Abstract
Macrophages are mononuclear phagocytes that are widely distributed throughout the body. These cells can contribute to development and homeostasis and participate in innate and adaptive immune responses. The physiology of macrophages can vary tremendously depending on the environment in which they reside and the local stimuli to which they are exposed. Macrophages are prodigious secretory cells, and in that role can promote and regulate immune responses and contribute to autoimmune pathologies. Macrophages are highly phagocytic, and in this capacity have long been considered to be essential immune effector cells. The important roles of macrophages in maintaining homeostasis and in contributing to tissue remodeling and wound healing is sometimes overlooked because of their vital role in host defense.
Keywords: colony stimulating factor, peritoneal cavity, lung, bone marrow, flow cytometry, biomarkers
INTRODUCTION
Tissue macrophages are derived from monocytes or from the local proliferation of tissue-resident macrophage colony–forming unit cells (Gordon and Taylor, 2005; Strauss-Ayali et al., 2007). Monocytes originate from progenitor cells in the bone marrow which undergo three divisions, into monoblasts, promonocytes, and monocytes, before their release into the circulation (Cohn, 1978; Gordon and Taylor, 2005). Monocytes differentiate further into macrophages as they leave the blood and enter the tissue. During inflammation, the majority of macrophages are derived from blood monocytes. Within tissue, macrophages can respond to activation stimuli and dramatically change their physiology. We describe macrophage activation in unit 14.2.
The peritoneal cavity provides an easily accessible site for the harvesting of moderate numbers of resident, nonmanipulated macrophages. These cells have been thoroughly studied, and most of our understanding of resident tissue macrophages comes from the study of these macrophages. Usually, the number of macrophages present in the peritoneum under nonelicited condition is insufficient for extensive biochemical studies. To increase macrophage yield, sterile eliciting agents, such as Brewer’s thioglycollate broth or proteose peptone, can be injected into the peritoneal cavity prior to cell harvest. These agents increase monocyte migration into the peritoneum, and therefore increase macrophage yield. Although these agents increase the number of peritoneal macrophages, concerns have been raised as to how these eliciting agents affect the physiology of these macrophages (also see unit 14.2). Isolation of murine peritoneal macrophages is described in Basic Protocol 1.
An alternative approach for isolating relatively large numbers of nonelicited macrophages takes advantage of the use of macrophage colony–stimulating factor (M-CSF) to differentiate macrophage progenitor cells from the bone marrow into mature macrophages in culture (Austin et al., 1971; Stanley, 1985). Basic Protocol 2 describes the isolation of bone marrow–derived progenitor cells and their propagation in M-CSF-containing medium. The main advantage of this isolation protocol is that it yields a homogenous population of macrophages in a relatively quiescent state that are responsive to activation stimuli in vitro.
Basic Protocol 3 describes isolation of macrophages from the lung. Macrophages are present in virtually all tissues, and in most cases the tissue environment exerts subtle alterations in macrophage physiology. This is especially true of alveolar macrophages, which have several unique features (Maus et al., 2001; Morimoto et al., 2001; Yoshikawa et al., 1991). For example, unlike most macrophage populations, alveolar macrophages utilize primarily aerobic oxidative phosphorylation rather than anaerobic glycolysis to generate energy. The isolation of these cells is a bit more cumbersome than the others because large numbers of these cells are not easily obtained from murine lungs.
Basic Protocol 4 describes some basic assays that are used to identify and characterize macrophages. Several good biochemical markers have been developed that can be used to identify macrophages, and many can also be used to characterize their physiology. The use of macrophage-specific surface markers combined with flow cytometry represents the preferred technique to identify macrophages in tissue and to characterize their activation state (Cohn, 1978; Edwards et al., 2006; Gordon, 2007).
NOTE: All solutions and equipment coming in contact with cells must be sterile, and proper sterile procedures should be employed. Solutions must be free of endotoxin because macrophages are extremely sensitive to its biological effects. Polypropylene plasticware should be used for maximal cell recovery, because macrophages tend to stick to most surfaces. All reagents and harvested cells should be kept at 4°C prior to use.
ISOLATION OF MURINE PERITONEAL MACROPHAGES
Murine peritoneal macrophages suitable for use in protocols outlined elsewhere in this chapter are isolated as described below. The peritoneal cavity provides an easily accessible site for harvesting moderate numbers of resident macrophages. Generally, macrophages isolated from the mouse peritoneal cavity will be mature quiescent macrophages (Cohn, 1978; Fortier et al., 1982). The yield, however, is typically only ~0.5–1 × 106 macrophages per mouse. The yield of macrophages can be increased by injecting eliciting agents into peritoneum several days prior to cell harvest. However, such elicitation will also alter the physiologic characteristics of the cells collected.
Specific pathogen-free mice are purchased from licensed commercial vendors. Mice should be bred and housed in a clean pathogen-free environment. It is important to use mice that are clean, stress-free, and uninfected. Chronic, endemic infectious diseases, such as Sendai virus or mouse hepatitis virus, can have profound effects on macrophage physiology. Mice should be housed in the facility for at least 1 week following delivery to allow them to recover from transit stress. BALB/c and C57Bl/6 mice have been the strains of choice. Both strains of mice yield macrophages that are fully responsive to exogenous stimuli.
Materials
Donor mice: BALB/c or C57B/6, specific-pathogen-free, typically 6 to 8 weeks old
3% Brewer thioglycollate medium (see recipe)
70% ethanol
Harvest medium: Dulbecco’s phosphate-buffered saline without calcium and magnesium (Mediatech, cat. no. 21-031-CV), cold
DMEM/F12-10 medium (see recipe)
Diff-Quik stain solutions: Differential Staining Set (B4132-1A, CardinalHealth, http://www.cardinal.com); includes fixative solution, solution I, and solution II.
