Abstract
Purpose
This study aimed to determine activity and safety of weekly bortezomib and rituximab in patients with relapsed/refractory Waldenström macroglobulinemia (WM).
Patients and Methods
Patients who had at least one previous therapy were eligible. All patients received bortezomib intravenously weekly at 1.6 mg/m2 on days 1, 8, and 15, every 28 days for six cycles and rituximab 375 mg/m2 weekly on cycles 1 and 4. The primary end point was the percentage of patients with at least a minor response.
Results
Thirty-seven patients were treated. The majority of patients (78%) completed treatment per protocol. At least minimal response (MR) or better was observed in 81% (95% CI, 65% to 92%), with two patients (5%) in complete remission (CR)/near CR, 17 patients (46%) in partial response, and 11 patients (30%) in MR. The median time to progression was 16.4 months (95% CI, 11.4 to 21.1 months). Death occurred in one patient due to viral pneumonia. The most common grade 3 and 4 therapy-related adverse events included reversible neutropenia in 16%, anemia in 11%, and thrombocytopenia in 14%. Grade 3 peripheral neuropathy occurred in only two patients (5%). The median progression-free (PFS) is 15.6 months (95% CI, 11 to 21 months), with estimated 12-month and 18-month PFS of 57% (95% CI, 39% to 75%) and 45% (95% CI, 27% to 63%), respectively. The median overall survival has not been reached.
Conclusion
The combination of weekly bortezomib and rituximab showed significant activity and minimal neurologic toxicity in patients with relapsed WM.
INTRODUCTION
Waldenström macroglobulinemia (WM) is a distinct lymphoproliferative disorder characterized by bone marrow infiltration with lymphoplasmacytic cells, along with an immunoglobulin M (IgM) monoclonal gammopathy.1–4 Although indolent, it remains incurable and most patients succumb to disease progression.2,3,5 However, the survival and outcome of therapy in patients with WM varies widely, and the 5-year survival of patients with WM may range from 36% in high-risk WM to 87% in low-risk patients based on the International Prognostic Scoring System in WM (ISSWM).6
In the United States, rituximab is one of the most widely used therapeutic agents in WM. It modulates the PI3K, nuclear-factor kappa-B (NF-kB), as well as the ERK signaling pathways.7 Rituximab triggered response rates including minimal responses (MR) of 35% to 48%.8–11 However, these studies all included rituximab-naïve patients. In addition, the IgM level may initially increase in response to rituximab, a phenomenon termed IgM flare that occurs in approximately 54% of patients.12,13 These levels may persist for up to 3 to 4 months and do not indicate treatment failure.
NF-kB comprises a family of transcription factors that regulate the transcription of hundreds of genes involved in inflammation, innate immunity, cell growth, and apoptosis.14 Previous studies in other plasma cell dyscrasias such as multiple myeloma have shown that NF-kB is highly activated in tumor cells, and targeting it with bortezomib leads to apoptosis in vitro and in vivo with high activity in clinical trials.15–17 In addition, we have previously demonstrated that NF-kB is highly activated in WM cells.18,19 The proteasome inhibitor bortezomib has also been shown to specifically target NF-kB in WM cells, induce apoptosis and cytotoxicity in preclinical studies,19 and enhance the cytotoxic activity of rituximab in antibody-dependent cellular cytotoxicity assays.18
Prior studies have examined the activity of single-agent bortezomib in WM. The use of bortezomib as a single agent in WM has been tested in relapsed WM.20–22 Chen et al20 treated 27 patients with bortezomib in both untreated (44%) and previously treated (56%) patients with WM. The overall response rate to bortezomib was 78%, with major responses (partial remission [PR] or better) observed in 44% of patients; however, sensory neuropathy occurred in 20 of 27 patients, five patients with grade worse than 3. In addition, the time to progression was short, with a median time to progression of 7.9 months.21
On the basis of our preclinical studies and prior clinical activity, we evaluated the efficacy and safety of weekly bortezomib at 1.6 mg/m2 in combination with rituximab in patients with relapsed and/or refractory WM.
PATIENTS AND METHODS
Eligibility
Study participants were at least 18 years of age with relapsed/refractory WM. Patients must have had prior therapy with at least one treatment regimen. Patients must have had symptomatic disease requiring therapy for WM according to the consensus recommendations for WM.23 Patients had measurable monoclonal IgM concentration on serum electrophoresis and IgM protein twice the upper limit of normal by nephelometry and evidence of relapsed or refractory disease, as well as the presence of lymphoplasmacytic cells in the bone marrow. Prior rituximab was permissible but must have been completed more than 3 months before enrollment, and CD20-positive disease must be present on prior bone marrow biopsy. Eligibility criteria also included a serum concentration of AST or ALT less than 3× the upper limit of the normal range, a serum total bilirubin less than 2 mg/dL, a measured creatinine less than 2.5× the upper limit of the normal range, a platelet count of ≥ 75,000/mm2, and an absolute neutrophil count of at least 1,000/mm2.
