 50 nM Munc18-308-BP and variable concentrations of synaptobrevin-61-Rho (C) or SNARE four-helix bundle containing synaptobrevin-61-TR and the SNARE motifs of syntaxin-1 and SNAP-25 (A). The diagrams next to the spectra represent Munc18-1 (purple) with a donor fluorescence probe (green), synaptobrevin(1-96) (red) with an acceptor fluorescence (red), and SNARE four-helix bundles containing the labeled synaptobrevin(1-96) (red) plus the SNARE motifs of syntaxin-1 (yellow) and SNAP-25 (green). (B,D) Plots of the fluorescence emission intensity at 512 nm as function of synaptobrevin-61-Rho concentration (D) or SNARE four-helix bundle concentration (B), derived from the spectra shown in (A) and (C), respectively. The data were fit to a standard single binding site model.
We also tested for binding of isolated synaptobrevin-61-Rho or -TR to Munc18-308-BP, and observed even more efficient FRET (Figure 2C). Titrations with different synaptobrevin-61-Rho concentrations (Figure 2D) also yielded kDs on the order of 6 μM. This similarity in affinities suggests that analogous residues mediate the interactions of Munc18-1 with synaptobrevin and with the SNARE four-helix bundle, and hence that synaptobrevin is largely responsible for binding of the SNARE four-helix bundle to Munc18-1. The observation that the FRET efficiency is higher for the synaptobrevin interaction (compare Figures 2A and 2C) is not inconsistent with this conclusion, since isolated synaptobrevin is known to be highly flexible (40). Therefore, if the region of synaptobrevin containing residue 61 remains flexible upon Munc18-1 binding, such flexibility can yield a range of distances between the donor and acceptor probes, and the r−6 dependence of the FRET efficiency is expected to yield ensemble-averaged values that are strongly biased in favor of the shorter distances. Note also that addition of 50 μM unlabeled synaptobrevin(1-96) led to a considerable decrease in the FRET between Munc18-1 and the SNARE four-helix bundle containing synatobrevin-61-TR (Suppl. Fig. 3A), showing that synaptobrevin and the SNARE four-helix bundle compete for Munc18-1 binding and strongly supporting the conclusion that both interactions involve similar residues.
The syntaxin-1 N-terminal region competes with the SNARE four-helix bundle and synaptobrevin for Munc18-1 binding
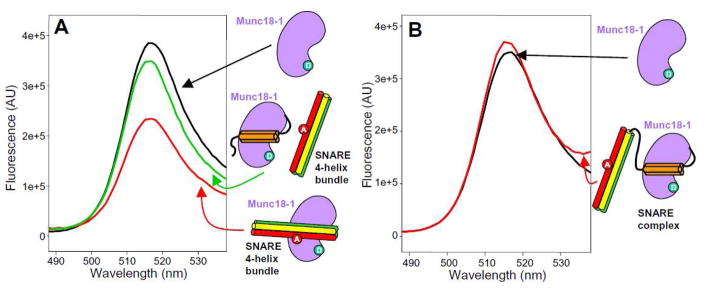
The syntaxin-1 closed conformation consists of a four-helix bundle formed by the Habc domain and the SNARE motif (14). Hence, it is natural to speculate that the SNARE four-helix bundle may bind to the same cavity of Munc18-1 as the syntaxin-1 closed conformation. Since the Habc domain likely remains bound at the same site when the SNARE complex interacts with Munc18-1 (32), this domain might hinder binding of the SNARE four-helix bundle to Munc18-1. To test this hypothesis, we first performed FRET experiments between Munc18-308-BP and the SNARE four-helix bundle containing synaptobrevin-61-Rho or -TR. Efficient FRET was observed when excess (10 μM) SNARE four-helix bundle was added to 50 nM Munc18-308-BP, but most of the FRET was lost when 10 μM syntaxin-1 N-terminal region (residues 1-180) was present (Figure 3A). Moreover, we observed no FRET between Munc18-308-BP and SNARE complex containing the syntaxin-1 N-terminal region and synaptobrevin-61-Rho or –TR (Figure 3B). Although we cannot rule out the possibility that these results arise from conformational changes, the data strongly support the hypothesis that the syntaxin-1 N-terminal region and the SNARE four-helix bundle compete for binding to the same Munc18-1 cavity. The syntaxin-1 N-terminal region also decreased FRET between synaptobrevin-61-Rho and Munc18-308-BP (Suppl. Fig. 3B), again suggesting that the same Munc18-1 cavity binds to synaptobrevin.
Figure 3.
