Abstract
Background
Targeting gene therapy vectors that can home in on desired cell and tissue types in vivo comprise the ultimate gene delivery system. We have previously developed targeting lentiviral vectors by pseudotyping vectors with modified Sindbis virus envelope proteins. The envelope protein contains the Fc-binding region of protein A (ZZ domain), so the virus can be conjugated with antibodies. The conjugated antibody mediates specific transduction of the cells and tissues expressing the target antigens, both in vitro and in vivo. However, more stable conjugation of targeting molecules would be optimal for use in immunocompetent animals, as well as in humans.
Methods
We inserted integrin-targeting peptides into two sites of the targeting envelope proteins and determined whether the peptides serve as receptor-binding regions of the envelope proteins and redirect the pseudotyped viruses.
Results
The integrin-targeting peptides can mediate binding to cells via the interaction with integrins on target cells and transduction. Peptides with a higher binding affinity increase titers of pseudotyped virus. We found two regions on the envelope protein that can accommodate insertion and serve as receptor-binding regions. Combining the peptides in two distinct regions increased the titers of the virus.
Conclusions
Successful incorporation of targeting molecules into the envelope protein will broaden the application of targeting vectors for a wide variety of experimental and clinical settings. Copyright © 2009 John Wiley & Sons, Ltd.
Keywords: integrin, lentiviral vector, RGD peptide, Sindbis virus envelope, targeting vector
Introduction
Because retroviral vectors (i.e. both oncoretroviral and lentiviral) integrate their transgenes into host cell chromosomes, the expression of transgenes is prolonged, which is ideal for therapy of chronic diseases, such as congenital disorders [1]. Thus, retroviral vectors have been successfully used for therapy of congenital hematopoietic diseases, including X-linked severe combined immunodeficiency (SCID) and adenosine deaminase deficiencies (ADA)-SCID [2,3]. Delivery of therapeutic genes for these diseases is performed using the ex vivo transduction method, whereby hematopoietic cells are isolated from patients, transduced in vitro, then re-infused. The ex vivo transduction method is ideal for introducing therapeutic genes into hematopoietic cells. However, this method is not suitable for cells of solid organs because it is difficult to isolate and replace these types of cells without causing them to lose their physiological functions. Development of efficient gene delivery methods for solid organs is necessary for broadening the applications of gene therapy.
One promising method for gene delivery to solid organs is injecting gene therapy vectors into the body. However, this results in transduction only in areas proximal to the injection sites [4]. This might suffice for disease models in small animals, but not in large animals, including man. Administration of vectors into the bloodstream is another means to deliver therapeutic genes to solid organs. Greater volumes of vectors can be injected into the bloodstream than the target organs, but transduction in various organs occurs nonspecifically [5]. Nonspecific transduction of multiple organs and tissues would reduce the therapeutic effects of transgenes on target cells and tissues if the therapeutic molecules need to be expressed at the sites of action [6–9]. In addition, integration and expression of transgenes in normal tissues and organs would increase the adverse effects of gene therapy [10]. Therefore, specific transduction and expression of therapeutic genes is necessary for gene therapy to be effective. One way to achieve specific gene delivery to target organs is by intravenous injection of vectors that can home in on and transduce specific cells and tissues. Such vectors are referred to as `targeting vectors', and many attempts have been made to develop targeting retroviral vectors [11]. A common strategy for redirecting gene therapy vectors to desired cells and tissues involves changing the binding specificity of the vectors for molecules abundantly expressed on target cells and tissues rather than their natural receptors.
To date, two strategies for changing the binding specificity of retroviral vectors have been reported. One strategy is to conjugate the vectors with adaptor molecules that specifically bind to target molecules [12,13]; the other is to pseudotype the vectors with chimeric proteins generated between the envelope proteins and targeting molecules, such as single-chain antibodies and growth factors [14–21].
