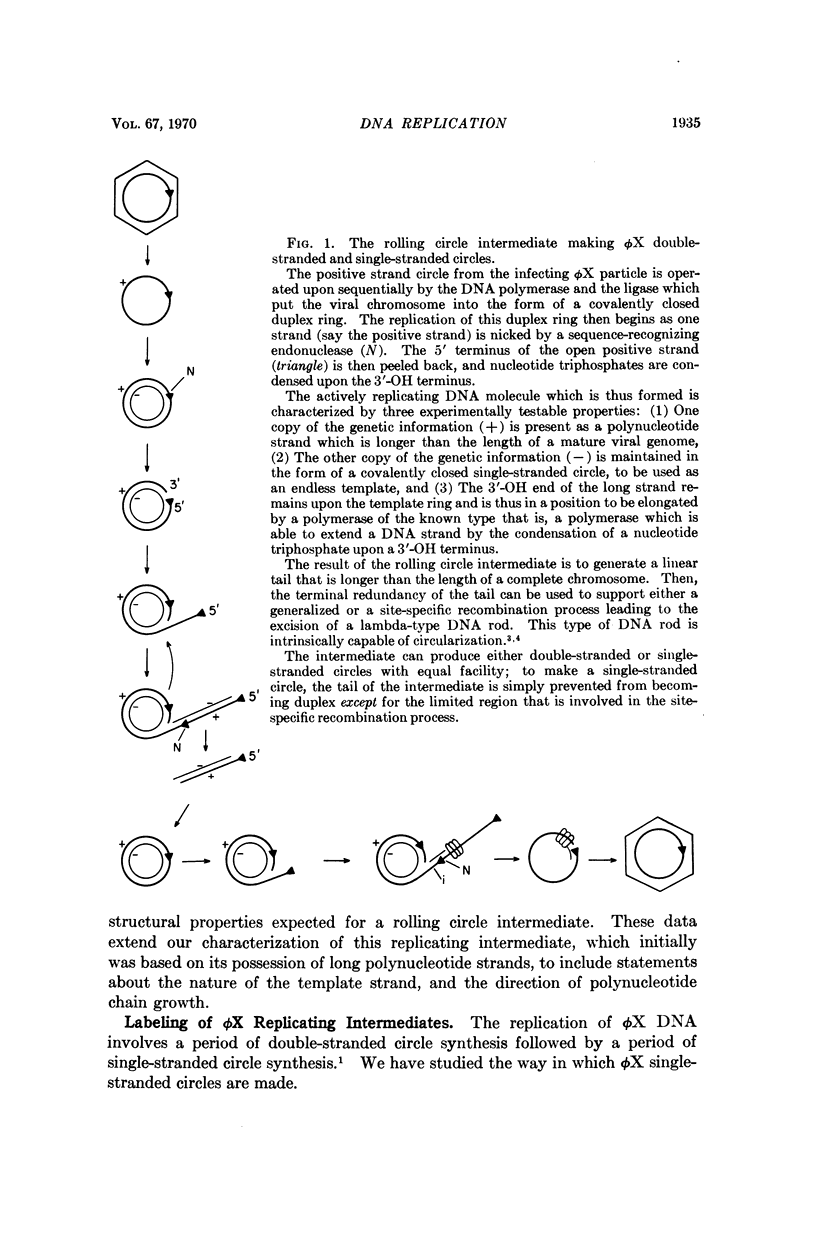
Abstract
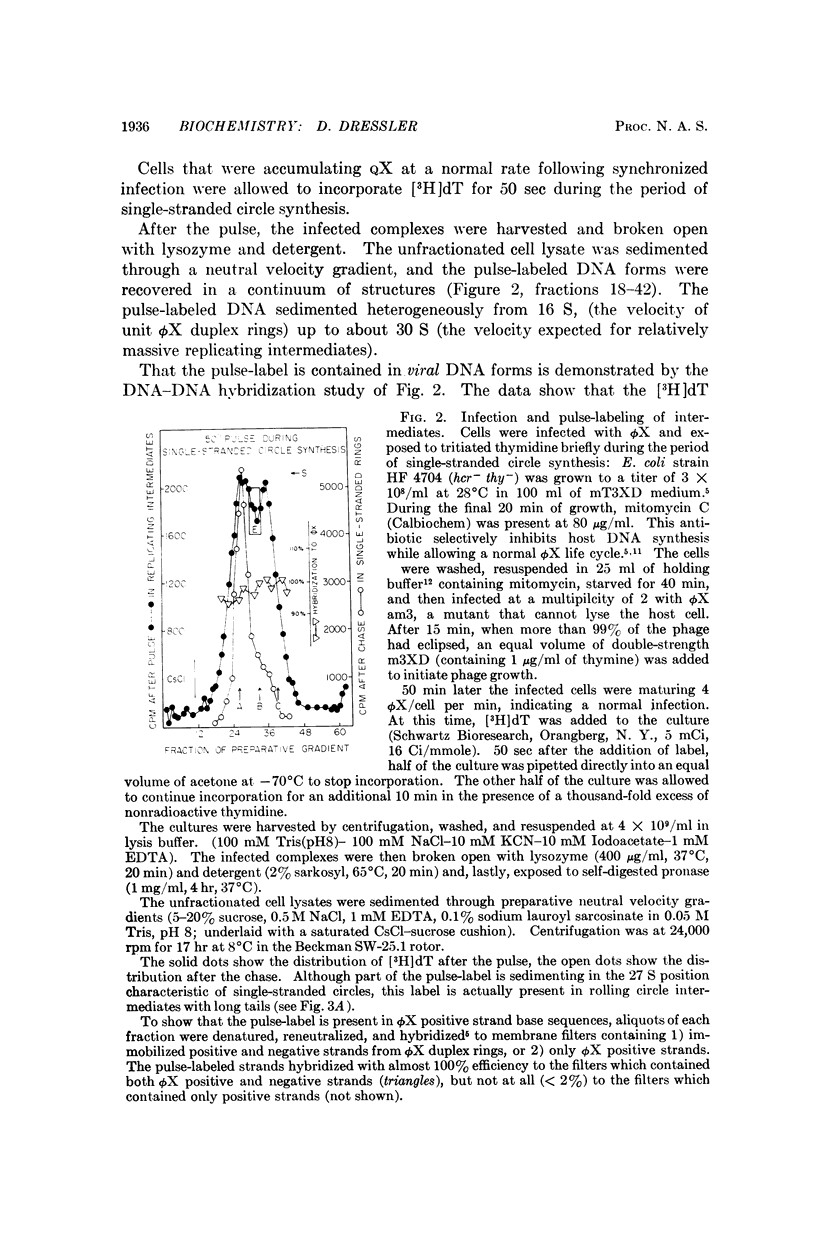
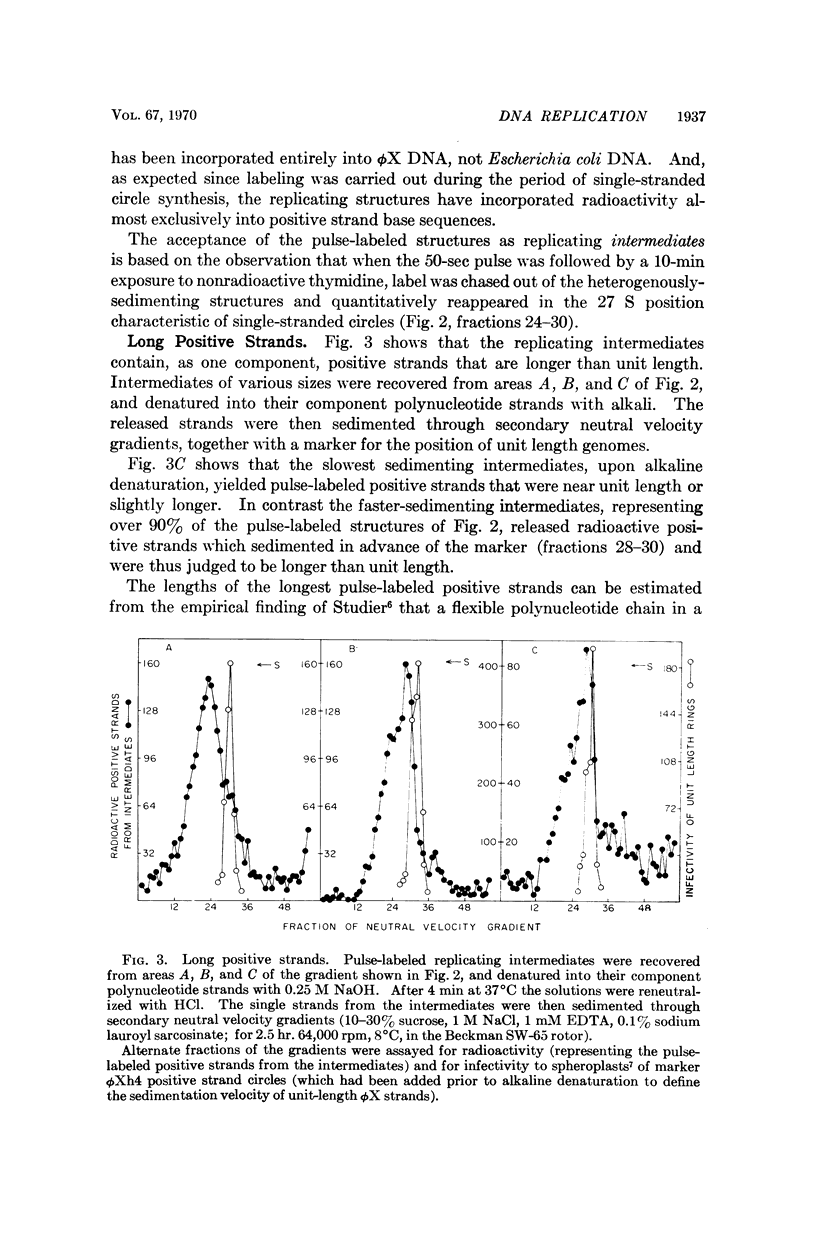
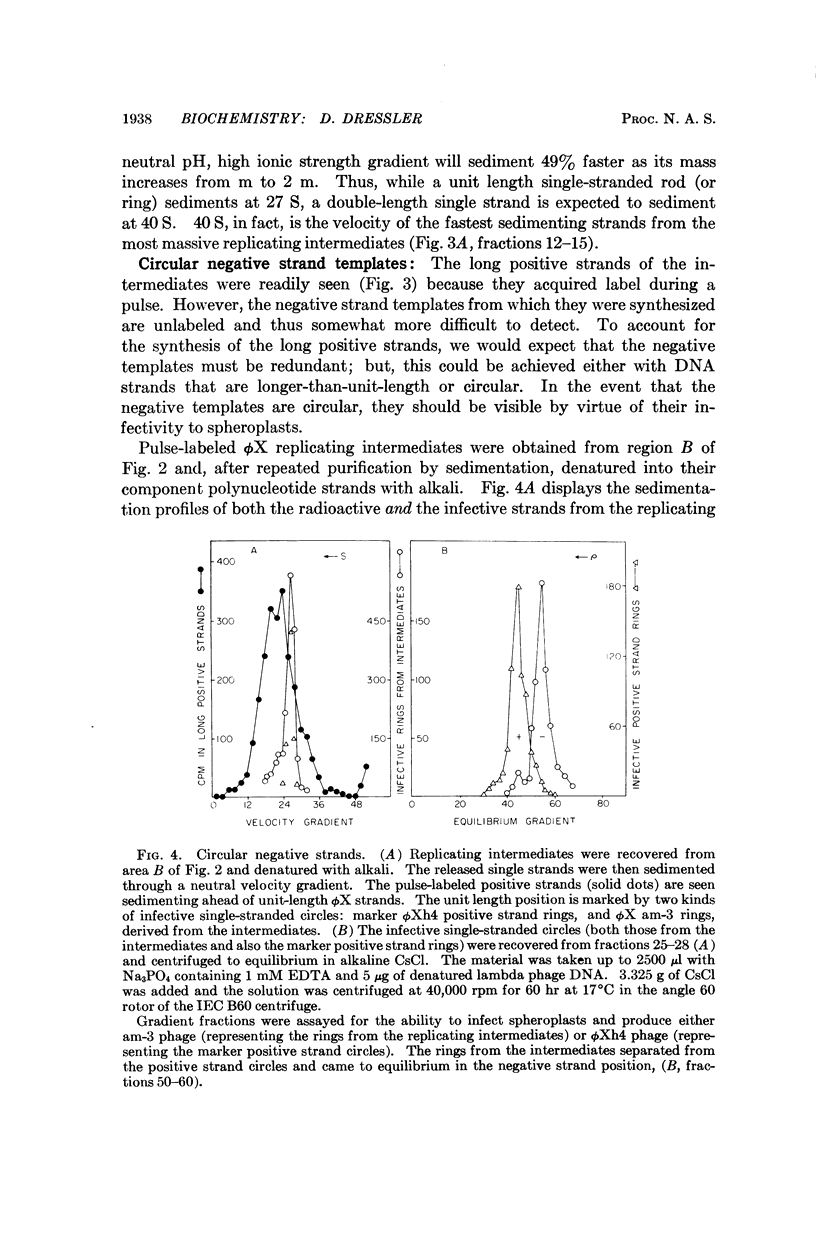
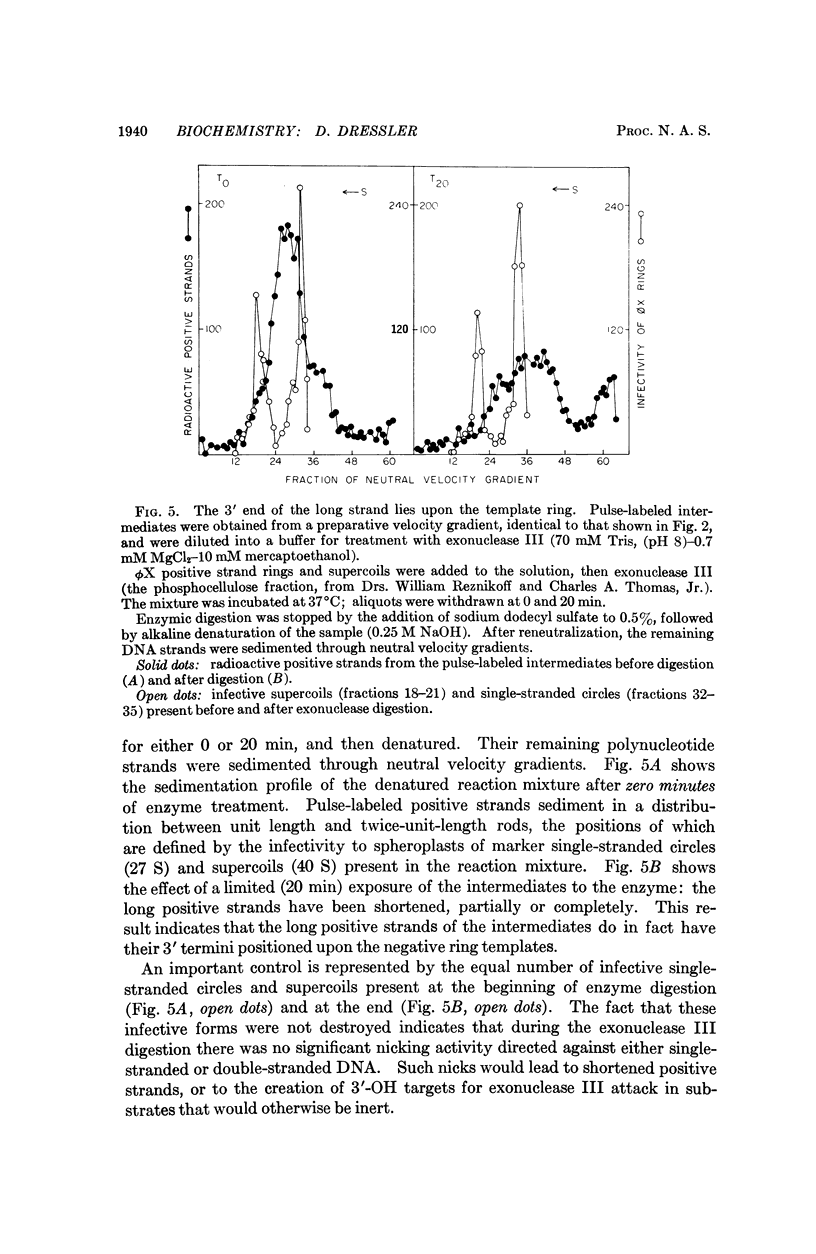
ϕX-infected cells have been allowed to incorporate tritiated thymidine late in the phage life cycle when single-stranded circles are the product of DNA synthesis. Virtually all of the radioactivity is recovered in a continuum of actively replicating viral DNA molecules. These molecules are termed rolling circle intermediates because they are characterized by three structural properties. They possess positive strands that are longer than the length of a mature viral genome, and negative strands that are covalently closed single-stranded circles. The 3′ termini of the long positive strands lie upon the template rings, while the 5′ ends are free in solution.
From these experimental data, the basic mode of synthesis is deduced to involve the continuous elongation of the open positive strand by endless copying around the circular negative strand template. As new