5- and 10-ml syringes
23-G and 20-G needles, 1.5-in. (3.75-cm) length
Forceps and small, straight surgical scissors (immerse in 70% ethanol)
50-ml polypropylene conical centrifuge tubes, on ice
Refrigerated centrifuge: Eppendorf 5810R (or equivalent)
Cytocentrifuge (Shandon Cytospin 4, cat no. A7830002, ThermoScientific)
Cytofunnels (Shandon EZ Cytofunnels, cat. no. A78710003, ThermoScientific)
Additional reagents and equipment for mouse euthanasia (unit 1.8), intraperitoneal injection (unit 1.6), and counting cells with a hemacytometer (appendix 3a)
-
1a.
For resident peritoneal cells: Euthanize untreated mice by rapid cervical dislocation (unit 1.8).
Euthanasia should be performed by skilled technicians to avoid excessive bleeding into the peritoneal cavity.
CO2 euthanasia (also described in unit 1.8) can also be used with good effect.
-
1b.
For elicited peritoneal cells: Fill a 5-ml syringe with 3% Brewer thioglycollate medium (Hoover and Nacy, 1984). Attach 23-G needle and inject 1 ml of the solution per mouse into the peritoneal cavity. Be sure to avoid the bladder. Allow inflammatory response to proceed for 4 days, then euthanize as in step 1a.
Alternatively, 3% proteose peptone (see recipe in Reagents and Solutions) can be used, but the yield of macrophages is ~30% lower. Thioglycollate-treated mice will yield ~1 × 107 macrophages per mouse. Proteose peptone–treated mice will yield ~4 × 106 macrophages per mouse. Both eliciting agents recruit immature macrophages into the peritoneum.
Thioglycollate-elicited cells usually internalize a considerable amount of agar from the broth and consequently have an atypical appearance that is distinct from resident macrophages. This internalized material may complicate studies on phagocytosis by these cells. These cells tend to spread more than resident cells, and, because the broth is generally contaminated with LPS, these cells can often respond to IFN-γ priming without further stimulation.
Bio-Gel-elicited macrophages (see recipe for 2% Bio-Gel in Reagents and Solutions) are free of intracellular debris because Bio-Gel beads cannot be phagocytosed by macrophages (Mahoney et al., 2000). These cells may be more suitable for the studies pertaining to phagocytosis. The yield is typically ~1 × 107 macrophages per mouse.
Different experiments may lend themselves to different irritants (see Critical Parameters).
-
2.
Soak the abdomen of each mouse with 70% alcohol and then make a small incision along the midline with sterile scissors. Retract the abdominal skin manually to expose the intact peritoneal wall.
-
3.
Fill a 10-ml syringe with cold harvest medium. With the beveled end of a 20-G needle facing inward, insert needle through peritoneal wall along the mouse’s left side (spleen side) and inject 10 ml of the cold harvest medium into each mouse.
unit 1.6 provides additional detail on intraperitoneal injection.
Be careful not to puncture the intestine. If intestine is punctured, remove mouse and syringe from procedure and discard.
-
4.
Using the same syringe and needle, aspirate fluid from peritoneum. Move needle away from the viscera to cause tenting of the peritoneal wall, and withdraw peritoneal fluid slowly.
Expect fluid recovery of ~8 ml per mouse.
-
5.
Remove needle from syringe and dispense peritoneal fluid into a 50-ml conical polypropylene centrifuge tube on ice.
Cells should be kept cold throughout the procedure.
Three or five mice can be euthanized at the same time, and the procedure is performed sequentially using three or five 10-ml syringes. For large-scale isolations, several successive repetitions of this procedure can be performed using the same syringes and needles.
-
6.
Centrifuge the peritoneal exudate cells (PEC) in a refrigerated centrifuge 10 min at 400 × g (~1000 rpm in Eppendorf 5810R), 4°C. Discard supernatant and resuspend cell pellet in cold DMEM/F12-10 by gently tapping the bottom of the tube and pipetting up and down.
A good general rule of thumb is to resuspend macrophages in a volume of 1 ml per five mice.
Some RBC contamination of the cell mixture will not have an adverse affect.
-
7.
Count cells a hemacytometer as described in appendix 3a, first diluting 20 µl of cell suspension into 180 µl of DMEM/F12-10 (final dilution of 10×) and then applying 10 µl to a hemacytometer.
-
8.
Optional: Transfer 0.2 ml of the cell suspension to a cytocentrifuge cytofunnel containing a slide, to prepare a cell smear for differential stain. Follow manufacturer’s instructions for collecting cells using cytocentrifuge. Cytospin 3 min at 600 rpm. Remove slide and allow to air dry. Stain cytocentrifuged sample with Diff-Quik staining solutions and perform a differential cell count according to the manufacturer’s instructions.
For additional information about cell counting, see Critical Parameters.
The differential staining step is generally not required for routine macrophage isolation procedures.
-
9.
Adjust cell concentration in harvest medium to ~1–3 × 106 total peritoneal cells/ml and keep on ice.
Peritoneal macrophages are now ready for phenotypic characterization (Basic Protocol 4) or functional assays (e.g., unit 14.2).
To obtain monolayers of peritoneal macrophages, the cell concentration is adjusted to 2–3 × 106 total nucleated cells/ml in DMEM/F12-10 medium. A total of 1–2 × 105 cells/well are added to 48-well tissue culture plates. For 24-well plates, this number is doubled. A total of 1–2 × 106 cells are added to 6-well tissue culture plates. These numbers vary depending on the degree of monolayer confluency desired. The cells are allowed to adhere to the substrate by culturing them for 1 to 2 hr at 37°C. Nonadherent cells are removed by gently washing three times with warm PBS. At this time, cells should be greater than 90% macrophages. Macrophages can be analyzed at this time or placed back into culture in DMEM/F12–10 medium and maintained for several days.
