Abstract
Involuntary images and visual memories are prominent in many types of psychopathology. Patients with posttraumatic stress disorder, other anxiety disorders, depression, eating disorders, and psychosis frequently report repeated visual intrusions corresponding to a small number of real or imaginary events, usually extremely vivid, detailed, and with highly distressing content. Both memory and imagery appear to rely on common networks involving medial prefrontal regions, posterior regions in the medial and lateral parietal cortices, the lateral temporal cortex, and the medial temporal lobe. Evidence from cognitive psychology and neuroscience implies distinct neural bases to abstract, flexible, contextualized representations (C-reps) and to inflexible, sensory-bound representations (S-reps). We revise our previous dual representation theory of posttraumatic stress disorder to place it within a neural systems model of healthy memory and imagery. The revised model is used to explain how the different types of distressing visual intrusions associated with clinical disorders arise, in terms of the need for correct interaction between the neural systems supporting S-reps and C-reps via visuospatial working memory. Finally, we discuss the treatment implications of the new model and relate it to existing forms of psychological therapy.
Keywords: memory, imagery, neuroscience, psychopathology, treatment
Recurrent and intrusive images feature in the diagnostic criteria for posttraumatic stress disorder (PTSD), acute stress disorder, and obsessive–compulsive disorder (OCD) but are not mentioned in relation to the majority of other mental disorders (American Psychiatric Association, 2000). Consistent with this, psychological theories of mental disorders have given much more attention to the role of various kinds of negative verbal thought than to the role of visual intrusions (Brewin, 1998; Hackmann & Holmes, 2004). In contrast, there is increasing empirical research suggesting that intrusive visual images and memories are a common feature of many disorders (i.e., a transdiagnostic process; A. G. Harvey, Watkins, Mansell, & Shafran, 2004; Hirsch & Holmes, 2007). In this article we relate observations of intrusive memories and images across a variety of disorders to models of memory and imagery drawn from cognitive psychology and cognitive neuroscience. This material is then used to update and expand the dual representation theory of PTSD (Brewin, 2001, 2003) by placing it within the context of a neurobiological model of normal memory and imagery (Burgess, Becker, King, & O’Keefe, 2001; Byrne, Becker, & Burgess, 2007). Finally, we consider the implications for psychological treatment.
Intrusions are instances of involuntary or direct, as opposed to voluntary, retrieval in that their appearance in consciousness is spontaneous rather than following a deliberate effort or search (Berntsen, 2009; Mace, 2007). The vast majority of psychological research on memory and imagery has involved voluntary, effortful processes to the neglect of involuntary ones. Although involuntary remembering appears to be a common, everyday phenomenon (Berntsen, 2007), little is known about other types of intrusive imagery. Much of what is known comes from observations of individuals with psychological disorders, which suggest that visual intrusions tend to be repetitive, uncontrollable, and distressing. Experimental research indicates that imagery may elicit stronger emotional responses than do corresponding verbal cognitions (Holmes & Mathews, 2005; Holmes, Mathews, Mackintosh, & Dalgleish, 2008), which makes these intrusions of particular theoretical and clinical interest. Consistent with this, the importance of intrusive imagery in understanding emotional distress has periodically been noted (e.g., Beck, 1970; Horowitz, 1970; Lang, 1977), particularly in theoretical writing on the anxiety disorders (e.g., Clark & Wells, 1995; Salkovskis, 1985), and images have occasionally featured as therapeutic targets in their own right (e.g., Beck, Emery, & Greenberg, 1985; Edwards, 2007; Hackmann, 1998).
In the clinical domain, theorizing about visual intrusions has largely been restricted to PTSD. Most theories have in common the idea that a disturbance in memory is a central component of the disorder and have identified the existence of decrements in voluntary trauma memory that coexist with enhanced involuntary memory for the trauma (see Brewin & Holmes, 2003, and Brewin, 2007, for reviews). Some influential clinical theories have therefore augmented a standard autobiographical memory model by proposing a role for a second memory system that processes lower level, sensory information (see, for example, Brewin, Dalgleish, & Joseph, 1996) or for increased stimulus priming (Ehlers & Clark, 2000). In contrast, models of trauma memory put forward by cognitive psychologists tend to be single-system accounts in which traumatic memory is essentially similar to normal memory in being the product of a high-level constructive process associated with activity in the medial temporal lobes (MTLs; see, e.g., Rubin, Berntsen, & Bohni, 2008, with ensuing commentaries from Monroe & Mineka, 2008, and Berntsen, Rubin, & Bohni, 2008).
The fact that intrusions are not restricted to PTSD suggests that there could be important lessons from studying their role in other psychological disorders, and this material is reviewed at the beginning of this article. The conclusions are then integrated into a revised and expanded version of the dual representation theory of PTSD (Brewin, 2001, 2003; Brewin, Dalgleish, & Joseph, 1996) that draws on recent developments in understanding the neural mechanisms of memory and imagery (Byrne et al., 2007). The revised model attempts to be more comprehensive by (a) encompassing both intrusive memories and images, (b) grounding clinical observations in specific brain pathways and processes, and (c) considering an increased range of psychological disorders.
Intrusive Images and Memories
Definitions and Characteristics
Images can be defined as “contents of consciousness that possess sensory qualities as opposed to those that are purely verbal or abstract” (Hackmann, 1998, p. 301) and can occur in many different forms, such as dreams, nightmares, and pleasant fantasies (Horowitz, 1967). They can possess various sensory qualities—including visual, auditory, olfactory, gustatory, touch, and movement (Kosslyn, 1994)—and can be described in terms of their content, vividness, clarity, color, shading, shapes, movement, foreground and background characteristics, and other spatial relationships (Horowitz, 1970). Furthermore, Horowitz (1970) stated that “a person can often tell how the image entered awareness, its duration, associated emotions, the relationship of the emotion to the external world, efforts to change or dispel it, and the sequential or simultaneous arrangement of a series of images” (p. 3). Although images can involve all the senses, the vast majority of the literature is concerned with visual images, and this is the focus of the current article.
Images are experienced “on a continuum from the near veridical reconstruction in the mind of a real event to the construction of an entirely hypothetical situation” (Martin & Williams, 1990, p. 268). Theories of autobiographical memory in particular emphasize the central role played by sensory images, which provide context and detail during the recollective process (e.g., Conway 2001). Many clinical investigations of naturally occurring imagery have not distinguished images based on fantasy from visual autobiographical memories. Others have classified imagery reports as either detailed autobiographical memories or other kinds of images. “Other” images would include, for example, a detail from a memory devoid of any surrounding context (e.g., the disembodied face of an attacker) or a hypothetical situation that had never happened (see, e.g., Patel et al., 2007, for further discussion).
From a clinical perspective, vividness is an important quality of imagery. Reliving is a general property of autobiographical memory, although one that can vary considerably in degree, and corresponds to Tulving’s (1985) idea of autonoetic consciousness, a particularly vivid form of imagery. Descriptions of PTSD emphasize the importance of flashbacks—powerful memories in which traumatic events are not just relived but reexperienced as occurring in the present (Brewin, 2003; Ehlers & Clark, 2000; Ehlers, Hackmann, & Michael, 2004). Flashbacks are considered to involve dissociative distortions to the perception of time, place, and the self and can vary from relatively mild (there is a transient sense of the event reoccurring in the present) to severe (the person loses all connection with his or her current autobiographical self and present surroundings while reexperiencing the memory). These features appear to be related to the presence of PTSD rather than to be general characteristics of trauma memories (Brewin, 2007).
Where traumatic events are concerned, vulnerability to flashbacks may relate to dissociative responses at the time of the trauma in which the person experienced an altered sense of time passing, a loss of emotional responsivity, or an out-of-body experience (observing events as they happen from an external perspective, for example as though looking down from the ceiling). It has been variously argued that these dissociative reactions may correspond to a biologically hard-wired freezing response (Nijenhuis, Vanderlinden, & Spinhoven, 1998) or to a defensive strategy for reducing the emotional and physiological impact of the event (van der Kolk, van der Hart, & Marmar, 1996).
There is a well-known and related memory alteration that does not imply any dissociative process but has also been argued to serve the function of reducing unwanted emotional arousal. This involves seeing oneself from an observer perspective rather than recalling an event from one’s original point of view (field perspective). Freud (1953) is one of many to have noted this process, but it has since been extensively researched (Rubin et al., 2008). Nigro and Neisser (1983) reported that when participants were asked to generate autobiographical memories, those seen from a field perspective tended to be more recent, whereas those seen from an observer perspective tended to be characterized by situations involving high self-awareness (e.g., self-consciousness induced by having to speak in public). In another experiment, Nigro and Neisser asked participants to recall autobiographical events and focus on either their feelings or the objective circumstances. The former instruction produced more field memories, and the latter more observer memories. The evidence reviewed below indicates that, like dissociative reactions, observer memories may occur involuntarily as well as voluntarily.
Methodology of Assessment
A semistructured interview is the main methodology used to investigate the phenomenology of intrusive images. Following Beck, Laude, and Bohnert (1974), in most investigations researchers have asked participants to report the images that come to mind when they are feeling anxious and to focus on a typical image and describe what they could see, hear, smell, taste, and feel in their body. Some investigators have asked the participants about their perspective in the image, their emotions, what led up to the scene, and its meaning in terms of themselves, other people, and the world. Finally, some have asked the participants about their earliest recollection of an incident involving the same images, sensations, and feelings (see, e.g., Hackmann, Clark, & McManus, 2000; Wells & Hackmann, 1993). Often the frequency of intrusions, their duration and vividness, the extent of reliving them, and any accompanying emotions would also be recorded.
Slightly different procedures have been adopted for different clinical populations. In studies of depression, participants have mainly been asked to report any recurrent visual memories of stressful autobiographical events that occurred during a specified time period, usually the past week (see, e.g., Brewin, Hunter, Carroll, & Tata, 1996). In most PTSD research, participants are specifically asked to report upon trauma-related memories, without necessarily being restricted to a specific time frame (see, e.g., Hackmann, Ehlers, Speckens, & Clark, 2004). In other clinical domains, the interview mainly varies in terms of the situation that people are orientated toward and may start with an exercise to familiarize participants with discussing imagery (Day, Holmes, & Hackmann, 2004). For example, in a study of OCD, participants were asked to think about a time when their symptoms were severe (Speckens, Hackmann, Ehlers, & Cuthbert, 2007); in a study of eating disorders, a time when they worried about their eating, weight, or shape (Somerville, Cooper, & Hackmann, 2007); and in a study of body dysmorphic disorder, a time when they worried about their appearance (Osman, Cooper, Hackmann, & Veale, 2004).
In common with related research in cognitive psychology, detailed phenomenological investigations such as these have generally sidestepped issues of validity, because no independent sources of validation are readily available. The emphasis has been more on covering multiple dimensions, even if single-item measures are the only ones practical, than on restricting the range of inquiry but using more reliable methods. As a result, we know little about how accurately these interview questions assess and discriminate the different dimensions of experience. Also lacking is information about test–retest reliability and about the internal and external conditions that may affect intrusion reports. As interest in intrusions becomes more widespread, it will be important to pay more attention to the measurement issues that have hitherto been largely neglected.
Naturalistic Studies of Intrusive Images and Memories in Psychological Disorders
Articles that provide systematic empirical data on the phenomenology of intrusive visual memories or images in samples meeting diagnostic criteria for a psychological disorder are presented in Table 1. The studies describe intrusions experienced under normal rather than experimental conditions. The table identifies studies that recorded any image, whether reality- or fantasy-based, and those that distinguished visual autobiographical memories from other images. The table also gives details of the samples studied: whether the focus was on intrusive images or memories, the prevalence of the phenomenon, and the typical number of images or memories that were reported to intrude. In the following sections we describe the content of the intrusions, their qualities (including sensory aspects, reliving, and observer/field perspective), associations with other variables, and their relation to the course of disorder or to treatment for each of a number of separate disorders.
Table 1. Recurrent Intrusive Images and Visual Memories in Psychological Disorders.
Anxiety Neurosis and Panic Disorder
The first systematic study of intrusive imagery was conducted by Beck et al. (1974, Study 2). Prefiguring later findings, they reported that 90% of their sample of patients with anxiety neurosis (a variety of mixed anxiety disorders) had intrusive images that, like the patients’ intrusive thoughts, concerned situations involving harm or danger. Other early studies were conducted with samples largely consisting of panic patients. Although rates of intrusive images were low in the studies by Hibbert (1984) and Breitholtz, Westling, and Öst (1998), this may have been because the interview was centered mainly on verbal cognition and was limited to the previous 3 weeks. Studies reporting higher rates of intrusive images typically asked respondents to think about times when they felt anxious or panicky (e.g., Ottaviani & Beck, 1987). There was agreement between studies that images were generally concerned with mental or physical catastrophe.
Health-Related Anxiety
Wells and Hackmann (1993) found that the imagery of patients with health anxiety tended to depict physical catastrophe while also reflecting negative beliefs about both the self and the nature of health and illness. This was the first study of recurrent imagery to note that the content of the images often connected to an earlier aversive experience and contained recognizable elements that were transformed in the image. For example, one patient had an image of himself as dead but trapped in a magic barrel that did not allow him to move or escape. He associated the image with seeing funerals as a young boy, when he had not understood that the people being buried were dead but had thought they were being buried alive as a punishment.
More recently, Whitaker, Brewin, and Watson (2008) compared rates of intrusive images and memories in matched groups of anxious and nonanxious men with prostate cancer. Levels of anxiety were lower than in other studies conducted with psychiatric patients, and perhaps as a result, the number of intrusions was lower. Nevertheless, the frequency of both images and memories was linearly related to anxiety. Intrusions were associated with poorer coping even after levels of anxiety were statistically controlled. Whitaker, Watson, and Brewin (2009) reported that in a mixed group of cancer patients, visual intrusions were significantly more distressing than were verbal intrusions.
Depression
Spenceley and Jerrom (1997); Reynolds and Brewin (1998); and Brewin, Watson, McCarthy, Hyman, and Dayson (1998a) have compared depressed and nondepressed samples, confirming that there are significantly higher levels of intrusion and avoidance of memories in the clinical groups. In the first study of its kind Kuyken and Brewin (1994) found that many depressed female inpatients reported memories involving childhood physical or sexual abuse. These findings were replicated by Brewin, Hunter, et al. (1996), who carried out a similar study with a mixed-gender sample of depressed patients, asking them about memories linked to a variety of life events. Most memories could be classified in one of three categories—illness and death, relationship and family problems, or abuse and assault—a finding that has since been repeated a number of times (see, e.g., Patel et al., 2007; Reynolds & Brewin, 1999). Like Wells and Hackmann (1993), Brewin, Hunter, et al. (1996) found that intrusions frequently involved events dating from many years prior to the onset of symptoms.
