Abstract
Renal disease leads to perturbations in calcium and phosphate homeostasis and vitamin D metabolism. Dietary fructose aggravates chronic kidney disease (CKD), but whether it also worsens CKD-induced derangements in calcium and phosphate homeostasis is unknown. Here, we fed rats diets containing 60% glucose or fructose for 1 mo beginning 6 wk after 5/6 nephrectomy or sham operation. Nephrectomized rats had markedly greater kidney weight, blood urea nitrogen, and serum levels of creatinine, phosphate, and calcium-phosphate product; dietary fructose significantly exacerbated all of these outcomes. Expression and activity of intestinal phosphate transporter, which did not change after nephrectomy or dietary fructose, did not correlate with hyperphosphatemia in 5/6-nephrectomized rats. Intestinal transport of calcium, however, decreased with dietary fructose, probably because of fructose-mediated downregulation of calbindin 9k. Serum calcium levels, however, were unaffected by nephrectomy and diet. Finally, only 5/6-nephrectomized rats that received dietary fructose demonstrated marked reductions in 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 levels, despite upregulation of 1α-hydroxylase. In summary, excess dietary fructose inhibits intestinal calcium absorption, induces marked vitamin D insufficiency in CKD, and exacerbates other classical symptoms of the disease. Future studies should evaluate the relevance of monitoring fructose consumption in patients with CKD.
In the past 20 yr, the cases of end stage renal disease (ESRD) in the United States quadrupled to 382,000 in 2000, and its prevalence is expected to increase by 85% to 713,000 patients in 2015, so reducing the incidence of ESRD is widely recognized as a major public health goal.1 When patients have been identified as having the major risk factors (diabetes, hypertension, and uricemia) for rapid progression from chronic kidney disease (CKD) to ESRD,2,3 efforts are made to slow the rate of progression, and these efforts involve tight management of these risk factors. Dietary fructose may be a major contributor to the rapid development of all of these chronic diseases considered risk factors for ESRD. For example, diabetes via hyperinsulinemia, hypertriglyceridemia via hepatic lipogenesis,4,5 and hypertension via uricemia6,7 each are exacerbated by excessive fructose consumption. Despite these documented linkages to symptoms of metabolic syndrome, fructose consumption instead has increased in the overall population and is not restricted or even monitored in patients with CKD. The average rate of fructose consumption is now approximately 80 g/d in the United States.4,8,9 In addition, more recent work indicated a strong correlation between excessive fructose consumption and renal disease. Chronic fructose but not glucose or starch consumption in 5/6-nephrectomized (5/6Nx) rats leads to a marked decrease in creatinine clearance as well as to sharp increases in blood urea nitrogen (BUN), renal mass index, and histologic biomarkers of renal pathology.10 Fructose-induced pathology seems to be caused by increases in levels of oxidative stress and inflammation, as demonstrated in immortalized proximal tubular cells (HK-2) incubated in fructose.11
Other complications of CKD, such as osteodystrophy, hyperphosphatemia, hyperparathyroidism, and deficiency in 1,25-dihydroxyvitamin D3 [1,25-(OH)2D3] levels,12–14 may also be affected by dietary fructose. For example, dietary fructose perturbs intestinal phosphate (Pi)15 and calcium (Ca) absorption16 as well as Ca and Pi balance.17,18 Excessive consumption of carbonated beverages known to contain high-fructose corn syrups have been strongly associated with increased incidence of bone fractures in the adolescent population.19 It seems clear from these studies that fructose could exacerbate the mineral imbalance usually associated with CKD; however, the studies investigating fructose effects on mineral metabolism have focused on young, actively growing individuals whereas ESRD and CKD are diseases that generally affect the aged population.
We first tested the hypothesis that dietary fructose consumption exacerbates the classical symptoms of CKD that can lead to ESRD. Using sham-operated rats as controls, we allowed 5/6Nx rats to develop clear symptoms of CKD for 6 wk, then subsequently fed either a high-fructose or -glucose diet for an additional 4 wk and determined the classical blood and bone markers of renal disease. No study has related fructose consumption to vitamin D metabolism, yet the liver and the kidney, the main sites of vitamin D synthesis, are organs adversely affected by both chronic fructose consumption20,21 and renal failure10,11; therefore, we next tested the hypothesis that fructose consumption aggravates the already adverse effects of ESRD on the synthesis of the major active form of vitamin D, 1,25-(OH)2D3. Because 1,25-(OH)2D3 regulates intestinal Ca and Pi absorption,22,23 we also examined rates of transepithelial transport of these minerals as well as determined expression levels of transporters and proteins involved in their uptake. Expression and activity levels of sugar transporters were also monitored as positive control because these transporters are affected by dietary sugars and as negative control because they are not affected by nephrectomy.
Results
Biomarkers of CKD
Rats (250 to 275 g) were randomly assigned to either the group that underwent 5/6 nephrectomy or the group that had the sham operation. Compared with sham-operated rats, 5/6Nx rats had a slower (approximately 10%) increase in body weight after surgery (Table 1); however, daily food intake relative to body weight was similar between the two groups. BUN and creatinine levels increased by approximately 50% in 5/6Nx compared with sham-operated rats 2 and 6 wk after surgery. Pi level in the blood was not significantly different between 5/6Nx and sham-operated rats at wk 2; however, after 6 wk, the 5/6Nx rats started to develop hyperphosphatemia (P = 0.08). In contrast, serum Ca concentrations were similar between 5/6Nx and sham-operated rats at 2 or 6 wk after surgery.
Table 1.
Clinical and biochemical parameters in 5/6Nx and sham-operated rats 2 and 6 weeks after surgery
| Parameter | 2 Wk |
6 Wk |
Significance |
||||
|---|---|---|---|---|---|---|---|
| Sham | 5/6Nx | Sham | 5/6Nx | Surgery | Time after Surgery | Surgery × Time Interaction | |
| Food type | Chow | Chow | Chow | Chow | |||
| Body weight (g) | 399 ± 9 | 361 ± 6 | 526 ± 12 | 459 ± 14 | <0.001 | <0.001 | 0.205 |
| Food intake (g/d per kg body wt) | 68 ± 9 | 66 ± 8 | 88 ± 12 | 90 ± 14 | 0.981 | <0.001 | 0.723 |
| BUN (mg/dl) | 27.9 ± 3.7 | 54.3 ± 5.6 | 22.7 ± 1.8 | 51.2 ± 4.3 | <0.001 | 0.261 | 0.775 |
| Creatinine (mg/dl) | 0.46 ± 0.07 | 0.71 ± 0.11 | 0.43 ± 0.15 | 0.86 ± 0.02 | <0.001 | 0.358 | 0.958 |
| Pi (mg/dl) | 6.4 ± 0.1 | 6.7 ± 0.2 | 6.3 ± 0.2 | 7.0 ± 0.3 | 0.077 | 0.708 | 0.389 |
| Ca (mg/dl) | 10.4 ± 0.3 | 9.8 ± 0.1 | 10.7 ± 0.5 | 9.8 ± 0.1 | 0.120 | 0.442 | 0.959 |
Data are means ± SEM ; n = 10 per group. Statistical significance refers to two-way ANOVA.
Table 2 shows the body and blood parameters at week 10 after surgery, after the rats were fed glucose or fructose diets for 4 wk. The diets had no significant effect on body weight and food intake; however, 5/6Nx rats had significantly lower body weights compared with sham-operated rats. The kidney mass index increased with 5/6 nephrectomy, and within the 5/6Nx group, the rats fed fructose (5/6Nx-F) had a significantly higher kidney mass than rats fed glucose (5/6Nx-G). 5/6Nx rats had greater BUN and creatinine serum levels than sham-operated rats, and both biomarkers of CKD were significantly exacerbated by fructose feeding. After 10 wk, 5/6 nephrectomy also induced a significant hyperphosphatemia that likewise was significantly aggravated by dietary fructose. Even in the absence of significant effects of the diet and nephrectomy on serum Ca, the Pi-Ca product ([Pi] × [Ca]) was significantly higher in 5/6Nx than in sham-operated rats, with a tendency to increase further with fructose feeding (P = 0.09, one-way ANOVA least significant difference [LSD]). Serum fructose was detectable in our samples only in fructose-fed rats, and the concentration was highest in 5/6Nx-F.