ISOLATION OF MURINE BONE MARROW–DERIVED MACROPHAGES
In this protocol, bone marrow cells are harvested and cultured in the medium containing macrophage colony–stimulating factor (M-CSF; Stanley, 1985). After 7 days in culture, contaminating nonadherent cells are eliminated and adherent cells are harvested for assays. Adherent bone marrow–derived macrophages are usually better than 90% pure, and, therefore, precisely defined numbers of macrophages can be plated for subsequent assays. This protocol has been used to examine the functional capabilities of macrophages under different external stimulation conditions (Warren and Vogel, 1985; Anderson and Mosser, 2002). Virtually all of the cells in this population of macrophages have the ability to respond to exogenous stimuli, and, therefore, relatively uniform populations of macrophages can be generated and harvested for biochemical analysis.
Materials
Donor mice: BALB/c or C57B/6, specific-pathogen-free
70% ethanol
Dulbecco’s phosphate-buffered saline without calcium and magnesium (Mediatech, cat. no. 21-031-CV), cold
Macrophage complete medium (see recipe)
Cellstripper nonenzymatic cell dissociation solution (Mediatech)
DMEM/F12-10 medium (see recipe)
Forceps and scissors (keep in sterile beaker containing 70% ethanol)
10-ml syringes with 25-G needles
50-ml conical polypropylene centrifuge tubes, on ice
Eppendorf 5810R (or equivalent) centrifuge
100 × 15–mm petri dishes (Falcon, BD Labware)
37°C, 5% CO2 incubator
Sterile rubber cell scraper (optional)
Additional reagents and equipment for mouse euthanasia (unit 1.8) and counting cells with a hemacytometer (appendix 3a)
Prepare mouse bone marrow suspension
-
1.
Euthanize mice by rapid cervical dislocation (unit 1.8). Using aseptic technique, peel the skin from the top of each hind leg and down over the foot. Cut off the foot along with the skin and discard. Cut off the hind legs at the hip joint with scissors, leaving the femur intact. Place femurs in plastic dish containing sterile PBS.
Use only mice bred and housed in clean environments (preferably barrier facilities). As described for peritoneal macrophages (see Basic Protocol 1), chronic, endemic infectious diseases will have a profound effect on macrophage physiology and will even affect bone marrow–derived macrophages that are grown in culture for 7 days.
-
2.
Remove excess muscle from legs by holding end of bone with forceps and using scissors to push muscle downward away from forceps.
Alternatively, excess muscle can be removed from the legs by holding the end of the bone with a piece of Clorox Disinfecting Wipe and pushing muscle away from bones with fingers.
-
3.
Using sharp scissors or razor blade soaked in ethanol, carefully sever leg bones proximal to each joint.
-
4.
Attach 10-ml syringe to 25-G needle and fill with cold sterile wash medium (Dulbecco’s phosphate-buffered saline without calcium and magnesium).
-
5.
Insert needle into bone marrow cavity of femur. Flush bone cavity with 2 to 5 ml of the wash medium, until bone cavity appears white. Allow wash medium to collect in a sterile 50-ml conical centrifuge tube on ice.
-
6.
Centrifuge cells 10 min at 500 × g, room temperature.
-
7.
Discard supernatant. Resuspend cell pellet in macrophage complete medium by tapping tube and pipetting up and down.
Culture macrophages
-
8.
Count total bone marrow progenitor cells in a hemacytometer (appendix 3a) and adjust cells to a concentration of ~4 × 106 /ml in macrophage complete medium. Add a total of 4 × 105 cells per sterile plastic petri dish in 10 ml macrophage complete medium. Incubate in 37°C, 5% CO2 incubator.
For optimal yields, both femurs and even both tibias can be harvested from a single mouse. Bone marrow cells derived from a single mouse can typically be divided into fifteen petri dishes (100 × 15 mm). After 7 days, each dish will yield ~7–10 × 106 macrophages, for a total of ~70–100 × 106 macrophages per single mouse.
-
9.
On day 3, add another 5 ml of macrophage complete medium to each dish.
Harvest macrophages
-
10.
On day 7, remove and discard culture supernatants. Wash remaining adherent cells with 5 ml prewarmed Dulbecco’s phosphate-buffered saline without calcium or magnesium. Discard wash, add 3 ml Cellstripper nonenzymatic cell dissociation solution to each dish, and incubate 5 min at 37°C.
Cells can be gently scraped to dislodge, but this should not be necessary if cultivated on non–tissue culture treated petri plates.
-
11.
Pool dislodged cells into a 50-ml conical centrifuge tube, and add an equal volume of cold DMEM/F12-10 medium. Centrifuge cells 10 min at 400 × g, 4°C, and remove supernatant.
-
12.
Resuspend cells in 3 to 5 ml DMEM/F12-10 medium and count cells using a hemacytometer (appendix 3a).
The volume of medium will vary with the number of dishes harvested. The resuspension volume should be determined based on volume necessary for subsequent assays. Cells should be essentially 100% macrophages, which can be analyzed directly at this time or replated for adherence and further experimentation.
At this time the cells should be essentially 100% macrophages, with the yield being 7.5–15 × 107 macrophages per mouse. Macrophages can be analyzed directly at this time or they can be replated for adherence and further experimentation. Typically, the cells are plated in DMEM/F12-10 medium (see recipe) at 1 × 105 cells/well in 48-well tissue culture plates, 2 × 105 cells/well in 24-well tissue culture plates, or 1 × 106 cells/well in 6-well tissue culture plates.
