Abstract
Acute kidney injury (AKI) associates with higher in-hospital mortality, but whether it also associates with increased long-term mortality is unknown, particularly after accounting for residual kidney function after hospital discharge. We retrospectively analyzed data from US veteran patients who survived at least 90 d after discharge from a hospitalization. We identified AKI events not requiring dialysis from laboratory data and classified them according to the ratio of the highest creatinine during the hospitalization to the lowest creatinine measured between 90 d before hospitalization and the date of discharge. We estimated mortality risks using multivariable Cox regression models adjusting for demographics, comorbidities, medication use, primary diagnosis of admission, length of stay, mechanical ventilation, and postdischarge estimated GFR (residual kidney function). Among the 864,933 hospitalized patients in the study cohort, we identified 82,711 hospitalizations of patients with AKI. In the study population of patients who survived at least 90 d after discharge, 17.4% died during follow-up (AKI 29.8%, without AKI 16.1%). The adjusted mortality risk associated with AKI was 1.41 (95% confidence interval [CI] 1.39 to 1.43) and increased with increasing AKI stage: 1.36 (95% CI 1.34 to 1.38), 1.46 (95% CI 1.42 to 1.50), and 1.59 (95% CI 1.54 to 1.65; P < 0.001 for trend). In conclusion, AKI that does not require dialysis associates with increased long-term mortality risk, independent of residual kidney function, for patients who survive 90 d after discharge. Long-term mortality risk is highest among the most severe cases of AKI.
Acute kidney injury (AKI) affects up to 15.3% of all hospitalized patients.1,2 Regardless of the underlying cause, AKI is associated with significantly increased in-hospital morbidity, mortality, and costs.2–16 The majority of previous studies linking AKI to mortality examined in-hospital mortality only and did not address postdischarge morbidity and mortality.2,4,7,8,10,11,13–16 Studies examining postdischarge mortality have focused primarily on critically ill patients with AKI that requires dialysis.9 Consequently, it remains unclear whether AKI that does not require dialysis is associated with a higher long-term risk for all-cause mortality.
One of the challenges of long-term mortality studies is to estimate the mortality risk independently associated with AKI from risk associated with chronic kidney disease (CKD). Some patients have incomplete recovery of their kidney function after AKI, and CKD is associated with a higher risk for mortality.17,18 To evaluate the independent long-term mortality risk of AKI, it is essential to adjust for postdischarge kidney function. The objective of this study was to estimate the postdischarge, long-term mortality risk associated with AKI while adjusting for residual kidney function in a large cohort of US veterans.
Results
We identified 864,933 patients who had a first hospitalization during our study period and met the inclusion criteria. The cohort was predominantly male (4.9% female), and the mean age was 61.8 yr. From this cohort, we identified 82,711 hospitalizations of patients with AKI (9.6% of the cohort), categorized as follows: Acute Kidney Injury Network (AKIN) stage I, 52,338; stage II, 19,771; and stage III, 10,602. Baseline characteristics for patients with and without AKI are presented in Table 1. As expected, patients with AKI had more comorbidities compared with patients without AKI. Mechanical ventilation was also more prevalent and the hospital length of stay was longer for patients with AKI.
Table 1.
Baseline characteristics of study participants
| Baseline Characteristic | With AKI (n = 82,711) | No AKI (n = 782,222) |
|---|---|---|
| Demographics | ||
| age (yr; mean ± SD) | 66.3 ± 12.4 | 61.3 ± 13.7 |
| female gender (%) | 3.2 | 5.0 |
| race (%) | ||
| white | 71.6 | 72.0 |
| black | 21.7 | 20.5 |
| Hispanic | 4.0 | 4.0 |
| other | 1.9 | 2.0 |
| Follow-up time (yr; mean ± SD) | 2.08 ± 1.42 | 2.36 ± 1.43 |
| eGFR (ml/min per 1.73 m2; median [first and third quartiles]) | ||
| baseline | 93.8 (71.0, 122.2) | 86.3 (70.3, 103.8) |
| after discharge | 75.0 (56.3, 96.7) | 81.5 (65.8, 97.9) |
| Concurrent conditions before admission (%) | ||
| previous AKIa | 1.1 | 0.3 |
| cancer | 14.6 | 10.5 |
| cardiac and vascular diseasesb | 37.5 | 28.9 |
| CKD | 4.5 | 2.0 |
| chronic obstructive pulmonary disease | 4.3 | 4.3 |
| diabetes | 33.6 | 22.9 |
| hypertension | 61.1 | 48.7 |
| neurodegenerative diseasesc | 5.0 | 3.9 |
| mental health disordersd | 25.2 | 31.3 |
| hospitalization in previous month | 0.9 | 0.4 |
| hospitalization in previous yeare | 8.0 | 6.1 |
| Medication use before admission (%) | ||
| ACEIs/ARBs | 40.8 | 28.5 |
| β blockers | 29.5 | 23.7 |
| diuretics | 35.4 | 23.0 |
| NSAIDs | 25.6 | 26.9 |
| other nephrotoxic drugs | 13.8 | 10.9 |
| platelet aggregation inhibitors | 26.9 | 21.6 |
| Hospitalization details | ||
| hospitalization primary diagnosis (%) | ||
| circulatory | 20.8 | 20.2 |
| digestive | 11.8 | 8.3 |
| infectious | 2.3 | 1.1 |
| neoplasms | 7.6 | 5.1 |
| respiratory | 10.2 | 6.5 |
| other | 47.2 | 58.9 |
| mechanical ventilationf | 6.45 | 0.4 |
| length of stay (d; median [first and third quartiles]) | 10 (5, 27) | 4 (2, 8) |
| Hospitalization between 30 and 90 d after discharge (%) | 13.7 | 10.0 |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; NSAID, nonsteroidal anti-inflammatory drug.
