Abstract
There is a growing number of patients returning to dialysis after a failed kidney transplant, and there is increasing evidence of higher mortality among this population. Whether removal of the failed renal allograft affects survival while receiving long-term dialysis is not well understood. We identified all adults who received a kidney transplant and returned to long-term dialysis after renal allograft failure between January 1994 and December 2004 from the US Renal Data System. Among 10,951 transplant recipients who returned to long-term dialysis, 3451 (31.5%) received an allograft nephrectomy during follow-up. Overall, 34.6% of these patients died during follow-up. Receiving an allograft nephrectomy associated with a 32% lower adjusted relative risk for all-cause death (adjusted hazard ratio 0.68; 95% confidence interval 0.63 to 0.74) after adjustment for sociodemographic characteristics, comorbidity burden, donor characteristics, interim clinical conditions associated with receiving allograft nephrectomy, and propensity to receive an allograft nephrectomy. In conclusion, within a large, nationally representative sample of high-risk patients returning to long-term dialysis after failed kidney transplant, receipt of allograft nephrectomy independently associated with improved survival.
The prevalence and incidence of ESRD are projected to increase substantially in the United States during the next several decades.1 Although kidney transplantation improves survival in patients with ESRD,2,3 risk for death after renal allograft failure is an underappreciated problem. Crude death rates of US dialysis patients after renal allograft loss exceed that of patients on the kidney transplantation waiting list,4,5 and high mortality rates after allograft loss have also been observed in Canadian registries.6 Repeat transplantation is associated with improved survival among patients with failed renal allografts,4 but only approximately 15% of these patients undergo repeat transplantation.4 Thus, optimal treatment of the large majority of these patients who do not receive repeat kidney transplantation is a challenging problem. Given that approximately 2000 patients annually enter dialysis in the United States after allograft failure, there is a growing population of long-term dialysis patients at increased risk for death.
The presence of a failed allograft at dialysis initiation is associated with anemia and hypoalbuminemia—predictors of poor outcomes among dialysis patients.7,8 It has been postulated that these abnormalities may be explained by inadequate predialysis care,8,9 but they may also reflect chronic inflammation, which is a major risk factor for death among dialysis patients.10 A chronic inflammatory syndrome characterized by hypoalbuminemia, elevated C-reactive protein, anemia, and elevated serum ferritin in association with retained renal allografts among dialysis patients can occur even in patients without symptomatic allograft rejection, and this inflammation can be ameliorated after allograft nephrectomy11; however, few data exist about whether allograft nephrectomy leads to improved survival in these patients, because there have been no randomized clinical trials and only one previous published observational study, which had several methodologic limitations.12
To address these issues, we examined the impact of failed allograft removal on risk for death in a large, representative cohort of patients returning to dialysis after failed kidney transplant. We hypothesized that removal of failed renal allografts would be associated with improved survival, even after adjustment for potential confounders and treatment selection bias.13
Results
Patient Characteristics
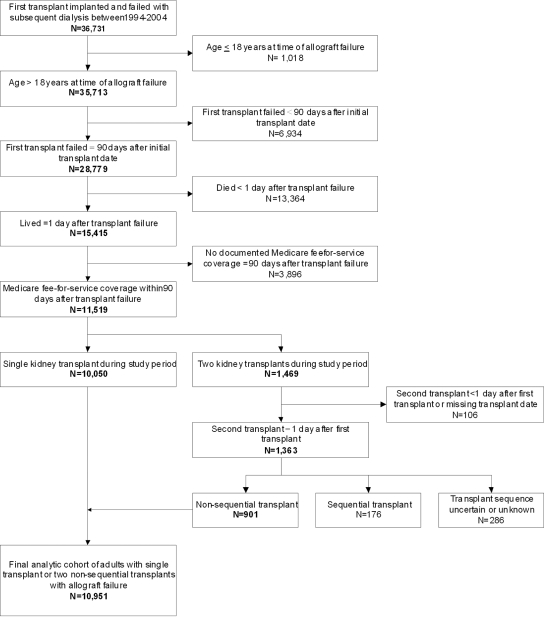
Assembly of the analytic cohort is summarized in Figure 1. Among 10,951 patients who returned to dialysis after failed kidney transplant, 3451 (31.5%) received nephrectomy of the transplanted kidney during follow-up. In patients receiving allograft nephrectomy, median time between return to dialysis and nephrectomy was 1.66 yr (interquartile range 0.73 to 3.02 yr).
Figure 1.