The N-terminal region of syntaxin-1 competes with the SNARE four-helix bundle for Munc18-1 binding. (A) Emission fluorescence spectra of 50 nM Munc18-308-BP alone (black) or in the presence of 10 μM SNARE four-helix bundle containing synaptobrevin-61-TR and the SNARE motifs of syntaxin-1 and SNAP-25, before (red) or after (green) addition of 10 μM syntaxin-1 N-terminal region (residues 1-180). The diagrams next to the spectra represent Munc18-1 (purple) with a donor fluorescence probe (green), the syntaxin-1 N-terminal region (Habc domain in orange), and SNARE four-helix bundles containing the labeled synaptobrevin(1-96) (red) with an acceptor fluorescence (red) plus the SNARE motifs of syntaxin-1 (yellow) and SNAP-25 (green). (B) Emission fluorescence spectra of 50 nM Munc18-308-BP alone (black) or in the presence of 20 μM SNARE complex formed with synaptobrevin-61-Rho, syntaxin(2-253) and the SNARE motifs of SNAP-25.
Munc18-1 binds to the C-terminus of the synaptobrevin SNARE motif
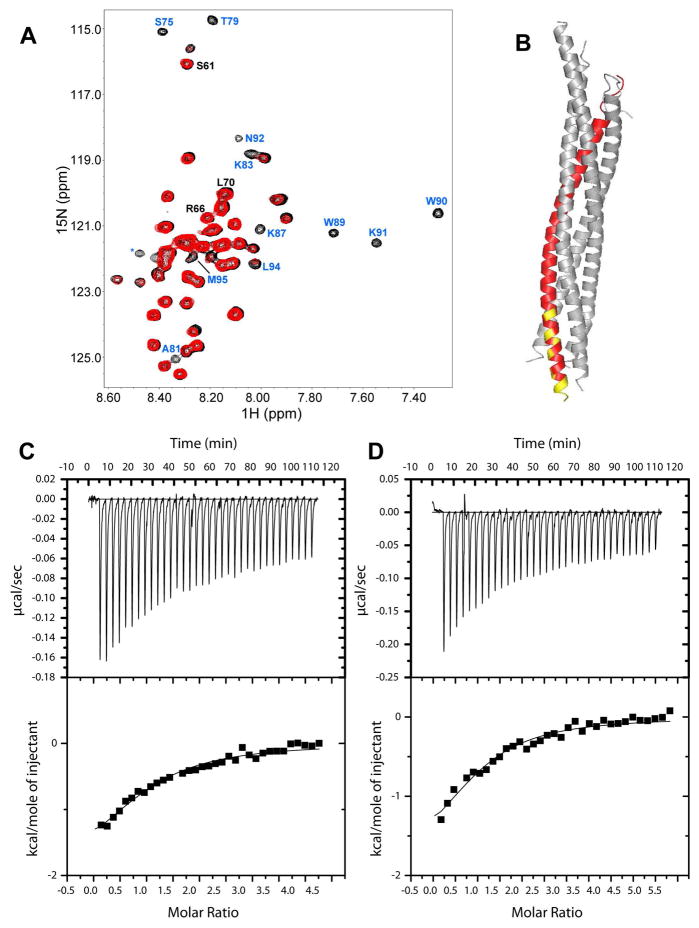
To obtain further insights into the residues involved in Munc18-1/synaptobrevin binding, we first performed chemical cross-linking experiments between WT Munc18-1 and a synaptobrevin fragment spanning its SNARE motif (residues (29-96)). No cross-linking was observed when we used EDC, an agent that links carboxyl groups to primary amines (Figure 4A), but efficient cross-linking was obtained upon addition of BS3, an agent that cross-links primary amines with primary amines (see red arrow in Figure 4A). Trypsin digestion of the cross-linked product and MS analysis revealed one peptide containing a sequence from Munc18-1 (KMPQYQK, residues 333-339) cross-linked to a sequence from synaptobrevin (KYWWK, residues 87-91). The sequence of Munc18-1 is indeed located at the cavity that binds to closed syntaxin-1 (Figure 4B). Intriguingly, residues 87-91 of syntaptobrevin are at the very C-terminus of the SNARE motif (Figure 4B). This finding could be of fundamental important for the mechanism of membrane fusion, since an interaction with the C-terminus of the synaptobrevin SNARE motif would place Munc18-1 right at the site where membrane fusion occurs.
Figure 4.
Cross-linking of synaptobrevin and Munc18-1. (A) SDS PAGE of samples containing synaptobrevin((29-96)) or Munc18-1 or both after cross-linking with EDC or BS3. The relative concentrations of both proteins are indicated above the lanes. The positions of molecular weight markers are indicated on the left. The red arrow on the right indicates the position of the cross-linked product. (B) Ribbon diagrams of the crystal structure of the SNARE complex (8) and the Munc18-1/syntaxin-1 complex (14) with synaptobrevin in red and Munc18-1 in purple (other proteins in gray). The sequences of synaptobrevin and Munc18-1 that were cross-linked are colored in yellow.