Intrusive memories are often triggered by rumination (Birrer, Michael, & Munsch, 2007) and can be embedded within longer ruminative sequences (Pearson, Brewin, Rhodes, & McCarron, 2008). They are associated with a variety of emotions (most commonly anger and sadness), tend to be vivid and distressing, and are often accompanied by physical sensations and a sense of reliving the event (Birrer et al., 2007; Patel et al., 2007; Reynolds & Brewin, 1999).
Kuyken and Brewin (1994) found that higher intrusion levels were correlated with indices of more severe abuse and with more severe depression. Intrusive memories are also positively correlated with numerous aspects of depressive cognition, including lower self-esteem, a negative attributional style, and dysfunctional coping (Kuyken & Brewin, 1999); anxious preoccupation, cognitive avoidance, and helplessness/hopelessness (Brewin et al., 1998a); and difficulty in supplying specific autobiographical memories to supplied cue words (Kuyken & Brewin, 1995; Raes et al., 2006).
To test the involvement of intrusions in maintenance, Brewin, Watson, McCarthy, Hyman, and Dayson (1998b) examined the ability of baseline measures of intrusive autobiographical memory to predict anxiety or depression in a sample of depressed cancer patients at 6 months follow-up, controlling for baseline anxiety or depression as well as the stage of the illness. The presence of intrusive memories either at baseline or prior to follow-up made an additional significant contribution to anxiety but not to depression at follow-up. Brewin, Reynolds, and Tata (1999) investigated similar processes in 62 depressed psychiatric patients. Having high-frequency involuntary intrusive memories at baseline was significantly predictive of later depression, even when controlling for the severity of symptoms at baseline.
Posttraumatic Stress Disorder
In Hackmann et al.’s (2004) study, patients typically described the intrusion of between one and four highly repetitive trauma memories, mainly consisting of sensory experiences of short duration (see also Speckens, Ehlers, Hackmann, Ruths, & Clark, 2007). Consistent with the observations of Ehlers et al. (2002), only 17% of these corresponded to the worst period of the trauma, the majority consisting of moments signaling that the traumatic event was about to happen or that the meaning of the event had become more threatening. In contrast, Holmes, Grey, and Young (2005) found that 77% of intrusions could be matched to the subjectively reported worst moments of the trauma (hotspots). Differences in methodology between studies means that questions about the precise timing of intrusion content relative to the traumatic event remain unresolved. It is also important to note that, as with Reynolds and Brewin (1998), Hackmann et al. noted a small proportion of repetitive intrusions that did not correspond to actual events. Rather, the intrusions consisted of imagined scenarios that nevertheless had a strong thematic connection to the traumatic event.
One of the earliest studies to provide empirical support for the observation that in PTSD intrusive memories contain prominent sensory/perceptual features, are highly emotional, and involve an intense reliving of the event in the present was that of van der Kolk and Fisler (1995). They noted that narrative memories of the traumatic event developed more slowly, with trauma victims initially struggling to put their experience into words. Reynolds and Brewin (1999) found additionally that intrusive memories were most likely to last for a period of minutes, were highly distressing, and were accompanied by physical sensations. They were associated with a variety of emotions, most commonly, anger, sadness, fear, helplessness, and guilt.
Consistent with the evidence for elevated levels of dissociation in PTSD (Brewin & Patel, in press; Waller, Putnam, & Carlson, 1996), 42% of Reynolds and Brewin’s (1999) PTSD patients reported that at least one of their intrusions involved an out-of-body experience in which during the trauma they had had the sensation of observing themselves from an external perspective. Similarly, McIsaac and Eich (2004) found that 36% of their PTSD sample experienced intrusive memories from an external perspective. In their study this perspective was associated with more information about spatial relations, self-observations, and peripheral details. In contrast, those patients who reported seeing the event through their own eyes described more emotional reactions, physiological sensations, and psychological states.
A key issue is the association between intrusive memories and the course of disorder. Michael, Ehlers, Halligan, and Clark (2005) showed that there were relatively small differences between traumatized samples with and without PTSD in the number of intrusive memories individuals reported and the likelihood that these contained sensory experiences. What did strongly reflect PTSD severity was increased distress associated with the memories, a lack of context, and the sense that the memories were being relived in the present. These intrusion characteristics predicted the severity of PTSD 6 months later over and above initial diagnostic status. Hackmann et al. (2004) reported that during therapy the frequency of intrusions diminished, as did their vividness, the associated distress, and the sense of how much the events appeared to be happening again in the present (see also Speckens, Ehlers, Hackmann, & Clark, 2006).
Other Anxiety Disorders
Hackmann, Surawy, and Clark (1998) reported that significantly more socially anxious patients than nonanxious controls experienced an intrusive image when in a stressful social situation. As in Wells and Hackmann’s (1993) study of health anxiety, Hackmann et al. (2000) further noted that intrusive images in social phobia were generally associated (by the patient) with early adverse social events that clustered in time around the onset of the disorder. They commented that the image appeared to correspond to the abstracted essence of the memory. For example, in her recurrent image, one of their participants reported seeing herself lurching around out of control, vocalizing noises that did not make sense. In the linked memory she saw herself in the playground at school, pretending to be a horse, galloping and neighing unconvincingly, while being mocked by her peers. Another participant recurrently saw herself as looking stupid with a red face and a closed posture. In the memory, she reported seeing herself in a classroom being harshly criticized by a teacher for a stupid answer and looking red with a closed body posture.
Thus, Hackmann et al. (2000) suggested that in most cases the central aspect of both the image and the memory was a negative impression of the observable self, with the memory containing additional material about the context. Importantly, they noted that patients had often not made the link between the image and the corresponding memory and appeared to become aware of it for the first time during the study. Hackmann et al. (1998) found that, compared with those of controls, patients’ images were more negative, often involving them looking embarrassed or humiliated, and they more often incorporated an external, observer perspective rather than seeing the situation through their own eyes.
Day et al. (2004) found that all of their agoraphobia sample, but none of the controls, reported intrusive images. These images were often linked to an autobiographical memory from childhood involving being victimized or being in danger. Seventy-five percent of the patients stated that they had not been anxious in agoraphobic-type situations before experiencing the event that occurred in their memory. Patients were most likely to report images that alternated between a field perspective and an observer perspective.
As documented by de Silva (1986), there are many clinical accounts of imagery accompanying OCD. In one of the few systematic studies, Speckens, Hackmann, et al. (2007) found vivid and distressing recurrent images in 78% of their inpatient sample. Many patients spontaneously reported initiating rituals when they became aware of the image. Images often corresponded to, or were related to, memories of earlier adverse events. These events tended to immediately precede the onset of or an exacerbation of OCD symptoms. Lipton, Brewin, Linke, and Halperin (2008) compared intrusive images in OCD patients with a control sample of patients suffering from other anxiety disorders. OCD intrusions were more likely to contain ideas of unacceptable harm and of a dangerous self. The majority of OCD intrusions were seen from a field rather than an observer perspective. Lipton et al. (2008) found that OCD patients reported more frequent intrusive images, as well as a lesser degree of similarity between their images and corresponding memories of earlier events.
Eating and Body Perception Disorders
Cooper, Todd, and Wells (1998) found that just over half of a sample of patients with anorexia or bulimia described intrusive images that came to mind when they thought about eating or weight issues. Somerville et al. (2007) noted that their participants with bulimia reported more spontaneous images than did nondieting control participants. One participant reported: “I see myself as if in a photo. I’m wearing baggy clothes. I’m spotty with greasy hair. I’m two stone overweight. Fat stomach, fat thighs, double chin. I’m eating a chocolate bar. My clothes feel tight. I’m hot, sweaty, sticky. My stomach feels full, uncomfortable, nauseous. My mouth tastes sour and horrible, cloying after eating lots of sweet things. I can smell the sweat from my own body.” A number of images were linked to a specific childhood memory that was similar in emotional and sensory characteristics.
The images of Somerville et al.’s (2007) participants with bulimia were recurrent and significantly more negative and anxiety-provoking than were those of controls. Patients’ images involved more sensory modalities than did dieting controls’ images and were more vivid than were nondieting controls’ images. Once depression was controlled, however, many of the between-groups differences became nonsignificant.
Intrusive images related to a person’s appearance are significantly more common in individuals with body dysmorphic disorder than in nonpatient controls (Osman et al., 2004). Images were more often linked to early memories in the patient sample, and the median age of this memory was 11.5 years. Typically the memories concerned being teased or bullied or feeling self-conscious about the patient’s appearance in adolescence. Recurrent images were significantly more vivid and detailed in the patient sample and were more often accompanied by bodily sensations. All patients saw their images from an observer perspective, whereas all nonpatients saw their images as though they were looking at themselves in a mirror.
Psychotic Disorders
Thirty-five patients with a diagnosis of schizophrenia were asked by Morrison et al. (2002) about the occurrence of images associated with their hallucinations and delusions. Just over half reported recurrent images. Common themes included images about feared catastrophes associated with paranoia, traumatic memories, and images about the perceived source or content of voices. Images were associated with a variety of emotions, of which the most common was fear.
Consistent with an experimental study by Mansell and Lam (2004), Tzemou and Birchwood’s (2007) study found that just under half of samples of unipolar depressed and bipolar patients reported distressing intrusive memories. There were no differences in the prevalence of intrusions between the groups, and no members of a control sample reported this phenomenon. Gregory, Brewin, Mansell, and Donaldson (2008) interviewed 29 bipolar patients in remission concerning their experience of intrusive visual memories and other images when in a depressed, a euthymic, and a hypomanic state. Their main finding was that intrusive memories were more common in euthymic and depressed states, whereas other images were more common in depressed and hypomanic states. Compared with intrusive memories in the euthymic state, intrusive memories in depression were more distressing and more vivid and involved more reliving the experience. During hypomania the intrusive images were as vivid as those the person experienced when depressed but were rated as much less distressing. On the contrary, most were highly positive.
Summary
Intrusive images and memories appear to be a common feature of many disparate psychological conditions. Where the data have been collected, it is generally found that there are a relatively small number of recurrent intrusions, rarely exceeding six per person and more often ranging between one and three per person. Memories tend to be linked in thematic groups, and intrusive images are frequently associated with specific adverse events in ways that often come as a surprise to patients when their attention is directed to it (Holmes & Hackmann, 2004). Recurrent intrusions also occur in healthy controls, but they are significantly less common, and when they do occur they are less frequent and less distressing.
One of the most obvious ways in which intrusions differ between disorders is in their theme (e.g., Lipton et al., 2008; Reynolds & Brewin, 1999). By and large, the content of these intrusions matches the specific content of verbal thoughts associated with each disorder. Thus, in panic and agoraphobia, intrusions center around physical and mental catastrophe; in depression and bipolar disorder, around negative life events including interpersonal problems; in health-related anxiety, around illness and death; in social phobia, around public failure and humiliation; and so on.
Table 1 indicates that in certain disorders, particularly the anxiety disorders, images involving dreaded future situations or outcomes have usually been studied, whereas in depression and PTSD visual memories of painful or traumatic events have been the main focus. It is premature, however, to conclude that memory- and fantasy-based images can be unambiguously distinguished or are more important in some disorders than others. For example, intrusive images concerning the future have been recorded in depression, PTSD, and the hypomanic phase of bipolar disorder (Gregory et al., 2008; Holmes et al., 2007; Patel et al., 2007; Reynolds & Brewin, 1998), although they were generally found to be less common. In many studies of intrusive imagery, no attempt was made to report which images corresponded exactly to autobiographical memories, and it was frequently found that fantasy-based images nevertheless contained memory-related material. Thus, the relation between images and memories in these clinical conditions remains one in which a great deal more research is needed.
Despite most studies’ reporting the existence of strongly negative intrusions, positive images appear to feature in the hypomanic phase of bipolar disorder (Gregory et al., 2008). Similarly, the elaborated intrusion model of craving (May, Andrade, Panabokke, & Kavanagh, 2004) proposes that when reminded about their desires for substances such as tobacco, certain people create images that are pleasurable in the short-term but exacerbate their sense of deficit and bring about a vicious circle of desire, positive imagery, and planning to fulfill that desire. Mental images, rather than verbal thoughts, of the substance or the context of its consumption create the strongest positive emotions of reward and relief. Consistent with this, May et al. (2004) reported data linking individual craving episodes with positive substance-related imagery.
It is striking that virtually all studies describe these recurrent intrusions as being vivid, detailed, and accompanied by (often extremely strong) physical sensations and emotions. Across disorders the visual modality is almost invariably the most common, but it is clear that sound, taste, and smell are also frequently involved.
Among the differences found are that in patients with PTSD, compared with those with depression, the intrusions are associated with greater helplessness and have more dissociative qualities such as out-of-body experiences or a sense of reliving the event in the present (Birrer et al., 2007; Reynolds & Brewin, 1999). Reynolds and Brewin (1999) conducted an exploratory factor analysis that suggested, consistent with this, that feeling helpless during an intrusive memory was associated with dissociation, whereas feeling fear was related to reliving the event in the present. A more recent study has confirmed that levels of dissociation are much higher in PTSD than in depression (Brewin & Patel, in press).
Of considerable importance are the accumulating observations that these intrusions are closely related to other aspects of disorders, including their course. Thus, intrusions and/or their characteristics have been found to be related to coping and other negative cognitions in depression and OCD; to outcome in cancer-related anxiety, depression, and PTSD; and to overgeneral memory recall in depression. Observations in samples with depression, PTSD, agoraphobia, social anxiety, and OCD are strongly suggestive that the events figuring in these memories were associated with either the onset or an exacerbation of the disorder. Hirsch, Clark, Mathews, and Williams (2003) provided further evidence for the functional role of imagery by experimentally manipulating the images that social phobia patients held in mind when interacting in a social situation. Holding a negative rather than a neutral image in mind led to greater anxiety and more negative interpretations of performance.
The review has also identified many areas in need of further research. For example, in PTSD, flashbacks are sometimes described as like video clips (Ehlers & Steil, 1995; Hackmann et al., 2004), implying that they consist of temporally extended events. However, the exact nature of how the scenes and incidents are chained together, and whether this is similar to that found in other temporally extended memories, has not been studied. Similarly, the subjective report of the same memories recurring repeatedly has not been subject to empirical verification, and it is unclear whether these phenomena occur as exact or approximate repetitions. Finally, research is needed into why memories appear to occur more frequently from an observer viewpoint in some disorders than in others.
Psychology and Neuroscience of Memory and Imagery
Memory
Involuntary memory
Intrusive memories combine two areas of research in cognitive psychology: involuntary memory and autobiographical memory. The study of involuntary memory, although minimal compared with that of voluntary memory, has a long history in psychology, stretching back at least as far as Ebbinghaus (Berntsen, 2009). It is generally seen as an associative process, in which the cue alone is sufficient to prompt retrieval, in contrast to the strategic, deliberative nature of voluntary recall. A number of researchers have proposed that involuntary retrieval results in memory contents remaining more consistent over time, as the alterations introduced by deliberate search and reconstruction are absent (e.g., Estes, 1997).
