Abstract
Acute allograft rejection has often been correlated with Th1 differentiation, whereas transplantation tolerance is frequently associated with induction of regulation. The discovery of the Th17 phenotype has prompted its scrutiny in transplant rejection. Although IL-17 has recently been observed in settings of acute allograft rejection and drives rejection in T-bet-deficient mice that have impaired type 1 T cell responses, there is little evidence of its requirement during acute rejection in wild-type animals. We and others have previously shown that TLR9 signaling by exogenous CpG at the time of transplantation is sufficient to abrogate anti-CD154-mediated acceptance of fully mismatched cardiac allografts. In this study, we investigated the mechanism by which acute rejection occurs in this inflammatory context. Our results indicate that CpG targets recipient hematopoietic cells and that its pro-rejection effects correlate both with prevention of anti-CD154-mediated conversion of conventional CD4+ T cells into induced regulatory T cells (iTregs) and with the expression of IFN-γ and IL-17 by intra-graft CD4+ T cells. Moreover, the combined elimination of IL-6 and IL-17 signaling abrogated the ability of CpG to promote acute cardiac allograft rejection. Thus, pro-inflammatory signals at the time of transplantation can change the quality of the effector immune response and reveal a pathogenic function for IL-6 and IL-17 in wild-type recipients.
Keywords: rodent, T cells, transplantation, cell differentiation, cytokines
Introduction
Acute allograft rejection is a T cell-dependent phenomenon. Effector CD4+ T cells were traditionally thought to segregate into Th1 and Th2 subsets with T cells exposed to IL-12 differentiating into IFN-γ-producing Th1 cells and exposure to IL-4 resulting in IL-4-producing Th2 cells. More recently, two other pathways of differentiation have been described, namely induced regulatory CD4+ T cells (iTregs) upon TCR stimulation during TGF-β/IL-2 signaling and Th17 cells for murine T cells activated in the presence of TGF-β/IL-6 (1). Acute allograft rejection has traditionally been associated with Th1 differentiation, as rejection often correlates with expression of IFN-γ in allografts and production of IFN-γ upon restimulation of peripheral T cells with alloantigen (2). However, we and others have shown that other pathways of allograft rejection must exist inasmuch as kinetics of rejection are unaltered in mice genetically deficient in STAT4 that have defective Th1 differentiation (3, 4).
The role of Th17 cells in acute allograft rejection has been less widely studied. IL-17 transcripts have been described in the early phase of rat and human kidney allograft rejection (5, 6) and IL-17-producing cells were sufficient to mediate rejection of rat lung allografts (7). In addition, IL-17 was found to play a major role in cardiac allograft rejection by mice deficient in T-bet that have reduced IFN-γ production and type 1 T cell differentiation (8, 9). However, antagonism of IL-17 only modestly delayed acute rejection in wild-type recipients of aortic (10) and cardiac transplantation (11). These results suggest that IL-17-producing cells become dominant when other pathways to rejection are dampened. Interestingly, there appears to be reciprocal control of Th17 and iTreg differentiation (12), both subsets requiring TGF-β signaling, but the concomitant presence of inflammatory cytokines antagonizing iTreg and promoting Th17 differentiation and IL-2 exerting converse effects. Whether this equilibrium occurs in transplantation models in vivo and if so, whether it can be skewed in one direction or the other, remains to be determined.
Toll-like receptors (TLRs) recognize molecular patterns expressed on microorganisms and signal the production of pro-inflammatory cytokines necessary for the elimination of pathogens (13). In particular, TLR signaling can induce the production of IL-6 , a potent Th17 differentiation cytokine and IL-12, a driver of Th1 differentiation (14, 15). In addition, a main effect of TLR signals in vivo may be to relieve effector T cells from suppression by Tregs (16). We and others have shown that peri-operative administration of synthetic TLR agonists can prevent the induction of transplantation tolerance (17–19). In our fully mismatched model of cardiac transplantation, injection of the TLR9 agonist CpG or of the TLR2 agonist Pam3CysK4 at the time of transplantation prevented anti-CD154-mediated long-term allograft acceptance and reduced intra-graft recruitment of CD4+FoxP3+ Tregs (18), an event thought to be necessary for anti-CD154-mediated tolerance (20). We hypothesized that TLR signals at the time of transplantation result in the production of pro-inflammatory cytokines by donor or recipient APCs that should both promote the differentiation of alloreactive T cells into Th1 and Th17 cells, and allow their escape from Treg suppression. In addition, TLR signals may prevent iTreg generation in anti-CD154 mAbs-treated recipients by further antagonizing the development of dominant suppression thereby resulting in allograft rejection. The purpose of our study was to identify the mechanisms of action of CpG by studying the effects of CpG on iTregs and monitoring the impact of TLR9 signaling on effector T cells and mechanisms of rejection. Our results demonstrate that CpG targets hematopoietic cells of recipient origin and promotes acute cardiac allograft rejection in a novel manner that depends on IL-6 and IL-17 and is distinct from acute rejection in untreated wildtype recipients.
Materials and Methods
Mice
C57Bl/6 (B6, H-2b), BALB/c (H-2d), B6 CD45.1 and B6 IL-6−/− mice were purchased from The Jackson Laboratory (Bar Harbor, ME). FoxP3-GFP knock-in mice (FoxP3GFP) on a 129/J background and TEa TCR transgenic (TEa-Tg) mice on a B6 background, whose T cells indirectly recognize a peptide of I-Ed presented on I-Ab, were obtained from Dr. Alexander Rudensky (University of Washington, Seattle, WA). FoxP3GFP mice were backcrossed to B6 mice for 6 generations. TEa-Tg mice were crossed to FoxP3GFP. MyD88−/− mice on a BALB/c and a B6 background were obtained from Dr. Shizuo Akira (Osaka University, Osaka, Japan). B6 CD45.1, B6 IL-6−/−, FoxP3GFP, MyD88−/− and TEa-FoxP3GFP mice were housed under specific pathogen-free conditions and used in agreement with our Institutional Animal Care and Use Committee, according to the National Institutes of Health guidelines for animal use.
Generation of bone marrow chimeric mice
Marrow from femurs and tibias were obtained by flushing. Erythrocytes were lysed from the marrow suspension, and T cells were depleted using anti-Thy1.2 mAb and negative selection over magnetic beads according to the instructions of the manufacturer (StemCell Technologies). Bone marrow cells (2 × 106) from B6 MyD88−/− and control mice (CD45.2) were injected i.v. to reconstitute lethally irradiated (11Gy 18h prior) congenic (CD45.1) recipients. Reconstitution was verified after 8 weeks by flow cytometry on peripheral blood and chimeric mice were then transplanted with fully allogeneic BALB/c hearts.
Heart transplantation
Transplantation of fully mismatched cardiac allografts was performed using a technique adapted from that originally described by Corry et al. (21). Cardiac allografts were transplanted in the abdominal cavity by anastomosing the aorta and pulmonary artery of the graft end-to-side to the recipient's aorta and vena cava, respectively. Recipient mice were treated with anti-CD154 (MR1 purified in our laboratories, 1 mg/mouse i.v. on day 0, and i.p. on days 7 and 14 post-transplantation) alone or in combination with CpG (CpG-B ODN 1826, Coley Pharmaceutical Group Ltd, 100 µg i.v. day 0, and 50 µg i.p. days 1 and 2) or anti-IL-17A mAb ( R&D Systems Inc., 100 µg/mouse i.v. on day 0 and i.p. on days 1, 2, 3, 4, 7, 9, 11 and 13), or control IgG (Jackson ImmunoResearch Laboratories). The day of rejection was defined as the last day of a detectable heartbeat in the graft. Graft rejection was verified in selected cases by necropsy and pathological examination of H&E-stained graft sections.
Isolation of intra-graft infiltrating leukocytes
Cardiac grafts were rinsed in situ with HBSS/1‰ heparin. Explanted hearts were cut into small pieces and digested for 40 min at 37°C with 400 U/mL collagenase IV (Sigma), 10 mM Hepes (Cellgro) and 0.01% DNase I (MP Biomedicals) in HBSS (Cellgro). Digested suspensions were passed through a nylon mesh and centrifuged and the cell pellet was resuspended in 5 mL of 45.5% Nycodenz solution (Sigma). Three milliliters of complete Dulbecco's Modified Eagle medium (DMEM) were added to the top of the Nycodenz and a centrifugation gradient was performed (1700g for 15 min at 4°C). The cells at the interface were recovered, washed with complete DMEM, stained according to the manufacturer's instructions and analyzed by flow cytometry (BD LSR-II; BD Biosciences).