To investigate the Munc18-1 binding site on synaptobrevin by a different method, we used NMR spectroscopy. We first attempted to acquire 1H-15N heteronuclear single quantum coherence (HSQC) spectra of 15N-labeled synaptobrevin((29-96)) in the presence and absence of rat Munc18-1. While the spectra suggested that rat Munc18-1 indeed binds to the C-terminus of synaptobrevin((29-96)), obtaining high quality data was hindered by the insolubility of rat Munc18-1 at the concentrations required for these NMR experiments. To solve this problem, we turned to squid Munc18-1 (sMunc18-1), which shares a high sequence identity with rat Munc18-1 (66.4%) and is more soluble [this better behavior allowed crystallization of isolated sMunc18-1 (38)]. Gel filtration experiments showed that sMunc18-1 co-elutes with mammalian syntaxin-1 and the SNARE complex (Suppl. Fig. 4), showing that sMunc18-1 binds tightly to the mammalian SNAREs similar to rat Munc18-1. Moreover, we were able to obtain 1H-15N HSQC spectra of 40 μM synaptobrevin((29-96)) in the absence and presence of sMunc18-1. Since synaptobrevin is unstructured (40), binding to a large protein such as sMunc18-1 is expected to lead to selective broadening of the cross-peaks from the sequences involved in binding, while regions that do not participate in the interaction are expected to remain flexible and still yield observable cross-peaks. Indeed, we observed that addition of sMunc18-1 caused broadening of some synaptobrevin((29-96)) cross-peaks, while many others were unaffected (Figure 5A). Importantly, the majority of broadened cross-peaks correspond to the region encompassing residues 75-95 of synaptobrevin (Figure 5A) at the very C-terminus of its SNARE motif (Figure 5B), in correlation with the cross-linking results obtained with rat Munc18-1. Note that the binding region does not include residues 60-70, which were previously suggested to mediate Munc18-1/synaptobrevin interactions (24), although we cannot rule that this region might contribute to Munc18-1/SNARE four-helix bundle interactions.
Figure 5.
sMunc18-1 binds to the C-terminus of the synaptobrevin SNARE motif. (A) 1H-15N HSQC spectra of 40 μM synaptobrevin((29-96)) in the absence (black) and presence (red) of 40 μM sMunc18-1. Well-resolved cross-peaks that were strongly broadened and correspond to residues at the C-terminus of the synaptobrevin SNARE motif are labeled in blue. Three well-resolved cross-peaks that do not exhibit such strong broadening and correspond to the region spanning residues 60–70 are labeled in black. (B) Ribbon diagram of the SNARE complex with synaptobrevin in red; residues corresponding to the cross-peaks labeled in blue in (A) are colored in yellow. (C,D) ITC analysis of binding of synaptobrevin(49-96) (C) or synaptobrevin(77-96) (D) to sMunc18-1.
To further confirm our conclusions, we obtained a synthetic peptide corresponding to residues 77-96 of synaptobrevin, and acquired 1H NMR spectra of the peptide alone, sMunc18-1 alone, and a mixture of the peptide and sMunc18-1, comparing the intensities in the methyl region of the spectra before and after applying a Carr Purcell Meiboom Gill (CPMG) pulse sequence for 100 ms. The stronger T2 relaxation of the peptide in the presence of sMunc18-1 showed that the peptide binds to sMunc18-1 (Suppl. Fig. 5). Finally, we also compared the affinity of sMunc18-1 for a fragment containing the C-terminal half of the synaptobrevin SNARE motif (residues 49-96) and the synaptobrevin(77-96) peptide using ITC, obtaining kDs of 13.3 and 13.5 μM, respectively (Figure 5C,D). These values are comparable to the kDs obtained by FRET for binding of rat Munc18-1 to synaptobrevin(1-96) and the SNARE four-helix bundle (Figure 2), and their similarity confirms that C-terminus of the SNARE motif is responsible for synaptobrevin-binding to Munc18-1.