Differences between voluntary and involuntary recall were reviewed by Berntsen (2007). Compared with the former, the latter were more likely to involve specific episodes, to influence mood, and to come with an accompanying bodily reaction. Involuntary memories have also been found to be retrieved more quickly and to be more likely following negative cues (Schlagman & Kvavilashvili, 2008). Studies comparing different types of recall for the same experimental material have generally found that the accuracy of voluntary recall is unrelated to the frequency of involuntary intrusions (Brewin & Saunders, 2001; Holmes, Brewin, & Hennessy, 2004).
Moscovitch (1995) proposed that associative (involuntary) retrieval has its neural basis in the functioning of the hippocampus and surrounding medial temporal cortex, which reinstate a previous conscious experience by activating a network of areas involved in the original perception of the event. Voluntary retrieval, by contrast, depends on the instigation of an appropriate strategy through activity in the prefrontal cortex, which then, if successful, also produces MTL activation. Subsequent research has indicated that, of the temporoparietal and prefrontal networks identified with episodic memory, the more medial structures such as the hippocampus, the retrosplenial cortex, the precuneus, and the ventromedial prefrontal cortex appear to support memory for the context of episodic events (Burgess, Maguire, Spiers, & O’Keefe, 2001) and can be activated by both voluntary and involuntary recall (Hall, Gjedde, & Kupers, 2008). By contrast, activation of more lateral and anterior prefrontal areas appears to reflect top-down modulation by variables such as effort, deliberate choice of strategy, and postretrieval processing in dealing with, for example, interference between multiple potentially relevant memories (e.g., Fletcher & Henson, 2001; Hall et al., 2008; King, Hartley, Spiers, Maguire, & Burgess, 2005).
Autobiographical memory
Traditionally, autobiographical memory and episodic memory have been treated as virtually interchangeable terms for a single memory system, with autobiographical memory referring to the specific subset of knowledge relating to the self and its personal history (Baddeley, 2001). From the time of Janet (1904), however, many of those studying the recall of highly emotional events have proposed the existence of an image-based memory system that captures sensory and perceptual details of significant scenes and is distinct from a verbally based narrative memory system. This image-based system is believed to be accessed involuntarily and to support the recollective experience revealed by flashbulb memories (Brown & Kulik, 1977) and the spontaneous switching into the present tense when recounting an emotional narrative (Pillemer, 1998; Pillemer & White, 1989). A perceptual memory system supporting conscious experience was also part of the more general memory framework proposed by Johnson (1983).
Drawing on these theories, Brewin, Dalgleish, and Joseph (1996) put forward a dual representation theory of PTSD, in which the traumatic event was represented in two parallel memory systems. The situationally accessible memory (SAM) system contains detailed sensory and perceptual images that can be accessed only involuntarily and that form the basis for flashbacks and nightmares relating to the traumatic moments themselves. This system is thought to be supported primarily by subcortical structures and by areas of the brain directly involved in perception rather than in higher order cognitive control. The lack of involvement of structures such as the hippocampus results in a memory that is not contextualized but experienced as happening again in the present. The verbally accessible memory (VAM) system contains records of conscious experience that can be either automatically or deliberately retrieved and that support communication, reappraisal, and consequent alteration of life goals. This system requires the ability to flexibly access and manipulate a variety of information, can situate such information in its appropriate spatial and temporal context, and is assumed to depend on prefrontal areas of the brain as well as MTL structures such as the hippocampus (Brewin, 2001). Both systems are conceived of as part of normal memory but as functioning abnormally in PTSD.
Conway (2001, 2005) proposed a similar separation between episodic and autobiographical memory systems. The episodic system retains highly detailed sensory and perceptual knowledge over relatively brief retention intervals (measured in minutes and hours). It is an image-based system possessing little if any conceptual organization and is located in posterior temporo-occipital areas. Retrieval is involuntary (i.e., associative) rather than generative (i.e., strategic) and corresponds to a recollective experience or reliving. Retention of this episodic information usually depends on its integration within autobiographical memory, a knowledge-based, conceptually organized system located in prefrontal anterior-temporal areas that can be accessed either automatically or strategically. Autobiographical memories are given their context by the individual’s personal history but are associated with recollection only to the extent that they are linked with episodic memories.
Conway’s (2001, 2005) formulation of episodic memory is similar to the episodic buffer proposed by a number of theorists, including Baddeley (2000) and Kosslyn, Thompson, and Ganis (2006). Its context is the immediate past and egocentric orientation of the individual perceiver. A similar distinction between a short-term episodic memory and a long-term autobiographical memory system forms part of a computational model of consciousness described by Baars, Ramamurthy, and Franklin (2007). More recently, however, Conway (2009) put forward evidence that episodic memory is not restricted to short-term storage and that its contents, even though relatively inaccessible without the necessary retrieval cues, may even be permanent. He further proposed that PTSD may be characterized by the intrusion into consciousness of episodic elements that are lacking a frame of conceptual, contextualizing knowledge.
Neuroscience of Fearful Memories
Finally, recent advances in the neuroscience of fearful memories—specifically the acquisition and reconsolidation of involuntary responses—appear poised to become relevant to the clinical disorders reviewed earlier. Although our main focus here concerns conscious imagery, the neural mechanisms of involuntary fearful responses are relevant to the revised dual representation model introduced later and also offer the future prospect of alternative therapies to those reviewed here. Put briefly, acquisition of the association between a (visual or auditory) sensory stimulus and an electric shock leads to subsequent involuntary fearful responses to the stimulus, such as freezing in rodents (LeDoux, 1996) or fear-potentiated startle in humans (e.g., Kindt, Soeter, & Vervliet, 2009). These changes depend on molecular processes in the basolateral amygdala (e.g., Monfils, Cowansage, Klann, & LeDoux, 2009) that effectively route low-level (subcortical) sensory representations to internal representations of emotional state, most likely supported by the insula (e.g., Craig, 2002). These findings relate to the low-level sensation-bound representations considered later in the section titled A Neurocognitive Model of Healthy Memory and Imagery. Of relevance to the alternative contextually bound representations considered later in the same section, the expression of these fearful responses is modulated by the hippocampus so that they occur only when the subject is within the same physical context in which the shock was experienced (Kim & Fanselow, 1992).
Reactivation of the initial memory, by a single presentation of the stimulus, renders its association to fearful responses vulnerable to disruption by pharmacological (Kindt et al., 2009; Nader, Schafe, & LeDoux, 2000) or behavioral (Monfils et al., 2009) intervention. Interestingly, this manipulation has no effect on the conscious recollection of the training events (Kindt et al., 2009), which is thought to depend on the hippocampus. One possibility is that the reactivated memory requires reconsolidation and that if this process is disrupted, then the learned association is lost. If correct, this potentially offers an alternative to the learning of a new positive or null association for the stimulus that then competes with the existing negative association (as in extinction training). Reconsolidation offers a theoretical advantage over extinction, following which the older negative association can recur spontaneously or after reexposure to the shock (e.g., Monfils et al., 2009).
Images
The clinical studies reviewed previously have demonstrated a close correspondence between intrusive images of dreaded future outcomes and intrusive memories of past upsetting or dangerous events. Apart from the similarities in content, both are described in terms of being vivid, persistent, hard to control, and accompanied by strong emotional reactions. This is consistent with an increased recent focus on the importance of future simulation. For example, Barsalou (2003) proposed that a primary function of the cognitive system is to run a series of simulations of future events so as to equip the individual to make better informed behavioral choices. Similarly, in their constructive episodic simulation hypothesis, Schacter, Addis, and Buckner (2007) proposed that future simulation is facilitated by having a memory system that can flexibly draw upon and recombine details of past events. This system, they suggested, may be involved in a range of tasks requiring mental simulation of alternative perspectives, including alternative perspectives on the present.
The core temporoparietal network for context retrieval has been associated with the related processes of the generation of imagery (Burgess, Becker, et al., 2001; Byrne et al., 2007; Hassabis, Kumaran, Vann, & Maguire, 2007; Schacter et al., 2007) and associative encoding (Peters, Daum, Gizewski, Forsting, & Suchan, 2009). Byrne et al. (2007), Kosslyn et al. (2006), and Schacter et al. (2007) have gone further in suggesting that the neural machinery for experiencing the past and imagining the future is similar if not identical. Schacter et al. (2007) proposed a core brain system mediating past and future thinking, consisting predominantly of medial prefrontal regions, posterior regions in the medial and lateral parietal cortices, the lateral temporal cortex, and the MTL (including the hippocampus). They suggested that this system integrates information from past experiences and uses it to construct mental simulations about possible future events.
Given the clinical evidence for taking alternative perspectives when visualizing images and memories, it is of considerable interest that there are thought to be a number of parallel neural systems that can represent objects and spatial information in different ways. For example, Marsolek (1999) presented evidence that object information is processed by one neural system, more prominent in the right hemisphere, that captures viewpoint-dependent information about the specific object exemplar presented on one occasion. He contrasted this type of episodic memory with another system, more prominent in the left hemisphere, that processes an abstract structural description of the object influenced by past exemplars.
Hitch, Brandimonte, and Walker (1995); Kosslyn et al. (2006); Schacter, Cooper, and Delaney (1990); and Stankiewicz, Hummel, and Cooper (1998) have all distinguished object representations consisting of abstract structural descriptions from representations depicting the spatial relations between object and perceiver as experienced in a particular episode. Kosslyn et al. (2006) proposed the existence of a visual buffer that contains more information from an episode than can be processed in detail. An attention window selects some of the information in the buffer for further processing. Information in the attention window is sent to an object-properties processing system in the inferior temporal lobe, where an abstract structural description is created on the basis of features such as shape and color and is compared with previously stored representations. In contrast, information from the entire visual field, not just the attention window, is sent to a spatial-properties processing system located in the parietal lobe. Within this system, object maps are topographically organized and depict objects or scenes relative to the perceiver.
Burgess, Becker, et al. (2001) and Byrne et al. (2007) proposed a related computational model in which spatial information derived from either perception or imagery is represented in memory in both a less flexible egocentric form (viewpoint-dependent information stored relative to the body) and a more flexible allocentric form (viewpoint-independent information stored relative to the environment and able to be manipulated in novel ways). In this model, egocentric representations are supported by the precuneus, a medial parietal structure generally considered to play an important role in imagery and autobiographical memory (e.g., Burgess, Becker, et al., 2001; Fletcher et al., 1995; Schacter et al., 2007). These egocentric representations form the basis of short-term spatial memory and imagery and can be modulated by directed attention in the form of planned eye movements (e.g., Wallentin, Roepstorff, & Burgess, 2008). Lateral parietal areas also participate in the storage aspects of spatial working memory, with prefrontal areas contributing to control aspects, such as planning or active maintenance.
Byrne et al. (2007) proposed that, whereas the parietal representation system is important for immediate action, long-term memory storage is facilitated by allocentric representations produced by activity in the hippocampus and surrounding MTL structures (see also Milner, Dijkerman, & Carey, 1999). These MTL representations are not dependent on a specific viewpoint and can be more flexibly accessed. Normal encoding and retrieval require communication between the parietal and MTL areas, accomplished by pathways that involve the posterior parietal and retrosplenial cortices, including a translation process that integrates these two sets of representations. As well as representing immediate sensory inputs, therefore, the precuneus provides an arena to which stored allocentric representations in long-term memory can be transferred and in which they can be recreated, inspected, and manipulated.
Summary: Dual Representations in Memory
It has been proposed that voluntary and involuntary memory depend on similar networks in the brain, with voluntary memory additionally recruiting prefrontal areas as part of the strategic search process. Both memory and imagery appear to rely on common networks involving medial prefrontal regions, posterior regions in the medial and lateral parietal cortices, the lateral temporal cortex, and the MTL. A common theme running through the work reviewed in the previous section is the distinction between representations that capture relatively unprocessed sensory data and representations that are more abstract. Although the precise terminology and distinctions differ owing to the varying phenomena under study, we suggest that there is a core difference that is common to all theories.
On the one hand there is a memory system that is designed to code sensations, as far as the brain will allow, in the unique form in which they were experienced (e.g., egocentric, viewpoint-dependent, depictive, SAM). Information in this system can be activated by matching inputs, with cues often consisting of low-level sensory features. The second system codes what is perceived in terms of more abstract properties that are common across experiences (e.g., allocentric, viewpoint-independent, structural, VAM). These representations can be used to (re)construct images consistent with the experienced event from any desired viewpoint. Information in this system can also be combined flexibly to respond to novel situations and, in humans, to support higher cognitive functions such as planning, narration, and communication. Although not the focus here, the parallels with the dual process models of recognition memory (e.g., Rugg & Yonelinas, 2003) are noteworthy.
A Revised Dual Representation Theory of Visual Intrusions
The dual representation theory of PTSD (Brewin, 2001, 2003; Brewin, Dalgleish, & Joseph, 1996) was concerned with explaining the nature of intrusive memories in this disorder in terms of VAMs, SAMs, and their interrelation. Here we present a revised dual representation theory, taking into account the recent clinical, neuroscientific, and psychological advances regarding intrusive memories and images reviewed earlier. The revised model is concerned with a broader spectrum of visual intrusions occurring across different disorders and offers considerably greater specificity in describing the underlying neural processes.
The background to the extension described here is provided by the main features of dual representation theory as it stands, which we can briefly summarize as follows. The VAM system supports abstract declarative representations within their associated autobiographical context and in a form accessible to deliberate retrieval and manipulation. The SAM system holds low-level representations tightly bound to their sensory and affective qualities. In PTSD, extremely stressful events are stored as SAMs without the usual association to the VAM system. This allows retrieval of SAMs to be triggered involuntarily by environmental or internal (situational) cues reminiscent of the original trauma without retrieval of the appropriate autobiographical context.
What function does this loss of association between VAM and SAM systems serve in PTSD? It has been proposed that the narrowing of attention brought about by extreme stress, as well as the loss of hippocampal function, means that less information about a potentially life-threatening event can be stored in a consciously available form (Brewin, 2003). This reduction in encoding may be increased by peritraumatic reactions such as dissociation or feelings of mental defeat (Ehlers, Maercker, & Boos, 2000). Because the information may be critical for future survival, it is captured in the form of sensory images, allowing maximally large amounts of data to be processed in parallel and recorded (although not provided with a spatial or temporal context). Flashbacks are an adaptive process in which stored information can be re-presented and processed in greater depth once the danger is past. PTSD, according to this account, reflects the failure of the re-presented information to be attended to. Instead, traumatic images are avoided and never become associated with their proper context.
The VAM system is identified with the hippocampus, whose function may be modulated by conditions of extreme stress (Metcalfe & Jacobs, 1998; Pitman, Shalev, & Orr, 2000), whereas SAMs are associated with sensory and interoceptive cortical areas. We outline, as the starting point for a revised dual representation theory, a neurocognitive model of healthy memory and then seek to define how intrusive memories and images arise within such a system.
