Abstract
Cell penetrating peptides are useful delivery tools for introducing molecules of interest into cells. A new class of cell penetrating molecules has been recently reported--cell penetrating, prenylated peptides. In this study a series of such peptides was synthesized to examine the relationship between peptide sequence and level of peptide internalization and to probe their mechanism of internalization. This study revealed that prenylated peptides internalize via a non-endocytotic pathway regardless of sequence. Sequence length and identity was found to play a role in peptide uptake but prenylated sequences as short as two amino acids were found to exhibit significant cell penetrating properties.
Prenylation is a post-translational modification resulting in the addition of a 15 (farnesyl) or 20 (geranylgeranyl) carbon isoprenoid chain near a protein’s C-terminus.1–3 The 1% of the proteome that is prenylated4 consists of proteins that end in specific amino acid sequences which flag the protein for enzymatic modification. Prenyl modification is important for the localization of the protein inside of cells and is crucial to the protein’s role in cellular signaling.5,6 Recently we discovered that prenylated peptides have intrinsic cell penetrating properties.7 These previous studies determined that the prenyl moiety on these peptides was crucial for their internalization but did not examine the relationship between the peptide length and sequence in modulating their levels of internalization.
Most cell penetrating peptides are from five to twenty amino acids long and have several positively charged amino acids.8 Both a net positive charge and degree of hydrophobicity play crucial roles in CPP internalization.9 The size and charge of many of these molecules hinder their attractiveness as molecular transporters. This study examines the role that prenylation plays in reducing both the size and charge needed for cellular entry. A series of geranylgeranylated peptides differing in charge and length were synthesized to study the contribution prenylation plays on cell penetrating ability relative to sequence identity and charge.
Peptides were assembled by automated Fmoc SPPS, starting with Rink-amide (peptides 1–6, 9) or Rink acid (peptides 7–8) resin using an ABI 430 Synthesizer (Figure 1). A 5-Fam fluorophore was coupled to either the N-terminus or to lysine side chains through the use of an orthogonal protection strategy employing a Dde group (1-(4,4-dimethyl-2,6-dioxocyclo-hexylidene)ethyl). Peptides were then cleaved from the resin and prenylated using geranylgeranyl bromide and Zn(OAc)2.10 For peptide 8 the sequence Fam-KKSRRC(Acm)VLL was prepared and the Acm protecting group (acetamidomethyl) was converted to a Scm (S-carboxymethyl) group by treatment with CH3O(CO)SCl under acidic conditions.11 The more reactive Scm group then underwent disulfide exchange upon treatment with penetratin (Pnt) at pH 2.
Figure 1.
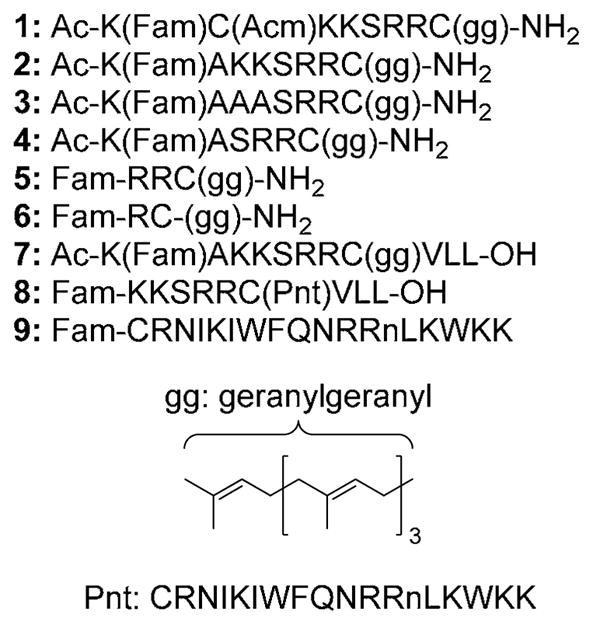
Cell penetrating peptides tested in this study. Compounds 1–7 have prenyl modifications on cysteine via thioether linkages. The linkage between Pnt and Cys in compound 8 is via a mixed disulfide. Lower case n in Pnt sequence encodes a norleucyl residue.