DISCUSSION
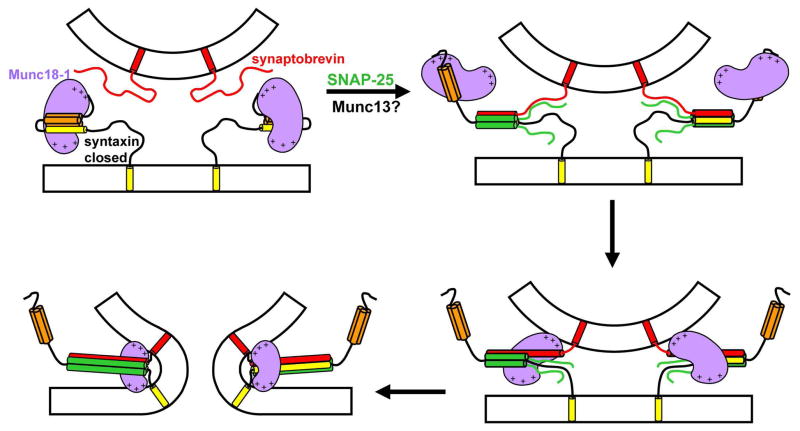
SM proteins are central components of the intracellular membrane fusion machinery, but their main role is still highly unclear. This uncertainty arises in part because of the diversity of SM protein/SNARE interactions that have been identified, and in part because no definitive evidence has been presented for the various models of SM protein function that have been proposed. To explain the very strong blocks in membrane fusion observed in the absence of SM proteins (4;34), one of these models predicted that SM proteins bind to the SNARE four-helix bundle, enabling application of force to the membranes by the SNAREs (29) (Suppl. Fig. 1). Reconstitution experiments showed that Munc18-1 can strongly enhance the ability of the SNAREs to induce lipid mixing and suggested that this ability depends on Munc18-1/synaptobrevin interactions, but no biochemical evidence for such interactions was described (24). We now show that Munc18-1 indeed binds directly to both synaptobrevin and to the SNARE four-helix bundle with an affinity in the low micromolar range. Our data suggest that these interactions involve the same cavity of Munc18-1 where the closed syntaxin-1 binds. Furthermore, we find that the C-terminus of synaptobrevin is responsible for Munc18-1 binding, placing Munc18-1 right at the site where membrane fusion occurs. These results reinforce the notion that Munc18-1, and SM proteins in general, play a direct role in membrane fusion, and suggest a speculative model whereby a sequence of distinct Munc18-1/SNARE interactions occurs during synaptic vesicle exocytosis (Figure 6).
Figure 6.
Proposed model of neurotransmitter release involving three types of Munc18-1/SNARE interactions. The model assumes that Munc18-1 (purple) is initially bound to closed syntaxin-1 (Habc domain, orange; SNARE motif, yellow) (upper left panel). Partial assembly of SNARE complexes of syntaxin-1 with synaptobrevin (red) and SNAP-25 (green) occurs by an unknown mechanism that likely involves Munc13 (not shown); here we propose that Munc18-1 is bound only to the N-terminal region of syntaxin-1 at this stage (upper right panel). The next step is proposed to involve transition of Munc18-1 from the syntaxin-1 N-terminal region to the C-terminus of the synaptobrevin SNARE motif, which could be favored by cooperativity with other interactions such as Munc18-1 binding to the vesicle membrane (bottom right panel). The central aspect of this model is that membrane fusion results from the cooperative action of Munc18-1 and the SNAREs, which would be favored by the binding of Munc18-1 to synaptobrevin and could involve interactions of basic residues of Munc18-1 (indicated by the + signs) with both membranes (bottom left panel; see text for further details). Other proteins involved in triggering release and conferring its Ca2+ sensitivity are not represented for simplicity, but they are expected to cooperated with Munc18-1 and the SNAREs to trigger release.
It is likely that several of the models of SM protein function that have been proposed over the years (4;34) are at least partially correct, since SM proteins may play several roles, but the key question is: why are SM proteins so critical for fusion? The binary Munc18-1/syntaxin-1 imposes a roadblock that gates entry of syntaxin-1 into SNARE complexes (13;15;33;41), but this interaction might still play positive roles by stabilizing both proteins in vivo (10;15), and by assisting in vesicle docking, since syntaxin-1 and Munc18-1 function in docking in chromaffin cells, while synaptobrevin does not (15). Importantly, overexpression of SNAP-25 rescues the docking defect in chromaffin cells from Munc18-1 KO mice, likely by promoting formation of syntaxin-1/SNAP-25 heterodimers, but does not rescue the secretion defect (42). Hence, Munc18-1 must play an additional function downstream of docking and syntaxin-1/SNAP heterodimer assembly. There is in fact evidence for participation of Munc18-1 in more than one of the steps that lead to exocytosis (4;15;32).