Assembly of analysis cohort of 10,951 patients who returned to maintenance dialysis after a failed kidney transplant between 1994 and 2004 in the USRDS is shown.
Compared with patients who did not receive nephrectomy, those who received nephrectomy were on average 4.6 yr younger and more likely to be black (Supplementary Table 1). Patients receiving a nephrectomy were less likely to be Hispanic; be male; use tobacco; and have previous myocardial infarction or other known coronary heart disease, heart failure, cerebrovascular disease, peripheral vascular disease, diabetes, insulin use, chronic obstructive pulmonary disease, cancer, and inability to ambulate or transfer. Patients who received nephrectomy were more likely to have higher serum creatinine concentration and higher serum albumin. Only minor absolute differences were noted between those who did and did not receive nephrectomy with regard to donor cause of death, cold ischemia time, peak panel reactivity, HLA antigen mismatches, and pretransplantation dialysis status. Overall use of OKT3/thymoglobulin and steroids after transplantation was slightly more common among patients who received nephrectomy. During follow-up, patients who received nephrectomy were much more likely than those who did not receive nephrectomy to have a hospitalization with a discharge diagnosis of fever, anemia, sepsis, urinary tract infection, or complication of the transplanted kidney (Supplementary Table 1).
Receipt of Allograft Nephrectomy and Death from Any Cause
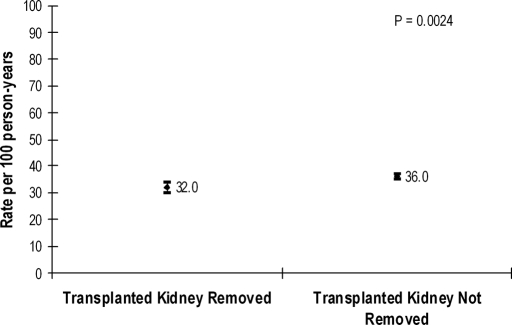
Overall, the mean follow-up was 2.93 ± 2.26 yr, and 3785 patients of the cohort were identified as having died by the end of follow-up (1106 in nephrectomy group, 2679 in non-nephrectomy group) in December 2004. Only 124 patients met criteria for being lost to follow-up during the study period. The unadjusted rate of death was significantly lower with receipt of nephrectomy compared with no nephrectomy (Figure 2). In multivariable extended Cox regression analyses, after adjustment for the propensity score to receive nephrectomy and other potential confounders, receipt of allograft nephrectomy was associated with a 32% reduction (95% confidence interval [CI] 26 to 37%) in the relative rate of death compared with not receiving nephrectomy (Table 1). We conducted six additional sensitivity analyses including or excluding specific patient subgroups that may have influenced estimates of treatment effectiveness for allograft nephrectomy, but there were no clinically relevant differences in the favorable point estimates associated with receipt of nephrectomy (Table 1).
Figure 2.
Unadjusted rate of death from any cause associated with or without receipt of renal allograft nephrectomy in 10,951 patients returning to maintenance dialysis after a failed kidney transplant between January 1, 1994, and December 31, 2004, is shown.
Table 1.
Multivariable association of receipt of allograft nephrectomy with death from any cause in patients who had a failed kidney transplant and returned to dialysis
| Analysis | Adjusted HR (95% CI) for Death for Nephrectomy versus Non-nephrectomy |
|---|---|
| Main | |
| original cohort (n = 10,951) | 0.68 (0.63 to 0.74) |
| original cohort with adjustment for transplant center (n = 10,951) | 0.68 (0.63 to 0.74) |
| Sensitivity | |
| original cohort + patients whose transplants failed <90 d after initial transplant date (n = 13,702) | 0.67 (0.63 to 0.71) |
| subset of original cohort who survived ≥30 d after transplant failure (n = 10,886) | 0.69 (0.66 to 0.74) |
| original cohort + patients without documented Medicare fee-for-service coverage within 90 d after transplant failure (n = 14,352) | 0.67 (0.63 to 0.72) |
| original cohort + patients with two transplants in which the transplant sequence was uncertain or unknown (n = 11,237) | 0.68 (0.63 to 0.73) |
| subset of original cohort whose duration of transplant before failure was <12 mo (n = 1545) | 0.76 (0.65 to 0.90) |
| subset of original cohort whose duration of transplant before failure was ≥12 mo (n = 9318) | 0.65 (0.60 to 0.71) |
Results are given for the overall cohort and six sensitivity analyses examining the potential influence of inclusion or exclusion of specific patient subgroups. All models were adjusted for quartile of propensity score, age, gender, race, lack of medical insurance, coronary disease, previous myocardial infarction, previous cardiac arrest, congestive heart failure, cerebrovascular disease, diabetes, diagnosed hypertension, chronic obstructive pulmonary disease, cancer, inability to ambulate, inability to transfer, obesity, serum creatinine, serum albumin, hemoglobin, donor age, donor race, anoxia, donor cause of death, cold ischemia time, year of transplantation, and interim hospitalizations for any of the following: Complication of anemia, abdominal pain, urinary obstruction, sepsis, urinary tract infection, malnutrition, or complication of transplanted kidney. HR, hazard ratio.