aDefined as the presence of at least one ICD-9-CM diagnostic code 584.xx in the 2 yr before the index admission date.
bInclude congestive heart failure, ischemic disease, arrhythmia, peripheral vascular disease, stroke, and valvular disease.
cInclude Parkinson disease, Alzheimer disease, other dementia, and multiple sclerosis.
dInclude major depression, posttraumatic stress disorder, anxiety disorder, schizophrenia, bipolar disorder, alcohol abuse, and drug abuse.
eExcludes hospitalizations in the previous month.
fDefined by the presence of ICD-9-CM procedure code 96.04 or 96.7x during hospitalization.
The mean number of creatinine values for each patient was 3.5 at baseline and 1.1 after discharge. Baseline creatinine values were missing in 5.0% of patients without AKI (by definition, no baseline creatinine values was missing in the AKI group), and 57.2% had at least one creatinine value before admission (versus 60.5% in the AKI group). The proportions of patients with postdischarge values in the group without AKI were 23, 38, and 48% within 30, 60, and 90 d respectively, whereas the corresponding proportions were 38, 55, and 65% in the AKI group. The latest creatinine value before discharge was ≤1.3 mg/dl (115 μmol/L) in 89% of patients with at least one inpatient value in the group without AKI and 78% in the AKI group. Because we included the latest inpatient value in the postdischarge creatinine assessment when a postdischarge creatinine was missing, 89.8% of those without AKI and 100% of the AKI group had a “postdischarge” value before imputation.
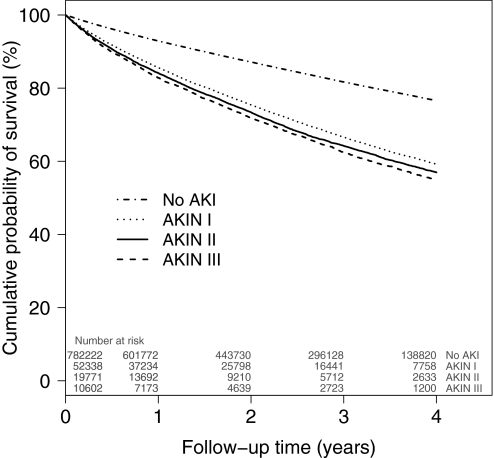
During follow-up (mean duration 2.34 yr [1.43]), 150,231 (17.4%) patients died. The crude cumulative risk for death was higher in the group with AKI (29.8%) than in the group without AKI (16.1%). Crude and adjusted hazard ratios (HRs) obtained from the Cox proportional hazards regression models are presented in Table 2. Whereas each successive addition of covariates to the model reduced the mortality risk estimate, AKI remained associated with a 41% increased risk for all-cause mortality in the fully adjusted model (adjusted HR 1.41; 95% confidence interval [CI] 1.39 to 1.43). A risk gradient was found among the three AKI categories (adjusted HR 1.39 versus 1.51 versus 1.71, for AKIN stages I, II, and III, respectively, compared with no AKI), with severity of AKI associated with increasing risk for death (P < 0.001 for trend). Unadjusted Kaplan-Meier survival curves stratified by AKIN categories of AKI are presented in Figure 1.
Table 2.
HR (95% CI) of all-cause mortality associated with AKI
| Parameter | Crude | +Demographicsa | +Comorbidities and Medication Useb | +Hospitalization Detailsc | +Postdischarge eGFR Categoriesd |
|---|---|---|---|---|---|
| No AKI (n = 782,222) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| With AKI (n = 82,711) | 2.10 (2.07 to 2.13) | 1.70 (1.68 to 1.73) | 1.55 (1.53 to 1.57) | 1.45 (1.43 to 1.47) | 1.41 (1.39 to 1.43) |
| By AKIN stage of AKIe | |||||
| I (n = 52,338) | 2.01 (1.98 to 2.04) | 1.62 (1.59 to 1.65) | 1.48 (1.45 to 1.50) | 1.39 (1.37 to 1.42) | 1.36 (1.34 to 1.38) |
| II (n = 19,771) | 2.20 (2.15 to 2.26) | 1.80 (1.75 to 1.84) | 1.61 (1.57 to 1.66) | 1.51 (1.47 to 1.55) | 1.46 (1.42 to 1.50) |
| III (n = 10,602) | 2.35 (2.27 to 2.43) | 1.98 (1.92 to 2.05) | 1.81 (1.74 to 1.87) | 1.71 (1.65 to 1.77) | 1.59 (1.54 to 1.65) |
aModel adjusted for age, gender, race, and VA priority status.
