Abstract
Polycystic kidney disease (PKD) can arise from either developmental or postdevelopmental processes. Recessive PKD, caused by mutations in PKHD1, is a developmental defect, whereas dominant PKD, caused by mutations in PKD1 or PKD2, occurs by a cellular recessive mechanism in mature kidneys. Oriented cell division is a feature of planar cell polarity that describes the orientation of the mitotic axes of dividing cells during development with respect to the luminal vector of the elongating nephron. In polycystic mutant mice, the loss of oriented cell division may also contribute to the pathogenesis of PKD. Here, we examined the role of oriented cell division in mouse models based on mutations in Pkd1, Pkd2, and Pkhd1. Precystic tubules after kidney-selective inactivation of either Pkd1 or Pkd2 did not lose oriented division before cystic dilation but lost oriented division after tubular dilation began. In contrast, Pkhd1del4/del4 mice lost oriented cell division but did not develop kidney cysts. Increased intercalation of cells into the plane of the tubular epithelium maintained the normal tubular morphology in Pkhd1del4/del4 mice, which had more cells present in transverse tubular profiles. In conclusion, loss of oriented cell division is a feature of Pkhd1 mutation and cyst formation, but it is neither sufficient to produce kidney cysts nor required to initiate cyst formation after mutation in Pkd1 or Pkd2.
Defective three-dimensional tissue organization is a phenotypic hallmark of polycystic kidney disease (PKD). Polycystic kidneys are permeated by fluid-filled cysts that grow and deform the organ in a process associated with a decline in glomerular filtration and ESRD. Positional cloning has discovered genes for autosomal dominant (ADPKD; PKD1, PKD2) and autosomal recessive (ARPKD; PKHD1) PKD. The protein products of these PKD genes along with other diseases manifesting with fibrocystic changes in the kidney (e.g., nephronophthisis, Bardet-Biedl syndrome) are expressed in the primary cilia and basal body complex in kidney epithelial cells.1 In addition, the gene products mutated in spontaneous or induced kidney cystic models in nonprimate vertebrates are associated with cilia.2–4 The role of cilia in PKD was shown prospectively by the occurrence of cysts after disruption of cilia structure in the kidney by inactivation of Kif3a, a gene not previously known to cause PKD.5 In aggregate, these findings have validated the role of cilia in the pathogenesis of fibrocystic diseases in the kidney. There are polycystic disease proteins, including those causing isolated human autosomal dominant polycystic liver disease6,7 and pronephric cysts in zebrafish,8 that do not localize directly to cilia; however, even in these cases, a functional interconnection with cilia has either been shown8 or proposed.7
Whereas many of the mutated genes and the central organelle for the pathogenesis of PKD have been identified, the effecter pathways for cyst formation remain less well defined. Among these, defects in planar cell polarity (PCP), a central determinant of tissue organization (reviewed in reference9), have been proposed as fundamental to the pathogenesis of PKD. Discovery that inv, a cystic disease– and cilia-related protein, acts as a switch between canonical and PCP-related noncanonical Wnt signaling10 led to the hypothesis that cyst growth may be associated with defective polarity within the plane of the tubule epithelium.11 The understanding that orientation of cell division (OCD) is a consequence of planar polarity12 led Fischer et al.13 to test whether defective OCD underlies at least part of the pathogenesis of PKD. Loss of OCD was observed in advance of cyst formation in the pck rat, an orthologous Pkhd1 model, and in cystic disease as a result of mutation of Hnf1β.13 More recently, tubules in postnatal Kif3a mutant kidneys showed loss of OCD in the absence of cilia.14 The converse hypothesis, that loss of PCP proteins can result in PKD, was recently demonstrated with inactivation of PCP-related protocadherin Fat4, resulting in both loss of OCD and PKD.15 These data support the hypothesis that loss of OCD is associated with mutations that affect cilia function or structure, and mutations in PCP proteins can be associated with kidney cyst formation.
In this study, we examined the role of OCD in PKD using orthologous mouse models of human ADPKD (Pkd1, Pkd2) and ARPKD (Pkhd1). We found that after kidney-selective inactivation of either Pkd1 or Pkd2, precystic tubules did not show evidence of loss of OCD in advance of cystic dilation; however, OCD was lost once the tubules began to dilate. By contrast, our Pkhd1del4 model of recessive PKD, like its rat ortholog,13 showed loss of OCD but, unlike the rat model, did not develop kidney cysts.16 The normal-appearing tubule morphology in Pkhd1del4/del4 is maintained by increased intercalation of cells into the plane of the epithelium that is associated with a small but significant increase in the number of cells present in transverse tubular profiles. Pkhd1 functions in a PCP pathway to maintain OCD. Loss of OCD is a feature of dilating cysts but is neither a prerequisite for initiation of cyst formation nor sufficient to produce cysts in elongating tubules.