Table 2.
Clinical and biochemical parameters in 5/6Nx and sham-operated rats 10 wk after surgery and after 4 wk of special sugar diet
| Parameter | Sham |
5/6Nx |
Significance |
|||||
|---|---|---|---|---|---|---|---|---|
| Two-Way |
One-Way | |||||||
| Glucose | Fructose | Glucose | Fructose | Diet | Surgery | Diet × Surgery | ||
| Body weight (g) | 644 ± 26 | 596 ± 16a,b | 505 ± 39c | 538 ± 29b,c | 0.959 | 0.005 | 0.242 | 0.034 |
| Food intake (g/d/kg body wt) | 86 ± 7 | 84 ± 8 | 80 ± 4 | 83 ± 7 | 0.951 | 0.450 | 0.548 | 0.311 |
| Kidney mass index (g/kg body wt) | 3.02 ± 0.20a | 3.05 ± 0.20a | 4.21 ± 0.20b | 5.39 ± 0.40c | 0.086 | 0.002 | 0.105 | 0.007 |
| BUN (mg/dl) | 13.9 ± 7.2a | 28.7 ± 8.7a,b | 40.1 ± 6.2b | 56.0 ± 3.1c | 0.022 | 0.0002 | 0.554 | 0.0004 |
| Creatinine (mg/dl) | 0.72 ± 0.11a | 0.75 ± 0.05a | 1.13 ± 0.04b | 1.45 ± 0.07c | 0.146 | 0.0004 | 0.197 | 0.0005 |
| Pi (mg/dl) | 6.3 ± 0.2a | 6.1 ± 0.2a | 7.1 ± 0.5a | 9.1 ± 0.6b | 0208 | 0.016 | 0.143 | 0.017 |
| Ca (mg/dl) | 10.1 ± 0.6 | 8.8 ± 1.1 | 11.2 ± 0.3 | 11.5 ± 0.3 | 0.521 | 0.090 | 0.527 | 0.211 |
| [Pi] × [Ca] (mg2/dl2) | 64 ± 5a | 54 ± 7a | 81 ± 7a,b | 102 ± 10b | 0.520 | 0.005 | 0.117 | 0.007 |
| Fructose (mM) | ND | 1.4 ± 0.6 | ND | 3.9 ± 1.6 | 0.027 | 0.274 | 0.274 | 0.120 |
Data are means ± SEM; n = 4 to 5 per group. Superscript letters refer to results of LSD post hoc tests after one-way ANOVA (P < 0.05). Means with different superscript letters are significantly different. ND, not detectable.
Sugar, Pi, and Ca Transporter Activity and Expression after 4 Wk of Fructose Feeding
Sugars.
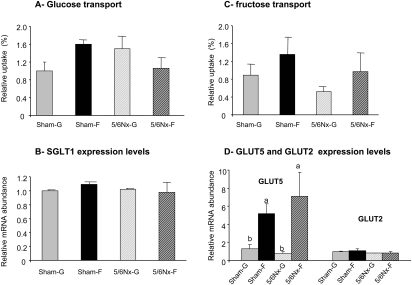
The intestinal uptake of glucose was independent of nephrectomy (P > 0.8) and diet (P > 0.7; Figure 1A). Fructose feeding induced a significant increase in fructose uptake (P < 0.05; Figure 1C), resulting from a specific increase in GLUT5 but not GLUT2 expression (P < 0.01; Figure 1D). The expression level of the Na+/glucose co-transporter (SGLT1) remained unchanged (Figure 1B). The 5/6 nephrectomy did not affect the rate of fructose uptake and the expression of GLUT5 and GLUT2 (P > 0.8).
Figure 1.
Dietary sugar effects on the regulation of intestinal glucose and fructose absorption in sham-operated and 5/6Nx rats. (A and C) Glucose (A) and fructose (C) uptakes were measured from the jejunum of sham-operated or 5/6Nx rats that had been fed a glucose or fructose diet for 4 wk. (B and D) The effects of dietary sugar and nephrectomy were measured on the mRNA levels of the intestinal apical glucose transporter, SGLT 1 (B), and the intestinal apical fructose transporter, GLUT5 and the basolateral glucose and fructose transporter GLUT2 (D), by real-time PCR using EF1α as a reference gene. Data are expressed relative to the level seen in Sham-G rats (Sham-G = 1.0). Data are means ± SEM (n = 4 to 5 per group). For glucose uptakes and for SGLT1 and GLUT2 expression, a two-way ANOVA showed no significant diet or surgery effects. For fructose uptake and GLUT5 mRNA, there were significant diet but not nephrectomy effects by two way ANOVA; a,bSignificant differences (P < 0.05).
Phosphate.
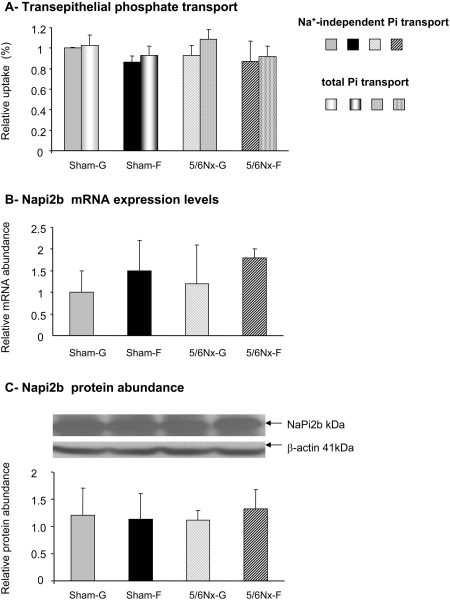
Pi uptake in the jejunum is mainly Na+ independent (Figure 2A). Thus, the active Na+-dependent Pi transport system mediated by NaPi2b contributes little to total Pi transport in adult rats, as would be expected from the dramatic decrease in NaPi2b expression with age that we15 and others24 found in rats. Total (P > 0.8) and Na+-independent (P > 0.8) Pi uptake did not change in sham-operated compared with 5/6Nx rats. Likewise, dietary sugar did not affect total (P > 0.3) Na+-independent (P > 0.5) Pi uptake. NaPi2b mRNA levels were not affected by 5/6 nephrectomy or dietary sugar (P > 0.8 for both factors; Figure 2B), and so were NaPi2b protein levels (P > 0.7 for both factors; Figure 2C). In this study, we used older rats exhibiting insignificant rates of active Na+-dependent Pi transport, and this minor transport pathway must not be regulated by fructose, as was observed in weaning pups.15,25
Figure 2.
Dietary sugar effects on the regulation of intestinal Pi absorption in sham-operated and 5/6Nx rats. Pi transport was measured using everted intestinal sacs formed from the jejunum of sham or 5/6Nx rats fed glucose or fructose diet for 4 wk. (A) The total and Na+-independent Pi transport from the apical to the basolateral compartments was expressed as a ratio of the final quantity of (33Pi inside/33Pi outside) of the everted gut sacs. Data are means ± SEM (n = 4 to 5 per group). (B) NaPi2b mRNA expression was analyzed by real-time PCR using EF1α as a reference. (C) The expression level of NaPi2b protein was analyzed by Western blot, and the protein abundance was expressed as the ratio of NaPi2b to β-actin. Data are normalized relative to levels seen in Sham-G rats (Sham-G = 1.0). A two-way ANOVA did not show significant diet and surgery effects.
Calcium.