bModel adjusted for demographics and previous AKI, rheumatoid arthritis, arrhythmia, cerebrovascular disease, congestive heart failure, CKD, previous cancer, chronic obstructive pulmonary disease, cardiovascular disease, diabetes, hyperlipidemia, hypertension, liver disease, osteoarthritis, peptic ulcer disease, peripheral vascular disease, valvular disease, low back pain, inflammatory bowel disease, multiple sclerosis, history of seizures, Parkinson disease, Alzheimer disease, other dementia, major depression, posttraumatic stress disorder, anxiety disorder, schizophrenia, bipolar disorder, alcohol abuse, drug abuse, tobacco use disorder, previous hospitalizations (previous month and previous year), angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, β blockers, calcium-channel blockers, corticosteroids, diuretics, immunosuppressants, nitrates, nonsteroidal anti-inflammatory drugs, oral anticoagulants, platelet aggregation inhibitors, and other nephrotoxic drugs.
cModel adjusted for demographics, comorbidities, and medication use and index admission primary diagnosis, mechanical ventilation use, and hospital length of stay.
dModel fully adjusted for demographics, comorbidities and medication use, and hospitalization details and postdischarge eGFR categories.
eAKIN categories are defined by the ratio of the highest creatinine to the lowest creatinine. AKIN I is a ratio ≥1.5 and <2, AKIN II is a ratio ≥2 and <3, and AKIN III is a ratio ≥3.
Figure 1.
Unadjusted Kaplan-Meier survival curves by AKIN categories are shown. Time is calculated from 90 d after discharge of the index admission. AKIN categories are defined by the ratio of the highest creatinine to the lowest creatinine. AKIN I is a ratio ≥1.5 and <2, AKIN II is a ratio ≥2 and <3, and AKIN III is a ratio ≥3. Log-rank test: P < 0.001.
As shown in Table 3, mortality risks were lower in patients who had lower baseline estimated GFR (eGFR; adjusted HR 1.45 versus 1.35 versus 1.31 versus 1.23 for eGFR ≥90, 60 to 89, 45 to 59, and 30 to 44 ml/min per 1.73 m2, respectively). AKI-associated mortality HR also decreased with older age and was slightly lower in patients with diabetes compared with those without diabetes (Table 3).
Table 3.
Adjusted all-cause mortality HRs associated with AKI for various subgroups
| Parameter | With AKI (n = 82,711) | No AKI (n = 782,222) | Adjusted Mortality HRa | 95% CI |
|---|---|---|---|---|
| Baseline eGFR (ml/min per 1.73 m2) | ||||
| ≥90 | 44,641 (54.0%) | 365,520 (46.7%) | 1.45 | 1.42 to 1.48 |
| ≥60, <90 | 26,042 (31.5%) | 322,818 (41.3%) | 1.35 | 1.32 to 1.39 |
| ≥45, <60 | 7983 (9.7%) | 68,361 (8.7%) | 1.31 | 1.25 to 1.36 |
| ≥30, <45 | 4045 (4.9%) | 25,523 (3.3%) | 1.23 | 1.16 to 1.30 |
| Age | ||||
| <50 | 7992 (9.7%) | 165,965 (21.2%) | 1.84 | 1.72 to 1.96 |
| 50 to 59 | 19,897 (24.1%) | 225,033 (28.8%) | 1.62 | 1.56 to 1.68 |
| 60 to 69 | 18,910 (22.9%) | 153,699 (19.7%) | 1.42 | 1.37 to 1.46 |
| 70 to 79 | 24,176 (29.2%) | 162,688 (20.8%) | 1.31 | 1.28 to 1.34 |
| ≥80 | 11,736 (14.2%) | 74,837 (9.6%) | 1.24 | 1.20 to 1.28 |
| Gender | ||||
| female | 2683 (3.2%) | 39,405 (5.0%) | 1.49 | 1.34 to 1.65 |
| male | 80,028 (96.8%) | 742,817 (95.0%) | 1.41 | 1.39 to 1.43 |
| Diabetes status | ||||
| no diabetes | 54,897 (66.4%) | 603,326 (77.1%) | 1.44 | 1.42 to 1.47 |
| diabetes | 27,814 (33.6%) | 178,896 (22.9%) | 1.33 | 1.29 to 1.36 |
aAdjusted for demographics, comorbidities and medication use, hospitalization details, and postdischarge eGFR categories.
Between baseline and postdischarge periods, eGFR decreased by a median of 1.5% (0.4 to 16.7%). In 56.4% of the patients, this decrease, if any, was ≤ 10%, and they were considered to have recovered from AKI or to have maintained their kidney function. Among those patients, AKI was also associated with a higher mortality risk (adjusted HR 1.47; 95% CI 1.43 to 1.51). Mortality risk also varied with baseline eGFR (Table 4) with risk decreasing with lower baseline eGFR.
Table 4.