Results
Mutations in Pkhd1 Are Associated with Defective OCD during Tubule Elongation
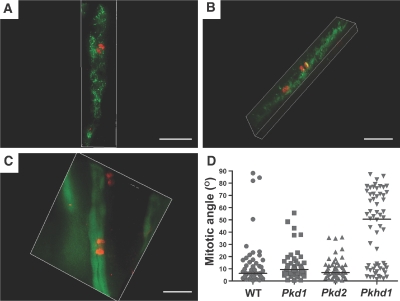
Defects in OCD have been identified in precystic tubules in the pck rat model with mutation in Pkhd1.13 We sought to determine whether similar loss of OCD occurs in the Pkhd1del4/del4 mouse model, which does not develop cystic kidneys.16 Mitotic spindles of dividing cells in Pkhd1del4/del4 mice at postnatal days 7 to 10 (P7 to P10) were marked with antiphosphorylated Ser10 in histone H3 (anti-H3pS10), and the angle of the spindle poles relative to unambiguous luminal vector of medullary collecting tubules was measured.13 In kidneys from wild-type (WT) mice, OCD was evidenced with 92% of mitotic angles (n = 48) deviating <30° from the vector of the tubule lumen (median 6°; Figure 1D). Pkhd1del4/del4 mice showed loss of OCD with 58% of tubules (n = 58) having mitotic angles >30° relative to the luminal vector (median 51°; Figure 1, A and D; Supplemental Videos 1 and 2). Loss of OCD was also apparent in a mouse model of Pkhd1 that forms kidney cysts (Pkhd1lacZ/lacZ)17 with 67% of mitotic angles >30° (n = 49; median 41°; Supplemental Figure 1). The comparable extent of loss of OCD in Pkhd1del4/del4 and Pkhd1lacZ/lacZ mice suggests that absence of cyst formation in Pkhd1del4/del4 is not the result of a lesser degree of loss of OCD and may, instead, be related to other functional differences between the specific mutant alleles. Interestingly, whereas WT and Pkd1 and Pkd2 mutant tubules (see next section) had <10% of mitoses with loss of OCD, heterozygous Pkhd1lacZ/+ mice showed 32% (n = 50; median, 16°; Supplemental Figure 1) and Pkhd1del4/+ showed 23% (n = 47; median 9.5°; data not shown) of mitotic angles >30°. Neither of these differed significantly in distribution from WT (data not shown); however, the trend toward slightly greater occurrence of misoriented mitotic angles in heterozygous Pkhd1 mice does suggest a possible correlation of gene dosage with occurrence of misoriented mitotic angles. Pkhd1 is required to maintain OCD during postnatal tubule elongation; however, loss of OCD is not sufficient to result in kidney tubule cyst formation.
Figure 1.
Mitotic orientation in nondilated tubular segments of orthologous mouse models of Pkhd1, Pkd1, and Pkd2 is shown. (A through C) OCD relative to the longitudinal axis of elongating collecting tubules in Pkhd1del4/del4 (A), Pkd1flox/−;Ksp-Cre (B), and Pkd2flox/flox;Pkhd1-Cre:RA/EG (C) mice at ages P7 through P10, P3, and P8 through P10, respectively. Chromosomal separation in anaphase was marked by anti-H3pS10 immunofluorescence (red), and the orientation of the corresponding tubules was determined after marking by anti–aquaporin-2 (A and B; green) or EGFP Cre reporter activity (C; green). Representative images of a mitotic angle >30° in a Pkhd1del4/del4 tubule (A) and <30° in Pkd1flox/−;Ksp-Cre (B), and Pkd2flox/flox;Pkhd1-Cre:RA/EG (C) tubules. Bars = 30 μm. Three-dimensional projections are available in Supplemental Videos 1 through 4. (D) Aggregate data of mitotic angles for WT (n = 48), Pkd1flox/−;Ksp-Cre (Pkd1; n = 52), Pkd2flox/flox;Pkhd1-Cre:RA/EG (Pkd2; n = 48), and Pkhd1del4/del4 (Pkhd1; n = 58). The distribution of mitotic angles in Pkhd1 mutants was significantly different from all other groups (P < 0.001), whereas Pkd1 and Pkd2 mutants did not differ from each other or from WT (horizontal line, median).
Mutations in Pkd1 and Pkd2 Do not Result Loss of OCD before Cyst Initiation
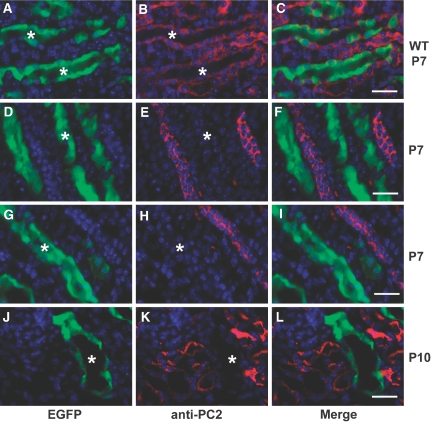
We next investigated whether mouse models of ADPKD based on mutations of Pkd1 and Pkd2, which form kidney cysts, exhibit defective OCD. We analyzed mitotic spindle axes in aquaporin-2–positive linear medullary nephron segments at P3 in Pkd1flox/−;Ksp-Cre mice (Figure 1B).18 The distribution of mitotic angles (n = 52) was not significantly different from WT kidneys, with 90% of the angles deviating <30° from the tubular axes (median 9°; Figure 1, B, and D; Supplemental Video 3). We next used a novel conditional model of ADPKD based on a Pkd2flox allele (X.T. and S.S., manuscript in preparation) and the Pkhd1-Cre transgenic mouse.14 Pkd2flox/flox;Pkhd1-Cre mice develop cystic dilation of collecting duct segments beginning at P8 to P10 (Figures 2, J through L, and 3, E through G, and data not shown), so we chose P7 to examine OCD in tubules before the onset of cystic dilation. Absence of detectable PC2 protein expression was confirmed using the RA/EG EGFP Cre reporter mouse line19 and anti-PC2 antisera (Figure 2). OCD was maintained in precystic tubules lacking PC2 expression, because 94% of cells undergoing anaphase (n = 48) had mitotic angles <30° (median 7°; Figure 1, C and D; Supplemental Video 4). Loss of OCD is not necessary to initiate cyst formation in ADPKD as a result of mutations of Pkd1 or Pkd2.
