Synopsis
This review focuses on the development of fluorine-18 radiolabeled PET tracers for imaging the dopamine transporter (DAT), serotonin transporter (SERT), and norepinephrine transporter (NET). All successful DAT PET tracers reported to date are members of the 3β-phenyl tropane class and are synthesized from cocaine. Currently available carbon-11 SERT PET tracers come from both the diphenylsulfide and 3β-phenyl nortropane class, but so far only the nortropanes have found success with fluorine-18 derivatives. NET imaging has so far employed carbon-11 and fluorine-18 derivatives of reboxetine but due to defluorination of the fluorine-18 derivatives further research is still necessary.
Keywords: Fluorine-18 PET, Tropanes, Dopamine Transporter, Serotonin Transporter, Norepinephrine Transporter
Introduction
The dopamine transporter (DAT), serotonin transporter (SERT), and norepinephrine transporter (NET) are plasma membrane biogenic monoamine transporters which belong to the family of Na+/Cl− dependent transporters.[1–8] In the central nervous system (CNS) the DAT, SERT, and NET are located on presynaptic neurons and function to remove their respective neurotransmitter from the synapse thereby terminating the action of that neurotransmitter. These three transporters have each been implicated in numerous psychiatric disorders such as depression, suicide, schizophrenia, Parkinson’s Disease (PD), and attention-deficit hyperactivity disorder (ADHD) and are also the target of drugs of abuse such as cocaine, amphetamines, and MDMA (Ecstasy). As such, these transporters have become therapeutic targets to treat psychiatric disorders and drug addiction.[9–11] The ability to image the DAT, SERT, and NET with positron emission tomography (PET) or single-photon emission computed tomography (SPECT) may aid in the diagnosis and management of psychiatric disease by providing a means to measure the density of these transporters in specific brain regions.[12–17] Additionally, the availability of radiolabeled tracers for these transporters may aid in the development of new therapeutics by enabling the occupancy of the therapeutic to be measured.[18–24]
Numerous PET tracers for the DAT, SERT, and NET have been developed that are radiolabeled with carbon-11 but these are limited to use in the location where they are prepared and only allow for imaging times of up to about 2 hours due to the short half-life of 11C (t1/2 = 20.4 min).[25–27] The longer half-life of 18F (t1/2 = 109.8 min) allows for longer synthesis times and imaging sessions as well as for the transport of the 18F-labeled tracer to locations away from the cyclotron facility which allows for PET imaging centers without onsite cyclotrons to employ these tracers. In addition to the longer half-life of 18F, the positrons emitted from 18F-nuclides have a lower maximum energy (0.64 MeV)[26] than the positrons emitted from 11C-nuclides (0.97 MeV) which therefore deposits less energy into tissue and also results in a shorter linear range that allows for higher spatial resolution.[28,29] Radiolabeling tracers with 11C is convenient due to the ubiquitous nature of carbon in organic compounds whereas fluorine is far less common. Fluorine, though, has been shown to impart unique properties to organic molecules and is now being exploited extensively in medicinal chemistry.[30–36] Thus, numerous methods have been developed to introduce 18F or 19F into molecules.[37–41] This review will focus on fluorine-18 radiolabeled PET tracers for imaging the DAT, SERT, and NET. Several carbon-11 PET tracers are also included to allow for comparisons in instances where a tracer can be radiolabeled with either isotope or where fluorinated analogs of existing 11C-labeled tracers have been developed.
There are several performance criteria that should be met in order for a candidate brain PET tracer to become a useful tracer. High binding affinity to the target, especially if the target is of low density, will enable highly specific and selective binding to the target. The goal is to obtain the highest possible target-to-nontarget uptake ratios which will result in PET images with high signal-to-noise ratios. If the tracer does not bind strong enough to the target then the tracer will not be retained in the tissue of interest and will just pass through. But, if the tracer binds too strongly then it will not dissociate from the target during the course of the PET study and will accumulate in the tissue of interest and only blood flow will be measured. A balance must, therefore, be obtained which will allow for the achievement of peak uptake in the target tissue in a short time frame (18F allows for longer time frames compared to 11C) followed by a steady washout to allow for kinetic modeling of the behavior of the tracer.[42–47] Moderate lipophilicity in the range of logP = ~1–3 is necessary to allow for rapid entry into the brain and to limit nonspecific binding. [48–50] Low binding to plasma proteins is necessary to make as many tracer molecules as possible available for brain entry. Metabolism of the tracer is unavoidable but the resulting metabolites that are generated in the periphery, if they are radiolabeled, should be hydrophilic so that they cannot enter the brain. The generation of radiolabeled metabolites in the brain is also undesirable and any radiolabeled metabolites that are produced should have little or no affinity for the target of interest. As will become apparent below, meeting all of these criteria simultaneously is a difficult task.
Dopamine Transporter (DAT)
The human DAT is a 620-amino acid transmembrane protein which is 98.9% homologous to the monkey DAT and 92% homologous to the rat DAT.[4,51–53] The DAT is found in high densities in the caudate, putamen, nucleus accumbens, and olfactory tubercle with lower densities in the substantia nigra, amygdala, and hypothalamus.[54–57] The DAT has been associated with numerous neuropsychiatric diseases including PD,[58–60] supranuclear palsy,[59] ADHD,[61] and Tourette’s Syndrome.[62] The ability to image the DAT with PET may therefore aid in the diagnosis, monitoring, and treatment of these diseases.[13,15,17,63]
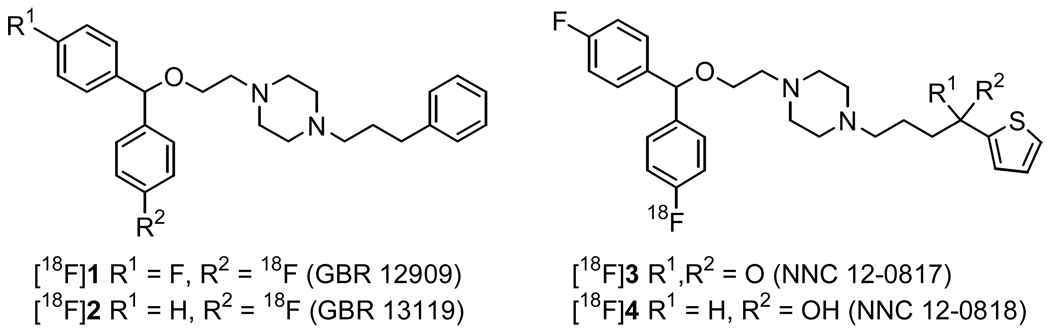
Early work towards developing an 18F-labeled DAT imaging agent focused on the GBR-compounds[64,65] [18F]1 and [18F]2 (Figure 1).[66–71] PET imaging with [18F]2 in monkeys[72] showed rapid uptake into the striatum and cerebellum with maximum levels achieved within 2–3 min followed by a slow but steady washout. Washout was faster from the cerebellum than the striatum which resulted in a striatum-to-cerebellum ratio of 1.76 at the end of the study. Radiotracer uptake was highest in the liver and kidneys due to metabolism of the tracer but low bone uptake indicated that the aryl-fluorine bond was stable to metabolic defluorination. The imaging results with [18F]2 were promising but the tracer still suffered from high lipophilicity and non-specific binding. The synthesis and radiolabeling of the GBR-derivatives [18F]3 and [18F]4 has also been reported but imaging data was not included.[73]
Figure 1.
Structures of GBR-based DAT PET tracers.
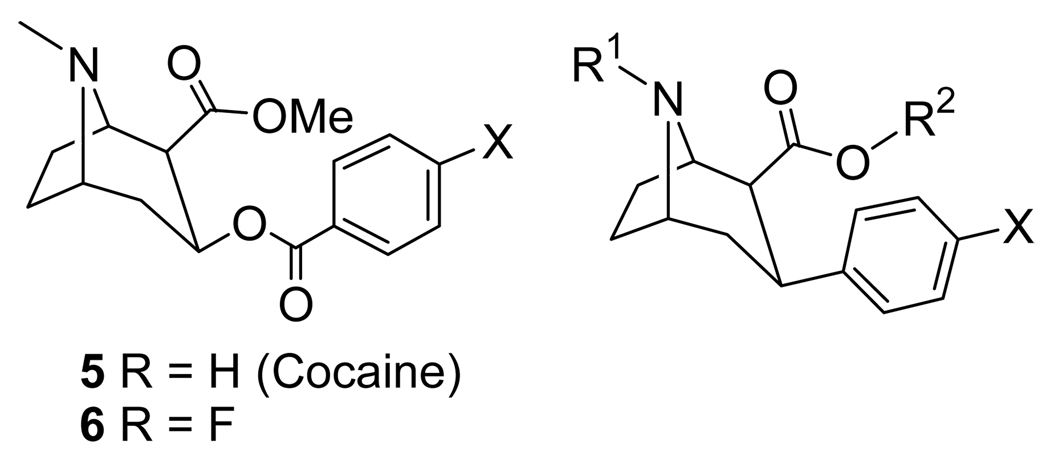
Cocaine (5) (Fig 2, Table 1) binds to the DAT, SERT, and NET[74,75] but the DAT was initially implicated as the site related to substance abuse.[76–78] As a tool to study cocaine addiction [11C]cocaine PET imaging has been employed[79–83] but imaging times are limited due to the short half-life of 11C. 4′-[18F]Fluorococaine ([18F]6) allows for extended imaging studies due to the longer half-life of 18F and [18F]6 was shown to have identical kinetic behavior to [11C]cocaine in PET imaging studies.[82]
Fig 2.
Cocaine, 4′-Fluorococaine, and 3β-Phenyl Tropane-Based Dopamine Transporter Ligands.
Table 1.
| Compound | R1 = | R2 = | X = | |
|---|---|---|---|---|
| 7 | WIN 35,065-2 | Me | Me | H |
| 8 | WIN 35,428; β-CFT | Me | Me | F |
| 9 | RTI-31 | Me | Me | Cl |
| 10 | RTI-51; CBT | Me | Me | Br |
| 11 | RTI-55; β-CIT | Me | Me | I |
| 12 | RTI-32 | Me | Me | Me |
| 13 | β-CFT-FE | CH2CH2F | Me | F |
| 14 | β-CFT-FP | CH2CH2CH2F | Me | F |
| 15 | FE-β-CIT; β-CIT-FE | CH2CH2F | Me | I |
| 16 | FP-β-CIT; β-CIT-FP | CH2CH2CH2F | Me | I |
| 17 | CH2CH2CH2F | i-Pr | I | |
| 18 | FECNT | CH2CH2F | Me | Cl |
| 19 | FPCT | CH2CH2CH2F | Me | Cl |
| 20 | FPCMT | CH2CH2CH2F | Me | Me |
| 21 | FPCBT | CH2CH2CH2F | Me | Br |
| 22 | FETT | Me | CH2CH2F | Me |
| 23 | FECT | Me | CH2CH2F | Cl |
| 24 | MCL322 | Me | CH2CH2F | Br |
| 25 | MCL301; FE@CIT | Me | CH2CH2F | I |
Cocaine metabolism can take several possible routes: (a) ester hydrolysis, (b) N-demethylation, (c) N-oxidation, (d) aryl hydroxylation and/or epoxidation, and (e) dehydrobenzoylation.[82,84–89] The benzoic ester hydrolysis route was rendered obsolete when Clarke and co-workers demonstrated that replacement of the benzoyloxy group with a phenyl group attached directly to the 3β-position of the tropane skeleton produced compounds with higher binding affinities at the DAT than cocaine itself.[90] This new class of 3β-phenyl tropanes has been exploited extensively in the search for therapeutics to treat cocaine addiction[10,11,91–95] and hundreds of compounds have already been reported in the past[96] and more continue to be reported to this day.[97,98] The structures of some of the first 3β-phenyl tropane compounds prepared, 7–12, are shown in Fig 2 and Table 1.[90–92,99] The high affinity of these compounds for the DAT naturally made them candidates for use as PET tracers when labeled with 11C as they can all be labeled on either the N-methyl group or the methyl ester.[88,100–104] Unfortunately, these 11C-labeled compounds do not reach binding equilibrium during the course of the imaging study which limits their usefulness in kinetic modeling. Furthermore, compound 11 has a similar affinity for the DAT and SERT and compounds 9 and 10 are only slightly selective for the DAT over the SERT[94,96,105] thus limiting the utility of these compounds as DAT-selective imaging agents.
Out of compounds 7–12, only compound 8 contains a 19F atom that could be replaced with 18F. Additionally, 8 is more selective for the DAT over the SERT and NET than compounds 9–11,[94,96] and previous work with [11C]8 had demonstrated that it was capable of imaging the DAT.[100,101] Thus, [18F]8 was synthesized and evaluated by biodistribution studies in rats[106] and then by PET imaging in humans.[107] In rat brain the striatum-to-cerebellum ratio reached a maximum of ~9.6 after 2 h and this uptake could be blocked by pretreatment with the DAT ligand GBR 12909 (1).[64,65] In rat biodistribution studies the highest uptake at all times was observed in the liver and urine whereas uptake in the kidney, spleen, and lungs was initially high but then decreased. Very little bone uptake was observed indicating that the aryl-fluorine bond of [18F]8 is stable to metabolic defluorination, similar to what was observed for [18F]2. In human subjects the total activity of [18F]8 in the caudate and putamen peaked at 225 min and then slowly declined whereas the specific binding remained at a plateau from 3–5 h post-injection. Application of [18F]8 to studying human subjects with early PD demonstrated that [18F]8 was capable of detecting presynaptic dopaminergic hypofunction.[108]
In an effort to reduce the time required to reach peak uptake of [18F]8, the N-[18F]fluoroethyl[105] and N-[18F]fluoropropyl[109,110] derivatives, [18F]13 and [18F]14, respectively, were prepared. In vitro binding assays demonstrated that both 13 and 14 had a reduced affinity for the DAT, SERT, and NET relative to 8, but 13 and 14 both had a slightly improved selectivity for the DAT over the SERT relative to 8.[105] PET imaging with [18F]13 in conscious rhesus monkeys demonstrated that [18F]13 reaches peak uptake after about 20 min followed by a washout phase, thus demonstrating reversible binding. A comparison study with [11C]8 showed only irreversible binding during the 91 min PET scan as the radioactivity continuously increased throughout the study. Therefore, replacing the N-methyl group of 8 with an N-fluoroethyl group as in 13 resulted in improved tracer performance. A blocking study with 1 demonstrated that [18F]13 binding was DAT-specific. Metabolite analysis of [18F]13 in plasma showed only polar metabolites and no lipophilic metabolites that could cross the blood-brain barrier. Compound [18F]14 was evaluated ex vivo in rats and demonstrated rapid entry into the brain with a striatum-to-cerebellum ratio of ~3.1 after 5 min followed by a continuous decrease. This uptake could be blocked by pretreatment with 1. A continuous uptake was observed in the skull indicating a slow defluorination of the [18F]fluoropropyl group.
Compound 11 has a nearly equal affinity for the DAT and SERT[94,96,105] and PET imaging with [11C]11 showed uptake in the striatum as well as the thalamus and neocortex.[88,103] This uptake in the thalamus and neocortex could be displaced by the SERT ligand citalopram[111,112] thus demonstrating binding to both the DAT and SERT in vivo, which is in agreement with the in vitro binding data.[96,105,113] In an effort to obtain 18F-labeled derivatives of 11 the N-fluoroalkyl compounds 15 and 16 were prepared.[113,114] Replacement of the N-methyl group of 11 with an N-fluoroethyl group (15) or an N-fluoropropyl group (16) resulted in a reduced binding affinity at the DAT and NET but an increased binding affinity at the SERT relative to 11.[113] Replacement of the methyl ester group of 16 with an isopropyl ester to give 17 increased the binding affinity at the DAT relative to 11 and significantly reduced the binding affinity at both the SERT and NET.[113,115] A SPECT imaging comparison[116] of [123I]15, [123I]16, and [123I]17 in baboons demonstrated that [123I]15 and [123I]16 had higher peak uptake in the striatum than [123I]17 thus ruling out [123I]17 for adaptation to PET imaging as [18F]17. Additionally, [123I]15 had more rapid kinetics than [123I]16. Comparison between [123I]11 and [123I]16 in humans with SPECT imaging showed that [123I]16 had higher non-specific binding than [123I]11 but a lower radiation burden to the basal ganglia.[117]
PET studies in anesthetized cynomolgus monkeys with [11C]15 showed high uptake in the putamen with lesser uptake in the thalamus and neocortex.[118] A blocking study with 1 reduced the uptake of [11C]15 in the putamen by 75 % but did not affect the uptake in the thalamus or neocortex thus indicating that SERT binding was still an issue. Compound [18F]16 has been radiolabeled and evaluated in anesthetized cynomolgus monkeys[119] and conscious humans.[120] In monkeys high uptake of [18F]16 was observed in the putamen (this was blockable with 1) with peak uptake achieved in 30–40 min followed by only a very slight washout. After 70 min the striatum-to-cerebellum ratio was 4.5–5. Uptake in the thalamus was at levels similar to the cerebellum thus demonstrating an improvement over [11C]11, [11C]15, and [11C]16. Metabolite analysis did not detect any lipophilic metabolites but [18F]fluoride was detected in plasma indicating that defluorination was occurring. Initial studies in humans with [18F]16 showed high uptake in the putamen of a healthy normal volunteer whereas uptake was reduced 65% in a PD patient.[120] Additional studies[121] in healthy normal humans with [18F]16 showed that uptake in the striatum increased rapidly after injection but then leveled off after 40 min and did not wash out. Uptake in the thalamus peaked at ~20 min and then began to wash out. In PD patients uptake in the putamen peaked at 30 min and then slowly washed out. Additional studies have been performed with [18F]16 to acquire data for parametric mapping,[122] dosimetry,[123] and modeling.[124] A one-step high-yield radiosynthesis of [18F]16 has been developed.[125]
Compounds 18 and 19 are the N-fluoroalkyl derivatives of 9 which has been previously radiolabeled as [11C]9 and evaluated in rats.[102] PET studies with [18F]19 in anesthetized rhesus monkeys showed high uptake in the striatum with putamen-to-cerebellum and caudate-to-cerebellum ratios of 3.35 and 2.28, respectively, after 115 min.[126] The uptake in the cerebellum washed out after an initial peak at 13.5 min but the uptake in the caudate and putamen remained nearly constant after peaking thus indicating irreversible binding to the DAT. Biodistribution studies in rats showed continuously increasing bone uptake indicating that the fluoropropyl group was not resistant to defluorination which is in agreement with the detection of [18F]fluoride in plasma reported for the metabolism of [18F]16 and the observed skull uptake in studies with [18F]14. PET imaging with [18F]18 in an anesthetized rhesus monkey showed high uptake in the caudate and putamen (displaceable with 11) with peak uptake achieved in 60–75 min followed by washout at a rate of 8%/h.[127] At 115 min the putamen-to-cerebellum ratio was 12.7 and the caudate-to-cerebellum ratio was 12.3. When an imaging study with [18F]19 was performed in the same rhesus monkey the uptake in the caudate and putamen continuously increased throughout the course of the study,[127] similar to what was previously reported.[126] Thus, [18F]18 displays reversible binding and can achieve binding equilibrium whereas [18F]19 binds irreversibly. Compound [18F]18 has since been used to measure DAT occupancy of cocaine[128] and radiation dosimetry studies have been performed in rats and monkeys.[129,130] Two different automated radiosynthesis methods of [18F]18 have been reported.[131,132] Initial studies[133] in 6 healthy humans with [18F]18 showed that the time for peak uptake in the caudate and putamen was in the range of 70–130 min with caudate-to-cerebellum ratios of 7.6–10.5 and putamen-to-cerebellum ratios of 7.1–9.3. Comparison of the data obtained in healthy humans to that obtained in PD patients demonstrated that [18F]18 has the potential to measure DAT density in the brain and to detect the reduction in DAT density that is associated with PD. Metabolism studies in humans using arterial samples after injection of [18F]18 identified a polar non-ether-extractable component.[133] Subsequent studies[134] in rats, monkeys, and humans identified this polar metabolite as either [18F]fluoroethanol, [18F]fluoroacetaldehyde, or [18F]fluoroacetic acid (or a combination of all three as they are metabolically interchangeable). This work demonstrated that the polar metabolite is generated in the periphery but then passes into the brain and distributes evenly throughout the brain. The authors therefore suggest that the tissue reference method should not be used for analyzing PET data obtained with [18F]18 but rather an arterial input function should be used.
This N-dealkylation of [18F]18 is not unique to [18F]18 but has also been observed with cocaine,[84] [11C]cocaine (to give [11C]CO2 in baboons),[79,82] and [11C]11,[88] as well as other tracers.[135–139] Thus, metabolic cleavage of radiolabeled N-methyl and N-fluoroethyl groups is unavoidable and it should be expected that any tracer radiolabeled with an N-[18F]fluoroethyl group will be metabolized in the periphery to [18F]fluoroethanol, [18F]fluoroacetaldehyde, or [18F]fluoroacetic acid, and such an effect has recently been reported with the amyloid imaging agent [18F]FDDNP.[140] Switching to an N-[18F]fluoropropyl group is not necessarily a better alternative because the N-[18F]fluoropropyl group is not stable to defluorination as was demonstrated with [18F]14, [18F]16, and [18F]19.