We have developed targeting lentiviral vectors using the first strategy [22]. The vectors are pseudotyped with modified Sindbis virus envelope proteins. The envelope proteins contain the Fc-binding region of protein A (ZZ domain) in the original receptor-binding region of the Sindbis virus envelope protein. Vectors pseudotyped with the envelope proteins can be conjugated with monoclonal antibodies through the interaction between the Fc region of antibodies and the ZZ domain. The antigen-binding regions of conjugated antibodies mediate binding of the vectors. Therefore, the binding specificity of the vectors is determined by the specificity of conjugated antibodies. Using antibodies against various antigens, we have demonstrated targeted transduction with both oncoretroviral and lentiviral vectors, both in vitro and in vivo [22–26].
Although effective in in vivo experiments with immunodeficient mice, which do not have serum immunoglobulin, conjugation of the viruses with antibodies would not be stable in immunocompetent animals because serum immunoglobulin will compete with conjugated antibodies for binding to the ZZ domain of the envelope protein. Covalent conjugation of targeting molecules would overcome this problem. However, creating fusion proteins can change the entire structure of the proteins, which could result in decreased expression levels of the proteins and/or loss of their functions. Additionally, if the targeting molecules are inserted into the regions of envelope proteins, which are difficult to access, the chimeric proteins would not be able to bind the targeted molecules on cells.
In the present study, we investigated the feasibility of covalent incorporation of targeting peptides into our targeting envelope proteins instead of the ZZ domain. We inserted two types of peptides containing arginineglycine-aspartic acid (RGD), which bind to integrins [27,28]. One does not contain disulfide bonds, and the other contains two disulfide bonds, which will aid in investigating the effects of the secondary structures of inserted molecules on the entire structure of chimeric proteins. We also inserted the targeting peptides into two different sites of the envelope proteins to determine whether multiple regions of the envelope protein can serve as receptor-binding regions of chimeric proteins.
Materials and methods
Plasmid construction
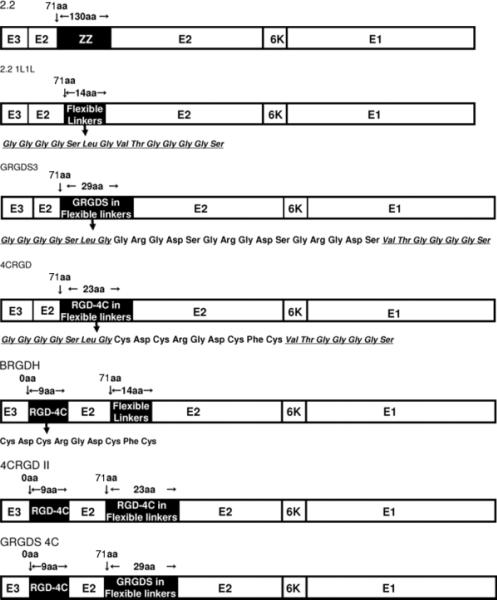
2.2 1L1 L was constructed from 2.2 by replacing the ZZ domain in the E2 protein with two sets of flexible linker peptides (GGGGS). GRGDS3 was constructed by inserting three sets of the GRGDS peptide between the two flexible linkers. 4CRGD was constructed by inserting the RGD-4C peptide (CDCRGDCFC) between the two flexible linkers. To construct BRGDH, the BbVC-1 and HindIII sites were introduced into the junction of E3 and E2 of 2.2 1L1 L by site-directed mutagenesis, followed by insertion of the RGD-4C peptide between the BbVC-1 and HindIII sites. 4CRGD II was constructed from 4CRGD and BRGD by combining the RGD-4C peptide insertion of each construct into one construct. GRGDS 4C was constructed from GRGDS3 and BRGDH by combining the three sets of the GRGDS peptide of GRGDS3 and the 4CRGD peptide of BRGDH into one construct.