Exclusion criteria included cytotoxic chemotherapy ≤ 3 weeks, biologic therapy ≤ 2 weeks, or corticosteroids ≤ 2 weeks before registration. All patients gave written informed consent before entering the study, which was performed in accordance with the Declaration of Helsinki; approval was obtained from the institutional review board at each of the participating centers.
Study Design and Treatment
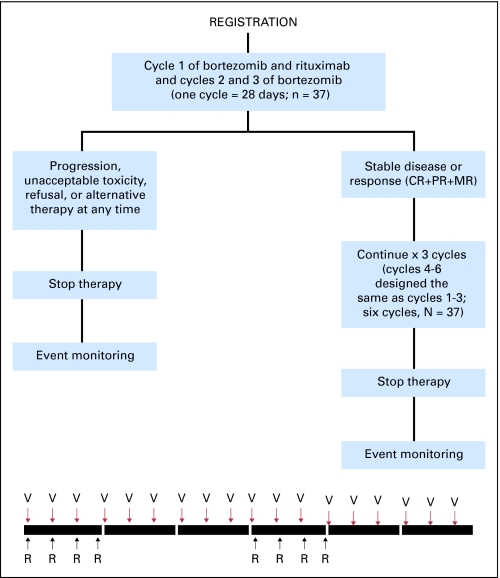
Patients received intravenous bortezomib weekly at 1.6 mg/m2 on days 1, 8, and 15 every 28 days for six cycles and rituximab 375 mg/m2 on days 1, 8, 15, and 22 on cycles 1 and 4. Patients with progressive disease after two cycles were taken off therapy. Patients with stable or responsive disease continued on therapy for a total of six cycles (Fig 1). There was no maintenance therapy. At the time of initiation of the study, the incidence of herpes zoster reactivation with bortezomib in WM was not well documented; therefore, antiviral prophylaxis was not mandated.
Fig 1.
CONSORT diagram. CR, complete remission; PR, partial remission; MR, minimal response; V, bortezomib 1.6 mg/m2 days 1, 8, 15 every 28 days × six cycles; R, rituximab 375 mg/m2 days 1, 8, 15, 22 on cycles 1 and 4.
Dose modifications for attributable toxicities were allowed. Bortezomib could be reduced from 1.6 mg/m2 to 1.3 mg/m2 to 1.0 mg/m2. No dose reductions were allowed for rituximab; however, the rate of infusion of rituximab could be modified for hypersensitivity or infusion related events. No dose re-escalation was allowed.
Efficacy and Safety Assessments
Tumor assessment was performed using the consensus panel recommendations.24 Patients were assessed every 28 days during the six cycles of therapy and every 3 months thereafter. Patients who came off therapy were monitored every 3 months until they experienced disease progression, were treated with another therapy, or died. We have also included analysis for near complete remission (nCR), which is not currently part of the WM criteria. We felt it was important to report the patients who did not have monoclonal protein in their serum but remained positive by immunofixation. This response criterion is currently present in the European Group for Blood and Marrow Transplant response criteria for multiple myeloma.25
Subjects were considered to have experienced relapse if they showed 25% increase in their M spike on two consecutive measurements after the last therapy. Patients were considered refractory to therapy, as defined by progression during treatment or within 60 days after the completion of salvage treatment. Patients refractory to rituximab were patients who had no response for 3 months after the last infusion of rituximab.
Adverse events were assessed at each visit and graded according to the National Cancer Institute Common Toxicity Criteria (version 3.0) from the first dose until 30 days after the last dose of therapy.
Statistical Analysis
The primary end point was the percentage of patients with at least a minor response (MR), and secondary end points included safety, time to progression (TTP), progression-free survival (PFS), overall survival (OS) and time to next therapy (TTNT). TTNT is an important end point for patients with WM because often these patients meet criteria for progression (> 25% increase in IgM protein), but remain asymptomatic and clinically are not treated until they become symptomatic based on the consensus recommendations for treatment of WM patients.23,24 Therefore, we wanted to capture both the TTP and the TTNT because they both provide information that is important in clinical practice.
A two-stage design was used, with 23 eligible patients entered on the first stage and an additional 14 eligible patients added to the second stage if at least 11 of the 22 patients achieved an MR. Patient characteristics were summarized and compared between responders and nonresponders using Fisher's exact test for binary end points and Wilcoxon rank sum test for continuous end points. Estimated response proportions were reported along with exact two-stage binomial 95% CIs. Median time to response (TTR), defined as the time to first response, and duration of response (DOR) were reported among responding patients. The time to event end points were estimated using Kaplan-Meier methodology. TTP, PFS, and OS are measured from the time of registration to the time of first event (progressive disease for TTP, progressive disease or death for PFS, and death for OS). Patients without the event are censored at the date they were last known to be in remission for TTP, in remission and alive for PFS, in remission, and alive for OS. TTNT is measured from the end of treatment to the initiation of next therapy, censored at date last known alive without initiation of next therapy. A Cox proportional hazards model was used to evaluate the impact of multiple factors on time to event end points. All P values are two-sided. Statistical analyses were performed using SAS statistical software (version 8.2, SAS Institute, Cary, NC).
