Abstract
PURPOSE
Variability in menstrual cycle length (largely determined by variation in follicular phase length) is related to several health outcomes, yet the causes of this variability are incompletely understood. We sought to identify characteristics associated with follicular phase length.
METHODS
We used the North Carolina Early Pregnancy Study to describe factors correlated with timing of ovulation (follicular phase length). Women collected daily urine specimens and recorded vaginal bleeding. Specimens were assayed for estrone 3-glucuronide and pregnanediol 3-glucuronide, which in turn were used to estimate the day of ovulation. All other variables were assessed through interview. Associations with follicular phase length were evaluated using a multiple regression model.
RESULTS
We determined follicular phase length for the first cycles of 201 women. Women with a history of miscarriage tended to have shorter follicular phases (2.2 days). Longer duration of oral contraceptive use and recent use (in the last 90 days) were both correlated with longer follicular phase. Occasional marijuana users (up to 3 times in the last 3 months) had a longer follicular phase than non-users (3.5 days); frequent users (>3) were almost two days longer than non-users.
CONCLUSIONS
The association between marijuana use and longer follicular phase is consistent with prior rhesus monkey research that shows ovulatory delay or inhibition.
Introduction
Variations in menstrual cycle length have been associated with risk factors for cardiovascular disease1 and with health outcomes such as breast cancer 2, myocardial infarction3, and hip fractures 4. Identifying factors that affect menstrual cycle length may provide insight into the biological mechanisms underlying those associations. The menstrual cycle is divided into two phases; the follicular phase begins with menstruation and ends just prior to ovulation while the luteal phase begins after ovulation and ends with the subsequent onset of menses. The timing of ovulation can be extremely variable, both within and between women, and the sources of this variance are essentially unknown. In contrast, the time between ovulation and the onset of the next menses is relatively stable 5. Thus, variability in the length of the follicular phase is the major contributor to menstrual cycle variability6.
In the literature, the most consistent predictor of follicular phase length and cycle length is the woman’s age: cycles become shorter as women get older, and then become markedly more variable at perimenopause7–18. In Western populations, longer menstrual cycles have been associated with higher BMI 8, later age at menarche 8, 19, increased parity 8, 18, and recent use of oral contraceptives20, 21. Shorter menstrual cycles and shorter follicular phases have been associated with lower education 18, heavy caffeine intake 22 and alcohol consumption 10. In two studies current smoking was associated with shorter cycle lengths 8, 23 while one study found no effect 24. A history of ever having smoked more than 100 cigarettes showed no association in one study 25 while in another, women with a history of ten or more pack-years of smoking were more likely to have shorter cycles 23.
Most of these analyses considered cycle length but did not specifically examine the follicular phase, which requires more intensive longitudinal data collection (usually including hormone assays). The goal of our descriptive analysis was to identify demographic, behavioral, and reproductive characteristics associated with follicular phase length. In addition to characteristics examined in previous studies, we also investigated the potential influence of marijuana smoking, which has been associated with alterations in menstrual cycle hormones in both humans and laboratory animals 26–29.
Materials and Methods
The North Carolina Early Pregnancy Study (NCEPS) was a prospective cohort study designed to investigate the risk of early pregnancy loss among healthy women. The details of the study design and laboratory methods are described elsewhere 30. Briefly, 221 women who were planning to become pregnant were recruited from local communities and enrolled at the time they discontinued using birth control in order to become pregnant. Women were asked about their demographic, reproductive, medical, and behavioral characteristics. Potential participants were excluded if they had a serious chronic illness or if they or their partners had a history of fertility problems. Women collected first-morning urine specimens and recorded presence or absence of bleeding every day until they became clinically pregnant or until 6 months had passed with no clinically-apparent pregnancy. All participants gave written informed consent and the study protocol was approved by the Institutional Review Board of the National Institute of Environmental Health Sciences.
To estimate the day of ovulation, urine specimens were assayed for estrone 3-glucuronide (a metabolite of estrogen) and pregnanediol 3-glucuronide (a metabolite of progesterone), as previously described 31. We used these ovulation data in conjunction with bleeding information to define follicular phase length as the number of days from the first day of menses up to (but not including) the estimated day of ovulation. Because the distribution of follicular phase length is non-normal and right-skewed, we analyzed the natural logarithm of follicular phase length and we present geometric means.