Adjusted all-cause mortality HRs associated with AKI for patients with renal recovery or without deterioration of kidney function
| Baseline eGFR (ml/min per 1.73 m2) | With AKI (n [%]) | No AKI (n [%]) | Adjusted Mortality HRa | 95% CI |
|---|---|---|---|---|
| All | 25,564 | 462,394 | 1.47 | 1.43 to 1.51 |
| ≥90 | 9983 (39.1) | 176,409 (38.2) | 1.52 | 1.46 to 1.59 |
| ≥60, <90 | 9630 (37.7) | 217,931 (47.1) | 1.39 | 1.33 to 1.45 |
| ≥45, <60 | 3915 (15.3) | 50,694 (11.0) | 1.39 | 1.31 to 1.48 |
| ≥30, <45 | 2036 (8.0) | 17,360 (3.8) | 1.21 | 1.12 to 1.30 |
A patient is considered to have recuperated or not had deterioration of kidney function when there is no decrease from the baseline eGFR to the eGFR after discharge > 10% of the baseline eGFR. When postdischarge creatinine was missing, postdischarge eGFR was calculated using imputed creatinine by multiple imputation; however, a baseline eGFR value was required to be included. A patient may have been included in the “no AKI” group because the ratio of the highest to lowest creatinine within the baseline period was <1.5 but still not included in this analysis when eGFR decrease was >10% when baseline and postdischarge eGFR values were compared.
aAdjusted for demographics, comorbidities and medication use, and hospitalization details. The reference is the “no AKI” group.
Both sensitivity analyses in which we included patients who were readmitted in the first 30 d after discharge or in which we did not include the latest inpatient creatinine value as a postdischarge value showed similar results to the main model (adjusted HR 1.40 [95% CI 1.38 to 1.42] and 1.44 [95% CI 1.42 to 1.46], respectively). The complete case analysis showed similar results (adjusted HR 1.33; 95% CI 1.31 to 1.35). The fourth sensitivity analysis, in which we limited the sample to survivors at 6 mo after discharge (n = 201,879) and started follow-up at that time, also showed a higher mortality risk associated with AKI (crude HR 1.19 [95% CI 1.18 to 1.21]; HR adjusted for demographics, comorbidities, and hospitalization details 1.14 [95% CI 1.12 to 1.16]; and fully adjusted HR 1.13 [95% CI 1.11 to 1.14]).
Discussion
This study evaluated long-term mortality risk with AKI not requiring dialysis in a large adult cohort with relatively long follow-up. To our knowledge, this is the first study to attempt to disentangle the direct association of AKI with mortality independent of the short-term loss of kidney function induced by AKI. We have shown that AKI is associated with a higher mortality risk in patients who survived for at least 90 d after hospital discharge and that the risk increases continuously with increasing severity of AKI.
The association of AKI and higher in-hospital mortality has been clearly demonstrated, and the magnitude of the association seems to depend on the population.2–16 Postdischarge mortality has also been evaluated in patients with severe AKI requiring renal replacement therapies.9 Our results are consistent with the few studies that reported long-term mortality in patients with less severe forms of AKI.3,5,12,15,19,20 In patients admitted to an intensive care unit, the 1-yr mortality was found to be higher in patients with mild and severe renal dysfunction but not with moderate dysfunction.3 In the same study, when the sample was restricted to patients who were discharged alive, the crude cumulative risk for death was more than two times higher in patients with moderate and severe AKI than in those without AKI (4.5, 11.4, and 12.6% for no AKI, moderate AKI, and severe AKI, respectively); however, those risks were not evaluated in a multivariate model and were not adjusted for kidney function after the event. Among elderly Medicare beneficiaries who were discharged alive after an acute myocardial infarction, patients with AKI had a 10 to 39% increased risk for death during a 10-yr period, depending on the severity and definition of AKI, compared with patients without AKI.19,20 AKI after percutaneous coronary intervention5,12 or cardiothoracic surgery21,22 has also been associated with long-term mortality. In another study, patients with temporary worsening of renal function after abdominal aortic aneurysm surgery (defined as a return to within 10% of the estimated baseline creatinine clearance 3 d after surgery) had 50% higher long-term mortality risk compared with those with no AKI.15 In two studies in which data on patients without AKI were not available, the 3-yr survival ranged from 50 to 73% in patients who were discharged alive.23,24 Early studies have shown that kidney function can remain impaired after AKI even when the creatinine had returned to baseline levels.25 This lower “kidney reserve” combined with an ongoing progressive damage caused by “rarefaction” of peritubular capillaries may be possible biologic pathways explaining our findings.26 Moreover, experimental studies have shown that AKI can injure other organs such as the heart and the lungs27–29; however, until further studies evaluate long-term eGFR decline and causes of death after AKI, these pathways underlying the long-term mortality risk remain speculative.
Our results showing an adjusted mortality risk increase from 1.36 to 1.59 with AKIN stage I to stage III is consistent with previous evaluations of the correlation between AKI classification and in-hospital mortality.11,13,21,30 This suggests that the predictive power of AKI classification is persistent with time, as we show in Figure 1. In one study, AKI classification did not predict 60-d and 6-mo mortality, but it was probably due to lack of statistical power.30
Because CKD is a strong predictor of mortality, we expected a dramatic lowering of the mortality risk estimate when postdischarge eGFR was added to the multivariate model, but we found that the HR dropped only from 1.45 to 1.41. The effect was greater in those with AKIN stage III (from 1.71 to 1.59), for which the proportion of patients with CKD induced by AKI is probably higher. Comparable results in the sample restricted to the patients who recovered or maintained their kidney function compared with the whole sample (1.41 versus 1.47) provide further evidence that adjustment for residual kidney function did work effectively in the main model.