Repeat Transplantation and Perioperative Mortality Rates
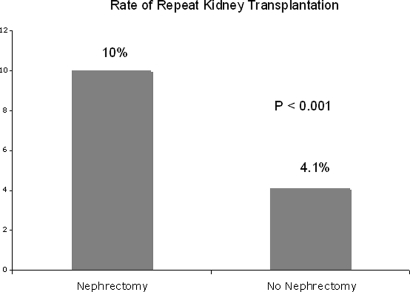
Those receiving transplant nephrectomy were more than twice as likely to receive a second transplant during the follow-up period than those who did not undergo nephrectomy of the initial failed allograft (10.0 versus 4.1%; P < 0.001; Figure 3). Among the 3451 patients undergoing transplant nephrectomy during the follow-up period, the rate of death within 30 d of the transplant nephrectomy was only 1.5% (53 deaths).
Figure 3.
Unadjusted rate of repeat transplantation associated with or without receipt of previous renal allograft nephrectomy in 10,951 patients returning to maintenance dialysis after a failed kidney transplant between January 1, 1994, and December 31, 2004, is shown.
Discussion
With larger numbers of patients entering long-term dialysis after failed kidney transplant in the United States and worldwide, optimal management of the failed allograft is an increasingly important question. Within a nationally representative sample of patients with ESRD, we observed a high absolute mortality rate overall and that receipt of renal allograft nephrectomy was associated with a lower rate of death from any cause compared with retaining the renal allograft, even after adjustment for potential confounders and selection bias. Our findings were also consistent in various sensitivity analyses (Figure 2, Table 1).
Lack of consensus exists about the optimal management of the failed renal allograft. In the United States, the traditional approach has been to perform allograft nephrectomy only for patients who have a clear clinical indication such as hyperacute allograft rejection, hematuria, serious infection, graft thrombosis, or graft intolerance syndrome.14,15 The practice of retaining failed allografts in situ is also partially predicated on the historically high perioperative mortality rates that have been associated with allograft nephrectomy (reported range 6 to 37%)13,16; however, in this study, we report a large representative sample and report a much lower mortality rate (1.5%) in association with allograft nephrectomy. Previous authors have argued against allograft nephrectomy because of increased recipient immunoreactivity presumably as a result of increased exposure to foreign antigens during the nephrectomy operation.17–19 We examined this outcome in our study cohort and surprisingly found that rates of repeat transplantation were significantly higher in the nephrectomy group than in the non-nephrectomy group (Figure 3). The reason for this increased rate of repeat transplantation cannot be determined from our analysis, although it may reflect better health in the nephrectomy group through either lower comorbidity burden or improved health status after nephrectomy as a result of reduced chronic inflammation. Either way, this finding argues against withholding transplant nephrectomy because of a presumed reduced chance of repeat transplantation.