RESULTS
Patients and Treatment
From August 2006 to August 2008, 37 patients were enrolled in four centers. Table 1 shows selected characteristics of all 37 patients. The median age at enrollment was 64 years (range, 42 to 81 years). The median IgM level was 3,660 mg/dL (range, 722 to 10,800 mg/dL), and the median M spike by serum protein electrophoresis was 2.4 g/dL (range, 0.7 to 5.3 g/dL). The median hemoglobin at enrollment was 11.4 g/dL (range, 6.2 to 16.7 g/dL), with 44% (n = 16) of the patients with anemia (< 11 g/dL). Median β2-microglobulin at enrollment was 3.2 mg/dL (range, 1.3 to 7.3 mg/dL) with 38% (13 of 34 patients) of the patients with β2-microglobulin more than 3.5 mg/dL. Four (11%) of the patients had platelets less than 100,000/mm2. The median percent bone marrow involvement was 50% (range, 2% to 95%). There was evidence of disease in soft tissue assessment including organomegaly or lymphadenopathy in 23 patients (62%). Almost 50% of the patients were intermediate or high risk by the ISSWM staging system at the time of enrollment. Prior types of therapies are included in Table 1. Seventy-eight percent of the patients received prior rituximab alone or in combination with other agents, and 17% received prior bortezomib.
Table 1.
Baseline Characteristics
| Characteristic | No. (N = 37) | % |
|---|---|---|
| Age, years | ||
| Median | 64 | |
| Range | 42-81 | |
| Male sex | 26 | 70 |
| Race, white | ||
| Disease status | ||
| Relapsed | 21 | 57 |
| Refractory | 5 | 14 |
| Relapsed and refractory | 11 | 30 |
| No. of prior treatments | ||
| 1 | 11 | 30 |
| 2 | 8 | 22 |
| 3 | 7 | 19 |
| > 3 | 11 | 30 |
| Prior treatment | ||
| Chlorambucil, chlorambucil/prednisone, melphalan/prednisone | 6 | 16 |
| Cladribine, fludarabine, pentostatin | 17 | 46 |
| CHOP, CVP, cyclophosphamide, mitoxantrone, cyclophosphamide/mitoxantrone | 10 | 27 |
| Rituximab alone or with others | 29 | 78 |
| Bortezomib | 6 | 16 |
| Thalidomide or lenalidomide | 7 | 19 |
| Prednisone, dexamethasone, methylprednisolone single agents | 5 | 14 |
| Clinical trials agents* | 14 | 38 |
| Others† | 3 | 8 |
| ECOG PS | ||
| 0 | 30 | 81 |
| 1 | 7 | 19 |
| ISSWM | ||
| High risk | 6 | 16 |
| Intermediate risk | 11 | 30 |
| Low risk | 18 | 49 |
| NA‡ | 2 | 5 |
Abbreviations: CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; CVP, cyclophosphamide, vincristine, and prednisone; ECOG PS, Eastern Cooperative Oncology Group performance status; ISSWM, International Prognostic Scoring System in Waldenström Macroglobulinemia; NA, not applicable.
Clinical trials include sildenafil citrate and perifosine, RAD001, imatinib mesylate, and oblimersen sodium.
Others include radiation therapy, alemtuzumab, autologous bone marrow transplantation.
Serum B2 was not measured in two patients at screening.
Among 37 patients, 29 (78%) completed treatment per protocol. Treatment was discontinued in the remaining eight patients due to unacceptable toxicity (n = 5; 14%), death as a result of viral pneumonia (n = 1; 3%), patient withdrawal in cycle 1 due to travel (n = 1; 3%), and physician decision (n = 1; 3%). Three patients did not receive all doses of rituximab, and six patients did not complete all six cycles of bortezomib.
Efficacy
Of the 37 patients, one patient (3%) achieved complete remission (CR), one patient (3%) achieved an nCR, 17 patients (46%) achieved PR, and 11 (30%) achieved MR (Table 2). At least MR or better was observed in 81% of the patients (95% CI, 65% to 92%). At least PR or better was observed in 51% of the patients (95% CI, 35% to 69%), with a median duration of response of 16.5 months (range, 5 to 30 months). The median time to first response was 2 months (range, 1 to 3 months), and the median time to best response was 6 months (range, 2 to 14 months). Using IgM response evaluated by nephelometry, 32 patients (87%; 95% CI, 71% to 96%) achieved at least an MR (Table 2).
Table 2.
Response Measured by M Spike and Using IgM Measured by Nephelometry
| Response | M Spike |
IgM by Nephelometry |
||
|---|---|---|---|---|
| No. | % | No. | % | |
| CR | 1 | 3 | 2 | 5 |
| nCR | 1 | 3 | NA | |
| PR | 17 | 46 | 21 | 57 |
| MR | 11 | 30 | 9 | 24 |
| SD | 4 | 11 | 2 | 5 |
| PD | 1 | 3 | 1 | 3 |
| Unevaluable | 2 | 5 | 2 | 5 |
| CR + nCR + PR + MR* | 30 | 81 | 32 | 87 |
| CR + nCR + PR† | 19 | 51 | 23 | 62 |
Abbreviations: IgM, immunoglobulin M; CR, complete remission; nCR, near CR; NA, not applicable; PR, partial remission; MR, minimal response; SD, stable disease; PD, progressive disease.