Figure 2.
Loss of PC2 expression after Cre-mediated inactivation of Pkd2 is shown. (A through L) Kidney sections from Pkhd1-Cre:RA/EG (WT; A through C) and Pkd2flox/flox;Pkhd1-Cre;RA/EG (D through L) mice showing EGFP reporter gene activity (green) and anti-PC2 immunoreactivity (red) at P7 (A through I) and P10 (J through L). In WT kidneys (A through C), PC2 immunoreactivity is present in segments with EGFP reporter activity (*) as well as in adjacent segments negative for EGFP. (D through I) In Pkd2flox/flox mice at P7, there is no detectable PC2 in tubules expressing Cre as indicated by presence of EGFP signal (*); PC2 expression persists in adjacent tubules in which Cre is not active (no EGFP). (J through L) Early dilated tubules at P10 have no detectable PC2 expression (*) whereas adjacent noncystic tubules lacking Cre expression have persistent PC2 expression. Bars = 20 μm.
Figure 3.
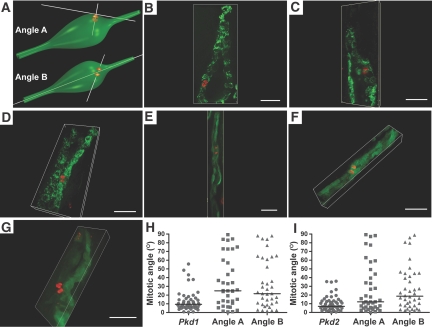
Mitotic orientation in early dilated tubular segments of Pkd1 and Pkd2 mouse models is shown. (A) Schematic representation showing angle A as the angle of the mitotic vector relative to the tangential plane of the dilated tubule wall and angle B as the angle of the mitotic vector relative to the tubule luminal vector. (B through D) Representative images of early dilated Pkd1flox/−;Ksp-Cre tubules at P3 stained with anti-H3pS10 (red) and anti–aquaporin 2 (green). (E through G) Representative images of Pkd2flox/flox;Pkhd1-Cre:RA/EG tubules at P8 through P10 stained with anti-H3pS10 (red) and EGFP (green). (B and F) Mitotic angles oriented within <30° of angle A and >30° of angle B. (C, D, and G) Mitotic angles at >30° for both angles A and B. (E) Mitotic angle <30° with respect to both angles A and B. Bars = 30 μm. (H) Aggregate data of angle A and angle B in Pkd1flox/−;Ksp-Cre (Pkd1; n = 35) compared with the respective nondilated Pkd1 mutant tubule data reproduced from Figure 1D. The distribution of angles A and B did not differ from each other but differed significantly from the mitotic angle distribution in nondilated tubules (P < 0.001). (I) Aggregate data of angle A and angle B in Pkd2flox/flox;Pkhd1-Cre:RA/EG mice (Pkd2; n = 38) compared with the respective nondilated Pkd2 mutant tubule data reproduced from Figure 1D. The distribution of angles A and B did not differ from each other but differed significantly from the mitotic angle distribution in nondilated tubules (angle A, P < 0.05; angle B, P < 0.01).
Pkd1- and Pkd2-Deficient Cells Lose OCD Once Tubules Begin to Dilate
Because we did not observe loss of OCD in precystic tubules after inactivation of Pkd1 and Pkd2, we evaluated whether OCD is lost after the early stages of tubule dilation had begun. We selected mildly dilated tubule segments for which we were able to define the luminal vector and the tangential plane for the tubule wall in the region of the dividing cells (Figure 3A). We defined angle A as the angle of the mitotic spindles with respect to the tangential plane and angle B as the mitotic angle relative to the luminal vector (Figure 3A). Mitotic angles were <30° for both angle A and angle B in 17 of 35 tubules in Pkd1flox/−;Ksp-Cre mice at P3 and in 24 of 38 tubules in Pkd2flox/flox;Pkhd1-Cre mice at P8 through P10 (Figure 3E). Mitotic angles were <30° for angle A but not angle B in three of 35 tubules from Pkd1flox/−;Ksp-Cre mice (Figure 3B) and one of 38 tubules from Pkd2flox/flox;Pkhd1-Cre mice (Figure 3F). Conversely, angle B but not angle A was <30° in five of 35 Pkd1flox/−;Ksp-Cre mice and one of 38 Pkd2flox/flox;Pkhd1-Cre mice. Ten of 35 mitoses in dilating tubules in Pkd1flox/−;Ksp-Cre mice (Figure 3, C and D) and 12 of 38 in Pkd2flox/flox;Pkhd1-Cre mice (Figure 3G) had mitotic angles >30° for both angle A and angle B. The median values for angle A and angle B in dilated tubules from Pkd1flox/−;Ksp-Cre mice were 25 and 22°, respectively, and from Pkd2flox/flox;Pkhd1-Cre mice were 12 and 18°, respectively (Figure 3, H and I). In aggregate 51% (n = 35) and 37% (n = 38) of early Pkd1 and Pkd2 cysts, respectively, showed loss of OCD in either or both angles A and B. Loss of OCD occurs once tubules start to dilate after inactivation of Pkd1 and Pkd2.