bases are added to the template-bound (3′) end of the positive strand, the distal (5′) end is displaced from the template ring as a single-stranded tail of increasing length. It is the tail which serves as the source of material for progeny chromosomes.
These data confirm our characterization of this ϕX intermediate, which initially was based only on the possession of long positive strands, and extend this characterization to include experimental statements about the circular nature of the template DNA strand, and the 5′ to 3′ direction of polynucleotide chain growth within the intermediate. Moreover, the description can now be applied to all of the molecules which acquire label during a pulse.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Denhardt D. T., Sinsheimer R. L. The process of infection with bacteriophage phi-X174. 3. Phage maturation and lysis after synchronized infection. J Mol Biol. 1965 Jul;12(3):641–646. doi: 10.1016/s0022-2836(65)80318-7. [DOI] [PubMed] [Google Scholar]
- Dressler D. H., Denhardt D. T. Mechanism of replication of phi-X-174 single stranded DNA. Nature. 1968 Jul 27;219(5152):346–351. doi: 10.1038/219346a0. [DOI] [PubMed] [Google Scholar]
- Dressler D., Wolfson J. The rolling circle for phi X DNA replication. 3. Synthesis of supercoiled duplex rings. Proc Natl Acad Sci U S A. 1970 Sep;67(1):456–463. doi: 10.1073/pnas.67.1.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton C. Continuous chromosome transfer in Escherichia coli. Genetics. 1965 Jul;52(1):55–74. doi: 10.1093/genetics/52.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUTHRIE G. D., SINSHEIMER R. L. Observations on the infection of bacterial protoplasts with the deoxyribonucleic acid of bacteriophage phi X174. Biochim Biophys Acta. 1963 Jun 25;72:290–297. [PubMed] [Google Scholar]
- Gilbert W., Dressler D. DNA replication: the rolling circle model. Cold Spring Harb Symp Quant Biol. 1968;33:473–484. doi: 10.1101/sqb.1968.033.01.055. [DOI] [PubMed] [Google Scholar]
- Hershey A. D., Burgi E., Ingraham L. COHESION OF DNA MOLECULES ISOLATED FROM PHAGE LAMBDA. Proc Natl Acad Sci U S A. 1963 May;49(5):748–755. doi: 10.1073/pnas.49.5.