95% exact two-stage CIs for proportion of patients with CR + nCR + PR + MR by M spike is 65 to 92 and with a CR + PR + MR by nephelometry is 71 to 96.
95% exact two-stage CIs for proportion of patients with a CR + nCR + PR by M spike is 35 to 69 and with a CR + PR by nephelometry is 45 to 78.
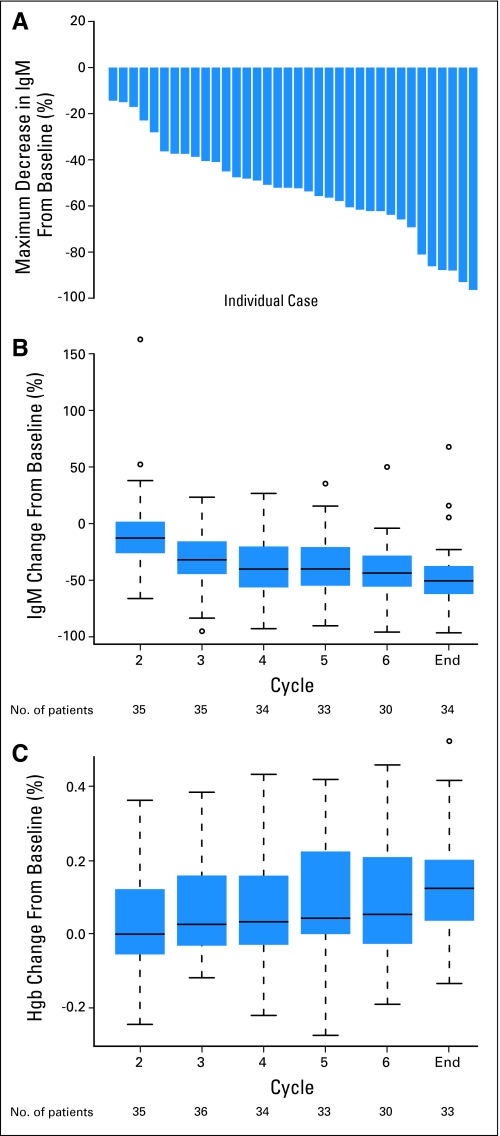
Figure 2A shows the maximum percent change from baseline in IgM over all cycles among responding patients (n = 30). Figures 2B and 2C show the median and interquartile range for IgM and hemoglobin values, respectively, in response to therapy per each cycle. The increase in hemoglobin shown in Figure 2C is indicative of the clinical response in these patients.
Fig 2.
(A) The maximum percent decrease in immunoglobulin M (IgM) over all cycles in response to therapy per patient in responding patients. (B) The median and interquartile range for IgM values in response to therapy per each cycle. (C) The median and interquartile range for hemoglobin (Hgb) values in response to therapy per each cycle.
Among the seven patients who did not achieve at least an MR, four patients (11%) were stable, one patient (3%) progressed at cycle 4 of therapy, one patient (3%) could not be evaluated because they withdrew within the first cycle due to travel, and one patient (3%) could not be evaluated because of death within the first cycle of therapy due to viral pneumonia. The patient who experienced disease progression on therapy continued on therapy until cycle 6, as it was presumed that the increase in his IgM was due to a rituximab flare. However, the IgM continued to increase and he was treated with a subsequent therapy after completion of the protocol.