Effects of loss of OCD in noncystic tubules
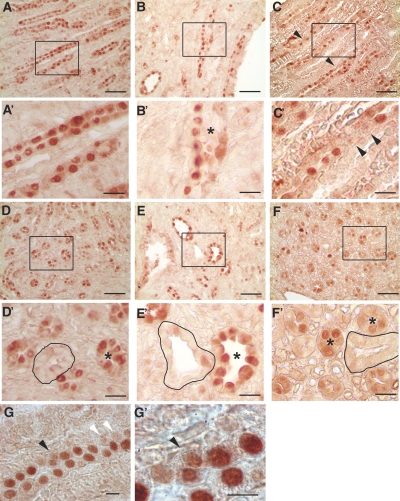
Pkhd1del4/del4 kidneys retain normal-appearing tubular morphology, suggesting that daughter cells generated by mitoses oriented out of the tubular epithelial plane undergo either programmed cell death or reintegration into the epithelial layer. We quantified apoptosis by terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling staining in collecting duct segments marked by Dolichos biflorus agglutinin that express fibrocystin16 and show loss of OCD. We found uniformly low rates and no discernible differences in apoptosis between WT and Pkhd1del4/del4 kidneys (data not shown), excluding increased apoptosis as the mechanism for normal-appearing collecting tubules in the Pkhd1del4/del4 model. Because we were not able to visualize cellular migration and integration events directly (i.e., convergent extension movements) in the newborn mouse kidney collecting duct, we sought indirect evidence for increased occurrence of such events in the Pkhd1del4/del4 model. We examined the pattern of synchronous cell proliferation observed in elongating tubules in postnatal mouse kidneys.13 WT kidneys in newborn mice show contiguous clusters of cells that stain positive with PCNA in longitudinal and transverse profiles (Figure 4, A, A′, D, and D′). Kidneys from newborn Pkd1flox/−;Ksp-Cre mice retain similar synchronous tracts of proliferating cells despite the appearance of early tubular dilation (Figure 4, B, B′, E, and E′). Whereas the overall degree of PCNA expression and kidney growth in Pkhd1 mutant mice does not appear different from WT, Pkhd1del4/del4 newborn mice show a more discontinuous and interrupted pattern of staining with PCNA (Figure 4, C, C′, F, and F′). This interrupted pattern of PCNA staining may reflect a more active planar migration and intercalation phenotype in the setting of loss of OCD in Pkhd1del4/del4 kidneys.
Figure 4.
Synchronous cell division in Pkd1 and Pkhd1 mutant mice. (A through F′) Longitudinal (A through C and A′ through C′) and transverse (D through F and D′ through F′) sections from kidneys of newborn mice stained with PCNA. Contiguous stretches of PCNA-positive nuclei suggestive of synchronous cell divisions are present in WT (A, A′, D, and D′) and Pkd1flox/−;Ksp-Cre (B, B'′, E, and E′) kidneys. Pkhd1del4/del4 kidneys (C, C′, F, and F′) show a more interrupted and discontinuous pattern of PCNA-positive nuclei in elongating tubules (C and C′, arrowheads). Tubules in transverse section were often uniformly negative (outlined) or uniformly positive (*) for PCNA in WT and Pkd1flox/−;Ksp-Cre kidneys (D, D′, E, and E′) but not in Pkhd1del4/del4 kidneys (F and F′, *). Dilating tubules of early cysts in Pkd1flox/−;Ksp-Cre kidneys (B′ and E′, *) continue to show a synchronous pattern of PCNA expression. A′ through F′ are digitally magnified views of the respective boxed regions in A through F. PCNA incorporation in Pkhd1del4/del4 kidneys occasionally showed misoriented cell (G and G′, black arrowhead); white arrowheads show discontinuous pattern of PCNA staining. Bars = 50 μm in A through F, 15 μm in A′ through F′, and 5 μm in G and G′.