Women’s characteristics and exposures were based on self-report at the time of enrollment. We restricted our analysis to each woman’s first cycle in the study, corresponding most closely with the exposure information at enrollment. (Some women may have changed their behaviors after failing to conceive in their first cycles.)
Women reported their alcohol intake in “servings” per month, with one serving being equal to either 12 ounces of beer, 4 ounces of wine, or 1.5 ounces of hard liquor. We treated all servings as equivalent and combined them to create an index of total servings per month. Body mass index was calculated as weight in kilograms divided by height in meters squared, and categorized into underweight (<18.5), normal (18.5–<25.0), overweight (25.0–<30.0), and obese (>=30.0) 32. Caffeine intake was calculated by multiplying the amount of caffeine (in mg) typically found in a serving of brewed coffee, instant coffee, tea or soft drinks by the number of servings of each beverage consumed over the previous month. The average reported frequency of intercourse per week was divided into four categories. We dichotomized (“yes/no”) participants’ prenatal exposure to cigarette smoke, recent oral contraceptive use (within the 90 days preceding onset of their first cycle), and history of miscarriage. Duration of oral contraceptive use was measured in years, and set to 0.5 for those who had used OCs for less than one year. The same was done for IUD use. Tobacco use was categorized into current, former, and never. Marijuana use was categorized based on the participant’s response to the question: “How many times did you smoke marijuana during the last three months?” Women who reported smoking marijuana at least once but less than three times were classified as “low” frequency users, while women who smoked more than three times were considered “high” frequency users. All continuous variables were categorized based on the approximate 25th and 75th percentiles.
We modeled follicular phase length (log-transformed) using linear regression with SAS software (SAS Institute, Cary, NC). The p-values obtained from these models test the null hypothesis that all categories of a variable have the same overall mean follicular phase length; the alternative hypothesis is that at least one category differs from the others. Each characteristic of interest was modeled separately, adjusted for both age and recent oral contraceptive use. We also categorized follicular phase length into three groups: short (≤11 days), average (12–18 days), and long (≥18 days) and performed a polytomous logistic regression using each woman’s first cycle. The results were not qualitatively different from those using continuous follicular phase length (log transformed), and therefore are not presented.
Results
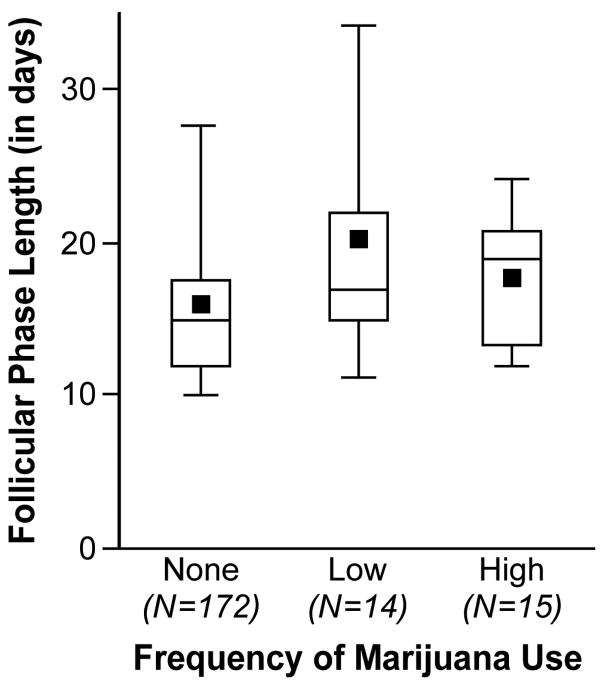
There were 201 women with a measurable follicular phase length in the first cycle of study participation. The geometric mean follicular phase length was 15.5 days, with an interquartile range of 12.0 to 19.0 days. The women ranged in age from 21–42 with a mean of 29. Age and recent oral contraceptive use showed a univariate association with follicular phase length, and thus were included in all subsequent models. The effect of age, however, was weakened when adjusted for recent oral contraceptive use, while recent oral contraceptive use remained important after adjustment for age (Table 1). Women with recent oral contraceptive use had a longer follicular phase (2.3 days, p=0.04). After adjusting for age and recent OC use, the only factors associated with follicular phase length were years of oral contraceptive use, a history of miscarriage and marijuana use. Follicular phase length generally increased with increasing duration of oral contraceptive use, with most of the increase occurring after 1 year of use. Among women who had ever been pregnant, those with a history of miscarriage had a shorter follicular phase (2.2 days, p=0.04). Marijuana use was associated with longer follicular phase length (p=0.04). Compared with non-users, occasional marijuana users had a follicular phase longer by 3.5 days, and more frequent users had a follicular phase longer by 1.7 days (Table 1, Figure 1).