Intracellular staining and flow cytometric analysis
Lymphocytes were isolated from spleens, lymph nodes (LNs) and heart grafts and processed into single cell suspensions. Cells were stained with APC-coupled anti-CD4 (L3T4), PE-Cy7-or PerCP-coupled anti-CD8 (Ly2), PE-Cy7-coupled anti-CD45.1 (A20), APC-Alexa Fluor 750-coupled anti-CD45.2 (104), and PE-Cy7-coupled anti-Thy1.2 (53-2.1) mAbs. For FoxP3 intracellular staining, surface-stained cells were fixed with 1% PFA in PBS/0.1% BSA/0.01M Hepes/0.1% saponin for 2–18 h and then incubated with FITC-coupled anti-FoxP3 (FJK-16s) for 30 min. After washing twice with PBS/0.1% BSA/0.01M Hepes/0.1% saponin, cells were resuspended in PBS/1% FCS and analyzed by flow cytometry. For IL-17 and IFN-γ intracellular staining, cells were stimulated with PMA (Sigma), ionomycin (Sigma) and GogiPlug (BD Pharmingen) for 6 h, prior to staining for surface markers. Cells were then stained intracellularly using PE-coupled anti-IFN-γ (XMG1.2), APC-coupled anti-IL-17A (eBio17B7) and analyzed by flow cytometry. All mAbs were from BD Pharmingen or eBioscience.
iTreg conversion in vivo
Spleens from TEa-FoxP3GFP mice were used to sort CD3+CD4+GFP−naïve T cells. Purity was greater than 99%. Sorted cells (5×106) were stained with PKH26 and injected i.v. into syngeneic B6 mice 1 day prior to transplantation with BALB/c hearts. Recipients were left untreated or injected with anti-CD154+CpG as described above. Animals were sacrificed on day 10 post transplantation and spleens, LNs and cardiac allografts were analyzed by flow cytometry for expression of GFP (FoxP3) within CD4-gated live events.
Generation and culture of bone marrow-derived dendritic cells (BMDCs)
BMDCs were generated from BM progenitors as previously described (22). Briefly, BM cells (5 × 106) from B6 WT or B6 IL-6−/− mice were cultured in 10 ml complete medium supplemented with 10 ng/ml of recombinant murine GM-CSF (R&D Systems). Culture medium was renewed every other day. On day 7 of culture, DCs were harvested, and irradiated (2500 rads) before in vitro culture.
Generation of Tregs in vitro and cytokine analysis
CD4+ T cells from pooled spleens and LNs of FoxP3GFP-knock-in, B6 or IL-6−/− mice were enriched by negative selection over magnetic beads according to the instructions of the manufacturer (StemCell Technologies). Enriched CD4+ T cells were stained with APC-coupled anti-CD4 (L3T4), APC-Alexa-Fluor750-coupled anti-CD25 (PC61.5) and PE-coupled anti-CD44 (1M7) and were FACS-sorted in a high-speed cell sorter (Dakocytomation MoFlo) to obtain FoxP3-GFP−, FoxP3-GFP+, or CD44lowCD25− CD4+ T cells. Naïve CD44lowCD25− CD4+ T conventional cells were resuspended in complete DMEM [supplemented with 10% FBS, penicillin (100 U/ml), streptomycin (100 µg/ml), HEPES, 2-ME (50 µM), and additional amino acids], plated on flat-bottom 96-well plates (2 × 105/well) and stimulated with plate-bound anti-CD3 (2 µg/ml) and anti-CD28 (2 µg/ml) mAbs, TGF-β̣ (2 ng/ml) and recombinant human IL-2 (50 U/ml) in the presence or absence of syngeneic irradiated (2000 rads) splenocytes or syngeneic BMDCs +/− CpG for five days. Results were similar whether syngeneic splenocytes or BMDCs were used as APCs. The cells were harvested, stained with anti-CD4 Ab, and analyzed for FoxP3-GFP+ or intracellular FoxP3+ expression by flow cytometry. Supernatants were collected and the concentration of IL-17A in each sample was detected by ELISA using Ab pairs as instructed by the manufacturer (BD Pharmingen; San Diego, CA). Absorbance was detected in a 96-well spectrophotometer (µQuant; Bio-Tek Instruments), and data were analyzed using KC4 software (Bio-Tek Instruments) by comparison to a standard curve generated using recombinant cytokines at known concentrations.
Analysis of serum cytokines
B6 mice were injected with CpG (100 mg i.p.) and serum was collected serially at 0, 2, 5 and 10 hrs post injection. The concentration of IL-12 and IL-6 in the serum were determined by multiplex bead analysis (Invitrogen, CA) and ELISA (eBioScience, CA), respectively, according to the instructions by the manufacturer.
Real-time RT-PCR
Lymphocytes were isolated from spleens, LNs and heart grafts and processed into single cell suspensions. Total cellular RNA was isolated using RNeasy Plus Mini Kit (Qiagen) and subjected to reverse transcription (RT), using iScript cDNA Synthesis Kit (Bio-Rad Laboratories) prior to PCR amplification. For RT reactions, 1 µg of total RNA was used per sample, and RT was conducted for 30 min at 42°C. For PCR, the primers used were as follows: IL-17, forward: TCCAGAAGGCCCTCAGACTA; reverse: AGCATCTTCTCGACCCTGAA; β-actin, forward: TGGAATCCTGTGGCAT-CCATGAAAC; reverse: TAAAACGCAGCTCAGTAACAGTCCG; CD3ε, forward: GATGCGGTGGAACACTTTCT; reverse: ACTGTCCTCGACTTCC-GAGA;
IFN-γ ELISPOTs
Splenocytes (106/well) from untransplanted mice or from transplanted mice harvested following rejection by the anti-CD154+CpG-treated group were restimulated with irradiated syngeneic (B6), donor (BALB/c) or third party (C3H) splenocytes (4 × 105/well) for 24 h. The ELISPOT assay was conducted according to the instructions of the manufacturer (BD Biosciences), and the numbers of spots per well were enumerated using the ImmunoSpot Analyzer (CTL Analyzers LLC, Cleveland, OH).
Statistical analysis
Comparisons of means were performed using the Student’s t test, the Mann-Whitney test, or the Tukey test for multiple comparisons, when appropriate. Graft mean survival time (MST) and p-values were calculated using Kaplan-Meier/log rank test methods. p values <0.05 were considered significant.
Results
CpG promotes rejection that is dependent on expression of MyD88 on recipient hematopoietic cells
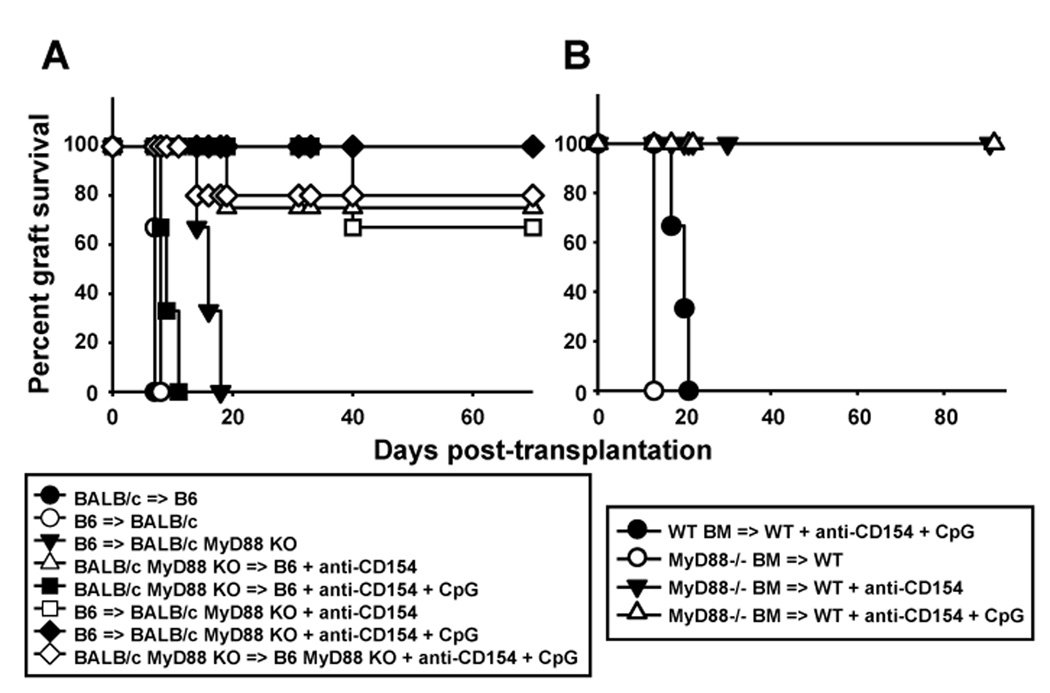
We have previously shown that elimination of MyD88-dependent signaling in both donor skin grafts and recipient mice, but not in either one alone, enables anti-CD154 therapy to induce long-term survival or fully mismatched skin allografts (18). Conversely, administration of the TLR9 agonist, CpG, which also signals via MyD88, prevents long-term transplant acceptance of cardiac allografts mediated by anti-CD154, or their tolerance induced by anti-CD154+donor-specific transfusion (DST) (18). However, the cell types required for CpG to prevent anti-CD154-dependent acceptance of cardiac allografts are not known. To determine whether CpG mediates its pro-rejection effects on heart allografts by targeting cells of donor or host origin, we utilized MyD88−/− mice on BALB/c and B6 backgrounds. Elimination of MyD88 in the recipient but not the donor abrogated the ability of CpG to promote cardiac allograft rejection (Figure 1A), thus contrasting with our previous results in the skin graft model.