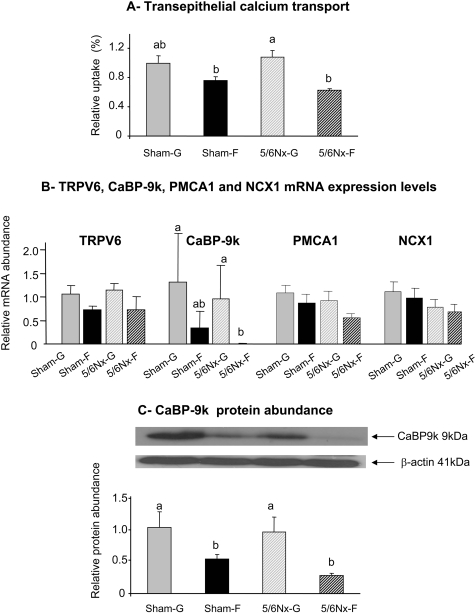
Fructose feeding induced a highly significant (P < 0.004) decrease in intestinal Ca uptake (Figure 3A). Interestingly, there was no effect of nephrectomy on intestinal Ca uptake (P > 0.7). In sham-operated (P = 0.08 by one-way ANOVA LSD) and 5/6Nx (P < 0.05 by one-way ANOVA LSD) rats, there was a 23 and 42% decrease, respectively, in Ca transport with fructose feeding.
Figure 3.
Dietary sugar effects on the regulation of intestinal Ca transport in sham-operated and 5/6Nx rats. Ca transport was measured using everted intestinal sacs formed from the duodenum of sham or 5/6Nx rats that had been fed glucose or fructose diet for 4 wk. (A) Trans epithelial Ca transport from the apical to the basolateral compartment was expressed as a ratio of the final quantity of (45Ca inside/45Ca outside) of the everted gut sacs. (B) mRNA expression of TRPV6, CaBP-9k, PMCA1, and NCX1 was analyzed by real-time PCR using EF1α as a reference. (C) The protein abundance of CaBP-9k was expressed as the ratio of calbindin to β-actin protein. All data were normalized relative to levels seen in Sham-G rats (Sham-G = 1.0). Data are means ± SEM (n = 4 to 5 per group). Differences (P < 0.05) among means, indicated by differences in superscript letters, were analyzed by one-way ANOVA LSD after a two-way analysis showed significant diet or surgery effects.
Nephrectomy (P > 0.8) and dietary fructose (P = 0.1) had no effect on mRNA expression of TRPV6 (Ca transporter 1), which mediates the uptake of dietary Ca from the lumen across the apical membrane into the cytosol (Figure 3B). Dietary fructose (P < 0.05) but not nephrectomy (P > 0.10) significantly decreased the mRNA expression of the intestinal intracellular Ca binding protein 9k (CaBP-9k) that mediates the transfer of dietary Ca from the apical to the basolateral pole of the enterocyte. By one-way ANOVA LSD, CaBP-9k expression in 5/6Nx-F was less than that in sham-operated rats that were fed glucose (Sham-G) and 5/6Nx-G (P < 0.05). The mRNA expression levels of the two basolateral Ca transporters, Ca-ATPase 1 (PMCA1) and the Na/Ca exchanger 1 (NCX1), each were not affected by nephrectomy (P > 0.6 and P > 0.5, respectively) or by diet (P = 0.09 and P > 0.2, respectively). Dietary fructose (P < 0.005) but not nephrectomy (P > 0.4) diminished the protein expression of CaBP-9k, which decreased by 50% in sham-F compared with sham-G (P < 0.05, one-way ANOVA LSD) and by 70% in 5/6Nx-F compared with 5/6Nx-G (P < 0.02, one-way ANOVA LSD; Figure 3C). No specific antibody for TRPV6 is available. Likewise, after several attempts, we found no suitable antibody for PMCA1 and NCX1.
Vitamin D
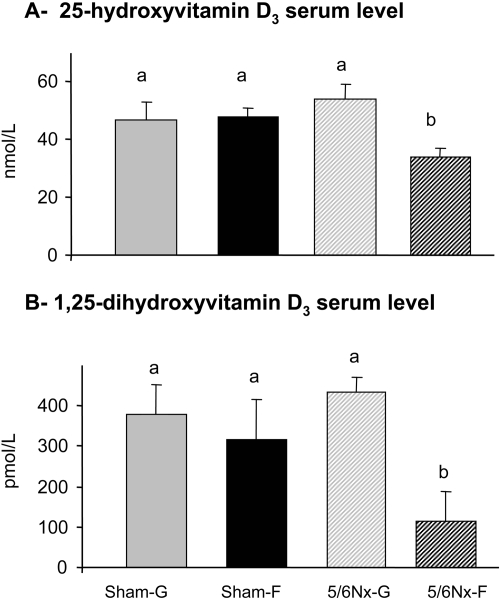
The serum concentration of 25-hydroxyvitamin D3 [25-(OH)D3]was not significantly altered by dietary fructose (P = 0.07 by two-way ANOVA) and by nephrectomy (P > 0.5; Figure 4A); however, the significant interaction (P = 0.05) indicated that the effect of diet depended on the type of surgery. Indeed, when analyzed by one-way ANOVA LSD, there clearly was a significantly lower 25-(OH)D3 serum level (by 30 to 40%) in 5/6Nx-F rats compared with those in the other three groups (P < 0.05). Diet (P < 0.02) but not nephrectomy (P > 0.1) affected the levels of 1,25-(OH)2D3 in the serum. Subsequent one-way ANOVA LSD indicated that the 5/6Nx-F rats had 2.5 to four-fold lower 1,25-(OH)2D3 serum levels than those of the other three groups (P ≤ 0.05; Figure 4B). Hence, dietary fructose decreased 25-(OH)D3 and 1,25-(OH)2D3 only in 5/6Nx rats.
Figure 4.
Dietary sugar effects on levels of vitamin D in the serum. (A) Levels of 25-(OH)D3 and (B) 1,25-(OH)2D3 in the serum of sham and 5/6Nx rats that had been fed glucose or fructose diet for 4 wk. Data are means ± SEM (n = 4 to 5 per group). Differences among means, indicated by differences in letters, were analyzed by one-way ANOVA LSD after a two-way analysis showed significant diet and surgery effects (P < 0.05).
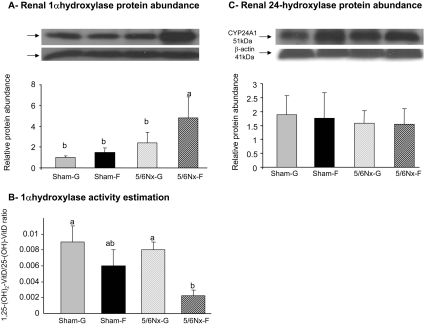
In the kidney, the protein expression level of 1α-hydroxylase [CYP27B1, which mediates the 1α-hydroxylation of 25-(OH)D3 to 1,25-(OH)2D3] is significantly affected by diet (P < 0.005) but not by surgery (P > 0.3; Figure 5A). By one-way ANOVA, 1α-hydroxylase protein abundance increased (two- to five-fold) significantly in the 5/6Nx-F group compared with that in the other three groups (P ≤ 0.05; Figure 5A). The relative 1α-hydroxylase activity, evaluated by the ratio [1,25-(OH)2D3/25-(OH)D3] shows the same pattern as that of serum levels of 1,25-(OH)2D3: The ratio [1,25-(OH)2D3/25-(OH)D3] was significantly affected by diet (P < 0.04) but not by surgery (P > 0.1; Figure 5B). In contrast, the protein level of 24-hydroxylase [CYP24A1 involved in the degradative pathway of 25-(OH)D3 and 1,25-(OH)2D3] remained similar among the four groups (Figure 5C).
Figure 5.