Our results of improved survival after allograft nephrectomy challenge the traditional practice of retaining renal allografts after transplant failure. Our study extends beyond a recent analysis using US Renal Data System (USRDS) data that focused on observed differential outcomes associated with allograft nephrectomy in patients with transplant failure categorized as early (<12 mo after transplantation) versus late (≥12 mo after transplantation)12; however, that study included a more limited set of potential confounders, did not account for clinical events during follow-up that could have affected the likelihood of receiving nephrectomy and associated outcomes, and had likely misclassification of a large number of deaths attributed to “transplant failures” (i.e., occurring within <1 d after “transplant failure”). In contrast, our study observed a beneficial association of allograft nephrectomy on mortality regardless of the timing of transplant failure after addressing several of these issues.12
The reasons for the high mortality rate seen in patients with failed renal allografts are not known; however, we previously showed that the chronically rejected renal allograft is a nidus of immunoreactivity11 that can perpetuate chronic inflammation—a major risk factor for cardiovascular mortality in patients receiving long-term dialysis. This situation is analogous to the presence of clotted arteriovenous grafts with subclinical infection in patients on hemodialysis, which can contribute to a chronic inflammatory state characterized by hypoalbuminemia, elevated C-reactive protein, and increased anemia20,21—all markers of poor outcomes in these patients.10,22 Thus, we postulate that chronic inflammation may be playing a role in the excess mortality in patients with a failed kidney transplant. An additional potential deleterious effect of retention of a failed renal allograft may be continued routine use of low-dosage immunosuppressive therapies after return to maintenance dialysis, because immunosuppressive drugs may delay the need for ultimate nephrectomy and contribute to an increased risk for cardiovascular and infectious complications.11,13
Our study was strengthened by use of the nationally representative USRDS population across the past decade, with systematic follow-up for death and collection of standardized data elements for all patients at the time of initiating or returning to dialysis. Thus, our results are highly generalizable to our target population of interest. We attempted to control for potential biases using several approaches to examine mortality associated with receipt of nephrectomy in those with failed kidney transplant. Furthermore, in our primary cohort analyses, we selected strict inclusion criteria to exclude patients in whom the allograft did not survive at least 3 mo, because they may have had classical indications for nephrectomy, such as hyperacute rejection and graft thrombosis, and to enhance uniform availability of relevant covariates and follow-up. Next, we applied a propensity score method that considered as candidate variables both baseline characteristics and interim clinical events to address residual treatment selection bias from measured confounders. Finally, we performed several sensitivity analyses to demonstrate the robustness of our findings. Despite this, as an observational study of clinical practice, our analysis remains susceptible to the effects of residual confounding and treatment selection bias. We did not have information on longitudinal use of pharmacologic and other therapies or updated comorbidity data included on the USRDS medical evidence form. Of note, there were many differences in the baseline characteristics in many important comorbidities between the nephrectomy and non-nephrectomy groups, and this may have had an impact on patient survival and the decision to perform transplant nephrectomy. This factor may have biased the study outcome, despite the steps taken to mitigate bias in our analysis. In summary, our results should be viewed in light of these methodologic limitations inherent to registry studies.
In conclusion, patients who underwent allograft nephrectomy after renal allograft failure had improved survival after adjustment for potential confounders and likelihood of receiving nephrectomy. This was coupled with very low perioperative mortality rates and higher crude rates of retransplantation in association with allograft nephrectomy. Our results raise questions about the current clinical paradigm and suggest that routine allograft nephrectomy in stable dialysis patients with a failed renal allograft should be evaluated against current management strategies in a randomized trial as a possible strategy for improving outcomes among this growing population of high-risk patients with ESRD.
Concise Methods
Study Sample
Using data from the USRDS,23 we assembled a cohort of patients who returned to long-term hemodialysis or peritoneal dialysis after failed kidney transplant. We identified from USRDS files all patients who were older than 18 yr, underwent a single kidney transplant or two nonsequential kidney transplants, and returned to long-term dialysis after renal allograft failure between January 1, 1994, and December 31, 2004. We excluded patients whose renal allograft failed within 90 d after transplantation (n = 6934), patients who died within <1 d after renal allograft failure (n = 13,364), those who did not have Medicare fee-for-service insurance after the first 90 d after the return to dialysis (n = 3896), and those without confirmed sequential transplants (n = 568; Figure 1).
The study was approved by the University of Texas Health Science Center at San Antonio institutional review board. Waiver of informed content was obtained because of the nature of the study.
Receipt of Renal Allograft Nephrectomy
Receipt of nephrectomy for the transplanted kidney during follow-up was ascertained from USRDS files for Medicare claims for any acute hospitalizations with Current Procedural Terminology codes for allograft nephrectomy.24
Mortality
The primary outcome was death from any cause through December 31, 2004, which was identified from USRDS files. Patients were considered lost to follow-up when there was no evidence of dialysis billing claims received by the USRDS for 12 consecutive months in the absence of an identified death date.25
Covariates
Information from the USRDS 2728 Medical Evidence Form24 completed closest to the start of dialysis was used to identify the following variables: Age, gender, race/ethnicity, primary modality of dialysis, insurance status, tobacco use, known ischemic heart disease, previous acute myocardial infarction, previous cardiac arrest, congestive heart failure, cerebrovascular disease, peripheral vascular disease, diabetes, use of insulin, diagnosed hypertension, chronic obstructive pulmonary disease, malignancy, documented inability to transfer or ambulate, body mass index, serum creatinine, blood urea nitrogen, serum albumin, and hemoglobin.