The long-term mortality risk in patients who recover from AKI has not been studied extensively. In most studies in which renal recovery was assessed, it was defined as being dialysis independent, which has no bearing in our study population.31 As previously mentioned, a temporary worsening of kidney function was associated with mortality.15 Similarly, long-term mortality risks in patients who had AKI after cardiac surgery and recovered at discharge were previously found to be comparable to patients who did not recover.22 In another study, recovery of renal function occurred in 92.5% of the AKI cases.30 This is considerably higher than the 30.9% we found in our study, mostly because our definition of renal recovery was more restrictive.
Age is a known risk factor for AKI10,16; however, our results suggest that experiencing AKI is more strongly associated with mortality in younger than older age groups. This may be explained by the fact that inducing AKI in a younger individual requires a more severe insult than in an older one, and this severe insult is also probably associated with a higher risk for mortality despite extensive covariate adjustment. Moreover, because age is in itself a strong risk factor for mortality among all patients, AKI would have a smaller attributable fraction in older patients and therefore a smaller observed relative risk for mortality. For all of these reasons, various age distributions in different studies should be taken into account when comparing estimates of association between AKI and long-term mortality. A similar pattern to age can be seen with eGFR before AKI. Indeed, CKD is also recognized as a risk factor for AKI, and CKD stages are independently associated with mortality.18,32
Heterogeneous definitions of AKI are an important issue in research pertaining to AKI. Traditionally, large observational studies used International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes to identify AKI, but it is recognized that use of codes alone has poor sensitivity.1 Moreover, they depend on the provider to define AKI, and, without a standard clinical definition, this leads to heterogeneity among cases and inability to classify AKI severity beyond requirement for dialysis. Although the AKIN definition is now widely accepted in prospective or intensive care unit studies, it is not readily applicable to observational studies using health care or administrative databases, because timing of creatinine tests is variable and urine output is often not available. To mimic clinical practice, in which, before diagnosing AKI, physicians examine previous creatinine values before admission to assess baseline kidney function, we extend the period of the baseline creatinine assessment to 90 d before admission. This definition is probably slightly more sensitive than a definition limited to in-hospital values, because it increases the probability of finding a lower baseline value, mostly in patients in whom AKI started before hospitalization. This higher sensitivity may decrease the bias caused by misclassification of AKI, reducing the bias of the HR toward the null. This extended period for baseline creatinine assessment may also increase the likelihood of including less severe cases, which would dilute the overall effect. This is not a bias but only an effect of choosing different definitions of AKI; however, this dilution with less severe cases should not affect considerably the risk in the more severe AKI categories.
Our study has several limitations. Even though increasing evidence shows that AKI is independently associated with long-term mortality in different clinical settings, AKI is often viewed as a proxy of disease severity.27,33 Despite our careful and extensive adjustment for covariates, we cannot exclude the possibility of residual confounding as a result of the presence of an unmeasured confounder or measurement error on included factors. Similarly, sicker patients may have had more creatinine measurements, leading to a higher probability of being identified with AKI (ascertainment bias). We selected a 90-d period after discharge for assessing residual kidney function to optimize availability of at least one creatinine value in the period and limit the possibility that renal function would significantly improve after our assessment. Had we extended the period excessively, we would have had to exclude a greater number of patients who died during this period. It is known that kidney function continues to improve after hospital discharge, mainly in the first 3 to 6 mo24,31; therefore, it is possible that, for some patients, the latest creatinine value that we used in our models may not completely capture the new kidney function status. The long-term mortality association remained true in our sensitivity analysis at 6 mo, however, but the magnitude of the risk was lower, probably because of the highly selected population in this analysis (survivors and still followed at 6 mo) that counted for <25% of the main sample. Finally, because of the ascertainment period after discharge, the results of our study can be applied only to patients who survived at least 90 d after discharge.
In summary, we found that AKI not requiring dialysis is associated with an increased risk for postdischarge mortality for patients who survived ≥90 d after hospital discharge. This association is independent of residual kidney function after hospital discharge. The mortality risk is higher in more severe cases of AKI. Prospective studies that evaluate the clinical course (including changes in kidney function after 90 d) and optimal treatment of patients discharged after AKI are needed to improve outcomes.
Concise Methods
We conducted a retrospective cohort study of adult veterans receiving care in the US Department of Veterans Affairs (VA) health care system.