Cells and viruses
293T cells were cultured in IMDM (Sigma-Aldrich, St Louis, MO, USA) containing 10% fetal calf serum (FCS) and antibiotics. Human T-cell leukemia cells (Jurkat) were cultured in RPMI (Invitrogen, Carlsbad, CA, USA) containing 10% FCS. Human breast cancer cells (MDAMB435) were cultured in Dulbecco's modofied Eagle's medium (Invitrogen) containing 10% FCS. Human umbilical vein endothelial cells (HUVEC) were purchased from AllCells (Emeryville, CA, USA) and Lonza (Walkersville, MD, USA). HUVEC were cultured in EBM-2 (Lonza) supplemented with EGM-2 singlequots (Lonza). When HUVEC started growing slowly, new lots of HUVEC were purchased and used for the experiments. Lentiviral vectors were produced in 293T cells, using the calcium phosphate transfection method, as described previously [22]. Briefly, 293T cells (1.8 × 107) were transfected with one of the envelope protein expression vectors (10 μg), packaging plasmid 8.2 delta VPR (12.5 μg) and lentiviral vector cppt2e (12.5 μg). After transfection, the cells were cultured in Opti-MEM containing 2% FCS. The supernatant was subjected to ultracentrifugation, and the pellet containing the virus was resuspended in Hepes-buffered saline. The viruses were concentrated 100-fold. The concentrations of virus were quantified by measuring amounts of viral capsid protein p24. The virus was frozen at −70 °C until use.
Flow cytometry for assessing expression levels of integrins
HUVEC, MDAMB435 and Jurkat cells were stained with anti-integrin αVβ3 antibody (Chemicon, Temucula, CA, USA), anti-integrin αVβ5 antibody (Chemicon) or isotype control (eBioscience, San Diego, CA, USA), followed by staining with Alexa 488-conjugated rabbit anti-mouse IgG (Invitrogen). Expression of the integrins was monitored by flow cytometry.
Transduction of cells
2.2 pseudotype vectors were conjugated with 2 μg/ml of either anti-integrin αVβ3 or anti-integrin αVβ5 antibody before transduction. HUVEC (2 × 104), MDAMB435 (5 × 104) and Jurkat cells (5 × 104) were incubated with lentiviral vectors pesudotyped with various types of envelope proteins at a high multiplicity of infection (MOI) (40 ng of HIV p24) and a low MOI (4 ng of HIV p24) for 2 h. Enhanced green fluorescent protein (EGFP) expression was assayed by flow cytometry 3 days post-transduction.
Blocking of transduction by soluble RGD peptide and anti-integrin antibodies
HUVEC (2 × 104), MDAMB435 (5 × 104) and Jurkat cells (5 × 104) were incubated with soluble RGD peptides (1000 or 100 μg/ml; Sigma-Aldrich), control RGE peptides (1000 or 100 μg/ml; Sigma-Aldrich), a mixture of anti-integrin antibodies (20 μg/ml of both anti-integrin αVβ3 and αVβ5 antibody) (Chemicon), or isotype control antibody (40 μg/ml) for 30 min before transduction. The cells were then infected with either the 2.2 pseudotype conjugated with anti-HLA class I antibody (Sigma-Aldrich), VSV-G pseudotype, GRGDS 4C pseudotype, or 4CRGD pseudotype for 2 h in the presence of the peptides or antibodies.
The amounts of virus used for transduction were 60 ng of HIV p24 of the VSV-G pseudotype. EGFP expression was assayed 3 days post-transduction by flow cytometry. The relative transduction efficiency was calculated as:
Titration of GRGDS 4C pseudotypes
HUVEC (2 × 104) were transduced with unconcentrated and concentrated viruses (1 ng of HIV p24) to investigate the effect of ultracentrifugation on the titers of the virus. EGFP expression was analysed by flow cytometry 3 days post-transduction. HUVEC (2 × 104) were transduced with concentrated GRGDS 4C pseduotype (1 ng of HIV p24) and VSV-G pseudotype (250 pg of HIV p24) in the presence (8 μg/ml) or absence of polybrene (Sigma-Aldrich) to investigate the effect of polybrene on the titers of the viruses. EGFP expression was analysed by flow cytometry 3 days post-transduction. HUVEC (2 × 104) were transduced with GRGDS 4C or VSV-G pseudotypes at various dilutions in the absence of polybrene. EGFP expression was analysed by flow cytometry 3 days post-transduction.