A Neurocognitive Model of Healthy Memory and Imagery
We first define terms for each of the two types of representation, in order to avoid the potential confusion arising from the multiple previous terminologies. Their properties are summarized in Table 2. Henceforth we refer to abstract, contextually bound representations as contextual memory (C-memory) and its representations as C-reps rather than as VAMs. The new terminology acknowledges that verbal accessibility is not their defining property, although the ability to deliberately retrieve and manipulate C-reps will certainly aid in their verbal expression. C-memory could provide the basis for narrative memory as described by Janet (1904) and Pillemer (1998). Similarly, we will refer to low-level sensation-based memory (S-memory) and its corresponding representations (S-reps) rather than as SAMs. The new terminology acknowledges that they play a role in healthy memory in addition to being reactivated as flashbacks in sufferers with PTSD. S-memory is a candidate for Conway’s (2009) reconceptualized episodic memory system and could support the image-based memory systems proposed by Brown and Kulik (1977) and Pillemer (1998).
Table 2. Characteristics of Contextual and Sensation-Based Memory.
We assume that C-reps are supported by the classical MTL system for declarative memory (Scoville & Milner, 1957; Squire & Zola-Morgan, 1991), including the hippocampal provision of the spatio-temporal contextual information associated with an episodic memory for an event (Cohen & Eichenbaum, 1993; Kinsbourne & Wood, 1975; O’Keefe & Nadel, 1978; Tulving, 1983). A more detailed model of C-reps would involve an initial contextual representation that becomes integrated into personal semantic memory over time, following Marr’s (1970, 1971) model of the relationship between the hippocampus and the neocortex (see also McClelland, McNaughton, & O’Reilly, 1995). With their association to semantic memory, C-reps can also be used to generate meaningful interpretations of an event, abstracted or “gist” representations, and novel images that combine object and conceptual information in flexible ways, such as images from nonexperienced viewpoints.
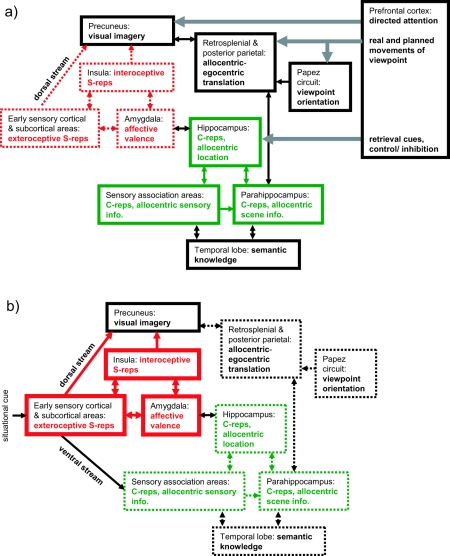
The neural mechanisms underlying the retrieval of relatively abstract long-term C-reps into short-term visuospatial imagery have been modeled in some detail (Burgess, Becker, et al., 2001; Byrne et al., 2007). In these models, the C-reps include the spatial layout of the scene comprising the event (i.e., its spatial context), encoded as allocentric representations in the hippocampal and parahippocampal regions (i.e., the distances of scene elements north, south, east or west of the person). Retrieval corresponds to the construction of a short-term egocentric representation for inspection or manipulation (i.e., the distances of scene elements to the left or right of or ahead of the subject). The required process of translating scene information between egocentric and allocentric reference frames is assumed to be supported by the retrosplenial and posterior parietal cortices, with imagery supported in the precuneus. The need for the imposition of a viewing direction to produce an egocentric representation potentially explains the involvement of Papez’s circuit in episodic recollection, because this circuit supports a representation of head direction in rodents (e.g., Taube, 1998).
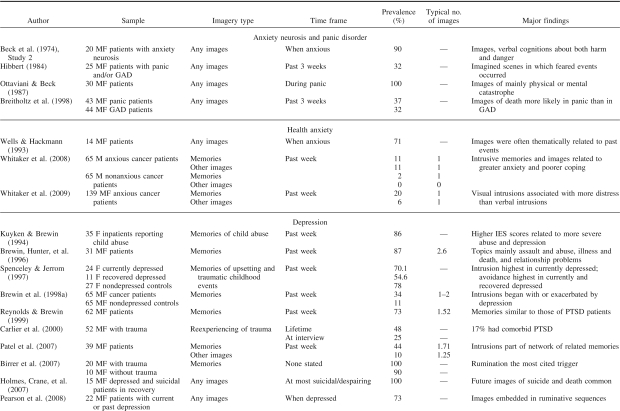
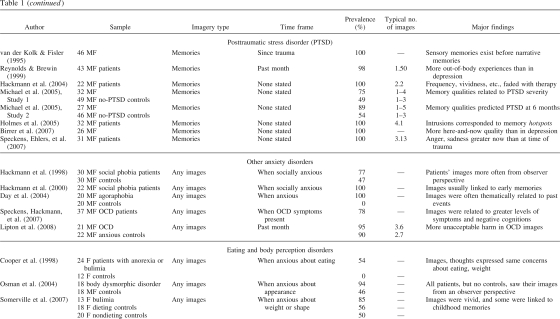
During the encoding of normal episodic memories, temporary activation of lower level sensory cortices, driven by perception, allows the initial formation of the higher level representations described earlier in the parietal and MTL associative cortices.1 The same mechanism that translates from egocentric perceptual representations to contribute to allocentric MTL representations during encoding also translates from allocentric long-term memory to generate egocentric imagery during deliberate retrieval of an event (see Figures 1a and 1b). For normal events, these low-level sensory representations, or S-reps, will quickly decay and become relatively inaccessible.
Figure 1. A schematic model of memory encoding, showing the approximate regions and pathways involved in, and the areas supporting, abstracted contextual representations (C-reps, in green) and sensory-bound representations (S-reps, in red). a: Normal encoding of a traumatic event. b: Pathological encoding of a traumatic event, showing up-regulation of S-reps, down-regulation of C-reps, and disconnection between S-reps and C-reps. Heavy lines indicate stronger representations and pathways; dashed lines, weaker representations and pathways. Note the necessarily schematic style (e.g., many pathways and regions are omitted, and Hippocampus refers to the extended hippocampal formation and its subcortical connections).
The effects of emotion on memory are well established, if far from being completely understood (Cahill & McGaugh, 1998; McGaugh, Roozendaal, & Okuda, 2006). According to dual representation theory, perception of a moderately stressful or emotionally salient event results in the creation of more enduring C-reps and S-reps. Such S-reps would also include autonomic markers of affective values such as fear or disgust mediated by the insula (Craig, 2002; Critchley, Wiens, Rotshtein, Öhman, & Dolan, 2004) that become associated to the low-level sensory characteristics of the event via the amygdala (LeDoux, 1996). The S-rep can be reactivated bottom-up by experiencing a similar perceptual input or top-down via associations from the higher level representation of the original event (e.g., a reconstructed egocentric image with features and viewpoint similar to those experienced at encoding) in the precuneus.
In healthy individuals, the S-rep for an extreme event is associated, via higher level representations in the precuneus, to a corresponding C-rep in the MTLs. The association to the corresponding C-rep has two consequences: (a) allowing the event to be correctly integrated with its semantic and autobiographical context, thereby preventing it from being reexperienced in the present, and (b) allowing for increased top-down control via connections from the prefrontal cortex to the MTL, such as the provision of specific retrieval cues, verification of the products of retrieval (see, e.g., Burgess & Shallice, 1996; Fletcher & Henson, 2001), strategies for disambiguating events with similar contexts (King et al., 2005), and deliberate suppression of retrieval if required (Anderson et al., 2004). It is of interest, however, that there is also evidence for neuroanatomically distinct pathways exerting separate top-down control over areas associated with the sensory aspects of memory (visual cortex, thalamus) and areas associated with multimodal and emotional aspects (hippocampus, amygdala; Depue, Curran, & Banich, 2007).
How Do Intrusive Images Arise Within This Model?
According to this revised dual representation theory, many of the intrusive memories found in clinical disorders such as depression arise within the normal working of the autobiographical memory system as instances of involuntary recall producing combined reactivation of corresponding C-reps and S-reps. As such, intrusive images can arise in two ways. Long-lasting S-reps, formed by emotionally salient experiences, may be retrieved by corresponding emotional states or by sensory cues in the external environment, with this sensory reliving component given a context and modulated by activation of the corresponding C-reps. Alternatively, images in the precuneus formed from C-reps may involuntarily activate associated S-reps that contribute additional sensory and emotional aspects to retrieval. For example, a depressed person might have a negative thought about being rejected that was associated with a C-rep of a time when he or she was jilted by a lover. Activation of the C-rep could then trigger the reactivation of an associated S-rep that would contribute sensory details of the rejection event, along with associated feelings of anger and despair. Consistent with this, visual memories of upsetting episodes have been observed to intrude into the verbal ruminations of depressed individuals (Birrer et al., 2007; Pearson et al., 2008).
As noted by Berntsen (2007), the descriptions given by many patients of their intrusions tend to involve specific episodes and emphasize reexperiencing, the impact on their mood, and the associated bodily reactions. Although there is no particular reason to think this process has a different neural basis from the involuntary memories of a nonclinical sample (cf. Berntsen & Rubin, 2008), an explanation is required for why intrusions are so much more frequent and distressing in clinical groups. Likely candidates are the processes of selective attention (patients attend to specific features of the environment that trigger negative memories), negative appraisals (they interpret events in a way that increases negative mood and the likelihood of recalling related negative events), cognitive and behavioral avoidance (they monitor the environment for negative reminders in a way that unintentionally prolongs the salience of negative memories), and distorted reasoning (they overgeneralize from other negative events or take excessive responsibility for them, thereby creating associative links to negative events in the past). All these processes have been identified as present across many different disorders (A. G. Harvey et al., 2004).
As part of the process of deliberately simulating possible future outcomes, some individuals will construct images (e.g., of worst possible scenarios) based on information in C-memory and generate images in the precuneus. These images may be influenced by related information held in C-memory or in addition the images may be altered by the involuntary retrieval of related material from S-memory. Novel images may also arise spontaneously in S-memory through processes of association. If sufficiently emotional, these internally generated images will, like externally generated images, bring about long-lasting S-reps. As a result, internally generated images may come to behave much like intrusive memories, being automatically triggered and accompanied by a strong sense of reliving.
Images deliberately created from long-term memory will have corresponding C-reps that provide an anchoring context, whereas spontaneous images arising from S-reps may not. In this case, some patients may have difficulty in retrieving any autobiographical events that form the basis of their images, as described by Holmes and Hackmann (2004). In other respects, nonveridical spontaneous images will tend to behave in the same way as those closely reflecting actual experience. Indeed, in some cases confabulatory memories may be confused with actual experiences, as in the case of perceived alien abduction (McNally et al., 2004).
Intrusive Images in Posttraumatic Stress Disorder
As we have seen, there is evidence that there are systematic differences between some intrusive memories characteristic of depression and those occurring in PTSD, with PTSD flashbacks being retrievable only involuntarily and involving reliving the event as though it were occurring in the present. To our knowledge the more extreme forms of reexperiencing observed in some PTSD patients have never been described in someone with a primary diagnosis of depression or of another anxiety disorder. In previous articles it was proposed that this was because (a) details of the traumatic event were initially encoded better into the SAM than into the VAM system and (b) some trauma-related information remained isolated in the SAM system and was not copied into the hippocampally dependent VAM system, where it could be provided with a spatial and temporal context (Brewin, 2001). This transfer process was assumed to require deliberate attention being paid to the content of flashbacks.
The idea that extreme stress may have different effects on different memory systems is not new. There is accumulating evidence that high levels of stress have deleterious effects on hippocampal functioning while simultaneously potentiating amygdala functioning (Elzinga & Bremner, 2002; Metcalfe & Jacobs, 1998; Payne et al., 2006; Vyas, Mitra, Rao, & Chattarji, 2002). Theoretically, this should produce strong S-reps but weaker or impoverished C-reps.
In the revised dual representation model, we suggest accordingly that flashbacks in PTSD result from the creation of an S-rep without the usual association to a corresponding C-rep. The contrast between normal and pathological encoding of a traumatic event is summarized in Figures 1a and 1b. Normal encoding involves the creation of C-reps and S-reps with connections between the two. Pathological encoding involves relatively stronger S-reps, relatively weaker C-reps, and impaired connections between them.
The desired contextual representation is not primarily defined by being verbal (although it would support conscious manipulation and generation of verbal descriptions more easily than would an S-rep) but by its properties being coded within the ventral stream in a way that permits integration with other autobiographical memories.
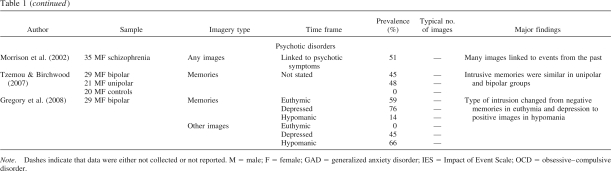
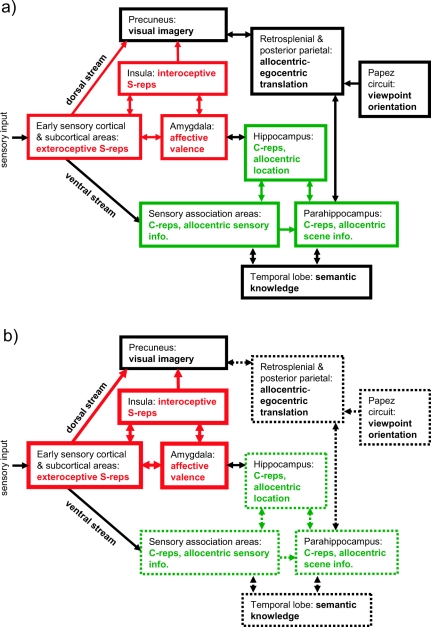
When such an S-rep is automatically reactivated bottom-up or top-down, it is vividly reexperienced in the present. The contrast between normal and pathological retrieval of a traumatic event is summarized in Figures 2a and 2b. Normal visual recall is driven top-down by C-reps, with the generation of egocentric visual imagery allowing reactivation of matching information contained in S-reps. Flashbacks involve visual imagery being driven bottom-up by S-reps, relatively uninfluenced by corresponding C-reps. This reactivation is more like perception than the retrieval of a memory from a past context and occurs without reference to the encoding context or associated autobiographical knowledge. Note that the absence of a C-rep (as, for example, in amnesia caused by MTL damage) is not sufficient on its own to cause flashbacks but only in the context of an enduring S-rep caused by an extreme event.
Figure 2. A schematic model of the generation of memory-related imagery, showing the approximate regions and pathways involved in, and the areas supporting, abstracted contextual representations (C-reps, in green) and sensory-bound representations (S-reps, in red). a: Deliberate visual recall. Visual imagery is driven top-down by C-reps, under the direction of the prefrontal cortex. Reactivation of S-reps is weak and depends on attention to matching details in imagery. b: Involuntary flashback of a traumatic event. Visual imagery is driven bottom-up by S-reps triggered by a situational cue and unaffected by weakly activated C-reps. Heavy lines indicate stronger representations and pathways; dashed lines, weaker representations and pathways. Note the necessarily schematic style (e.g., many pathways and regions are omitted, and Hippocampus refers to the extended hippocampal formation and its subcortical connections).