We also ascertained selected variables related to their transplant from the USRDS United Network for Organ Sharing transplant registration forms. These included donor age, donor race, living versus cadaveric donor, transplant center, cause of donor death, cold ischemia time of the transplanted kidney, occurrence of delayed graft function, peak panel-reactivity antibody level, HLA donor–recipient mismatch, year of transplantation, receipt of pretransplantation dialysis, and immunosuppressive therapies used.
During follow-up, we also identified from Medicare claims any acute hospitalizations that included primary or secondary discharge International Classification of Diseases, Ninth Edition codes for the following clinical conditions that may be associated with receiving allograft nephrectomy: Fever (780.6), anemia (285.2, 285.21, 285.29, 285.9), hematuria (599.7), abdominal pain (789.0, 789.0x), urinary obstruction (593.89, 599.6, 599.60, 599.69), sepsis (785.52, 790.7, 995.9, 995.90, 995.91, 995.92), urinary tract infection (590.1x, 590.2, 590.3, 590.8x, 590.9, 599.0), cachexia or malnutrition (783.2, 783.7, 799.4), and complications of the transplanted kidney (996.81).
Statistical Analysis
Analyses were conducted using SAS 9.3 (SAS Institute, Cary, NC). Continuous variables were compared between groups using t test or Wilcoxon rank test, and categorical variables were compared using χ2 or Fisher exact test.
We examined the association between removal of the renal allograft and risk for death using several approaches. First, unadjusted rates (per 100 person-years) of death from any cause with associated 95% CIs were calculated for periods with or without allograft nephrectomy. Next, to address possible treatment selection bias, we constructed a propensity score26 for the likelihood of receiving allograft nephrectomy during follow-up using logistic regression (c statistic = 0.76) and included as covariates variables known to be associated with a clinical indication for nephrectomy and any other characteristics that differed between the two groups. We next performed multivariable extended Cox regression comparing the risk for death associated with receipt of allograft nephrectomy during follow-up that adjusted for quartile of propensity score, variables previously shown to be associated with mortality after failed transplant, and any differences in any other characteristics between those who did and did not die during follow-up. Finally, to test the robustness of our estimates, we adjusted for initial transplant center and conducted a series of separate sensitivity analyses that tested the impact of including the subgroup of patients whose initial transplants failed <90 d after transplantation, restricting to only patients who survived at least 30 d after initial transplant failure, including patients who did not have evidence of Medicare fee-for-service coverage after 90 d after allograft failure, including the subset of patients with two transplants in which the sequence was uncertain or unknown and in subgroups of patients with both early (<12 mo) and late (>12 mo) transplant failure.
Disclosures
None.
Supplementary Material
Acknowledgments
J.C.A. was supported by National Institutes of Health (NIH) grant U01 DK066481, and S.G.A. was supported by an NIH T32 Training Grant. A.S.G. has received research support from NIH grants U01 DK60902, R01 DK067126, and R01 DK58411.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
The data reported here were supplied by the US Renal Data System. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US government.
See related editorial, “Allograft Nephrectomy after Transplant Failure: Should It Be Performed in All Patients Returning to Dialysis?” on pages 207–208.
Supplemental information for this article is available online at http://www.jasn.org/.