Time-to-Event Analysis
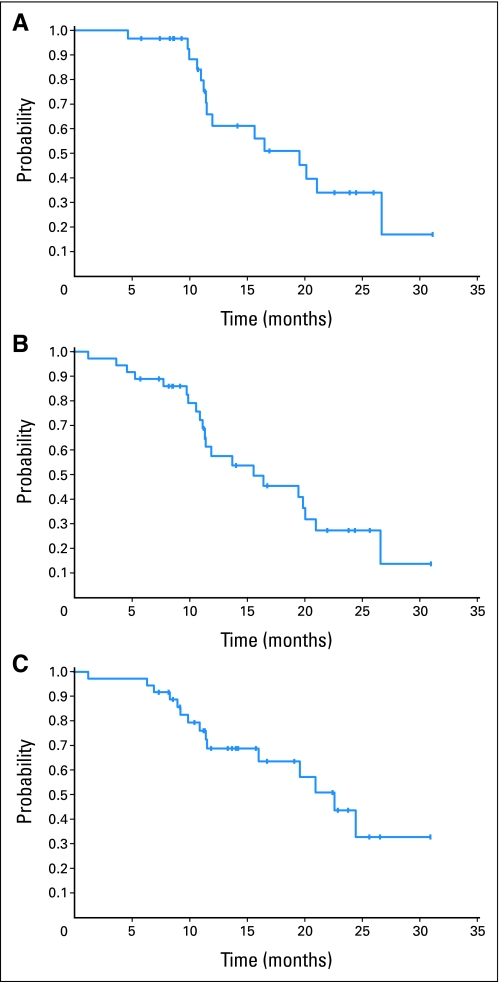
After a median follow-up of 16 months, three patients have died and 34 remain alive as of June 2009. A total of 18 patients have experienced disease progression, of whom 15 have started another therapy and three have died. Death occurred as a result of treatment toxicity or disease progression in these three patients, one during therapy on this trial and two others on follow-up while on other subsequent therapies. The median duration of response (MR or better) was 19.5 months (range, 5 to 30 months; Fig 3A). The median PFS is 15.6 months (95% CI, 11.2 to 21.1; Fig 3B), with estimated 12-month and 18-month PFS of 58% (95% CI, 39% to 75%) and 45% (95% CI, 27% to 63%). The median TTP is 16.4 months (95% CI, 11.4 to 21.1 months), with estimated 12-month and 18-month TTP of 59% (95% CI, 40% to 78%) and 46% (95% CI, 26% to 66%). The median OS has not been reached, with an estimated 12-month OS of 94% (95% CI, 86% to 100%). The median TTNT was 17.6 months, with a range of 1 to 25 months (Fig 3C).
Fig 3.
(A) Duration of response (DOR), minimal response or better. The median DOR for all 30 responders was 19.5 months (95% CI, 11.5 to 26.7 months; range, 5 to 30 months). (B) The median progression-free survival (PFS) is 15.6 months (95% CI, 11.2 to 21.1 months), with estimated 12-month and 18-month PFS of 58% (95% CI, 39% to 75%) and 45% (95% CI, 27% to 63%). (C) The median time to next therapy was 17.6 months (range, 1 to 25 months).
Prognostic Factors
We evaluated the prognostic value of clinical characteristics and laboratory results on PFS, including age (≤ 65 v > 65 years), ISSWM, number of prior treatments (one or fewer v more than one), percent marrow involvement (dichotomized at the median value of 50%), and β2-microglobulin (dichotomized at ≤ 3.5 v > 3.5) at screening. Univariate analyses show a significant increase in risk for failure (death, or PD) for those who are older than 65 years of age (relative risk [RR] = 2.6; 95% CI, 1.1 to 6.3; P = .02) and a marginal increase in risk for those who received more than one prior treatment (RR = 2.7; 95% CI, 0.9 to 8.3; P = .08), and those with more than 50% plasma cells in BM (RR = 2.4; 95% CI, 0.9 to 6.1; P = .08). However, after adjusting for other variables in a multivariate model none of the factors were significant (P > .10).
Safety
Adverse events that were related to therapy (possible, probable or definite) included 12 grade 3 toxicities (32%), 6 grade 4 toxicities (16%), and 1 grade 5 toxicity (3%). A summary of all toxicities related to therapy is shown in Table 3. The grade 5 toxicity was viral pneumonia that occurred in 1 patient during the first cycle of therapy. This patient did not receive antiviral prophylactic therapy. He developed a grade 1 zoster infection. This was followed by a viral pneumonia, which progressively worsened. The family opted for comfort care and the patient passed away. Autopsy showed evidence of disease and viral pneumonia.
Table 3.
Summary of Treatment-Related (possible, probably, definitely) Adverse Events in > 10% of Patients (N = 37)
| Toxicity | Grade 1 to 2 |
Grade 3 to 4 |
Grade 5 |
|||
|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | |
| Hematologic | ||||||
| Hemoglobin | 30 | 81 | 4 | 11 | ||
| Leukocytes | 19 | 51 | 5 | 14 | ||
| Lymphopenia | 3 | 8 | 9 | 24 | ||
| Neutrophils | 11 | 30 | 6 | 16 | ||
| Thrombocytopenia | 14 | 37 | 5 | 13 | ||
| Gastrointestinal | ||||||
| Diarrhea | 14 | 37 | ||||
| Constipation | 4 | 11 | ||||
| Nausea | 11 | 30 | ||||
| Vomiting | 4 | 11 | ||||
| Infections | ||||||
| Infection, conjunctivitis | 6 | 16 | ||||
| Infection, respiratory | 3 | 8 | 1 | 3 | ||
| Herpes Zoster reactivation | 4 | 11 | ||||
| Electrolytes and liver function studies | ||||||
| Hyponatremia | 4 | 11 | ||||
| Hyperglycemia | 16 | 43 | ||||
| Alkaline phosphatase | 6 | 16 | ||||
| AST | 6 | 16 | ||||
| Neurologic/pain/others | ||||||
| Peripheral neuropathy | 15 | 41 | 2 | 5 | ||
| Muscle pain | 4 | 11 | ||||
| Fatigue | 25 | 68 | ||||
| Dizziness | 4 | 11 | ||||
| Allergic reaction | 11 | 30 | ||||
The most common grade 3 and 4 therapy-related adverse events included leucopenia in five patients (14%), neutropenia in six patients (16%), anemia in four patients (11%), lymphopenia in nine patients (24%), and thrombocytopenia in five patients (14%). Grade 3 neuropathy occurred in two patients (5%), and grade 1 and 2 occurred in 16 patients (43%). Grade 3 neuropathy occurred at cycle 5 and cycle 6 in the two patients and completely resolved in one patient and improved to grade 2 neuropathy in the second patient after 3 months of follow-up. Herpes zoster reactivation grade 2 occurred in three patients and grade 1 occurred in one patient. All patients who developed zoster reactivation were either not on prophylactic antiviral therapy or stopped taking it. Conjunctivitis/chalazia occurred in six patients and has been previously observed with bortezomib.