Cell lineage analysis during kidney development shows that tubular cells retain an uninterrupted contiguous linear relationship after cell division during elongation in approximately 75% of medullary segments examined.13 We sought to determine whether loss of OCD in Pkhd1del4/del4 kidneys affected this property of elongating tubules. We produced mice with the pCX-CreERTM tamoxifen-inducible Cre transgene20 in combination with the ROSA26R reporter on either WT or Pkhd1del4/del4 background. Cre recombinase was activated using a single tamoxifen injection in newborn mice, and kidneys were analyzed at 5 wk of age. Vibratome sections (100 μm; 10 to 20 sections per kidney) from five mice of each genotype were stained for β-galactosidase activity. We examined >500 tubules showing β-galactosidase reporter activity in pCX-CreERTM;ROSA26R mice on the WT background. More than 70% of tubules showed uninterrupted contiguous linear arrays of β-galactosidase–positive cells (Figure 5A). In comparison, <40% of tubule segments showed uninterrupted linear stretches of β-galactosidase–expressing cells in a comparable number of β-galactosidase–positive tubules from Pkhd1del4/del4;pCX-CreERTM;ROSA26R mice. The majority of tubules showed interposition of cells not expressing β-galactosidase into the plane of the tubule epithelium or migration of cells expressing β-galactosidase out of the epithelial plane (Figure 5, B and B′). Single tubules with evidence of both events were detectable in Pkhd1del4/del4;pCX-CreERTM;ROSA26R mice (Figure 5, B and B′) but were never seen on the WT background. These data are consistent with an increased occurrence of migration and intercalation of cells after mitosis in elongating tubules and may represent increased convergent extension movements, another manifestation of PCP, in mice with loss of OCD as a result of the Pkhd1del4 mutation.
Figure 5.
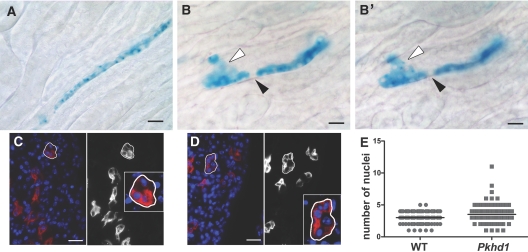
Disruptions in the pattern of tubular elongation with loss of OCD are shown. (A through B′) Representative sections from pCX-CreERTM:ROSA26R (A) and Pkhd1del4/del4:pCX-CreERTM:ROSA26R (B and B′) mice induced with a single dose of tamoxifen (100 μg) at the newborn stage and examined at 5 wk by X-gal staining for β-galactosidase activity. In the medulla, X-gal–positive blue cells in pCX-CreERTM:ROSA26R mice on a WT background appear as largely uninterrupted linear segments (A). In Pkhd1del4/del4:pCX-CreERTM:ROSA26R mice, these clonal rows of cells are often interrupted by cells not stained by X-gal (B and B′, black arrowhead) or by blue staining cells migrating out of the linear segment (B and B′, white arrowhead). The same tubule is shown in different focal planes in B and B′. (C and D) Representative images of 1-mo-old WT (C) and Pkhd1del4/del4 (D) kidney sections stained with Dolichos biflorus agglutinin (red) and DAPI (blue) and used to count the number of nuclei in transverse sections of medullary collecting ducts. Images are shown both in color (left) and in grayscale (red channel only, right), and a representative tubule in each is outlined and magnified in the insets. (E) Collecting ducts from Pkhd1del4/del4 kidneys (Pkhd1) had a larger mean number of cells (3.68 ± 0.18 SEM; n = 80) in transverse section than WT kidneys (3.06 ± 0.10 SEM; n = 84); P = 0.0106. Bars = 20 μm.
Given these findings, we asked whether the observed PCP defects result in any discernible effect on the geometry of the collecting tubules along the minor (transverse) axis in Pkhd1del4/del4 kidneys. The number of nuclei visible around the circumference of collecting ducts in 5-μm sections from adult mouse WT kidneys is 3.06 ± 0.10 SEM (n = 84; Figure 5, C and E). In Pkhd1del4/del4 kidneys, the mean number of nuclei in transverse sections of collecting duct tubules increases to 3.68 ± 0.18 SEM (n = 80; P = 0.0106; Figure 5, D and E). These findings support the hypothesis that Pkhd1 functions in a PCP pathway that is essential for maintaining OCD but that disruption of this pathway is not sufficient to promote cyst formation in the mouse kidney.
Discussion
Loss of OCD is a common feature of mutations in Pkhd1, but it is not sufficient to produce kidney cysts. By contrast, germline inactivation of Pkd1 and Pkd2, which results in cyst formation, shows normal OCD in precystic tubules despite the absence of PC1 or PC2. Loss of OCD does manifest in Pkd1 and Pkd2 models after tubules start to dilate. This latter occurrence is either part of the transition from normal-appearing tubules to cysts or a consequence of this transition. Support for the latter interpretation is suggested by the observation in early Pkd1 and Pkd2 cysts: >50% of the mitoses show normal OCD, and median mitotic angles range from 12 to 25° compared with 51° in noncystic Pkhd1del4/del4 tubules. Nephron segments specifically lacking Pkd1 in the Pkd1flox/flox;Ksp-Cre model have excess proliferation documented by quantification of bromodeoxyuridine incorporation,18 and this has been confirmed in Pkd2flox/flox;Pkhd1-Cre mice as well (X.T. and S.S., unpublished observations). Bromodeoxyuridine incorporation in postnatal kidneys may be more sensitive than whole-organ proliferative indices that failed to detect increased proliferation in a different early-onset Pkd1 model.21 We propose that postnatal cyst formation in Pkd1 and Pkd2 mutant mice results from increased proliferation in the absence of compensatory increased elongation but does not require loss of OCD. The contribution of subsequent loss of OCD to cyst growth in the Pkd1 and Pkd2 models is uncertain. For example, despite mitoses in which angle A is misoriented, cyst linings in these models remain a single-cell layer throughout.18 This may reflect successful reintegration of cells into the cyst lining monolayer through signals related to convergent extension pathways even as another PCP-related pathway, OCD, is lost. Such separation of the PCP phenotypes of convergent extension and OCD during kidney development were recently described.22 Convergent extension processes in the absence of OCD set tubule diameters during in utero kidney development; subsequently, OCD manifests in postnatal kidney development to maintain tubule diameters during elongation.22 Our data suggest that both Pkhd1del4/del4 tubules and ADPKD cysts retain PCP information related to convergent extension processes but lose OCD information as either a primary (Pkhd1del4) or a secondary (Pkd1flox, Pkd2flox) defect.