Data Sources
The VA provides care to >7 million veterans in the United States through an integrated national health care system. Data from electronic medical records are collected and maintained in local facilities using standardized methods and quality control protocols before being sent to regional and national centers. For this study, we used linked research data sets, containing patient characteristics, diagnosis and procedure codes, prescriptions dispensed, and laboratory test results, created as part of the Diabetes Epidemiology Cohorts.34,35 This is a national registry of linked VA and Medicare data going back more than a decade that was established by our research team. Previous work included evaluation of methods for disease identification, including diabetes,36 CKD,37 and other conditions.38–40 It also includes a death registry combining the VA Vital Status File41 with information from the Beneficiary Identification and Record Location files, a national database of veterans who applied for VA death benefits, VA inpatient records, Medicare files, and Social Security Administration files.
Cohort Definition
The study sample included all adult veterans who had at least one hospitalization between October 2000 and September 2005 and at least 1 yr of health care in the VA before that hospitalization. We defined the first hospitalization in this period as the index admission, with follow-up beginning 90 d after discharge (index date). We excluded patients who had dialysis treatments. Dialysis was defined as the presence of at least one of the following codes: ICD-9-CM diagnostic code V45.1, V56.0, V56.3, or V56.8; ICD-9-CM inpatient procedure code 39.95 or 54.98; or CPT-4 outpatient procedure code 90935, 90937, 90945, 90947, or 90999. We also excluded patients who died before the index date or were readmitted within 30 d after discharge (10.6 and 14.7% of those without AKI and with AKI, respectively) or had an eGFR <30 ml/min per 1.73 m2 before the index admission. This last exclusion criterion was applied because the performance of the AKI definition is not known in patients with stages 4 and 5 CKD.42 Patients were followed until the earliest of the following events: Leaving VA health care, end of the study (September 2005), or death.
Definition of AKI, Outcome, and Covariates
Each index admission was classified as “with AKI” or “no AKI.” Because no definition for AKI is widely accepted for retrospective research involving databases, we adapted the AKI definition recently proposed by the AKIN. This definition classifies AKI according to change in creatinine values and urine output and has been validated with hospitalized patients.13,42 Using the AKIN definition, we identified and classified AKI within the hospitalization by comparing the highest creatinine value found between the admission and discharge dates with the lowest value recorded between 3 mo before the index admission and the discharge date (baseline creatinine). The baseline creatinine assessment period was extended to include outpatient values before admission, because the AKI process may have started before admission and this would have led to an elevated creatinine value at admission. When the ratio of those two values was ≥1.5, the hospitalization was classified as “with AKI.” AKI events were further classified by AKIN stage (I, II, and III) according to this ratio (1.5 to 2, 2 to 3, and >3, respectively). When the creatinine increased by ≥0.5 mg/dl and reached at least 4.0 mg/dl, the hospitalization was also classified as “with AKI” (AKIN stage III). Dialysis-requiring AKI events were excluded from this study because we excluded patients who received dialysis treatments before the index date (90 d after discharge; see the Cohort Definition section). A primary goal of this study was to estimate mortality risk with adjustment for residual kidney function after discharge, and including dialysis-requiring AKI and its various clinical courses would have made this more difficult. The primary outcome in this study was all-cause mortality.
Covariates included demographics, comorbidities, medication use, index admission details (primary diagnosis of admission, mechanical ventilation use, and length of stay), and postdischarge eGFR categories. Concurrent diseases were defined using ICD-9-CM codes found in the medical records in the 2 yr before the index admission date. The patient was considered exposed to a specific drug when a prescription for that drug was dispensed in the 6 mo before the index admission date. The primary diagnosis of admission was categorized by using ICD-9-CM main categories (infectious and parasitic diseases, neoplasms, diseases of the circulatory system, diseases of the respiratory system, diseases of the digestive system, or other causes). Mechanical ventilation was used as a proxy for intensive care and disease severity.
In accordance with the National Kidney Foundation Kidney Disease Outcomes Quality Initiative (KDOQI) guideline, eGFR was calculated with the four-variable version of the Modification of Diet in Renal Disease equation.43,44 eGFR after discharge was calculated using the most recent creatinine value before the index date and then categorized following the KDOQI CKD classification (≥90, 60 to 89, 30 to 59, 15 to 29, or <15 ml/min per 1.73 m2).43 When no creatinine value was found in the 3 mo after discharge, the last creatinine measured during the hospitalization was taken. Not doing so would bias the results, because it is expected that a patient with a normal creatinine level before discharge would not have a control value in the next 3 mo. To evaluate the mortality risk in patients who had renal recovery (for the group with AKI) or who had maintained their kidney function (for group with no AKI), we calculated the percentage of eGFR decrease between baseline and after discharge ([baseline eGFR − eGFR after discharge]/baseline eGFR). We defined renal recovery or maintenance of kidney function as a percentage of eGFR decrease ≤10%.