Dietary sugar effects on protein levels of hydroxylases involved in vitamin D metabolism. (A and C) Protein expression level of 1α-hydroxylase (CYP27B1; A) and of 24-hydroxylase (CYP24A1; C) was analyzed on the whole kidney. (B) Relative 1α-hydroxylase activities expressed as ratio of 1,25-(OH)2D3/25-(OH)D3 in sham or 5/6Nx rats that had been fed glucose or fructose diet for 4 wk. The protein abundance values corresponded to a ratio of CYP27B1 (A) or CYP24A1 (B) to β-actin, and the data are normalized relative to levels seen in Sham-G rats (Sham-G = 1.0). Data are means ± SEM (n = 4 to 5 per group). Differences among means, indicated by differences in letters, were analyzed by one-way ANOVA LSD after a two-way analysis showed significant diet and surgery effects (P < 0.05).
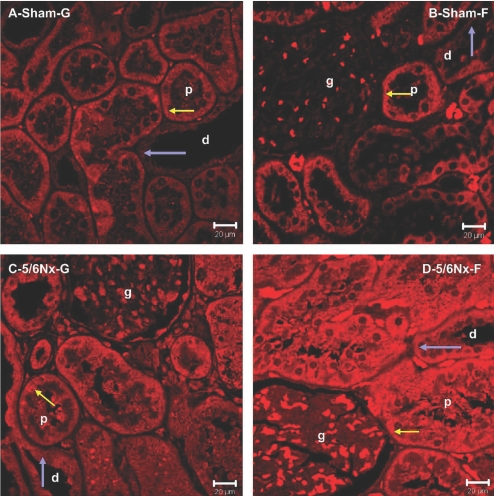
Renal Histology and 1α-Hydroxylase Immunohistology
1α-Hydroxylase is expressed at high levels only in the proximal tubule of normal kidneys (Figure 6, A and B, yellow arrow for proximal tubule, which is brighter red compared with the distal tubule [blue arrow]). In 5/6Nx rats, however, 1α-hydroxylase becomes equally expressed in both proximal and distal tubules (Figure 6, C and D, yellow arrow versus blue arrow), suggesting that distal tubular cells were recruited to express 1α-hydroxylase. The intensity of the staining in both the proximal and distal tubules is higher in the 5/6Nx-F rats (Figure 6D, yellow and blue arrows, respectively) than in the other three groups.
Figure 6.
1α-Hydroxylase (CYP27B1) is immunolocalized in kidney of sham or 5/6Nx rats fed glucose or fructose diets. (A through D) Analysis of 1α-hydroxylase expression in the distal tubules (d), proximal tubules (p), and glomerulus (g) in the Sham-G rats (A), Sham-F rats (B), 5/6Nx-G rats (C), and 5/6Nx-F rats (D). Yellow arrows indicate 1α-hydroxylase occupying the basolateral cytosolic pole of cells lining the proximal tubules (A through D). Blue arrows indicate the absence of 1α-hydroxylase in the cells of the distal tubules in sham rats (A and B) or its presence in the distal tubules of 5/6Nx rats (C and D).
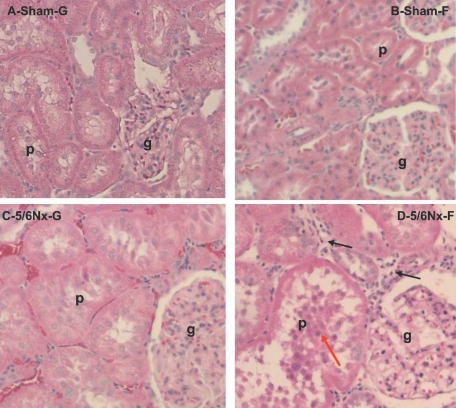
In association with the increase in kidney mass index, hematoxylin and eosin staining showed significantly larger and seemingly more deformed glomeruli and proximal tubules in 5/6Nx (Figure 7, C and D) compared with sham-operated (Figure 7, A and B) rats. The increase in proximal tubule diameter has been shown to originate from hypertrophy and not hyperplasia of tubular cells.26 There may be a greater incidence of proximal tubular damage as well as an increase in cellular debris in the tubular lumen (Figure 7, D versus C, red arrow) of 5/6Nx-F rats compared with glucose controls. The number of periglomerular interstitium that was infiltrated by mononuclear cells (inflammatory signals) was observed to be higher in 5/6Nx-F rats than that in the other three groups (Figure 7D, black arrows); however, the increase in levels of serum [Ca] × [Pi], as is often the case in long-term ESRD patients, did not lead to its accumulation in tissues, as shown by the absence of hydroxyapatite deposits in the kidney of hyperphosphatemic 5/6Nx-F and 5/6Nx-G rats (Supplemental Figure S1).
Figure 7.
The effect of diet on the progression of CKD damage in 5/6Nx rats is histologically assessed. Renal tissue was obtained from Sham-G (A), Sham-F (B), 5/6Nx-G (C), or 5/6Nx-F (D) rats. Hematoxylin and eosin staining shows marked glomeruli (g) and proximal tubular (p) hypertrophy in 5/6Nx rats; the tubular hypertrophy is much higher in the 5/6Nx-F rats (D) than in the 5/6Nx-G rats (C). (D) The red arrow shows the accumulation of cell debris in the lumen of the proximal tubules of 5/6Nx-F rats; the black arrows show increased infiltration of macrophages in 5/6Nx-F rats.
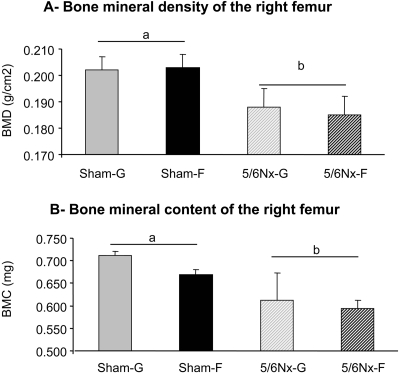
Bone Mineral Density and Content after Nephrectomy
Bone mineral density (BMD) of the left femur decreased significantly with nephrectomy (P < 0.05) but was independent of diet (P > 0.9; Figure 8A). Bone mineral content (BMC) was also significantly affected by nephrectomy (P < 0.02) but not by diet (P > 0.4; Figure 8B). No significant interaction between surgery and diet were observed.
Figure 8.
Dietary sugar effects on BMD (A) and BMC (B) of the right femur determined 10 wk after surgery from Sham-G, Sham-F, 5/6Nx-G or 5/6Nx-F rats. Data are means ± SEM (n = 4 to 5 per group). Differences between means, indicated by differences in letters, were analyzed by two-way ANOVA (P < 0.05).
Discussion
This study has three major findings. First, chronic consumption of a high-fructose diet when renal function is already compromised drastically reduces serum levels of 25-(OH)D3 and 1,25-(OH)2D3. Second, chronic consumption of a high-fructose diet has a marked deleterious effect on intestinal Ca absorption mediated mainly by a decrease in CaBP-9k expression. Finally, dietary fructose worsens the progression of renal dysfunction, as indicated by increases in most of the blood parameters (BUN, creatinine, Pi, [Pi] × [Ca]), indicative of CKD. We also confirmed the augmentation of kidney mass and the changes in renal histologic structure in 5/6Nx-F rats first mentioned by Gersch et al.10
Fructose Reduces Serum Vitamin D Levels in 5/6Nx Rats
The conversion of vitamin D to the active form 1,25-(OH)2D3 involves hepatic 25-hydroxylation by cytochrome P450 enzymes of vitamin D3 to 25-(OH)D3. There are still controversies about the specific enzymes mediating this step.27,28 The second step involves the hydroxylation of 25-(OH)D3 by 1α-hydroxylase in renal proximal tubule cells, leading to the formation of 1,25-(OH)2D3.
The 25-Hydroxylation Step.