Compounds 20 and 21 are the N-fluoropropyl derivatives of 12 and 10, respectively. A procedure for preparing [18F]20 has been reported but imaging data was not included.[141] PET imaging with [18F]21 in baboons showed the highest uptake in the putamen with peak uptake achieved in 7–10 min. The washout half-life was 54 min for the striatum whereas it was 19 min for the midbrain and 16 min for the cerebellum. A striatum-to-cerebellum ratio of 3.3 was achieved at 45–60 min post injection. Metabolite analysis detected a “less-lipophilic” metabolite which was presumed to be the 2β-carboxylic acid resulting from hydrolysis of the methyl ester.
As an alternative to radiolabeling with N-fluoroalkyl groups several compounds containing fluoroethyl esters have been prepared (22–25). Biodistribution studies in rats with [18F]22 and [18F]23 showed high uptake in the striatum and olfactory tubercles.[102] Compound [18F]23 showed higher uptake in the striatum than [18F]22 but also significantly higher liver uptake. Bone uptake was minimal for both tracers indicating that the [18F]fluoroethyl ester was stable to defluorination. An improved synthesis and metabolic stability analysis in rats of [18F]23 has recently been reported.[142] This work found that after 3 h plasma radioactivity consisted of ~93% intact [18F]23 and the radioactivity in homogenized cerebrum and cerebellum extracts each consisted of ~96% intact [18F]23. Furthermore, there was no trace of [18F]fluoroacetaldehyde or [18F]fluoroacetic acid which would result from hydrolysis of the [18F]fluoroethyl ester.
Compounds 24 and 25 are the fluoroethyl ester derivatives of 10 and 11, respectively.[143,144] Biodistribution studies of [18F]24 in rats showed high uptake in the striatum (blockable with 1) with peak uptake around 60 min followed by a slow washout.[145] Uptake in the adrenals and kidneys was initially high but then washed out whereas uptake in the liver continuously increased throughout the study. The tracer was rapidly metabolized in rat plasma to a polar metabolite which accounted for 85% of radioactivity in plasma after 60 min. In rat striatal homogenates the radioactivity was >90% intact parent compound after 60 min. Low bone uptake in the biodistribution studies indicated that the [18F]fluoroethyl ester was stable to defluorination. The striatal uptake observed in the biodistribution studies was further confirmed by autoradiography. MicroPET studies with [18F]24 in rats showed a steady uptake in the striatum for the first 20 min which then peaked and remained stable for 30–100 min post-injection. At the end of the study (115 min p.i.) the striatum-to-cerebellum ratio was 2.8. Evaluation of [18F]25 in rats showed peak uptake in the striatum at 15 min followed by a steady washout.[146] At 60 min the striatum-to-cerebellum ratio was 3.73 and the striatum-to-thalamus ratio was 2.99, while at 120 min the thalamus-to-cerebellum ratio was 1.65. High uptake in the kidneys suggested a renal excretion route whereas low bone uptake indicated stability to defluorination. A metabolic stability study with porcine carboxyl esterase found that [18F]25 and [123I]11 have similar stabilities in regards to resistance to enzymatic hydrolysis of the ester but this stability was less than that found for [123I]16.[147] The authors suggest that the N-fluoropropyl group of [123I]16 is bulky enough to impede access to the ester bond by the enzyme thus inhibiting this route of metabolism. This bulkiness of the N-[18F]fluoropropyl group and the subsequent prevention of ester hydrolysis may, therefore, account for why defluorination of [18F]14, [18F]16, and [18F]19 is observed as the major metabolic route.
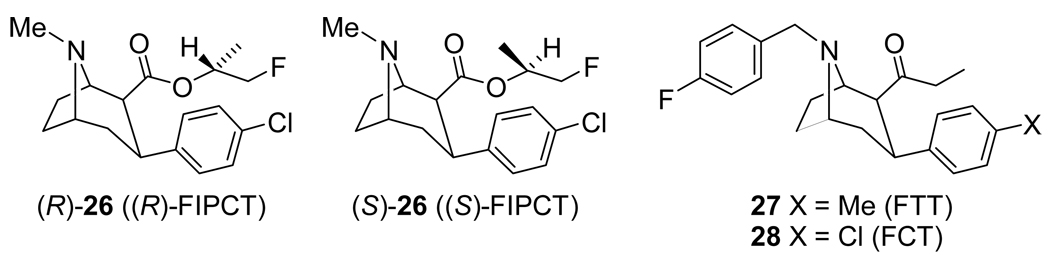
It had been previously shown that replacing the methyl ester of 9 with an isopropyl ester significantly reduced binding affinity at the SERT and NET while only slightly reducing DAT binding affinity.[115] Thus, the fluoroisopropyl derivatives (R)-26 and (S)-26 (Figure 3) were prepared and evaluated.[148] Competitive binding assays in murine kidney cells with transfected DAT or SERT showed that (S)-26 had a nearly 5-fold higher affinity (Ki = 0.67 nM) than (R)-26 (Ki = 3.2 nM) at the DAT and a lower affinity at the SERT (Ki’s = 85 nM (S) and 65 nM (R)). For comparison, 11 was included and had binding affinities of Ki = 0.48 nM (DAT) and Ki = 0.67 nM (SERT) which is in agreement with the previously reported nearly equal affinities at each transporter.[96,105,113] Thus, (S)-26 has only a slight reduction in DAT affinity compared to 11 but has a significant improvement in DAT vs. SERT selectivity. Biodistribution studies in rats with [18F](R/S)-26 showed highest uptake in the liver but very little bone uptake indicating that the fluoroisopropyl ester was stable to defluorination. In rat brain biodistribution studies [18F](R)-26 reached peak uptake in the striatum at 30 min and had a striatum-to-cerebellum ratio of 6.0 at 60 min, whereas [18F](S)-26 also reached peak uptake in the striatum at 30 min but at 60 min the striatum-to-cerebellum ratio was 11.8 and at 120 min it was 12.7. This higher uptake ratio for [18F](S)-26 compared to [18F](R)-26 is in agreement with the higher in vitro DAT binding affinity observed for [18F](S)-26. PET imaging in rhesus monkeys with [18F](R)-26 and [18F](S)-26 showed that for [18F](R)-26 uptake peaked in the putamen and caudate around 45 min and remained nearly stable until the end of the study (115 min) while uptake of [18F](S)-26 was still increasing at 115 min when the study ended. At 115 min [18F](R)-26 had a putamen-to-cerebellum ratio of 3.5 and a caudate-to-cerebellum ratio of 2.5 whereas [18F](S)-26 had a putamen-to-cerebellum ratio of 2.5 and a caudate-to-cerebellum ratio of 2.5. Additionally, the cerebellum uptake washed out significantly faster for [18F](R)-26 than for [18F](S)-26 which allowed [18F](R)-26 to achieve a transient equilibrium at 75 min. Metabolite analysis of rhesus monkey arterial plasma indicated that both [18F](R)-26 and [18F](S)-26 are metabolized to a non-ether-extractable polar compound but that [18F](R)-26 is metabolized more rapidly with 25% unmetabolized [18F](R)-26 remaining after 14 min but 30% unmetabolized [18F](S)-26 remaining after 30 min.
Figure 3.
3β-Phenyl Tropane-Based Dopamine Transporter Ligands.
Other variations on the tropane structure have included the N-benzyl 2β-ethyl ketones 27 and 28.[149] PET studies in rhesus monkeys with [18F]27 and [18F]28 demonstrated that both tracers reached peak uptake in the basal ganglia in ~20 min followed by a steady washout, thus indicating reversible binding. Compound [18F]28 was the superior of the two tracers with a basal ganglia-to-cerebellum uptake ratio of 2.6 vs. 1.5 for [18F]27, presumably due to the higher affinity [18F]28 has for the DAT.[149] Both tracers were rapidly metabolized in a rhesus monkey with <30% [18F]27 remaining and <20% [18F]28 remaining after 20 min. Metabolite analysis of arterial plasma samples from both a rhesus monkey and rats showed that [18F]28 was metabolized to a lipophilic metabolite but this metabolite was not observed in rat whole brain samples indicating that the metabolite cannot cross the blood-brain barrier.
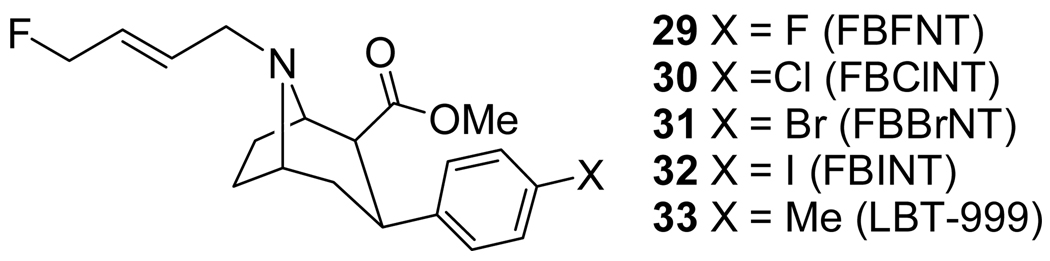
The N-(E)-fluorobutenyl tropanes 29–32 (Fig 4, Table 2) have been previously reported.[150,151] Subsequently, the tolyl-derivative 33 was also reported.[152–156] Compounds 29–33 are thus the N-(E)-fluorobutenyl derivatives of 8–12, respectively. The binding affinities of 29–32 are shown in Fig 4 and Table 2.[151] Compounds 31 and 32 have a high affinity at both the DAT and SERT with little or no selectivity, similar to what has been reported for 10 and 11.[11,96,105,113,148] Compound 30 has a reduced affinity at the DAT and SERT relative to 31 and 32 without a significant improvement in selectivity. Compound 29 has a 10-fold lower affinity at the DAT than 32 along with a greatly reduced affinity at the SERT which results in a significantly improved selectivity for the DAT over the SERT compared to 30–32.
Fig 4.
Binding Affinities of 29–32 At Transfected Human Monoamine Transporters.
Table 2.
|
Ki (nM) |
Selectivity |
||||
|---|---|---|---|---|---|
| Compound | DAT | SERT | NET | SERT/DAT | NET/DAT |
| 29 | 1.70 | 85.5 | >10,000 | 50.3 | >5882 |
| 30 | 2.54 | 24.2 | >10,000 | 9.5 | >3937 |
| 31 | 0.24 | 0.85 | 91 | 3.5 | 379 |
| 32 | 0.17 | 0.21 | 57 | 1.2 | 335 |
Our initial imaging work with [18F]30-[18F]32 was performed on a Siemens 951 PET scanner. We have since reevaluated [18F]30 and [18F]31 along with [18F]29 in anesthetized cynomolgus monkeys with a Concorde microPET P4 which allows for higher resolution images.[157] The highest uptake of [18F]29 is seen in the putamen with peak uptake achieved after 25 min followed by a steady washout. Uptake in the caudate is also high but less than that seen in the putamen. This uptake in the caudate peaks after ~30 min but then remains stable until ~100 min post-injection followed by only a slight washout. Uptake in the substantia nigra peaks after 10 min and then slowly washes out, whereas uptake in the cerebellum peaks after 10 min and rapidly washes out. Injection of the DAT ligand RTI-113[158–161] at 90 min post-injection completely displaces [18F]29 from the caudate and the putamen. Compound [18F]30 shows a rapid uptake in the putamen with peak uptake achieved after 20 min followed by only a slight washout. Uptake in the caudate increases rapidly for the first 20 min, then increases slowly for 20–90 min, and then remains level for 90–235 min. The uptake in the caudate is less than that observed in the putamen and remains less than in the putamen even after the slight washout of activity from the putamen. The uptake in the substantia nigra peaks after 15 min and then washes out at a slower rate than that observed for [18F]29. Uptake of [18F]31 in the putamen peaks after 30 min and only slightly washes out whereas uptake in the caudate peaks after 30 min and then remains constant for 30–235 min. The initial uptake in the putamen was 1.5x that observed in the caudate. Uptake in the substantia nigra peaked after 20 min and then washed out steadily but slower than that observed for [18F]29. This rapid and high uptake of [18F]30 and [18F]31 in the putamen and the caudate followed by a lack of washout is similar to that reported for [11C]33.[152,153,156]
Compounds [18F]29-[18F]31 and [11C]33 all show higher uptake in the putamen than in the caudate. It has been previously shown that anesthesia can influence the behavior of PET tracers and can cause trafficking of the DAT to the plasma membrane.[162–168] Therefore, in an effort to evaluate whether the uptake observed for [18F]29-[18F]31 was influenced by the anesthesia used in the microPET studies a PET study with [18F]29 was performed in an awake rhesus monkey on a Siemens/CTI high resolution research tomograph (HRRT).[157] The uptake of [18F]29 in the caudate and putamen was equal in each hemisphere of the brain in the awake rhesus monkey thus indicating that the higher uptake in the putamen relative to the caudate observed in the microPET studies with [18F]29-[18F]31 was most likely an anesthesia effect. This is presumably also the cause of the higher uptake observed in the putamen than in the caudate with [11C]33 in an anesthetized baboon as well as the reported putamen-to-cerebellum and caudate-to-cerebellum uptake ratios of 30 and 24.6, respectively, in one study[152] and 28.9 and 23.6, respectively, in another study.[153] The uptake of [18F]29 in the putamen and the caudate of the awake rhesus monkey peaked at 30 min and then slowly washed out until the end of the study (85 min), whereas the uptake ratios peaked around 60 min and then began to decline. Thus, [18F]29 is able to achieve peak uptake, reversible binding, and attain a transient equilibrium in an acceptable time frame while also achieving excellent uptake ratios vs. cerebellum uptake in an awake state.
The microPET images obtained with [18F]29-[18F]31 and the PET images obtained with [18F]29 all show skull uptake thus indicating defluorination of the [18F]-(E)-fluorobutenyl group. In an effort to by-pass this [18F]-defluorination and eliminate bone uptake of [18F]fluoride we are currently working towards radiolabeling [18F]29 on the aromatic ring similar to what has been reported for [18F]1-[18F]4, [18F]6, and [18F]8. Skull uptake of [18F]fluoride resulting from defluorination of [18F]33 can be avoided by using [11C]33 but both [18F]33 and [11C]33 presumably will still suffer from benzylic hydroxylation and the generation of radiolabeled metabolites similar to what has recently been reported for [11C]PE2I.[139,147] It is expected that the size of the N-(E)-fluorobutenyl group will prevent enzymatic hydrolysis of the methyl ester similar to what has been previously reported.[147]
Extensive research over the past 20 years directed at developing fluorine-18 radiolabeled PET tracers for imaging the DAT has identified numerous potential candidates from the 3β-phenyltropane class. The most promising are those that can achieve reversible binding and a transient equilibrium in a short time frame along with high specific binding and selectivity while also minimizing the interference from radiolabeled metabolites. So far both [18F]16 and [18F]18 have found use in human imaging and both have demonstrated their utility in detecting a reduction of DAT density in human PD subjects. But both compounds show a slower washout than may be desirable and both suffer from complications due to metabolism. Compound [18F]29 offers improved kinetics but it will need to be radiolabeled on the aromatic ring in order to avoid the skull uptake that results from defluorination of the [18F]fluorobutenyl group.
Serotonin Transporter (SERT)
The human SERT is a 630-amino acid protein[6] that is 98.6% homologous to rhesus monkey SERT, 93% homologous to mouse SERT, and 90% homologous to rat SERT.[4,169] The SERT is found in high densities in the dorsal and median raphe nuclei, putamen, caudate, thalamus, hypothalamus, and amygdala, with lower levels in the cortex and still lower levels in the cerebellar cortex.[170–177] The serotonin system has been associated with numerous psychiatric conditions including PD,[178,179] obsessive-compulsive and panic disorders,[180] stress,[181] and depression and suicide.[182–184] A reduced density of SERT has been observed postmortem in depressed individuals and victims of suicide.[185–187] The SERT is, therefore, the target of the selective serotonin reuptake inhibitor (SSRI) class of antidepressants.[111,112] The availability of specific and selective SERT PET tracers would allow for the measurement of SERT density in the brain and may allow for the detection and diagnosis of SERT-related psychiatric illness as well as a means of measuring SSRI occupancy and the monitoring of SSRI therapy.[16,188–192]
The SSRI Fluoxetine (Prozac) 34[193] (Figure 5) has been radiolabeled with both 11C[194] and 18F.[195] In both cases the uptake of the tracer was characterized by non-specific binding and high lipophilicity. A PET study with [18F]34 in rhesus monkeys showed uptake in all brain regions and an autoradiographic study in rats demonstrated irreversible binding due to subcellular uptake of the tracer.
Figure 5.
SERT Ligands.
The antidepressant (+)-McN5652[196] 35 has been radiolabeled with 11C and evaluated in rats and mice[197] and in humans.[198] In the human brain the uptake of [11C]35 in the midbrain reached a plateau after ~90 min but did not wash out before the end of the study (115 min) thus necessitating the need for the longer-lived 18F. The fluorinated derivatives 36 and 37 were therefore prepared and evaluated. Ex vivo autoradiography with [18F]37 in mice showed accumulation in the hypothalamus, substantia nigra, and raphe nuclei but whole-brain uptake, specific binding, and tissue-to-cerebellum ratios were significantly less than that observed for [11C]35. Replacement of the methyl group of 35 with a fluoromethyl group to give 36 resulted in about a 3-fold loss in binding affinity at the SERT.[199] Biodistribution studies in rats with [18F]36 showed uptake in the raphe nuclei, substantia nigra, locus coeruleus, hypothalamus, thalamus, and amygdala, and this uptake could be blocked with 34.[200] The uptake in these regions washed out slower than cerebellum washout and this resulted in uptake ratios of 7–9 after 3 h. High uptake was also observed in the adrenal gland, lung, intestine, spleen, kidney, liver, and bone marrow. A continuous increase of radioactivity in the skull indicated that [18F]36 was not stable to defluorination in rats. Additional studies with [18F]36 were performed in pigs.[201–203]
The fluorinated derivatives of 6-nitroquipazine,[204] 38 and 39, have been prepared and evaluated as possible SERT PET tracers.[205,206] In rats [18F]38 rapidly entered the brain and showed the highest accumulation in the frontal- and posterial cortex followed by the striatum and this uptake could be reduced 10–20% with the SSRI citalopram.[111,112] Lesser uptake was observed in the thalamus but this uptake could be reduced 20–30% with citalopram. PET imaging in a monkey showed rapid uptake into the brain with a frontal cortex-to-cerebellum ratio of 1.53 and a striatum-to-cerebellum ratio of 1.25 at 80 min post-injection. The uptake in the thalamus was less than that observed in the cerebellum indicating that [18F]38 was not a good candidate for imaging the SERT. Biodistribution studies in mice with [18F]39 showed the highest uptake after 60 min in the frontal cortex and the lowest uptake in the cerebellum. High uptake was also observed in the olfactory tubercle, hypothalamus, thalamus, hippocampus, and striatum. Continuous bone uptake was also observed indicating a slow defluorination of the fluoropropyl group.
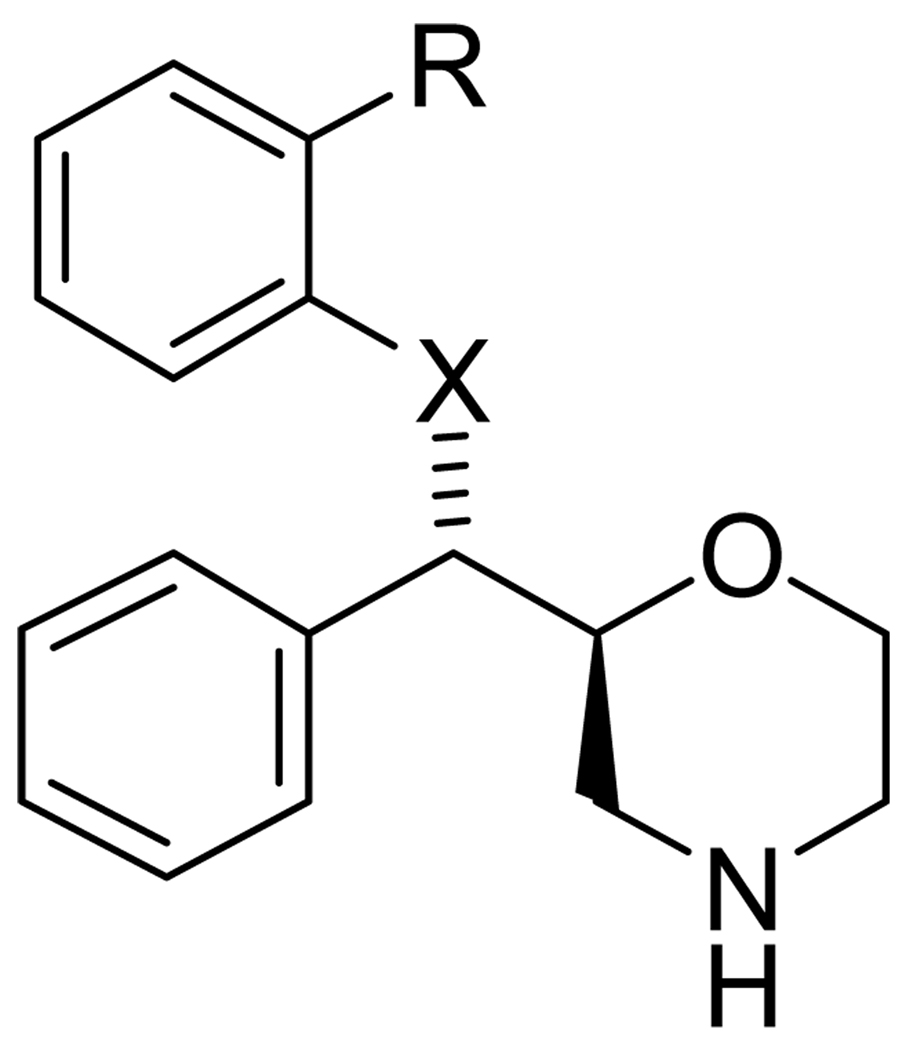
The diphenylsulfide 40 (Figure 6, Table 3) has been previously reported to be an inhibitor of the SERT and NET.[207,208] Numerous compounds have now been reported which exploit the diphenylsulfide motif in order to take advantage of its high binding affinity at the SERT and compounds 41–51 have been radiolabeled with 11C.[209–218] Comparison of [11C]35, [11C]41, [11C]42, [11C]44, and [11C]49 indicated that [11C]42 had the fastest kinetics whereas [11C]49 had the highest signal-to-noise ratio.[213] Comparison between [11C]42 and [11C]45 in the same anesthetized rhesus monkey indicated that [11C]45 achieved higher uptake ratios than [11C]42 and also reached peak uptake faster.[218] Both [11C]42 and [11C]45 have been employed in humans[219–221] and both have demonstrated that they are valuable tracers for imaging the SERT with PET, but both are 11C-labeled and are still, therefore, limited to use where they are prepared and to imaging sessions of less than 2 h.