Results
Insertion of integrin-targeting peptides into the modified Sindbis virus envelope proteins
We inserted integrin-targeting peptides into our targeting envelope protein, 2.2, which contains an insertion of the ZZ domain in its original receptor-binding region of the E2 protein, E2 amino acid 71. Insertion of the ZZ domain enables conjugation of targeting antibodies with the envelope protein through the interaction with the Fc region of antibodies, and also eliminates its original tropism (Figure 1). We replaced the ZZ domain with two flexible linkers consisting of four glycines and one serine, and designated this envelope protein as 2.2 1L1 L (Figure 1). Based upon 2.2 1L1 L, we created integrin-targeting envelope proteins. We inserted two types of integrin-binding peptides between the linkers of 2.2 1L1 L. We inserted three tandem repeats of integrin-binding peptides, arginine-glycine-aspartic acid (RGD), between the linkers, and designated this envelope protein as GRGDS3 (Figure 1). We also inserted another integrin-binding peptide, cysteine-aspartic acid-cysteine-arginine-glycine-aspartic acid-cysteine-phenylalanine-cysteine, which is referred to as the RGD-4C peptide [28,29–33]. The RGD-4C peptide contains four cysteines that form two disulfide bonds, which expose the RGD peptide and increase the affinity for integrins αVβ3 and αVβ5. We designated the envelope inserted into this peptide as 4CRGD.
Figure 1.
Schematic representation of chimeric Sindbis virus envelope proteins. 2.2 1L1 L was derived from the chimeric 2.2 envelope protein. 2.2 1L1 L has two flexible linkers (Gly Gly Gly Gly Ser) at amino acid 71 of the E2 protein. GRGDS3 contains three tandem repeats of the GRGDS peptide (Gly Arg-Gly Asp-Ser) between the two flexible linkers. 4CRGD contains the RGD-4C peptide (Cys-Asp-Cys-Arg-Gly Asp-Cys-Phe-Cys) between the two flexible linkers. BRGDH contains the RGD-4C peptide at the first amino acid of the E2 protein, and 4CRGD II contains an RGD-4C peptide at both insertion sites in the E2 protein. GRGDS 4C contains three tandem repeats of the GRGDS peptides at amino acid 71 of the E2 protein and the 4CRGD peptide at amino acid 1
Insertion of integrin-targeting peptides increased the transduction efficiencies of the vectors pseudotyped with the targeting envelope proteins
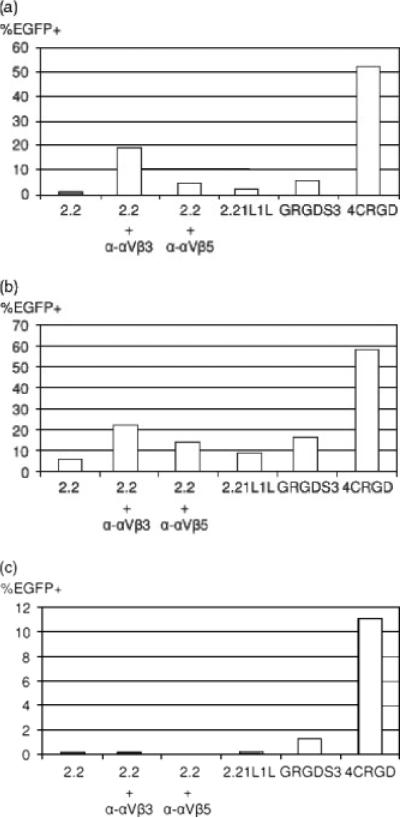
We investigated whether insertion of integrin-targeting peptides increases the titers of pseudotyped lentiviral vectors. We chose three types of target cells, HUVEC, Jurkat and MDAMB435, which have different levels of integrins (Table 1). As we have previously reported [25], HUVEC express high levels of integrins αVβ3 and αVβ5, and Jurkat cells express very low levels of integrin αVβ3. We cannot detect expression of integrin αVβ5 on Jurkat cells. MDAMB435 cells express integrin αVβ3 as abundantly as HUVEC, and integrin αVβ5 more abundantly than HUVEC. When MDAMB435 cells were transduced with the 2.2 or 2.2 1L1 L pseudotypes, the cells were transduced at minimal levels because those envelope proteins do not bind target cells efficiently (Figure 2a).