Levels of dietary vitamin D and of light exposure (factors regulating the serum levels of 25-(OH)D3) were similar in all groups of rats, so dietary precursors or substrate levels cannot explain the low level of 25-(OH)D3 in 5/6Nx-F rats. The six hepatic cytochrome P 450 having a 25-hydroxylase activity (CYP27A1, CYP2R1, CYP2C11, CYP2D25, CYP33A4, and CYP2J3)27,28 all are NADPH dependent. It has been shown that fructose induces the NADPH-consuming synthesis of fatty acids in the liver, as well as of reactive oxygen species production by NADPH oxidase (unpublished observations), thereby potentially reducing levels of NADPH available for the 25-hydroxylation step. Moreover, hepatic oxidative stress increases with renal failure,29 which may amplify the NADPH reduction in the 5/6Nx-F rats compared with the sham-F rats and explain why the decrease in 25-(OH)D3 level is observed only in 5/6Nx-F rats.
The 1,25-Hydroxylation Step.
Although 1α-hydroxylase is expressed in extrarenal tissues,30 their contribution to the levels of serum 1,25-(OH)2D3 is modest.31 Thus, there are likely three proximate factors regulating the 1,25-(OH)2D3 serum level: The amount of precursor 25-(OH)D3 from the liver, the rate of degradation from 24-hydroxylase, and the rate of synthesis arising from the activity of renal 1α-hydroxylase. First, the precursor 25-(OH)D3 is indeed already quite low in 5/6Nx-F rats. Second, there is no significant change in the amount of 24-hydroxylase, suggesting there is no diet- and nephrectomy-induced change in degradation rate of 1,25-(OH)2D3. Finally, the reduction of 1,25-(OH)2D3 levels in 5/6Nx-F rats was significant despite marked increases in 1α-hydroxylase protein abundance.32 The renal abundance of 1α-hydroxylase is inversely regulated by 1,25-(OH)2D3, a potent inhibitor of CYP27B1 gene expression.33 The paradoxic decrease in serum levels of 1,25-(OH)2D3 despite an increase in 1α-hydroxylase protein may also be due to decreases in 1α-hydroxylase activity. Indeed, the ratio [1,25-(OH)2D3/25-(OH)D3], commonly used as an index of the 1α-hydroxylase activity,34 is reduced in 5/6Nx-F rats. 1α-Hydroxylase is also a mitochondrial cytochrome P450, which, like the other hepatic CYP proteins mentioned, is NADPH dependent. During CKD, renal gluconeogenesis is strongly repressed, which likely leads to decreases in NADPH production. Moreover, as in the liver, fructose consumption as well as renal failure results in increased levels of oxidative stress in the kidney.35 Such increases would reduce NADPH levels, leading to marked decreases in 1α-hydroxylase activity in 5/6Nx-F rats.
Intestinal Ca Uptake and CaBP-9k Expression
The inhibitory effect of fructose on intestinal Ca transport is specific, because fructose had no effect on transepithelial Pi transport and transapical glucose uptake. Transepithelial Ca transport and CaBP-9k expression each are clearly reduced only by fructose in both 5/6Nx and sham-operated rats, suggesting the reduction is mainly a diet effect. Previous studies showed a strong correlation between Ca intestinal absorption and CaBP-9k expression levels36; however, this effect of fructose is a novel and unexpected observation. In accordance with our findings, previous studies on the interaction between dietary sugars and mineral absorption showed that lactose (a digestible disaccharide like fructose) decreased intestinal Ca absorption as well as CaBP-9k protein levels.37 In contrast, indigestible oligosaccharides enhanced intestinal Ca absorption,38 probably via an increase in the passive paracellular route.39 It would be interesting to determine whether the fructose-induced decrease in transepithelial Ca absorption is also associated with a change in paracellular intestinal Ca transport.
The link between the fructose-induced decrease in 1,25-(OH)2D3, which normally regulates intestinal Ca transport, and the fructose-induced decrease in Ca transport is not clear because fructose affected 1,25-(OH)2D3 only in 5/6Nx-F rats. The effect of fructose on Ca transport may be partly vitamin D independent. Indeed, other studies showed vitamin D receptor–independent regulation of intestinal Ca absorption and of CaBP-9k expression.40 Moreover, fructose metabolism leads to rapid reductions in ATP and Pi levels in hepatocytes41 and enterocytes,42 thereby affecting all ATP- and Pi-dependent processes in the cells, such as PMCA1 and NCX1. Further studies are needed to evaluate the contributions of PMCA1 and NCX1 as well as TRPV6 to fructose-induced changes in intestinal Ca transport.
Fructose Exacerbates Classical Symptoms of CKD
The mechanisms underlying the fructose-induced enlargement of the kidney and degeneration of renal function are unknown. The effect can be indirect because dietary fructose increases the incidence of hypertension, insulin resistance, and dyslipidemia,4 which are metabolic diseases that often lead to kidney dysfunction. The effect can also be direct, because fructose promotes the production of proinflammatory factors such as reactive oxygen species and monocyte chemoattractant protein 1 in cultured proximal tubular cells HK2.11 Because GLUT5 is expressed in the proximal tubular cells,43 they must be able to absorb fructose, possibly promoting fructose-induced inflammation.
Enlargement of the kidney usually reflects a compensation for a decrease in kidney filtration rates, as would be expected after nephrectomy. Fructose-induced increases in renal mass index therefore indicate further decreases in renal filtration rate, which may lead to fructose causing a further elevation of nephrectomy-induced changes in serum biomarkers related to renal function (e.g., Pi, fructose).
Other Consequences of Fructose Feeding: Bone Integrity and Tissue Calcification
Most likely, an experimental duration longer than 1 mo is required before significant effects of fructose on bone integrity can be observed. In fact, bone remodeling takes at least 4 wk to happen in rodents.44
There was no deposition of hydroxyapatite in the kidney (Supplemental Figure S1) of 5/6Nx-F rats, the group with the highest blood levels of phosphate and of [Ca] × [Pi]. In our study, in the 5/6Nx-F rats, the Pi was only 9 mg/dl (1 mM), well below the critical concentration required for calcification (e.g., 11.5 mg/dl Ca).45 Because serum Pi level increased by as much as 50% in the 5/6Nx-F rats after only 4 wk, we predict that the calcification of soft tissues and blood vessels would be another major complication of chronic fructose consumption in advanced renal failure.
Conclusion
Until now, the recommended diet for the patient with CKD is traditionally based on protein and phosphorus restriction. Excessive fructose intake may also be carefully avoided by the patient with CKD to slow the progression of the disease. Besides renal degradation, we show here that not only mineral but also vitamin D homeostasis is markedly altered by excessive fructose consumption. The decrease in 25-(OH)D3 level indicates that liver function during ESRD is also compromised by fructose feeding. Further studies need to be conducted to evaluate whether the impairment of vitamin D synthesis by fructose is direct or is a secondary, indirect effect of the deleterious consequence of fructose on renal function. Dietary fructose constitutes approximately 60% of our rat diet, but the average fructose content of American diets is 15 to 17%; therefore, further studies need to be done using lower levels of dietary fructose and longer durations of feeding to evaluate properly the effect of this sugar on the progression and complications of CKD.
Concise Methods
Animals and Treatment
All of the procedures conducted in this study were approved by the Institutional Animal Care and Use Committee, UMDNJ-New Jersey Medical School. Studies were conducted on 20 male Sprague-Dawley rats (approximately 3 mo old and 200 to 210 g at the beginning of the experiment). Male sham-operated and 5/6Nx rats were purchased from Charles River Laboratories US (Wilmington, MA). Five-sixths nephrectomy was performed by ligating two of three branches of the renal artery of the right kidney and then removing 1 wk later the left kidney. The rats were delivered 1 wk after the second surgery and at this time divided in two groups: The sham-operated rats (sham; n = 10 rats) and the 5/6Nx rats (n = 10). They all were housed in the research animal facility and kept under standard conditions, 12-h light/dark photoperiod and controlled temperatures (22 to 24°C), and fed for 5 wk a commercial rat chow (Purina Mills, Richmond, IN) and water ad libitum.