TABLE 1.
Results of the linear regressions predicting the natural log of follicular phase length, with fitted values presented in days.a
| Variable | n (%) (N=217) | Geometric Mean | p-valueb |
|---|---|---|---|
| Physical and Lifestyle factors | |||
| Age | |||
| <27 | 49 (24.4) | 15.4 | 0.52 |
| 27–31 | 110 (54.7) | 15.4 | |
| >31 | 42 (20.9) | 14.5 | |
| Height (in meters) | |||
| <1.63 | 43 (21.4) | 15.5 | 1 |
| 1.63–1.68 | 106 (52.7) | 15.4 | |
| >1.68 | 52 (25.9) | 15.4 | |
| BMI | |||
| Underweight | 20 (9.9) | 15.2 | 0.91 |
| Normal | 159 (79.1) | 15.4 | |
| Overweight | 22 (10.9) | 15.9 | |
| Marijuana use | |||
| None | 172 (85.6) | 15.1 | 0.04 |
| Low | 14 (7.0) | 18.6 | |
| High | 15 (7.5) | 16.8 | |
| Tobacco smoking | |||
| Current | 12 (6.0) | 15.1 | 0.98 |
| Former | 49 (24.4) | 15.4 | |
| Never | 140 (69.6) | 15.4 | |
| Alcohol intake (servings/month)c | |||
| <=1 | 51 (25.4) | 14.4 | 0.19 |
| >1–18 | 102 (50.7) | 15.7 | |
| >18 | 48 (23.9) | 16.1 | |
| Caffeine intake (mg/week)d | |||
| <=1480 | 58 (28.9) | 14.6 | 0.2 |
| >1480–<6900 | 102 (50.7) | 15.4 | |
| >=6900 | 41 (20.4) | 16.5 | |
| Reproductive factors | |||
| Recente Oral Contraceptive Use | |||
| Yes | 29 (14.4) | 17.7 | 0.04 |
| No | 172 (85.6) | 15.4 | |
| Duration of pill use | |||
| Never | 26 (13.0) | 13.9 | 0.0003 |
| >0–1 | 44 (22.0) | 13.2 | |
| >1–2 | 28 (14.0) | 15.7 | |
| >2–4 | 41 (20.5) | 16.3 | |
| >4 | 61 (30.5) | 17.3 | |
| Missing | 1 | ||
| Duration of IUD use | |||
| Never | 141 (70.1) | 15.4 | 0.09 |
| >0–1 | 25 (12.4) | 15.8 | |
| >1–2 | 10 (5.0) | 14.8 | |
| >2–4 | 9 (4.5) | 12.7 | |
| >4 | 16 (8.0) | 18.4 | |
| Age at menarche | |||
| <12 | 32 (15.9) | 15.0 | 0.83 |
| 12,13 | 117 (58.2) | 15.4 | |
| >13 | 52 (25.9) | 15.7 | |
| Frequency of Intercourse (per week) | |||
| ≤1 | 39 (19.4) | 15.3 | 0.13 |
| >1–2 | 45 (22.4) | 14.6 | |
| >2–3 | 78 (38.8) | 16.5 | |
| >3 | 39 (19.4) | 14.6 | |
| Gravidity | |||
| 0 | 70 (35.0) | 15.5 | 0.73 |
| 1,2 | 110 (55.0) | 15.5 | |
| ≥3 | 20 (10.0) | 14.6 | |
| Missing | 1 | ||
| History of Miscarriage (among gravid women) | |||
| Yes | 28 (14.0) | 14.0 | 0.04 |
| No | 102 (51.0) | 16.2 | |
| Never pregnant | 70 (35.0) | ||
| Missing | 1 | ||
| Prenatal Exposures | |||
| DES | |||
| Yes | 7 (4.8) | 14.1 | 0.42 |
| No | 139 (95.2) | 15.6 | |
| Missing | 55 | ||
| In utero smoke exposure | |||
| No | 111 (65.3) | 15.4 | 0.29 |
| Yes | 59 (34.7) | 16.2 | |
| Missing | 31 | ||
Each variable is adjusted for age and recent pill use. Predicted mean cycle lengths are presented for a woman age 27–31 and a non-recent pill user.
p-value testing the null hypothesis that the groups share a common mean, the alternative is that at least one group is different.