Table 1.
Expression levels of integrins
| Integrin αVβ3 | Integrin αVβ5 | |
|---|---|---|
| HUVEC (human umbilical vein endothelial cells) | + | + |
| MDAMB435 (human breast cancer) | + | ++ |
| Jurkat (acute T cell leukemia) | ± | − |
Expression levels of integrins in HUVEC, MDAMB435 and Jurkat cells were analysed by flow cytometry. The histograms of flow cytometry of HUVEC and Jurkat cells were previously reported [25].
Figure 2.
Transduction of cells with lentiviral vectors pseudo-typed with various types of chimeric envelope proteins. Cells were transduced with lentiviral vectors (40 ng of HIV p24) pseudotyped with various types of envelope proteins: (a) human breast carcinoma cell line, MDAMB435; (b) human umbilical vein endothelial cells (HUVEC); or (c) human T-cell line, Jurkat. 2.2 pseudotypes were conjugated with either anti-integrin αVβ3 or αVβ5 antibody (2 μg/ml). EGFP transgene expression was analysed 3 days post-transduction. Experiments were performed three times and representative data are shown
When the 2.2 pseudotype was conjugated with antibodies against either integrin αVβ3 or αVβ5, transduction was increased five- to 20-fold, demonstrating that integrins can serve as receptors of our targeting lentiviral vectors. When we infected the cells with the GRGDS3 or 4CRGD pseudotypes, the cells were transduced three- to 50-fold more efficiently than 2.2 1L1 L, suggesting that the inserted peptides increase the infectivity of its pseudotype. The 4CRGD pseudotype transduced ten-fold more efficiently than the GRGDS3 pseudotype, which indicates that insertion of a ligand with higher affinity for target molecules increases the affinity of the envelope protein and results in higher transduction efficiency. The transduction efficiency of the 4CRGD pseudotype is higher than that of the 2.2 pseudotype conjugated with anti-integrins αVβ3 or αVβ5, or the combined transduction efficiency of both, indicating that covalent conjugation of targeting molecules to envelopes more efficiently targets receptors than noncovalent conjugation of targeting antibodies through the interaction between the Fc regions of antibodies and the ZZ domain. We observed similar results when we transduced primary endothelial cells, HUVEC (Figure 2b). We next transduced Jurkat cells, which do not express high levels of integrin αVβ3 or αVβ5; because of this low expression, 2.2 pseudotypes conjugated with integrin αVβ3 or αVβ5 could not transduce Jurkat cells efficiently (Figure 2c). However, insertion of integrin-targeting peptides into 2.2 1L1 L increased the titers of pseudotyped vectors, which also indicates that covalent incorporation of targeting molecules is a better targeting strategy than noncovalent conjugation of targeting molecules. Of note, lentiviral vectors pseudotyped with the wild-type Sindbis virus envelope protein cannot transduce Jurkat cells (data not shown). Therefore, insertion of the targeting molecules into the envelope expanded the host range of the Sindbis virus envelope protein.
We next confirmed that the inserted RGD peptide served as a receptor-binding region of the 4CRGD envelope. We blocked transduction of the 4CRGD pseudotype with the soluble RGD peptide. As a control for the virus that does not use integrins as its receptors, we used the 2.2 pseudotype conjugated with anti-HLA class I antibody. Transduction of all types of target cells with the 4CRGD pseudotype was inhibited by the soluble RGD peptide in all target cells, whereas transduction with 2.2 pseudotype conjugated with anti-HLA class I antibody was not inhibited (Table 2). This result confirmed that the inserted RGD peptide serves as the receptor-binding region of the 4CRGD pseudotypes.
Table 2.
