Abstract
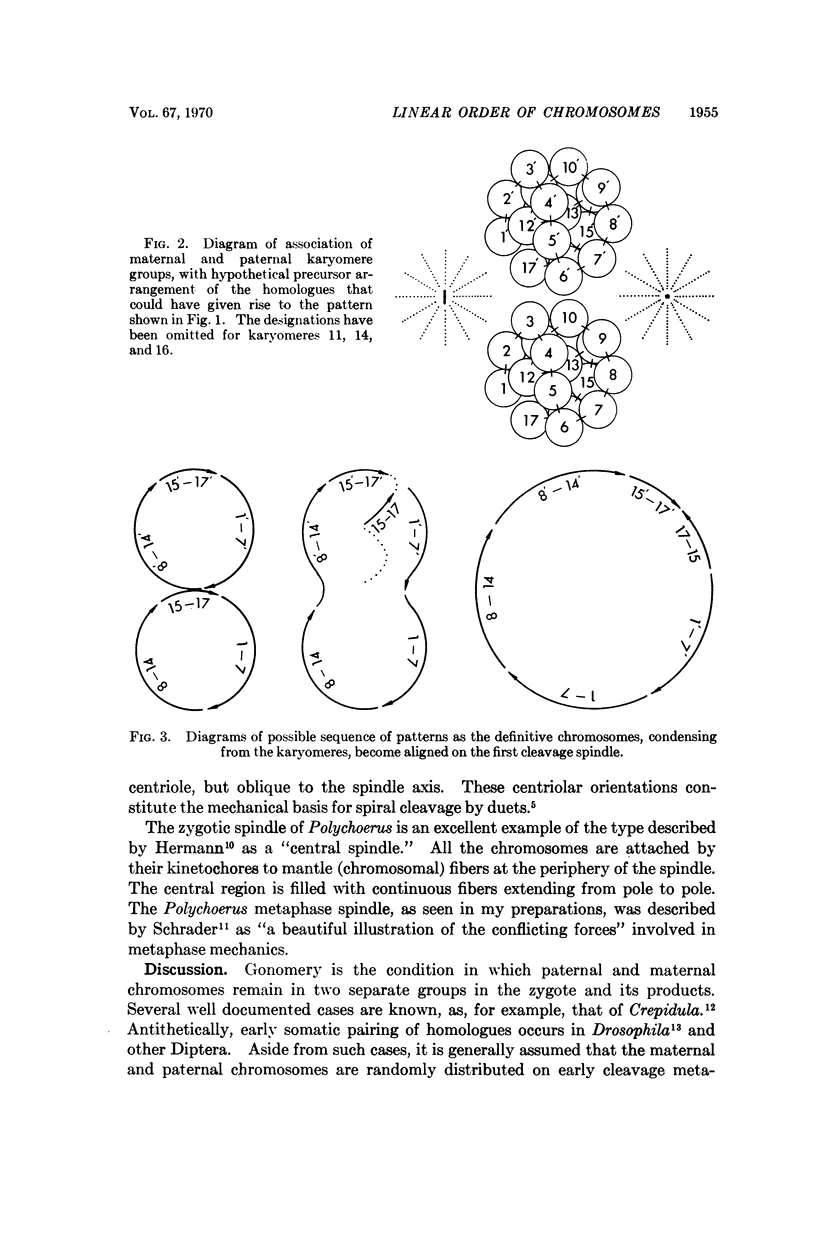
In an exceptionally well-defined first cleavage metaphase in a sectioned egg of Polychoerus carmelensis all 34 (2n) chromosomes were present in a single 8-micron section, and it was possible to identify the homologous chromosomes derived from the two parent gametes. Three groups of chromosomes from one parent, containing 7, 7, and 3 chromosomes, respectively, could be exactly matched by corresponding groups of homologues derived from the other gamete. The probability of such an ordered pattern occurring by chance is somewhat less than 1 in 248. The simplest explanation of this arrangement is that the 17 chromosomes contributed by the egg were in precisely the same linear order as those contributed by the spermatozoon. This suggests that during certain stages preceding first cleavage metaphase, the 17 members of each haploid set of chromosomes may have been attached, end-to-end, in a linear fashion, by highly specific bonds.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin R. L., Barrand P., Fritsch A., Goldthwait D. A., Jacob F. Cohesive sites on the deoxyribonucleic acids from several temperate coliphages. J Mol Biol. 1966 Jun;17(2):343–357. doi: 10.1016/s0022-2836(66)80146-8. [DOI] [PubMed] [Google Scholar]
- Berendes H. D., Meyer G. F. A specific chromosome element, the telomere of Drosophila polytene chromosomes. Chromosoma. 1968;25(2):184–197. doi: 10.1007/BF00327177. [DOI] [PubMed] [Google Scholar]
- Comings D. E. The rationale for an ordered arrangement of chromatin in the interphase nucleus. Am J Hum Genet. 1968 Sep;20(5):440–460. [PMC free article] [PubMed] [Google Scholar]
- Gowen J. W., Gay E. H. Gene Number, Kind, and Size in Drosophila. Genetics. 1933 Jan;18(1):1–31. doi: 10.1093/genetics/18.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGHES-SCHRADER S. HERMAPHRODITISM IN AN AFRICAN COCCID, WITH NOTES ON OTHER MARGARODIDS (COCCOUIDEA--HOMOPTERA). J Morphol. 1963 Sep;113:173–184. doi: 10.1002/jmor.1051130205. [DOI] [PubMed] [Google Scholar]
- Hershey A. D., Burgi E., Ingraham L. COHESION OF DNA MOLECULES ISOLATED FROM PHAGE LAMBDA. Proc Natl Acad Sci U S A. 1963 May;49(5):748–755. doi: 10.1073/pnas.49.5.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton T., Atwood K. C. Terminal Adhesions of Salivary Gland Chromosomes in Drosophila. Proc Natl Acad Sci U S A. 1941 Nov 15;27(11):491–496. doi: 10.1073/pnas.27.11.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser A. D., Wu R. Structure and function of DNA cohesive ends. Cold Spring Harb Symp Quant Biol. 1968;33:729–734. doi: 10.1101/sqb.1968.033.01.083. [DOI] [PubMed] [Google Scholar]
- Koshman R. W., Serra J. A. Spireme karyodieresis: a new type of reductional division. Can J Genet Cytol. 1967 Mar;9(1):31–37. doi: 10.1139/g67-004. [DOI] [PubMed] [Google Scholar]
- Yerganian G., Papoyan S. Isomorphic sex chromosomes, autosomal heteromorphism, and telomeric associations in the grey hamster of Armenia, Cricetulus migratorius, Pall. Hereditas. 1965;52(3):307–319. doi: 10.1111/j.1601-5223.1965.tb01963.x. [DOI] [PubMed] [Google Scholar]