A ”serving” was defined as 12 ounces of beer, 4 ounces of wine or 1.5 ounces of hard liquor.
100mg is approximately the amount of caffeine in one cup of brewed coffee
Within 90 days of cycle start.
Figure 1.
Discussion
In our data, recent use of oral contraceptives, extended use of oral contraceptives, and marijuana use were all associated with a longer follicular phase. Women with a history of miscarriage (among gravid women) tended to have shorter follicular phases.
One of the best documented factors affecting menstrual cycle length is women’s age. The absence of a clear age effect in our data is not so surprising, given the narrow age range of women. We also found no detectable effect of BMI, age at menarche, gravidity, caffeine intake, alcohol intake, or smoking on follicular phase length – all factors that have previously been associated with either menstrual cycle length or follicular phase length. The absence of such associations in our data may be due to the lack of variability in our sample. Also, our sample excluded women with chronic medical conditions or known reproductive problems; such conditions may be part of the causal pathway by which these variables affected cycle length in other studies.
Our results regarding longer follicular phases after recent oral contraceptive use are consistent with most previous studies. In two studies, women who discontinued oral contraceptives tended to have an increased cycle length for one to three cycles post-discontinuation 20, 21; in another, longer cycles persisted for 8 cycles33. In a recent study using ultrasonography, women in their first cycle after discontinuing oral contraceptives took more time to select and manifest a dominant follicle; ovulation was 5 days later than in unexposed cycles34. In contrast, one study reported no differences in cycle length between those who recently discontinued oral contraceptives and those who had not used them within three months of the study35. However, in this study there was a significantly longer median cycle length in one age group after discontinuation of oral contraceptives (ages 18–24), and non-significantly longer cycles in two other age groups (30–34 and 35–40). Thus, when stratified by age, there was some evidence of longer cycles. These findings are also consistent with a slightly longer time to pregnancy in women who recently discontinued oral contraceptives36–41. The cycle length studies considered only recent use; duration of use varied widely (from 4–10 years20, a few months to 13 years33, to >0 to 4 years21). However, in other studies duration of oral contraceptive use has shown no associations with such cycle-related outcomes as post-pill amenorrhea 20, 42 or inhibition of ovulation 43. There is some evidence that among women over 45 years of age, use of oral contraceptives for at least five years is associated with lower levels of follicle stimulating hormone (FSH) in the early follicular phase. Moreover, use of oral contraceptives within the past five years was associated with lower FSH levels regardless of age, which ranged from 26–5044. In turn, lower FSH levels were reported to be weakly associated with longer menstrual cycles in the same study sample45. In a recent randomized trial of continuous versus conventional oral contraceptive use, researchers found that as the duration of oral contraceptive use increased (from one to three cycles), the number of developing follicles decreased34. Thus, the authors suggest that the inhibition of follicle recruitment by oral contraceptives may compound over time, possibly through increased suppression of the hypothalamic-pituitary-ovarian axis. Whether this might have long term effects on future cycling is not known. The interpretation of our finding is complicated by the selective nature of women who choose to take oral contraceptives. For example, some women with irregular cycles may use oral contraceptives to help regulate their cycles, and such women may also have longer cycles.
We did not find any previous reports of shorter follicular phase length for women with a history of miscarriage, although short follicular phase and miscarriage might share hormonal determinants. One study has suggested that a shorter (<30 days) or longer (≥32 days) cycle length is associated with a spontaneous abortion in the following cycle 46, and another study found long cycles associated with history of miscarriage47. Previous analyses of our data did not find an association between usual cycle length (longer vs. other) or follicular phase length and risk of early pregnancy loss (occurring within six weeks of the last menstrual period). 48 However, early loss and clinical spontaneous abortion may be endpoints with distinct etiologies and risk factors.
Our finding of longer follicular phases with occasional marijuana use is intriguing. Animal research supports an effect of marijuana on ovulation. In rhesus monkeys, daily injections of 2.5 mg/kg Δ9-tetra-hydrocannabinol (THC) (the equivalent of 5–6 joints) during the follicular phase delayed or prevented ovulation49. In a longer-term study, the impact of THC on ovulation in rhesus monkeys appeared to decline over time, suggesting that tolerance may develop50.
