Abstract
Objectives
Sparse information on dose–response characteristics for initial antiepileptic drug monotherapy in children with idiopathic generalized epilepsy (IGE) is available. The aim of this study is to characterize the therapeutic dose of valproate in children with newly diagnosed IGE.
Materials and methods
Effect of initial valproate monotherapy and doses associated with seizure freedom were examined in consecutive children with IGE identified from a New Onset Seizure Clinic.
Results
Of 84 patients identified, 48 (57%) became seizure-free on valproate monotherapy and another 10 patients became seizure-free but discontinued VPA because of adverse effects. The mean dose in seizure-free children was 15.7 mg/kg/day and over 95% of IGE patients will respond below 25 mg/kg/day.
Conclusions
Half of children became seizure-free on valproate monotherapy and did so at modest doses.
Keywords: antiepileptic drugs, child neurology, epilepsy, treatment, neuropharmacology
Introduction
Idiopathic generalized epilepsy (IGE) accounts for approximately 20% children treated for epilepsy. The overall effectiveness of antiepileptic drug treatment in adults with newly diagnosed IGE is generally favorable. Almost two-thirds of patient presenting to an adult epilepsy clinical diagnosed with IGE became seizure-free on initial monotherapy and valproate (VPA) tended to be more effective than lamotrigine (1, 2). However, most IGE syndromes have an onset in childhood or adolescence but not as much is known about the response to therapy in children with newly diagnosed IGE.
Early studies on the effectiveness of VPA for treatment of absence seizures in children demonstrated VPA was effective at a wide range of doses (from to 15 to 60 mg/kg/day) (3–5). Dose–response relationships were evaluated in two studies (4, 5) and the effective doses differed widely (ranging from 16 to 66 mg/kg/day); however, both studies included children who had previously been treated (unsuccessfully) with other anticonvulsants. Little is known about the minimum dosages that should be considered effective in children with newly diagnosed generalized epilepsy. Similarly, little is known about whether increases above those doses will result in seizure control.
This study examines the dose–response to treatment in newly diagnosed children with IGE who were treated with a single anticonvulsant valproic acid (VPA). Because many people with newly diagnosed epilepsy can be treated optimally without monitoring serum drug levels (6, 7), to make this information practical for a wide variety of clinical practices, dose–response, rather than concentration–response relationships, were examined.
Materials and methods
A retrospective chart review was undertaken in a cohort of consecutive children aged 2–18 years of age identified from a New Onset Seizure Clinic in whom IGE was diagnosed and valproate treatment initiated from October 2003 to January 2007. These children had not previously been under the care of a neurologist. All had a comprehensive history and physical examination as well as an EEG and magnetic resonance imaging (MRI) as part of their initial evaluation. Information from the history and diagnostic testing was used to classify each child’s epilepsy. Patients were classified according to syndromes recognized by the ILAE in 1989 (8). This include seven IGE syndromes; benign myoclonic epilepsy in infancy (BMEI), generalized epilepsy with febrile seizures plus (GEFS +), epilepsy with myoclonic absences (EMA), epilepsy with myoclonic-astatic seizures (Doose’s syndrome; DS), childhood absence epilepsy (CAE), juvenile absence epilepsy (JAE), juvenile myoclonic epilepsy (JME) and epilepsy with generalized tonic–clonic seizures only (IGE with GTC only) (9). In addition, children with normal development and normal background EEG with generalized epileptiform discharges and generalized clinical seizure types (myoclonic seizures, absence seizures, generalized tonic–clonic seizures) who did not fit into the ILEA classification system were included and were categorized as IGE-other.
Children were evaluated in the clinic at 4- to 26-week intervals and were followed for a minimum of 1 year after initiation of therapy. Seizure control, adherence, random serum levels and adverse events were recorded at each visit. Valproate doses were adjusted based on clinical response and tolerability. Adverse events leading to discontinuation of VPA were determined on follow-up visits using patient and caregiver spontaneous reports, physical and neurological examinations and a validated pediatric epilepsy side effect tool (10). Adherence was assessed by direct questioning and by measurement of serum VPA concentration.
Valproate responsiveness was defined as clinical freedom from seizures with a minimum follow-up of 3 months on a stable dose of VPA in monotherapy or breakthrough seizures only with missed doses of medication in children who otherwise demonstrated evidence of adherence via measurement of serum VPA concentrations. EEG confirmation of seizure freedom was not performed for all patients; however, if a follow-up EEG was performed and prolonged generalized spike–wave discharges were present the patient was consider not seizure-free. Children who had less than one seizure per 6 months prior to treatment were not included for evaluation of VPA responsiveness.