Previous studies have shown that rituximab can cause a transient increase in the IgM (IgM flare) in up to 50% of patients.12,13 In this study, eight patients (22%) developed a transient increase in the IgM after the first cycle of therapy. Of these, four patients eventually developed a PR, two developed MR, and two developed stable disease.
DISCUSSION
Despite continuing advances in the therapy of WM, the disease remains incurable, thereby necessitating the development and evaluation of novel therapeutics.3,5,26 There is no standard of therapy for the treatment of WM.27 Most treatment options were originally derived from those used for other lymphoproliferative diseases, including multiple myeloma and chronic lymphocytic leukemia.28 Current therapies used in the upfront or relapsed settings include alkylator agents, nucleoside analogs, and the monoclonal antibody rituximab.8,23,29,30 In the salvage setting, the overall response rate is in the range of 30% to 40%, with a median response duration of 1 year or less.29,31 Therefore, there is a need for novel therapeutic agents that have less toxicity and achieve high responses.
On the basis of our preclinical studies and prior clinical trials, we examined the role of weekly bortezomib in combination with rituximab in patients with relapsed or relapsed/refractory WM. In this study, the proportion of patients with MR or better was 81%, and an additional 11% of patients had stabilization of their disease while on therapy. The estimated median PFS of 16.5 months was longer than that reported for single-agent bortezomib (7.9 months; 95% CI, 3 to 21.4+ months), and the median OS has not been reached. Only one patient showed progression while on therapy on this trial. One death occurred during the first cycle of therapy, which was due to viral pneumonia.
The combination of bortezomib and rituximab was well tolerated. There were only two cases of grade 3 neuropathy, both of which resolved. This is in comparison to more than 18% grade 3 and 4 neuropathies seen in prior studies using twice-a-week bortezomib. Therefore, we recommend that future studies use the weekly regimen of bortezomib. In this study, we did not mandate zoster prophylaxis. Herpes zoster reactivation occurred in four patients who were not on prophylactic therapy. Future studies using bortezomib should mandate the use of herpes zoster prophylaxis in patients with WM.
In addition, we did not use high-dose dexamethasone as part of the combination of therapy. Prior studies using bortezomib in the upfront setting have included dexamethasone.32 This study shows that high responses can occur even without the addition of dexamethasone. This regimen therefore spares patients from the toxicities of high-dose dexamethasone.
In summary, we demonstrate that the combination of weekly bortezomib and rituximab showed a high response rate that included CRs in patients with relapsed or refractory WM and was well tolerated with minimal peripheral neuropathy. Further studies using this combination along with other therapeutic agents may improve the depth of response in these patients. Similar studies using weekly bortezomib therapy are ongoing in the upfront setting in WM.
Acknowledgment
We thank Jennifer Stedman for editing and reviewing the manuscript.
Footnotes
Supported in part by the International Waldenström Macroglobulinemia Foundation (IWMF), and Michelle and Steven Kirsch lab for Waldenström Macroglobulinemia.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00422799.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Although all authors completed the disclosure declaration, the following author(s) indicated a financial or other interest that is relevant to the subject matter under consideration in this article. Certain relationships marked with a “U” are those for which no compensation was received; those relationships marked with a “C” were compensated. For a detailed description of the disclosure categories, or for more information about ASCO's conflict of interest policy, please refer to the Author Disclosure Declaration and the Disclosures of Potential Conflicts of Interest section in Information for Contributors.