We propose that Pkd1/Pkd2-dependent processes act as sensors for tubule diameter, whereas PCP-dependent processes, including Pkhd1 signaling, function as the effectors that establish and maintain tubule diameter. Worsening kidney cystic disease in Pkhd1 mutant mice on a Pkd1+/− background23 is consistent with the hypothesis that the Pkhd1 and Pkd1/Pkd2 pathways are distinct but interact functionally. For example, interaction of PCP with cell-proliferative processes is evident in neurulation in zebrafish, in which blocking cell division rescues PCP defects of convergence and extension.24 Human ARPKD is largely a developmental disorder. The predominant kidney presentation, severe perinatal disease, and juvenile-onset disease, respectively, coincide with in utero development and postnatal growth of the kidneys. These are periods when PCP processes are active. Notably, PKHD1 carrier parents do not develop adult-onset cysts despite the likely occurrence of somatic second-step mutations in the normal copy of PKHD1.25 One possible explanation for the absence of cysts from inactivation of PKHD1 in adulthood is that the PCP processes in which fibrocystin functions are no longer active at this postdevelopmental stage. The lack of a renal cystic phenotype in Pkhd1del4 and other Pkhd1 mutant mice26,27 may indicate the presence of mechanisms of PCP on the basis of convergence and extension that can compensate for loss of OCD. For example, inactivation of the protocadherin PCP protein Fat4 results in loss of OCD with a mild cystic phenotype that is exacerbated on a Vangl2+/− background,15 perhaps reflecting a relative reduction in convergent extension processes in Vangl2 heterozygotes.24 The distinct and complementary role of convergent extension in tubule formation and OCD in kidney tubule elongation22 and of Wnt signaling in OCD and tubule elongation28 establish the centrality of PCP processes in kidney development. Our lineage analyses in Pkhd1del4/del4 mice are consistent with the hypothesis that increased occurrence of reintegration of cells into the epithelial plane, perhaps reflective of convergent extension movements, compensates for the cell divisions out of the plane as a result of Pkhd1 mutation. The observed modest change in the number of cells in transverse tubular profiles suggests adequate but not complete rescue of the OCD defect.
In contrast to ARPKD, somatic inactivation of either Pkd1 or Pkd2 results in cyst formation.21,25,29 Our finding that loss of polycystins does not seem to affect a primary PCP-related mechanism suggests different primary processes underlie ADPKD. These mechanisms drive phenotypic alterations that include changes in cell shape from columnar to highly attenuated squamoid epithelia as well as the modest but significant proliferative changes.18,30 Evidence of a role for proliferation has also come from acute kidney injury models that exacerbate cyst formation in the setting of reparative proliferation.14,31,32 Cystic kidney disease mechanisms have converged on the primary cilium as a central organelle but have diverged with an expanding group of genes that can cause a broad spectrum of cystic phenotypes. This study shows that Pkhd1 functions in PCP-related pathways, whereas Pkd1 and Pkd2 do not. Defining the role of Pkhd1 in PCP and the pathway(s) in which Pkd1 and Pkd2 operate are essential steps in further understanding polycystic diseases.
Concise Methods
Mouse Strains and Breeding
The Pkd1− null allele,27 the Pkd1flox allele,18 and the Pkhd1del4 allele16 have been previously described. The Pkd2flox allele was made by inserting flanking loxP sites in IVS2 and IVS4 of Pkd2; Cre recombinase activity results in deletion of exons 3 and 4 and complete loss of function of Pkd2 (X.T. and S.S., manuscript in preparation). Kidney-selective inactivation was achieved using either the Ksp-Cre21 or Pkhd1-Cre14 transgenic line. Tamoxifen-inducible Cre recombinase–mediated gene inactivation used the pCX-CreERTM line.20 The ROSA26R Cre reporter strain was obtained from JAX (Bar Harbor, ME). The RA/EG EGFP Cre reporter mouse line19 was provided by Bernd Arnold. All experiments were conducted in accordance with Yale University institutional animal care and use committee guidelines and procedures.