Blocking of transdution by soluble RGD peptide
| Relative transduction efficiency (%) |
||||
|---|---|---|---|---|
| HUVEC | MDAMB435 | Jurkat | ||
| 2.2 + anti-HLA | No block | 100 | 100 | 100 |
| RGD peptide (1000 μg/ml) | 108 | 112 | 104 | |
| RGD peptide (100 μg/ml) | 110 | 104 | 87 | |
| 4CRGD | No block | 100 | 100 | 100 |
| RGD peptide (1000 μg/ml) | 32 | 9 | 18 | |
| RGD peptide (100 μg/ml) | 56 | 14 | 22 | |
The target cells were incubated with the soluble RGD peptide at either 1000 or 100 μg/ml before and during transduction. 2.2 pseudotype was conjugated with anti-HLA class I antibody before transduction. The cells were transduced with the vectors adjusted to 60 ng of HIV p24, and EGFP expression was analysed 3 days post-transduction. The relative transduction efficiency was calculated as:
%EGFP transduction with each blocking agent/%EGFP transduction without blocking agent) × 100
%EGFP transduction of HUVEC, MDAMB435 and Jurkat cells with the 2.2 pseudotype plus anti-HLA class I antibody without blocking reagents is 39.1%, 47% and 54.6%, respectively. %EGFP transduction of HUVEC, MDAMB435, and Jurkat cells with the 4CRGD pseudotype without blocking reagents is 20.6%, 22.2% and 6.9%, respectively.
Insertion of the RGD-4C peptide into another site of Sindbis virus envelope proteins
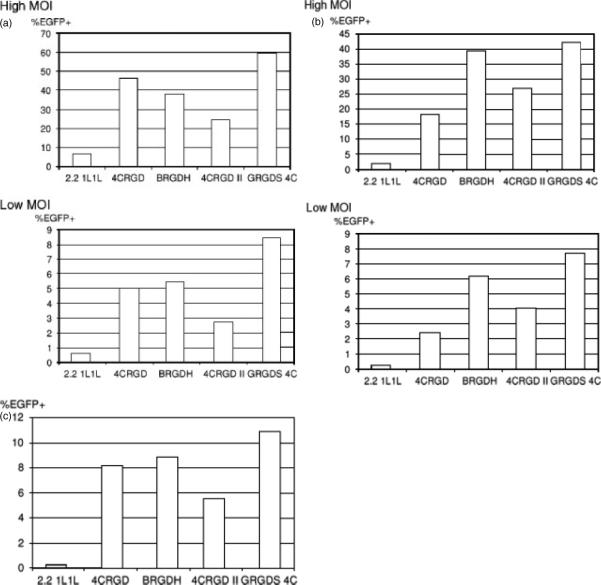
During the mutagenesis carried out to eliminate the original tropism of the Sindbis virus envelope protein, we found that the junction region between E3 and E2 can serve as a receptor-binding region [24]. Another study successfully inserted a large peptide derived from protein L into the same region of the Sindbis virus vector, demonstrating that this region is able to accommodate targeting molecules [34]. In addition, the same region of replication-competent Sindbis virus binds to heparan sulfate and mediates binding of virus to target cells [35]. We attempted to insert the RGD-4C peptide into this site of 2.2 1L1 L at the junction between E3 and E2, without flexible linkers, and the envelope protein was designated as BRGDH (Figure 1). We transduced MDAMB435 (Figure 3a), HUVEC (Figure 3b) and Jurkat cells (Figure 3c) with BRGDH. Insertion of the RGD-4C peptide increased transduction efficiency, suggesting that the inserted peptide serves as the receptor-binding region of the BRGDH pseudotype.
Figure 3.