Experimental Design
Body weights and food consumption of 5/6Nx and sham-operated rats were measured weekly. Serum biochemical parameters were measured at 2 and 6 wk. Blood was collected from the tail vein in anesthetized (0.2 to 0.4 ml/100g body wt, intraperitoneally, of ketamine 20 mg/ml and xylazine 2.5 mg/ml), unconscious rats. Six weeks after surgery, the sham and 5/6Nx groups were each randomly divided in two groups fed either a 60% glucose or a 60% fructose AIN-93M–based diet46 (Research Diets; Supplemental Table S1). Rats were fed the high-sugar diets for 4 wk ad libitum because the weekly measurement of food intake and body weight showed similar food consumption rates (when normalized to body weight) among the four groups. After 4 wk of fructose or glucose diets (total 10 wk after surgery), rats were anesthetized and killed.
Tissue Sampling
After opening the abdominal cavity, a small incision was made 5 cm distal to the stomach, and a catheter was inserted into the lumen of the small intestine. After its contents were flushed with ice-cold KRB, the small intestine was removed entirely and placed in ice-cold KRB and divided into five segments. Segment 1 was the proximal 5 cm of the duodenum for use in Ca uptakes. Segment 2 was the subsequent 3 cm, which was quickly frozen in liquid nitrogen and then stored at −80°C for subsequent protein and mRNA analyses. Segment 3 was the first 4 cm of the jejunum and was stored in ice-cold KRB for glucose and fructose uptakes. Segments 4 and 5 were the following 10 cm divided in two equal pieces and reserved in ice-cold KRB for measurements of total and Na+-independent Pi uptake, respectively. The entire right kidney was removed, weighed, and either fixed in 4% paraformaldehyde (for two rats in each group) or quickly frozen in liquid nitrogen and then stored at −80°C for subsequent protein extractions (for three rats in each group). The blood was then sampled by cardiac puncture, and the serum was separated in a BD Vacutainer (BD Scientific) by centrifugation for 10 min at 5000 rpm and stored at −80°C. The left femur was dissected, the muscle fibers were removed, and the bone was stored at −80°C until later analyses.
Intestinal In Vitro Uptake Measurements
All of the segments were everted quickly after isolation and prepared as everted sacs or sleeves to determine nutrient function as described next.
Ca Uptake.
The intestinal Ca transport determination was modified from the classical everted sac method described by Benn et al.47 The everted gut sacs were made by using the first 5-cm segment of proximal duodenum, where active transport of Ca is localized. The everted intestinal segment was filled with 400 μl of transport buffer (125 mM NaCl, 10 mM fructose, 1.3 mM HEPES, and 0.25 mM CaCl2 [pH 7.4]), and two ligations were tied at both extremities of the segment. The sacs were incubated in tubes containing 30 ml of transport buffer containing 45CaCl2 (40,000 cpm/ml) and kept in a water bath at 37°C for 1 h aerated continuously with 95% O2/5% CO2. At the end of the incubation, sacs were removed from the flask, gently washed in cold KRB, blotted dry, and cut open. A total of 50 μl of internal fluid from the sac and 50 μl from the external incubation medium were assayed in duplicate for 45Ca using a scintillation counter. The active accumulation of 45Ca in the inside fluid was expressed as a ratio of the final quantity of 45Ca inside/outside. The everted gut sac assay selectively measures active transepithelial Ca transport, which occurs primarily at low concentrations of Ca and is saturable.47
Fructose and Glucose Uptake.
From the first 4 cm of everted jejunum, four 1-cm jejunal segments were prepared and preincubated at 37°C for 5 min in Ringer bubbled with 95% O2/5% CO2 as described previously.48 The sleeves were then incubated in 50-mM sugar solutions containing either 10 μCi d-[14C]glucose (NEN, Boston, MA) for 1 min or 10 μCi of d-[14C]fructose for 2 min. We used 20 μCi of l-[3H]glucose to correct for adherent fluid and passive diffusion of glucose or fructose.
Pi Uptake.
Intestinal Pi transport was determined by a modification of the everted gut sac assay using two consecutive 5-cm segments of medial jejunum. The everted intestinal segment dedicated to the measurement of total Pi transport was filled with 400 μl of Na+-containing transport buffer (in mM: 128 NaCl, 4.7 KCl, 2.5 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, and 19 NaHCO3 [pH 7.3]). The everted intestinal segment dedicated to the measurement of Na+-independent Pi transport was filled with 400 μl of Na+-free transport buffer (128 mM choline chloride replacing NaCl). Then two ligations were tied at both extremities, thereby trapping the buffer within the segment. The everted sac with Na+-containing buffer was incubated in a tube with 30 ml of Na+-containing buffer with 33Pi (40,000 cpm/ml) and kept in a water bath at 37°C for 1 h and aerated continuously with 95% O2/5% CO2. The everted sac with Na+-free transport buffer was likewise incubated in a tube containing Na+-free transport buffer. At the end of the incubation, sacs were removed from the flask, gently washed in cold KRB, blotted dry, and cut open. A total of 50 μl of internal fluid from each sac and 50 μl from the external incubation medium were assayed in duplicate for 33Pi using a scintillation counter. The active accumulation of 33Pi in the inside fluid was expressed as a ratio of the final concentration of 33Pi inside/outside.
Measurements of Serum Clinical Parameters
Creatinine concentrations in serum samples were determined by the Jaffe method using QuantiChrom Creatinine Assay Kit (BioAssay Systems, Hayward, CA), serum urea nitrogen (BUN) concentrations were determined by the Jung method using QuantiChrom Urea Assay Kit (BioAssay Systems), and the total serum Pi concentrations were determined by the Malachite en method using QuantiChrom Pi Assay Kit (BioAssay Systems). The total serum Ca concentrations were determined by previously described techniques using flame atomic absorption spectrophotometry (Model 603; Perkin-Elmer, Norwalk, CT).
Vitamin D Assays
1,25-(OH)2D3 levels were measured from serum by enzyme immunoassay according the manufacturer's protocol (ImmunoDiagnosticSystems). Briefly, serum samples are delipidated and 1,25-(OH)2D3 immunoextracted before assaying. 25-(OH)2D3 levels was measured directly from serum by enzyme immunoassay according to the manufacturer's protocol (ImmunoDiagnosticSystems).
Western Blot Analysis.
For Western blot analysis, supernatants were prepared from 100 mg of tissue (liver, kidney, or intestine) using T-Per Tissue Protein Extraction Reagent (Thermo Scientific, Rockford, IL) and analyzed for protein concentration by the Bradford method. For calbindin-D9k (Swant Swiss Antibodies, Bellinzona, Switzerland), 50 μg of intestinal protein was analyzed by Western blot using precast gradient gel 4 to 20% Tris-HCl (BioRad, Hercules, CA). For other proteins, CYP27B1 (CYP27B1 M-100, Santa Cruz Biotechnology, Santa Cruz, CA) CYP24A1 (CYP24 S-20; Santa Cruz Biotechnology), Napi2B (Chemicon International), and 50 μg of renal or intestinal proteins were analyzed using a precast gel 12% Tris-HCl. All membranes were stripped and reprobed with β-actin antibody (Chemicon International). The relative OD obtained using the test antibody was divided by the relative OD obtained after probing with β-actin to normalize for variations in sample loading and transfer.
Extraction of mRNA and Reverse Transcription Reaction.
Total RNA was isolated from 100 mg of frozen small intestine tissue (−70°C) using TRIzol reagent (Invitrogen, Carlsbad, CA). Reverse transcription was performed with 5 μg of total RNA and 200 U of SuperScript III reverse transcriptase (Invitrogen, Carlsbad, CA) at 37°C for 1 h as described by the manufacturer.
Real-Time PCR.