Measurement of Mitotic Angles
The method for measuring orientation of mitotic angles was adopted from Fischer et al.13 Kidneys were fixed in 4% paraformaldehyde, washed in PBS, then embedded in 4% agarose. Sections of 100 μm were incubated with antibodies to aquaporin 2 (Santa Cruz) and H3pS1,32 in PBS, 10% FCS, and 0.1% Triton X-100 for 24 h at 4°C. Sections were rinsed in PBS, then incubated with secondary antibody under the same conditions. Sections were mounted, and images were obtained with a Nikon TE2000U inverted microscope. Approximately 60 frames at 0.25-μm-interval z steps were acquired using MetaMorph acquisition software (Molecular Devices) and were processed with AutoDeblur/AutoVisualize deconvolution software (MediaCybernetics; Supplemental Videos 1 through 4). The three-dimensional coordinates defining the vectors for the tubular lumen and for the mitotic axis perpendicular to the interface between separating chromosomes in late anaphase were determined using the software and were entered into an Excel worksheet14 to calculate angle of mitosis between the two vectors.
Immunofluorescence, β-Galactosidase Staining, and Immunohistochemistry
For immunofluorescence, tissues were fixed in 4% paraformaldehyde in PBS and embedded in OCT. Kidney sections (4 to 5 μm thick) were incubated with primary antibodies overnight at 4°C followed by the secondary antibodies for 1 h at room temperature. Images were obtained using a Nikon TE2000U inverted microscope using MetaMorph acquisition software.
For β-galactosidase staining, the kidneys were fixed in 2.7% formaldehyde, 0.02% NP-40, and 0.2% glutaraldehyde in PBS (pH 7.4) for 30 min at 4°C and washed. Processing was carried out through a graded series of sucrose concentrations from 15 to 30% in PBS at 4°C for 5 to 12 h for each step. Kidneys were then embedded in OCT and frozen in 2-methyl-butane submerged in liquid nitrogen. Sections (5 to 8 μm thick) were prepared, mounted on slides, washed in PBS for 5 min, and subsequently stained in X-gal solution (1 mg/ml X-gal in DMSO, 2 mM MgCl2, 20 mM potassium ferricyanide, and 0.02% NP-40 in PBS) at 37°C overnight. For PCNA staining, paraffin-embedded sections (5 μm) were stained using Histomouse Plus Kit (Invitrogen) and a mouse mAb to PCNA (Sigma).
Statistical Analysis
Multiple group comparisons were analyzed by Kruskal-Wallis nonparametric one-way ANOVA with Dunn multiple comparison post test. Pair-wise comparisons were performed by nonparametric Mann Whitney U test.
Disclosures
None.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (DK51041, DK54053, and DK57328) and the Joseph Leroy and Ann C. Warner Fund to S.S. and from the PKD Foundation to A.R.G. (149a2r). S.N., X.T., A.R.G., Z.Y., and S.S. are members of the Yale Center for the Study of Polycystic Kidney Disease (DK57328).
We thank Marco Pontoglio for critical reading of the manuscript and Zhaoning Liu for providing artwork.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “What Drives Cyst Formation in PKD?” on pages 200–202.
References
- 1.Harris PC, Torres VE: Polycystic kidney disease. Annu Rev Med 60: 321–337, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taulman P, Haycraft C, Balkovetz D, Yoder B: Polaris, a protein involved in left-right axis patterning, localizes to basal bodies and cilia. Mol Biol Cell 12: 589–599, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun Z, Amsterdam A, Pazour GJ, Cole DG, Miller MS, Hopkins N: A genetic screen in zebrafish identifies cilia genes as a principal cause of cystic kidney. Development 131: 4085–4093, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Hou X, Mrug M, Lefkowitz E, Yoder B, D'Eustachio P, Beier D, Guay-Woodford L: Cystin, a novel cilial-associated protein, is disrupted in the cpk mouse model of polycystic kidney disease. J Am Soc Nephrol 109: 533–540, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin F, Hiesberger T, Cordes K, Sinclair AM, Goldstein LS, Somlo S, Igarashi P: Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci U S A 100: 5286–5291, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li A, Davila S, Furu L, Qian Q, Tian X, Kamath PS, King BF, Torres VE, Somlo S: Mutations in PRKCSH cause isolated autosomal dominant polycystic liver disease. Am J Hum Genet 72: 691–703, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davila S, Furu L, Gharavi AG, Tian X, Onoe T, Qian Q, Li A, Cai Y, Kamath PS, King BF, Azurmendi PJ, Tahvanainen P, Kaariainen H, Hockerstedt K, Devuyst O, Pirson Y, Martin RS, Lifton RP, Tahvanainen E, Torres VE, Somlo S: Mutations in SEC63 cause autosomal dominant polycystic liver disease. Nat Genet 36: 575–577, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Kishimoto N, Cao Y, Park A, Sun Z: Cystic kidney gene seahorse regulates cilia-mediated processes and Wnt pathways. Dev Cell 14: 954–961, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Simons M, Mlodzik M: Planar cell polarity signaling: From fly development to human disease. Annu Rev Genet 42: 517–540, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, Schermer B, Benzing T, Cabello OA, Jenny A, Mlodzik M, Polok B, Driever W, Obara T, Walz G: Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet 37: 537–543, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Germino GG: Linking cilia to Wnts. Nat Genet 37: 455–457, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Gong Y, Mo C, Fraser SE: Planar cell polarity signalling controls cell division orientation during zebrafish gastrulation. Nature 430: 689–693, 2004 [DOI] [PubMed] [Google Scholar]