ISOLATION OF MURINE ALVEOLAR MACROPHAGES
Because the lung is exposed to pathogens and various environmental stimuli, infections and inflammation in the lung are common. Alveolar macrophages are an important component of host defense against invading microorganisms, and they can play a critical role in the initiation and the resolution of inflammation in the lung. In this protocol, a method for isolating murine alveolar macrophages is described. These harvested macrophages can be examined in vitro for their roles in phagocytosis, cytokine and mediator production, and other biological functions (Yoshikawa et al., 1991; Maus et al., 2001; Steele et al., 2003).
Materials
Ketamine/xylazine cocktail: 80 to 100 mg/kg body weight ketamine (Ketaset, Fort Dodge)/10 mg/kg body weight xylazine (Rompun, Bayer) per 100 to 200 µl Dulbecco’s phosphate-buffered saline without calcium or magnesium (Mediatech, cat. no. 21-031-CV)
Dulbecco’s phosphate-buffered saline without calcium or magnesium (Mediatech, cat. no. 21-031-CV) supplemented with 0.5 mM EDTA
Surgical instruments: forceps and small, straight surgical scissors (keep in 70% ethanol)
Surgical suture material: Ethilon black 18-in. PS-1 cutting (3-0, cat. no. 1663, http://www.eSutures.com)
5-ml syringes with 18-G needles
50-ml conical polypropylene centrifuge tubes
Eppendorf 5810R (or equivalent) centrifuge, 4°C (or equivalent)
Additional reagents and equipment for intraperitoneal injection (unit 1.6), euthanasia of the mouse (unit 1.8), counting cells (see appendix 3a and Basic Protocol 1, step 7), and differential staining of Cytospin cells (see Basic Protocol 1, step 8)
-
Anesthetize mice by intraperitoneal injection of 100 to 200 µl of ketamine/xylazine cocktail. Euthanize by exsanguination (unit 1.8).
The use of cervical dislocation is not indicated since it can damage the trachea, contaminating the sample with blood.
Surgically expose the trachea on the ventral side of the neck and insert an 18-G needle attached to a 5-ml syringe containing 4 ml of prewarmed (37°C) calcium- and magnesium-free Dulbecco’s phosphate-buffered saline containing 0.5 mM EDTA through the tracheal wall into the lumen just below the larynx.
Tie surgical suture material (or sterilized sewing thread) around the trachea to secure the needle in place.
-
Gently infuse the prewarmed (37°C) calcium and magnesium-free Dulbecco’s phosphate-buffered saline supplemented with 0.5 mM EDTA into the lung.
A total volume of not more than 2 ml of saline can generally be instilled into the lung at any one time.
Gently withdraw fluid into the syringe and reinfuse back into the lung three times in succession. Express the final lung fluid from the syringe into a 50-ml conical polypropylene tube on ice.
-
Repeat the above steps on several mice and pool lung fluid into a 50-ml conical centrifuge tube. Centrifuge 10 min at 400 × g, 4°C.
Typically, a total of ~3–5 × 105 alveolar macrophages cells can be obtained per mouse. Therefore this procedure is often performed on groups of 10 mice or more to provide sufficient alveolar macrophages for functional assays.
-
Resuspend cells in 1 to 3 ml medium and count in a hemacytometer (appendix 3a; also see Basic Protocol 1, step 7).
The resuspension volume should be determined based on volume necessary for subsequent assays. Alveolar macrophages are adherent and can be replated for in vitro experiments. Following an adhesion step, the cells are typically greater than 90% macrophages.
-
To ensure that the suspension cell preparation is enriched for alveolar macrophages, perform a differential staining on Cytospin cells as described in Basic Protocol 1, step 8.
Briefly, a total of 0.25 × 105 cells are added to Cytospin funnels and stained with Diff-Quik staining solutions.
CHARACTERIZATION OF MURINE MACROPHAGES WITH DIRECTLY CONJUGATED ANTIBODIES
This protocol describes the phenotypic characterization of macrophages using fluorescently labeled monoclonal antibodies (mAbs) that specifically recognize proteins expressed by macrophages. These sets of surface markers can be used to distinguish macrophages from other cell types in a heterogeneous population of cells. Through the use of flow cytometry, these cells can be identified and sorted from a heterogeneous population for further analysis.
Monoclonal antibodies used to characterize specific target cell designations are chosen based upon the ability to distinguish macrophages from other hematopoietic cells. Generally, a combination of antibodies against several cell-specific antigens is necessary to identify a specific cell type with confidence. Because there are some excellent markers for macrophages, often a 2-color combination of surface markers is adequate (see Fig. 14.1.1A). However for some procedures, a conservative three-color panel can be used to definitively identify macrophages. This panel includes two surface antigens (F4/80 and CD11b) and an intracellular antigen (CD68). The three main antigens that are unique to macrophages, and the mAbs used to identify them, are described in Table 14.1.1. Often this approach can be complemented by parallel staining for surface markers on non-macrophage cells (see Table 14.1.2). Macrophages would be expected to be negative for these markers, and the inclusion of some of these markers in the analysis can provide information about the degree of non-macrophage contamination in the cell suspension. In this three-color immunofluorescence protocol, each antibody is used alone, and then all three are used together in a cocktail. The inclusion of single-color samples is important to optimize instrumentation compensation, a procedure that is necessary for three-color flow cytometry. Antibodies that are directly conjugated with a different fluorescent tags are used for this procedure. After the cell suspension is incubated with the appropriate mAb cocktail, cells are processed for flowcytometry as described in detail in units 5.3 & 5.4.