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Replicating molecules of polyoma virus DNA. J Mol Biol. 1969 Feb 28;40(1):141–144. doi: 10.1016/0022-2836(69)90302-7. [DOI] [PubMed] [Google Scholar]
- Inselburg J., Fuke M. Replicating DNA: structure of colicin factor E1. Science. 1970 Aug 7;169(3945):590–592. doi: 10.1126/science.169.3945.590. [DOI] [PubMed] [Google Scholar]
- Kiger J. A., Jr, Sinsheimer R. L. Vegetative lambda DNA. IV. Fractionation of replicating lambda DNA on benzoylated-naphthoylated DEAE cellulose. J Mol Biol. 1969 Mar 28;40(3):467–490. doi: 10.1016/0022-2836(69)90166-1. [DOI] [PubMed] [Google Scholar]
- Knippers R., Razin A., Davis R., Sinsheimer R. L. The process of infection with Bacteriophage phi-X174. XXIX. In vivo studies on the synthesis of the single-stranded DNA of progeny phi-X174 bacteriophage. J Mol Biol. 1969 Oct 28;45(2):237–263. doi: 10.1016/0022-2836(69)90103-x. [DOI] [PubMed] [Google Scholar]
- Lee C. S., Davidson N. Physicochemical studies on the minicircular DNA in Escherichia coli 15. Biochim Biophys Acta. 1970 Apr 15;204(2):285–295. doi: 10.1016/0005-2787(70)90146-2. [DOI] [PubMed] [Google Scholar]