Figure 1. The pro-rejection effect of CpG requires MyD88 on recipient hematopoietic cells.
A. Wild-type and MyD88-deficient hearts of BALB/c origin were transplanted into wild-type and MyD88−/− mice on the B6 background. Recipients were either left untreated or treated with anti-CD154±CpG and cardiac allograft survival was examined over time (n=3–5 mice/group). B. T-depleted bone marrow cells from wild-type or MyD88−/− mice on a B6 background (CD45.2+) were injected into congenic (CD45.1+) lethally irradiated B6 recipients and allowed to reconstitute the immune system for 8 weeks prior to transplantation with BALB/c hearts. Recipients were either left untreated or treated with anti-CD154±CpG and cardiac allograft survival was examined over time (n=3 mice/group).
Cells of both parenchymal (endothelial and epithelial) and hematopoietic origin (T cells and APCs in particular) are known to express TLRs and MyD88 (23). To determine whether host cells of hematopoietic or non-hematopoietic origin were required for CpG to prevent cardiac allograft acceptance, bone marrow chimeras were generated using wild-type or MyD88−/− bone marrow to reconstitute lethally irradiated congenic MyD88-competent mice. Resulting animals harbored MyD88-competent parenchymal cells and hematopoietic cells either competent or deficient in MyD88. Bone marrow chimeric animals were transplanted, 8–10 weeks following reconstitution, with BALB/c hearts and treated with anti-CD154 with or without CpG. Administration of CpG+anti-CD154 resulted in acute rejection in recipients reconstituted with MyD88-expressing but not MyD88-deficient bone marrow cells (Figure 1B), indicating that host cells of hematopoietic origin expressing MyD88 are required for CpG to prevent anti-CD154-mediated graft acceptance.
CpG prevents conversion of graft-reactive T cells into iTregs
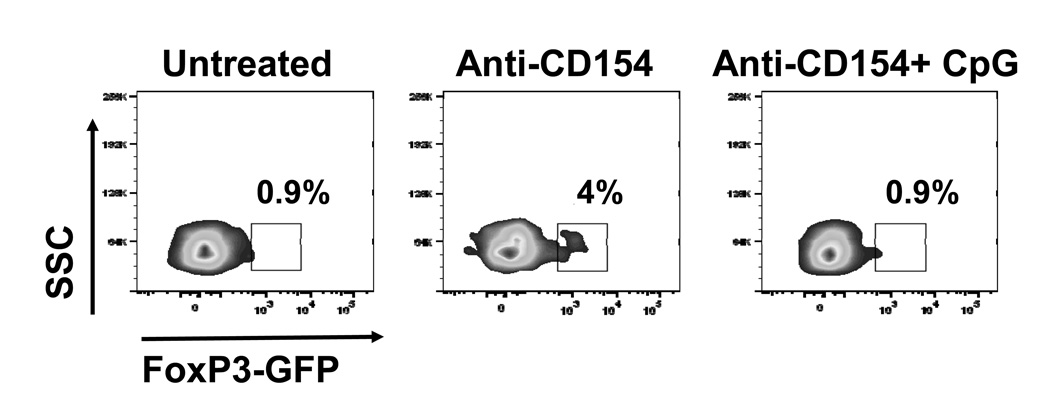
If CpG targets recipient hematopoietic cells, it is likely to alter the differentiation of T cells exposed to alloantigen. Anti-CD154 is known to promote conversion of naïve T cells into iTregs (24). We have previously shown that animals treated with anti-CD154+CpG displayed both reduced numbers of intra-graft CD4+FoxP3+ cells and a decreased intra-graft ratio of CD4+FoxP3+ to CD4+FoxP3− cells (18) when compared with mice treated with anti-CD154 alone. We had concluded that this was due in part to reduced migration of Tregs into the graft as intra-graft expression levels of the CCR4 ligands CCL17 (TARC) and CCL22 (MDC) were diminished in animals treated with CPG (18). However, many graft-infiltrating Tregs were CCR4− (data not shown), such that it remained possible that part of the decreased accumulation of FoxP3+ cells in the allografts of CpG-treated mice was a consequence of reduced Treg proliferation or inhibited conversion of “conventional” T cells into iTregs. To test whether CpG could prevent conversion of naïve T cells into iTregs in vivo, TEa+CD4+FoxP3-negative cells that indirectly recognize a peptide from I-Ed presented on I-Ab were sorted from TEa-FoxP3GFP knock-in mice, labeled with PKH26 and adoptively transferred into syngeneic B6 mice one day before cardiac transplantation with BALB/c hearts. Treatment with anti-CD154 resulted in the conversion of a subset of the transferred CD4+ cells into FoxP3+ cells, which accumulated only in the allograft (Figure 2), and were not detected in either the spleen or LNs (data not shown). In contrast, injection of CpG at the time of transplantation prevented detection of FoxP3 expression among the TEa+CD4+FoxP3-negative transferred cells (Figure 2). This was not due to reduced recruitment of TEa+ cells into cardiac allografts of CpG-treated recipients as similar percentages of PKH26+CD4+ cells were observed in the allografts of tolerant and CpG-treated recipients (data not shown). Together, these results suggest that CpG can prevent conversion of naïve T cells into iTregs in vivo.
Figure 2. CpG prevents conversion of naïve CD4+ T cells into iTregs in vivo.
CD4+GFP− cells were sorted from TEa-Tg-FoxP3GFP knock-in mice, labeled with PKH26 and injected i.v. (5×106) into syngeneic B6 recipients one day prior to transplantation with BALB/c hearts and indicated treatments. Allografts were harvested on day 10 post transplantation and infiltrating leukocytes analyzed by flow cytometry for expression of GFP among CD4-gated events. Numbers in the plots represent percentages of GFP+ cells among CD4+ cells.
CpG opposes TGF-β-mediated iTreg differentiation and promotes IL-17 production in an IL-6-dependent manner in vitro
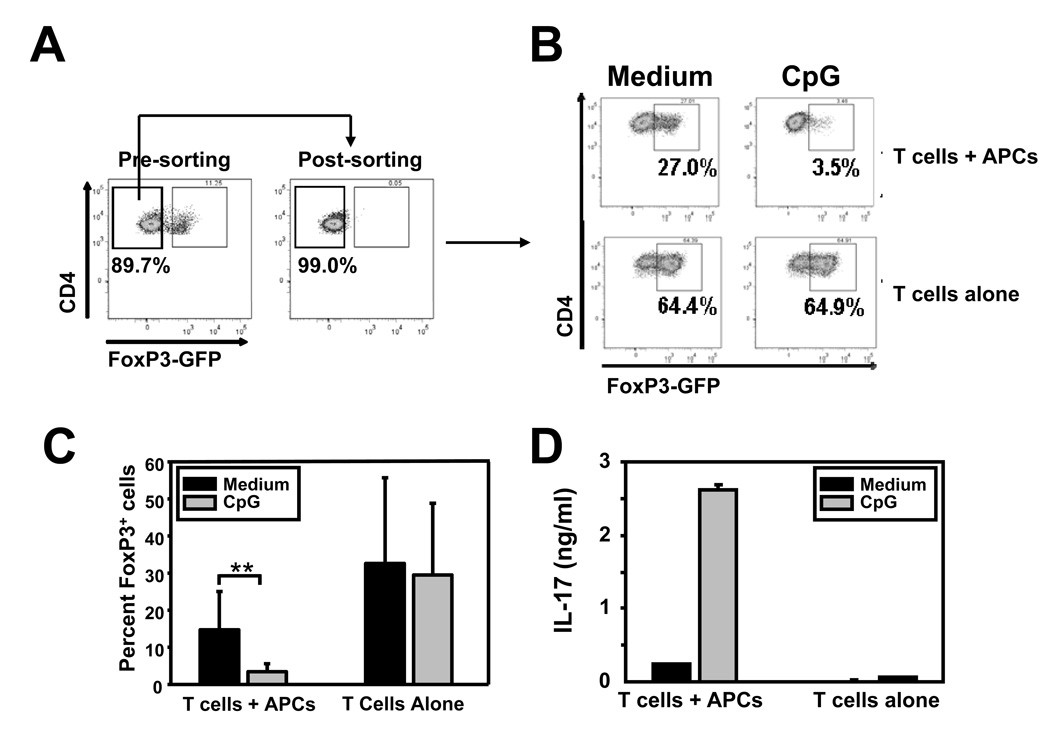
iTregs can be generated in vitro from naïve conventional CD4+ T cells upon TCR stimulation in the presence of TGF-β and IL-2 (25). To investigate the mechanisms by which CpG can prevent iTreg differentiation under more controlled conditions, sorted naïve (CD4+CD25−CD44lowGFP−) conventional T cells from FoxP3GFP-knock-in mice (Figure 3A) were stimulated in vitro under iTreg differentiation conditions with immobilized anti-CD3/CD28 in the presence or absence of T-depleted APCs and CpG. Stimulation of T/APC mixtures in the presence of TGF-β/IL-2 resulted in the conversion of 27% naïve T cells into FoxP3+(GFP+) cells (Figure 3B). Addition of CpG almost completely abrogated iTreg differentiation (Figures 3B and 3C). Importantly, the effect of CpG required the presence of APCs, as iTreg differentiation was not affected in cultures containing T cells alone (Figures 3B and 3C). In fact, conversion of naïve T cells into FoxP3+ cells was more efficient in this setting, presumably because fewer inflammatory factors are produced in the absence of APCs even without exogenous TLR stimulation.