The internalization of fluorescein-linked peptides was investigated using a combination of confocal laser scanning microscopy (CLSM) and fluorescence activated cell sorting (FACS) analysis. For microscopy HeLa cells were treated for 1 h, washed, fixed in 3.7% formaldehyde and imaged. Live cell images were obtained from similarly prepared cells that were not subjected to the formaldehyde treatment. For microscopy, Hoechst 34850 and wheat germ agglutinin Alexa Fluor 594 conjugate were used to identify the nucleus and plasma membrane, respectively (Figure 2). Live images of treated cells were comparable to fixed images. This observation, along with the observation of significant peptide uptake from flow cytometry analysis of live cell samples, suggests that fixing cell samples did not render them more permeable to uptake. Examples of fixed and live cell images are shown in panels A–D and E–H in Figure 2. To quantify the internalization levels of the various peptides, cells were treated with peptide (0.3 μM – 3.0 μM) for 1 h at 37 °C, trypsinized, and washed to remove any peptide adhered to the cell surface before FACS analysis.
Figure 2.
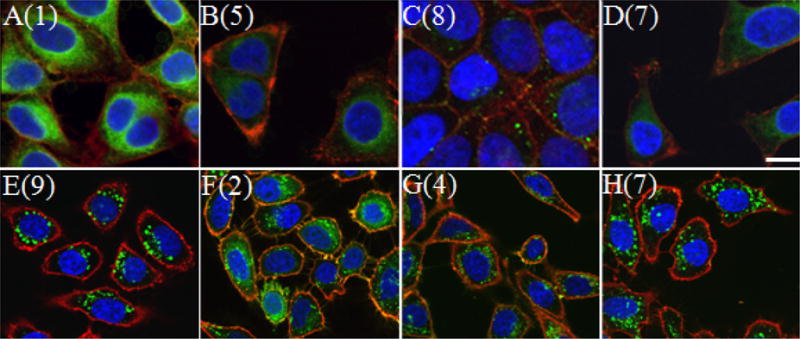
Confocal microscopy images of HeLa cells treated with cell-penetrating peptides. Cells in panels A–D were treated with 1 μM peptide for 1h and fixed before imaging. Cells in panels E–H were treated with 3 μM peptide and imaged live. Panel A: Cells treated with peptide 1. Panel B: Cells treated with peptide 5. Panel C: Cells treated with peptide 8. Panel D: Cells treated with peptide 7. Panel E: Cells treated with peptide 9. Panel F: Cells treated with peptide 2. Panel G: Cells treated with peptide 4. Panel H: Cells treated with peptide 7. Hoechst 34850 blue DNA staining dye was used to stain the nucleus blue and wheat germ agglutinin Alexa Fluor 594 was used to stain the plasma membrane red. The size bar represents a distance of 20 μm.
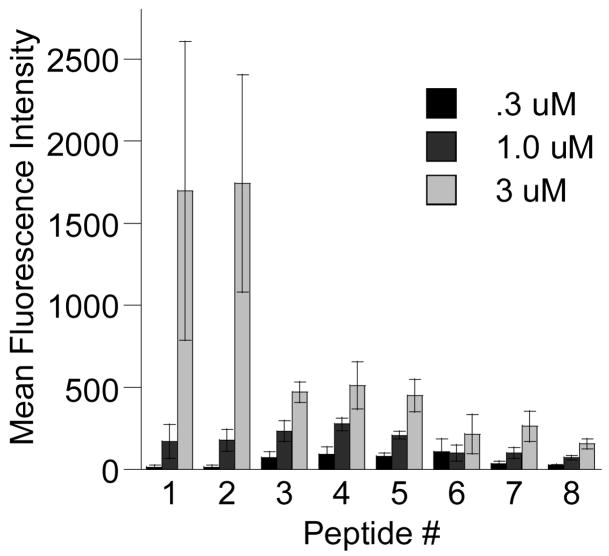
Using the above techniques, the contribution from various amino acids to cellular uptake of prenylated peptide 1 was investigated using the series of peptides shown in Figure 1. Peptide 2 showed that removing the Cys(Acm) residue from 1 did not affect internalization at any treated concentration (Student’s t-test, p > 0.05, Figure 3). Lysine’s contribution to peptide uptake was investigated using peptides 3 and 4. Removing lysines (peptide 4) or changing lysines to alanine (peptide 3) caused a decrease in peptide internalization for 3.0 μM treatments (p < 0.05), but did not hinder uptake when treated at lower concentrations (compare peptides 3 and 4 to peptide 2 in Figure 3). Prenylated tripeptide 5 showed that truncating the peptide even further causes no statistically significant decreases in uptake (p > 0.05 for all concentrations, compare peptide 4 to 5 in Figure 3). However truncating the sequence further caused statistically significant drops in internalization for 1.0 μM and 3.0 μM treatments (p < 0.05, compare peptide 6 to peptide 4 in Figure 3). This result may be attributed to small amounts of peptide precipitation observed for this peptide after incubation with cells for 1 h.