Thus, dual representation theory proposes two conditions for the occurrence of flashbacks: the creation of an enduring S-rep by an extremely emotional or stressful event, combined with an absence of integration with a corresponding C-rep, and thus a lack of appropriate contextualization or top-down control for this S-rep. If flashbacks are to persist, there must be mechanisms that perpetuate this lack of integration. PTSD sufferers show marked behavioral and cognitive avoidance and find their intrusions (or certain parts of them) too unpleasant to attend to, which could plausibly account for the fact that the corresponding C-rep remains incomplete. We propose that the C-rep is rarely completely absent and that it may be only certain traumatic moments (hotspots) that are missing from the C-rep. However, extreme reexperiencing, in which all contact with the current environment is temporarily suspended, would follow from the complete absence of a C-rep (e.g., because the S-rep being retrieved was of a childhood experience that had not been consciously thought about in later life).
Delayed-Onset Posttraumatic Stress Disorder
Many studies have noted that the onset of full PTSD may not occur until months or years after the corresponding traumatic event. The Diagnostic and Statistical Manual of Mental Disorders (4th ed., text rev.; American Psychiatric Association, 2000) requires a 6-month period to elapse if PTSD is to qualify as delayed-onset PTSD. On average this occurs in 15.3% of civilian cases of PTSD and in 38.2% of cases of combat-related PTSD, with initial PTSD symptoms gradually increasing in number and intensity (Andrews, Brewin, Philpott, & Stewart, 2007). Delayed-onset PTSD is associated with gradually increasing levels of arousal and is often preceded by episodes of depression (Andrews, Brewin, Stewart, Philpott, & Hejdenberg, 2009).
Following Becker and Wojtowicz (2007), it may be that conditions such as long-term stress or depression impair the ability of the hippocampus to continue to provide distinct C-reps for traumatic events. In combination with low mood and cognitive biases, trauma memories may trigger further negative reactions, which are in turn encoded as S-reps. In the absence of distinctive C-reps to strongly associate negative memories with their past context rather than current experience, this could produce a downward spiral or negative feedback loop, eventually creating an enduring S-rep associated with extreme negative emotion.
One possible mechanism by which the hippocampus could differentiate the past and present context of reexperiencing trauma is by association to a continually changing population of new, easily excitable, neurons in dentate gyrus, created by a process of neurogenesis (Aimone, Wiles, & Gage, 2006). One of the consequences of depression is a down-regulation of neurogenesis (Becker & Wojtowicz, 2007), which might lead to prolonged difficulties in establishing a clear temporal context. Thus, the ability of the hippocampus to contextualize trauma memories may change over time. Although this account is necessarily speculative, it is consistent with observations that late-life PTSD onset appears frequently to be related to the start of neurodegeneration (Hiskey, Luckie, Davies, & Brewin, 2008).
Field and Observer Memories
The naturalistic studies reviewed earlier have shown that recurrent intrusions involve memories that may be perceived from a field (i.e., first-person perspective) or an observer perspective. In these studies, field memories would correspond to egocentric representations in the precuneus generated bottom-up by specific S-reps, being more recent, more strongly associated with emotion, and from an experienced viewpoint. By contrast, observer memories would correspond to egocentric representations in the precuneus generated top-down from C-reps, in that they require generation of an image from a nonexperienced viewpoint and relate to older and more abstracted information (see also Conway, 2009). However, as noted by Nigro and Neisser (1983), some observer memories may correspond to the person’s perception at the time (i.e., they may reflect observer experiences, in which the observer perspective was present during the event, elicited by factors such as acute self-consciousness).
The suggestion that the observer perspective can be employed strategically to reduce emotion (e.g., McIsaac & Eich, 2004; Nigro & Neisser, 1983) fits well with a long history of clinical observations and theory concerning the role of dissociative reactions such as out-of-body experiences in protecting some individuals from the short-term emotional impact of extreme stress. In terms of the revised model, individuals can be seen as bringing online a scene that is constructed from information in C-memory. Compared with the corresponding S-rep, this will have preferential access to contextual information stored in the MTL and to MTL structures like the hippocampus—explaining the association of these structures with the reduction of fear (Douglas, 1972; McCormick & Thompson, 1982).
Under the revised model, the generation of imagery in the precuneus from an observer perspective may occur either during the stressful event itself or subsequently, via a stored C-rep. The observer perspective makes these images less likely to lead to the retrieval of corresponding S-reps, because these low-level sensory representations would not match the observer viewpoint. Findings suggest this is a mechanism particularly associated with PTSD (McIsaac & Eich, 2004; Reynolds & Brewin, 1999), social phobia (Hackmann et al., 2000, 1998), and body dysmorphic disorder (Osman et al., 2004). Strikingly, 89% of McIsaac and Eich’s (2004) sample who adopted the observer vantage point said they did so to spare themselves the horror of having to relive the trauma again through their own eyes. Similarly, Williams and Moulds (2007) found that, in a high dysphoric sample, seeing an intrusive memory from an observer perspective was associated with feeling more detached and numb.
Summary
The conceptual basis of the dual representation theory of PTSD, which was inspired largely by clinical observation, has been revised to anchor it more firmly in cognitive neuroscience. Thus, in terms of visual imagery, we have identified the flexible, consciously accessible, allocentric, context-dependent representations (C-reps) with the projection of the ventral visual stream to the inferior temporal cortex and with its connections to MTL structures such as the hippocampus and parahippocampus. We have identified the inflexible, sensation-bound, viewpoint-dependent, involuntarily reactivated representations accompanied by strong emotional and autonomic components (S-reps) with the projection of the dorsal visual stream to the superior parietal areas and with their connections to the amygdala and insula. It is proposed that these two types of representation can interact via egocentric imagery in the precuneus, which could be activated either top-down or bottom-up by either system, respectively.
We have used the insights afforded by this process to extend its range to cover intrusions in other disorders as well as to phenomena such as observer memories. There continue to be important differences, however, between PTSD and other disorders characterized by intrusions. It is only in PTSD, for example, that the disturbance in memory (or, more accurately, the failure of this disturbance to be corrected) is seen as a causal factor in the creation of the disorder as well as a contributor to the maintenance of the disorder. In other disorders, in which no corresponding dissociation between memory systems is implicated, the disturbance is viewed as contributing much more to maintenance than to onset. In depression or eating disorders, for example, intrusions may reflect the effects of high levels of emotion on the entire memory system rather than any qualitative alteration in the way the system is operating.
Pursuing this point further, in our earlier review of naturalistic studies of intrusions we also identified that intrusive images and memories were sometimes associated with events that appeared to occur at the onset of a wide variety of disorders. Humiliating childhood experiences were sometimes linked with the onset of social phobia, for instance. It is well established that in disorders such as PTSD, stress may act more as a primary cause but that in other disorders, such as schizophrenia, stress acts more as a provoking factor in a person with a suitable predisposition. The focus on intrusions may help to explain why events that are not in themselves overwhelmingly stressful can be associated with the onset of these disorders. According to this account, it is because certain of these events lead to the creation of vivid memories or images that are hard to dismiss and continue to exert an influence on subsequent mood regulation.
Although the revised model points to the common neural underpinning of memory and imagery, in the future researchers need to explore whether past memories of adversity and dreaded images of future adversity share the same functions. Researchers could also profitably address the origin of distortions introduced into the representation of past events as they are projected into the future.
Implications for Psychological Treatment
The finding that intrusive images derived, to a greater or lesser degree, from autobiographical events are common across a wide variety of disorders is consistent with the claims of many social psychologists (e.g., Srull & Wyer, 1990, 1993) that knowledge about the self does not exist just in a generalized, semantic form (cf. trait knowledge about the worthlessness or unlovability of the self; Beck, Rush, Shaw, & Emery, 1979) but in the form of episodic memories of specific events. Such events (e.g., a child being told by a parent that he or she was not wanted) may form turning points (Pillemer, 1998) that help to define or provide evidence for the conclusions about the self at which patients may have arrived.
The data reviewed in this article demonstrate that such events, and their accompanying emotions, can be repeatedly present in awareness, providing opportunities to shape appraisal and coping processes. These memories often have prominent visual components that amplify emotional reactions and contribute to the difficulties they cause (Holmes, Geddes, Colom, & Goodwin, 2008). Moreover, we have presented evidence that these intrusions are causally related to the course of disorders. Thus, an appreciation of the importance of these intrusions and of their neural basis will be important for understanding current therapies as well as suggesting opportunities for novel forms of therapy.
A general theoretical framework that can suggest suitable interventions for intrusive images, regardless of their veracity or modality, is the retrieval competition hypothesis (Brewin, 2006). Much research in human and animal learning has suggested that emotions and behavior are under the control of alternative memory representations that compete for retrieval (Gold, 2004; Jacobs & Nadel, 1985; Poldrack & Packard, 2003). It is further assumed that the dominant negative representations that support disordered mood and behavior cannot be directly altered but that it is possible to create or strengthen alternative, more positive, representations. Importantly, these alternative representations do not have to be more accurate or free of cognitive distortion. Recovery from an episode of disorder occurs when positive thoughts, memories, and images are regularly retrieved in preference to negative ones. The function of the therapist is to produce new positive representations, or strengthen existing positive representations, so that they are highly memorable and highly accessible and can compete effectively with previously dominant negative representations.
The role of imagery in psychological therapy has previously been reviewed in some detail by Hackmann (1998) and Holmes, Arntz, and Smucker (2007). Here, we focus on the implications of the revised dual representation theory for understanding two evidence-based treatments for intrusive memories in PTSD (National Institute for Clinical Excellence, 2005)—exposure and eye-movement desensitization and reprocessing (EMDR)—and one relatively understudied treatment, imagery rescripting, that is potentially applicable to many different disorders.
Treatments for Posttraumatic Stress Disorder
Exposure
Dual representation theory has previously been used to provide an explanation for the effectiveness of exposure-based treatments for PTSD (Brewin, 2001, 2003). It was proposed that narrowed attention during a traumatic event results in more detailed perceptual representations being created in the image-based memory (SAM) system compared with the verbally accessible memory (VAM) system. Exposure to trauma cues, particularly sensory cues, is therefore more likely to result in the SAM winning the retrieval competition. The fact that the SAM is not contextualized leads to the brain responding as though the trauma is in fact happening again in the present, producing a powerful sense of reliving as well as intense emotional reactions. Normal recovery depends on holding intrusive images automatically retrieved from the SAM in focal attention for sufficient time so that the details they contain are copied into the VAM, where they are provided with an autobiographical context. This process is interrupted in PTSD, for example because of other demands on attention, such as anxiety about the health of self and loved ones, or altered states such as shock.
The revised theory is more specific about a common neural basis for these processes, proposing that the contents of the SAM correspond to long-lasting sensory representations in parieto-occipital networks that have no counterpart in the contextual representations stored in MTL structures. The effects of exposure treatment are now related to two underlying processes. First, holding images in focal attention in the precuneus facilitates the transfer of successive parts of S-reps into more elaborated C-reps that can be integrated with existing autobiographical information. Second, it facilitates the association of these representations with one another by means of a pathway via the posterior parietal and retrosplenial cortices. In this way, the expression of S-reps can benefit from the contextualization provided by the hippocampus whereas C-reps can benefit from modification of their associations to autobiographical and semantic knowledge and from coming under greater inhibitory control by the prefrontal cortex.
Exposure-based treatments require PTSD patients to give an extremely detailed oral or written narrative of their traumatic event and to come into deliberate contact with trauma reminders, for example by revisiting the scene of the trauma. Some treatments, such as prolonged exposure (Foa & Rothbaum, 1998), involve repeating these procedures on numerous occasions until fear diminishes. The treatment thus tends to both elicit involuntary traumatic images and encourage patients to direct their attention to them in a systematic way. This is puzzling from the perspective of a conventional theory of memory, according to which such a procedure should further consolidate the memory and make the condition worse. The proposal that there are two sets of memories—one that primarily acts to excite fear in preparation for action and one that primarily acts to modulate fear by contextualizing it—helps to resolve this conundrum.
Eye-movement desensitization and reprocessing
EMDR (Shapiro, 2001) involves having patients retrieve and hold in focal attention an image of the worst moment of their trauma, together with any associated negative thoughts, while the therapist repeatedly introduces bilateral stimulation for periods of about 20 s (called sets). This stimulation may take the form of requiring patients to track the therapist’s hand with their eyes backward and forward across the visual field, or of repeated taps on alternate hands, or of playing sounds that alternate between left and right. Of these, the most common and empirically validated method is the visual tracking of the therapist’s hand. During and between each set, patients are instructed to allow images and thoughts to enter and leave awareness or change their form, observing them but not attempting to influence the process.
EMDR is a complex treatment containing elements of exposure that should facilitate memory contextualization. Another of its central components involves directing patients to focus attention on traumatic moments that they may previously have avoided while simultaneously using a secondary task to reduce the intensity of the experienced images. We suppose that, while the traumatic image is being held in the precuneus’s working memory system (Byrne et al., 2007; Kosslyn et al., 2006), patients can scan and direct their attention to specific parts of the image via projections from the frontal eye fields (e.g., Wallentin et al., 2008). Carrying out a series of eye movements at the request of the therapist will interfere with this process and use up some of the resources available for maintaining and elaborating the image. Baddeley and Andrade (2000) and Postle, Idzikowski, Della Sala, Logie, and Baddeley (2006) have shown that carrying out eye movements selectively interferes with the visuospatial sketchpad and reduces the emotionality and vividness of images.
Thus, eye movements are sufficient but not necessary to interfere with trauma imagery, and the direction of the eye movements is not critical (see Gunter & Bodner, 2008). We speculate that the emotion-reducing properties of EMDR are an alternative method of facilitating the elaboration of C-reps and the mapping of S-reps and C-reps onto one another. The resulting C-reps will also contain, superimposed on the traumatic image, disambiguating information (the therapist’s hand movements) that identify the new representations with the (safe) present rather than the (dangerous) past. These memories will now be available for retrieval and may compete effectively with the original S-reps when the individual subsequently encounters traumatic reminders.
Imagery Rescripting
Imagery rescripting involves having patients focus on the contents of their intrusive image or memory and vividly construct an alternative, more positive outcome that they have previously generated and rehearsed with their therapist (Hackmann, 1998). Common positive images involve fantasies of overcoming aggressors, humiliating enemies, being rescued, communicating with the dead, being comforted, and being treated compassionately. Fantasies often include impossible elements, such as superhuman feats and supernatural beings, or impossible transformations of the physical world (Smucker & Dancu, 2005).
Rescripting can be used to support standard cognitive–behavioral interventions, such as cognitive challenging of negative beliefs, or alternatively as a stand-alone intervention. Although there have been no major randomized controlled trials concerning rescripting, it has shown promising effects, either alone or in combination with other interventions, in the treatment of the sequelae of child sexual abuse (Smucker, Dancu, Foa, & Niederee, 1995), borderline personality disorder (Arntz & Weertman, 1999), bulimia (Ohanian, 2002), PTSD (Grunert, Smucker, Weis, & Rusch, 2003), depression (Brewin et al., 2009), social phobia (Wild, Hackmann, & Clark, 2008), and a variety of other disorders (Holmes, Crane, Fennell, & Williams, 2007).