(a) MDAMD435 and (b) HUVEC cells were infected with lentiviral vectors pseudotyped with chimeric Sindbis virus envelope proteins containing integrin-binding peptides at a high (40 ng of HIV p24) and a low (4 ng of HIV p24) MOI. (c) Jurkat cells were infected only at the high MOI. EGFP transgene expression was analysed 3 days post-transduction. Experiments were performed three times and representative data are shown
Combination of integrin-targeting peptides in two different sites of the envelope protein
To further increase the titers of vectors pseudotyped with integrin-targeting envelope proteins, we next attempted to combine the integrin-targeting peptides inserted into both the E3–E2 junction region and at E2 amino acid 71 (Figure 1). We combined BRGDH with 4CRGD, designated 4CRGD II. We also combined BRGDH with GRGDS3, designated GRGDS 4C. The vectors pseudotyped with these envelope proteins were used for transduction of the above-mentioned cell types, and their titers were compared with the titers of the 4CRGD and BRGDH pseudotypes. Transduction was performed using two different MOI to rigorously compare the titers of the viruses (Figures 3a, 3b and 3c). The titers of the 4CRGD II pseudotype were lower than those of the BRGDH pseudotype at both MOIs and in all cells tested, and were lower than those of the 4CRGD pseudotype for transduction of MDAMB435 and Jurkat cells. By contrast, GRGDS 4C demonstrated the highest titers of all the pseudotypes in all cells and MOIs tested. Therefore, we successfully increased the titers of the targeting vector by combining two types of targeting peptides inserted into different sites of the envelope protein.
The GRGDS 4C pseudotype transduces cells through the interaction of inserted RGD peptides and integrins expressed on target cells
We attempted to confirm that the GRGDS 4C pseudotype binds and transduces cells through the interaction between the inserted RGD peptides and integrins expressed on target cells. Transduction of HUVEC and Jurkat cells with GRGDS 4C was blocked by the soluble RGD peptide, whereas the control peptide did not have significant inhibitory effects (Table 3). The RGD peptide did not block transduction by the 2.2 pseudotype conjugated with anti-HLA class I antibody or the VSV-G pseudotype, confirming that the RGD peptides in GRGDS 4C functioned as receptor-binding regions of the envelope protein. We also attempted to block transduction by the GRGDS 4C pseudotype, using antibodies against integrins. Incubation of target cells with anti-integrins αVβ3 and αVβ5 antibody before transduction with GRGDS 4C inhibited transduction, whereas transduction with the VSV-G pseudotype was not affected by those antibodies. These results confirm that the GRGDS 4C pseudotypes bind target cells through the interaction of the inserted RGD peptides and integrins.
Table 3.
Blocking of transduction by soluble RGD peptide and anti-integrin antibodies
| Relative transduction efficiency (%) |
|||
|---|---|---|---|
| HUVEC | Jurkat | ||
| 2.2 + anti-HLA | No block | 100 | 100 |
| Control peptide | 82 | 150 | |
| RGD peptide | 105 | 151 | |
| VSV-G | No block | 100 | 100 |
| Control peptide | 139 | 116 | |
| RGD peptide | 139 | 112 | |
| Isotype control | 96 | 106 | |
| Anti-integrins | 88 | 165 | |
| GRGDS 4C | No block | 100 | 100 |
| Control peptide | 94 | 87 | |
| RGD peptide | 12 | 19 | |
| Isotype control | 93 | 92 | |
| Anti-integrins | 15 | 29 | |
Cells were incubated with soluble RGD peptides(1000 μg/ml), control RGE peptides (1000 μg/ml), the mixture of anti-integrin antibodies (20 μg/ml of both anti-integrin αVβ3 and αVβ5 antibody), or isotype control antibody (40 μg/ml) before and during transduction. The cells were then infected with 60 ng of HIV p24 of either VSV-G, GRGDS 4C or 4C RGD pseudotype. EGFP expression was assayed 3 days post-transduction by flow cytometry. The transduction The relative transduction efficiency was calculated as:
%EGFP transduction with each blocking agent/%EGFP transduction without blocking agent) × 100
%EGFP transduction of HUVEC and Jurkat cells with the 2.2 pseudotype plus anti-HLA class I antibody without blocking reagents is 13.4% and 6%, respectively. %EGFP transduction of HUVEC and Jurkat cells with the VSV-G pseudotype without blocking reagents is 62.7% and 47%, respectively. %EGFP transduction of HUVEC and Jurkat with the GRGDS 4C pseudotype is 18.2% and 2.9%, respectively.