References
- 1.US Renal Data System: USRDS 2005 Annual Data Report: Atlas of End-Stage Renal Disease in the United States, Bethesda, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2005 [Google Scholar]
- 2.Schnuelle P, Lorenz D, Trede M, Van Der Woude FJ: Impact of renal cadaveric transplantation on survival in end-stage renal failure: Evidence for reduced mortality risk compared with hemodialysis during long-term follow-up. J Am Soc Nephrol 9: 2135–2141, 1998 [DOI] [PubMed] [Google Scholar]
- 3.Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK: Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341: 1725–1730, 1999 [DOI] [PubMed] [Google Scholar]
- 4.Ojo A, Wolfe RA, Agodoa LY, Held PJ, Port FK, Leavey SF, Callard SE, Dickinson DM, Schmouder RL, Leichtman AB: Prognosis after primary renal transplant failure and the beneficial effects of repeat transplantation: Multivariate analyses from the United States Renal Data System. Transplantation 66: 1651–1659, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Kaplan B, Meier-Kriesche HU: Death after graft loss: An important late study endpoint in kidney transplantation. Am J Transplant 2: 970–974, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Knoll G, Muirhead N, Trpeski L, Zhu N, Badovinac K: Patient survival following renal transplant failure in Canada. Am J Transplant 5: 1719–1724, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Almond MK, Tailor D, Marsh FP, Raftery MJ, Cunningham J: Increased erythropoietin requirements in patients with failed renal transplants returning to a dialysis programme. Nephrol Dial Transplant 9: 270–273, 1994 [PubMed] [Google Scholar]
- 8.Gill JS, Abichandani R, Khan S, Kausz AT, Pereira BJ: Opportunities to improve the care of patients with kidney transplant failure. Kidney Int 61: 2193–2200, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Gill JS, Abichandani R, Kausz AT, Pereira BJ: Mortality after kidney transplant failure: The impact of non-immunologic factors. Kidney Int 62: 1875–1883, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Wanner C, Metzger T: C-reactive protein a marker for all-cause and cardiovascular mortality in haemodialysis patients. Nephrol Dial Transplant 17[ Suppl 8]: 29–32, 2002 [DOI] [PubMed] [Google Scholar]
- 11.López-Gómez JM, Pérez-Flores I, Jofré R, Carretero D, Rodríguez-Benitez P, Villaverde M, Pérez-García R, Nassar GM, Niembro E, Ayus JC: Presence of a failed kidney transplant in patients who are on hemodialysis is associated with chronic inflammatory state and erythropoietin resistance. J Am Soc Nephrol 15: 2494–2501, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Johnston O, Rose C, Landsberg D, Gourlay WA, Gill JS: Nephrectomy after transplant failure: Current practice and outcomes. Am J Transplant 7: 1961–1967, 2007 [DOI] [PubMed] [Google Scholar]
- 13.Ayus JC, Achinger SG: At the peril of dialysis patients: Ignoring the failed transplant. Semin Dial 18: 180–184, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Vanrenterghem Y, Khamis S: The management of the failed renal allograft. Nephrol Dial Transplant 11: 955–957, 1996 [PubMed] [Google Scholar]
- 15.Silberman H, Fitzgibbons TJ, Butler J, Berne TV: Renal allografts retained in situ after failure. Arch Surg 115: 42–43, 1980 [DOI] [PubMed] [Google Scholar]
- 16.Sharma DK, Pandey AP, Nath V, Gopalakrishnan G: Allograft nephrectomy: A 16-year experience. Br J Urol 64: 122–124, 1989 [DOI] [PubMed] [Google Scholar]
- 17.Freier DT, Haines RF, Rosenzweig J, Neiderhuber J, Konnak J, Turcotte JG: Sequential renal transplants: Some surgical and immunological implications on management of the first homograft. Surgery 79: 262–267, 1976 [PubMed] [Google Scholar]
- 18.Opelz G, Gustafsson LA, Terasaki PI: Influence of interval between first graft removal and retransplantation on outcome of second cadaver kidney grafts. Transplantation 22: 521–522, 1976 [PubMed] [Google Scholar]
- 19.Sumrani N, Delaney V, Hong JH, Daskalakis P, Sommer BG: The influence of nephrectomy of the primary allograft on retransplant graft outcome in the cyclosporine era. Transplantation 53: 52–55, 1992 [DOI] [PubMed] [Google Scholar]
- 20.Ayus JC, Sheikh-Hamad D: Silent infection in clotted hemodialysis access grafts. J Am Soc Nephrol 9: 1314–1317, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Nassar GM, Fishbane S, Ayus JC. Occult infection of old nonfunctioning arteriovenous grafts: A novel cause of erythropoietin resistance and chronic inflammation in hemodialysis patients. Kidney Int Suppl49–54, 2002 [DOI] [PubMed] [Google Scholar]
- 22.Barany P, Divino Filho JC, Bergstrom J: High C-reactive protein is a strong predictor of resistance to erythropoietin in hemodialysis patients. Am J Kidney Dis 29: 565–568, 1997 [DOI] [PubMed] [Google Scholar]
- 23.US Renal Data System: USRDS 2007 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2007 [Google Scholar]
- 24.Researchers Guide to the USRDS Database. The National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2005. Available at: http://www.usrds.org Accessed September 1, 2006
- 25.Kestenbaum B, Andress DL, Schwartz SM, Gillen DL, Seliger SL, Jadav PR, Sherrard DJ, Stehman-Breen C: Survival following parathyroidectomy among United States dialysis patients. Kidney Int 66: 2010–2016, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Rosenbaum PR, Dubin RD: The central role of the propensity score in observational studies for causal effects. Biometrika 70: 41–45, 1983 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.