According to the revised dual representation theory, rescripting involves first retrieving the C-rep corresponding to the intrusive memory or image, projecting it to the precuneus, and then, while it is being held in mind, reporting on the content of associated S-reps that are retrieved in consequence. This ensures that all relevant material in S-reps have the opportunity to be fully contextualized by association to a new C-rep. A new and more elaborated C-rep is then constructed that blends this negative material with novel positive elements. If this process is successful, the projection of this new C-rep to the precuneus will lead to the spontaneous retrieval of positive sensations and emotions from S-memory, thereby helping to consolidate it and make it more accessible. It may be necessary to try a number of alternative scenarios before a successful C-rep is found that is sufficiently positive to neutralize the existing negative emotions as well as being sufficiently memorable to win the retrieval competition with the original C-rep.
Summary
Several therapies—including exposure, EMDR, and imagery rescripting—that focus primarily on images and memories already exist. We suggest that these therapies can be understood within the context of the revised model and that the scope for intervention is potentially much wider than has hitherto been realized. For example, techniques aimed at abolishing the influence of negative images could be adapted to disorders involving craving or to the hypomanic phase of bipolar disorder, where it appears to be excessively positive images that are problematic. Therapists could attempt to rescript these images to incorporate negative elements that would inhibit inappropriate approach behaviors.
Another area involves inhibiting unwanted intrusions by introducing competing visuospatial task demands. In the area of food cravings, there are already experimental studies showing that carrying out visuospatial tasks reduces the vividness of food-related images and the intensity of cravings (K. Harvey, Kemps, & Tiggemann, 2005; Kemps, Tiggemann, & Christianson, 2008). Other research has shown that the development of intrusive images following exposure to a traumatic film can be inhibited by visuospatial task demands either at encoding (Brewin & Saunders, 2001; Holmes et al., 2004) or shortly thereafter (Holmes, James, Coode-Bate, & Deeprose, 2009). In addition, potential alternative therapies may develop out of recent advances in disrupting the reconsolidation of fearful responses (Kindt et al., 2009; Monfils et al., 2009).
Conclusion
Involuntary memory-related imagery appears to play a more significant role than has previously been thought across a wide range of psychological disorders. It represents a mechanism whereby high-impact events in a person’s history, whether real or imagined, can continue to affect thought, emotion, and behavior, both in stressful situations and in episodes of disorder. This suggests that it should be given greater prominence as a target of psychological therapy than has often been the case. This is particularly relevant for disorders such as depression, for which psychological therapies have to date achieved only moderate levels of effectiveness (Fava, Ruini, & Belaise, 2007; Roth & Fonagy, 2004).
Of importance are the many indications that the retrieval of memories concerning the past and images concerning the future appear to share the same neural structures. By interpreting the dual representation theory of PTSD (Brewin, 2001, 2003) within the framework of a neurobiological model of normal memory and imagery (Burgess, Becker, et al., 2001; Byrne et al., 2007) we have outlined some of the neural mechanisms potentially underlying the observation of involuntary visual intrusions within a range of clinical disorders.
Among the testable predictions that follow from the model are that asking patients to describe a trauma they have witnessed or experienced should be more likely to elicit intrusive images if they describe it from a field perspective than if they describe it from an observer perspective. Further, the ability to retrieve information from a viewpoint other than the perceived one, and to suppress involuntary field memories, should indicate the greater health of or greater activity in structures, particularly the hippocampus, associated with C-memory (Abrahams, Pickering, Polkey, & Morris, 1997; Holdstock et al., 2000; King, Burgess, Hartley, Vargha-Khadem, & O’Keefe, 2002). Thus, behavioral or pharmacological manipulations that lead to an increased likelihood of subsequent intrusive imagery should also be associated with a specific impairment in C-memory-related functions.
We hope that this approach will allow advances in cognitive neuroscience to inform a wide spectrum of psychological treatments that are applicable to many different disorders. We are confident that, by constantly updating and integrating findings from both neuroscience and clinical psychology, researchers will reach the realistic goal of creating more sophisticated models with more possibilities of benefiting clinical practice.
Acknowledgments
This research was supported in part by grants from the Wellcome Trust and the United Kingdom Medical Research Council. We gratefully acknowledge useful comments from John King, Neil Harrison, Hugo Critchley, and John O’Keefe.
Footnotes
Here we use lower level to indicate more sensory-bound representations and lower perceptual processing areas, as opposed to more abstract, less perceptually bound representations and higher association areas.
References
- Abrahams S., Pickering A., Polkey C. E., & Morris R. G. (1997). Spatial memory deficits in patients with unilateral damage to the right hippocampal formation. Neuropsychologia, 35, 11–24. [DOI] [PubMed] [Google Scholar]
- Aimone J. B., Wiles J., & Gage F. H. (2006). Potential role for adult neurogenesis in the encoding of time in new memories. Nature Neuroscience, 9, 723–727. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders (4th ed., text rev.). Washington, DC: Author. [Google Scholar]
- Anderson M. C., Ochsner K. N., Kuhl B., Cooper J., Robertson E., Gabrieli S. W., . . . Gabrieli J. D. E. (2004, January9). Neural systems underlying the suppression of unwanted memories. Science, 303, 232–235. [DOI] [PubMed] [Google Scholar]
- Andrews B., Brewin C. R., Philpott R., & Stewart L. (2007). Delayed onset posttraumatic stress disorder: A systematic review of the evidence. American Journal of Psychiatry, 164, 1319–1326. [DOI] [PubMed] [Google Scholar]
- Andrews B., Brewin C. R., Stewart L., Philpott R., & Hejdenberg J. (2009). Comparison of immediate and delayed-onset posttraumatic stress disorder in military veterans. Journal of Abnormal Psychology 118, 767–777. [DOI] [PubMed] [Google Scholar]
- Arntz A., & Weertman A. (1999). Treatment of childhood memories: Theory and practice. Behaviour Research and Therapy, 37, 715–740. [DOI] [PubMed] [Google Scholar]
- Baars B. J., Ramamurthy U., & Franklin S. (2007). How deliberate, spontaneous, and unwanted memories emerge in a computational theory of consciousness. In Mace J. H. (Ed.), Involuntary memory (pp. 177–207). Oxford, England: Blackwell. [Google Scholar]
- Baddeley A. D. (2000). The episodic buffer: A new component of working memory? Trends in Cognitive Sciences, 4, 417–423. [DOI] [PubMed] [Google Scholar]
- Baddeley A. D. (2001). The concept of episodic memory. In Baddeley A. D., Conway M. A., & Aggleton J. (Eds.), Episodic memory: New directions in research (pp. 1–10). Oxford, England: Oxford University Press. [Google Scholar]
- Baddeley A. D., & Andrade J. (2000). Working memory and the vividness of imagery. Journal of Experimental Psychology: General, 129, 126–145. [DOI] [PubMed] [Google Scholar]
- Barsalou L. W. (2003). Situated simulation in the human conceptual system. Language and Cognitive Processes, 18, 513–562. [Google Scholar]
- Beck A. T. (1970). Role of fantasies in psychotherapy and psychopathology. Journal of Nervous and Mental Disease, 150, 3–17. [DOI] [PubMed] [Google Scholar]
- Beck A. T., Emery G., & Greenberg R. L. (1985). Anxiety disorders and phobias: A cognitive perspective. New York, NY: Basic Books. [Google Scholar]
- Beck A. T., Laude R., & Bohnert M. (1974). Ideational components of anxiety neurosis. Archives of General Psychiatry, 31, 319–325. [DOI] [PubMed] [Google Scholar]
- Beck A. T., Rush A. J., Shaw B. F., & Emery G. (1979). Cognitive therapy of depression. New York, NY: Wiley. [Google Scholar]
- Becker S., & Wojtowicz J. M. (2007). A model of hippocampal neurogenesis in memory and mood disorders. Trends in Cognitive Sciences, 11, 70–76. [DOI] [PubMed] [Google Scholar]
- Berntsen D. (2007). Involuntary autobiographical memories: Speculations, findings, and an attempt to integrate them. In Mace J. H. (Ed.), Involuntary memory (pp. 20–49). Oxford, England: Blackwell. [Google Scholar]
- Berntsen D. (2009). Involuntary autobiographical memories: An introduction to the unbidden past. Cambridge, England: Cambridge University Press. [Google Scholar]
- Berntsen D., & Rubin D. C. (2008). The reappearance hypothesis revisited: Recurrent involuntary memories after traumatic events and in everyday life. Memory & Cognition, 36, 449–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntsen D., Rubin D. C., & Bohni M. K. (2008). Contrasting models of posttraumatic stress disorder: Reply to Monroe and Mineka. Psychological Review, 115, 1099–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrer E., Michael T., & Munsch S. (2007). Intrusive images in PTSD and in traumatised and non-traumatised depressed patients: A cross-sectional clinical study. Behaviour Research and Therapy, 45, 2053–2065. [DOI] [PubMed] [Google Scholar]
- Breitholtz E., Westling B. E., & Öst L. -G. (1998). Cognitions in generalized anxiety disorder and panic disorder patients. Journal of Anxiety Disorders, 12, 567–577. [DOI] [PubMed] [Google Scholar]
- Brewin C. R. (1998). Intrusive autobiographical memories in depression and posttraumatic stress disorder. Applied Cognitive Psychology, 12, 359–370. [Google Scholar]
- Brewin C. R. (2001). A cognitive neuroscience account of posttraumatic stress disorder and its treatment. Behaviour Research and Therapy, 39, 373–393. [DOI] [PubMed] [Google Scholar]
- Brewin C. R. (2003). Posttraumatic stress disorder: Malady or myth? New Haven, CT: Yale University Press. [Google Scholar]
- Brewin C. R. (2006). Understanding cognitive-behaviour therapy: A retrieval competition account. Behaviour Research and Therapy, 44, 765–784. [DOI] [PubMed] [Google Scholar]
- Brewin C. R. (2007). Autobiographical memory for trauma: Update on four controversies. Memory, 15, 227–248. [DOI] [PubMed] [Google Scholar]
- Brewin C. R., Dalgleish T., & Joseph S. (1996). A dual representation theory of post-traumatic stress disorder. Psychological Review, 103, 670–686. [DOI] [PubMed] [Google Scholar]
- Brewin C. R., & Holmes E. A. (2003). Psychological theories of posttraumatic stress disorder. Clinical Psychology Review, 23, 339–376. [DOI] [PubMed] [Google Scholar]
- Brewin C. R., Hunter E., Carroll F., & Tata P. (1996). Intrusive memories in depression. Psychological Medicine, 26, 1271–1276. [DOI] [PubMed] [Google Scholar]
- Brewin C. R., & Patel T. (in press). Trauma and voice-hearing: Auditory pseudo-hallucinations in posttraumatic stress disorder. Journal of Clinical Psychiatry. [Google Scholar]
- Brewin C. R., Reynolds M., & Tata P. (1999). Autobiographical memory processes and the course of depression. Journal of Abnormal Psychology, 108, 511–517. [DOI] [PubMed] [Google Scholar]
- Brewin C. R., & Saunders J. (2001). The effect of dissociation at encoding on intrusive memories for a stressful film. British Journal of Medical Psychology, 74, 467–472. [DOI] [PubMed] [Google Scholar]
- Brewin C. R., Watson M., McCarthy S., Hyman P., & Dayson D. (1998a). Intrusive memories and depression in cancer patients. Behaviour Research and Therapy, 36, 1131–1142. [DOI] [PubMed] [Google Scholar]
- Brewin C. R., Watson M., McCarthy S., Hyman P., & Dayson D. (1998b). Memory processes and the course of psychopathology in cancer patients. Psychological Medicine, 28, 219–224. [DOI] [PubMed] [Google Scholar]
- Brewin C. R., Wheatley J., Patel T., Fearon P., Hackmann A., Wells A., . . . Myers S. (2009). Imagery rescripting as a brief stand-alone treatment for depressed patients with intrusive memories. Behaviour Research and Therapy, 47, 569–576. [DOI] [PubMed] [Google Scholar]
- Brown R., & Kulik J. (1977). Flashbulb memories. Cognition, 5, 73–99. [Google Scholar]
- Burgess N., Becker S., King J. A., & O’Keefe J. (2001). Memory for events and their spatial context: Models and experiments. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences, 356, 1493–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess N., Maguire E. A., Spiers H. J., & O’Keefe J. (2001). A temporoparietal and prefrontal network for retrieving the spatial context of lifelike events. NeuroImage, 14, 439–453. [DOI] [PubMed] [Google Scholar]
- Burgess P. W., & Shallice T. (1996). Confabulation and the control of recollection. Memory, 4, 359–411. [DOI] [PubMed] [Google Scholar]
- Byrne P., Becker S., & Burgess N. (2007). Remembering the past and imagining the future: A neural model of spatial memory and imagery. Psychological Review, 114, 340–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L., & McGaugh J. L. (1998). Mechanisms of emotional arousal and lasting declarative memory. Trends in Neurosciences, 21, 294–299. [DOI] [PubMed] [Google Scholar]
- Carlier I. V. E., Voerman B. E., & Gersons B. P. R. (2000). Intrusive traumatic recollections and comorbid posttraumatic stress disorder in depressed patients. Psychosomatic Medicine, 62, 26–32. [DOI] [PubMed] [Google Scholar]
- Clark D. M., & Wells A. (1995). A cognitive model of social phobia. In Heimberg R., Liebowitz M., Hope D. A., & Schneier F. R. (Eds.), Social phobia: Diagnosis, assessment and treatment (pp. 69–93). New York, NY: Guilford Press. [Google Scholar]
- Cohen N. J., & Eichenbaum H. (1993). Memory, amnesia and the hippocampal system. Cambridge, MA: MIT Press. [Google Scholar]