These observations have not been definitively replicated in humans. Twenty-six women who used marijuana at least three times a week over the previous six months had shorter menstrual cycles, specifically shorter luteal phases, when compared to 17 non-marijuana users 51. We found little evidence in our study for an association between short luteal phase and marijuana-use (data not shown). The 26 marijuana users had lower prolactin levels, a slightly lower peak level of luteinizing hormone (LH), and a slower post-ovulatory rise in estrogen and progesterone 51. Another study that included 17 female chronic marijuana users (at least weekly for more than two years) found no differences in testosterone, LH, FSH, prolactin or cortisol levels when stratified by frequency of use or when comparing users and non-users 29. Studies of the acute effects of marijuana suggest that the timing of marijuana exposure in the menstrual cycle may modulate its effects on LH 26, 28. Marijuana use had no acute effect on progesterone or estradiol levels 26, 28. We do not have data on the timing of marijuana use in the menstrual cycle.
There are several possible reasons for the disparate human epidemiologic results. Marijuana users in the four previous (human) studies were, on average, heavier users than those in our study. The average frequency of marijuana use in earlier studies ranged from approximately 4 to 14 times per month26, 28, 29, 51 whereas the average among users in our sample was about 3 times per month. None of the studies, including ours, has accounted for the amount smoked at each exposure or the timing of the exposure in the menstrual cycle, which may also differ among the studies.
In the study of chronic users there was a slightly higher proportion of anovulatory cycles among marijuana users compared with non-users, but anovulation was rare and the difference was non-significant51. We also found an excess of anovulatory cycles among marijuana smokers: three of seven (43%) confirmed anovulatory cycles were to marijuana smokers, compared with 15% marijuana smokers in the study population. In the rhesus studies, the monkeys had not been exposed to marijuana prior to the investigation, whereas in the human studies women had been marijuana smokers for an unknown (and possibly long) period of time. It could be that infrequent marijuana use (as in our study), more closely resembles the exposure pattern of the rhesus studies, and therefore that lengthening of follicular phase in our data is consistent with delayed ovulation.
All exposure information was obtained by self-report at enrollment and participants’ responses at enrollment may not represent their actual behavior after enrollment. We attempted to control this source of error by using only the first menstrual cycle under study. Our study sample was mostly white, which made it impossible to consider racial differences reported in other studies 10, 18. Similarly, we were unable to investigate stress or physical activity as these characteristics were not included in the questionnaire. Finally, we were unable to investigate within-woman variance in follicular phase length; this would require longitudinal cycle-specific exposure data. A strength of our study is the detailed hormonal data, which allowed us to examine follicular phase length. In addition, the study participants were highly motivated and extremely careful in other aspects of their study participation, which suggests that their exposure data may also be of good quality.
In summary, we provide descriptive data on factors associated with follicular phase length, which was determined using hormonal estimates of ovulation in each menstrual cycle. Our findings suggest exposures (including oral contraceptive and marijuana use) that may lead to delayed ovulation. Future studies of follicular phase length should be more detailed in assessing marijuana exposure including measures of dose and timing of the exposure during the cycle.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences. We would like to thank Robert McConnaughey for his contributions to data management and statistical programming. We also thank Olga Basso and Dionne Law for their helpful comments on this manuscript.