- Ohki M., Tomizawa J. Asymmetric transfer of DNA strands in bacterial conjugation. Cold Spring Harb Symp Quant Biol. 1968;33:651–658. doi: 10.1101/sqb.1968.033.01.074. [DOI] [PubMed] [Google Scholar]
- RICHARDSON C. C., LEHMAN I. R., KORNBERG A. A DEOXYRIBONUCLEIC ACID PHOSPHATASE-EXONUCLEASE FROM ESCHERICHIA COLI. II. CHARACTERIZATION OF THE EXONUCLEASE ACTIVITY. J Biol Chem. 1964 Jan;239:251–258. [PubMed] [Google Scholar]
- Ray D. S. Replication of bacteriophage M13. II. The role of replicative forms in single-strand synthesis. J Mol Biol. 1969 Aug 14;43(3):631–643. doi: 10.1016/0022-2836(69)90364-7. [DOI] [PubMed] [Google Scholar]
- Rupp W. D., Ihler G. Strand selection during bacterial mating. Cold Spring Harb Symp Quant Biol. 1968;33:647–650. doi: 10.1101/sqb.1968.033.01.073. [DOI] [PubMed] [Google Scholar]
- STRACK H. B., KAISER A. D. ON THE STRUCTURE OF THE ENDS OF LAMBADA DNA. J Mol Biol. 1965 May;12:36–49. doi: 10.1016/s0022-2836(65)80280-7. [DOI] [PubMed] [Google Scholar]