Figure 3. CpG prevents conversion of naïve T cells into iTregs and promotes IL-17 production in an IL-6-dependent manner in vitro.
Sorted naïve CD4+ T cells (CD25− CD44lowGFP−) from FoxP3GFP-knock-in mice were stimulated with immobilized anti-CD3 and anti-CD28, in the presence of TGF-β and IL-2 and ± CpG for 5 days, with or without irradiated syngeneic splenocytes (APCs). A. Cells were analyzed by flow cytometry for expression of CD4 and GFP before and after cell-sorting. B and C. Cells were analyzed by flow cytometry for expression of CD4 and FoxP3-GFP on day 5 of the culture. Panel B shows a representative flow cytometry profile. Panel C shows the means and standard deviations of the percentage of FoxP3+ cells among CD4+ cells from 5 independent experiments. D. Supernatants were collected on day 5 and analyzed by ELISA for IL-17 content. Results represent means and standard deviations from triplicate determinations and are representative of 4 independent experiments.
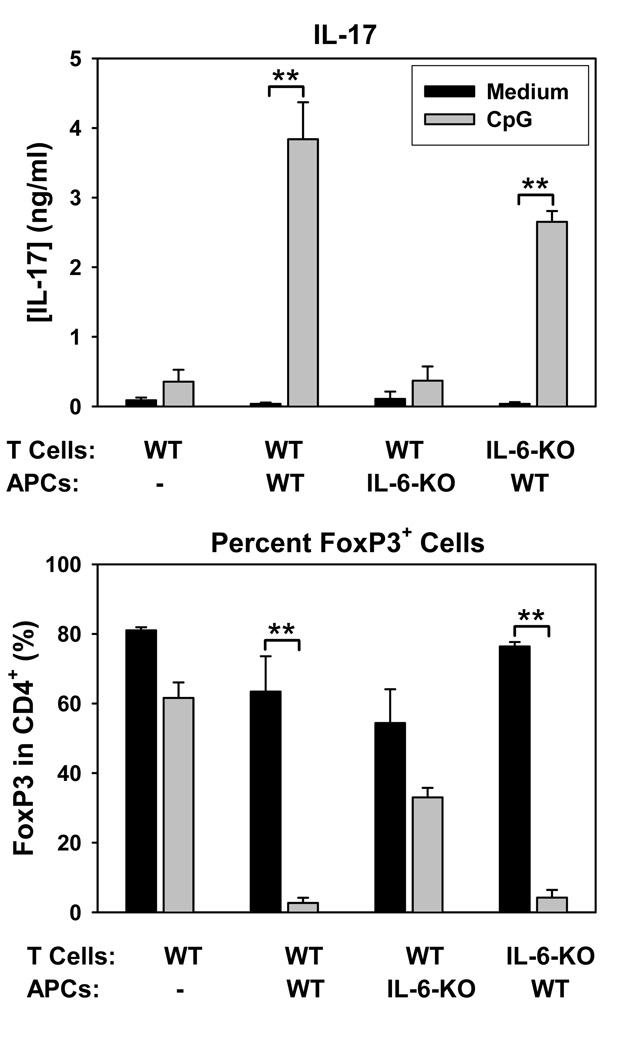
Analysis of supernatants in the same cultures by ELISA revealed that CpG not only prevented iTreg differentiation, but concomitantly promoted IL-17 production (Figure 3D). TLR signals can induce IL-6 production and IL-6 is a potent driver of Th17 differentiation when in the presence of TGF-β. To test whether the effects of CpG were due to IL-6 production, experiments were repeated using T cells or APCs from IL-6-deficient mice. As shown in Figure 4, CpG-mediated promotion of IL-17 production and prevention of FoxP3 induction required production of IL-6 by APCs but not T cells. These results suggest that in vitro, CpG activates APCs to produce IL-6, which in turn antagonizes iTreg differentiation and promotes the production of IL-17 cells by naïve CD4+ T cells.
Figure 4. CpG-mediated IL-17 production is dependent on APC-but not T cell-derived IL-6.
Naïve T cells from wild-type and IL-6−/− mice were sorted as for Figure 3 and stimulated as above except that APCs were syngeneic bone marrow-derived dendritic cells (BMDCs) of either wiltype or IL-6−/− origin. Cells and supernatants were harvested on day 5 for analysis of Foxp3 expression on CD4+ cells by flow cytometry and of IL-17 by ELISA, respectively. Results represent means and standard deviations from triplicate determinations and are representative of 3 independent experiments. **p<0.01 between the groups spanned by the horizontal bars.
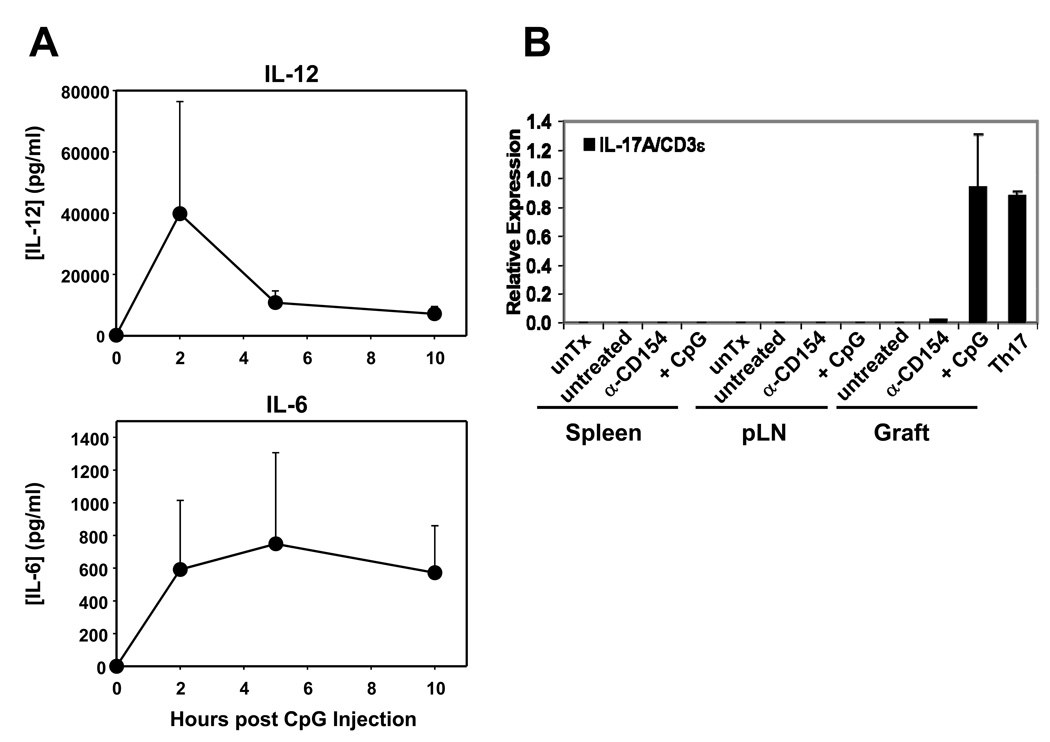
CpG promotes IL-17 production in cardiac allograft recipients
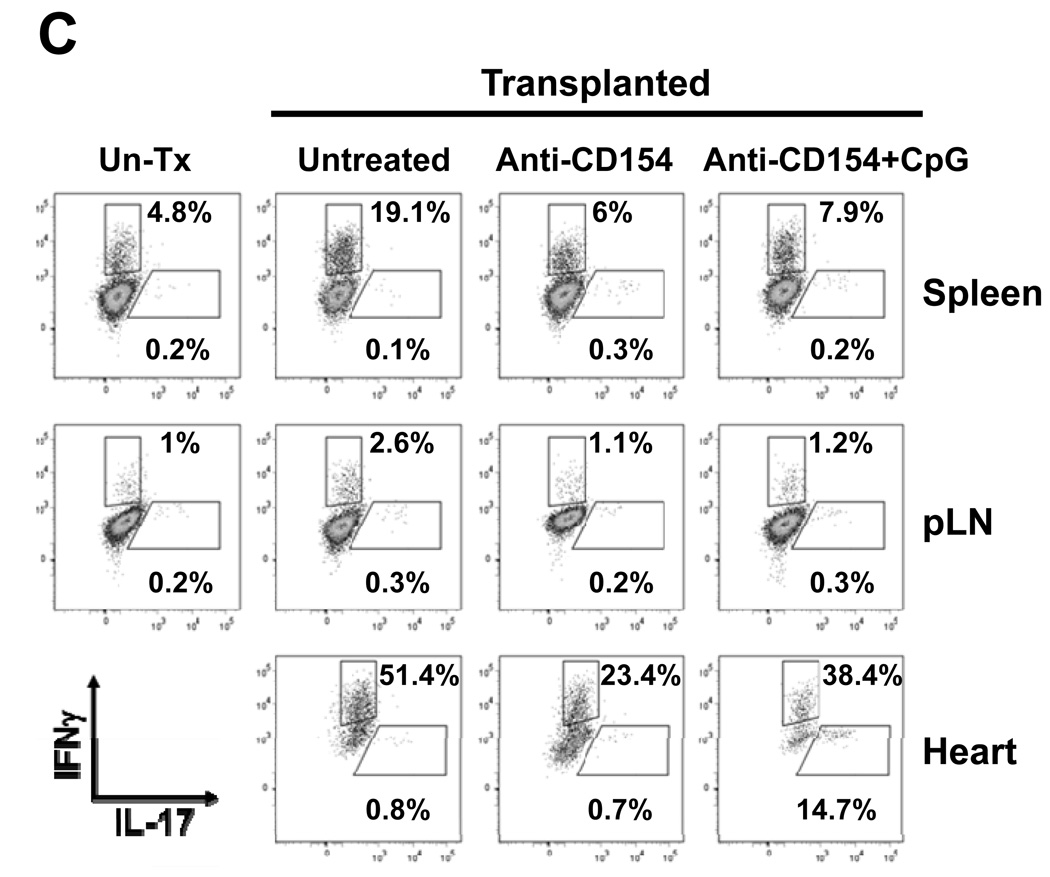
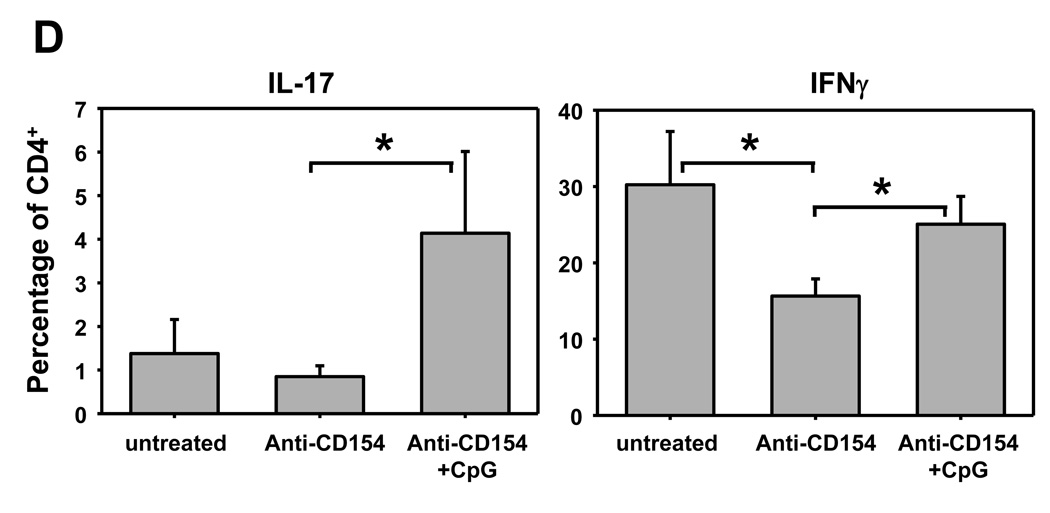