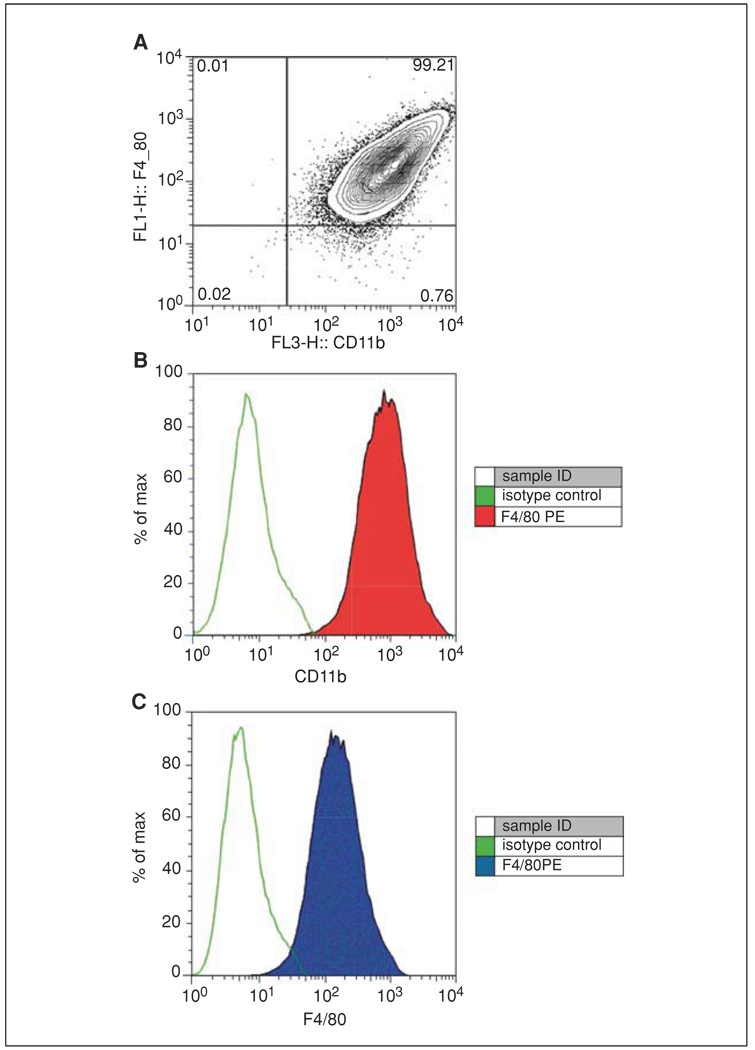
Figure 14.1.1.
F4/80 and CD11b expression on bone marrow–derived macrophages. BALB/c macrophages were cultivated in L-cell conditioned medium for 7 days and then processed for flow cytometry. A 1:200 dilution of PE-conjugated anti-mouse F4/80 mAb BM8 (eBioscience, cat no. 12-4801) and a 1:800 dilution of PerCP Cy5.5 M1/70 antibody to CD11b (BD Pharmingen, cat no. 550993) were added. Cells were examined on a BD FACScalibur flow cytometer. For the color version of this figure go to http://www.currentprotocols.com.
Table 14.1.1.
Monoclonal Antibodies Frequently Used to Characterize Murine Macrophages
| Antibody | Clone | Cell type | Reference |
|---|---|---|---|
| Anti-F4/80 | BM8 | Macrophages | McGarry and Stewart (1991) |
| Anti-CD11b (Mac-1 beta chain) | M1/70 | Macrophages | Ho and Springer (1982) |
| Anti-CD68 (macrosialin) | FA-11 | Macrophages | Rabinowitz and Gordon (1991) |
Materials
1.2 × 107 cell/ml test cell suspension: macrophages from any anatomical compartment (see Basic Protocols 1 to 3), or macrophages differentiated in culture from bone marrow precursors
Dulbecco’s phosphate-buffered saline without calcium and magnesium (Mediatech, cat. no. 21-031-CV), cold
Pharm Lyse Lysing Buffer (BD Biosciences cat. no. 555899)
Blocking solution: 5% (v/v) FBS in PBS
Fc receptor blocking antibody: anti-CD16/32 (eBioscence cat. no. 14-0161-86, clone 93; http://www.ebioscience.com)
Fluorescence-labeled monoclonal antibodies (MAbs; Table 14.1.1, Table 14.1.2, Table 14.1.3)
Cytofix/Cytoperm Kit (BD Biosciences cat. no. 554714)
2% paraformaldehyde (see recipe)
14-ml round-bottom polypropylene tubes, 17 × 100–mm (BD Biosciences, cat. no. 352030)
Clear 96-well U-bottom polystyrene assay plate (BD Microtest, BD Biosciences cat. no. 353910)
Refrigerated centrifuge with microtiter plate carrier
96-well polypropylene cluster tubes with rack (Corning Life Sciences cat. no. 4410)
Additional reagents and equipment for counting cells (appendix 3a) and flow cytometry (Chapter 5)
Table 14.1.2.
Surface Markers on Other Leukocytes
| Markers on cells other than macrophages | mAb /clone |
|---|---|
| CD11c (dendritic cells) | HL3, N418 |
| CD3 (T cells) | 17A2, 500A2 |
| CD19 (B cells and peritoneal mast cells) | 1D3, MB19-1 |
| CD49b (NK cells) | DX5 |
| GR-1 (neutrophils and subset of monocytes) | RB6-8C5 |
| CD193 (eosinophils) | 83101 |
| CD23 (eosinophils) | B3B4, 2G8 |
Table 14.1.3.