Although Munc18-1 binding to closed syntaxin-1 hinders SNARE complex formation from an energetic point of view (33), Munc18-1 could still play a role in assisting SNARE complex assembly in downstream events (5;13;14). Indeed, Munc18-1 enhances assembly of SNARE complexes between co-expressed syntaxin-1/SNAP-25 heterodimers and synaptobrevin, which might underlie the stimulation of SNARE-dependent lipid mixing in reconstitution assays (24). Since the co-expressed heterodimers appear to be in an inhibited state where the Habc domain hinders full interactions between the SNARE motifs (43), it seems likely that Munc18-1 disinhibits this state by binding to the syntaxin-1 N-terminal region. It is unknown whether these events occur in vivo, but in any case it seems clear that interactions of Munc18-1 with the syntaxin-1 N-terminal region are important for release (31;32). However, these interactions do not appear to play a role after vesicle priming (32), and it seems unlikely that a mere role for Munc18-1 in assisting SNARE complex assembly underlies its essential nature for release (10), as SNARE complexes can be readily assembled in vitro. Moreover, stimulation of SNARE-dependent lipid mixing by Munc18-1 appears to depend on interactions with synaptobrevin (24), and some evidence indicates that the SM protein Sec1p functions downstream of SNARE complex formation (35).
These observations suggest that the key function of Munc18-1 and SM proteins in membrane fusion involves interactions with the SNARE four-helix bundle, as initially inferred for Sec1p (16) and as predicted by our previous model of how SM proteins and SNAREs form the core fusion machinery (29) (Suppl. Fig. 1). Some results suggested the existence of such interactions (23;24;28;44), but ITC data indicated that Munc18-1 binding to the SNARE complex involves interactions with only the syntaxin-1 N-terminal region (36). Our results now show unambiguously that Munc18-1 indeed binds to the SNARE four-helix bundle and to the very C-terminus of the synaptobrevin SNARE motif (Figs. 2, 4–5). It is very likely that both interactions involve the same residues, since they have similar affinities and synaptobrevin competes with the SNARE four-helix bundle for Munc18-1 binding (Suppl. Fig. 3). Our data also show that these interactions involve the cavity of Munc18-1 where closed syntaxin-1 binds (Figs. 3,4), which explains the ITC data mentioned above (36). These results need to be interpreted with caution, since the interactions we report have moderate affinity and their physiological relevance remains to be established. However, the finding that Munc18-1 binds to the very C-terminus of the synaptobrevin SNARE motif is very intriguing, since it places Munc18-1 right at the site of fusion and hence suggests a fundamental new view whereby Munc18-1 is intimately and directly involved in fusion. Such a role could explain the critical nature of SM proteins for membrane fusion in vivo.
Our original idea of how Munc18-1 could help inducing fusion (29) (Suppl. Fig. 1) was based on a simple mechanical principle: flexibility in the linker between the SNARE motifs and TM regions could hinder transduction of the energy of SNARE complex assembly onto the membranes and allow assembled SNARE complexes to diffuse to the middle of the intermembrane space. However, binding of a bulky protein such as Munc18-1 to assembling SNARE complexes would prevent such diffusion and would help applying torque on the two membranes to induce fusion, since Munc18-1 would push the membranes away while SNARE complexes bring them together. Application of force by the SNAREs on the membranes would be particularly efficient with Munc18-1 bound to the synaptobrevin C-terminus (Fig. 6), as it would be impossible to fully assemble the SNARE complex without strongly bending the membranes (even if there is some flexibility in the linkers). Moreover, Munc18-1 contains two highly positive surfaces surrounding the SNARE-binding cavity (Suppl. Fig. 6) that could bind to the two membranes and help bending them to induce fusion (Figure 6), as proposed for synaptotagmin-1 (39). In this context, only weak binding of Munc18-1 to liposomes has been reported (43), but such interactions could be greatly strengthened by the intrinsic cooperativity of the system (see below).