Fig 6.
Diaryl Sulfide-Based Serotonin Transporter Ligands.
Table 3.
|
Ki (nM) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Compound | R1 | R2 | R3 | SERT | DAT | NET | Ref. | |
| 41 | ADAM | I | H | H | 0.013 | 840 | 699 | [209] |
| 42 | DASB | CN | H | H | 1.10 | 1423 | 1350 | [211] |
| 43 | DAPP | OMe | H | H | 1.89 | 2651 | 1992 | [211] |
| 44 | DAPA | Br | H | H | 0.38 | N/A | N/A | [213] |
| 45 | HOMADAM | CH2OH | H | H | 0.57 | >1000 | 144 | [218] |
| 46 | EADAM | Et | H | H | 0.17 | >1000 | 367 | [217] |
| 47 | AFA, | F | H | H | 1.46 | >10,000 | 141.7 | [215] |
| 4-F-ADAM | F | H | H | 0.08 | 2267 | 117 | [222] | |
| 48 | CF3 | H | H | 0.33 | 2038 | 1205 | [211] | |
| 49 | AFM | CH2F | H | H | 1.04 | >10,000 | 664 | [214] |
| 50 | AFE | CH2CH2F | H | H | 1.80 | >10,000 | 946 | [216] |
| 51 | AFP | CH2CH2CH2F | H | H | 3.58 | >10,000 | 505 | [223] |
| 52 | 5-F-ADAM | H | F | H | 0.47 | N/A | N/A | [224] |
| 53 | ACF | Cl | F | H | 0.05 | 3020 | 650 | [225] |
| 54 | H | H | OCH2CH2F | 0.25 | 340 | 7.5 | [226] | |
| 55 | F | H | OCH2CH2F | 0.10 | >1000 | 37 | [226] | |
| 56 | Cl | H | OCH2CH2F | 0.03 | 847 | 97 | [226] | |
| 57 | Br | H | OCH2CH2F | 0.05 | >1000 | 114 | [226] | |
| 58 | H | H | OCH2CH2CH2F | 1.4 | 299 | 12 | [226] | |
| 59 | F | H | OCH2CH2CH2F | 0.95 | >1000 | 95 | [226] | |
| 60 | Br | H | OCH2CH2CH2F | 0.15 | >1000 | >1000 | [226] | |
| 61 | CH2OH | H | F | 1.26 | >2000 | 618 | [227] | |
Compounds 47, 49, and 50 all have a high affinity for the SERT and are selective for the SERT over the DAT and NET. Compound 51 has a slightly reduced SERT affinity but still shows selectivity over the DAT and NET.[223] Compounds [11C]47, [11C]49, and [11C]50, have been evaluated in baboons.[214–216] Biodistribution studies in rats were performed to compare [18F]47, [18F]49, [18F]50, and [18F]51.[223] All four compounds rapidly entered the brain and accumulated in the thalamus, hypothalamus, frontal cortex, striatum, and hippocampus. This uptake could be blocked with citalopram thus demonstrating SERT-specific binding. Compound [18F]49 reached peak uptake after 30 min followed by washout and this uptake was the highest among the four tracers. Compounds [18F]47, [18F]50, and [18F]51 all reached peak uptake after 10 min followed by washout while the highest uptake observed was with [18F]47. PET imaging comparisons in the same baboon with [18F]47, [18F]49, and [18F]50 showed that [18F]47 and [18F]50 reached peak uptake in the thalamus in 15–35 min whereas [18F]49 reached peak uptake in 40–60 min but showed significantly higher specific binding.[228] Another reported biodistribution study in rats with [18F]47 had shown peak uptake at 30 min followed by washout.[222] This study also showed that [18F]47 accumulated rapidly in the muscle, lung, liver, and kidney but then cleared whereas bone uptake continuously increased indicating that, at least in rats, defluorination was a problem. In a PET study with [18F]47 in a baboon the radioactivity in the skull did not increase with time suggesting that defluorination was isolated to rats.[222] In this PET study peak uptake in the midbrain and striatum was reached in ~30–40 min with midbrain-to-cerebellum ratios of 3.2 at 2 h and 4.2 at 3 h and striatum-to-cerebellum ratios of 2.4 at 2 h and 2.7 at 3 h.
Compound 52 is an isomer of 47 where the fluorine atom has been moved from the 4-position (para to the sulfur atom) to the 5-position (meta to the sulfur atom). This change resulted in a significant loss in affinity for the SERT[224] compared to 47[222] as well as a slight decrease in lipophilicity (logP = 2.47 vs. 2.73). Biodistribution studies in rats with [18F]52 showed an initial high uptake in the lungs, kidneys, and heart but this cleared quickly whereas bone uptake continued to increase indicating defluorination.[229] Brain uptake was rapid with peak uptake at 2 min followed by a fast washout. The hypothalamus-to-cerebellum ratio was 2.97 at 60 min which is less than that observed with [18F]47. Thus, changing the position of the fluorine-atom did not produce an improvement in imaging properties compared to [18F]47.
Compound 53 retains the fluorine atom in the 5-position and places a chlorine atom in the 4-position which produced a compound with a high SERT affinity and selectivity.[225] Biodistribution studies with [18F]53 in rats showed initial high accumulation in lung, muscle, liver, kidney, and skin which then declined.[225] Bone uptake was slightly less than that observed with [18F]52[229] and significantly less than that observed with [18F]47.[222] Brain uptake of [18F]53 was rapid with the highest uptake observed at 2 min followed by washout. At 60 min the hypothalamus-to-cerebellum ratio was 3.5 (compared to 3 for [18F]52) and the striatum-to-cerebellum ratio was 2.9. Thus, [18F]52 and [18F]53 behave similarly in rats with regards to uptake ratios and defluorination but both have too rapid of kinetics when compared to [18F]47.
The fluoroalkyl ethers 54–60 have a high affinity for the SERT but 54 and 58 also have an appreciable affinity for the NET [226] which may cause interference in PET imaging if the cerebellum was to be used as the reference region. In rat biodistribution studies [18F]55-[18F]57, [18F]59, and [18F]60 showed high brain uptake but slow washout whereas [18F]54 and [18F]58 also showed high brain uptake but a faster washout. Of compounds [18F]54-[18F]60, the highest hypothalamus-to-cerebellum ratios were observed with [18F]54 and [18F]58 (~7.8 and ~7.7, respectively, at 120 min) due to the faster washout of these compounds.[226] Additional rat biodistribution studies with [18F]58 showed initial high uptake in the lung, skin, muscle, liver, and kidney which then slowly declined.[230] Bone uptake was high and remained high indicating defluorination. Brain uptake was rapid followed by a steady washout. Autoradiography showed uptake in the olfactory tubercles, thalamic nuclei, hypothalamic nuclei, substantia nigra, superior colliculus, dorsal raphe, medial raphe, and locus coeruleus, all of which could be blocked with the SSRI escitalopram[231,232] except for the locus coeruleus which could be blocked with the NET ligand nisoxetine.[233,234] Thus, some NET binding in the locus coeruleus is observed in conjunction with the desired binding in the SERT-rich brain regions when performing autoradiography but uptake in the cerebellum was not observed in the rat when PET imaging was performed with [18F]58 which will, therefore, allow for use of the cerebellum as the reference region. In the rat PET images obtained with [18F]58, clear localization was observed in the thalamus, midbrain, and striatum with peak uptake achieved in 10–20 min followed by a steady washout.[230] The region-to-cerebellum ratios peaked at 100–110 min with a value of ~4 followed by a slow decline. The uptake in the midbrain, thalamus, and striatum could be displaced by escitalopram (2 mg/kg) but also, to a lesser extent, by nisoxetine (10 mg/kg). A similar displacement of the SERT PET tracer [11C]mZIENT during a chase study with the NET ligand (±)-reboxetine•mesylate (1 mg/kg) has also been observed.[235] Both nisoxetine and reboxetine[236] have a weak affinity for the SERT (Ki = 30 and 60 nM, respectively) but are selective for the NET over the SERT (21.4 and 6.7, respectively).[237] This weak binding to the SERT may be the cause of the observed displacement of these two tracers but alternatively, the displacement may be an indirect effect mediated through interactions between the noradrenergic and serotonergic systems.[238–240]
Compound 61 is a fluorinated derivative of 45.[227] Introduction of the fluorine atom resulted in a slight reduction in SERT affinity but also a reduction in DAT and NET affinity which produced similar selectivities for the SERT over the DAT and NET. The presence of the fluorine atom also increased the lipophilicity somewhat (logP7.4 = 2.06 and 1.60, respectively). MicroPET imaging with [11C]61 in an anesthetized cynomolgus monkey showed high uptake in the midbrain, pons, thalamus, and medulla with little uptake in the frontal cortex and cerebellum. No uptake was observed in the caudate or putamen which is surprising since this brain region does contain SERT.[177] Peak uptake of [11C]61 in the midbrain occurred between 12.5 and 27.5 min post-injection followed by a steady washout. At 85 min the midbrain-to-cerebellum ratio was 3.4 and the thalamus-to-cerebellum ratio was 2.3. The observed uptake was displaceable with (R/S)-citalopram•HBr thus demonstrating SERT-selective binding. These promising results suggest that [18F]61 may be a viable SERT PET tracer.
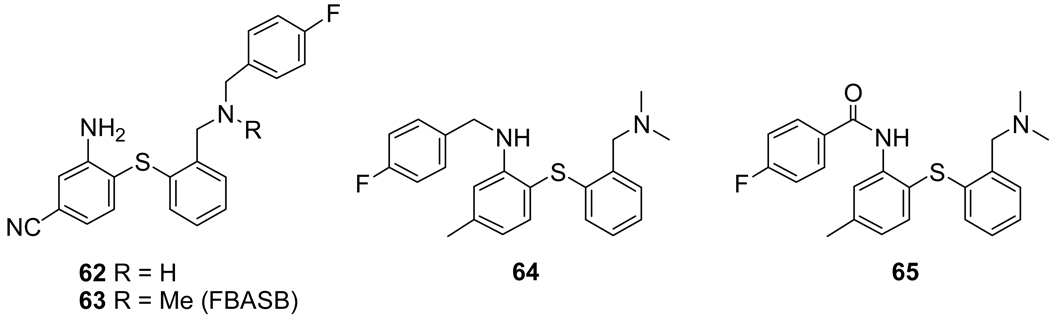
Replacement of one of the N-methyl groups of 41 with a fluoroethyl group resulted in a significant loss of SERT affinity (Ki = ~19 nM vs. 0.4 nM for 41)[241] indicating that functionalizing this position was detrimental to binding. Nevertheless, the para-fluorobenzyl derivatives 62 (SERT Ki = ~4 nM) and 63 (SERT Ki = ~510 nM) were prepared and evaluated.[242] As seen from the SERT binding affinities the large p-fluorobenzyl group can be tolerated to a certain extent if the nitrogen atom is substituted secondarily (62) but replacement of the hydrogen atom with a methyl group (63) completely abolished any affinity for the SERT. Biodistribution studies in rats with [18F]63 showed low brain uptake and low selectivity in SERT-rich brain regions. Placement of the p-fluorobenzyl group on the anilino-nitrogen (64) produced a compound with moderate SERT affinity (Ki = ~10 nM) and replacement of the p-fluorobenzyl group with a p-fluorobenzoyl group (65) further reduced the SERT affinity (Ki = ~14 nM).[243]
During the search for tropane-based cocaine addiction therapeutics as mentioned in the DAT section above, it was discovered that removal of the N-methyl group of a tropane to give a nortropane produced compounds with enhanced SERT and NET affinity.[244] Numerous compounds have been prepared in an attempt to obtain SERT-selective nortropane derivatives[245–251] and several have been radiolabeled with 11C.[235,252–255] The fluoroethyl ester nortropanes 66 and 67 (Figure 8) have been reported but, similar to 11, the affinities for the SERT and DAT were nearly equal.[144]
Figure 8.
Fluoroethyl Ester Nortropane-based SERT Ligands.
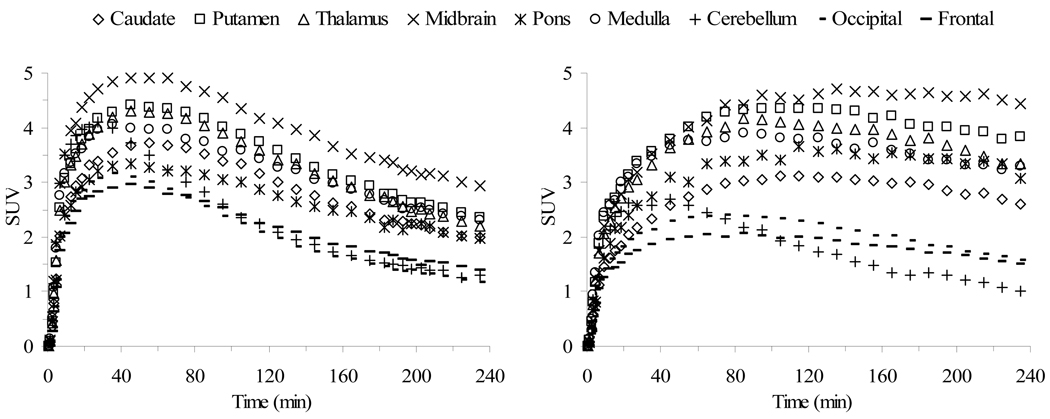
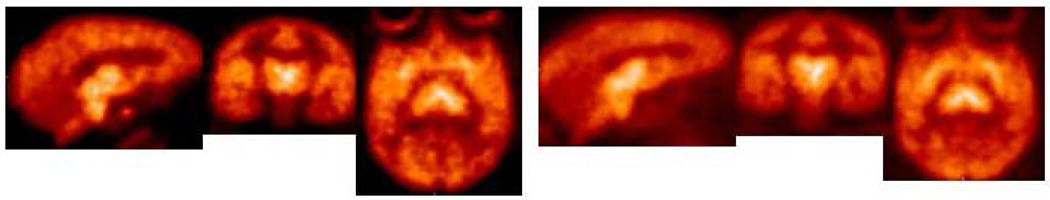
The results of microPET and HRRT PET imaging in monkeys with the meta- and para-vinyl iodides [18F]68 and [18F]69, respectively, have recently been reported.[256,257] Both 68 and 69 have a high affinity for the SERT (Ki = 0.43 and 0.08 nM, respectively) but substitution in the para-position provides about a 5-fold higher affinity. These differences in binding affinity to the SERT translate into significant differences in the imaging behavior of the compounds. MicroPET imaging with [18F]68 and [18F]69 was performed in anesthetized cynomolgus monkeys (Figure 9) and PET imaging on an HRRT with [18F]68 and [18F]69 was performed in an awake rhesus monkey (Figure 10). The time required to reach peak uptake is significantly different for the two isomers with the stronger binding para-isomer [18F]69 taking longer to reach peak uptake. Also, the times required for each tracer to reach peak uptake are about the same in both the anesthetized and awake states indicating that anesthesia does not significantly affect the performance of these tracers. After reaching peak uptake the activity of [18F]68 in the midbrain, putamen, thalamus, medulla, and caudate remains nearly steady for about 20 min followed by a constant washout during which time (65–175 min post-injection) a pseudo-equilibrium is achieved. Compound [18F]69 takes significantly longer to reach peak uptake in the SERT-rich brain regions and then only slightly washes out. The uptake of [18F]69 in the cerebellum reaches peak uptake after 45 min followed by a steady washout which provides ever increasing tissue-to-cerebellum ratios as the study progresses. Because the washout of [18F]68 from the SERT-rich brain regions is nearly parallel to the washout from the cerebellum the tissue-to-cerebellum ratios for [18F]68 slowly increase throughout the course of the study. Thus, [18F]68 provides superior imaging kinetics and achieves a pseudo-equilibrium whereas [18F]69 provides higher uptake ratios. Both of the tracers can be displaced with citalopram but the observed displacement is significantly greater for [18F]69 because of the minor washout that occurs (under baseline conditions) after peak uptake is achieved which results in only slightly declining time-activity curves. When a citalopram chase is then performed the slope of the time-activity curves of [18F]69 changes drastically. Thus, each of these tracers is a promising candidate for human use because each has its own unique imaging properties. Compound [18F]68 can be used for kinetic analysis because of its ability to achieve a pseudo-equilibrium whereas compound [18F]69 can be used for SSRI occupancy studies due to the large change in slope of the time-activity curves that occurs when an SSRI is administered.
Figure 9.
MicroPET time-activity curves obtained by injection of [18F]68 (left) and [18F]69 (right) into anesthetized cynomolgus monkeys.
Figure 10.
HRRT PET images obtained by injection of [18F]68 (left) and [18F]69 (right) into an awake rhesus monkey.
The goal of developing an 18F-radiolabeled PET tracer for imaging the SERT has benefited from the availability of high affinity and selective compounds from both the diphenylsulfide and nortropane classes. Compounds [11C]42 and [11C]45 have both demonstrated their utility in imaging normal human volunteers but the adaptation of these compounds to 18F-labeled tracers has so far proven difficult. The 18F-labeled nortropanes [18F]68 and [18F]69 have been shown to have improved kinetics relative to their 11C-labeled counterparts, [11C]mZIENT[235] and [11C]pZIENT,[255] respectively, and we look forward to evaluating [18F]68 and [18F]69 in healthy human volunteers.
Norepinephrine Transporter (NET)
The human NET is a 617-amino acid transmembrane protein and is 98.9% homologous to the monkey NET.[4,174,258] The NET is found in high densities in the locus coeruleus, cerebellum, dorsal raphe nuclei, thalamus, and hypothalamus.[233,234,239,259,260] The norepinephrine system has been associated with a variety of neuropsychiatric diseases including PD,[261] ADHD,[262,263] posttraumatic stress disorder (PTSD),[264] depression and anxiety,[240,265–267] and seizure,[268] and a reduction in NET density has been demonstrated postmortem in depressed subjects.[269] The availability of specific and selective NET PET imaging agents will allow for density measurements of the NET and may help to clarify the role of the NET in certain psychiatric diseases as well as help to identify and evaluate NET therapeutics.[270–272]
The antidepressant reboxetine 70 (Figure 11, Table 4) is a selective norepinephrine reuptake inhibitor (SNRI) which is marketed as the racemic mixture.[236,273,274] The ethyl group of 70 has been replaced with a [11C]methyl group to give [11C]71 and PET imaging comparisons in baboon between racemic-[11C]71, (S,S)-[11C]71, and (R,R)-[11C]71 has shown that (S,S)-[11C]71 is the active isomer based on a significantly higher distribution volume in the thalamus and the ability to block the uptake of (S,S)-[11C]71, but not (R,R)-[11C]71, in the thalamus and cerebellum with nisoxetine.[275] The uptake of (S,S)-[11C]71 in the striatum could not be blocked with nisoxetine thus demonstrating either low affinity binding or non-specific binding in this brain region. In biodistribution studies in rats (S,S)-[11C]71 showed a hypothalamus-to-striatum ratio of 2.5 at 60 min post-injection whereas (R,R)-[11C]71 showed only a homogenous distribution.[276] Plasma analysis indicated that (S,S)-[11C]71 was metabolized rapidly in the periphery with 50% unmetabolized radiotracer remaining after 30 min and 20% remaining after 60 min but whole rat brain extracts showed that 95% of radioactivity in the brain was unmetabolized (S,S)-[11C]71. PET imaging with (S,S)-[11C]71 in cynomolgus monkeys showed the highest uptake in the lower brainstem, mesencephalon, and thalamus while the lowest uptake was in the striatum.[277] Thus, three groups independently verified that (S,S)-[11C]71 could be used to image the NET in vivo with PET although specific peak binding equilibrium is not achieved during the course of these studies. Based on these results fluoroalkyl ether derivatives of reboxetine have been developed in pursuit of an 18F-labeled NET PET tracer which would enable longer imaging times and possibly the achievement of peak specific binding equilibrium.