Other Surface Markers Used for Macrophage Characterization
| Receptor (cluster designation) |
Other names | Receptor family | mAb Clone |
|---|---|---|---|
| CD204 | SR-A | Scavenger (collagenous) | 2F8 |
| CD36 | FAT | Scavenger (noncollagenous) | HM36 |
| CD14 | LPS-R | GPI-anchored | RmC5-3 |
| Sa14-2 | |||
| CD18 | CR3 β Chain | Integrin | C71/16 |
| M18/2 | |||
| CD11b | MAC-1, CR3 α | Integrin | M1-70 |
| chain | 3A33 | ||
| CD64, CD32, CD16 | FcRs | Ig superfamily | 93 |
| FCR4G8 | |||
| CD88 | C5aR | C5a receptor | 20/70 |
| 10/92 | |||
| CD303 | Dectin-2 | Type II-C-type lectin | D2.11E4 |
| CD209 | DC-SIGN | Type II-C-type lectin (single CTLD) |
ER-TR9 |
| CD205 | Mannose receptor | Type I-C-type lectin (multiple CTLD) |
NLDC-145 |
| CD282 | TLR2 | Toll-like receptors | T2.5 |
| CD284 | TLR4 | Toll-like receptors | MTS510 |
| CD80 | B7-1 | CD28/152 Receptor | 16-10A1 |
| 1G10 | |||
| CD86 | B7-2 | CD28/152 Receptor | PO3 |
| GL-1 |
NOTE: All antibodies should be diluted in blocking buffer unless otherwise indicated.
Prepare cells
-
1.
Add a total of 1.2 × 107 cells per sample to a 14-ml round-bottom polypropylene tube and pellet the cells by centrifugation for 10 min at 400 × g, 4°C.
-
2.
Resuspend the cells with 5 ml cold Dulbecco’s phosphate-buffered saline (without calcium and magnesium) per sample and centrifuge 10 min at 400 × g, 4°C. Remove supernatant and resuspend cells in 2 ml of room temperature 1 × BD Pharm Lyse lysing buffer. Incubate 2 min at room temperature.
This step is to lyse red blood cells. In some cases (e.g., bone marrow macrophages), this step is not necessary.
-
3.
Centrifuge the cells 10 min at 400 × g, 4°C. If the pellet is still red, repeat the lysis step. If not, discard the supernatant and resuspend the cells in 1 ml of blocking solution. Count cells with a hemacytometer (appendix 3a).
Some RBC contamination is not a problem because these cells are easily gated away during flow cytometry.
-
4.
For each test cell sample, add 100 µl of 1 × 107 cells/ml suspension to five wells of a U-bottom 96-well plate (1 × 106 cells total).
Cells in well no. 5 are the controls, which receive no antibody. Control wells are important because they are used to adjust for autofluorescence derived from the cells themselves. Macrophages typically exhibit high autofluorescence.
Stain cells with antibodies
In three of the five wells, cells will be stained with individual mAbs, and in the fourth well cells will be stained with a cocktail containing all three mAbs. The optimal concentrations of antibody indicated by the manufacturer should be considered reasonable starting points, but they should not be the only concentrations tested. The optimal amount of mAb can vary with the instrument and with the cell population, and therefore must be determined empirically by each laboratory. See Critical Parameters.
-
5.
Add 1 µl of Fc-receptor blocking antibody to each well and incubate for 15 min on ice.
This step is included to block the binding of labeled mAbs to Fc receptors, which are abundantly expressed on macrophages (see unit 14.8).
-
6.
Add the appropriate concentrations of anti-F4/80-PE to well no. 1, anti-CD11b-PerCP-Cy5.5 to well no. 2, and anti-F4/80-PE and anti-CD11b-PerCP-Cy5.5 to well no. 4.
This step will stain macrophage surface antigens. Often these two antigens are sufficient to identify macrophages within a population.
-
7.
Keep the 96-well plate in the dark at 4°C for 30 min.
-
8.
Centrifuge the plate for 5 to 10 min at 400 × g, 4°C, in a plate carrier.
-
9.
Discard the supernatants from the wells by rapidly “flicking” the plate contents into the sink or by careful aspiration. Be careful to do not disturb the pellets.
-
10.
Vortex briefly to loosen the pellets.
-
11.
Add 100 µl of Cytofix/Cytoperm reagent (from BD Cytofix/Cytoperm Kit) to each well and incubate for 20 min in the dark at 4°C.
This step will permeabilize cells for intracellular staining.
-
12.
Centrifuge 10 min at 400 × g, 4°C, and wash twice with 200 µl of 1× Perm/Wash buffer from the Cytofix/Cytoperm Kit.
-
13.
Centrifuge for 5 to 10 min at 400 × g, 4°C, and discard the supernatant.
-
14.
Vortex carefully to loosen pellets.
-
15.
Add 50 µl of 1× Perm/Wash buffer from the Cytofix/Cytoperm Kit to each well and add anti-CD68-FITC to wells no. 3 and 4.
-
16.
Keep in the dark at 4°C for 30 min.
-
17.
Centrifuge for 5 to 10 min at 400 × g, 4°C, and remove the supernatant. Wash the pellet twice with 200 µl of 1× Perm/Wash buffer, centrifuging 5 min at 400 × g, 4°C, and discarding the supernatant each time.
Analyze cells
-
18.
Resuspend the cells in PBS and transfer to 1.2-ml polypropylene cluster tubes for immediate analysis, or fix in 200 µl of 2% paraformaldehyde and store at 2° to 8°C in the dark.
Fixed cells should be analyzed within 18 hr.
-
19.
Perform flow cytometric analysis (see Chapter 5).
IMPORTANT NOTE: Dead cells can bind to antibodies and result in false positive results. There are several ways to detect dead cells by flow cytometry. Some reagents that can be used to determine cell viability are: propidium iodide (Sigma-Aldrich), Fixable Green Dead Cell Stain Kit (Invitrogen, cat. no. L23101), and 7-AAD (Calbiochem).