Our model involves three different types of interactions of Munc18-1 with the SNAREs (Figure 6): first the binary interaction with closed syntaxin-1; second the interaction with the syntaxin-1 N-terminal region as syntaxin-1 opens and SNARE complexes start to form via an as yet unknown mechanism that likely involves Munc13 (43); and third the binding to the C-terminus of synaptobrevin. Although the affinity of Munc18-1 for synaptobrevin is moderate, and weaker than its affinity for the syntaxin-1 N-terminal region in binding assays with isolated proteins, the transition from syntaxin-1 to synaptobrevin binding could be favored by strong cooperativity with the proposed interactions of Munc18-1 with both membranes, which would bring the membranes together and could therefore cooperate, in addition, with the pulling forces of the assembling SNARE complexes. Support for these ideas is provided by the finding that the HOPS complex, which includes the SM protein involved in yeast vacuolar fusion Vps33p, stabilizes trans SNARE complexes as opposed to cis SNARE complexes (44), since such stabilization must involve binding of HOPS to the two apposed membranes. Moreover, the functional importance of the synaptobrevin region that binds to Munc18-1 has been shown by the impairment in neurotransmitter release caused by mutation of two tryptophane residues in this region (45), although the molecular target of this region in vivo remains to be determined. Candidates for such targets are also Munc13s, complexins and syntaptotagmin-1, factors that play key roles in neurotransmitter release and also bind to the SNARE four-helix bundle (37;43;46). These factors could provide additional cooperativity to the system, but they might also compete with Munc18-1 for binding to the four-helix bundle. Clearly, our model remains highly speculative and, to understand the mechanisms of membrane fusion and neurotransmitter release, it will be particularly important to study the interplay between the interactions among all these proteins and the lipids in the context of trans-SNARE complexes formed between two apposed membranes. The data presented here have now yielded a hypothesis that can help guiding these very challenging studies and proposes a fundamentally new view of how Munc18-1 might actively cooperate with the SNAREs to induce membrane fusion.
Supplementary Material
Acknowledgments
We thank W. Weissenhorn for providing a expression vector for sMunc18-1, and the Protein Chemistry Facility of UT Southwestern for MS analysis of fluorescence labeling and cross-linking.
Abbreviations
- CPMG
Carr Purcell Meiboom Gill
- EDC
1-Ethyl-3-[3-dimethylaminopropyl]carbodiimide
- BS3
Bis(sulfosuccinimidyl)suberate
- E
FRET efficiency
- FRET
fluorescence resonance energy transfer
- HSQC
heteronuclear single quantum correlation
- MS
mass spectrometry
- NMR
nuclear magnetic resonance
- SNARE
soluble N-ethylmaleimide sensitive factor attachment protein receptor
- WT
wild type
Footnotes
This work was supported by a grant from the Welch Foundation (I-1304) and by NIH grant NS37200 (to JR).
SUPPORTING INFORMATION AVAILABLE
Additional models and results. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Jahn R, Scheller RH. SNAREs--engines for membrane fusion. Nat Rev Mol Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 2.Brunger AT. Structure and function of SNARE and SNARE-interacting proteins. Q Rev Biophys. 2005:1–47. doi: 10.1017/S0033583505004051. [DOI] [PubMed] [Google Scholar]
- 3.Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 4.Verhage M, Toonen RF. Regulated exocytosis: merging ideas on fusing membranes. Curr Opin Cell Biol. 2007;19:402–408. doi: 10.1016/j.ceb.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Rizo J, Rosenmund C. Synaptic vesicle fusion. Nat Struct Mol Biol. 2008;15:665–674. doi: 10.1038/nsmb.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sollner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell. 1993;75:409–418. doi: 10.1016/0092-8674(93)90376-2. [DOI] [PubMed] [Google Scholar]
- 7.Poirier MA, Xiao W, Macosko JC, Chan C, Shin YK, Bennett MK. The synaptic SNARE complex is a parallel four-stranded helical bundle. Nat Struct Biol. 1998;5:765–769. doi: 10.1038/1799. [DOI] [PubMed] [Google Scholar]