Fig 11.
(S,S)-Reboxetine Derivative-Based Norepinephrine Transporter Ligands.
Table 4.
| Compound | X = | R = | |
|---|---|---|---|
| 70 | Reboxetine | O | OCH2CH3 |
| 71 | MeNER | O | OCH3 |
| 72 | FMeNER | O | OCH2F |
| 73 | FMeNER-D2 | O | OCD2F |
| 74 | FERB | O | OCH2CH2F |
| 75 | FERB-D2 | O | OCD2CD2F |
| 76 | MENET | O | CH3 |
| 77 | MESNET | S | CH3 |
| 78 | FENET | O | CH2CH2F |
| 79 | FPNET | O | CH2CH2CH2F |
| 80 | S | OCH2CH2F | |
| 81 | S | OCH2CH2CH2F | |
Compound (S,S)-[18F]72 is a fluorinated derivative of (S,S)-[11C]71 but PET imaging in a cynomolgus monkey with (S,S)-[18F]72 showed rapid defluorination and skull uptake. Thus, the deuterated derivative (S,S)-[18F]73 was developed and PET imaging showed that defluorination was reduced but not totally inhibited.[278] During PET imaging the total brain uptake of (S,S)-[18F]72 peaked at 8 min and then washed out whereas total brain uptake of (S,S)-[18F]73 peaked at 12 min and then washed out. The uptake of both (S,S)-[18F]72 and (S,S)-[18F]73 could be blocked with desipramine but blocking with 1 or citalopram did not have any effect on the uptake of either tracer. At 110 min after injection of (S,S)-[18F]72 the tissue-to-striatum ratios were 1.2, 1.2, and 1.3 for the lower brainstem, mesencephalon, and thalamus, respectively. At 160 min after injection of (S,S)-[18F]73 the tissue-to-striatum ratios were 1.5, 1.6, 1.3, and 1.5 for the lower brainstem, mesencephalon, thalamus, and temporal cortex, respectively. Peak specific binding was achieved at 90–120 min for (S,S)-[18F]72 and at 120–160 min for (S,S)-[18F]73. Autoradiography with (S,S)-[18F]73 using post-mortem human brain slices showed the highest accumulation of radioactivity in the locus coeruleus with lower accumulation in the cerebellum, cortex, thalamus, and hypothalamus along with non-specific binding in the caudate nucleus and putamen.[279] A low signal-to-noise ratio was seen outside the locus coeruleus and the authors suggest that the binding affinity of (S,S)-[18F]73 (Ki = 3.1 nM) may not be sufficient enough for visualization and quantification of the NET in low density regions. Biodistribution and radiation dosimetry studies have been performed with (S,S)-[18F]73 in cynomolgus monkeys[280] and humans[281] and (S,S)-[18F]73 has been used to measure the occupancy of the SNRI atomoxetine.[282] Initial human imaging studies with (S,S)-[18F]73 have recently been reported.[283] Using 4 healthy male subjects the mean peak uptake in the whole brain was 2.6 ± 0.5% at 12.5 min after injection. Uptake in the thalamus achieved peak equilibrium with a thalamus-to-caudate ratio of 1.5. Cortical uptake was high due to high skull uptake resulting from defluorination. Thus, (S,S)-[18F]73 is a promising PET tracer for imaging the NET in humans but the inability to totally stop defluorination suggests that further structural improvements are still needed.
Compound 74 is a fluorinated derivative of 70 and compound 75 is the deuterated analog of 74, the deuteration strategy again being employed to inhibit defluorination.[284] Biodistribution studies in mice with (S,S)-[18F]74 and (S,S)-[18F]75 showed high accumulation of both tracers in the kidneys, liver, and intestines. Brain uptake was moderate and washout was slow for both (S,S)-[18F]74 and (S,S)-[18F]75. At 2 h post-injection the average bone uptake of (S,S)-[18F]74 was 0.83% ID/g and that of (S,S)-[18F]75 was 0.25% ID/g thus demonstrating a reduction of defluorination via the deuterium isotope effect. A PET imaging comparison in baboons with (S,S)-[11C]71 and (S,S)-[18F]75 (along with several other 11C-labeled NET ligands) determined that (S,S)-[11C]71 still has the best PET imaging characteristics of the prospective NET PET tracers available at that time due to the higher signal-to-noise ratio obtained with (S,S)-[11C]71 and its faster washout from the striatum.[271,285]
Compounds 76–81 have recently been developed as potential NET PET tracers.[286,287] Compounds 76–79 have directly connected the alkyl group to the aryl ring thereby eliminating the phenyl alkyl ether functionality and thus possibly enhancing the metabolic stability of the radiolabel. Compounds 77, 80, and 81 have incorporated a sulfur atom in replacement of the benzyl phenyl ether oxygen atom. Compounds 76 and 77 each have a high affinity for the NET (Ki = 1.02 ± 0.11 and 0.30 ± 0.03 nM, respectively) which is very similar to the NET affinity of 70 (Ki = 1.04 ± 0.16 nM) and 71 (0.95 ± 0.03 nM).[287] Thus, the phenyl methyl ether oxygen atom is not an important determinant of binding affinity at the NET and a slight enhancement in affinity results from replacing the benzyl phenyl ether oxygen atom with a sulfur atom. Compound 77 has an enhanced SERT affinity (Ki = 14.8 ± 2.83 nM) relative to 76 (Ki = 93 ± 20 nM) indicating that the benzyl phenyl ether oxygen atom is necessary for low SERT affinity. Compounds 78 and 79 each had a reduced NET affinity (Ki = 3.14 ± 0.17 and 3.68 ± 0.92 nM, respectively) compared to 76 indicating that larger alkyl groups, or fluorine substitution, or both, is detrimental to the NET affinity of this series of compounds. MicroPET imaging with [11C]76 in an anesthetized rhesus monkey showed peak uptake around 20 min followed by a steady washout with tissue-to-caudate ratios of 1.30, 1.45, 1.40, and 1.30 at 45 min post-injection and 1.30, 1.43, 1.44, and 1.25 at 85 min post-injection for the thalamus, midbrain, pons, and cerebellum, respectively, indicating that a quasi-equilibrium had been achieved. MicroPET imaging with [11C]77 in an anesthetized rhesus monkey showed peak uptake around 30–40 min followed by a steady washout (but slower than that of [11C]76) with a thalamus-to-caudate ratio of 1.34 and a midbrain-to-caudate ratio of 1.33 at 85 min post-injection. Because of the moderate affinity 77 has for the SERT, a chase study was performed with (R,S)-citalopram•HBr (1.5 mg/kg) at 40 min post-injection. The uptake of radioactivity in the caudate did not change indicating that this uptake was most likely the result of nonspecific binding rather than SERT binding. PET imaging on an HRRT was performed in awake rhesus monkeys with [11C]76 and [11C]77 to assess the effects of anesthesia on the performance of these tracers. With [11C]76 high uptake was observed in the thalamus, locus coeruleus, midbrain, and pons and the kinetics were very similar to that observed in the microPET study with an anesthetized rhesus monkey but the tissue-to-caudate ratios were slightly reduced in the awake state. Compound [11C]77, on the other hand, showed significantly different kinetics than that observed in the microPET study with an anesthetized rhesus monkey with prolonged retention observed over the course of the study and very little washout. The tissue-to-caudate ratios were also slightly reduced in the awake state. It is known that the anesthetic ketamine (which is used to initially anesthetize the animal before administration of isoflurane) binds to the NET[288] and this may be the cause of the differences observed between the anesthetized and awake states. In microPET studies with [18F]78 and [18F]79 in anesthetized rhesus monkeys peak uptake was achieved in all regions of interest after 15 min followed by a fast washout. High uptake was also observed in the caudate with tissue-to-caudate ratios for the thalamus, midbrain, and cerebellum of 1.13, 1.13, and 1.05, respectively, for [18F]78 and 1.18, 1.18, and 0.95, respectively, for [18F]79. Thus, the poor performance of [18F]78 and [18F]79 eliminates them as potential 18F-labeled NET PET tracers.
As mentioned in the DAT section above, cocaine binds to the NET and numerous tropane derivatives have been reported as ligands for the NET.[289–294] Preliminary microPET evaluation of a 11C-labeled NET tropane derivative showed both SERT and NET binding and, therefore, a lack of NET selectivity.[295] The NET ligands talopram[296] and talsupram have been radiolabeled with 11C and evaluated with PET but neither compound entered the brain in sufficient amounts to become a viable PET tracer.[297]
The goal of developing an 18F-labeled PET tracer for the NET is still a work-in-progress but progress is indeed being made. The results above with reboxetine derivatives demonstrate that some minor structural variation can be tolerated without a significant loss in NET affinity but it appears that placing an 18F-radiolabel on the methoxy or ethoxy group does reduce the NET affinity. A similar loss in affinity was observed when going from 35 to 36. Furthermore, defluorination also is a problem that can only be reduced, but not stopped, by incorporation of deuterium into these fluoroalkoxy groups. Defluorination was also observed with [18F]36 which, in combination with the 18F-NET ligand data above, suggests that radiolabeling with [18F]methyl and [18F]ethyl ethers and thioethers should be avoided if at all possible. Thus, the development of a reboxetine-based 18F-labeled PET tracer for the NET may require that the 18F-radiolabel be placed on one of the aromatic rings.
Summary
Progress is being made towards the development of fluorine-18 radiolabeled PET tracers for the DAT, SERT, and NET. The greatest number of potential tracers developed so far has been for the DAT due to the greater number of years that have been devoted to this target but also due in part to the concurrent research effort focused on developing a cocaine addiction therapeutic based on the tropane skeleton. The development of a library of potential therapeutic tropane compounds with high DAT affinity that could be radiolabeled with 11C aided in the discovery of PET tracers for the DAT as well as the SERT, and this knowledge was then successfully translated to 18F-labeled tropane derivatives for both the DAT and SERT. The diphenylsulfide class of SERT ligands has already found success with 11C-labeled PET tracers and a viable 18F-labeled PET tracer may soon be realized. Fewer tracers for the NET have been reported because PET imaging of the NET has received considerable less attention than the DAT or SERT but promising, though not optimal, compounds have already been reported. Further work towards this goal should also eventually provide a viable 18F-labeled NET PET tracer.
It is very difficult to predict if a proposed compound will have the desired properties of a PET tracer and so numerous compounds generally have to be synthesized and evaluated. As demonstrated with [18F]1-[18F]4, [18F]34, and [11C]35, radiolabeling an antidepressant will not necessarily produce a good PET tracer. On the other hand, derivatives of antidepressants may produce candidate PET tracers as evidenced by the derivatives of 40 shown in Figure 6 and Table 3 and the derivatives of 70 shown in Figure 11 and Table 4. A high binding affinity to the target and a selectivity for that target are the first requirements that need to be met and any compound that cannot meet these requirements will most likely not succeed. Compounds with a high binding affinity and selectivity must also have a lipophilicity in the range of logP = ~1–3 or they will also fail. Furthermore, compounds which appear to have the appropriate affinity, selectivity, and lipophilicity can still fail due to poor kinetics or problems resulting from metabolism. Thus, the development of suitable PET tracers for the DAT, SERT, and NET is not an easy task, but with continued hard work by many research groups the goal will eventually be realized. It should, in fact, be possible to develop more than one tracer for each target and, as shown above, this has already been done. Which of the available tracers is the “best” would then have to be determined from side-by-side comparisons in the same subject under the same conditions (preferably in a conscious state) while taking into consideration the influence of metabolites that are generated from each individual tracer. This has already been done with many of the tracers reported above and this should continue to be done as part of tracer development. Because the goals of different studies of the same target may vary, the availability of several different tracers with slightly different properties may be of greater benefit to the PET imaging community than having only one “best” tracer for a given target.
Figure 7.
Diaryl Sulfide-Based Serotonin Transporter Ligands.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rudnick G, Clark J. From Synapse to Vesicle: the Reuptake and Storage of Biogenic Amine Neurotransmitters. Biochim. Biophys. Acta. 1993;1144:249–263. doi: 10.1016/0005-2728(93)90109-s. [DOI] [PubMed] [Google Scholar]
- 2.Nelson N. The Family of Na+/Cl− Neurotransmitter Transporters. J. Neurochem. 1998;71:1785–1803. doi: 10.1046/j.1471-4159.1998.71051785.x. [DOI] [PubMed] [Google Scholar]
- 3.Eisenhofer G. The Role of Neuronal and Extraneuronal Plasma Membrane Transporters in the Inactivation of Peripheral Catecholamines. Pharmacol. Therap. 2001;91:35–62. doi: 10.1016/s0163-7258(01)00144-9. [DOI] [PubMed] [Google Scholar]
- 4.Miller GM, Yatin SM, De La Garza R, et al. Cloning of Dopamine, Norepinephrine and Serotonin Transporters from Monkey Brain: Relevance to Cocaine Sensitivity. Mol. Brain Res. 2001;87:124–143. doi: 10.1016/s0169-328x(00)00288-6. [DOI] [PubMed] [Google Scholar]
- 5.Gainetdinov RR, Caron MG. Monoamine Transporters: From Genes to Behavior. Annu. Rev. Pharmacol. Toxicol. 2003;43:261–284. doi: 10.1146/annurev.pharmtox.43.050802.112309. [DOI] [PubMed] [Google Scholar]
- 6.Torres GE, Gainetdinov RR, Caron MG. Plasma Membrane Monoamine Transporters: Structure, Regulation and Function. Nature Reviews Neuroscience. 2003;4:13–25. doi: 10.1038/nrn1008. [DOI] [PubMed] [Google Scholar]
- 7.Lester HA, Cao Y, Mager S. Listening to Neurotransmitter Transporters. Neuron. 1996;17:807–810. doi: 10.1016/s0896-6273(00)80213-5. [DOI] [PubMed] [Google Scholar]
- 8.Melikian HE. Neurotransmitter Transporter Trafficking: Endocytosis, Recycling, and Regulation. Pharmacol. Therap. 2004;104:17–27. doi: 10.1016/j.pharmthera.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Iversen L. Neurotransmitter Transporters: Fruitful Targets for CNS Drug Discovery. Mol. Psychiatry. 2000;5:357–362. doi: 10.1038/sj.mp.4000728. [DOI] [PubMed] [Google Scholar]
- 10.Carroll FI, Howell LL, Kuhar MJ. Pharmacotherapies for Treatment of Cocaine Abuse: Preclinical Aspects. J. Med. Chem. 1999;42:2721–2736. doi: 10.1021/jm9706729. [DOI] [PubMed] [Google Scholar]
- 11.Carroll FI. 2002 Medicinal Chemistry Division Award Address: Monoamine Transporters and Opioid Receptors. Targets for Addiction Therapy. J. Med. Chem. 2003;46:1775–1794. doi: 10.1021/jm030092d. [DOI] [PubMed] [Google Scholar]
- 12.Volkow ND, Fowler JS, Gatley SJ, et al. PET Evaluation of the Dopamine System of the Human Brain. J. Nucl. Med. 1996;37:1242–1256. [PubMed] [Google Scholar]
- 13.Laakso A, Hietala J. PET Studies of Brain Monoamine Transporters. Curr. Pharm. Des. 2000;6:1611–1623. doi: 10.2174/1381612003398799. [DOI] [PubMed] [Google Scholar]
- 14.Jaffer FA, Weissleder R. Molecular Imaging in the Clinical Arena. JAMA. 2005;293:855–862. doi: 10.1001/jama.293.7.855. [DOI] [PubMed] [Google Scholar]
- 15.Hammoud DA, Hoffman JM, Pomper MG. Molecular Neuroimaging: From Conventional to Emerging Techniques. Radiology. 2007;245:21–42. doi: 10.1148/radiol.2451060731. [DOI] [PubMed] [Google Scholar]
- 16.Meyer JH. Imaging the Serotonin Transporter During Major Depressive Disorder and Antidepressant Treatment. J. Psychiatry Neurosci. 2007;32:86–102. [PMC free article] [PubMed] [Google Scholar]
- 17.Brooks DJ. Neuroimaging in Parkinson's Disease. NeuroRx. 2004;1:243–254. doi: 10.1602/neurorx.1.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aboagye EO, Price PM, Jones T. In Vivo Pharmacokinetics and Pharmacodynamics in Drug Development Using Positron-Emission Tomography. Drug Discovery Today. 2001;6:293–302. doi: 10.1016/s1359-6446(01)01684-1. [DOI] [PubMed] [Google Scholar]
- 19.Laruelle M, Slifstein M, Huang Y. Positron Emission Tomography: Imaging and Quantification of Neurotransporter Availability. Methods. 2002;27:287–299. doi: 10.1016/s1046-2023(02)00085-3. [DOI] [PubMed] [Google Scholar]
- 20.Talbot PS, Laruelle M. The Role of In Vivo Molecular Imaging with PET and SPECT in the Elucidation of Psychiatric Drug Action and New Drug Development. Eur. Neuropsychopharmacol. 2002;12:503–511. doi: 10.1016/s0924-977x(02)00099-8. [DOI] [PubMed] [Google Scholar]
- 21.Guilloteau D, Chalon S. PET and SPECT Exploration of Central Monoaminergic Transporters for the Development of New Drugs and Treatments in Brain Disorders. Curr. Pharm. Design. 2005;11:3237–3245. doi: 10.2174/138161205774424744. [DOI] [PubMed] [Google Scholar]
- 22.Lee C-M, Farde L. Using Positron Emission Tomography to Facilitate CNS Drug Development. Trends Pharmacol. Sci. 2006;27:310–316. doi: 10.1016/j.tips.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Hargreaves RJ. The Role of Molecular Imaging in Drug Discovery and Development. Clin. Pharmacol. Ther. 2008;83:349–353. doi: 10.1038/sj.clpt.6100467. [DOI] [PubMed] [Google Scholar]