Data analysis was performed using commercially and freely available software (SAS; SAS Institute Inc., Cary, NC, USA and statpage; http://statpages.org/). To determine what VPA dose guarantees that 95% of patient with newly diagnosed IGE will responded (95% certainty) the 95% upper tolerance limit was calculated (11). In addition, a 99% upper tolerance limit was also determined. Logistic regression was used to determine the odds ratio (OR) and 95% confidence interval (CI) for factors associated with discontinuation of VPA. This study was approved by our institution’s Institutional Review Board.
Results
Patient characteristics
Ninety-five children were diagnosed with IGE. Childhood absence epilepsy was the most common diagnosis, occurring in 33 (35%) of the children (Table 1). This is probably an underestimate of the prevalence of CAE because during the last 30 months of this study Cincinnati Children’s Hospital Medical Center (CCHMC) was participating in a prospective blinded therapeutic trial of CAE. Prior to the initiation of that study CAE was diagnosed in 41% of the children. Other common epilepsy syndromes were IGE with GTC only, JAE and JME. There were two cases of EMA, one of Doose’s syndrome, and 15 children had IGE-other. No cases of GEFS+ or BMEI were identified. The pretreatment seizure frequency was difficult to determine because families often did not recognize ictal events. However, children with CAE appeared to have the highest seizure frequency prior to treatment with multiple seizures occurring everyday (median of three recognized seizures per day; range 1–13 per day). In contrast, children with IGE with GTC only had relatively few seizures (median one seizure per month; range two per year to five per month) at the time of treatment. Children with other idiopathic generalized epilepsies had a wide range of pretreatment seizure frequencies (median of six recognized seizures per month; range four per year to five per day).
Table 1.
Clinical characteristics of patients seizure-free on valproate compared with those not seizure-free
| All | Seizure-free | Not seizure-free | |
|---|---|---|---|
| Number | 84 | 58 | 13 |
| Age on onset (years; Mean ± SD) | 9.0 ± 3.9 | 9.0 ± 4.0 | 6.0 ± 2.4** |
| Age at initiation | 9.9 ± 4.0 | 10.1 ± 4.0 | 6.9 ± 3.2* |
| Pretreatment duration of epilepsy | 1.0 ± 1.9 | 1.1 ± 2.0 | 0.9 ± 2.2 |
| Classification | |||
| Childhood absence | 29 | 21 (36%) | 6 (46%) |
| Juvenile absence | 13 | 10 (17%) | 0 |
| Juvenile myoclonic | 13 | 6 (10%) | 1 (8%) |
| Idiopathic generalized with GTC only | 12 | 11 (19%) | 0 |
| All others (Doose, EMA, IGE-other) | 17 | 10 (17%) | 6 (46%) |
| Dose (mg/kg/day) | 18.7 ± 8.8 | 15.7 ± 4.2 | 34.5 ± 10.1** |
| Random level (mcg/ml) | 91.4 ± 33.2 | 86.3 ± 30.2 | 109.6 ± 32.8* |
Patients who continued to have seizures on low doses of VPA at the time of initiation of alternate AEDs are not included.
P < 0.05 compared with seizure-free patients; two-sided Student’s t-test
P ≤ 0.002 compared with seizure-free patients; two-sided Student’s t-test
Of the 95 patients identified with IGE, the response to VPA could be categorized in 84 children. Response to therapy could not be determined in four patients because of a low initial seizure frequency, three because of persistent non-adherence, two because they did not return for follow-up, and two because of a combination of epileptic and non-epileptic events.