We have previously shown that administration of CpG at the time of transplantation in anti-CD154-treated recipient mice indeed restores IFN-γ production by alloreactive splenocytes as determined by ELISpot (18), suggesting that CpG facilitates the generation of graft-specific type 1 T cells in anti-CD154-treated recipients. To determine whether administration of CpG in vivo resulted in production of IL-12 or IL-6 that may potentially drive Th1 and Th17 differentiation, B6 animals treated with one dose of CpG were bled serially over a 10h period. IL-12 and IL-6 were detected rapidly after CpG injection (Figure 5A). To determine if IL-17-producing cells are also generated in CpG-treated transplanted mice, allografts and secondary lymphoid organs were harvested 2 weeks after transplantation in animals untreated or treated with anti-CD154±CpG. Infiltrating leukocytes from the graft and secondary lymphoid organs were isolated for analysis by real time RT-PCR or flow cytometry. IL-17 mRNA expression was detected only in the cardiac allografts from CpG/anti-CD154-treated animals, but not in untreated or anti-CD154-treated recipients and not in secondary lymphoid organs from any group (Figure 5B). Similarly, IL-17 was detected at the protein level by flow cytometry in graft-infiltrating CD4+ T cells from CpG/anti-CD154-treated animals but not in any other location or treatment group (Figures 5C and 5D) and not in CD8+ T cells (data not shown). In contrast, IFN-γ expression by CD4+ cells was detected in all groups and locations, although, as expected, in lower proportions in anti-CD154-treated groups (Figure 5C and 5D). Together with Figure 2, these results are consistent with the hypothesis that TLR signals at the time of transplantation may favor Th17 differentiation while preventing iTreg differentiation in anti-CD154-treated recipients.
Figure 5. CpG promotes expression of IL-17 in cardiac allografts.
A. B6 mice were injected with CpG (100µg) i.p. and serum was collected serially at the indicated time points. Concentration of IL-12 and IL-6 were determined by multiplex bead analysis and ELISA, respectively. Results represent mean+SD from 3 animals per group. B. BALB/c hearts were transplanted into B6 or FoxP3GFP-knock-in recipients that were either left untreated or received anti-CD154 (MR1) or anti-CD154+CpG (CpG). Spleen, pLN and cardiac allografts were harvested 7–14 days post-transplantation. An untransplantated B6 mouse and Th17 cells differentiated in vitro from naïve CD4+ T cells were used as controls. Leukocytes were isolated from the different tissues and mRNA expression for IL-17 and CD3ε was determined by real time RT-PCR. The figure shows the ratio of IL-17A:CD3ε expression levels and the mean+SD of triplicate determinations. The plot is representative of 4 independent experiments. C. Spleen, pLN and heart allografts were harvested 8–12 days post transplantation. Leukocytes were isolated, stimulated for 6h with PMA and ionomycin in the presence of GolgiPlug and stained for analysis by flow cytometry. The plot represents intracellular expression of IL-17 and IFN-γ on CD4-gated events and is representative of 3 independent experiments. D. The percentage of IL-17 and IFN-g-producing cells among CD4-gated events is represented as mean+SD of 3 independent experiments and 5–7 mice per goup. *p<0.05 between the groups indicated by the horizontal bars.