- 24.Takano A, Suzuki K, Kosaka J, et al. A Dose-Finding Study of Duloxetine Based on Serotonin Transporter Occupancy. Psychopharmacology. 2006;185:395–399. doi: 10.1007/s00213-005-0304-0. [DOI] [PubMed] [Google Scholar]
- 25.Schlyer DJ. PET Tracers and Radiochemistry. Ann. Acad. Med. Singapore. 2004;33:146–154. [PubMed] [Google Scholar]
- 26.Ametamey SM, Honer M, Schubiger PA. Molecular Imaging with PET. Chem. Rev. 2008;108:1501–1516. doi: 10.1021/cr0782426. [DOI] [PubMed] [Google Scholar]
- 27.Miller PW, Long NJ, Vilar R, et al. Synthesis of 11C, 18F,15O, and 13N Radiolabels for Positron Emission Tomography. Angew. Chem. Int. Ed. 2008;47:8998–9033. doi: 10.1002/anie.200800222. [DOI] [PubMed] [Google Scholar]
- 28.Wernick MN, Aarsvold JN, editors. Emission Tomography: The Fundamentals of PET and SPECT. San Diego, CA, USA; London, UK: Elsevier Academic Press; 2004. [Google Scholar]
- 29.Levin CS, Hoffman EJ. Calculation of Positron Range and Its Effect on the Fundamental Limit of Positron Emission Tomography System Spatial Resolution. Phys. Med. Biol. 1999;44:781–799. doi: 10.1088/0031-9155/44/3/019. [DOI] [PubMed] [Google Scholar]
- 30.Smart BE. Fluorine Substituent Effects (on Bioactivity) J. Fluorine Chem. 2001;109:3–11. [Google Scholar]
- 31.Böhm H-J, Banner D, Bendels S, et al. Fluorine in Medicinal Chemistry. ChemBioChem. 2004;5:637–643. doi: 10.1002/cbic.200301023. [DOI] [PubMed] [Google Scholar]
- 32.DiMagno SG, Sun H. The Strength of Weak Interactions: Aromatic Fluorine in Drug Design. Curr. Top. Med. Chem. 2006;6:1473–1482. doi: 10.2174/156802606777951127. [DOI] [PubMed] [Google Scholar]
- 33.Schweizer E, Hoffmann-Röder A, Schärer K, et al. A Fluorine Scan at the Catalytic Center of Thrombin: C-F, C-OH, and C-OMe Bioisosterism and Fluorine Effects on pKa and log D Values. ChemMedChem. 2006;1:611–621. doi: 10.1002/cmdc.200600015. [DOI] [PubMed] [Google Scholar]
- 34.Sun S, Adejare A. Fluorinated Molecules as Drugs and Imaging Agents in the CNS. Curr. Med. Top. Chem. 2006;6:1457–1464. doi: 10.2174/156802606777951046. [DOI] [PubMed] [Google Scholar]
- 35.Müller K, Faeh C, Diederich F. Fluorine in Pharmaceuticals: Looking Beyond Intuition. Science. 2007;317:1881–1886. doi: 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]
- 36.Hagmann WK. The Many Roles for Fluorine in Medicinal Chemistry. J. Med. Chem. 2008;51:4359–4369. doi: 10.1021/jm800219f. [DOI] [PubMed] [Google Scholar]
- 37.Prakash GKS, Hu J. Selective Fluoroalkylations with Fluorinated Sulfones, Sulfoxides, and Sulfides. Acc. Chem. Res. 2007;40:921–930. doi: 10.1021/ar700149s. [DOI] [PubMed] [Google Scholar]
- 38.Kirk KL. Fluorination in Medicinal Chemistry: Methods, Strategies, and Recent Developments. Org. Proc. Res. Dev. 2008;12:305–321. [Google Scholar]
- 39.Lasne M-C, Perrio C, Rouden J, et al. Chemistry of β+-Emitting Compounds Based on Fluorine-18. Top. Curr. Chem. 2002;222:201–258. [Google Scholar]
- 40.Cai L, Lu S, Pike VW. Chemistry with [18F]Fluoride Ion. Eur. J. Org. Chem. 2008:2853–2873. [Google Scholar]
- 41.Bejot R, Fowler T, Carroll L, et al. Fluorous Synthesis of 18F Radiotracers with the [18F]Fluoride Ion: Nucleophilic Fluorination as the Detagging Process. Angew. Chem. Int. Ed. 2009;48:586–589. doi: 10.1002/anie.200803897. [DOI] [PubMed] [Google Scholar]
- 42.Logan J, Fowler JS, Volkow ND, et al. Distribution Volume Ratios Without Blood Sampling from Graphical Analysis of PET Data. J. Cereb. Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 43.Morris ED, Alpert NM, Fischman AJ. Comparison of Two Compartmental Models for Describing Receptor Ligand Kinetics and Receptor Availability in Multiple Injection PET Studies. J. Cereb. Blood Flow Metab. 1996;16:841–853. doi: 10.1097/00004647-199609000-00009. [DOI] [PubMed] [Google Scholar]
- 44.Laruelle M. The Role of Model-Based Methods in the Development of Single Scan Techniques. Nucl. Med. Biol. 2000;27:637–642. doi: 10.1016/s0969-8051(00)00142-6. [DOI] [PubMed] [Google Scholar]
- 45.Logan J. Graphical Analysis of PET Data Applied to Reversible and Irreversible Tracers. Nucl. Med. Biol. 2000;27:661–670. doi: 10.1016/s0969-8051(00)00137-2. [DOI] [PubMed] [Google Scholar]
- 46.Lammertsma AA. Radioligand Studies:Imaging and Quantitative Analysis. Eur. Neuropsychopharmacol. 2002;12:513–516. doi: 10.1016/s0924-977x(02)00100-1. [DOI] [PubMed] [Google Scholar]
- 47.Bentourkia M, Zaidi H. Tracer Kinetic Modeling in PET. PET Clin. 2007;2:267–277. doi: 10.1016/j.cpet.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 48.Dischino DD, Welch MJ, Kilbourn MR, et al. Relationship Between Lipophilicity and Brain Extraction of C-11-Labeled Radiopharmaceuticals. J. Nucl. Med. 1983;24:1030–1038. [PubMed] [Google Scholar]
- 49.Elfving B, Bjørnholm B, Ebert B, et al. Binding Characteristics of Selective Serotonin Reuptake Inhibitors With Relation to Emission Tomography Studies. Synapse. 2001;41:203–211. doi: 10.1002/syn.1076. [DOI] [PubMed] [Google Scholar]
- 50.Waterhouse RN. Determination of Lipophilicity and Its Use as a Predictor of Blood-Brain Barrier Penetration of Molecular Imaging Agents. Mol. Imag. Biol. 2003;5:376–389. doi: 10.1016/j.mibio.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 51.Kilty JE, Lorang D, Amara SG. Cloning and Expression of a Cocaine-Sensitive Rat Dopamine Transporter. Science. 1991;254:578–579. doi: 10.1126/science.1948035. [DOI] [PubMed] [Google Scholar]
- 52.Shimada S, Kitayama S, Lin C-L, et al. Cloning and Expression of a Cocaine-Sensitive Dopamine Transporter Complementary DNA. Science. 1991;254:576–578. doi: 10.1126/science.1948034. [DOI] [PubMed] [Google Scholar]
- 53.Giros B, El Mestikawy S, Godinot N, et al. Cloning, Pharmacological Characterization, and Chromosome Assignment of the Human Dopamine Transporter. Mol. Pharmacol. 1992;42:383–390. [PubMed] [Google Scholar]
- 54.Ciliax BJ, Heilman C, Demchyshyn LL, et al. The Dopamine Transporter: Immunochemical Characterization and Localization in Brain. J. Neurosci. 1995;15:1714–1723. doi: 10.1523/JNEUROSCI.15-03-01714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nirenberg MJ, Vaughan RA, Uhl GR, et al. The Dopamine Transporter is Localized to Dendritic and Axonal Plasma Membranes of Nigrostriatal Dopaminergic Neurons. J. Neurosci. 1996;16:436–447. doi: 10.1523/JNEUROSCI.16-02-00436.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ciliax BJ, Drash GW, Staley JK, et al. Immunocytochemical Localization of the Dopamine Transporter in Human Brain. J. Comp. Neurol. 1999;409:38–56. doi: 10.1002/(sici)1096-9861(19990621)409:1<38::aid-cne4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 57.Kaufman MJ, Spealman RD, Madras BK. Distribution of Cocaine Recognition Sites in Monkey Brain: I. In Vitro Autoradiography with [3H]CFT. Synapse. 1991;9:177–187. doi: 10.1002/syn.890090304. [DOI] [PubMed] [Google Scholar]
- 58.Niznik HB, Fogel EF, Fassos FF, et al. The Dopamine Transporter Is Absent in Parkinsonian Putamen and Reduced in the Caudate Nucleus. J. Neurochem. 1991;56:192–198. doi: 10.1111/j.1471-4159.1991.tb02580.x. [DOI] [PubMed] [Google Scholar]
- 59.Chinaglia G, Alvarez FJ, Probst A, et al. Mesostriatal and Mesolimbic Dopamine Uptake Binding Sites are Reduced in Parkinson's Disease and Progressive Supranuclear Palsy: A Quantitative Autoradiographic Study Using [3H]Mazindol. Neuroscience. 1992;49:317–327. doi: 10.1016/0306-4522(92)90099-n. [DOI] [PubMed] [Google Scholar]
- 60.Dawson TM, Dawson VL. Molecular Pathways of Neurodegeneration in Parkinson's Disease. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- 61.Mazei-Robison MS, Couch RS, Shelton RC, et al. Sequence Variation in the Human Dopamine Transporter Gene in Children with Attention Deficit Hyperactivity Disorder. Neuropharmacology. 2005;49:724–736. doi: 10.1016/j.neuropharm.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 62.Singer HS, Hahn I-H, Moran TH. Abnormal Dopamine Uptake Sites in Postmortem Striatum from Patients with Tourette's Syndrome. Ann. Neurol. 1991;30:558–562. doi: 10.1002/ana.410300408. [DOI] [PubMed] [Google Scholar]
- 63.Marek K, Jennings D, Tamagnan G, et al. Biomarkers for Parkinson's Disease: Tools to Assess Parkinson's Disease Onset and Progression. Ann. Neurol. 2008;64 Supplement:S111–S121. doi: 10.1002/ana.21602. [DOI] [PubMed] [Google Scholar]
- 64.Heikkila RE, Manzino L. Behavioral Properties of GBR 12909, GBR 13069 and GBR 13098: Specific Inhibitors of Dopamine Uptake. Eur. J. Pharmacol. 1984;103:241–248. doi: 10.1016/0014-2999(84)90483-7. [DOI] [PubMed] [Google Scholar]
- 65.Andersen PH. The Dopamine Uptake Inhibitor GBR 12909: Selectivity and Molecular Mechanism of Action. Eur. J. Pharmacol. 1989;166:493–504. doi: 10.1016/0014-2999(89)90363-4. [DOI] [PubMed] [Google Scholar]
- 66.Kilbourn MR, Haka MS, Mulholland GK, et al. Regional Brain Distribution of [18F]GBR 13119, a Dopamine Uptake Inhibitor, in CD-1 and C57BL/6 Mice. Eur. J. Pharmacol. 1989;166:331–334. doi: 10.1016/0014-2999(89)90078-2. [DOI] [PubMed] [Google Scholar]
- 67.Ciliax BJ, Kilbourn MR, Haka MS, et al. Imaging the Dopamine Uptake Site with Ex Vivo [18F]GBR 13119 Binding Autoradiography in Rat Brain. J. Neurochem. 1990;55:619–623. doi: 10.1111/j.1471-4159.1990.tb04178.x. [DOI] [PubMed] [Google Scholar]
- 68.Haka MS, Kilbourn MR. Synthesis of [18F]GBR 12909 for Human Studies of Dopamine Reuptake Sites. J. Nucl. Med. 1990;31 Abstract Book Supplement, 901, No. 836. [Google Scholar]
- 69.Koeppe RA, Kilbourn MR, Frey KA, et al. Imaging and Kinetic Modeling of [F-18]GBR 12909, A Dopamine Uptake Inhibitor. J. Nucl. Med. 1990;31 Abstract Book Supplement, 720, No. 55. [Google Scholar]
- 70.Van Dort ME, Chakraborty PK, Wieland DM, et al. Dopamine Uptake Inhibitors: Comparison of 125I- and 18F-Labeled Ligands. J. Nucl. Med. 1990;31 Abstract Book Supplement, 898, No. 823. [Google Scholar]
- 71.Kilbourn MR, Sherman PS, Pisani T. Repeated Reserpine Administration Reduces In Vivo [18F]GBR 13119 Binding to the Dopamine Uptake Site. Eur. J. Pharmacol. 1992;216:109–112. doi: 10.1016/0014-2999(92)90216-q. [DOI] [PubMed] [Google Scholar]
- 72.Kilbourn MR, Carey JE, Koeppe RA, et al. Biodistribution, Dosimetry, Metabolism and Monkey PET Studies of [18F]GBR 13119. Imaging the Dopamine Uptake System In Vivo. Nucl. Med. Biol. (Int. J. Radiat. Appl. Instrum. Part B) 1989;16:569–576. doi: 10.1016/0883-2897(89)90072-x. [DOI] [PubMed] [Google Scholar]
- 73.Müller L, Halldin C, Foged C, et al. Synthesis of [18F]NNC 12-0817 and [18F]NNC 12-0818; Two Potential Radioligands for the Dopamine Transporter. Appl. Radiat. Isot. 1995;46:323–328. doi: 10.1016/0969-8043(94)00004-j. [DOI] [PubMed] [Google Scholar]
- 74.Wolf WA, Kuhn DM. Cocaine and Serotonin Neurochemistry. Neurochem. Int. 1991;18:33–38. doi: 10.1016/0197-0186(91)90032-9. [DOI] [PubMed] [Google Scholar]
- 75.Uhl GR, Hall FS, Sora I. Cocaine, Reward, Movement and Monoamine Transporters. Mol. Psychiatry. 2002;7:21–26. doi: 10.1038/sj.mp.4000964. [DOI] [PubMed] [Google Scholar]
- 76.Ritz MC, Lamb RJ, Goldberg SR, et al. Cocaine Receptors on Dopamine Transporters Are Related to Self-Administration of Cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- 77.Scheffel U, Boja JW, Kuhar MJ. Cocaine Receptors: In Vivo Labeling with 3H-(-)-Cocaine, 3H-WIN 35,065-2, and 3H-WIN 35,428. Synapse. 1989;4:390–392. doi: 10.1002/syn.890040415. [DOI] [PubMed] [Google Scholar]
- 78.Boja JW, Kuhar MJ. [3H]Cocaine Binding and Inhibition of [3H]Dopamine Uptake is Similar in Both the Rat Striatum and Nucleus Accumbens. Eur. J. Pharmacol. 1989;173:215–217. doi: 10.1016/0014-2999(89)90524-4. [DOI] [PubMed] [Google Scholar]
- 79.Fowler JS, Volkow ND, Wolf AP, et al. Mapping Cocaine Binding Sites in Human and Baboon Brain In Vivo. Synapse. 1989;4:371–377. doi: 10.1002/syn.890040412. [DOI] [PubMed] [Google Scholar]
- 80.Fowler JS, Volkow ND, MacGregor RR, et al. Comparative PET Studies of the Kinetics and Distribution of Cocaine and Cocaethylene in Baboon Brain. Synapse. 1992;12:220–227. doi: 10.1002/syn.890120307. [DOI] [PubMed] [Google Scholar]
- 81.Yu D-W, Gatley SJ, Wolf AP, et al. Synthesis of Carbon-11 Labeled Iodinated Cocaine Derivatives and Their Distribution in Baboon Brain Measured Using Positron Emission Tomography. J. Med. Chem. 1992;35:2178–2183. doi: 10.1021/jm00090a005. [DOI] [PubMed] [Google Scholar]
- 82.Gatley SJ, Yu D-W, Fowler JS, et al. Studies with Differentially Labeled [11C]Cocaine, [11C]Norcocaine, [11C]Benzoylecgonine, and [11C]- and 4’-[18F]Fluorococaine to Probe the Extent to Which [11C]Cocaine Metabolites Contribute to PET Images of the Baboon Brain. J. Neurochem. 1994;62:1154–1162. doi: 10.1046/j.1471-4159.1994.62031154.x. [DOI] [PubMed] [Google Scholar]
- 83.Kimmel HL, Negus SS, Wilcox KM, et al. Relationship Between Rate of Drug Uptake in Brain and Behavioral Pharmacology of Monoamine Transporter Inhibitors in Rhesus Monkeys. Pharmacol. Biochem. Behavior. 2008;90:453–462. doi: 10.1016/j.pbb.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kloss MW, Rosen GM, Rauckman EJ. N-Demethylation of Cocaine to Norcocaine. Mol. Pharmacol. 1983;23:482–485. [PubMed] [Google Scholar]
- 85.Jindal SP, Lutz T. Mass Spectrometric Studies of Cocaine Disposition in Animals and Humans Using Stable Isotope-Labeled Analogues. J. Pharm. Sci. 1989;78:1009–1014. doi: 10.1002/jps.2600781208. [DOI] [PubMed] [Google Scholar]
- 86.Bergström KA, Halldin C, Kuikka JT, et al. The Metabolite Pattern of [123I]β-CIT Determined with a Gradient HPLC Method. Nucl. Med. Biol. 1995;22:971–976. doi: 10.1016/0969-8051(95)02019-5. [DOI] [PubMed] [Google Scholar]
- 87.Potter PM, Wadkins RM. Carboxylesterases - Detoxifying Enzymes and Targets for Drug Therapy. Curr. Med. Chem. 2006;13:1045–1054. doi: 10.2174/092986706776360969. [DOI] [PubMed] [Google Scholar]
- 88.Lundkvist C, Halldin C, Swahn C-G, et al. Different Brain Radioactivity Curves in a PET Study with [11C]β-CIT Labelled in Two Different Positions. Nucl. Med. Biol. 1999;26:343–350. doi: 10.1016/s0969-8051(98)00111-5. [DOI] [PubMed] [Google Scholar]
- 89.Larsen NA, Turner JM, Stevens J, et al. Crystal Structure of a Bacterial Cocaine Esterase. Nature Structural Biology. 2002;9:17–21. doi: 10.1038/nsb742. [DOI] [PubMed] [Google Scholar]
- 90.Clarke RL, Daum SJ, Gambino AJ, et al. Compounds Affecting the Central Nervous System. 4. 3β-Phenyltropane-2-carboxylic Esters and Analogs. J. Med. Chem. 1973;16:1260–1267. doi: 10.1021/jm00269a600. [DOI] [PubMed] [Google Scholar]
- 91.Boja JW, Carroll FI, Rahman MA, et al. New, potent cocaine analogs:ligand binding and transport studies in rat striatum. Eur. J. Pharmacol. 1990;184:329–332. doi: 10.1016/0014-2999(90)90627-i. [DOI] [PubMed] [Google Scholar]
- 92.Carroll FI, Gao Y, Rahman MA, et al. Synthesis, Ligand Binding, QSAR, and CoMFA Study of 3β-(p-Substituted phenyl)tropane-2β-carboxylic Acid Methyl Esters. J. Med. Chem. 1991;34:2719–2725. doi: 10.1021/jm00113a008. [DOI] [PubMed] [Google Scholar]
- 93.Carroll FI, Lewin AH, Boja JW, et al. Cocaine Receptor: Biochemical Characterization and Structure-Activity Relationships of Cocaine Analogues at the Dopamine Transporter. J. Med. Chem. 1992;35:969–981. doi: 10.1021/jm00084a001. [DOI] [PubMed] [Google Scholar]
- 94.Kuhar MJ, McGirr KM, Hunter RG, et al. Studies of Selected Phenyltropanes at Monoamine Transporters. Drug Alcohol Dependence. 1999;56:9–15. doi: 10.1016/s0376-8716(99)00005-8. [DOI] [PubMed] [Google Scholar]
- 95.Cook CD, Carroll FI, Beardsley PM. Cocaine-Like Discriminative Stimulus Effects of Novel Cocaine and 3-Phenyltropane Analogs in the Rat. Psychopharmacology. 2001;159:58–63. doi: 10.1007/s002130100891. [DOI] [PubMed] [Google Scholar]
- 96.Singh S. Chemistry, Design, and Structure-Activity Relationship of Cocaine Antagonists. Chem. Rev. 2000;100:925–1024. doi: 10.1021/cr9700538. [DOI] [PubMed] [Google Scholar]
- 97.Jin C, Navarro HA, Page K, et al. Synthesis and Monoamine Transporter Binding Properties of 2β-[3′-(substituted benzyl)isoxazol-5-yl]- and 2β-[3′-methyl-4′-(substituted phenyl)isoxazol-5-yl]-3β-(substituted phenyl)tropanes. Bioorg. Med. Chem. 2008;16:6682–6688. doi: 10.1016/j.bmc.2008.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jin C, Navarro HA, Carroll FI. Development of 3-Phenyltropane Analogues with High Affinity for the Dopamine and Serotonin Transporters and Low Affinity for the Norepinephrine Transporter. J. Med. Chem. 2008;51:8048–8056. doi: 10.1021/jm801162z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Boja JW, Patel A, Carroll FI, et al. [125I]RTI-55: a potent ligand for dopamine transporters. Eur. J. Pharmacol. 1991;194:133–134. doi: 10.1016/0014-2999(91)90137-f. [DOI] [PubMed] [Google Scholar]
- 100.Meltzer PC, Liang AY, Brownell A-L, et al. Substituted 3-Phenyltropane Analogs of Cocaine: Synthesis, Inhibition of Binding at Cocaine Recognition Sites, and Positron Emission Tomography Imaging. J. Med. Chem. 1993;36:855–862. doi: 10.1021/jm00059a010. [DOI] [PubMed] [Google Scholar]
- 101.Wong DF, Yung B, Dannals RF, et al. In Vivo Imaging of Baboon and Human Dopamine Transporters by Positron Emission Tomography Using [11C]WIN 35,428. Synapse. 1993;15:130–142. doi: 10.1002/syn.890150205. [DOI] [PubMed] [Google Scholar]
- 102.Wilson AA, DaSilva JN, Houle S. In Vivo Evaluation of [11C]- and [18F]-Labelled Cocaine Analogues as Potential Dopamine Transporter Ligands for Positron Emission Tomography. Nucl. Med. Biol. 1996;23:141–146. doi: 10.1016/0969-8051(95)02044-6. [DOI] [PubMed] [Google Scholar]