REAGENTS AND SOLUTIONS
Use deionized, distilled water in all recipes and protocol steps. For common stock solutions, see appendix 2a; for suppliers, see appendix 5.
Bio-Gel, 2%
Wash 2 g of Bio-Gel P-100 gel (BioRad, cat. no. 150-4174) three times with 30 ml of Dulbecco’s phosphate-buffered saline (without calcium and magnesium). Pellet Bio-Gel by centrifugation for 5 min at 400 × g, then resuspend in 100 ml Dulbecco’s phosphate-buffered saline (without calcium and magnesium) to yield 2% (w/v) Bio-Gel. Autoclave for 20 min before use.
Brewer thioglycollate medium, 3%
Prepare3%(w/v) Brewer thioglycollate medium (BD BBL Thioglycollate Medium, Brewer Modified, cat. no. 211716) in distilled water Bring solution to boil to dissolve all solids and autoclave to sterilize. Store in the dark for up to 3 months (or longer) at room temperature. Discard if turbidity develops (indicating bacterial contamination).
DMEM/F12 and DMEM/F12-10
DMEM/F12 medium
Purchase Dulbecco’s Modified Eagle Medium/F12 (DMEM/F12; Invitrogen, cat. no. 10565 (DMEM/F12). Supplement with 10 mM l-glutamine, 100 IU/ml penicillin, and 100 µg/ml streptomycin (penicillin and streptomycin can be replaced by 50 µg/ml gentamicin sulfate).
DMEM/F12-10 medium
To prepare DMEM/F12-10, additionally supplement DMEM/F12 medium with 10% (v/v) fetal bovine serum (FBS; Hyclone).
L-929 conditioned medium
L-929 is a murine fibroblast cell line that is used as a source of M-CSF (Austin et al., 1971; Stanley, 1985). L-929 cells (ATCC no. CCL-1) are cultured in DMEM/F12- 10 (see recipe) until confluent. L-929 conditioned medium is then harvested at confluence; cells are removed by centrifugation, and supernatants are stored at −80°C until use.
Macrophage complete medium
Supplement DMEM/F12-10 (see recipe) with either 100 U/ml recombinant M-CSF (mouse M-CSF, CYT-439, ProSpec-Tany TechnoGene Ltd., http://www.ProSpecBio.com) or 20% (v/v) L-929 conditioned medium (see recipe). The latter is less expensive and works quite well, but other growth factors may be present in low concentrations in L-929 conditioned medium.
Proteose peptone, 3%
Prepare 3% (w/v) Bacto Proteose Peptone (BD Difco, cat. no. 211684) in distilled water. Bring solution to a boil to dissolve all solids and autoclave to sterilize. Store up to 3 months at 4°C and warm to room temperature before use. Discard if turbidity develops (indicating bacterial contamination).
COMMENTARY
Background Information
Resident and elicited peritoneal macrophages and bone marrow–derived macrophages have been used by many groups to dissect the many complex functions of macrophages (Cohn, 1978; Gordon, 2007). The murine peritoneal cavity provides a convenient site to obtain either resident or elicited macrophages (Edwards et al., 2006). Sterile irritants such as thioglycollate, Bio-Gel, or proteose peptone are used to enhance the yield of peritoneal macrophages (Hoover and Nacy, 1984; Mahoney et al., 2000). These elicited “inflammatory” macrophages not only differ in number, but are also functionally different from resident cells. They have an increased rate of plasma membrane turnover as well as increased phagocytic and respiratory burst capacity, and can exhibit variable responses to cytokine stimulation. Cells isolated from the peritoneum are a more heterogeneous population than bone marrow–derived macrophages. Alveolar macrophages are functionally different from peritoneal macrophages. Peritoneal macrophages are F4/80high, CD11bhigh, and CD68+. Bone marrow–derived macrophages also express F4/80high, CD11bhigh, and CD68+. However, alveolar macrophages display a F4/80low, CD11blow, and CD68+ phenotype under normal physiological conditions.
Critical Parameters
Macrophages are acutely sensitive to the biological effects of endotoxin; thus, all reagents must be of high quality and endotoxin-free. Use of pathogen-free mice is equally critical, because macrophages responding to an ongoing infection may be refractory to in vitro stimuli. If the yield of peritoneal cells seems unusually high, then the possibility of an ongoing low level of infection in the colony should be considered. Cell suspensions from an unelicited peritoneal cavity will typically contain ~50% macrophages. Fluids from elicited peritoneal cavities will vary depending on the eliciting agent used, and can range from 50% to 75% macrophages. Diff-Quik staining is a fast and convenient way to determine differential cell counts. To quantitate the number of macrophages in peritoneal fluids, the total leukocyte count from the hemacytometer is multiplied by the percent of macrophages (determined from the differential cell count).
CD68 is a predominantly late endosomal glycoprotein that is also expressed on the cell surface. Permeablization enhances the staining of this (predominantly) intracellular antigen. The methanol modification of cell permeabilization can be considered if the antibody is FITC- conjugated, but it is not recommended when PE-conjugated antibodies are used.
Anticipated Results
Peritoneal macrophage
In general, one can expect to harvest 1–2 × 106 total peritoneal exudate cells from an unmanipulated, “clean” mouse. This harvest will contain ~50% macrophages. A mouse previously inoculated with thioglycollate will contain 1–2 × 107 peritoneal exudate cells, of which 70% may be macrophages. The yield of macrophages derived from bone marrow progenitor cells should be 5–20 × 107 per mouse following 7 days of liquid culture in M-CSF. This number may vary depending on the biological activity of M-CSF. The yield of alveolar macrophages is generally around 3–5 × 105 per mouse. Typically, 80% of these cells are alveolar macrophages.