Statistical Analysis
All statistical analyses were conducted using SAS 9.2 (SAS Institute, Cary, NC). Continuous variables are summarized with mean and SD or median with first and third quartiles. HRs of mortality were estimated using multivariable Cox proportional hazard regression models adjusting for demographics, comorbidities, medication use, index hospitalization details, and postdischarge eGFR categories. Use of continuous variables, such as creatinine or continuous eGFR, instead of eGFR categories for postdischarge kidney function gave similar results. A multiple imputation technique including all available variables was used in all analyses to impute missing creatinine values after discharge (11.2%).45 The proportional hazards assumption was verified using log-log survival plots. Unadjusted Kaplan-Meier survival curves were compared using a log-rank test. We conducted four sensitivity analyses: (1) Including patients who were readmitted in the first 30 d after discharge, (2) not including the last inpatient creatinine value when no creatinine value was found in the postdischarge period, (3) a complete case analysis in which only patients with actual baseline and postdischarge (not using the latest inpatient value or imputation) eGFR measurements were included, and (4) limiting the sample to survivors at 6 mo after discharge and initiating follow-up at that time point. The project was approved by the institutional review board at the VA Medical Center in (Bedford, MA), and patient informed consent was waived.
Disclosures
D.R.M. has received research grant funds (but not salary support) from Sanofi-Aventis.
Supplementary Material
Acknowledgments
J.-P.L. is supported by a KRESCENT Fellowship. The study was supported by a VA research grant.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Waikar SS, Wald R, Chertow GM, Curhan GC, Winkelmayer WC, Liangos O, Sosa M-A, Jaber BL: Validity of International Classification of Diseases, Ninth Revision, Clinical Modification Codes For Acute Renal Failure. J Am Soc Nephrol 17: 1688–1694, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Hou SH, Bushinsky DA, Wish JB, Cohen JJ, Harrington JT: Hospital-acquired renal insufficiency: A prospective study. Am J Med 74: 243–248, 1983 [DOI] [PubMed] [Google Scholar]
- 3.Bagshaw SM, Mortis G, Doig CJ, Godinez-Luna T, Fick GH, Laupland KB: One-year mortality in critically ill patients by severity of kidney dysfunction: a population-based assessment. Am J Kidney Dis 48: 402–409, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Clermont G, Acker CG, Angus DC, Sirio CA, Pinsky MR, Johnson JP: Renal failure in the ICU: Comparison of the impact of acute renal failure and end-stage renal disease on ICU outcomes. Kidney Int 62: 986–996, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Gupta R, Gurm HS, Bhatt DL, Chew DP, Ellis SG: Renal failure after percutaneous coronary intervention is associated with high mortality. Catheter Cardiovasc Interv 64: 442–448, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Lameire N, Van Biesen W, Vanholder R: Acute renal failure. Lancet 365: 417–430, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Liangos O, Wald R, O'Bell JW, Price L, Pereira BJ, Jaber BL: Epidemiology and outcomes of acute renal failure in hospitalized patients: A National Survey. Clin J Am Soc Nephrol 1: 43–51, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Liano F, Pascual J: Epidemiology of acute renal failure: A prospective, multicenter, community-based study. Madrid Acute Renal Failure Study Group. Kidney Int 50: 811–818, 1996 [DOI] [PubMed] [Google Scholar]
- 9.Morgera S, Schneider M, Neumayer HH: Long-term outcomes after acute kidney injury. Crit Care Med 36: S193–S197, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Nash K, Hafeez A, Hou S: Hospital-acquired renal insufficiency. Am J Kidney Dis 39: 930–936, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Ostermann M, Chang RRiyadh ICU Program Users Group: Correlation between the AKI classification and outcome. Crit Care 12: R144, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roghi A, Savonitto S, Cavallini C, Arraiz G, Angoli L, Castriota F, Bernardi G, Sansa M, De Servi S, Pitscheider W, Danzi GB, Reimers B, Klugmann S, Zaninotto M, Ardissino D: Impact of acute renal failure following percutaneous coronary intervention on long-term mortality. J Cardiovasc Med (Hagerstown) 9: 375–381, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C: An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med 34: 1913–1917, 2006 [DOI] [PubMed] [Google Scholar]
- 14.Waikar SS, Curhan GC, Wald R, McCarthy EP, Chertow GM: Declining mortality in patients with acute renal failure, 1988 to 2002. J Am Soc Nephrol 17: 1143–1150, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Welten GM, Schouten O, Chonchol M, Hoeks SE, Feringa HH, Bax JJ, Dunkelgrün M, van Gestel YR, van Domburg RT, Poldermans D: Temporary worsening of renal function after aortic surgery is associated with higher long-term mortality. Am J Kidney Dis 50: 219–228, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Xue JL, Daniels F, Star RA, Kimmel PL, Eggers PW, Molitoris BA, Himmelfarb J, Collins AJ: Incidence and mortality of acute renal failure in Medicare beneficiaries, 1992 to 2001. J Am Soc Nephrol 17: 1135–1142, 2006 [DOI] [PubMed] [Google Scholar]