- 8.Sutton RB, Fasshauer D, Jahn R, Brunger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- 9.Hanson PI, Roth R, Morisaki H, Jahn R, Heuser JE. Structure and conformational changes in NSF and its membrane receptor complexes visualized by quick-freeze/deep-etch electron microscopy. Cell. 1997;90:523–535. doi: 10.1016/s0092-8674(00)80512-7. [DOI] [PubMed] [Google Scholar]
- 10.Verhage M, Maia AS, Plomp JJ, Brussaard AB, Heeroma JH, Vermeer H, Toonen RF, Hammer RE, van den Berg TK, Missler M, Geuze HJ, Sudhof TC. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 2000;287:864–869. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- 11.Hata Y, Slaughter CA, Sudhof TC. Synaptic vesicle fusion complex contains unc-18 homologue bound to syntaxin. Nature. 1993;366:347–351. doi: 10.1038/366347a0. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez I, Ubach J, Dulubova I, Zhang X, Sudhof TC, Rizo J. Three-dimensional structure of an evolutionarily conserved N-terminal domain of syntaxin 1A. Cell. 1998;94:841. doi: 10.1016/s0092-8674(00)81742-0. [DOI] [PubMed] [Google Scholar]
- 13.Dulubova I, Sugita S, Hill S, Hosaka M, Fernandez I, Sudhof TC, Rizo J. A conformational switch in syntaxin during exocytosis: role of munc18. EMBO J. 1999;18:4372–4382. doi: 10.1093/emboj/18.16.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Misura KM, Scheller RH, Weis WI. Three-dimensional structure of the neuronal-Sec1-syntaxin 1a complex. Nature. 2000;404:355–362. doi: 10.1038/35006120. [DOI] [PubMed] [Google Scholar]
- 15.Gerber SH, Rah JC, Min SW, Liu X, de WH, Dulubova I, Meyer AC, Rizo J, Arancillo M, Hammer RE, Verhage M, Rosenmund C, Sudhof TC. Conformational switch of syntaxin-1 controls synaptic vesicle fusion. Science. 2008;321:1507–1510. doi: 10.1126/science.1163174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carr CM, Grote E, Munson M, Hughson FM, Novick PJ. Sec1p binds to SNARE complexes and concentrates at sites of secretion. J Cell Biol. 1999;146:333–344. doi: 10.1083/jcb.146.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dulubova I, Yamaguchi T, Wang Y, Sudhof TC, Rizo J. Vam3p structure reveals conserved and divergent properties of syntaxins. Nat Struct Biol. 2001;8:258–264. doi: 10.1038/85012. [DOI] [PubMed] [Google Scholar]
- 18.Dulubova I, Yamaguchi T, Gao Y, Min SW, Huryeva I, Sudhof TC, Rizo J. How Tlg2p/syntaxin 16 ‘snares’ Vps45. EMBO J. 2002;21:3620–3631. doi: 10.1093/emboj/cdf381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamaguchi T, Dulubova I, Min SW, Chen X, Rizo J, Sudhof TC. Sly1 binds to Golgi and ER syntaxins via a conserved N-terminal peptide motif. Dev Cell. 2002;2:295–305. doi: 10.1016/s1534-5807(02)00125-9. [DOI] [PubMed] [Google Scholar]
- 20.Bracher A, Weissenhorn W. Structural basis for the Golgi membrane recruitment of Sly1p by Sed5p. EMBO J. 2002;21:6114–6124. doi: 10.1093/emboj/cdf608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dulubova I, Yamaguchi T, Arac D, Li H, Huryeva I, Min SW, Rizo J, Sudhof TC. Convergence and divergence in the mechanism of SNARE binding by Sec1/Munc18-like proteins. Proc Natl Acad Sci U S A. 2003;100:32–37. doi: 10.1073/pnas.232701299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng R, Gallwitz D. Sly1 protein bound to Golgi syntaxin Sed5p allows assembly and contributes to specificity of SNARE fusion complexes. J Cell Biol. 2002;157:645–655. doi: 10.1083/jcb.200202006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dulubova I, Khvotchev M, Liu S, Huryeva I, Sudhof TC, Rizo J. Munc18-1 binds directly to the neuronal SNARE complex. Proc Natl Acad Sci U S A. 2007;104:2697–2702. doi: 10.1073/pnas.0611318104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen J, Tareste DC, Paumet F, Rothman JE, Melia TJ. Selective activation of cognate SNAREpins by Sec1/Munc18 proteins. Cell. 2007;128:183–195. doi: 10.1016/j.cell.2006.12.016. [DOI] [PubMed] [Google Scholar]
- 25.Stroupe C, Collins KM, Fratti RA, Wickner W. Purification of active HOPS complex reveals its affinities for phosphoinositides and the SNARE Vam7p. EMBO J. 2006;25:1579–1589. doi: 10.1038/sj.emboj.7601051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carpp LN, Ciufo LF, Shanks SG, Boyd A, Bryant NJ. The Sec1p/Munc18 protein Vps45p binds its cognate SNARE proteins via two distinct modes. J Cell Biol. 2006;173:927–936. doi: 10.1083/jcb.200512024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Latham CF, Lopez JA, Hu SH, Gee CL, Westbury E, Blair DH, Armishaw CJ, Alewood PF, Bryant NJ, James DE, Martin JL. Molecular dissection of the Munc18c/syntaxin4 interaction: implications for regulation of membrane trafficking. Traffic. 2006;7:1408–1419. doi: 10.1111/j.1600-0854.2006.00474.x. [DOI] [PubMed] [Google Scholar]