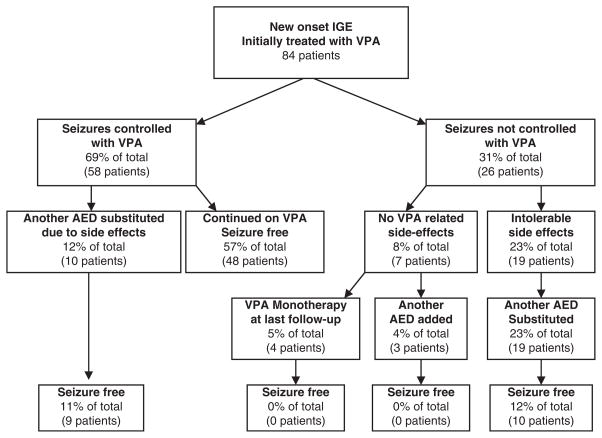
Response to VPA in newly diagnosed IGE patients is outlined in Fig. 1. Forty-eight (57%) were seizure-free on long-term (>12 months) VPA therapy. Another, 10 children became seizure-free VPA therapy but changed to another AED because of chronic adverse effects (7 weight gain; 1 fatigue; 1 behavioral problem and 1 sedation and declining school performance) after prolonged therapy with valproate. All but one (90%) of these children continued to be seizure-free on alternate AEDs. Alternative treatments (ethosuximide, lamotrigine, topiramate or levetiracetam) attempted in children who were not seizure-free on VPA tended to be less successful than those who achieved seizure control with VPA. Only 45% (10 of 22) of these patients became seizure-free with alternant AEDs (P = 0.024, Fisher’s exact test). In all, 67 of 84 (80%) children with newly diagnosed IGE eventually became seizure-free on antiepileptic therapy and slightly over half were maintained seizure-free on valproate monotherapy.
Figure 1.
Outcome of children with newly diagnosed idiopathic generalized epilepsy treated with valproate.
Valproate responders
Children who became seizure-free on VPA monotherapy did so at relatively low doses. The mean dose at which children responded to VPA treatment was 15.7 mg/kg/day (SD: 4.2 mg/kg/day). There were no significant differences in VPA doses needed to control seizures for the different epilepsy syndromes. From this, the likelihood of seizure freedom at selected VPA doses can be determined (from the tolerance interval). Ninety-five percent of children with newly diagnosed IGE will respond below VPA doses of 24.3 mg/kg/day (95% level of certainty). Similarly, 99% of patients with IGE will respond by 27.7 mg/kg/day. Only one seizure-free patient was taking a VPA dose greater than 28 mg/kg/day. Interestingly, this patient had CAE and did not become seizure-free until almost 12 years of age. Seizure freedom occurred at a VPA dose lower than the maximal dose attempted suggesting that the seizure freedom was related to the natural history of CAE rather than VPA therapy.
Valproate adverse events
Twenty-nine children discontinued valproate because of adverse effects. Seven discontinued VPA because of central nervous system side effects, 2 discontinued because of GI discomfort, 17 discontinued because of weight gain alone, 2 discontinued due both CNS side effects and weight gain, 1 because of hair loss and weight gain and 1 patient discontinued because of fatigue. Risk factors for discontinuation of VPA for weight gain (alone or in combination with other side effect) were an increase of BMI by one CDC defined BMI category (OR 3.35; CI 1.52–7.37; P = 0.002) and older age (OR 1.22; CI 1.02–1.46; P = 0.033). For those children who discontinued VPA of other reasons, higher doses were associated with greater risk of discontinuation (OR 1.07; CI 1.00–1.14; P = 0.044). Duration of therapy was not different in children who discontinued VPA because of weight gain (0.7 ± 0.6 years) when compared with the children who discontinued VPA for other reasons (0.6 ± 0.4 years; P = 0.62).
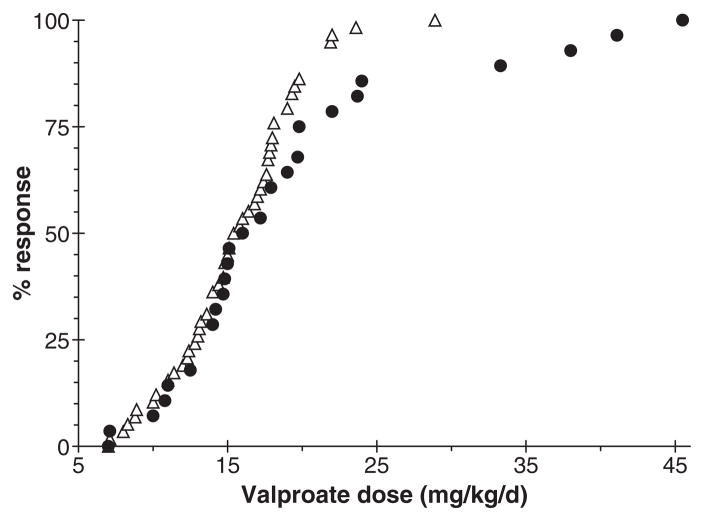
The average VPA dose taken by patients who discontinued VPA because of adverse effects was 19.5 mg/kg/day (SD 9.3 mg/kg/day). There was no significant difference in VPA doses taken by patients who cited CNS-related side effects as a reason for discontinuation (20.9 ± 11.8 mg/kg/day) compared with patients who did not report CNS-related side effects as a reason for discontinuation (19.2 ± 8.1 mg/kg/day). Side effects often occurred at modest VPA doses; 40% of CNS-related side effects occurred at doses below the average mean effective dose of VPA. A comparison of VPA doses associated with seizure control and adverse effects is shown in Fig. 2.