Figure 3.
Uptake of peptides 1–8 in HeLa cells treated at 37 °C determined by FACS analysis. After incubation with 0.3 μM, 1.0 μM, or 3.0 μM peptide, the cells were trypsinized and washed to remove any surface-bound peptide and subsequently had their fluorescence measured by flow cytometry. Each bar represents the fluorescence of 10,000 cells counted by FACS analysis and each experiment was performed in triplicate. The results are expressed as the geometric mean relative fluorescence ± standard deviation.12
The role of charge at the C-terminus was investigated by analyzing the uptake of peptide 7 which included the full “CAAX box” of a naturally prenylated protein that ends in a terminal acid. Peptide 7 manifested lower overall levels of internalization for 3.0 μM treatments when compared to peptides 1–4 (p < 0.05) but had similar levels of uptake to these peptides for lower (0.3 μM) concentrations (p > 0.05). Unprenylated versions of 1–7 did not exhibit cellular uptake when analyzed by CLSM. This indicates that prenylation is necessary for this series of peptides to penetrate cells. Peptide 8, a version of 7 in which the geranylgeranyl group was swapped with a penetratin moiety, had uptake levels similar to those of geranylgeranylated peptide 7 at all treated concentrations (p > 0.05). This indicates the geranylgeranyl group of peptide 7 contributes to cell penetrating ability as much as the penetratin peptide does for peptide 8.
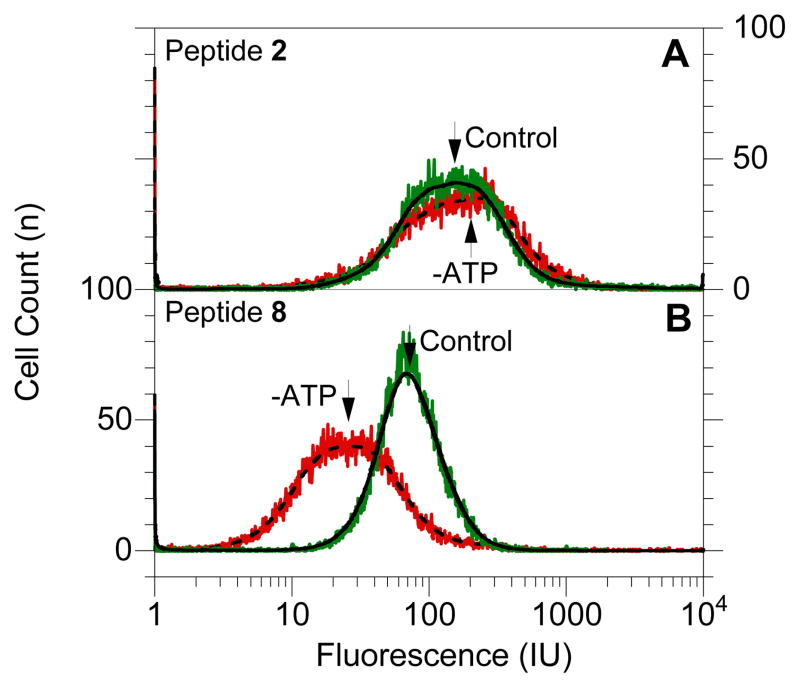
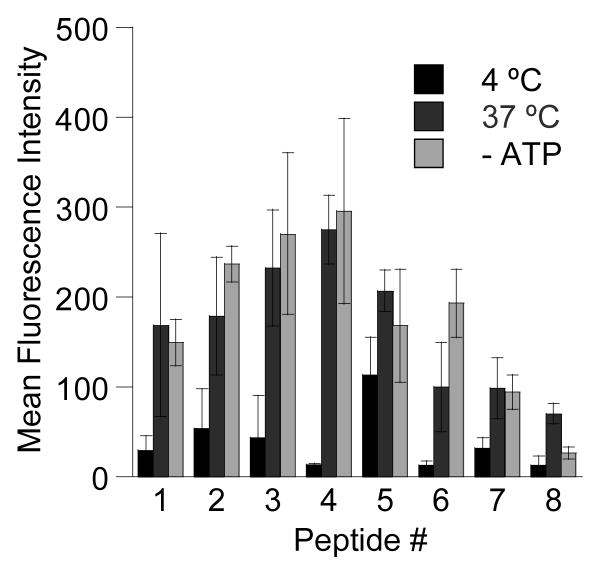
Using CLSM and FACS, the effects of temperature and reducing cellular ATP levels13 on the internalization of peptides 1–8 were also investigated to deduce information on the internalization mechanisms of these peptides (See CLSM images in S-1). Representative FACS data for HeLa cells treated with peptides 2 and 8 with and without ATP depletion is shown in Figure 4; under ATP depleting conditions, a significant shift in the mean fluorescence (peptide internalization) was observed for peptide 8 but not for peptide 2. A summary of the mean fluorescence obtained from FACS analysis is shown in Figure 5. All geranylgeranylated peptides (1–7) exhibited minimal changes in mean fluorescence upon ATP depletion. (p > 0.05). However, penetratin-linked peptide 8 showed substantially lower internalization levels when ATP was depleted (p < 0.005). In fact under those later conditions, the internalization of 8 was reduced to the levels comparable to those observed at 4 °C (p > 0.05). This suggests the prenylated peptides enter via an ATP-independent manner while penetratin linked peptide 8 enters via an ATP-dependant manner. All peptides showed reduced levels of internalization at 4 °C. Since cellular membranes have decreased fluidity at lower temperatures it is reasonable to expect lower internalization levels even for peptides that enter