- Conway M. A. (2001). Sensory-perceptual episodic memory and its context: Autobiographical memory. In Baddeley A. D., Conway M. A., & Aggleton J. (Eds.), Episodic memory: New directions in research (pp. 53–70). Oxford, England: Oxford University Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway M. A. (2005). Memory and the self. Journal of Memory and Language, 53, 594–628. [Google Scholar]
- Conway M. A. (2009). Episodic memories. Neuropsychologia, 47, 2305–2313. [DOI] [PubMed] [Google Scholar]
- Cooper M. J., Todd G., & Wells A. (1998). Content, origins, and consequences of dysfunctional beliefs in anorexia nervosa and bulimia nervosa. Journal of Cognitive Psychotherapy: An International Quarterly, 12, 213–230. [Google Scholar]
- Craig A. D. (2002). How do you feel? Interoception: The sense of the physiological condition of the body. Nature Reviews Neuroscience, 3, 655–666. [DOI] [PubMed] [Google Scholar]
- Critchley H. D., Wiens S., Rotshtein P., Öhman A., & Dolan R. J. (2004). Neural systems supporting interoceptive awareness. Nature Neuroscience, 7, 189–195. [DOI] [PubMed] [Google Scholar]
- Day S. J., Holmes E. A., & Hackmann A. (2004). Occurrence of imagery and its link with early memories in agoraphobia. Memory, 12, 416–427. [DOI] [PubMed] [Google Scholar]
- Depue B. E., Curran T., & Banich M. T. (2007, July13). Prefrontal regions orchestrate suppression of emotional memories via a two-phase process. Science, 317, 215–219. [DOI] [PubMed] [Google Scholar]
- de Silva P. (1986). Obsessional-compulsive imagery. Behaviour Research and Therapy, 24, 333–350. [DOI] [PubMed] [Google Scholar]
- Douglas R. J. (1972). Pavlovian conditioning and the brain. In Boakes R. A. & Halliday M. S. (Eds.), Inhibition and learning (pp. 529–553). London, England: Academic Press. [Google Scholar]
- Edwards D. (2007). Restructuring implicational meaning through memory-based imagery: Some historical notes. Journal of Behavior Therapy and Experimental Psychiatry, 38, 306–316. [DOI] [PubMed] [Google Scholar]
- Ehlers A., & Clark D. M. (2000). A cognitive model of posttraumatic stress disorder. Behaviour Research and Therapy, 38, 319–345. [DOI] [PubMed] [Google Scholar]
- Ehlers A., Hackmann A., & Michael T. (2004). Intrusive re-experiencing in post-traumatic stress disorder: Phenomenology, theory, and therapy. Memory, 12, 403–415. [DOI] [PubMed] [Google Scholar]
- Ehlers A., Hackmann A., Steil R., Clohessy S., Wenninger K., & Winter H. (2002). The nature of intrusive memories after trauma: The warning signal hypothesis. Behaviour Research and Therapy, 40, 995–1002. [DOI] [PubMed] [Google Scholar]
- Ehlers A., Maercker A., & Boos A. (2000). Posttraumatic stress disorder following political imprisonment: The role of mental defeat, alienation, and perceived permanent change. Journal of Abnormal Psychology, 109, 45–55. [PubMed] [Google Scholar]
- Ehlers A., & Steil R. (1995). Maintenance of intrusive memories in posttraumatic stress disorder: A cognitive approach. Behavioural and Cognitive Psychotherapy, 23, 217–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga B. M., & Bremner J. D. (2002). Are the neural substrates of memory the final common pathway in posttraumatic stress disorder (PTSD)? Journal of Affective Disorders, 70, 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes W. K. (1997). Processes of memory loss, recovery, and distortion. Psychological Review, 104, 148–169. [DOI] [PubMed] [Google Scholar]
- Fava G. A., Ruini C., & Belaise C. (2007). The concept of recovery in major depression. Psychological Medicine, 37, 307–317. [DOI] [PubMed] [Google Scholar]
- Fletcher P. C., Frith C. D., Baker S. C., Shallice T., Frackowiak R. S. J., & Dolan R. J. (1995). The mind’s eye—precuneus activation in memory-related imagery. NeuroImage, 2, 195–200. [DOI] [PubMed] [Google Scholar]
- Fletcher P. C., & Henson R. N. (2001). Frontal lobes and human memory: Insights from functional neuroimaging. Brain, 124, 849–881. [DOI] [PubMed] [Google Scholar]
- Foa E. B., & Rothbaum B. O. (1998). Treating the trauma of rape. New York, NY: Guilford Press. [Google Scholar]
- Freud S. (1953). Screen memories. In Strachey J. (Ed. & Trans.), The standard edition of the complete psychological works of Sigmund Freud (Vol. 6, pp. 43–63). London, England: Hogarth Press. (Original work published 1899) [Google Scholar]
- Gold P. E. (2004). Coordination of multiple memory systems. Neurobiology of Learning and Memory, 82, 230–242. [DOI] [PubMed] [Google Scholar]
- Gregory J. D., Brewin C. R., Mansell W., & Donaldson C. (2008). Intrusive memories and images in bipolar disorder. Manuscript submitted for publication. [DOI] [PubMed] [Google Scholar]
- Grunert B. K., Smucker M. R., Weis J. M., & Rusch M. D. (2003). When prolonged exposure fails: Adding an imagery-based cognitive restructuring component in the treatment of industrial accident victims suffering from PTSD. Cognitive and Behavioral Practice, 10, 333–346. [Google Scholar]
- Gunter R. W., & Bodner G. E. (2008). How eye movements affect unpleasant memories: Support for a working-memory account. Behaviour Research and Therapy, 46, 913–931. [DOI] [PubMed] [Google Scholar]
- Hackmann A. (1998). Working with images in clinical psychology. In Bellack A. S. & Hersen M. (Eds.), Comprehensive clinical psychology (Vol. 6, pp. 301–318). New York, NY: Elsevier. [Google Scholar]
- Hackmann A., Clark D. M., & McManus F. (2000). Recurrent images and early memories in social phobia. Behaviour Research and Therapy, 38, 601–610. [DOI] [PubMed] [Google Scholar]
- Hackmann A., Ehlers A., Speckens A., & Clark D. M. (2004). Characteristics and content of intrusive memories in PTSD and their changes with treatment. Journal of Traumatic Stress, 17, 231–240. [DOI] [PubMed] [Google Scholar]
- Hackmann A., & Holmes E. A. (2004). Reflecting on imagery: A clinical perspective and overview of the special issue of memory on mental imagery and memory in psychopathology. Memory, 12, 389–402. [DOI] [PubMed] [Google Scholar]
- Hackmann A., Surawy C., & Clark D. M. (1998). Seeing yourself through others’ eyes: A study of spontaneously occurring images in social phobia. Behavioural and Cognitive Psychotherapy, 26, 3–12. [Google Scholar]
- Hall N. M., Gjedde A., & Kupers R. (2008). Neural mechanisms of voluntary and involuntary recall: A PET study. Behavioural Brain Research, 186, 261–272. [DOI] [PubMed] [Google Scholar]
- Harvey A. G., Watkins E., Mansell W., & Shafran R. (2004). Cognitive behavioural processes across psychological disorders: A transdiagnostic approach to research and treatment. New York, NY: Oxford University Press. [Google Scholar]
- Harvey K., Kemps E., & Tiggemann M. (2005). The nature of imagery processes underlying food cravings. British Journal of Health Psychology, 10, 49–56. [DOI] [PubMed] [Google Scholar]
- Hassabis D., Kumaran D., Vann S. D., & Maguire E. A. (2007). Patients with hippocampal amnesia cannot imagine new experiences. Proceedings of the National Academy of Sciences USA, 104, 1726–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbert G. A. (1984). Ideational components of anxiety: Their origin and content. British Journal of Psychiatry, 144, 618–624. [DOI] [PubMed] [Google Scholar]
- Hirsch C. R., Clark D. M., Mathews A., & Williams R. (2003). Self-images play a causal role in social phobia. Behaviour Research and Therapy, 41, 909–921. [DOI] [PubMed] [Google Scholar]
- Hirsch C. R., & Holmes E. A. (2007). Mental imagery in anxiety disorders. Psychiatry, 6, 161–165. [Google Scholar]
- Hiskey S., Luckie M., Davies S., & Brewin C. R. (2008). Reactivated posttraumatic distress among older adults: A review. Journal of Geriatric Psychiatry and Neurology, 21, 232–241. [DOI] [PubMed] [Google Scholar]
- Hitch G. J., Brandimonte M. A., & Walker P. (1995). Two types of representation in visual memory: Evidence from the effects of stimulus contrast on image combination. Memory & Cognition, 23, 147–154. [DOI] [PubMed] [Google Scholar]
- Holdstock J. S., Mayes A. R., Cezayirli E., Isaac C. L., Aggleton J. P., & Roberts N. (2000). A comparison of egocentric and allocentric spatial memory in a patient with selective hippocampal damage. Neuropsychologia, 38, 410–425. [DOI] [PubMed] [Google Scholar]
- Holmes E. A., Arntz A., & Smucker M. R. (2007). Imagery rescripting in cognitive behaviour therapy: Images, treatment techniques and outcomes. Journal of Behavior Therapy and Experimental Psychiatry, 38, 297–305. [DOI] [PubMed] [Google Scholar]
- Holmes E. A., Brewin C. R., & Hennessy R. G. (2004). Trauma films, information processing, and intrusive memory development. Journal of Experimental Psychology: General, 133, 3–22. [DOI] [PubMed] [Google Scholar]
- Holmes E. A., Crane C., Fennell M. J. V., & Williams J. M. G. (2007). Imagery about suicide in depression: “Flash-forwards”? Journal of Behavior Therapy and Experimental Psychiatry, 38, 423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes E. A., Geddes J. R., Colom F., & Goodwin G. M. (2008). Mental imagery as an emotional amplifier: Application to bipolar disorder. Behaviour Research and Therapy, 46, 1251–1258. [DOI] [PubMed] [Google Scholar]
- Holmes E. A., Grey N., & Young K. A. D. (2005). Intrusive images and “hotspots” of trauma memories in posttraumatic stress disorder: An exploratory investigation of emotions and cognitive themes. Journal of Behavior Therapy and Experimental Psychiatry, 36, 3–17. [DOI] [PubMed] [Google Scholar]
- Holmes E. A., & Hackmann A. (2004). A healthy imagination? Memory, 12, 387–388. [DOI] [PubMed] [Google Scholar]
- Holmes E. A., James E. L., Coode-Bate T., & Deeprose C. (2009). Can playing the computer game “Tetris” reduce the build-up of flashbacks for trauma? A proposal from cognitive science. PLoS ONE, 4, e4153. doi:10.1371/journal.pone.0004153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes E. A., & Mathews A. (2005). Mental imagery and emotion: A special relationship? Emotion, 5, 489–497. [DOI] [PubMed] [Google Scholar]
- Holmes E. A., Mathews A., Mackintosh B., & Dalgleish T. (2008). The causal effect of mental imagery on emotion assessed using picture-word cues. Emotion, 8, 395–409. [DOI] [PubMed] [Google Scholar]
- Horowitz M. J. (1967). Visual imagery and cognitive organization. Archives of General Psychiatry, 123, 938–946. [DOI] [PubMed] [Google Scholar]
- Horowitz M. J. (1970). Image formation and cognition. New York, NY: Appleton-Century-Crofts. [Google Scholar]
- Jacobs W. J., & Nadel L. (1985). Stress-induced recovery of fears and phobias. Psychological Review, 92, 512–531. [PubMed] [Google Scholar]
- Janet P. (1904). L’amnésie et la dissociation des souvenirs par l’émotion [Amnesia and the dissociation of memories by emotion]. Journal de Psychologie, 1, 417–453. [Google Scholar]
- Johnson M. K. (1983). A multiple-entry, modular memory system. Psychology of Learning and Motivation: Advances in Research and Theory, 17, 81–123. [Google Scholar]
- Kemps E., Tiggemann M., & Christianson R. (2008). Concurrent visuo-spatial processing reduces food cravings in prescribed weight-loss dieters. Journal of Behavior Therapy and Experimental Psychiatry, 39, 177–186. [DOI] [PubMed] [Google Scholar]
- Kim J. J., & Fanselow M. S. (1992, May1). Modality-specific retrograde amnesia of fear. Science, 256, 675–677. [DOI] [PubMed] [Google Scholar]
- Kindt M., Soeter M., & Vervliet B. (2009). Beyond extinction: Erasing human fear responses and preventing the return of fear. Nature Neuroscience, 12, 256–258. [DOI] [PubMed] [Google Scholar]
- King J. A., Burgess N., Hartley T., Vargha-Khadem F., & O’Keefe J. (2002). The human hippocampus and viewpoint dependence in spatial memory. Hippocampus, 12, 811–820. [DOI] [PubMed] [Google Scholar]
- King J. A., Hartley T., Spiers H. J., Maguire E. A., & Burgess N. (2005). Anterior prefrontal involvement in episodic retrieval reflects contextual interference. NeuroImage, 28, 256–267. [DOI] [PubMed] [Google Scholar]
- Kinsbourne M., & Wood F. (1975). Short-term memory and the amnesic syndrome. In Deutsch D. & Deutsch J. A. (Eds.), Short term memory (pp. 257–291). New York, NY: Academic Press. [Google Scholar]
- Kosslyn S. M. (1994). Image and brain: The resolution of the imagery debate. Cambridge, MA: MIT Press. [Google Scholar]
- Kosslyn S. M., Thompson W. L., & Ganis G. (2006). The case for mental imagery. Oxford, England: Oxford University Press. [Google Scholar]
- Kuyken W., & Brewin C. R. (1994). Intrusive memories of childhood abuse during depressive episodes. Behaviour Research and Therapy, 32, 525–528. [DOI] [PubMed] [Google Scholar]
- Kuyken W., & Brewin C. R. (1995). Autobiographical memory functioning in depression and reports of early abuse. Journal of Abnormal Psychology, 104, 585–591. [DOI] [PubMed] [Google Scholar]
- Kuyken W., & Brewin C. R. (1999). The relation of early abuse to cognition and coping in depression. Cognitive Therapy and Research, 23, 665–677. [Google Scholar]
- Lang P. J. (1977). Imagery in therapy: An information-processing analysis of fear. Behavior Therapy, 8, 862–886. [DOI] [PubMed] [Google Scholar]
- LeDoux J. (1996). The emotional brain. New York, NY: Simon & Schuster. [Google Scholar]
- Lipton M., Brewin C. R., Linke S., & Halperin G. (2008). Distinguishing features of visual intrusions in obsessive-compulsive disorder. Manuscript submitted for publication. [DOI] [PubMed] [Google Scholar]
- Mace J. H. (2007). Involuntary memory: Concept and theory. In Mace J. H. (Ed.), Involuntary memory (pp. 1–19). Oxford, England: Blackwell. [Google Scholar]
- Mansell W., & Lam D. (2004). A preliminary study of autobiographical memory in remitted bipolar and unipolar depression and the role of imagery in the specificity of memory. Memory, 12, 437–446. [DOI] [PubMed] [Google Scholar]
- Marr D. (1970). A theory for cerebral cortex. Proceedings of the Royal Society of London Series B: Biological Sciences, 176, 161–234. [DOI] [PubMed] [Google Scholar]
- Marr D. (1971). Simple memory: A theory for archicortex. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences, 262, 23–81. [DOI] [PubMed] [Google Scholar]
- Marsolek C. J. (1999). Dissociable neural, subsystems underlie abstract and specific object recognition. Psychological Science, 10, 111–118. [Google Scholar]