Figure 2.
Valproate dose leading to seizure control (open triangles) or discontinuation because of adverse effects (filled circles) in children with idiopathic generalized epilepsy.
Valproate non-responders
Valproate non-response was defined as continuing seizures on doses of valproate above the 95% tolerance interval (24.3 mg/kg/day). Thirteen children were classified as VPA non-responders. An additional AED was added to or substituted for VPA in nine of these children and only one (11%) became seizure-free with exposure to additional AEDs. Thus, alternate AEDs are less effective in children who were VPA non-responders than in those became seizure-free on VPA but changed due to side effects (P = 0.001, Fisher’s exact test). In addition, children who were not seizure-free on VPA but were taking doses below the 95% tolerance interval at the time another AED was added also responded better to alternative AEDs than VPA non-responders. Nine of the 12 children in this group (75%) were seizure-free on alternative therapies (P = 0.008 compared with non-responders; Fisher’s exact test).
Children with uncommon (Doose’s syndrome, EMA) or undefined forms of IGE (IGE-other) were less likely to respond to therapy when compared with the more common pediatric epilepsy syndromes (CAE, IGE with GTC only, JME, JAE). Only 10 of the 17 children (69%) with IGE-other, Doose’s syndrome or EMA responded to therapy. In contrast, 88% of children (58 of 67) with common forms of IGE (CAE, JAE, JME and IGE–GTC only) achieved seizure control with AEDs (P = 0.016, Fisher’s exact test). Younger age was also associated with a poor response to therapy. This is likely due to an earlier onset of unclassified forms of IGE.
Discussion
The effectiveness of medical treatment in children with newly diagnosed IGE epilepsy was examined. To ensure the patients were representative of a general population of children with IGE-related epilepsy, a consecutive cohort of children who came to a new onset seizure clinic with suspected seizures were diagnosed and with IGE following that evaluation was studied. Overall, 80% of children with newly diagnosed IGE became free of clinical seizures on AED therapy. Children with less common or undefined IGE had more difficult to control seizures when compared with those have common IGE syndromes (CAE, JAE, JME, IGE with GTCs only).
Following the diagnosis of IGE, 69% children became seizure-free on VPA monotherapy; however, only 57% were maintained on VPA because of significant side effects with long-term therapy. Weight gain was the most common reason for discontinuation of VPA, accounting (at least in part) for over two-thirds of cases of VPA discontinuation. When another AED was substituted for VPA in children who were seizure-free on VPA, all but one remained seizure-free. However, if an alternate therapy was tried in a patient who was not seizure-free on VPA, it was less likely that seizures would be controlled on the alternate therapy. These observations are similar to those observed in children and adults with newly diagnosed partial epilepsy (12, 13), in adults with newly diagnosed primary generalized epilepsy (1, 14) and in children with absence epilepsy (15).
In general, children with newly diagnosed IGE responded to relatively low doses of VPA. The average dose of the responders was <16 mg/kg/day. Over 95% of children with IGE will respond to VPA by 24.3 mg/kg/day and almost all children with IGE will respond by 30 mg/kg/day. The risk of adverse effects continues to increase significantly at doses above 25 mg/kg/day while the chances of response are low. Thus, it is likely that the commonly employed strategy of titrating to clinical response (increasing the medication dose until seizures are controlled or until untoward side effects are seen) may expose children to unnecessary side effects as well as delay the identification of other potentially effective treatments.
Acknowledgments
We thank Dr Paul Horn, PhD, Professor of Statistics, University Cincinnati for advice as assistance with data analysis. Support by grants from the Cincinnati Children’s Hospital Medical Center Trustees and the National Institutes of Health (grants NS062756 and NS044956).