via energy-independent processes. This has been reported for other cell penetrating peptides.14 CLSM experiments (see Figure 2) also reveal morphological differences suggesting that the prenylated peptides enter cells via processes that are different than those for penetratin. Images of cells treated with penetratin-containing peptides (8 and 9) reveal a highly punctate pattern of fluorescence, characteristic of endocytotic uptake (for example, see Figure 2E). In contrast, images of cells treated with prenylated peptides (1–7) are characterized by a more diffuse pattern of fluorescence (see Figure 2F). Interestingly, variable amounts of punctate fluorescence are also observed with the prenylated peptides. The significance of those observations is not yet clear.
Figure 4.
FACS analysis of HeLa cells treated with peptides 2 or 8 under control (37 °C) and ATP-depleted conditions. Panel A: Analysis of cells treated with peptide 2. Panel B: Analysis of cells treated with peptide 8. In each panel, raw data is shown in color (control, 37°C: green; ATP-depleted: red) and smoothed data is shown as lines (37 °C: solid line; ATP-depleted: broken line).
Figure 5.
Uptake of peptides 1–8 in HeLa cells treated at 4 °C, 37 °C and under ATP-depleting conditions determined by FACS analysis. After incubation with 1.0 μM peptide, the cells were trypsinized and washed to remove any surface-bound peptide and subsequently had their fluorescence measured by flow cytometry. Each bar represents the fluorescence of 10,000 cells counted by FACS analysis and each experiment was performed in triplicate and the results are expressed as the geometric mean relative fluorescence ± standard deviation.12
In conclusion the cell-penetrating ability of peptides presented here is determined primarily by their prenylation state. While 1 and 2 showed the highest levels of uptake at 3 μM, all prenylated peptides were internalized at 1 μM at levels comparable to or greater than penetratin-linked peptide 8 including tetrapeptide 5 and dipeptide 6. This is especially significant since most known cell penetrating peptides are at least four amino acids long. For instance oligoarginines are required to be at least 5 residues long in order to exhibit cell penetrating ability.15 Another appealing feature of these peptides is their ability to enter cells though ATP-independent processes. This allows them to avoid endosomal entrapment. Finally, we note that modification of peptides with other hydrophobic moieties such as decyl9 or myristoyl groups16 has been shown to increase a peptide’s cell-penetrating ability; like geranylgeranylation, the hydrophobicity of these modifications may impart ATP-independent uptake. The size, uptake efficiency, and internalization mechanism make prenylated cell-penetrating molecules interesting candidates for further study as cellular transport vehicles.