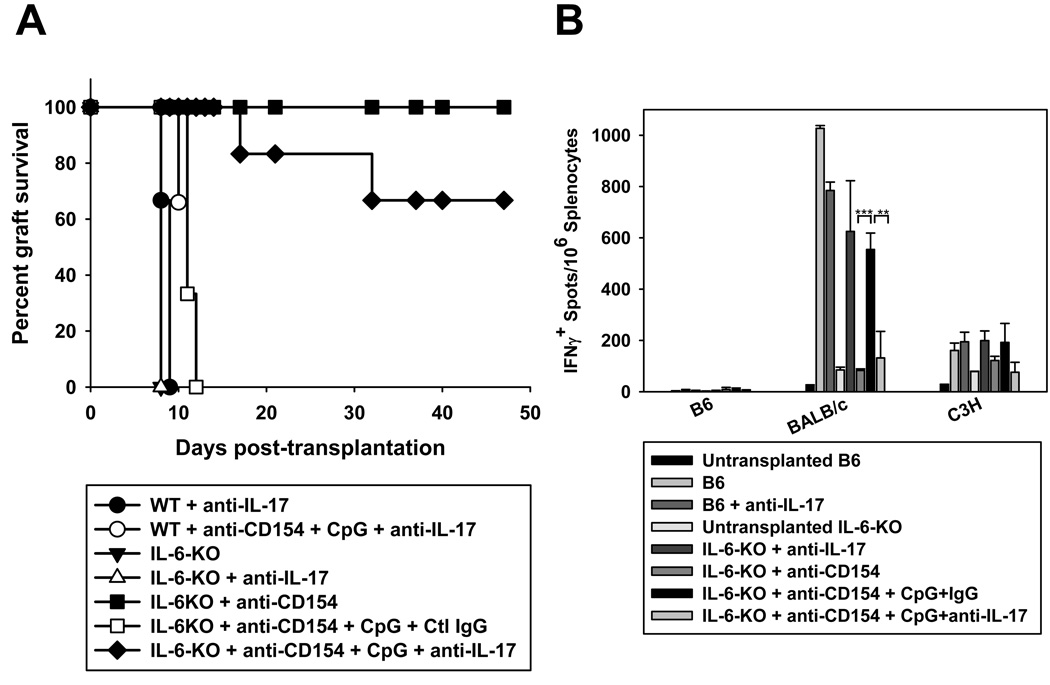
IL6/IL-17 signals are required for CpG to prevent anti-CD154-mediated allograft acceptance in vivo
To determine whether IL-17 was required for CpG-mediated cardiac allograft rejection in vivo, wild-type mice were transplanted with fully allogeneic BALB/c hearts and treated with blocking anti-IL-17 mAb in the presence or absence of anti-CD154+CpG. As shown in Figure 6A, blockade of IL-17 did not prevent CpG-mediated cardiac allograft rejection in anti-CD154-treated wild-type recipients. We reasoned that IL-17 is only one of the effector cytokines produced by Th17 cells and that to eliminate Th17-mediated rejection, it may be necessary to reduce Th17 differentiation in addition to blocking IL-17 function. To this end, we utilized IL-6-deficient mice on the B6 background as recipients of cardiac allografts, as our in vitro data indicated that IL-6 plays an essential in the ability of CpG to induce IL-17 production (Figure 4). IL-6-deficient mice rejected allografts promptly when left untreated and accepted cardiac allografts long-term when treated with anti-CD154 (Figure 6A). As in wild-type animals, peri-operative administration of CpG prevented anti-CD154-mediated allograft acceptance, indicating that lack of IL-6 is not sufficient to prevent CpG-mediated rejection. In contrast, blockade of IL-17 abrogated the pro-rejection effects of CpG in IL-6-deficient mice, indicating that CpG-mediated rejection is dependent on the combination of IL-6 and IL-17 signals. Intriguingly, lack of rejection in IL-6-deficient mice treated with anti-IL-17 correlated with reduced frequency of IFN-γ-producing alloreactive splenocytes (Figure 6B) suggesting also diminished Th1 differentiation. Together, our results suggest that pro-inflammatory signals induced by TLR agonists may promote Th1 and Th17 differentiation in vivo and indicate that they promote cardiac allograft rejection via a different effector mechanism, namely an IL6/IL-7-dependent mechanism, than that operating in non-immunosuppressed wild-type recipients.
Figure 6. IL-6 and IL-17 are required for CpG to prevent anti-CD154-mediated cardiac allograft acceptance.
Wild-type B6 (n=3 per control group) or IL-6−/− mice on a B6 background were transplanted with BALB/c hearts and left untreated or treated with anti-CD154 +/− CpG (n=3–5 for all control groups) in the presence of anti-IL-17 (n=6) or control IgG (n=3 but similar results were obtained in the absence of control isotype, n=5, not shown). A. Graft survival was examined over time. B. Animals were sacrificed on day 50–80 post-transplantation and splenocytes were restimulated with irradiated syngeneic (B6), donor (BALB/c) or third party (C3H) splenocytes for detection of IFN-γ-producing cells by ELISpot. ***p<0.001, **p<0.01, between the groups spanned by the horizontal bars.
Discussion
We have previously shown that TLR signals at the time of transplantation can prevent cardiac allograft acceptance normally induced by anti-CD154. Our current results demonstrate that this TLR-induced rejection depends on the combination of IL-6 and IL-17 signals. In addition, blockade of IL-6 and IL-17 does not prevent cardiac allograft rejection in non-immunosuppressed recipients that did not receive CpG, indicating that TLR signals at the time of transplantation trigger acute rejection in anti-CD154-treated recipients by a mechanism distinct from that in untreated controls.
Our data show that the pro-rejection effect of CpG depends on recipient hematopoietic cells and that administration of CpG results in the rapid systemic production of IL-12 and IL-6 and the subsequent expression of IFN-γ and IL-17 by graft-infiltrating CD4+ T cells despite anti-CD154 treatment. These observations suggest a model in which TLR signals activate host innate immune cells resulting in Th1 and Th17 differentiation by alloreactive T cells, perhaps also preventing anti-CD154-mediated iTreg differentiation, which collectively results in acute rejection by a distinct IL-6 and IL-17-dependent pathway. Whether IL-6 is only necessary for driving Th17 differentiation or whether other pro-inflammatory effects of IL-6 play a role in eliciting rejection after CpG remains to be elucidated. Similarly, we cannot exclude the possibility that CpG may expand or promote intra-graft recruitment of IL-17-producing cells rather than, or in addition to, helping Th17 differentiation. Nevertheless, our results clearly demonstrate that inflammatory signals produced at the time of transplantation can determine the cytokines, and subsequently, the quality of the cellular alloresponse driving acute allograft rejection.
Our results indicate that the cell type required for the pro-rejection effect of CpG is of recipient and of hematopoietic origin. Our in vitro data suggest that the cells targeted by CpG are likely host APCs rather than T cells, as CpG did not prevent iTreg differentiation or promote Th17 differentiation in T cell cultures devoid of APCs. However, we cannot exclude that CpG also targets T cells in vivo, as CpG has been shown to increase proliferation, prevent anergy and promote humoral responses when MyD88 was expressed only in T cells (30). In addition, donor hematopoietic cells and donor and/or recipient parenchymal cells may also participate in the pro-rejection effects of CpG, but our data indicate that MyD88-dependent signaling via these cells is not required. Consistent with an important effect of TLR signals on host APCs, selective deletion of MyD88 in dendritic cells has recently been shown to result in marked reduction of CpG-induced early production of pro-inflammatory cytokines thought to be involved in promoting Th1 and Th17 differentiation, including IL-12, IL-6, IL-23 and TNF (31). Thus, within APCs, the effects of CpG may be largely mediated by dendritic cells. The importance of recipient APCs in the effects of CpG in solid organ transplant settings is in marked contrast with the critical role of donor APCs in the ability of CpG to promote rejection of bone marrow transplants. In that model, CpG-mediated rejection depended on the expression of TLR9 by donor but not recipient cells and was IL-6-independent, suggesting that CpG may indirectly modulate the strength of the recipient anti-donor response (32).
The importance of the ratio of Tregs to T effector cells in target organs is being increasingly recognized both in tumor and allograft settings (26, 27). Our previous results had correlated CpG administration with reduced accumulation of Tregs in allografts from anti-CD154-treated mice, in association with reduced intra-graft expression of the CCR4-attracting chemokines, CCL17 and CCL22 (18). In parallel, it has been shown that anti-CD154 promotes the conversion of naïve alloreactive T cells into iTregs (24) suggesting that the accumulation of Tregs found in allografts from anti-CD154-treated mice may be secondary to recruitment of iTregs rather than to proliferation of natural Tregs. Indeed, BrdU incorporation by FoxP3+ cells was similar in our transplanted mice whether untreated, or receiving anti-CD154 with or without CpG (data not shown), suggesting similar global proliferating rates of all Tregs in the presence or absence of TLR engagement. In addition, the suppressive capacity of Tregs from transplanted mice treated with anti-CD154 or anti-CD154+CpG was comparable in vitro, as was the susceptibility to suppression of splenic conventional T cells from the different transplanted mice to control Tregs (data not shown). These results suggest that the pro-rejection effect of CpG in this case is not solely due to relieving effector T cells from Treg suppression as suggested in other models (16). Our current data indicate that CpG can prevent the conversion of naïve alloreactive T cells into iTregs in vivo. Whether this is only the case for T cells recognizing alloantigens indirectly such as the TEa cells utilized in this study or is a wider phenomenon applicable to all alloreactive T cells, remains to be established. Additionally, whether prevention of iTreg differentiation is necessary for the ability of CpG to promote rejection is not clear and difficult to evaluate in vivo as no current strategies are available to delete iTregs independently from nTregs.