- 17.Cerda J, Lameire N, Eggers P, Pannu N, Uchino S, Wang H, Bagga A, Levin A: Epidemiology of acute kidney injury. Clin J Am Soc Nephrol 3: 881–886, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Newsome BB, Warnock DG, McClellan WM, Herzog CA, Kiefe CI, Eggers PW, Allison JJ: Long-term risk of mortality and end-stage renal disease among the elderly after small increases in serum creatinine level during hospitalization for acute myocardial infarction. Arch Intern Med 168: 609–616, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Parikh CR, Coca SG, Wang Y, Masoudi FA, Krumholz HM: Long-term prognosis of acute kidney injury after acute myocardial infarction. Arch Intern Med 168: 987–995, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Hobson CE, Yavas S, Segal MS, Schold JD, Tribble CG, Layon AJ, Bihorac A: Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation 119: 2444–2453, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Loef BG, Epema AH, Smilde TD, Henning RH, Ebels T, Navis G, Stegeman CA: Immediate postoperative renal function deterioration in cardiac surgical patients predicts in-hospital mortality and long-term survival. J Am Soc Nephrol 16: 195–200, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Stevens PE, Tamimi NA, Al-Hasani MK, Mikhail AI, Kearney E, Lapworth R, Prosser DI, Carmichael P: Non-specialist management of acute renal failure. QJM 94: 533–540, 2001 [DOI] [PubMed] [Google Scholar]
- 24.Liano F, Felipe C, Tenorio MT, Rivera M, Abraira V, Saez-de-Urturi JM, Ocana J, Fuentes C, Severiano S: Long-term outcome of acute tubular necrosis: A contribution to its natural history. Kidney Int 71: 679–686, 2007 [DOI] [PubMed] [Google Scholar]
- 25.Bull G, Joekes A, Lowe K: Renal function studies in acute tubular necrosis. Clin Sci 9: 379–404, 1950 [PubMed] [Google Scholar]
- 26.Basile DP, Donohoe D, Roethe K, Osborn JL: Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol 281: F887–F899, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR: Long-term risk of mortality and other adverse outcomes after acute kidney injury: A systematic review and meta-analysis. Am J Kidney Dis 53: 961–973, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelly KJ: Distant effects of experimental renal ischemia/reperfusion injury. J Am Soc Nephrol 14: 1549–1558, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Kramer AA, Postler G, Salhab KF, Mendez C, Carey LC, Rabb H: Renal ischemia/reperfusion leads to macrophage-mediated increase in pulmonary vascular permeability. Kidney Int 55: 2362–2367, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Ali T, Khan I, Simpson W, Prescott G, Townend J, Smith W, Macleod A: Incidence and outcomes in acute kidney injury: A comprehensive population-based study. J Am Soc Nephrol 18: 1292–1298, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Macedo E, Bouchard J, Mehta RL: Renal recovery following acute kidney injury. Curr Opin Crit Care 14: 660–665, 2008 [DOI] [PubMed] [Google Scholar]
- 32.Hsu CY, Ordonez JD, Chertow GM, Fan D, McCulloch CE, Go AS: The risk of acute renal failure in patients with chronic kidney disease. Kidney Int 74: 101–107, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bagshaw SM: The long-term outcome after acute renal failure. Curr Opin Crit Care 12: 561–566, 2006 [DOI] [PubMed] [Google Scholar]
- 34.Miller DR, Pogach L: Longitudinal approaches to evaluate health care quality and outcomes: The Veterans Health Administration Diabetes Epidemiology Cohort. J Diabetes Sci Technol 2: 24–32, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boyko EJ, Koepsell TD, Gaziano JM, Horner RD, Feussner JR: US Department of Veterans Affairs medical care system as a resource to epidemiologists. Am J Epidemiol 151: 307–314, 2000 [DOI] [PubMed] [Google Scholar]
- 36.Miller DR, Safford MM, Pogach LM: Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care 27[ Suppl 2]: B10–B21, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Kern EF, Maney M, Miller DR, Tseng CL, Tiwari A, Rajan M, Aron D, Pogach L: Failure of ICD-9-CM codes to identify patients with comorbid chronic kidney disease in diabetes. Health Serv Res 41: 564–580, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Borzecki AM, Wong AT, Hickey EC, Ash AS, Berlowitz DR: Identifying hypertension-related comorbidities from administrative data: What's the optimal approach? Am J Med Qual 19: 201–206, 2004 [DOI] [PubMed] [Google Scholar]
- 39.Kashner TM: Agreement between administrative files and written medical records: A case of the Department of Veterans Affairs. Med Care 36: 1324–1336, 1998 [DOI] [PubMed] [Google Scholar]
- 40.Miller DR, Rogers WH, Kazis LE, Spiro A, 3rd, Ren XS, Haffer SC: Patients' self-report of diseases in the Medicare Health Outcomes Survey based on comparisons with linked survey and medical data from the Veterans Health Administration. J Ambul Care Manage 31: 161–177, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Arnold N, Sohn MW, Maynard C, Hynes DM: VIReC Technical Report 2: VA-NDI Mortality Data Merge Project, Hines, IL, VA Information Resource Center, 2006 [Google Scholar]
- 42.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A: Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit Care 11: R31, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39: S1–S266, 2002 [PubMed] [Google Scholar]
- 44.Levey AS, Greene T, Kusek J, Beck G: A simplified equation to predict glomerular filtration rate from serum creatinine [Abstract]. J Am Soc Nephrol 11: 155A, 2000 [Google Scholar]
- 45.Ake CF, Carpenter AL: Survival Analysis with PREG: Using MI and MIANALYZE to accommodate missing data. In: Proceedings of the 10th Annual Western Users of SAS Software Regional Users Group Conference, Cary, NC, SAS Institute, 2002, pp 102–107 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.