References
- 1.Matthews KA, Santoro N, Lasley B, et al. Relation of cardiovascular risk factors in women approaching menopause to menstrual cycle characteristics and reproductive hormones in the follicular and luteal phases. J Clin Endocrinol Metab. 2006;91(5):1789–95. doi: 10.1210/jc.2005-1057. [DOI] [PubMed] [Google Scholar]
- 2.Whelan EA, Sandler DP, Root JL, Smith KR, Weinberg CR. Menstrual cycle patterns and risk of breast cancer. Am J Epidemiol. 1994;140(12):1081–90. doi: 10.1093/oxfordjournals.aje.a117208. [DOI] [PubMed] [Google Scholar]
- 3.Bertuccio P, Tavani A, Gallus S, Negri E, La Vecchia C. Menstrual and reproductive factors and risk of non-fatal acute myocardial infarction in Italy. Eur J Obstet Gynecol Reprod Biol. 2007 doi: 10.1016/j.ejogrb.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Cooper GS, Sandler DP. Long-term effects of reproductive-age menstrual cycle patterns on peri- and postmenopausal fracture risk. Am J Epidemiol. 1997;145(9):804–9. doi: 10.1093/oxfordjournals.aje.a009173. [DOI] [PubMed] [Google Scholar]
- 5.Baird DD, McConnaughey DR, Weinberg CR, et al. Application of a method for estimating day of ovulation using urinary estrogen and progesterone metabolites. Epidemiology. 1995;6(5):547–50. doi: 10.1097/00001648-199509000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Fehring RJ, Schneider M, Raviele K. Variability in the phases of the menstrual cycle. J Obstet Gynecol Neonatal Nurs. 2006;35(3):376–84. doi: 10.1111/j.1552-6909.2006.00051.x. [DOI] [PubMed] [Google Scholar]
- 7.Fukuda M, Fukuda K, Andersen CY, Byskov AG. Characteristics of human ovulation in natural cycles correlated with age and achievement of pregnancy. Hum Reprod. 2001;16(12):2501–7. doi: 10.1093/humrep/16.12.2501. [DOI] [PubMed] [Google Scholar]
- 8.Kato I, Toniolo P, Koenig KL, et al. Epidemiologic correlates with menstrual cycle length in middle aged women. Eur J Epidemiol. 1999;15(9):809–14. doi: 10.1023/a:1007669430686. [DOI] [PubMed] [Google Scholar]
- 9.Klein NA, Battaglia DE, Fujimoto VY, Davis GS, Bremner WJ, Soules MR. Reproductive aging: accelerated ovarian follicular development associated with a monotropic follicle-stimulating hormone rise in normal older women. J Clin Endocrinol Metab. 1996;81(3):1038–45. doi: 10.1210/jcem.81.3.8772573. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Gold EB, Lasley BL, Johnson WO. Factors affecting menstrual cycle characteristics. Am J Epidemiol. 2004;160(2):131–40. doi: 10.1093/aje/kwh188. [DOI] [PubMed] [Google Scholar]
- 11.van Zonneveld P, Scheffer GJ, Broekmans FJ, et al. Do cycle disturbances explain the age-related decline of female fertility? Cycle characteristics of women aged over 40 years compared with a reference population of young women. Hum Reprod. 2003;18(3):495–501. doi: 10.1093/humrep/deg138. [DOI] [PubMed] [Google Scholar]
- 12.Sherman BM, Korenman SG. Hormonal characteristics of the human menstrual cycle throughout reproductive life. J Clin Invest. 1975;55(4):699–706. doi: 10.1172/JCI107979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ecochard R, Gougeon A. Side of ovulation and cycle characteristics in normally fertile women. Hum Reprod. 2000;15(4):752–5. doi: 10.1093/humrep/15.4.752. [DOI] [PubMed] [Google Scholar]
- 14.Munster K, Schmidt L, Helm P. Length and variation in the menstrual cycle--a cross-sectional study from a Danish county. Br J Obstet Gynaecol. 1992;99(5):422–9. doi: 10.1111/j.1471-0528.1992.tb13762.x. [DOI] [PubMed] [Google Scholar]
- 15.Lenton EA, Landgren BM, Sexton L, Harper R. Normal variation in the length of the follicular phase of the menstrual cycle: effect of chronological age. Br J Obstet Gynaecol. 1984;91(7):681–4. doi: 10.1111/j.1471-0528.1984.tb04830.x. [DOI] [PubMed] [Google Scholar]
- 16.A prospective multicentre trial of the ovulation method of natural family planning. III. Characteristics of the menstrual cycle and of the fertile phase. Fertil Steril. 1983;40(6):773–8. [PubMed] [Google Scholar]