- 103.Farde L, Halldin C, Müller L, et al. PET Study of [11C]β-CIT Binding to Monoamine Transporters in the Monkey and Human Brain. Synapse. 1994;16:93–103. doi: 10.1002/syn.890160203. [DOI] [PubMed] [Google Scholar]
- 104.Någren K, Halldin C, Müller L, et al. Comparison of [11C]Methyl Triflate and [11C]Methyl Iodide in the Synthesis of PET Radioligands such as [11C]β-CIT and [11C]β-CFT. Nucl. Med. Biol. 1995;22:965–970. doi: 10.1016/0969-8051(95)02018-7. [DOI] [PubMed] [Google Scholar]
- 105.Harada N, Ohba H, Fukumoto D, et al. Potential of [18F]β-CFT-FE (2β-Carbomethoxy-3β-(4-fluorophenyl)-8-(2-[18F]fluoroethyl)nortropane) as a Dopamine Transporter Ligand: A PET Study in the Conscious Monkey Brain. Synapse. 2004;54:37–45. doi: 10.1002/syn.20059. [DOI] [PubMed] [Google Scholar]
- 106.Haaparanta M, Bergman J, Laakso A, et al. [18F]CFT ([18F]WIN 35,428), A Radioligand to Study the Dopamine Transporter With PET: Biodistribution in Rats. Synapse. 1996;23:321–327. doi: 10.1002/(SICI)1098-2396(199608)23:4<321::AID-SYN10>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 107.Laakso A, Bergman J, Haaparanta M, et al. [18F]CFT ([18F]WIN 35,428), A Radioligand to Study the Dopamine Transporter With PET: Characterization in Human Subjects. Synapse. 1998;28:244–250. doi: 10.1002/(SICI)1098-2396(199803)28:3<244::AID-SYN7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 108.Rinne JO, Nurmi E, Ruottinen HM, et al. [18F]FDOPA and [18F]CFT Are Both Sensitive PET Markers to Detect Presynaptic Dopaminergic Hypofunction in Early Parkinson's Disease. Synapse. 2001;40:193–200. doi: 10.1002/syn.1042. [DOI] [PubMed] [Google Scholar]
- 109.Kämäräinen E-L, Kyllönen T, Airaksinen A, et al. Preparation of [18F]β-CFT-FP and [11C]β-CFT-FP, Selective Radioligands for Visualisation of the Dopamine Transporter Using Positron Emission Tomography (PET) J. Labelled Cpd. Radiopharm. 2000;43:1235–1244. [Google Scholar]
- 110.Koivula T, Marjamäki P, Haaparanta M, et al. Ex Vivo Evaluation of N-(3-[18F]fluoropropyl)-2β-carbomethoxy-3β-(4-fluorophenyl)nortropane in Rats. Nucl. Med. Biol. 2008;35:177–183. doi: 10.1016/j.nucmedbio.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 111.Owens MJ, Morgan WN, Plott SJ, et al. Neurotransmitter Receptor and Transporter Binding Profile of Antidepressants and Their Metabolites. J. Pharmacol. Exp. Ther. 1997;283:1305–1322. [PubMed] [Google Scholar]
- 112.Hiemke C, Härtter S. Pharmacokinetics of Selective Serotonin Reuptake Inhibitors. Pharmacol. Therap. 2000;85:11–28. doi: 10.1016/s0163-7258(99)00048-0. [DOI] [PubMed] [Google Scholar]
- 113.Neumeyer JL, Tamagnan G, Wang S, et al. N-Substituted Analogs of 2β-Carbomethoxy-3β-(4′-iodophenyl)tropane (β-CIT) with Selective Affinity to Dopamine or Serotonin Transporters in Rat Forebrain. J. Med. Chem. 1996;39:543–548. doi: 10.1021/jm9505324. [DOI] [PubMed] [Google Scholar]
- 114.Neumeyer JL, Wang S, Gao Y, et al. N-ω-Fluoroalkyl Analogs of (1R)-2β-Carbomethoxy-3β-(4-iodophenyl)-tropane (β-CIT): Radiotracers for Positron Emission Tomography and Single Photon Emission Computed Tomography Imaging of Dopamine Transporters. J. Med. Chem. 1994;37:1558–1561. doi: 10.1021/jm00037a004. [DOI] [PubMed] [Google Scholar]
- 115.Carroll FI, Abraham P, Lewin AH, et al. Isopropyl and Phenyl Esters of 3β-(4-Substituted phenyl)tropan-2β-carboxylic Acids. Potent and Selective Compounds for the Dopamine Transporter. J. Med. Chem. 1992;35:2497–2500. doi: 10.1021/jm00091a019. [DOI] [PubMed] [Google Scholar]
- 116.Baldwin RM, Zea-Ponce Y, Al-Tikriti M, et al. Regional Brain Uptake and Pharmacokinetics of [123I]N-ω-Fluoroalkyl-2β-carboxy-3β-(4-iodophenyl)nortropane Esters in Baboons. Nucl. Med. Biol. 1995;22:211–219. doi: 10.1016/0969-8051(94)00096-3. [DOI] [PubMed] [Google Scholar]
- 117.Kuikka JT, Bergström KA, Ahonen A, et al. Comparison of Iodine-123 Labelled 2β-Carbomethoxy-3β-(4-iodophenyl)tropane and 2β-Carbomethoxy-3β-(4-iodophenyl)-N-(3-fluoropropyl)nortropane for Imaging of the Dopamine Transporter in the Living Human Brain. Eur. J. Nucl. Med. 1995;22:356–360. doi: 10.1007/BF00941854. [DOI] [PubMed] [Google Scholar]
- 118.Halldin C, Farde L, Lundkvist C, et al. [11C]β-CIT-FE, a Radioligand for Quantitation of the Dopamine Transporter in the Living Brain Using Positron Emission Tomography. Synapse. 1996;22:386–390. doi: 10.1002/(SICI)1098-2396(199604)22:4<386::AID-SYN10>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 119.Lundkvist C, Halldin C, Ginovart N, et al. [18F]β-CIT-FP Is Superior to [11C]β-CIT-FP for Quantitation of the Dopamine Transporter. Nucl. Med. Biol. 1997;24:621–627. doi: 10.1016/s0969-8051(97)00077-2. [DOI] [PubMed] [Google Scholar]
- 120.Chaly T, Dhawan V, Kazumata K, et al. Radiosynthesis of [18F] N-3-Fluoropropyl-2-β-Carbomethoxy-3-β-(4-Iodophenyl) Nortropane and the First Human Study With Positron Emission Tomography. Nucl. Med. Biol. 1996;23:999–1004. doi: 10.1016/s0969-8051(96)00155-2. [DOI] [PubMed] [Google Scholar]
- 121.Kazumata K, Dhawan V, Chaly T, et al. Dopamine Transporter Imaging with Fluorine-18-FPCIT PET. J. Nucl. Med. 1998;39:1521–1530. [PubMed] [Google Scholar]
- 122.Ma Y, Dhawan V, Mentis M, et al. Parametric Mapping of [18F]FPCIT Binding in Early Stage Parkinson's Disease: a PET Study. Synapse. 2002;45:125–133. doi: 10.1002/syn.10090. [DOI] [PubMed] [Google Scholar]
- 123.Robeson W, Dhawan V, Belakhlef A, et al. Dosimetry of the Dopamine Transporter Radioligand 18F-FPCIT in Human Subjects. J. Nucl. Med. 2003;44:961–966. [PubMed] [Google Scholar]
- 124.Yaqub M, Boellaard R, van Berckel BNM, et al. Quantification of Dopamine Transporter Binding Using [18F]FP-β-CIT and Positron Emission Tomography. J. Cerebral Blood Flow Metab. 2007;27:1397–1406. doi: 10.1038/sj.jcbfm.9600439. [DOI] [PubMed] [Google Scholar]
- 125.Lee SJ, Oh SJ, Chi DY, et al. One-Step High-Radiochemical-Yield Synthesis of [18F]FP-CIT Using a Protic Solvent System. Nucl. Med. Biol. 2007;34:345–351. doi: 10.1016/j.nucmedbio.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 126.Goodman MM, Keil R, Shoup TM, et al. Fluorine-18-FPCT: A PET Radiotracer for Imaging Dopamine Transporters. J. Nucl. Med. 1997;38:119–126. [PubMed] [Google Scholar]
- 127.Goodman MM, Kilts CD, Keil R, et al. 18F-Labeled FECNT: A Selective Radioligand for PET Imaging of Brain Dopamine Transporters. Nucl. Med. Biol. 2000;27:1–12. doi: 10.1016/s0969-8051(99)00080-3. [DOI] [PubMed] [Google Scholar]
- 128.Votaw JR, Howell LL, Martarello L, et al. Measurement of Dopamine Transporter Occupancy for Multiple Injections of Cocaine Using a Single Injection of [F-18]FECNT. Synapse. 2002;44:203–210. doi: 10.1002/syn.10068. [DOI] [PubMed] [Google Scholar]
- 129.Deterding TA, Votaw JR, Wang CK, et al. Biodistribution and Radiation Dosimetry of the Dopamine Transporter Ligand [18F]FECNT. J. Nucl. Med. 2001;42:376–381. [PubMed] [Google Scholar]
- 130.Tipre DN, Fujita M, Chin FT, et al. Whole-Body Biodistribution and Radiation Dosimetry Estimates for the PET Dopamine Transporter Probe 18F-FECNT in Non-Human Primates. Nucl. Med. Commun. 2004;25:737–742. doi: 10.1097/01.mnm.0000133074.64669.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Voll RJ, McConathy J, Waldrep MS, et al. Semi-automated Preparation of the Dopamine Transporter Ligand [18F]FECNT for Human PET Imaging Studies. Appl. Radiat. Isot. 2005;63:353–361. doi: 10.1016/j.apradiso.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 132.Chen ZP, Wang SP, Li XM, et al. A One-Step Automated High-Radiochemical-Yield Synthesis of 18F-FECNT from Mesylate Precursor. Appl. Radiat. Isot. 2008;66:1881–1885. doi: 10.1016/j.apradiso.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 133.Davis MR, Votaw JR, Bremner JD, et al. Initial Human PET Imaging Studies with the Dopamine Transporter Ligand 18F-FECNT. J. Nucl. Med. 2003;44:855–861. [PubMed] [Google Scholar]
- 134.Zoghbi SS, Shetty U, Ichise M, et al. PET Imaging of the Dopamine Transporter with 18F-FECNT: A Polar Radiometabolite Confounds Brain Radioligand Measurements. J. Nucl. Med. 2006;47:520–527. [PubMed] [Google Scholar]
- 135.Cumming P, Yokoi F, Chen A, et al. Pharmacokinetics of Radiotracers in Human Plasma During Positron Emission Tomography. Synapse. 1999;34:124–134. doi: 10.1002/(SICI)1098-2396(199911)34:2<124::AID-SYN5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 136.Gillings NM, Bender D, Falborg L, et al. Kinetics of the Metabolism of Four PET Radioligands in Living Minipigs. Nucl. Med. Biol. 2001;28:97–104. doi: 10.1016/s0969-8051(00)00187-6. [DOI] [PubMed] [Google Scholar]
- 137.Greuter HNJM, van Ophemert PLB, Luurtsema G, et al. Optimizing an Online SPE-HPLC Method for Analysis of (R)-[11C]1-(2-Chlorophenyl)-N-methyl-N-(1-methylpropyl)-3-isoquinolinecarboxamide [(R)-[11C]PK11195] and its Metabolites in Humans. Nucl. Med. Biol. 2005;32:307–312. doi: 10.1016/j.nucmedbio.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 138.Luurtsema G, Molthoff CFM, Schuit RC, et al. Evaluation of (R)-[11C]Verapamil as PET Tracer of P-glycoprotein Function in the Blood-Brain Barrier: Kinetics and Metabolismin in the Rat. Nucl. Med. Biol. 2005;32:87–93. doi: 10.1016/j.nucmedbio.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 139.Shetty HU, Zoghbi SS, Liow J-S, et al. Identification and Regional Distribution in Rat Brain of Radiometabolites of the Dopamine Transporter PET Radioligand[11C]PE2I. Eur. J. Nucl. Med. Mol. Imaging. 2007;34:667–678. doi: 10.1007/s00259-006-0277-1. [DOI] [PubMed] [Google Scholar]
- 140.Luurtsema G, Schuit RC, Takkenkamp K, et al. Peripheral Metabolism of [18F]FDDNP and Cerebral Uptake of its Labelled Metabolites. Nucl. Med. Biol. 2008;35:869–874. doi: 10.1016/j.nucmedbio.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 141.Chaly T, Jr, Matacchieri R, Dahl R, et al. Radiosynthesis of [18F] N-3-Fluoropropyl-2-β-carbomethoxy-3-β(4′ methylphenyl) Nortropane (FPCMT) Appl. Radiat. Isot. 1999;51:299–305. doi: 10.1016/s0969-8043(99)00044-5. [DOI] [PubMed] [Google Scholar]
- 142.Chitneni SK, Garreau L, Cleynhens B, et al. Improved Synthesis and Metabolic Stability Analysis of the Dopamine Transporter Ligand [18F]FECT. Nucl. Med. Biol. 2008;35:75–82. doi: 10.1016/j.nucmedbio.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 143.Gu X-H, Zong R, Kula NS, et al. Synthesis and Biological Evaluation of a Series of Novel N- or O-Fluoroalkyl Derivatives of Tropane: Potential Positron Emission Tomography (PET) Imaging Agents for the Dopamine Transporter. Bioorg. & Med. Chem. Lett. 2001;11:3049–3053. doi: 10.1016/s0960-894x(01)00626-6. [DOI] [PubMed] [Google Scholar]
- 144.Peng X, Zhang A, Kula NS, et al. Synthesis and Amine Transporter Affinities of Novel Phenyltropane Derivatives as Potential Positron Emission Tomography (PET) Imaging Agents. Bioorg. & Med. Chem. Lett. 2004;14:5635–5639. doi: 10.1016/j.bmcl.2004.08.049. [DOI] [PubMed] [Google Scholar]
- 145.Wuest F, Berndt M, Strobel K, et al. Synthesis and Radiopharmacological Characterization of 2β-carbo-2′-[18F]fluoroethoxy-3β-(4-bromo-phenyl)tropane ([18F]MCL-322) as a PET Radiotracer for Imaging the Dopamine Transporter (DAT) Bioorg. Med. Chem. 2007;15:4511–4519. doi: 10.1016/j.bmc.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 146.Mitterhauser M, Wadsak W, Mien L-K, et al. Synthesis and Biodistribution of [18F]FE@CIT, a New Potential Tracer for the Dopamine Transporter. Synapse. 2005;55:73–79. doi: 10.1002/syn.20095. [DOI] [PubMed] [Google Scholar]
- 147.Ettlinger DE, Häusler D, Wadsak W, et al. Metabolism and Autoradiographic Evaluation of [18F]FE@CIT: a Comparison with [123I]β-CIT and [123I]FP-CIT. Nucl. Med. Biol. 2008;35:475–479. doi: 10.1016/j.nucmedbio.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 148.Xing D, Chen P, Keil R, et al. Synthesis, Biodistribution, and Primate Imaging of Fluorine-18 Labeled 2β-Carbo-1′-fluoro-2-propoxy-3β-(4-chlorophenyl)tropanes. Ligands for the Imaging of Dopamine Transporters by Positron Emission Tomography. J. Med. Chem. 2000;43:639–648. doi: 10.1021/jm9902234. [DOI] [PubMed] [Google Scholar]
- 149.Mach RH, Nader MA, Ehrenkaufer RL, et al. Fluorine-18-Labeled Tropane Analogs for PET Imaging Studies of the Dopamine Transporter. Synapse. 2000;37:109–117. doi: 10.1002/1098-2396(200008)37:2<109::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 150.Chen P, Kilts CD, Camp VM, et al. Synthesis, characterization and in vivo evaluation of (N-(E)-4-[18F]fluorobut-2-en-1-yl)-2β-carbomethoxy-3β-(4-substituted-phenyl)nortropanes for imaging DAT by PET. J. Labelled. Cpd. Radiopharm. 1999;42 Suppl. 1:S400. [Google Scholar]
- 151.Goodman MM, Chen P. Fluoroalkenyl Nortropanes. 2000 WO2000064490. [Google Scholar]
- 152.Chalon S, Hall H, Saba W, et al. Pharmacological Characterization of (E)-N-(4-Fluorobut-2-enyl)-2β-carbomethoxy-3β-(4’-tolyl)nortropane (LBT-999) as a Highly Promising Fluorinated Ligand for the Dopamine Transporter. J. Pharmacol. Exp. Ther. 2006;317:147–152. doi: 10.1124/jpet.105.096792. [DOI] [PubMed] [Google Scholar]
- 153.Dollé F, Emond P, Mavel S, et al. Synthesis,radiosynthesis and in vivo preliminary evaluation of [11C]LBT-999, a selective radioligand for the visualisation of the dopamine transporter with PET. Bioorg. Med. Chem. 2006;14:1115–1125. doi: 10.1016/j.bmc.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 154.Dollé F, Hinnen F, Emond P, et al. Radiosynthesis of [18F]LBT-999, A Selective Radioligand for the Visualization of the Dopamine Transporter with PET. J. Label. Compd. Radiopharm. 2006;49:687–698. doi: 10.1016/j.bmc.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 155.Dollé F, Helfenbein J, Hinnen F, et al. One-Step Radiosynthesis of [18F]LBT-999: A Selective Radioligand for the Visualization of the Dopamine Transporter with PET. J. Label. Compd. Radiopharm. 2007;50:716–723. [Google Scholar]
- 156.Saba W, Valette H, Schöllhorn-Peyronneau M-A, et al. [11C]LBT-999: A Suitable Radioligand for Investigation of Extra-Striatal Dopamine Transporter with PET. Synapse. 2007;61:17–23. doi: 10.1002/syn.20337. [DOI] [PubMed] [Google Scholar]
- 157.Stehouwer JS, Chen P, Voll RJ, et al. PET Imaging of the Dopamine Transporter with [18F]FBFNT. J. Labelled. Cpd. Radiopharm; Aachen, Germany. Presented at the 17th International Symposium on Radiopharmaceutical Sciences; 2007. p. S335. Poster #248. [Google Scholar]
- 158.Carroll FI, Kotian P, Dehghani A, et al. Cocaine and 3β-(4′-Substituted phenyl)tropane-2β-carboxylic Acid Ester and Amide Analogues. New High-Affinity and Selective Compounds for the Dopamine Transporter. J. Med. Chem. 1995;38:379–388. doi: 10.1021/jm00002a020. [DOI] [PubMed] [Google Scholar]
- 159.Dworkin SI, Lambert P, Sizemore GM, et al. RTI-113 Administration Reduces Cocaine Self-Administration at High Occupancy of Dopamine Transporter. Synapse. 1998;30:49–55. doi: 10.1002/(SICI)1098-2396(199809)30:1<49::AID-SYN6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 160.Cook CD, Carroll FI, Beardsley PM. RTI 113, a 3-Phenyltropane Analog, Produces Long-Lasting Cocaine-Like Discriminative Stimulus Effects in Rats and Squirrel Monkeys. Eur. J. Pharmacol. 2002;442:93–98. doi: 10.1016/s0014-2999(02)01501-7. [DOI] [PubMed] [Google Scholar]
- 161.Wilcox KM, Lindsey KP, Votaw JR, et al. Self-Administration of Cocaine and the Cocaine Analog RTI-113: Relationship to Dopamine Transporter Occupancy Determined by PET Neuroimaging in Rhesus Monkeys. Synapse. 2002;43:78–85. doi: 10.1002/syn.10018. [DOI] [PubMed] [Google Scholar]
- 162.Tsukada H, Nishiyama S, Kakiuchi T, et al. Isoflurane Anesthesia Enhances the Inhibitory Effects of Cocaine and GBR12909 on Dopamine Transporter: PET Studies in Combination with Microdialysis in the Monkey Brain. Brain Research. 1999;849:85–96. doi: 10.1016/s0006-8993(99)02018-1. [DOI] [PubMed] [Google Scholar]
- 163.Miyamoto S, Leipzig JN, Lieberman JA, et al. Effects of Ketamine, MK-801, and Amphetamine on Regional Brain 2-Deoxyglucose Uptake in Freely Moving Mice. Neuropsychopharmacology. 2000;22:400–412. doi: 10.1016/S0893-133X(99)00127-X. [DOI] [PubMed] [Google Scholar]
- 164.Tsukada H, Harada N, Nishiyama S, et al. Ketamine Decreased Striatal [11C]Raclopride Binding With No Alterations in Static Dopamine Concentrations in the Striatal Extracellular Fluid in the Monkey Brain: Multiparametric PET Studies Combined With Microdialysis Analysis. Synapse. 2000;37:95–103. doi: 10.1002/1098-2396(200008)37:2<95::AID-SYN3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 165.Mizugaki M, Nakagawa N, Nakamura H, et al. Influence of Anesthesia on Brain Distribution of [11C]Methamphetamine in Monkeys in Positron Emission Tomography (PET) Study. Brain Research. 2001;911:173–175. doi: 10.1016/s0006-8993(01)02669-5. [DOI] [PubMed] [Google Scholar]
- 166.Tsukada H, Nishiyama S, Kakiuchi T, et al. Ketamine Alters the Availability of Striatal Dopamine Transporter as Measured by [11C]β-CFT and [11C]β-CIT-FE in the Monkey Brain. Synapse. 2001;42:273–280. doi: 10.1002/syn.10012. [DOI] [PubMed] [Google Scholar]
- 167.Elfving B, Bjørnholm B, Knudsen GM. Interference of Anaesthetics With Radioligand Binding in Neuroreceptor Studies. Eur. J. Nucl. Med. Mol. Imaging. 2003;30:912–915. doi: 10.1007/s00259-003-1171-8. [DOI] [PubMed] [Google Scholar]