Time Considerations
The harvesting peritoneal macrophages from 10 mice should take less than 1 hr.
The harvesting of bone marrow macrophages depends on how quickly femurs and tibias are obtained and the bone marrow extracted. This harvesting (four bones per mouse) should take ~1 hr. Cultures must be fed on day 3 and harvested on day 7. The isolation of alveolar macrophages will take more time because of the modest yields, which require the harvesting of macrophages from 10 or more mice. This procedure becomes much faster with experience, but in general one should plan to spend a minimum of 2 hr to collect alveolar lavages from 10 mice. It is therefore important to store lavaged cells on ice prior to centrifugation.
Literature Cited
- Anderson CF, Mosser DM. Cutting edge: Biasing immune responses by directing antigen to macrophage Fc gamma receptors. J. Immunol. 2002;168:3697–3701. doi: 10.4049/jimmunol.168.8.3697. [DOI] [PubMed] [Google Scholar]
- Austin PE, McCulloch EA, Till JE. Characterization of the factor in L-cell conditioned medium capable of stimulating colony formation by mouse marrow cells in culture. J. Cell Physiol. 1971;77:121–134. doi: 10.1002/jcp.1040770202. [DOI] [PubMed] [Google Scholar]
- Cohn Z. The activation of mononuclear phagocytes: Fact, fancy, and future. J. Immunol. 1978;121:813–816. [PubMed] [Google Scholar]
- Edwards JP, Zhang X, Frauwirth KA, Mosser DM. Biochemical and functional characterization of three activated macrophage populations. J. Leuk. Biol. 2006;80:1298–1307. doi: 10.1189/jlb.0406249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier AH, Hoover DL, Nacy CA. Intracellular replication of Leishmania tropica in mouse peritoneal macrophages: Amastigote infection of resident cells and inflammatory exudate macrophages. Infect. Immun. 1982;38:1304–1308. doi: 10.1128/iai.38.3.1304-1308.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S. The macrophage: Past, present and future. Eur. J. Immunol. 2007;37:S9–S17. doi: 10.1002/eji.200737638. [DOI] [PubMed] [Google Scholar]
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- Ho MK, Springer TA. Mac-1 antigen: Quantitative expression in macrophage populations and tissues, and immunofluorescent localization in spleen. J. Immunol. 1982;128:2281–2286. [PubMed] [Google Scholar]
- Hoover DL, Nacy CA. Macrophage activation to kill Leishmania tropica: Defective intracellular killing of amastigotes by macrophages elicited with sterile inflammatory agents. J. Immunol. 1984;132:1487–1491. [PubMed] [Google Scholar]
- Mahoney JA, Haworth R, Gordon S. Monocytes and macrophages. In: Dallman MJ, Lamb JR, editors. Haematopoietic and Lymphoid Cell Culture. Cambridge: Cambridge University Press; 2000. pp. 121–146. [Google Scholar]
- Maus U, Herold S, Muth H, Maus R, Ermert L, Ermert M, Weissmann N, Rosseau S, Seeger W, Grimminger F, Lohmeyer J. Monocytes recruited into the alveolar air space of mice show a monocytic phenotype but upregulate CD14. Am. J. Physiol. Lung Cell Mol. Physiol. 2001;280:L58–L68. doi: 10.1152/ajplung.2001.280.1.L58. [DOI] [PubMed] [Google Scholar]
- McGarry MP, Stewart CC. Murine eosinophil granulocytes bind the murine macrophage-monocyte specific monoclonal antibody F4/80. J. Leukoc. Biol. 1991;50:471–478. doi: 10.1002/jlb.50.5.471. [DOI] [PubMed] [Google Scholar]
- Morimoto K, Amano H, Sonoda F, Baba M, Senba M, Yoshimine H, Yamamoto H, Ii T, Oishi K, Nagatake T. Alveolar macrophages that phagocytose apoptotic neutrophils produce hepatocyte growth factor during bacterial pneumonia in mice. Am. J. Respir. Cell Mol. Biol. 2001;24:608–615. doi: 10.1165/ajrcmb.24.5.4292. [DOI] [PubMed] [Google Scholar]
- Rabinowitz SS, Gordon S. Macrosialin, a macrophage-restricted membrane sialoprotein differentially glycosylated in response to inflammatory stimuli. J. Exp. Med. 1991;174:827–836. doi: 10.1084/jem.174.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss-Ayali D, Conrad SM, Mosser DM. Monocyte subpopulations and their differentiation patterns during infection. J. Leukoc. Biol. 2007;82:244–252. doi: 10.1189/jlb.0307191. [DOI] [PubMed] [Google Scholar]
- Stanley ER. The macrophage colony-stimulating factor, CSF-1. Methods Enzymol. 1985;116:564–587. doi: 10.1016/s0076-6879(85)16044-1. [DOI] [PubMed] [Google Scholar]
- Steele C, Marrero L, Swain S, Harmsen AG, Zheng M, Brown GD, Gordon S, Shellito JE, Kolls JK. Alveolar macrophage-mediated killing of Pneumocystis carinii f. sp. muris involves molecular recognition by the Dectin-1 beta-glucan receptor. J. Exp. Med. 2003;198:1677–1688. doi: 10.1084/jem.20030932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren MK, Vogel SN. Bone marrow-derived macrophages: Development and regulation of differentiation markers by colony stimulating factor and interferon. J. Immunol. 1985;134:982–989. [PubMed] [Google Scholar]
- Yoshikawa K, Suzuki Y, Kawai M, Fukada M, Yokochi T. Novel cell surface antigens expressed on mouse alveolar macrophages. Microbiol. Immunol. 1991;35:803–807. doi: 10.1111/j.1348-0421.1991.tb01613.x. [DOI] [PubMed] [Google Scholar]