Primers were designed using primer3 (http://frodo.wi.mit.edu/cgi-bin/primer3/) and were purchased from Integrated DNA Technologies (Coralville, IA). Real-time PCR was performed using a Stratagene Mx3000P (Stratagene, La Jolla, CA) as follows: The reactions were performed in 12.5 μl with 300 nM of each primer and 5 μl of a 1:30 dilution of the real-time reaction and the SYBR-Green PCR Master Mix (Stratagene) according to the manufacturer's instructions. The thermal cycling protocol was as follows: 95°C for 10 min, followed by 40 cycles of PCR at 95°C for 30 s, then annealing step at adequate temperature in function of the primers (Supplemental Table S2) and 72°C for 1 min for extension. After the final cycle, melting curves were monitored from 55 to 95°C (0.05°C/s). The relative expression ratio of target genes was calculated as described previously.49 In all of the experiments, the control group was the Sham-G rats. The target gene expression was also normalized by a reference gene, α-elongation factor 1 (EF1α), which we already validated as having a stable expression level in our previous experiments.
Immunohistochemistry.
After overnight fixation in fresh 4% paraformaldehyde in PBS (pH 7.35), rat kidneys were then embedded in paraffin. Some slides were then processed for routine hematoxylin-eosin staining. For immunohistochemistry, heat-induced antigen retrieval was performed in a programmable pressure cooker (Retriever, Electron Microscopy Services) on 5-μm sections in 0.01 M Na citrate buffer (pH 6.0). The sections were rinsed in PBS and blocked in 1% normal goat serum for 1 h at room temperature. The kidney sections were incubated in rabbit anti-mouse CYP27B1 (Santa Cruz Biotechnology; 1:200 in 1% BSA in PBS) overnight at 4°C, then washed in PBS. The secondary antibody was goat anti-rabbit IgG labeled with Cy3 (1:100; Chemicon International), diluted in 1% BSA in PBS and applied for 1 h at room temperature. After PBS wash, the stained sections were mounted in Vectashield (Vector Laboratories) and examined at ×20 or ×40 with a laser scanning confocal microscope (Radiance 2100; BioRad). All images of sections being compared were obtained with the same settings of the microscope. Nonspecific staining with secondary antibodies was consistently negligible.
Determinations of BMC and BMC.
BMD and BMC were evaluated using dual-energy x-ray absorptiometry (GE-Lunar PIXImus; GE Healthcare). Excised bone measurements were obtained by placing the tibia or femur on a Delrin block. The total tibia or femur was scanned. The PIXImus densitometer uses a 14° stationary anode x-ray tube generator with a 0.25 × 0.25-mm focal spot that generates a cone beam x-ray (55/80 kVp at 400 μA). The right femurs were analyzed for the whole bone “total” BMD and BMC. The age coefficient of variation (CV) for three repeated BMD and BMC measurements for four samples was calculated. The CV for the tibia and femur BMD was 2.2 and 1.4%, respectively. The BMC values for CV for tibia and femur were 3.5 and 1.5%, respectively.
Statistical analyses.
Data are presented as means ± SEM. A two-way ANOVA was first used to determine the significance of differences as a function of surgery and of diet. When there was a significant difference, a one-way ANOVA was used followed by a Fisher paired LSD test to determine the particular effect that caused that difference (STATVIEW; Abacus Concepts). Differences were considered significant at P < 0.05.
Disclosures
Y. Sabbagh is an employee of Genzyme Corporation and owns stock in the company.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grant DK-075617 (to R.P.F.), National Science Foundation grant 722365 (to R.P.F.), National Institutes of Health grant AG-12161 (to S.A.S.), and Diabetes Action Research and Education Foundation (to D.M.C.).
This study was presented at the Annual Meeting of the Federation of American Societies for Experimental Biology; April 9, 2009; New Orleans, LA.
We are grateful to Dr. D. Lagunoff, Prof. S. Christakos, Dr. L.G. Meggs, Dr. A.S. Reddi, D. Ajibade, H. Ambia-Sobhan, and F. Kemp for valuable advice and help in experiments.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Gilbertson DT, Liu J, Xue JL, Louis TA, Solid CA, Ebben JP, Collins AJ: Projecting the number of patients with end-stage renal disease in the United States to the year 2015. J Am Soc Nephrol 16: 3736–3741, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Luk AO, So WY, Ma RC, Kong AP, Ozaki R, Ng VS, Yu LW, Lau WW, Yang X, Chow FC, Chan JC, Tong PC: Metabolic syndrome predicts new onset of chronic kidney disease in 5,829 patients with type 2 diabetes: A 5-year prospective analysis of the Hong Kong Diabetes Registry. Diabetes Care 31: 2357–2361, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lea J, Cheek D, Thornley-Brown D, Appel L, Agodoa L, Contreras G, Gassman J, Lash J, Miller ER, 3rd, Randall O, Wang X, McClellan W: Metabolic syndrome, proteinuria, and the risk of progressive CKD in hypertensive African Americans. Am J Kidney Dis 51: 732–740, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Le KA, Tappy L: Metabolic effects of fructose. Curr Opin Clin Nutr Metab Care 9: 469–475, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Havel PJ: Dietary fructose: Implications for dysregulation of energy homeostasis and lipid/carbohydrate metabolism. Nutr Rev 63: 133–157, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Higashiura K, Ura N, Takada T, Agata J, Yoshida H, Miyazaki Y, Shimamoto K: Alteration of muscle fiber composition linking to insulin resistance and hypertension in fructose-fed rats. Am J Hypertens 12: 596–602, 1999 [DOI] [PubMed] [Google Scholar]
- 7.Johnson RJ, Segal MS, Sautin Y, Nakagawa T, Feig DI, Kang DH, Gersch MS, Benner S, Sanchez-Lozada LG: Potential role of sugar (fructose) in the epidemic of hypertension, obesity and the metabolic syndrome, diabetes, kidney disease, and cardiovascular disease. Am J Clin Nutr 86: 899–906, 2007 [DOI] [PubMed] [Google Scholar]
- 8.Bray GA, Nielsen SJ, Popkin BM: Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity. Am J Clin Nutr 79: 537–543, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Douard V, Ferraris RP: Regulation of the fructose transporter GLUT5 in health and disease. Am J Physiol Endocrinol Metab 295: E227–E237, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gersch MS, Mu W, Cirillo P, Reungjui S, Zhang L, Roncal C, Sautin YY, Johnson RJ, Nakagawa T: Fructose, but not dextrose, accelerates the progression of chronic kidney disease. Am J Physiol Renal Physiol 293: F1256–F1261, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Cirillo P, Gersch MS, Mu W, Scherer PM, Kim KM, Gesualdo L, Henderson GN, Johnson RJ, Sautin YY: Ketohexokinase-dependent metabolism of fructose induces proinflammatory mediators in proximal tubular cells. J Am Soc Nephrol 20: 545–553, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caudarella R, Vescini F, Buffa A, Francucci CM: Hyperphosphatemia: Effects on bone metabolism and cardiovascular risk. J Endocrinol Invest 30: 29–34, 2007 [PubMed] [Google Scholar]