Employment or Leadership Position: None Consultant or Advisory Role: Irene M. Ghobrial, Celgene (C), Millennium (C), Novartis (C); Swaminathan Padmanabhan, Genentech (C); Paul G. Richardson, Millennium (C), Celgene (C), Gentium (C); Steven P. Treon, Millennium (U), Genentech (U) Stock Ownership: None Honoraria: Irene M. Ghobrial, Celgene, Millennium, Novartis; Swaminathan Padmanabhan, Genentech; Kenneth C. Anderson, Millennium, Celgene; Steven P. Treon, Millennium, Genentech; Jeffrey Matous, Celgene, Millennium Research Funding: Swaminathan Padmanabhan, Millennium; Kenneth C. Anderson, Millennium; Steven P. Treon, Millennium Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Irene M. Ghobrial
Administrative support: Meghan Rourke, Renee Leduc, Diane Warren, Brianna Harris, Amy Sam
Provision of study materials or patients: Irene M. Ghobrial, Swaminathan Padmanabhan, Ashraf Badros, Jeffrey Matous, Steven P. Treon
Collection and assembly of data: Irene M. Ghobrial, Meghan Rourke, Renee Leduc, Stacey Chuma, Janet Kunsman, Brianna Harris, Amy Sam
Data analysis and interpretation: Irene M. Ghobrial, Fangxin Hong, Kenneth C. Anderson, Paul G. Richardson, Steven P. Treon, Edie Weller
Manuscript writing: Irene M. Ghobrial
Final approval of manuscript: Irene M. Ghobrial, Jeffrey Matous
REFERENCES
- 1.Dimopoulos MA, Panayiotidis P, Moulopoulos LA, et al. Waldenstrom's macroglobulinemia: Clinical features, complications, and management. J Clin Oncol. 2000;18:214–226. doi: 10.1200/JCO.2000.18.1.214. [DOI] [PubMed] [Google Scholar]
- 2.Ghobrial IM, Witzig TE. Waldenstrom macroglobulinemia. Curr Treat Options Oncol. 2004;5:239–247. doi: 10.1007/s11864-004-0015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dimopoulos MA, Kyle RA, Anagnostopoulos A, et al. Diagnosis and management of Waldenstrom's macroglobulinemia. J Clin Oncol. 2005;23:1564–1577. doi: 10.1200/JCO.2005.03.144. [DOI] [PubMed] [Google Scholar]
- 4.Owen RG, Treon SP, Al-Katib A, et al. Clinicopathological definition of Waldenstrom's macroglobulinemia: Consensus panel recommendations from the Second International Workshop on Waldenstrom's Macroglobulinemia. Semin Oncol. 2003;30:110–115. doi: 10.1053/sonc.2003.50082. [DOI] [PubMed] [Google Scholar]
- 5.Ghobrial IM, Gertz MA, Fonseca R. Waldenstrom macroglobulinaemia. Lancet Oncol. 2003;4:679–685. doi: 10.1016/s1470-2045(03)01246-4. [DOI] [PubMed] [Google Scholar]
- 6.Morel P, Duhamel A, Gobbi P, et al. International prognostic scoring system for Waldenstrom's macroglobulinemia. Blood. 2009;113:4163–4170. doi: 10.1182/blood-2008-08-174961. [DOI] [PubMed] [Google Scholar]
- 7.Bonavida B. Rituximab-induced inhibition of antiapoptotic cell survival pathways: Implications in chemo/immunoresistance, rituximab unresponsiveness, prognostic and novel therapeutic interventions. Oncogene. 2007;26:3629–3636. doi: 10.1038/sj.onc.1210365. [DOI] [PubMed] [Google Scholar]
- 8.Treon SP, Emmanouilides C, Kimby E, et al. Extended rituximab therapy in Waldenstrom's macroglobulinemia. Ann Oncol. 2005;16:132–138. doi: 10.1093/annonc/mdi022. [DOI] [PubMed] [Google Scholar]
- 9.Dimopoulos MA, Zervas C, Zomas A, et al. Extended rituximab therapy for previously untreated patients with Waldenstrom's macroglobulinemia. Clin Lymphoma. 2002;3:163–166. doi: 10.3816/clm.2002.n.022. [DOI] [PubMed] [Google Scholar]
- 10.Dimopoulos MA, Zervas C, Zomas A, et al. Treatment of Waldenstrom's macroglobulinemia with rituximab. J Clin Oncol. 2002;20:2327–2333. doi: 10.1200/JCO.2002.09.039. [DOI] [PubMed] [Google Scholar]
- 11.Gertz MA, Rue M, Blood E, et al. Multicenter phase 2 trial of rituximab for Waldenstrom macroglobulinemia (WM): An Eastern Cooperative Oncology Group Study (E3A98) Leuk Lymphoma. 2004;45:2047–2055. doi: 10.1080/10428190410001714043. [DOI] [PubMed] [Google Scholar]
- 12.Ghobrial I, Fonseca R, Greipp PR, et al. Initial immunoglobulin M ‘flare’ after rituximab therapy in patients diagnosed with Waldenström macroglobulinemia: An Eastern Cooperative Oncology Group Study. Cancer. 2004;101:2593–2598. doi: 10.1002/cncr.20658. [DOI] [PubMed] [Google Scholar]
- 13.Treon S, Branagan AR, Hunter Z, et al. Paradoxical increases in serum IgM and viscosity levels following rituximab in Waldenström's macroglobulinemia. Ann Oncol. 2004;15:1481–1483. doi: 10.1093/annonc/mdh403. [DOI] [PubMed] [Google Scholar]