- STUDIER F. W. SEDIMENTATION STUDIES OF THE SIZE AND SHAPE OF DNA. J Mol Biol. 1965 Feb;11:373–390. doi: 10.1016/s0022-2836(65)80064-x. [DOI] [PubMed] [Google Scholar]
- Sinsheimer R. L., Knippers R., Komano T. Stages in the replication of bacteriophage phi X174 DNA in vivo. Cold Spring Harb Symp Quant Biol. 1968;33:443–447. doi: 10.1101/sqb.1968.033.01.051. [DOI] [PubMed] [Google Scholar]
- Smith M. G., Skalka A. Some properties of DNA from phage-infected bacteria. J Gen Physiol. 1966 Jul;49(6):127–142. doi: 10.1085/jgp.49.6.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VINOGRAD J., MORRIS J., DAVIDSON N., DOVE W. F., Jr The bouyant behavior of viral and bacterial DNA in alkaline CsCl. Proc Natl Acad Sci U S A. 1963 Jan 15;49:12–17. doi: 10.1073/pnas.49.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westmoreland B. C., Szybalski W., Ris H. Mapping of deletions and substitutions in heteroduplex DNA molecules of bacteriophage lambda by electron microscopy. Science. 1969 Mar 21;163(3873):1343–1348. doi: 10.1126/science.163.3873.1343. [DOI] [PubMed] [Google Scholar]