- Martin M., & Williams R. (1990). Imagery and emotion: Clinical and experimental approaches. In Hampson P. J., Marks D. F., & Richardson J. T. E. (Eds.), Imagery: Current developments (pp. 268–307). London, England: Routledge. [Google Scholar]
- May J., Andrade J., Panabokke N., & Kavanagh D. (2004). Images of desire: Cognitive models of craving. Memory, 12, 447–461. [DOI] [PubMed] [Google Scholar]
- McClelland J. L., McNaughton B. L., & O’Reilly R. C. (1995). Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychological Review, 102, 419–457. [DOI] [PubMed] [Google Scholar]
- McCormick D. D., & Thompson R. F. (1982). Locus coeruleus lesions and resistance to extinction of a classically conditioned response: Involvement of the neocortex and hippocampus. Brain Research, 245, 239–249. [DOI] [PubMed] [Google Scholar]
- McGaugh J. L., Roozendaal B., & Okuda S. (2006). Role of stress hormones and the amygdala in creating lasting memories. In Kato N., Kawata M., & Pitman R. K. (Eds.), PTSD: Brain mechanisms and clinical implications (pp. 89–103). Tokyo: Springer–Verlag. [Google Scholar]
- McIsaac H. K., & Eich E. (2004). Vantage point in traumatic memory. Psychological Science, 15, 248–253. [DOI] [PubMed] [Google Scholar]
- McNally R. J., Lasko N. B., Clancy S. A., Macklin M. L., Pitman R. K., & Orr S. P. (2004). Psychophysiological responding during script-driven imagery in people reporting abduction by space aliens. Psychological Science, 15, 493–497. [DOI] [PubMed] [Google Scholar]
- Metcalfe J., & Jacobs W. J. (1998). Emotional memory: The effects of stress on ’cool’ and ’hot’ memory systems. In Bower G. H. (Ed.), The psychology of learning and motivation (Vol. 38, pp. 187–222). New York, NY: Academic Press. [Google Scholar]
- Michael T., Ehlers A., Halligan S. L., & Clark D. M. (2005). Unwanted memories of assault: What intrusion characteristics are associated with PTSD? Behaviour Research and Therapy, 43, 613–628. [DOI] [PubMed] [Google Scholar]
- Milner A. D., Dijkerman H. C., & Carey D. P. (1999). Visuospatial processing in a case of visual form agnosia. In Burgess N., Jeffery K. J., & O’Keefe J. (Eds.), The hippocampal and parietal foundations of spatial cognition (pp. 443–466). Oxford, England: Oxford University Press. [Google Scholar]
- Monfils M. H., Cowansage K. K., Klann E., & LeDoux J. E. (2009, May15). Extinction-reconsolidation boundaries: Key to persistent attenuation of fear memories. Science, 324, 951–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe S. M., & Mineka S. (2008). Placing the mnemonic model in context: Diagnostic, clinical, and theoretical considerations. Psychological Review, 115, 1084–1098. [DOI] [PubMed] [Google Scholar]
- Morrison A. P., Beck A. T., Glentworth D., Dunn H., Reid G. S., Larkin W., & Williams S. (2002). Imagery and psychotic symptoms: A preliminary investigation. Behaviour Research and Therapy, 40, 1053–1062. [DOI] [PubMed] [Google Scholar]
- Moscovitch M. (1995). Models of consciousness and memory. In Gazzaniga M. S. (Ed.), The cognitive neurosciences (pp. 1341–1356). Cambridge, MA: MIT Press. [Google Scholar]
- Nader K., Schafe G. E., & LeDoux J. E. (2000). The labile nature of consolidation theory. Nature Reviews Neuroscience, 1, 216–219. [DOI] [PubMed] [Google Scholar]
- National Institute for Clinical Excellence. (2005). Post-traumatic stress disorder: The management of PTSD in adults and children in primary and secondary care. London, England: Gaskell. [PubMed] [Google Scholar]
- Nigro G., & Neisser U. (1983). Point of view in personal memories. Cognitive Psychology, 15, 467–482. [Google Scholar]
- Nijenhuis E. R. S., Vanderlinden J., & Spinhoven P. (1998). Animal defensive reactions as a model for trauma-induced dissociative reactions. Journal of Traumatic Stress, 11, 243–260. [DOI] [PubMed] [Google Scholar]
- Ohanian V. (2002). Imagery rescripting within cognitive behavior therapy for bulimia nervosa: An illustrative case report. International Journal of Eating Disorders, 31, 352–357. [DOI] [PubMed] [Google Scholar]
- O’Keefe J., & Nadel L. (1978). The hippocampus as a cognitive map. Oxford, England: Oxford University Press. [Google Scholar]
- Osman S., Cooper M., Hackmann A., & Veale D. (2004). Spontaneously occurring images and early memories in people with body dysmorphic disorder, Memory, 12, 428–436. [DOI] [PubMed] [Google Scholar]
- Ottaviani R., & Beck A. T. (1987). Cognitive aspects of panic disorders. Journal of Anxiety Disorders, 1, 15–28. [Google Scholar]
- Patel T., Brewin C. R., Wheatley J., Wells A., Fisher P., & Myers S. (2007). Intrusive images and memories in major depression. Behaviour Research and Therapy, 45, 2573–2580. [DOI] [PubMed] [Google Scholar]
- Payne J. D., Jackson E. D., Ryan L., Hoscheidt S., Jacobs W. J., & Nadel L. (2006). The impact of stress on neutral and emotional aspects of episodic memory. Memory, 14, 1–16. [DOI] [PubMed] [Google Scholar]
- Pearson M., Brewin C. R., Rhodes J., & McCarron G. (2008). Frequency and nature of rumination in chronic depression: A preliminary study. Cognitive Behavior Therapy, 37, 160–168. [DOI] [PubMed] [Google Scholar]
- Peters J., Daum I., Gizewski E., Forsting M., & Suchan B. (2009). Associations evoked during memory encoding recruit the context-network. Hippocampus, 19, 141–151. [DOI] [PubMed] [Google Scholar]
- Pillemer D. B. (1998). Momentous events, vivid memories. Cambridge MA: Harvard University Press. [Google Scholar]
- Pillemer D. B., & White S. H. (1989). Childhood events recalled by children and adults. In Reese H. W. (Ed.), Advances in child development and behavior (Vol. 21, pp. 297–340). Orlando, FL: Academic Press. [DOI] [PubMed] [Google Scholar]
- Pitman R. K., Shalev A. Y., & Orr S. P. (2000). Posttraumatic stress disorder: Emotion, conditioning, and memory. In Gazzaniga M. S. (Ed.), The new cognitive neurosciences (2nd ed.; pp. 1133–1147). Cambridge, MA: MIT Press. [Google Scholar]
- Poldrack R. A., & Packard M. G. (2003). Competition among multiple memory systems: Converging evidence from animal and human brain studies. Neuropsychologia, 41, 245–251. [DOI] [PubMed] [Google Scholar]
- Postle B. R., Idzikowski C., Della Sala S., Logie R. H., & Baddeley A. D. (2006). The selective disruption of spatial working memory by eye movements. Quarterly Journal of Experimental Psychology, 59, 100–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raes F., Hermans D., Williams J. M. G., Brunfaut E., Hamelinck L., & Eelen P. (2006). Reduced autobiographical memory specificity and trauma in major depression: On the importance of post-trauma coping versus mere trauma exposure. In Sturt S. M. (Ed.), New developments in child abuse research (pp. 61–72). New York, NY: Nova Science. [Google Scholar]
- Reynolds M., & Brewin C. R. (1998). Intrusive cognitions, coping strategies and emotional responses in depression, post-traumatic stress disorder, and a non-clinical population. Behaviour Research and Therapy, 36, 135–147. [DOI] [PubMed] [Google Scholar]
- Reynolds M., & Brewin C. R. (1999). Intrusive memories in depression and posttraumatic stress disorder. Behaviour Research and Therapy, 37, 201–215. [DOI] [PubMed] [Google Scholar]
- Roth A., & Fonagy P. (2004). What works for whom? (2nd ed.). New York, NY: Guilford Press. [Google Scholar]
- Rubin D. C., Berntsen D., & Bohni M. K. (2008). A memory-based model of posttraumatic stress disorder: Evaluating basic assumptions underlying the PTSD diagnosis. Psychological Review, 115, 985–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugg M. D., & Yonelinas A. P. (2003). Human recognition memory: A cognitive neuroscience perspective. Trends in Cognitive Sciences, 7, 313–319. [DOI] [PubMed] [Google Scholar]
- Salkovskis P. M. (1985). Obsessional-compulsive problems: A cognitive-behavioural analysis. Behaviour Research and Therapy, 23, 571–583. [DOI] [PubMed] [Google Scholar]
- Schacter D. L., Addis D. R., & Buckner R. L. (2007). Remembering the past to imagine the future: The prospective brain. Nature Reviews Neuroscience, 8, 657–661. [DOI] [PubMed] [Google Scholar]
- Schacter D. L., Cooper L. A., & Delaney S. M. (1990). Implicit memory for unfamiliar objects depends on access to structural descriptions. Journal of Experimental Psychology: General, 119, 5–24. [DOI] [PubMed] [Google Scholar]
- Schlagman S., & Kvavilashvili L. (2008). Involuntary autobiographical memories in and outside the laboratory: How different are they from voluntary autobiographical memories? Memory & Cognition, 36, 920–932. [DOI] [PubMed] [Google Scholar]
- Scoville W. B., & Milner B. (1957). Loss of recent memory after bilateral hippocampal lesions. Journal of Neurology, Neurosurgery, and Psychiatry, 20, 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro F. (2001). Eye movement desensitization and reprocessing: Basic principles, protocols and procedures (2nd ed.). New York, NY: Guilford Press. [Google Scholar]
- Smucker M. R., & Dancu C. (2005). Cognitive-behavioral treatment for adult survivors of childhood trauma: Imagery rescripting and reprocessing. Lanham, MD: Rowman & Littlefield. (Original work published 1999) [Google Scholar]
- Smucker M. R., Dancu C., Foa E. B., & Niederee J. L. (1995). Imagery rescripting: A new treatment for survivors of childhood sexual abuse suffering from posttraumatic stress. Journal of Cognitive Psychotherapy, 9, 3–17. [Google Scholar]
- Somerville K., Cooper M., & Hackmann A. (2007). Spontaneous imagery in women with bulimia nervosa: An investigation into content, characteristics and links to childhood memories. Journal of Behavior Therapy and Experimental Psychiatry, 38, 435–446. [DOI] [PubMed] [Google Scholar]
- Speckens A. E. M., Ehlers A., Hackmann A., & Clark D. M. (2006). Changes in intrusive memories associated with imaginal reliving in posttraumatic stress disorder. Journal of Anxiety Disorders, 20, 328–341. [DOI] [PubMed] [Google Scholar]
- Speckens A. E. M., Ehlers A., Hackmann A., Ruths F. A., & Clark D. M. (2007). Intrusive memories and rumination in patients with post-traumatic stress disorder: A phenomenological comparison. Memory, 15, 249–257. [DOI] [PubMed] [Google Scholar]
- Speckens A. E. M., Hackmann A., Ehlers A., & Cuthbert B. (2007). Intrusive images and memories of earlier adverse events in patients with obsessive compulsive disorder. Journal of Behavior Therapy and Experimental Psychiatry, 38, 411–422. [DOI] [PubMed] [Google Scholar]
- Spenceley A., & Jerrom W. (1997). Intrusive traumatic childhood memories in depression: A comparison between depressed, recovered, and never depressed women. Behavioural and Cognitive Psychotherapy, 25, 309–318. [Google Scholar]
- Squire L. R., & Zola-Morgan S. (1991, September20). The medial temporal lobe memory system. Science, 253, 1380–1386. [DOI] [PubMed] [Google Scholar]
- Srull T. K., & Wyer R. S. (Eds.). (1990). Advances in Social Cognition: Vol. 3. Content and process specificity in the effects of prior experiences. Hillsdale, NJ: Erlbaum. [Google Scholar]
- Srull T. K., & Wyer R. S. (Eds.). (1993). Advances in Social Cognition: Vol. 5. The mental representation of trait and autobiographical knowledge about the self. Hillsdale, NJ: Erlbaum. [Google Scholar]
- Stankiewicz B. J., Hummel J. E., & Cooper E. E. (1998). The role of attention in priming for left-right reflections of object images: Evidence for a dual representation of object shape. Journal of Experimental Psychology: Human Perception and Performance, 24, 732–744. [DOI] [PubMed] [Google Scholar]
- Taube J. S. (1998). Head direction cells and the neuropsychological basis for a sense of direction. Progress in Neurobiology, 55, 225–256. [DOI] [PubMed] [Google Scholar]
- Tulving E. (1983). Elements of episodic memory. Oxford, England: Clarendon Press. [Google Scholar]
- Tulving E. (1985). Memory and consciousness. Canadian Psychologist, 26, 1–12. [Google Scholar]
- Tzemou E., & Birchwood M. (2007). A prospective study of dysfunctional thinking and the regulation of negative intrusive memories in Bipolar I disorder: Implications for affect regulation theory. Psychological Medicine, 37, 689–698. [DOI] [PubMed] [Google Scholar]
- van der Kolk B. A., & Fisler R. (1995). Dissociation and the fragmentary nature of traumatic memories: Overview and exploratory study. Journal of Traumatic Stress, 8, 505–525. [DOI] [PubMed] [Google Scholar]
- van der Kolk B. A., van der Hart O., & Marmar C. R. (1996). Dissociation and information processing in posttraumatic stress disorder. In van der Kolk B. A., McFarlane A. C., & Weisaeth L. (Eds.), Traumatic stress (pp. 303–327). New York, NY: Guilford Press. [Google Scholar]
- Vyas A., Mitra R., Rao B. S. S., & Chattarji S. (2002). Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. Journal of Neuroscience, 22, 6810–6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallentin M., Roepstorff A., & Burgess N. (2008). Frontal eye fields involved in shifting frame of reference within working memory for scenes. Neuropsychologia, 46, 399–408. [DOI] [PubMed] [Google Scholar]
- Waller N. G., Putnam F. W., & Carlson E. B. (1996). Types of dissociation and dissociative types: A taxometric analysis of dissociative experiences. Psychological Methods, 1, 300–321. [Google Scholar]
- Wells A., & Hackmann A. (1993). Imagery and core beliefs in health anxiety: Content and origins. Behavioural and Cognitive Psychotherapy, 21, 265–274. [Google Scholar]
- Whitaker K., Brewin C. R., & Watson M. (2008). Intrusive cognitions and anxiety in cancer patients. Journal of Psychosomatic Research, 64, 509–517. [DOI] [PubMed] [Google Scholar]
- Whitaker K., Watson M., & Brewin C. R. (2009). Intrusive cognitions and their appraisal in anxious cancer patients. Psycho-Oncology, 18, 1147–1155. [DOI] [PubMed] [Google Scholar]
- Wild J., Hackmann A., & Clark D. M. (2008). Rescripting early memories linked to negative images in social phobia: A pilot study. Behavior Therapy, 39, 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A. D., & Moulds M. L. (2007). Cognitive avoidance of intrusive memories: Recall vantage perspective and associations with depression. Behaviour Research and Therapy, 45, 1141–1153. [DOI] [PubMed] [Google Scholar]