- 17.Treloar AE, Boynton RE, Behn BG, Brown BW. Variation of the human menstrual cycle through reproductive life. Int J Fertil. 1967;12(1 Pt 2):77–126. [PubMed] [Google Scholar]
- 18.Waller K, Swan SH, Windham GC, Fenster L, Elkin EP, Lasley BL. Use of urine biomarkers to evaluate menstrual function in healthy premenopausal women. Am J Epidemiol. 1998;147(11):1071–80. doi: 10.1093/oxfordjournals.aje.a009401. [DOI] [PubMed] [Google Scholar]
- 19.Jarett LR. Psychosocial and biological influences on menstruation: synchrony, cycle length, and regularity. Psychoneuroendocrinology. 1984;9(1):21–8. doi: 10.1016/0306-4530(84)90018-0. [DOI] [PubMed] [Google Scholar]
- 20.Balogh A, Ditroi F, Lampe LG. LH, FSH, estradiol and progesterone levels after discontinuation of hormonal contraception. Acta Univ Palacki Olomuc Fac Med. 1981;101:95–101. [PubMed] [Google Scholar]
- 21.Taylor RN, Jr, Berger GS, Treloar AE. Changes in menstrual cycle length and regularity after use of oral contraceptives. Int J Gynaecol Obstet. 1977;15(1):55–9. doi: 10.1002/j.1879-3479.1977.tb00645.x. [DOI] [PubMed] [Google Scholar]
- 22.Fenster L, Quale C, Waller K, et al. Caffeine consumption and menstrual function. Am J Epidemiol. 1999;149(6):550–7. doi: 10.1093/oxfordjournals.aje.a009851. [DOI] [PubMed] [Google Scholar]
- 23.Windham GC, Elkin EP, Swan SH, Waller KO, Fenster L. Cigarette smoking and effects on menstrual function. Obstet Gynecol. 1999;93(1):59–65. doi: 10.1016/s0029-7844(98)00317-2. [DOI] [PubMed] [Google Scholar]
- 24.Hornsby PP, Wilcox AJ, Weinberg CR. Cigarette smoking and disturbance of menstrual function. Epidemiology. 1998;9(2):193–8. [PubMed] [Google Scholar]
- 25.Santoro N, Lasley B, McConnell D, et al. Body size and ethnicity are associated with menstrual cycle alterations in women in the early menopausal transition: The Study of Women’s Health across the Nation (SWAN) Daily Hormone Study. J Clin Endocrinol Metab. 2004;89(6):2622–31. doi: 10.1210/jc.2003-031578. [DOI] [PubMed] [Google Scholar]
- 26.Mendelson JH, Mello NK, Ellingboe J, Skupny AS, Lex BW, Griffin M. Marihuana smoking suppresses luteinizing hormone in women. J Pharmacol Exp Ther. 1986;237(3):862–6. [PubMed] [Google Scholar]
- 27.Lex BW, Mendelson JH, Bavli S, Harvey K, Mello NK. Effects of acute marijuana smoking on pulse rate and mood states in women. Psychopharmacology (Berl) 1984;84(2):178–87. doi: 10.1007/BF00427443. [DOI] [PubMed] [Google Scholar]
- 28.Mendelson JH, Mello NK, Cristofaro P, Ellingboe J, Benedikt R. Acute effects of marijuana on pituitary and gonadal hormones during the periovulatory phase of the menstrual cycle. NIDA Res Monogr. 1984;55:24–31. [PubMed] [Google Scholar]
- 29.Block RI, Farinpour R, Schlechte JA. Effects of chronic marijuana use on testosterone, luteinizing hormone, follicle stimulating hormone, prolactin and cortisol in men and women. Drug Alcohol Depend. 1991;28(2):121–8. doi: 10.1016/0376-8716(91)90068-a. [DOI] [PubMed] [Google Scholar]
- 30.Wilcox AJ, Weinberg CR, O’Connor JF, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988;319(4):189–94. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- 31.Baird DD, Weinberg CR, Wilcox AJ, McConnaughey DR, Musey PI. Using the ratio of urinary oestrogen and progesterone metabolites to estimate day of ovulation. Stat Med. 1991;10(2):255–66. doi: 10.1002/sim.4780100209. [DOI] [PubMed] [Google Scholar]
- 32.WHO. Report of the World Health Organization Consultation of Obesity. Geneva: World Health Organization; 1997. Jun, Preventing and Managing the Global Epidemic of Obesity. [PubMed] [Google Scholar]
- 33.Gnoth C, Frank-Herrmann P, Schmoll A, Godehardt E, Freundl G. Cycle characteristics after discontinuation of oral contraceptives. Gynecol Endocrinol. 2002;16(4):307–17. [PubMed] [Google Scholar]