- 168.Votaw JR, Byas-Smith MG, Voll R, et al. Isoflurane Alters the Amount of Dopamine Transporter Expressed on the Plasma Membrane in Humans. Anesthesiology. 2004;101:1128–1135. doi: 10.1097/00000542-200411000-00012. [DOI] [PubMed] [Google Scholar]
- 169.Hoffman BJ, Mezey E, Brownstein MJ. Cloning of a Serotonin Transporter Affected by Antidepressants. Science. 1991;254:579–580. doi: 10.1126/science.1948036. [DOI] [PubMed] [Google Scholar]
- 170.Cortés R, Soriano E, Pazos A, et al. Autoradiography of Antidepressant Binding Sites in the Human Brain: Localization Using [3H]Imipramine and [3H]Paroxetine. Neuroscience. 1988;27:473–496. doi: 10.1016/0306-4522(88)90282-5. [DOI] [PubMed] [Google Scholar]
- 171.Hrdina PD, Foy B, Hepner A, et al. Antidepressant Binding Sites in Brain: Autoradiographic Comparison of [3H]Paroxetine and [3H]Imipramine Localization and Relationship to Serotonin Transporter. J. Pharmacol. Exp. Ther. 1990;252:410–418. [PubMed] [Google Scholar]
- 172.Fujita M, Shimada S, Maeno H, et al. Cellular Localization of Serotonin Transporter mRNA in the Rat Brain. Neurosci. Lett. 1993;162:59–62. doi: 10.1016/0304-3940(93)90559-4. [DOI] [PubMed] [Google Scholar]
- 173.Austin MC, Bradley CC, Mann JJ, et al. Expression of Serotonin Transporter Messenger RNA in the Human Brain. J. Neurochem. 1994;62:2362–2367. doi: 10.1046/j.1471-4159.1994.62062362.x. [DOI] [PubMed] [Google Scholar]
- 174.Blakely RD, De Felice LJ, Hartzell HC. Molecular Physiology of Norepinephrine and Serotonin Transporters. J. Exp. Biol. 1994;196:263–281. doi: 10.1242/jeb.196.1.263. [DOI] [PubMed] [Google Scholar]
- 175.Gurevich EV, Joyce JN. Comparison of [3H]Paroxetine and [3H]Cyanoimipramine for Quantitative Measurement of Serotonin Transporter Sites in Human Brain. Neuropsychopharm. 1996;14:309–323. doi: 10.1016/0893-133X(95)00139-5. [DOI] [PubMed] [Google Scholar]
- 176.Stockmeier CA, Shapiro LA, Haycock JW, et al. Quantitative Subregional Distribution of Serotonin-1A Receptors and Serotonin Transporters in the Human Dorsal Raphe. Brain Res. 1996;727:1–12. doi: 10.1016/0006-8993(96)00239-9. [DOI] [PubMed] [Google Scholar]
- 177.Kish SJ, Furukawa Y, Chang L-J, et al. Regional Distribution of Serotonin Transporter Protein in Postmortem Human Brain. Is the Cerebellum a SERT-Free Brain Region? Nucl. Med. Biol. 2005;32:123–128. doi: 10.1016/j.nucmedbio.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 178.Chinaglia G, Landwehrmeyer B, Probst A, et al. Serotonergic Terminal Transporters are Differentially Affected in Parkinson's Disease and Progressive Supranuclear Palsy: An Autoradiographic Study with [3H]Citalopram. Neuroscience. 1993;54:691–699. doi: 10.1016/0306-4522(93)90240-g. [DOI] [PubMed] [Google Scholar]
- 179.Cash R, Raisman R, Ploska A, et al. High and Low Affinity [3H]Imipramine Binding Sites in Control and Parkinsonian Brains. Eur. J. Pharmacol. 1985;117:71–80. doi: 10.1016/0014-2999(85)90473-x. [DOI] [PubMed] [Google Scholar]
- 180.Blier P, de Montigny C. Serotonin and Drug-Induced Therapeutic Responses in Major Depression, Obsessive-Compulsive and Panic Disorders. Neuropsychopharm. 1999;21:91S–98S. doi: 10.1016/S0893-133X(99)00036-6. [DOI] [PubMed] [Google Scholar]
- 181.Chaouloff F, Berton O, Mormede P. Serotonin and Stress. Neuropsychopharm. 1999;21:28S–32S. doi: 10.1016/S0893-133X(99)00008-1. [DOI] [PubMed] [Google Scholar]
- 182.Lucki I. The Spectrum of Behaviors Influenced by Serotonin. Biol. Psychiatry. 1998;44:151–162. doi: 10.1016/s0006-3223(98)00139-5. [DOI] [PubMed] [Google Scholar]
- 183.Mann JJ. Role of the Serotonergic System in the Pathogenesis of Major Depression and Suicidal Behavior. Neuropsychopharm. 1999;21:99S–105S. doi: 10.1016/S0893-133X(99)00040-8. [DOI] [PubMed] [Google Scholar]
- 184.Mann JJ, Brent DA, Arango V. The Neurobiology and Genetics of Suicide and Attempted Suicide: A Focus on the Serotonergic System. Neuropsychopharm. 2001;24:467–477. doi: 10.1016/S0893-133X(00)00228-1. [DOI] [PubMed] [Google Scholar]
- 185.Owens MJ, Nemeroff CB. Role of Serotonin in the Pathophysiology of Depression: Focus on the Serotonin Transporter. Clin. Chem. 1994;40:288–295. [PubMed] [Google Scholar]
- 186.Owens MJ, Nemeroff CB. The Serotonin Transporter and Depression. Depression and Anxiety. 1998;8 Suppl. 1:5–12. [PubMed] [Google Scholar]
- 187.Purselle DC, Nemeroff CB. Serotonin Transporter: A Potential Substrate in the Biology of Suicide. Neuropsychopharmacology. 2003;28:613–619. doi: 10.1038/sj.npp.1300092. [DOI] [PubMed] [Google Scholar]
- 188.Benmansour S, Cecchi M, Morilak DA, et al. Effects of Chronic Antidepressant Treatments on Serotonin Transporter Function, Density, and mRNA Level. J. Neurosci. 1999;19:10494–10501. doi: 10.1523/JNEUROSCI.19-23-10494.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Benmansour S, Owens WA, Cecchi M, et al. Serotonin Clearance in vivo is Altered to a Greater Extent by Antidepressant-Induced Downregulation of the Serotonin Transporter than by Acute Blockade of this Transporter. J. Neurosci. 2002;22:6766–6772. doi: 10.1523/JNEUROSCI.22-15-06766.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ramsey IS, De Felice LJ. Serotonin Transporter Function and Pharmacology Are Sensitive to Expression Level. J. Biol. Chem. 2002;277:14475–14482. doi: 10.1074/jbc.M110783200. [DOI] [PubMed] [Google Scholar]
- 191.Kugaya A, Sanacora G, Staley JK, et al. Brain Serotonin Transporter Availability Predicts Treatment Response to Selective Serotonin Reuptake Inhibitors. Biol. Psychiatry. 2004;56:497–502. doi: 10.1016/j.biopsych.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 192.Mirza NR, Nielsen EØ, Troelsen KB. Serotonin Transporter Density and Anxiolytic-like Effects of Antidepressants in Mice. Prog. Neuro-Psychopharm. Biol. Psych. 2007;31:858–866. doi: 10.1016/j.pnpbp.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 193.Stokes PE. Ten Years of Fluoxetine. Depression and Anxiety. 1998;8 Supplement 1:1–4. [PubMed] [Google Scholar]
- 194.Shiue C-Y, Shiue GG, Cornish KG, et al. PET Study of the Distribution of [11C]Fluoxetine in a Monkey Brain. Nucl. Med. Biol. 1995;22:613–616. doi: 10.1016/0969-8051(94)00146-b. [DOI] [PubMed] [Google Scholar]
- 195.Mukherjee J, Das MK, Yang Z-Y, et al. Evaluation of the Binding of the Radiolabeled Antidepressant Drug, 18F-Fluoxetine in the Rodent Brain: An In Vitro and In Vivo Study. Nucl. Med. Biol. 1998;25:605–610. doi: 10.1016/s0969-8051(98)00043-2. [DOI] [PubMed] [Google Scholar]
- 196.Shank RP, Vaught JL, Pelley KA, et al. McN-5652: A Highly Potent Inhibitor of Serotonin Uptake. J. Pharmacol. Exp. Ther. 1988;247:1032–1038. [PubMed] [Google Scholar]
- 197.Suehiro M, Scheffel U, Dannals RF, et al. A PET Radiotracer for Studying Serotonin Uptake Sites: Carbon-11-McN-5652Z. J. Nucl. Med. 1993;34:120–127. [PubMed] [Google Scholar]
- 198.Szabo Z, Kao PF, Scheffel U, et al. Positron Emission Tomography Imaging of Serotonin Transporters in the Human Brain Using [11C](+)McN5652. Synapse. 1995;20:37–43. doi: 10.1002/syn.890200107. [DOI] [PubMed] [Google Scholar]
- 199.Zessin J, Eskola O, Brust P, et al. Synthesis of S-([18F]fluoromethyl)-(+)-McN5652 as a Potential PET Radioligand for the Serotonin Transporter. Nucl. Med. Biol. 2001;28:857–863. doi: 10.1016/s0969-8051(01)00248-7. [DOI] [PubMed] [Google Scholar]
- 200.Marjamäki P, Zessin J, Eskola O, et al. S-[18F]fluoromethyl-(+)-McN5652, a PET Tracer for the Serotonin Transporter: Evaluation in Rats. Synapse. 2003;47:45–53. doi: 10.1002/syn.10150. [DOI] [PubMed] [Google Scholar]
- 201.Brust P, Hinz R, Kuwabara H, et al. In vivo Measurement of the Serotonin Transporter with (S)-([18F]fluoromethyl)-(+)-McN5652. Neuropsychopharmacology. 2003;28:2010–2019. doi: 10.1038/sj.npp.1300281. [DOI] [PubMed] [Google Scholar]
- 202.Brust P, Zessin J, Kuwabara H, et al. Positron Emission Tomography Imaging of the Serotonin Transporter in the Pig Brain Using [11C](+)-McN5652 and S-([18F]fluoromethyl)-(+)-McN5652. Synapse. 2003;47:143–151. doi: 10.1002/syn.10163. [DOI] [PubMed] [Google Scholar]
- 203.Kretzschmar M, Brust P, Zessin J, et al. Autoradiographic Imaging of the Serotonin Transporter in the Brain of Rats and Pigs Using S-([18F]fluoromethyl)-(+)-McN5652. Eur. Neuropsychopharmacol. 2003;13:387–397. doi: 10.1016/s0924-977x(03)00039-7. [DOI] [PubMed] [Google Scholar]
- 204.Hashimoto K, Goromaru T. High-Affinity [3H]6-Nitroquipazine Binding Sites in Rat Brain. Eur. J. Pharmacol. 1990;180:273–281. doi: 10.1016/0014-2999(90)90310-3. [DOI] [PubMed] [Google Scholar]
- 205.Karramkam M, Dollé F, Valette H, et al. Synthesis of a Fluorine-18-labelled Derivative of 6-Nitroquipazine as a Radioligand for the In Vivo Serotonin Transporter Imaging with PET. Bioorg. Med. Chem. 2002;10:2611–2623. doi: 10.1016/s0968-0896(02)00098-6. [DOI] [PubMed] [Google Scholar]
- 206.Lee BS, Chu S, Lee KC, et al. Syntheses and Binding Affinities of 6-Nitroquipazine Analogues for Serotonin Transporter: Part 3. A Potential 5-HT Transporter Imaging Agent,3-(3-[18F]Fluoropropyl)-6-nitroquipazine. Bioorg. Med. Chem. 2003;11:4949–4958. doi: 10.1016/j.bmc.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 207.Mehta NB, Hollingsworth CEB, Brieaddy LE, et al. Halogen Substituted Diphenylsulfides. 0 402 097 A401. European Patent Application. 1990
- 208.Ferris RM, Brieaddy L, Mehta N, et al. Pharmacological Properties of 403U76, a New Chemical Class of 5-Hydroxytryptamine-and Noradrenaline-reuptake Inhibitor. J. Pharm. Pharmacol. 1995;47:775–781. doi: 10.1111/j.2042-7158.1995.tb06740.x. [DOI] [PubMed] [Google Scholar]
- 209.Oya S, Choi SR, Hou C, et al. 2-((2-((Dimethylamino)methyl)phenyl)thio)-5-iodophenylamine (ADAM): An Improved Serotonin Transporter Ligand. Nucl. Med. Biol. 2000;27:249–254. doi: 10.1016/s0969-8051(00)00084-6. [DOI] [PubMed] [Google Scholar]
- 210.Houle S, Ginovart N, Hussey D, et al. Imaging the Serotonin Transporter with Positron Emission Tomography: Initial Human Studies with [11C]DAPP and [11C]DASB. Eur. J. Nucl. Med. 2000;27:1719–1722. doi: 10.1007/s002590000365. [DOI] [PubMed] [Google Scholar]
- 211.Wilson AA, Ginovart N, Schmidt M, et al. Novel Radiotracers for Imaging the Serotonin Transporter by Positron Emission Tomography: Synthesis, Radiosynthesis, and in Vitro and ex Vivo Evaluation of 11C-Labeled 2-(Phenylthio)araalkylamines. J. Med. Chem. 2000;43:3103–3110. doi: 10.1021/jm000079i. [DOI] [PubMed] [Google Scholar]
- 212.Ginovart N, Wilson AA, Meyer JH, et al. Positron Emission Tomography Quantification of [11C]-DASB Binding to the Human Serotonin Transporter: Modeling Strategies. J. Cereb. Blood Flow Metab. 2001;21:1342–1353. doi: 10.1097/00004647-200111000-00010. [DOI] [PubMed] [Google Scholar]
- 213.Huang Y, Hwang D-R, Narendran R, et al. Comparative Evaluation in Nonhuman Primates of Five PET Radiotracers for Imaging the Serotonin Transporters: [11C]McN5652, [11C]ADAM, [11C]DASB, [11C]DAPA, and [11C]AFM. J Cereb Blood Flow Metab. 2002;22:1377–1398. doi: 10.1097/01.WCB.0000040948.67415.05. [DOI] [PubMed] [Google Scholar]
- 214.Huang Y, Hwang D-R, Bae S, et al. A New Positron Emission Tomography Imaging Agent for the Serotonin Transporter: Synthesis, Pharmacological Characterization, and Kinetic Analysis of [11C]2-[2-(dimethylaminomethyl)phenylthio]-5-fluoromethylphenylamine ([11C]AFM) Nucl. Med. Biol. 2004;31:543–556. doi: 10.1016/j.nucmedbio.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 215.Huang Y, Narendran R, Bae S, et al. A PET Imaging Agent with Fast Kinetics: Synthesis and In Vivo Evaluation of the Serotonin Transporter Ligand [11C]2-[2-dimethylaminomethylphenylthio)]-5-fluorophenylamine ([11C]AFA) Nucl. Med. Biol. 2004;31:727–738. doi: 10.1016/j.nucmedbio.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 216.Zhu Z, Guo N, Narendran R, et al. The New PET Imaging Agent [11C]AFE is a Selective Serotonin Transporter Ligand with Fast Brain Uptake Kinetics. Nucl. Med. Biol. 2004;31:983–994. doi: 10.1016/j.nucmedbio.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 217.Jarkas N, McConathy J, Votaw JR, et al. Synthesis and Characterization of EADAM: A Selective Radioligand for Mapping the Brain Serotonin Transporters by Positron Emission Tomography. Nucl. Med. Biol. 2005;32:75–86. doi: 10.1016/j.nucmedbio.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 218.Jarkas N, Votaw JR, Voll RJ, et al. Carbon-11 HOMADAM: A Novel PET Radiotracer for Imaging Serotonin Transporters. Nucl. Med. Biol. 2005;32:211–224. doi: 10.1016/j.nucmedbio.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 219.Frankle WG, Huang Y, Hwang D-R, et al. Comparative Evaluation of Serotonin Transporter Radioligands 11C-DASB and 11C-McN 5652 in Healthy Humans. J. Nucl. Med. 2004;45:682–694. [PubMed] [Google Scholar]
- 220.Frankle WG, Narendran R, Huang Y, et al. Serotonin Transporter Availability in Patients with Schizophrenia: A Positron Emission Tomography Imaging Study with [11C]DASB. Biol. Psychiatry. 2005;57:1510–1516. doi: 10.1016/j.biopsych.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 221.Nye JA, Votaw JR, Jarkas N, et al. Compartmental Modeling of 11C-HOMADAM Binding to the Serotonin Transporter in the Healthy Human Brain. J. Nucl. Med. 2008;49:2018–2025. doi: 10.2967/jnumed.108.054262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Shiue GG, Choi S-R, Fang P, et al. N,N-Dimethyl-2-(2-Amino-4-18F-Fluorophenylthio)-Benzylamine (4-18F-ADAM): An Improved PET Radioligand for Serotonin Transporters. J. Nucl. Med. 2003;44:1890–1897. [PubMed] [Google Scholar]
- 223.Huang Y, Bae S, Zhu Z, et al. Fluorinated Diaryl Sulfides as Serotonin Transporter Ligands: Synthesis, Structure-Activity Relationship Study, and in Vivo Evaluation of Fluorine-18-Labeled Compounds as PET Imaging Agents. J. Med. Chem. 2005;48:2559–2570. doi: 10.1021/jm0400808. [DOI] [PubMed] [Google Scholar]
- 224.Oya S, Choi SR, Hou C, et al. 18F(2-[2-Amino-5-fluorophenyl)thio]-N,N-dimethyl-benzenmethanamine) as a PET Imaging Agent for Serotonin Transporters. J. Label. Compd. Radiopharm. 2003;46 Supplement 1:S164. [Google Scholar]
- 225.Oya S, Choi SR, Coenen H, et al. New PET Imaging Agent for the Serotonin Transporter: [18F]ACF (2-[(2-Amino-4-chloro-5-fluorophenyl)thio]-N,N-dimethyl-benzenmethanamine) J. Med. Chem. 2002;45:4716–4723. doi: 10.1021/jm020167y. [DOI] [PubMed] [Google Scholar]
- 226.Parhi AK, Wang JL, Oya S, et al. 2-(2’-((Dimethylamino)methyl)-4’-(fluoroalkoxy)-phenylthio)benzenamine Derivatives as Serotonin Transporter Imaging Agents. J. Med. Chem. 2007;50:6673–6684. doi: 10.1021/jm070685e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Jarkas N, Voll RJ, Williams L, et al. Synthesis and In Vivo Evaluation of Halogenated N,N-Dimethyl-2-(2′-amino-4′-hydroxymethylphenylthio)benzylamine Derivatives as PET Serotonin Transporter Ligands. J. Med. Chem. 2008;51:271–281. doi: 10.1021/jm0707929. [DOI] [PubMed] [Google Scholar]
- 228.Huang Y, Zhu Z, Bae S-A, et al. Comparison of Three F-18 Labeled PET Ligands for the Serotonin Transporter: Radiosynthesis and In Vivo Imaging Studies in Baboon. J. Label. Compd. Radiopharm. 2003;46 Supplement 1:S57. [Google Scholar]
- 229.Fang P, Shiue GG, Shimazu T, et al. Synthesis and Evaluation of N,N-Dimethyl-2-(2-amino-5-[18F]fluorophenylthio)benzylamine (5-[18F]-ADAM) as a Serotonin Transporter Imaging Agent. Appl. Radiat. Isot. 2004;61:1247–1254. doi: 10.1016/j.apradiso.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 230.Wang JL, Parhi AK, Oya S, et al. 2-(2′-((Dimethylamino)methyl)-4′-(3-[18F]fluoropropoxy)-phenylthio)benzenamine for Positron Emission Tomography Imaging of Serotonin Transporters. Nucl. Med. Biol. 2008;35:447–458. doi: 10.1016/j.nucmedbio.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Owens MJ, Knight DL, Nemeroff CB. Second-Generation SSRIs: Human Monoamine Transporter Binding Profile of Escitalopram and R-Fluoxetine. Biol. Psychiatry. 2001;50:345–350. doi: 10.1016/s0006-3223(01)01145-3. [DOI] [PubMed] [Google Scholar]
- 232.Elfving B, Wiborg O. Binding of S-Citalopram and Paroxetine Discriminates Between Species. Synapse. 2005;55:280–282. doi: 10.1002/syn.20116. [DOI] [PubMed] [Google Scholar]
- 233.Tejani-Butt SM. [3H]Nisoxetine: A Radioligand for Quantitation of Norepinephrine Uptake Sites by Autoradiography or by Homogenate Binding. J. Pharmacol. Exp. Ther. 1992;260:427–436. [PubMed] [Google Scholar]
- 234.Cheetham SC, Viggers JA, Butler SA, et al. [3H]Nisoxetine - A Radioligand for Noradrenaline Reuptake Sites: Correlation With Inhibition of [3H]Noradrenaline Uptake and Effects of DSP-4 Lesioning and Antidepressant Treatments. Neuropharmacology. 1996;35:63–70. doi: 10.1016/0028-3908(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 235.Stehouwer JS, Jarkas N, Zeng F, et al. Synthesis, Radiosynthesis, and Biological Evaluation of Carbon-11 Labeled 2β-Carbomethoxy-3β-(3′-((Z)-2-haloethenyl)phenyl)nortropanes: Candidate Radioligands for In Vivo Imaging of the Serotonin Transporter with Positron Emission Tomography. J. Med. Chem. 2006;49:6760–6767. doi: 10.1021/jm060641q. [DOI] [PubMed] [Google Scholar]