Our current results suggest that within graft-infiltrating conventional (FoxP3−) CD4+ T cells, both IFN-γ-producing and IL-17-producing cells coexist in CpG-treated animals. Although quantitative differences in the percentage of IFN-γ-producing cells existed between mice treated with anti-CD154 and those with anti-CD154+CpG, the main difference triggered by TLR engagement was a qualitative one as IL-17-producing cells became detectable only in the grafts from CpG-treated animals. In addition, although Th1 cells may participate in CpG-mediated rejection, our results indicate that rejection in this setting strictly depended on IL-6/IL-17. CpG-mediated IL-17 production in vitro was completely IL-6-dependent. However, the fact that both IL-6 and IL-17 needed to be blocked in vivo suggests either that additional inflammatory factors other than IL-6 may promote acquisition of IL-17 production in vivo, or that IL-6 exerts pro-rejection effects independent from its ability to promote Th17 differentiation. Indeed, pro-inflammatory cytokines such as TNF and IL-1, as well as IL-23 have been shown to enhance Th17 differentiation (28) and it is possible that these or other cytokines were produced in vivo either at levels below detection in the serum (data not shown) or at priming sites in secondary lymphoid organs. In parallel, IL-6 is known to enhance T cell proliferation {Pasare, 2003 #2544}. Porrett and colleagues (19) have recently reproduced the CpG-mediated rejection in a different model of cardiac allograft bearing a single MHC class II mismatch (bm12 allografts into B6 recipients). In this setting, CpG-mediated rejection correlated with IFN-γ production by restimulated alloreactive splenocytes but was independent of IL-6. However, it remains possible that rejection in this bm12 model also depends on IL6+IL-17, as elimination of both cytokines was not performed in these experiments and cytokines in the allograft were not investigated.
Although IL-17 has been detected in wild-type recipient animals undergoing acute allograft rejection, or in grafts of transplanted patients, rejection of solid organs did not depend on IL-17. Similarly, IL-17-production by CD4+ cells has recently been shown to contribute to early pathogenesis in a model of graft versus host disease (GVHD), but disease progression and mortality was unaffected by the lack of IL-17 in T cells (29). In contrast to wild-type animals, acute rejection of cardiac allografts in T-bet-deficient animals was IL-17-dependent, indicating that the quality of the rejection mechanism can change when type 1 T cell differentiation is prevented (8, 9). Our model of CpG-mediated rejection is to our knowledge the first model utilizing wild-type animals in which acute rejection depends on IL-17,.
Most T cell subsets have been shown to be capable of producing IL-17, including, CD4+, CD8+, NKT, γδT cells and Tregs (33–38). Porrett and colleagues have shown that injection of CpG in vivo did not induce Tregs to produce IL-17 (19), although in vitro both mouse and human Tregs have been reported to be able to become IL-17-producing cells in the presence of IL-6 (37, 38). Our results identify the CpG-induced IL-17-producing cells as a subset of intra-graft infiltrating αβCD4+ T cells, whereas we could not detect any IL-17-producing CD8+ T cells by intracellular staining. It has been previously shown that selective deletion of MyD88 in dendritic cells can result in defective CpG-mediated activation of NK and NKT cells (31), such that it is conceivable that CpG promotes IL-17 production by NKT cells in some settings. However, we have previously shown that CpG-mediated rejection occurs in CD1-deficient animals and is therefore independent of invariant NKT cells (18). We have also reported that NK but not NKT cells can help T cells mediate cardiac allograft rejection in CD28-deficient mice (39), but the potential contribution of NK cells in the pro-rejection effect of CpG remains to be investigated.
In conclusion, our results indicate that TLR ligation at the time of transplantation can shift the effector cytokines produced by alloreactive T cells and result in acute rejection that is crucially dependent on IL-6 and IL-17, although IFN-γ is a likely contributors as well (19). Thus, the stimulation of innate immunity can induce specific pro-inflammatory events that can alter the effector mechanisms of rejection. As infections with microorganisms of different origins can trigger TLR activation, it is conceivable that peri-operative infections may similarly skew cytokine production by effector T cells and modify the mechanism of acute rejection. However, not all infections may trigger IL-6 and IL-17 production. For instance, we have recently shown that peri-operative infection with Listeria monocytogenes (LM) results in prevention of anti-CD154/DST-mediated cardiac allograft acceptance (40). Of interest, rejection in this setting was independent of MyD-88 expression but rather required type I IFN signaling, as mice deficient in IFNα/βR were resistant to the pro-rejection effects of LM. In contrast, although TLR9 engagement can signal the production of type I IFN, CpG-mediated rejection of cardiac allografts in anti-CD154-treated recipients occurred with normal kinetics in IFNα/βR-deficient mice (data not shown). This is distinct from the TLR4 ligand LPS and the TLR3 agonist poly I:C, which have been found to prevent skin allograft acceptance induced by anti-CD154+DST in a type I IFN signaling-dependent manner (41). These results highlight the fact that inflammatory signals may lead to activation of different pathways that can all promote acute allograft rejection. Future therapies may need to take several of these pathways into consideration for prevention or treatment of acute rejection.
Acknowledgments
We thank A. Rudensky for providing the TEa-Tg and FoxP3GFP knock-in mice, S. Uematsu and S. Akira for providing MyD88-deficient mice and the University of Chicago Flow Cytometry Core Facility for expert technical help.
Footnotes
This work was supported by grants AHA 0620026Z to LC, NIAID RO1 AI071080 to MLA and NIAID R01 AI072630 to ASC.
References
- 1.Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y, Wood KJ. Interleukin-23 and TH17 cells in transplantation immunity: does 23+17 equal rejection? Transplantation. 2007;84:1071–1074. doi: 10.1097/01.tp.0000287126.12083.48. [DOI] [PubMed] [Google Scholar]
- 3.Zhou P, Szot GL, Guo Z, Kim O, He G, Wang J, Grusby MJ, Newell KA, Thistlethwaite JR, Bluestone JA, Alegre ML. Role of STAT4 and STAT6 signaling in allograft rejection and CTLA4-Ig-mediated tolerance. J Immunol. 2000;165:5580–5587. doi: 10.4049/jimmunol.165.10.5580. [DOI] [PubMed] [Google Scholar]
- 4.Kishimoto K, Dong VM, Issazadeh S, Fedoseyeva EV, Waaga AM, Yamada A, Sho M, Benichou G, Auchincloss H, Jr, Grusby MJ, Khoury SJ, Sayegh MH. The role of CD154-CD40 versus CD28-B7 costimulatory pathways in regulating allogeneic Th1 and Th2 responses in vivo. J Clin Invest. 2000;106:63–72. doi: 10.1172/JCI9586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loong CC, Hsieh HG, Lui WY, Chen A, Lin CY. Evidence for the early involvement of interleukin 17 in human and experimental renal allograft rejection. J Pathol. 2002;197:322–332. doi: 10.1002/path.1117. [DOI] [PubMed] [Google Scholar]
- 6.Hsieh HG, Loong CC, Lui WY, Chen A, Lin CY. IL-17 expression as a possible predictive parameter for subclinical renal allograft rejection. Transpl Int. 2001;14:287–298. doi: 10.1007/s001470100344. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida S, Haque A, Mizobuchi T, Iwata T, Chiyo M, Webb TJ, Baldridge LA, Heidler KM, Cummings OW, Fujisawa T, Blum JS, Brand DD, Wilkes DS. Anti-type V collagen lymphocytes that express IL-17 and IL-23 induce rejection pathology in fresh and well-healed lung transplants. Am J Transplant. 2006;6:724–735. doi: 10.1111/j.1600-6143.2006.01236.x. [DOI] [PubMed] [Google Scholar]