- 34.Birtch RL, Olatunbosun OA, Pierson RA. Ovarian follicular dynamics during conventional vs. continuous oral contraceptive use. Contraception. 2006;73(3):235–43. doi: 10.1016/j.contraception.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 35.Duijkers I, Engels L, Klipping C. Length of the menstrual cycle after discontinuation of oral contraceptives. Gynecol Endocrinol. 2005;20(2):74–9. doi: 10.1080/09513590400021011. [DOI] [PubMed] [Google Scholar]
- 36.Hassan MA, Killick SR. Is previous use of hormonal contraception associated with a detrimental effect on subsequent fecundity? Hum Reprod. 2004;19(2):344–51. doi: 10.1093/humrep/deh058. [DOI] [PubMed] [Google Scholar]
- 37.Vessey MP, Wright NH, McPherson K, Wiggins P. Fertility after stopping different methods of contraception. Br Med J. 1978;1(6108):265–7. doi: 10.1136/bmj.1.6108.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pardthaisong T, Gray RH. The return of fertility following discontinuation of oral contraceptives in Thailand. Fertil Steril. 1981;35(5):532–4. [PubMed] [Google Scholar]
- 39.Bracken MB, Hellenbrand KG, Holford TR. Conception delay after oral contraceptive use: the effect of estrogen dose. Fertil Steril. 1990;53(1):21–7. [PubMed] [Google Scholar]
- 40.Axmon A, Rylander L, Albin M, Hagmar L. Factors affecting time to pregnancy. Hum Reprod. 2006;21(5):1279–84. doi: 10.1093/humrep/dei469. [DOI] [PubMed] [Google Scholar]
- 41.Wiegratz I, Mittmann K, Dietrich H, Zimmermann T, Kuhl H. Fertility after discontinuation of treatment with an oral contraceptive containing 30 microg of ethinyl estradiol and 2 mg of dienogest. Fertil Steril. 2006;85(6):1812–9. doi: 10.1016/j.fertnstert.2005.11.052. [DOI] [PubMed] [Google Scholar]
- 42.Evrard JR, Buxton BH, Jr, Erickson D. Amenorrhea following oral contraception. Am J Obstet Gynecol. 1976;124(1):88–91. doi: 10.1016/0002-9378(76)90017-x. [DOI] [PubMed] [Google Scholar]
- 43.Portuondo JA, Sarasola R, Echanojauregui AD, de los Rios A. Ovulation recovery after hormonal contraception. Endoscopy. 1979;11(2):114–5. doi: 10.1055/s-0028-1098333. [DOI] [PubMed] [Google Scholar]
- 44.Barbieri RL, Gao X, Xu H, Cramer DW. Effects of previous use of oral contraceptives on early follicular phase follicle-stimulating hormone. Fertil Steril. 1995;64(4):689–92. doi: 10.1016/s0015-0282(16)57839-x. [DOI] [PubMed] [Google Scholar]
- 45.Cramer DW, Barbieri RL, Xu H, Reichardt JK. Determinants of basal follicle-stimulating hormone levels in premenopausal women. J Clin Endocrinol Metab. 1994;79(4):1105–9. doi: 10.1210/jcem.79.4.7962282. [DOI] [PubMed] [Google Scholar]
- 46.Small CM, Manatunga AK, Klein M, et al. Menstrual cycle characteristics: associations with fertility and spontaneous abortion. Epidemiology. 2006;17(1):52–60. doi: 10.1097/01.ede.0000190540.95748.e6. [DOI] [PubMed] [Google Scholar]
- 47.Rowland AS, Baird DD, Long S, et al. Influence of medical conditions and lifestyle factors on the menstrual cycle. Epidemiology. 2002;13(6):668–74. doi: 10.1097/00001648-200211000-00011. [DOI] [PubMed] [Google Scholar]
- 48.Wilcox AJ, Weinberg CR, Baird DD. Risk factors for early pregnancy loss. Epidemiology. 1990;1(5):382–5. doi: 10.1097/00001648-199009000-00008. [DOI] [PubMed] [Google Scholar]
- 49.Asch RH, Smith CG, Siler-Khodr TM, Pauerstein CJ. Effects of delta 9-tetrahydrocannabinol during the follicular phase of the rhesus monkey (Macaca mulatta) J Clin Endocrinol Metab. 1981;52(1):50–5. doi: 10.1210/jcem-52-1-50. [DOI] [PubMed] [Google Scholar]
- 50.Smith CG, Almirez RG, Berenberg J, Asch RH. Tolerance develops to the disruptive effects of delta 9-tetrahydrocannabinol on primate menstrual cycle. Science. 1983;219(4591):1453–5. doi: 10.1126/science.6298938. [DOI] [PubMed] [Google Scholar]
- 51.Bauman J. Marijuana and the female reproductive system. Washington D.C: U.S. Government Printing Office; 1980. [Google Scholar]