Footnotes
Disclosure
The authors have reported no conflicts of interest.
References
- 1.Mohanraj R, Brodie MJ. Outcomes of newly diagnosed idiopathic generalized epilepsy syndromes in a non-pediatric setting. Acta Neurol Scand. 2007;115:204–8. doi: 10.1111/j.1600-0404.2006.00791.x. [DOI] [PubMed] [Google Scholar]
- 2.Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SA-NAD study of effectiveness of valproate, lamotrigine, or topiramate for generalised and unclassifiable epilepsy: an unblinded randomised controlled trial. Lancet. 2007;369:1016–1026. doi: 10.1016/S0140-6736(07)60461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sato S, White BG, Penry JK, Dreifuss FE, Sackellares JC, Kupferberg HJ. Valproic acid versus ethosuximide in the treatment of absence seizures. Neurology. 1982;32:157–63. doi: 10.1212/wnl.32.2.157. [DOI] [PubMed] [Google Scholar]
- 4.Erenberg G, Rothner AD, Henry CE, Cruse RP. Valproic acid in the treatment of intractable absence seizures in children: a single-blind clinical and quantitative EEG study. Am J Dis Child. 1982;136:526–9. doi: 10.1001/archpedi.1982.03970420050011. [DOI] [PubMed] [Google Scholar]
- 5.Braathen G, Theorell K, Persson A, Rane A. Valproate in the treatment of absence epilepsy in children: a study of dose-response relationships. Epilepsia. 1988;29:548–52. doi: 10.1111/j.1528-1157.1988.tb03759.x. [DOI] [PubMed] [Google Scholar]
- 6.Guidelines for Therapeutic Monitoring on Antiepileptic Drugs. Commission on Antiepileptic Drugs, International League Against Epilepsy. Epilepsia. 1993;34:585–7. doi: 10.1111/j.1528-1157.1993.tb00430.x. [DOI] [PubMed] [Google Scholar]
- 7.Jannuzzi G, Cian P, Fattore C, et al. A multicenter randomized controlled trial on the clinical impact of therapeutic drug monitoring in patients with newly diagnosed epilepsy. The Italian TDM Study Group in Epilepsy. Epilepsia. 2000;41:222–30. doi: 10.1111/j.1528-1157.2000.tb00144.x. [DOI] [PubMed] [Google Scholar]
- 8.Proposal for Revised Classification of Epilepsies and Epileptic Syndromes. Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia. 1989;30:389–99. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- 9.Nordli DR., Jr Idiopathic generalized epilepsies recognized by the International League Against Epilepsy. Epilepsia. 2005;46(Suppl 9):48–56. doi: 10.1111/j.1528-1167.2005.00313.x. [DOI] [PubMed] [Google Scholar]
- 10.Morita DA, Glauser TA, Fordyce S, Holder D, Altaye M. Development and validation of a tool to objectively measure antiepileptic drug side effects in children with epilepsy: Cincinnati Antiepileptic Drug Side Effect Scale. Ann Neurol. 2003;54(Suppl 7):S136–S137. [Google Scholar]
- 11.Walpole RE, Myers RH, Myers SL, Ye K. Probability and statistics for engineers & scientists. 8. Upper Saddle River, NJ: Prentice Hall; 2007. pp. 284–85. [Google Scholar]
- 12.Holland KD, Glauser TA. Response to carbamazepine in children with newly diagnosed partial onset epilepsy. Neurology. 2007;69:596–9. doi: 10.1212/01.wnl.0000267274.69619.f3. [DOI] [PubMed] [Google Scholar]
- 13.Mohanraj R, Brodie MJ. Pharmacological outcomes in newly diagnosed epilepsy. Epilepsy Behav. 2005;6:382–7. doi: 10.1016/j.yebeh.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Nicolson A, Appleton RE, Chadwick DW, Smith DF. The relationship between treatment with valproate, lamotrigine, and topiramate and the prognosis of the idiopathic generalised epilepsies. J Neurol Neurosurg Psychiatry. 2004;75:75–9. [PMC free article] [PubMed] [Google Scholar]
- 15.Wirrell E, Camfield C, Camfield P, Dooley J. Prognostic significance of failure of the initial antiepileptic drug in children with absence epilepsy. Epilepsia. 2001;42:760–3. doi: 10.1046/j.1528-1157.2001.02401.x. [DOI] [PubMed] [Google Scholar]