Supplementary Material
Acknowledgments
The authors thank Mr. Gregg Amundson, Mr. Jerry Sedgewick and Mr. John Oja of the University of Minnesota Biomedical Image Processing Lab for technical assistance regarding cell imaging. We would like to acknowledge the assistance of the Flow Cytometry Core Facility of the Masonic Cancer Center, a comprehensive cancer center designated by the National Cancer Institute, supported in part by P30 CA77598. This work was supported by the National Institutes of Health (Grant No GM058842, CA104609 and T32GM008347).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schafer WR, Rine J. Annu Rev Genet. 1992;26:209–37. doi: 10.1146/annurev.ge.26.120192.001233. [DOI] [PubMed] [Google Scholar]
- 2.Zhang FL, Casey PJ. Annu Rev Biochem. 1996;65:241–269. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]
- 3.Chow M, Der CJ, Buss JE. Curr Opin Cell Biol. 1992;4:629–36. doi: 10.1016/0955-0674(92)90082-n. [DOI] [PubMed] [Google Scholar]
- 4.Barbacid M. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- 5.Roberts MJ, Troutman JM, Chehade KAH, Cha HC, Kao JPY, Huang X, Zhan CG, Peterson YK, Subramanian T, Kamalakkannan S, Andres DA, Spielmann HP. Biochemistry. 2006;45:15862–15872. doi: 10.1021/bi061704+. [DOI] [PubMed] [Google Scholar]
- 6.Michaelson D, Silletti J, Murphy G, D’Eustachio P, Rush M, Philips MR. J Cell Biol. 2001;152:111–126. doi: 10.1083/jcb.152.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wollack JW, Zeliadt NA, Mullen DG, Amundson G, Geier S, Falkum S, Wattenberg EV, Barany G, Distefano MD. J Am Chem Soc. 2009;131:7293–7303. doi: 10.1021/ja805174z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansen M, Kilk K, Langel U. Adv Drug Delivery Rev. 2008;60:572–579. doi: 10.1016/j.addr.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Carrigan CN, Imperiali B. Anal Biochem. 2005;341:290–298. doi: 10.1016/j.ab.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 10.Xue CB, Becker JM, Naider F. Tetrahedron Lett. 1992;33:1435–8. [Google Scholar]
- 11.Hiskey RG, Muthukumaraswamy N, Vunnam RR. J Org Chem. 1975;40:950–3. doi: 10.1021/jo00895a600. [DOI] [PubMed] [Google Scholar]
- 12.Macey MG. Flow Cytometry: Principals and Applications. Humana Press; Totowa, NJ: 2007. [Google Scholar]
- 13.Meriin AB, Yaglom JA, Gabai VL, Zon L, Ganiatsas S, Mosser DD, Zon L, Sherman MY. Mol Cell Biol. 1999;19:2547–55. doi: 10.1128/mcb.19.4.2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Letoha T, Gaal S, Somlai C, Czajlik A, Perczel A, Penke B. J Mol Recognit. 2003;16:272–279. doi: 10.1002/jmr.637. [DOI] [PubMed] [Google Scholar]
- 15.Wender PA, Mitchell DJ, Pattabiraman K, Pelkey ET, Steinman L, Rothbard JB. Proc Natl Acad Sci. 2000;97:13003–13008. doi: 10.1073/pnas.97.24.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ensenat-Waser R, Martin F, Barahona F, Vazquez J, Soria B, Reig JA. IUBMB Life. 2002;54:33–36. doi: 10.1080/15216540213823. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.