- 13.Ganesh SK, Stack AG, Levin NW, Hulbert-Shearon T, Port FK: Association of elevated serum PO(4), Ca x PO(4) product, and parathyroid hormone with cardiac mortality risk in chronic hemodialysis patients. J Am Soc Nephrol 12: 2131–2138, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Marks J, Churchill LJ, Srai SK, Biber J, Murer H, Jaeger P, Debnam ES, Unwin RJ: Intestinal phosphate absorption in a model of chronic renal failure. Kidney Int 72: 166–173, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Kirchner S, Muduli A, Casirola D, Prum K, Douard V, Ferraris RP: Luminal fructose inhibits rat intestinal sodium-phosphate cotransporter gene expression and phosphate uptake. Am J Clin Nutr 87: 1028–1038, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsanzi E, Light HR, Tou JC: The effect of feeding different sugar-sweetened beverages to growing female Sprague-Dawley rats on bone mass and strength. Bone 42: 960–968, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Ivaturi R, Kies C: Mineral balances in humans as affected by fructose, high fructose corn syrup and sucrose. Plant Foods Hum Nutr 42: 143–151, 1992 [DOI] [PubMed] [Google Scholar]
- 18.Milne DB, Nielsen FH: The interaction between dietary fructose and magnesium adversely affects macromineral homeostasis in men. J Am Coll Nutr 19: 31–37, 2000 [DOI] [PubMed] [Google Scholar]
- 19.Wyshak G: Teenaged girls, carbonated beverage consumption, and bone fractures. Arch Pediatr Adolesc Med 154: 610–613, 2000 [DOI] [PubMed] [Google Scholar]
- 20.Lee O, Bruce WR, Dong Q, Bruce J, Mehta R, O'Brien PJ: Fructose and carbonyl metabolites as endogenous toxins. Chem Biol Interact 178: 332–339, 2009 [DOI] [PubMed] [Google Scholar]
- 21.Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, McGahan JP, Seibert A, Krauss RM, Chiu S, Schaefer EJ, Ai M, Otokozawa S, Nakajima K, Nakano T, Beysen C, Hellerstein MK, Berglund L, Havel PJ: Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest 119: 1322–1334, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perez AV, Picotto G, Carpentieri AR, Rivoira MA, Peralta Lopez ME, Tolosa de Talamoni NG: Minireview on regulation of intestinal calcium absorption: Emphasis on molecular mechanisms of transcellular pathway. Digestion 77: 22–34, 2008 [DOI] [PubMed] [Google Scholar]
- 23.Fuchs R, Peterlik M: Vitamin D-induced phosphate transport in intestinal brush border membrane vesicles. Biochem Biophys Res Commun 93: 87–92, 1980 [DOI] [PubMed] [Google Scholar]
- 24.Xu H, Bai L, Collins JF, Ghishan FK: Age-dependent regulation of rat intestinal type IIb sodium-phosphate cotransporter by 1,25-(OH)(2) vitamin D(3). Am J Physiol Cell Physiol 282: C487–C493, 2002 [DOI] [PubMed] [Google Scholar]
- 25.Cui XL, Soteropoulos P, Tolias P, Ferraris RP: Fructose-responsive genes in the small intestine of neonatal rats. Physiol Genomics 18: 206–217, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Penaranda C, Garcia-Ocana A, Sarasa JL, Esbrit P: Hypertrophy of rabbit proximal tubule cells is associated with overexpression of TGF beta. Life Sci 59: 1773–1782, 1996 [DOI] [PubMed] [Google Scholar]
- 27.Ohyama Y, Yamasaki T: Eight cytochrome P450s catalyze vitamin D metabolism. Front Biosci 9: 3007–3018, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Prosser DE, Jones G: Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem Sci 29: 664–673, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Golab F, Kadkhodaee M, Zahmatkesh M, Hedayati M, Arab H, Schuster R, Zahedi K, Lentsch AB, Soleimani M: Ischemic and non-ischemic acute kidney injury cause hepatic damage. Kidney Int 75: 783–792, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, Hewison M: Extrarenal expression of 25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J Clin Endocrinol Metab 86: 888–894, 2001 [DOI] [PubMed] [Google Scholar]
- 31.Hewison M, Zehnder D, Chakraverty R, Adams JS: Vitamin D and barrier function: A novel role for extra-renal 1 alpha-hydroxylase. Mol Cell Endocrinol 215: 31–38, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG: Using serum creatinine to estimate glomerular filtration rate: Accuracy in good health and in chronic kidney disease. Ann Intern Med 141: 929–937, 2004 [DOI] [PubMed] [Google Scholar]
- 33.Sakaki T, Kagawa N, Yamamoto K, Inouye K: Metabolism of vitamin D3 by cytochromes P450. Front Biosci 10: 119–134, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Inaba M, Yukioka K, Furumitsu Y, Murano M, Goto H, Nishizawa Y, Morii H: Positive correlation between levels of IL-1 or IL-2 and 1,25(OH)2D/25-OH-D ratio in synovial fluid of patients with rheumatoid arthritis. Life Sci 61: 977–985, 1997 [DOI] [PubMed] [Google Scholar]
- 35.Cachofeiro V, Goicochea M, de Vinuesa SG, Oubina P, Lahera V, Luno J: Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int SupplS4–S9, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Armbrecht HJ, Boltz MA, Bruns ME: Effect of age and dietary calcium on intestinal calbindin D-9k expression in the rat. Arch Biochem Biophys 420: 194–200, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Pansu D, Bellaton C, Bronner F: Effect of lactose on duodenal calcium-binding protein and calcium absorption. J Nutr 109: 508–512, 1979 [DOI] [PubMed] [Google Scholar]
- 38.Mineo H, Hara H, Kikuchi H, Sakurai H, Tomita F: Various indigestible saccharides enhance net calcium transport from the epithelium of the small and large intestine of rats in vitro. J Nutr 131: 3243–3246, 2001 [DOI] [PubMed] [Google Scholar]
- 39.Younes H, Demigne C, Remesy C: Acidic fermentation in the caecum increases absorption of calcium and magnesium in the large intestine of the rat. Br J Nutr 75: 301–314, 1996 [DOI] [PubMed] [Google Scholar]
- 40.Song Y, Kato S, Fleet JC: Vitamin D receptor (VDR) knockout mice reveal VDR-independent regulation of intestinal calcium absorption and ECaC2 and calbindin D9k mRNA. J Nutr 133: 374–380, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Rawson NE, Blum H, Osbakken MD, Friedman MI: Hepatic phosphate trapping, decreased ATP, and increased feeding after 2,5-anhydro-D-mannitol. Am J Physiol 266: R112–R117, 1994 [DOI] [PubMed] [Google Scholar]
- 42.Kles KA, Wallig MA, Tappenden KA: Luminal nutrients exacerbate intestinal hypoxia in the hypoperfused jejunum. JPEN J Parenter Enteral Nutr 25: 246–253, 2001 [DOI] [PubMed] [Google Scholar]
- 43.Sugawara-Yokoo M, Suzuki T, Matsuzaki T, Naruse T, Takata K: Presence of fructose transporter GLUT5 in the S3 proximal tubules in the rat kidney. Kidney Int 56: 1022–1028, 1999 [DOI] [PubMed] [Google Scholar]
- 44.Vignery A, Baron R: Dynamic histomorphometry of alveolar bone remodeling in the adult rat. Anat Rec 196: 191–200, 1980 [DOI] [PubMed] [Google Scholar]
- 45.Reynolds JL, Joannides AJ, Skepper JN, McNair R, Schurgers LJ, Proudfoot D, Jahnen-Dechent W, Weissberg PL, Shanahan CM: Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: A potential mechanism for accelerated vascular calcification in ESRD. J Am Soc Nephrol 15: 2857–2867, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Reeves PG, Rossow KL, Lindlauf J: Development and testing of the AIN-93 purified diets for rodents: Results on growth, kidney calcification and bone mineralization in rats and mice. J Nutr 123: 1923–1931, 1993 [DOI] [PubMed] [Google Scholar]
- 47.Benn BS, Ajibade D, Porta A, Dhawan P, Hediger M, Peng JB, Jiang Y, Oh GT, Jeung EB, Lieben L, Bouillon R, Carmeliet G, Christakos S: Active intestinal calcium transport in the absence of transient receptor potential vanilloid type 6 and calbindin-D9k. Endocrinology 149: 3196–3205, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jiang L, Ferraris RP: Developmental reprogramming of rat GLUT-5 requires de novo mRNA and protein synthesis. Am J Physiol Gastrointest Liver Physiol 280: G113–G120, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Douard V, Cui XL, Soteropoulos P, Ferraris RP: Dexamethasone sensitizes the neonatal intestine to fructose induction of intestinal fructose transporter (Slc2A5) function. Endocrinology 149: 409–423, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.