- 13.Fischer E, Legue E, Doyen A, Nato F, Nicolas JF, Torres V, Yaniv M, Pontoglio M: Defective planar cell polarity in polycystic kidney disease. Nat Genet 38: 21–23, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Patel V, Li L, Cobo-Stark P, Shao X, Somlo S, Lin F, Igarashi P: Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum Mol Genet 17: 1578–1590, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saburi S, Hester I, Fischer E, Pontoglio M, Eremina V, Gessler M, Quaggin SE, Harrison R, Mount R, McNeill H: Loss of Fat4 disrupts PCP signaling and oriented cell division and leads to cystic kidney disease. Nat Genet 40: 1010–1015, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Gallagher A, Esquivel E, Briere T, Tian X, Mitobe M, Menezes L, Markowitz G, Jain D, Onuchic L, Somlo S: Biliary and pancreatic dysgenesis in mice harboring a mutation in Pkhd1. Am J Pathol 172: 417–429, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams S, Cobo-Stark P, James L, Somlo S, Igarashi P: Kidney cysts, pancreatic cysts, and biliary disease in a mouse model of autosomal recessive polycystic kidney disease. Pediatr Nephrol 23: 733–741, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Shibazaki S, Yu Z, Nishio S, Tian X, Thomson RB, Mitobe M, Louvi A, Velazquez H, Ishibe S, Cantley LG, Igarashi P, Somlo S: Cyst formation and activation of the extracellular regulated kinase pathway after kidney specific inactivation of Pkd1. Hum Mol Genet 17: 1505–1516, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Forde A, Constien R, Grone HJ, Hammerling G, Arnold B: Temporal Cre-mediated recombination exclusively in endothelial cells using Tie2 regulatory elements. Genesis 33: 191–197, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Guo C, Yang W, Lobe CG: A Cre recombinase transgene with mosaic, widespread tamoxifen-inducible action. Genesis 32: 8–18, 2002 [DOI] [PubMed] [Google Scholar]
- 21.Piontek K, Menezes LF, Garcia-Gonzalez MA, Huso DL, Germino GG: A critical developmental switch defines the kinetics of kidney cyst formation after loss of Pkd1. Nat Med 13: 1490–1495, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karner CM, Chirumamilla R, Aoki S, Igarashi P, Wallingford JB, Carroll TJ: Wnt9b signaling regulates planar cell polarity and kidney tubule morphogenesis. Nat Genet 41: 793–799, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia-Gonzalez M, Menezes L, Piontek K, Kaimori J, Huso D, Watnick T, Onuchic L, Guay-Woodford L, Germino G: Genetic interaction studies link autosomal dominant and recessive polycystic kidney disease in a common pathway. Hum Mol Genet 16: 1940–1950, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ciruna B, Jenny A, Lee D, Mlodzik M, Schier AF: Planar cell polarity signalling couples cell division and morphogenesis during neurulation. Nature 439: 220–224, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qian F, Watnick TJ, Onuchic LF, Germino GG: The molecular basis of focal cyst formation in human autosomal dominant polycystic kidney disease type I. Cell 87: 979–987, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Moser M, Matthiesen S, Kirfel J, Schorle H, Bergmann C, Senderek J, Rudnik-Schoneborn S, Zerres K, Buettner R: A mouse model for cystic biliary dysgenesis in autosomal recessive polycystic kidney disease (ARPKD). Hepatology 41: 1113–1121, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Woollard J, Punyashtiti R, Richardson S, Masyuk T, Whelan S, Huang B, Lager D, vanDeursen J, Torres V, Gattone V, LaRusso N, Harris P, Ward C: A mouse model of autosomal recessive polycystic kidney disease with biliary duct and proximal tubule dilatation. Kidney Int 72: 328–336, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Yu J, Carroll TJ, Rajagopal J, Kobayashi A, Ren Q, McMahon AP: A Wnt7b-dependent pathway regulates the orientation of epithelial cell division and establishes the cortico-medullary axis of the mammalian kidney. Development 136: 161–171, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lantinga-van Leeuwen IS, Leonhard WN, van der Wal A, Breuning MH, de Heer E, Peters DJ: Kidney-specific inactivation of the Pkd1 gene induces rapid cyst formation in developing kidneys and a slow onset of disease in adult mice. Hum Mol Genet 16: 3188–3196, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Nishio S, Hatano M, Nagata M, Horie S, Koike T, Tokuhisa T, Mochizuki T: Pkd1 regulates immortalized proliferation of renal tubular epithelial cells through p53 induction and JNK activation. J Clin Invest 115: 910–918, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takakura A, Contrino L, Zhou X, Bonventre JV, Sun Y, Humphreys BD, Zhou J: Renal injury is a third hit promoting rapid development of adult polycystic kidney disease. Hum Mol Genet 18: 2523–2531, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Happe H, Leonhard WN, van der Wal A, van de Water B, Lantinga-van Leeuwen IS, Breuning MH, de Heer E, Peters DJ: Toxic tubular injury in kidneys from Pkd1-deletion mice accelerates cystogenesis accompanied by dysregulated planar cell polarity and canonical Wnt signaling pathways. Hum Mol Genet 18: 2532–2542, 2009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.