- 28.Togneri J, Cheng YS, Munson M, Hughson FM, Carr CM. Specific SNARE complex binding mode of the Sec1/Munc-18 protein, Sec1p. Proc Natl Acad Sci U S A. 2006 doi: 10.1073/pnas.0605448103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rizo J, Chen X, Arac D. Unraveling the mechanisms of synaptotagmin and SNARE function in neurotransmitter release. Trends Cell Biol. 2006;16:339–350. doi: 10.1016/j.tcb.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Rodkey TL, Liu S, Barry M, McNew JA. Munc18a Scaffolds SNARE Assembly to Promote Membrane Fusion. Mol Biol Cell. 2008 doi: 10.1091/mbc.E08-05-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khvotchev M, Dulubova I, Sun J, Dai H, Rizo J, Sudhof TC. Dual modes of Munc18-1/SNARE interactions are coupled by functionally critical binding to syntaxin-1 N terminus. J Neurosci. 2007;27:12147–12155. doi: 10.1523/JNEUROSCI.3655-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deak F, Xu Y, Chang WP, Dulubova I, Khvotchev M, Liu X, Sudhof TC, Rizo J. Munc18-1 binding to the neuronal SNARE complex controls synaptic vesicle priming. J Cell Biol. 2009;184:751–764. doi: 10.1083/jcb.200812026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen X, Lu J, Dulubova I, Rizo J. NMR analysis of the closed conformation of syntaxin-1. J Biomol NMR. 2008;41:43–54. doi: 10.1007/s10858-008-9239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rizo J, Sudhof TC. Snares and munc18 in synaptic vesicle fusion. Nat Rev Neurosci. 2002;3:641–653. doi: 10.1038/nrn898. [DOI] [PubMed] [Google Scholar]
- 35.Grote E, Carr CM, Novick PJ. Ordering the final events in yeast exocytosis. J Cell Biol. 2000;151:439–452. doi: 10.1083/jcb.151.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burkhardt P, Hattendorf DA, Weis WI, Fasshauer D. Munc18a controls SNARE assembly through its interaction with the syntaxin N-peptide. EMBO J. 2008;27:923–933. doi: 10.1038/emboj.2008.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen X, Tomchick DR, Kovrigin E, Arac D, Machius M, Sudhof TC, Rizo J. Three-dimensional structure of the complexin/SNARE complex. Neuron. 2002;33:397–409. doi: 10.1016/s0896-6273(02)00583-4. [DOI] [PubMed] [Google Scholar]
- 38.Bracher A, Perrakis A, Dresbach T, Betz H, Weissenhorn W. The X-ray crystal structure of neuronal Sec1 from squid sheds new light on the role of this protein in exocytosis. Structure Fold Des. 2000;8:685–694. doi: 10.1016/s0969-2126(00)00156-8. [DOI] [PubMed] [Google Scholar]
- 39.Arac D, Chen X, Khant HA, Ubach J, Ludtke SJ, Kikkawa M, Johnson AE, Chiu W, Sudhof TC, Rizo J. Close membrane-membrane proximity induced by Ca(2+)-dependent multivalent binding of synaptotagmin-1 to phospholipids. Nat Struct Mol Biol. 2006;13:209–217. doi: 10.1038/nsmb1056. [DOI] [PubMed] [Google Scholar]
- 40.Hazzard J, Sudhof TC, Rizo J. NMR analysis of the structure of synaptobrevin and of its interaction with syntaxin. J Biomol NMR. 1999;14:203–207. doi: 10.1023/a:1008382027065. [DOI] [PubMed] [Google Scholar]
- 41.Yang B, Steegmaier M, Gonzalez LC, Jr, Scheller RH. nSec1 binds a closed conformation of syntaxin1A. J Cell Biol. 2000;148:247–252. doi: 10.1083/jcb.148.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de WH, Walter AM, Milosevic I, Gulyas-Kovacs A, Riedel D, Sorensen JB, Verhage M. Synaptotagmin-1 docks secretory vesicles to syntaxin-1/SNAP-25 acceptor complexes. Cell. 2009;138:935–946. doi: 10.1016/j.cell.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 43.Guan R, Dai H, Rizo J. Binding of the Munc13-1 MUN Domain to Membrane-Anchored SNARE Complexes. Biochemistry. 2008;47:1474–1481. doi: 10.1021/bi702345m. [DOI] [PubMed] [Google Scholar]
- 44.Starai VJ, Hickey CM, Wickner W. HOPS proofreads the trans-SNARE complex for yeast vacuole fusion. Mol Biol Cell. 2008;19:2500–2508. doi: 10.1091/mbc.E08-01-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maximov A, Tang J, Yang X, Pang ZP, Sudhof TC. Complexin controls the force transfer from SNARE complexes to membranes in fusion. Science. 2009;323:516–521. doi: 10.1126/science.1166505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dai H, Shen N, Arac D, Rizo J. A Quaternary SNARE-Synaptotagmin-Ca(2+)-Phospholipid Complex in Neurotransmitter Release. J Mol Biol. 2007;367:848–863. doi: 10.1016/j.jmb.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.