- 14.Hayden MS GS. Signaling to NF-kappaB. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 15.Hideshima T, Mitsiades C, Akiyama M, et al. Molecular mechanisms mediating antimyeloma activity of proteasome inhibitor PS-341. Blood. 2003;101:1530–1534. doi: 10.1182/blood-2002-08-2543. [DOI] [PubMed] [Google Scholar]
- 16.Mitsiades N, Mitsiades CS, Poulaki V, et al. Biologic sequelae of nuclear factor-kappaB blockade in multiple myeloma: Therapeutic applications. Blood. 2002;99:4079–4086. doi: 10.1182/blood.v99.11.4079. [DOI] [PubMed] [Google Scholar]
- 17.Richardson PG, Barlogie B, Berenson J, et al. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 18.Leleu X, Eeckhoute J, Jia X, et al. Targeting NF-{kappa}B in Waldenstrom macroglobulinemia. Blood. 2008;111:5068–5077. doi: 10.1182/blood-2007-09-115170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roccaro AM, Leleu X, Sacco A, et al. Dual targeting of the proteasome regulates survival and homing in Waldenstrom's Macroglobulinemia. Blood. 2008;111:4752–4763. doi: 10.1182/blood-2007-11-120972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen CI, Kouroukis CT, White D, et al. Bortezomib is active in patients with untreated or relapsed Waldenstrom's macroglobulinemia: A phase II study of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1570–1575. doi: 10.1200/JCO.2006.07.8659. [DOI] [PubMed] [Google Scholar]
- 21.Treon SP, Hunter ZR, Matous J, et al. Multicenter clinical trial of bortezomib in relapsed/refractory Waldenstrom's macroglobulinemia: Results of WMCTG Trial 03-248. Clin Cancer Res. 2007;13:3320–3325. doi: 10.1158/1078-0432.CCR-06-2511. [DOI] [PubMed] [Google Scholar]
- 22.Dimopoulos MA, Anagnostopoulos A, Kyrtsonis MC, et al. Treatment of relapsed or refractory Waldenstrom's macroglobulinemia with bortezomib. Haematologica. 2005;90:1655–1658. [PubMed] [Google Scholar]
- 23.Gertz MA, Anagnostopoulos A, Anderson K, et al. Treatment recommendations in Waldenstrom's macroglobulinemia: Consensus panel recommendations from the Second International Workshop on Waldenstrom's Macroglobulinemia. Semin Oncol. 2003;30:121–126. doi: 10.1053/sonc.2003.50039. [DOI] [PubMed] [Google Scholar]
- 24.Kimby E, Treon SP, Anagnostopoulos A, et al. Update on recommendations for assessing response from the Third International Workshop on Waldenstrom's Macroglobulinemia. Clin Lymphoma Myeloma. 2006;6:380–383. doi: 10.3816/CLM.2006.n.013. [DOI] [PubMed] [Google Scholar]
- 25.Bladé J, Samson D, Reece D, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation: Myeloma Subcommittee of the EBMT—European Group for Blood and Marrow Transplant. Br J Haematol. 1998;102:1115–1123. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 26.Kyle RA, Treon SP, Alexanian R, et al. Prognostic markers and criteria to initiate therapy in Waldenstrom's macroglobulinemia: Consensus panel recommendations from the Second International Workshop on Waldenstrom's Macroglobulinemia. Semin Oncol. 2003;30:116–120. doi: 10.1053/sonc.2003.50038. [DOI] [PubMed] [Google Scholar]
- 27.Treon SP, Gertz MA, Dimopoulos M, et al. Update on treatment recommendations from the Third International Workshop on Waldenstrom's macroglobulinemia. Blood. 2006;107:3442–3446. doi: 10.1182/blood-2005-02-0833. [DOI] [PubMed] [Google Scholar]
- 28.Vijay A, Gertz MA. Waldenstrom Macroglobulinemia. Blood. 2007;109:5096–5103. doi: 10.1182/blood-2006-11-055012. [DOI] [PubMed] [Google Scholar]
- 29.Treon SP, Morel P, Leblond V, et al. Report of the Third International Workshop on Waldenstrom's macroglobulinemia. Clin Lymphoma. 2005;5:215–216. doi: 10.3816/clm.2005.n.001. [DOI] [PubMed] [Google Scholar]
- 30.Dimopoulos MA, O'Brien S, Kantarjian H, et al. Fludarabine therapy in Waldenstrom's macroglobulinemia. Am J Med. 1993;95:49–52. doi: 10.1016/0002-9343(93)90231-d. [DOI] [PubMed] [Google Scholar]
- 31.Dimopoulos MA, Weber D, Delasalle KB, et al. Treatment of Waldenstrom's macroglobulinemia resistant to standard therapy with 2-chlorodeoxyadenosine: Identification of prognostic factors. Ann Oncol. 1995;6:49–52. doi: 10.1093/oxfordjournals.annonc.a059040. [DOI] [PubMed] [Google Scholar]
- 32.Treon SP, Ioakimidis L, Soumerai JD, et al. Primary therapy of Waldenstrom macroglobulinemia with bortezomib, dexamethasone, and rituximab: WMCTG Clinical Trial 05-180. J Clin Oncol. 2009;27:3830–3835. doi: 10.1200/JCO.2008.20.4677. [DOI] [PMC free article] [PubMed] [Google Scholar]