- 8.Burrell BE, Csencsits K, Lu G, Grabauskiene S, Bishop DK. CD8+Th17 mediate costimulation blockade-resistant allograft rejection in T-bet-deficient mice. J Immunol. 2008;181:3906–3914. doi: 10.4049/jimmunol.181.6.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuan X, Paez-Cortez J, Schmitt-Knosalla I, D'Addio F, Mfarrej B, Donnarumma M, Habicht A, Clarkson MR, Iacomini J, Glimcher LH, Sayegh MH, Ansari MJ. A novel role of CD4 Th17 cells in mediating cardiac allograft rejection and vasculopathy. J Exp Med. 2008;1:1. doi: 10.1084/jem.20081937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang JL, Subbotin VM, Antonysamy MA, Troutt AB, Rao AS, Thomson AW. Interleukin-17 antagonism inhibits acute but not chronic vascular rejection. Transplantation. 2001;72:348–350. doi: 10.1097/00007890-200107270-00035. [DOI] [PubMed] [Google Scholar]
- 11.Antonysamy MA, Fanslow WC, Fu F, Li W, Qian S, Troutt AB, Thomson AW. Evidence for a role of IL-17 in organ allograft rejection: IL-17 promotes the functional differentiation of dendritic cell progenitors. J Immunol. 1999;162:577–584. [PubMed] [Google Scholar]
- 12.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. Epub 2006 Apr 2030. [DOI] [PubMed] [Google Scholar]
- 13.Goldstein DR. Toll like receptors and acute allograft rejection. Transpl Immunol. 2006;17:11–15. doi: 10.1016/j.trim.2006.09.012. Epub 2006 Oct 2010. [DOI] [PubMed] [Google Scholar]
- 14.Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 15.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 16.Pasare C, Medzhitov R. Toll-dependent control mechanisms of CD4 T cell activation. Immunity. 2004;21:733–741. doi: 10.1016/j.immuni.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Thornley TB, Brehm MA, Markees TG, Shultz LD, Mordes JP, Welsh RM, Rossini AA, Greiner DL. TLR Agonists Abrogate Costimulation Blockade-Induced Prolongation of Skin Allografts. J Immunol. 2006;176:1561–1570. doi: 10.4049/jimmunol.176.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen L, Wang T, Zhou P, Ma L, Yin D, Shen J, Molinero L, Nozaki T, Phillips T, Uematsu S, Akira S, Wang CR, Fairchild RL, Alegre ML, Chong A. TLR engagement prevents transplantation tolerance. Am J Transplant. 2006;6:2282–2291. doi: 10.1111/j.1600-6143.2006.01489.x. [DOI] [PubMed] [Google Scholar]
- 19.Porrett PM, Yuan X, LaRosa DF, Walsh PT, Yang J, Gao W, Li P, Zhang J, Ansari JM, Hancock WW, Sayegh MH, Koulmanda M, Strom TB, Turka LA. Mechanisms underlying blockade of allograft acceptance by TLR ligands. J Immunol. 2008;181:1692–1699. doi: 10.4049/jimmunol.181.3.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee I, Wang L, Wells AD, Dorf ME, Ozkaynak E, Hancock WW. Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J Exp Med. 2005;201:1037–1044. doi: 10.1084/jem.20041709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Corry RJ, Winn HJ, Russell PS. Primarily vascularized allografts of hearts in mice. The role of H-2D, H-2K, and non-H-2 antigens in rejection. Transplantation. 1973;16:343–350. doi: 10.1097/00007890-197310000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Chun T, Page MJ, Gapin L, Matsuda JL, Xu H, Nguyen H, Kang HS, Stanic AK, Joyce S, Koltun WA, Chorney MJ, Kronenberg M, Wang CR. CD1d-expressing dendritic cells but not thymic epithelial cells can mediate negative selection of NKT cells. J Exp Med. 2003;197:907–918. doi: 10.1084/jem.20021366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alegre ML, Leemans J, Le Moine A, Florquin S, De Wilde V, Chong A, Goldman M. The Multiple Facets of Toll-Like Receptors in Transplantation Biology. Transplantation. 2008;86:1–9. doi: 10.1097/TP.0b013e31817c11e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ochando JC, Homma C, Yang Y, Hidalgo A, Garin A, Tacke F, Angeli V, Li Y, Boros P, Ding Y, Jessberger R, Trinchieri G, Lira SA, Randolph GJ, Bromberg JS. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7:652–662. doi: 10.1038/ni1333. Epub 2006 Apr 2023. [DOI] [PubMed] [Google Scholar]
- 25.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei S, Kryczek I, Zou W. Regulatory T cell compartmentalization and trafficking. Blood. 2006;14:14. doi: 10.1182/blood-2006-01-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Graca L, Cobbold SP, Waldmann H. Identification of regulatory T cells in tolerated allografts. J Exp Med. 2002;195:1641–1646. doi: 10.1084/jem.20012097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stockinger B, Veldhoen M, Martin B. Th17 T cells: linking innate and adaptive immunity. Semin Immunol. 2007;19:353–361. doi: 10.1016/j.smim.2007.10.008. Epub 2007 Nov 2026. [DOI] [PubMed] [Google Scholar]
- 29.Kappel LW, Goldberg GL, King CG, Suh DY, Smith OM, Ligh C, Holland AM, Grubin J, Mark NM, Liu C, Iwakura Y, Heller G, van den Brink MR. IL-17 contributes to CD4-mediated graft-versus-host disease. Blood. 2008;17:17. doi: 10.1182/blood-2008-08-172155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gelman AE, LaRosa DF, Zhang J, Walsh PT, Choi Y, Sunyer JO, Turka LA. The adaptor molecule MyD88 activates PI-3 kinase signaling in CD4+ T cells and enables CpG oligodeoxynucleotide-mediated costimulation. Immunity. 2006;25:783–793. doi: 10.1016/j.immuni.2006.08.023. Epub 2006 Oct 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hou B, Reizis B, Defranco AL. Toll-like Receptors Activate Innate and Adaptive Immunity by using Dendritic Cell-Intrinsic and -Extrinsic Mechanisms. Immunity. 2008;23:23. doi: 10.1016/j.immuni.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor PA, Ehrhardt MJ, Lees CJ, Panoskaltsis-Mortari A, Krieg AM, Sharpe AH, Murphy WJ, Serody JS, Hemmi H, Akira S, Levy RB, Blazar BR. TLR agonists regulate alloresponses and uncover a critical role for donor APCs in allogeneic bone marrow rejection. Blood. 2008;9:9. doi: 10.1182/blood-2007-09-113670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Intlekofer AM, Banerjee A, Takemoto N, Gordon SM, Dejong CS, Shin H, Hunter CA, Wherry EJ, Lindsten T, Reiner SL. Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science. 2008;321:408–411. doi: 10.1126/science.1159806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pichavant M, Goya S, Meyer EH, Johnston RA, Kim HY, Matangkasombut P, Zhu M, Iwakura Y, Savage PB, DeKruyff RH, Shore SA, Umetsu DT. Ozone exposure in a mouse model induces airway hyperreactivity that requires the presence of natural killer T cells and IL-17. J Exp Med. 2008;205:385–393. doi: 10.1084/jem.20071507. Epub 2008 Feb 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rachitskaya AV, Hansen AM, Horai R, Li Z, Villasmil R, Luger D, Nussenblatt RB, Caspi RR. Cutting edge: NKT cells constitutively express IL-23 receptor and RORgammat and rapidly produce IL-17 upon receptor ligation in an IL-6-independent fashion. J Immunol. 2008;180:5167–5171. doi: 10.4049/jimmunol.180.8.5167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006;177:4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 37.Zheng SG, Wang J, Horwitz DA. Cutting edge: Foxp3+CD4+CD25+regulatory T cells induced by IL-2 and TGF-beta are resistant to Th17 conversion by IL-6. J Immunol. 2008;180:7112–7116. doi: 10.4049/jimmunol.180.11.7112. [DOI] [PubMed] [Google Scholar]
- 38.Koenen HJ, Smeets RL, Vink PM, Rijssen EV, Boots AM, Joosten I. Human CD25highFoxp3pos regulatory T-cells differentiate into IL-17 producing cells. Blood. 2008;10:10. doi: 10.1182/blood-2008-01-133967. [DOI] [PubMed] [Google Scholar]
- 39.McNerney ME, Lee KM, Zhou P, Molinero L, Mashayekhi M, Guzior D, Sattar H, Kuppireddi S, Wang CR, Kumar V, Alegre ML. Role of natural killer cell subsets in cardiac allograft rejection. Am J Transplant. 2006;6:505–513. doi: 10.1111/j.1600-6143.2005.01226.x. [DOI] [PubMed] [Google Scholar]
- 40.Wang T, Chen L, Ahmed EM, Ma L, Yin D, Zhou P, Shen J, Xu H, Wang CR, Alegre ML, Chong A. Prevention of allograft tolerance by bacterial infection with Listeria monocytogenes. J Immunol. 2008;180:5991–5999. doi: 10.4049/jimmunol.180.9.5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thornley TB, Phillips NE, Beaudette-Zlatanova BC, Markees TG, Bahl K, Brehm MA, Shultz LD, Kurt-Jones EA, Mordes JP, Welsh RM, Rossini AA, Greiner DL. Type 1 IFN mediates cross-talk between innate and adaptive immunity that abrogates transplantation tolerance. J Immunol. 2007;179:6620–6629. doi: 10.4049/jimmunol.179.10.6620. [DOI] [PMC free article] [PubMed] [Google Scholar]