- 236.Wong EHF, Sonders MS, Amara SG, et al. Reboxetine: A Pharmacologically Potent, Selective, and Specific Norepinephrine Reuptake Inhibitor. Biol. Psychiatry. 2000;47:818–829. doi: 10.1016/s0006-3223(99)00291-7. [DOI] [PubMed] [Google Scholar]
- 237.Goodman MM, Chen P, Plisson C, et al. Synthesis and Characterization of Iodine-123 Labeled 2β-Carbomethoxy-3β-(4′-((Z)-2-iodoethenyl)phenyl)nortropane. A Ligand for in vivo Imaging of Serotonin Transporters by Single-Photon-Emission Tomography. J. Med. Chem. 2003;46:925–935. doi: 10.1021/jm0100180. [DOI] [PubMed] [Google Scholar]
- 238.Plaznik A, Danysz W, Kostowski W, et al. Interaction Between Noradrenergic and Serotonergic Brain Systems as Evidenced by Behavioral and Biochemical Effects of Microinjections of Adrenergic Agonists and Antagonists into the Medial Raphe Nucleus. Pharmacol. Biochem. Behavior. 1983;19:27–32. doi: 10.1016/0091-3057(83)90306-4. [DOI] [PubMed] [Google Scholar]
- 239.Ordway GA, Stockmeier CA, Cason GW, et al. Pharmacology and Distribution of Norepinephrine Transporters in the Human Locus Coeruleus and Raphe Nuclei. J. Neurosci. 1997;17:1710–1719. doi: 10.1523/JNEUROSCI.17-05-01710.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Ressler KJ, Nemeroff CB. Role of Serotonergic and Noradrenergic Systems in the Pathophysiology of Depression and Anxiety Disorders. Depression and Anxiety. 2000;12 Supplement 1:2–19. doi: 10.1002/1520-6394(2000)12:1+<2::AID-DA2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 241.Emond P, Vercouillie J, Innis R, et al. Substituted Diphenyl Sulfides as Selective Serotonin Transporter Ligands: Synthesis and In Vitro Evaluation. J. Med. Chem. 2002;45:1253–1258. doi: 10.1021/jm010939a. [DOI] [PubMed] [Google Scholar]
- 242.Garg S, Thopate SR, Minton RC, et al. 3-Amino-4-(2-((4-[18F]fluorobenzyl)methylamino)methylphenylsulfanyl)benzonitrile, an F-18 Fluorobenzyl Analogue of DASB: Synthesis, in Vitro Binding, and in Vivo Biodistribution Studies. Bioconj. Chem. 2007;18:1612–1618. doi: 10.1021/bc070112g. [DOI] [PubMed] [Google Scholar]
- 243.Mavel S, Vercouillie J, Garreau L, et al. Docking Study, Synthesis, and In Vitro Evaluation of Fluoro-MADAM Derivatives as SERT Ligands for PET Imaging. Bioorg. Med. Chem. 2008;16:9050–9055. doi: 10.1016/j.bmc.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 244.Boja JW, Kuhar MJ, Kopajtic T, et al. Secondary Amine Analogues of 3β-(4′-Substituted phenyl)tropane-2β-carboxylic Acid Esters and N-Norcocaine Exhibit Enhanced Affinity for Serotonin and Norepinephrine Transporters. J. Med. Chem. 1994;37:1220–1223. doi: 10.1021/jm00034a021. [DOI] [PubMed] [Google Scholar]
- 245.Blough BE, Abraham P, Lewin AH, et al. Synthesis and Transporter Binding Properties of 3β-(4′-Alkyl-, 4′-alkenyl-, and 4′-alkynylphenyl)nortropane-2β-carboxylic Acid Methyl Esters: Serotonin Transporter Selective Analogs. J. Med. Chem. 1996;39:4027–4035. doi: 10.1021/jm960409s. [DOI] [PubMed] [Google Scholar]
- 246.Blough BE, Abraham P, Mills AC, et al. 3β-(4-Ethyl-3-iodophenyl)nortropane-2β-carboxylic Acid Methyl Ester as a High-Affinity Selective Ligand for the Serotonin Transporter. J. Med. Chem. 1997;40:3861–3864. doi: 10.1021/jm970492z. [DOI] [PubMed] [Google Scholar]
- 247.Tamagnan G, Baldwin RM, Kula NS, et al. Synthesis and Monoamine Transporter Affinity of 2β-Carbomethoxy-3β-(2″-, 3″- or 4″-substituted) Biphenyltropanes. Bioorg. & Med. Chem. Lett. 2000;10:1783–1785. doi: 10.1016/s0960-894x(00)00317-6. [DOI] [PubMed] [Google Scholar]
- 248.Emond P, Helfenbein J, Chalon S, et al. Synthesis of Tropane and Nortropane Analogues with Phenyl Substitutions as Serotonin Transporter Ligands. Bioorg. & Med. Chem. 2001;9:1849–1855. doi: 10.1016/s0968-0896(01)00083-9. [DOI] [PubMed] [Google Scholar]
- 249.Bois F, Baldwin RM, Kula NS, et al. Synthesis and Monoamine Transporter Affinity of 3′-Analogs of 2-β-Carbomethoxy-3-β-(4′-iodophenyl)tropane (β-CIT) Bioorg. & Med. Chem. Lett. 2004;14:2117–2120. doi: 10.1016/j.bmcl.2004.02.043. [DOI] [PubMed] [Google Scholar]
- 250.Tamagnan G, Alagille D, Fu X, et al. Synthesis and Monoamine Transporter Affinity of New 2β-Carbomethoxy-3β-[4-(Substituted Thiophenyl)]Phenyltropanes: Discovery of a Selective SERT Antagonist with Picomolar Potency. Bioorg. & Med. Chem. Lett. 2005;15:1131–1133. doi: 10.1016/j.bmcl.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 251.Tamagnan G, Alagille D, Fu X, et al. Synthesis and Monoamine Transporter Affinity of New 2β-Carbomethoxy-3β-[aryl or heteroaryl]phenyltropanes. Bioorg. Med. Chem. Lett. 2006;16:217–220. doi: 10.1016/j.bmcl.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 252.Bergström KA, Halldin C, Hall H, et al. In Vitro and In Vivo Characterisation of Nor-β-CIT: A Potential Radioligand for Visualisation of the Serotonin Transporter in the Brain. Eur. J. Nucl. Med. 1997;24:596–601. doi: 10.1007/BF00841395. [DOI] [PubMed] [Google Scholar]
- 253.Helfenbein J, Sandell J, Halldin C, et al. PET Examination of Three Potent Cocaine Derivatives as Specific Radioligands for the Serotonin Transporter. Nucl. Med. Biol. 1999;26:491–499. doi: 10.1016/s0969-8051(99)00004-9. [DOI] [PubMed] [Google Scholar]
- 254.Stehouwer JS, Plisson C, Jarkas N, et al. Synthesis, Radiosynthesis, and Biological Evaluation of Carbon-11 and Fluorine-18 (N-Fluoroalkyl) Labeled 2β-Carbomethoxy-3β-(4′-(3-furyl)phenyl)-tropanes and -nortropanes: Candidate Radioligands for in Vivo Imaging of the Serotonin Transporter with Positron Emission Tomography. J. Med. Chem. 2005;48:7080–7083. doi: 10.1021/jm0504095. [DOI] [PubMed] [Google Scholar]
- 255.Plisson C, Jarkas N, McConathy J, et al. Evaluation of Carbon-11-Labeled 2β-Carbomethoxy-3β-[4′-((Z)-2-iodoethenyl)phenyl]nortropane as a Potential Radioligand for Imaging the Serotonin Transporter by PET. J. Med. Chem. 2006;49:942–946. doi: 10.1021/jm050799v. [DOI] [PubMed] [Google Scholar]
- 256.Plisson C, Stehouwer JS, Voll RJ, et al. Synthesis and In Vivo Evaluation of Fluorine-18 and Iodine-123 Labeled 2β-Carbo(2-fluoroethoxy)-3β-(4′-((Z)-2-iodoethenyl)phenyl)nortropane as a Candidate Serotonin Transporter Imaging Agent. J. Med. Chem. 2007;50:4553–4560. doi: 10.1021/jm061303s. [DOI] [PubMed] [Google Scholar]
- 257.Stehouwer JS, Jarkas N, Zeng F, et al. Synthesis, Radiosynthesis, and Biological Evaluation of Fluorine-18-Labeled 2β-Carbo(fluoroalkoxy)-3β-(3′-((Z)-2-haloethenyl)phenyl)nortropanes: Candidate Radioligands for In Vivo Imaging of the Serotonin Transporter with Positron Tomography. J. Med. Chem. 2008;51:7788–7799. doi: 10.1021/jm800781a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Pacholczyk T, Blakely RD, Amara SG. Expression Cloning of a Cocaine- and Antidepressant-Sensitive Human Noradrenaline Transporter. Nature. 1991;350:350–354. doi: 10.1038/350350a0. [DOI] [PubMed] [Google Scholar]
- 259.Schroeter S, Apparsundaram S, Wiley RG, et al. Immunolocalization of the Cocaine-and Antidepressant-Sensitive 1-Norepinephrine Transporter. J. Comp. Neurol. 2000;420:211–232. [PubMed] [Google Scholar]
- 260.Sara SJ. The Locus Coeruleus and Noradrenergic Modulation of Cognition. Nature Reviews Neuroscience. 2009;10:211–223. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- 261.Rommelfanger KS, Weinshenker D. Norepinephrine: The Redheaded Stepchild of Parkinson's Disease. Biochemical Pharmacology. 2007;74:177–190. doi: 10.1016/j.bcp.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 262.Biederman J, Spencer T. Attention-Deficit/Hyperactivity Disorder (ADHD) as a Noradrenergic Disorder. Biol. Psychiatry. 1999;46:1234–1242. doi: 10.1016/s0006-3223(99)00192-4. [DOI] [PubMed] [Google Scholar]
- 263.Bymaster FP, Katner JS, Nelson DL, et al. Atomoxetine Increases Extracellular Levels of Norepinephrine and Dopamine in Prefrontal Cortex of Rat: A Potential Mechanism for Efficacy in Attention Deficit/Hyperactivity Disorder. Neuropsychopharmacology. 2002;27:699–711. doi: 10.1016/S0893-133X(02)00346-9. [DOI] [PubMed] [Google Scholar]
- 264.Southwick SM, Bremner DJ, Rasmusson A, et al. Role of Norepinephrine in the Pathophysiology and Treatment of Posttraumatic Stress Disorder. Biol. Psychiatry. 1999;46:1192–1204. doi: 10.1016/s0006-3223(99)00219-x. [DOI] [PubMed] [Google Scholar]
- 265.Ressler KJ, Nemeroff CB. Role of Norepinephrine in the Pathophysiology and Treatment of Mood Disorders. Biol. Psychiatry. 1999;46:1219–1233. doi: 10.1016/s0006-3223(99)00127-4. [DOI] [PubMed] [Google Scholar]
- 266.Van Moffaert M, Dierick M. Noradrenaline (Norepinephrine) and Depression: Role in Aetiology and Therapeutic Implications. CNS Drugs. 1999;12:293–305. [Google Scholar]
- 267.Zhu M-Y, Klimek V, Dilley GE, et al. Elevated Levels of Tyrosine Hydroxylase in the Locus Coeruleus in Major Depression. Biol. Psychiatry. 1999;46:1275–1286. doi: 10.1016/s0006-3223(99)00135-3. [DOI] [PubMed] [Google Scholar]
- 268.Ahern TH, Javors MA, Eagles DA, et al. The Effects of Chronic Norepinephrine Transporter Inactivation on Seizure Susceptibility in Mice. Neuropsychopharmacology. 2006;31:730–738. doi: 10.1038/sj.npp.1300847. [DOI] [PubMed] [Google Scholar]
- 269.Klimek V, Stockmeier C, Overholser J, et al. Reduced Levels of Norepinephrine Transporters in the Locus Coeruleus in Major Depression. J. Neurosci. 1997;17:8451–8458. doi: 10.1523/JNEUROSCI.17-21-08451.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Logan J, Ding Y-S, Lin K-S, et al. Modeling and Analysis of PET Studies with Norepinephrine Transporter Ligands: The Search for a Reference Region. Nucl. Med. Biol. 2005;32:531–542. doi: 10.1016/j.nucmedbio.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 271.Ding Y-S, Lin K-S, Logan J. PET Imaging of Norepinephrine Transporters. Curr. Pharm. Design. 2006;12:3831–3845. doi: 10.2174/138161206778559687. [DOI] [PubMed] [Google Scholar]
- 272.Schou M, Pike VW, Halldin C. Development of Radioligands for Imaging of Brain Norepinephrine Transporters In Vivo with Positron Emission Tomography. Curr. Topics. Med. Chem. 2007;7:1806–1816. doi: 10.2174/156802607782507411. [DOI] [PubMed] [Google Scholar]
- 273.Patient Selection and Antidepressant Therapy With Reboxetine, A New Selective Norepinephrine Reuptake Inhibitor. J. Clin. Psychiatry. 1998;59 Supplement 14 [Google Scholar]
- 274.Brenner E, Baldwin RM, Tamagnan G. Asymmetric Synthesis of (+)-(S,S)-Reboxetine via a New (S)-2-(Hydroxymethyl)morpholine Preparation. Org. Lett. 2005;7:937–939. doi: 10.1021/ol050059g. [DOI] [PubMed] [Google Scholar]
- 275.Ding Y-S, Lin K-S, Garza V, et al. Evaluation of a New Norepinephrine Transporter PET Ligand in Baboons, Both in Brain and Peripheral Organs. Synapse. 2003;50:345–352. doi: 10.1002/syn.10281. [DOI] [PubMed] [Google Scholar]
- 276.Wilson AA, Johnson DP, Mozley D, et al. Synthesis and In Vivo Evaluation of Novel Radiotracers for the In Vivo Imaging of the Norepinephrine Transporter. Nucl. Med. Biol. 2003;30:85–92. doi: 10.1016/s0969-8051(02)00420-1. [DOI] [PubMed] [Google Scholar]
- 277.Schou M, Halldin C, Sóvágó J, et al. Specific In Vivo Binding to the Norepinephrine Transporter Demonstrated with the PET Radioligand, (S,S)-[11C]MeNER. Nucl. Med. Biol. 2003;30:707–714. doi: 10.1016/s0969-8051(03)00079-9. [DOI] [PubMed] [Google Scholar]
- 278.Schou M, Halldin C, Sóvágó J, et al. PET Evaluation of Novel Radiofluorinated Reboxetine Analogs as Norepinephrine Transporter Probes in the Monkey Brain. Synapse. 2004;53:57–67. doi: 10.1002/syn.20031. [DOI] [PubMed] [Google Scholar]
- 279.Schou M, Halldin C, Pike VW, et al. Post-Mortem Human Brain Autoradiography of the Norepinephrine Transporter Using (S,S)-[18F]FMeNER-D2. Eur. Neuropsychopharmacol. 2005;15:517–520. doi: 10.1016/j.euroneuro.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 280.Seneca N, Andree B, Sjöholm N, et al. Whole-Body Biodistribution, Radiation Dosimetry Estimates for the PET Norepinephrine Transporter Probe (S,S)-[18F]FMeNER-D2 in Non-Human Primates. Nucl. Med. Commun. 2005;26:695–700. doi: 10.1097/01.mnm.0000171780.72908.e7. [DOI] [PubMed] [Google Scholar]
- 281.Takano A, Halldin C, Varrone A, et al. Biodistribution and Radiation Dosimetry of the Norepinephrine Transporter Radioligand (S,S)-[18F]FMeNER-D2: A Human Whole-Body PET Study. Eur. J. Nucl. Med. Mol. Imaging. 2008;35:630–636. doi: 10.1007/s00259-007-0622-z. [DOI] [PubMed] [Google Scholar]
- 282.Seneca N, Gulyás B, Varrone A, et al. Atomoxetine Occupies the Norepinephrine Transporter in a Dose-Dependent Fashion: A PET Study in Nonhuman Primate Brain Using (S,S)-[18F]FMeNER-D2. Psychopharmacology. 2006;188:119–127. doi: 10.1007/s00213-006-0483-3. [DOI] [PubMed] [Google Scholar]
- 283.Takano A, Gulyás B, Varrone A, et al. Imaging the Norepinephrine Transporter with Positron Emission Tomography: Initial Human Studies with (S,S)-[18F]FMeNER-D2. Eur. J. Nucl. Med. Mol. Imaging. 2008;35:153–157. doi: 10.1007/s00259-007-0598-8. [DOI] [PubMed] [Google Scholar]
- 284.Lin K-S, Ding Y-S, Kim S-W, et al. Synthesis, Enantiomeric Resolution, F-18 Labeling and Biodistribution of Reboxetine Analogs: Promising Radioligands for Imaging the Norepinephrine Transporter with Positron Emission Tomography. Nucl. Med. Biol. 2005;32:415–422. doi: 10.1016/j.nucmedbio.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 285.Ding Y-S, Lin K-S, Logan J, et al. Comparative Evaluation of Positron Emission Tomography Radiotracers for Imaging the Norepinephrine Transporter: (S,S) and (R,R) Enantiomers of Reboxetine Analogs ([11C]Methylreboxetine, 3-Cl-[11C]Methylreboxetine and [18F]Fluororeboxetine), (R)-[11C]Nisoxetine, [11C]Oxaprotiline and [11C]Lortalamine. J. Neurochem. 2005;94:337–351. doi: 10.1111/j.1471-4159.2005.03202.x. [DOI] [PubMed] [Google Scholar]
- 286.Zeng F, Jarkas N, Stehouwer JS, et al. Synthesis, In Vitro Characterization, and Radiolabeling of Reboxetine Analogs as Potential PET Radioligands for Imaging the Norepinephrine Transporter. Bioorg. Med. Chem. 2008;16:783–793. doi: 10.1016/j.bmc.2007.10.025. [DOI] [PubMed] [Google Scholar]
- 287.Zeng F, Mun J, Jarkas N, et al. Synthesis Radiosynthesis, and Biological Evaluation of Carbon-11 and Fluorine-18 Labeled Reboxetine Analogues: Potential Positron Emission Tomography Radioligands for in Vivo Imaging of the Norepinephrine Transporter. J. Med. Chem. 2009;52:62–73. doi: 10.1021/jm800817h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Hara K, Yanagihara N, Minami K, et al. Ketamine Interacts with the Noradrenaline Transporter at a Site Partly Overlapping the Desipramine Binding Site. Naunyn-Schmiedeberg's Arch. Pharmacol. 1998;358:328–333. doi: 10.1007/pl00005261. [DOI] [PubMed] [Google Scholar]
- 289.Blough BE, Holmquist CR, Abraham P, et al. 3α-(4-Substituted Phenyl)nortropane-2β-carboxylic Acid Methyl Esters Show Selective Binding at the Norepinephrine Transporter. Bioorg. & Med. Chem. Lett. 2000;10:2445–2447. doi: 10.1016/s0960-894x(00)00480-7. [DOI] [PubMed] [Google Scholar]
- 290.Hoepping A, Johnson KM, George C, et al. Novel Conformationally Constrained Tropane Analogues by 6-endo-trig Radical Cyclization and Stille Coupling - Switch of Activity toward the Serotonin and/or Norepinephrine Transporter. J. Med. Chem. 2000;43:2064–2071. doi: 10.1021/jm0001121. [DOI] [PubMed] [Google Scholar]
- 291.Tamiz AP, Smith MP, Kozikowski AP. Design, Synthesis and Biological Evaluation of 7-Azatricyclodecanes: Analogues of Cocaine. Bioorg. & Med. Chem. Lett. 2000;10:297–300. doi: 10.1016/s0960-894x(99)00685-x. [DOI] [PubMed] [Google Scholar]
- 292.Zhou J, Zhang A, Kläss T, et al. Biaryl Analogues of Conformationally Constrained Tricyclic Tropanes as Potent and Selective Norepinephrine Reuptake Inhibitors: Synthesis and Evaluation of Their Uptake Inhibition at Monoamine Transporter Sites. J. Med. Chem. 2003;46:1997–2007. doi: 10.1021/jm020596w. [DOI] [PubMed] [Google Scholar]
- 293.Carroll FI, Tyagi S, Blough BE, et al. Synthesis and Monoamine Transporter Binding Properties of 3α-(Substituted phenyl)nortropane-2β-carboxylic Acid Methyl Esters. Norepinephrine Transporter Selective Compounds. J. Med. Chem. 2005;48:3852–3857. doi: 10.1021/jm058164j. [DOI] [PubMed] [Google Scholar]
- 294.Zhou J, Kläβ T, Johnson KM, et al. Discovery of Novel Conformationally Constrained Tropane-Based Biaryl and Arylacetylene Ligands as Potent and Selective Norepinephrine Transporter Inhibitors and Potential Antidepressants. Bioorg. Med. Chem. Lett. 2005;15:2461–2465. doi: 10.1016/j.bmcl.2005.03.083. [DOI] [PubMed] [Google Scholar]
- 295.Zeng F, Jarkas N, Owens MJ, et al. Synthesis and Monoamine Transporter Affinity of Front Bridged Tricyclic 3β-(4′-halo or 4′-methyl)phenyltropanes Bearing Methylene or Carbomethoxymethylene on the Bridge to the 2β-Position. Bioorg. Med. Chem. Lett. 2006;16:4661–4663. doi: 10.1016/j.bmcl.2006.05.098. [DOI] [PubMed] [Google Scholar]
- 296.Eildal JNN, Andersen J, Kristensen AS, et al. From the Selective Serotonin Transporter Inhibitor Citalopram to the Selective Norepinephrine Transporter Inhibitor Talopram: Synthesis and Structure-Activity Relationship Studies. J. Med. Chem. 2008;51:3045–3048. doi: 10.1021/jm701602g. [DOI] [PubMed] [Google Scholar]
- 297.McConathy J, Owens MJ, Kilts CD, et al. Synthesis and Biological Evaluation of [11C]Talopram and [11C]Talsupram: Candidate PET Ligands for the Norepinephrine Transporter. Nucl. Med. Biol. 2004;31:705–718. doi: 10.1016/j.nucmedbio.2003.05.001. [DOI] [PubMed] [Google Scholar]