The titers of the GRGDS 4C pseudotype lentiviral vectors
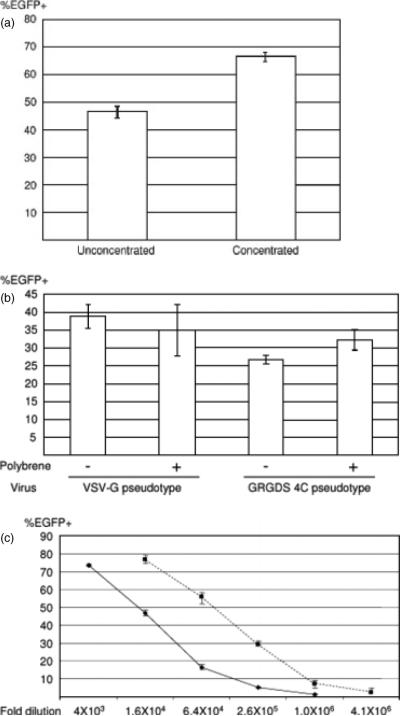
We attempted to investigate the titers of GRGDS 4C pseudotypes on HUVEC. We first compared the titers of the virus before and after concentration by ultracentrifugation. Lentiviral and oncoretroviral vectors pseudotyped with certain types of envelope proteins are reported to have reduced titers after ultracentrifugation as a result of their instability. Although the targeting vectors that we previously created can be concentrated without reduced titers, insertion of different molecules into different sites of the Sindbis virus envelope protein can result in frailty of the chimeric proteins. The same amount of unconcentrated and concentrated virus, adjusted by p24, was used to infect HUVEC. We found that the concentrated virus had slightly higher titers than the unconcentrated, demonstrating that the virus can be concentrated by simple ultracentrifugation without reduced titers (Figure 4a).
Figure 4.
(a) HUVEC (2 × 104) were transduced with unconcentrated and concentrated viruses (1 ng of HIV p24) to investigate the effect of ultracentrifugation on the titers of the virus. EGFP expression was analysed by flow cytometry 3 days post-transduction. Percent EGFP-positive is shown as the mean ± SD of triplicate experiments. (b) HUVEC (2 × 104) were transduced with concentrated GRGDS 4C pseduotype (1 ng of HIV p24) or VSV-G pseudotype (250 pg of HIV p24) in the presence (8 μg/ml) or absence of polybrene to investigate the effect of polybrene on the titers of the viruses. EGFP expression was analysed by flow cytometry 3 days post-transduction. Percent EGFP-positive is shown as the mean ± SD of triplicate experiments. (c) HUVEC (2 × 104) were transduced with GRGDS 4C and VSV-G pseudotypes at various dilutions in the absence of polybrene. EGFP expression was analysed by flow cytometry 3 days post-transduction. The titers (EGFP transduction unit/ml) are calculated at the dilution of 2.6 105 for the GRGDS 4C pseudotype and at 1.0 × 106 for the VSV-G pseudotype
Because polybrene has been known to increase the in vitro titers of lentiviral vectors pseudotyped with several different envelope proteins, we also attempted to test the effect of polybrene on the infectivity of the GRGDS 4C pseudotype. Polyberene did not have any significant effect on the titers of the targeting vectors that we previously developed (data not shown) and did not significantly affect the transduction of HUVEC by either the GRGDS 4C or VSV-G pseudotypes (Figure 4b).
We then quantified the titers of concentrated GRGDS 4C pseudoetypes and compared them with that of the VSV-G pseudotyped vector. We used 100-fold concentrated GRGDS 4C and VSV-G pseudotypes. We used the same fold of dilutions instead of adjusting the amounts of both virus by the amount of p24 to calculate absolute titers (EGFP transduction units/ml of virus stock) in the concentrated virus stock. HUVEC were transduced with both viruses at various dilutions (Figure 4c). The titers of the viruses were calculated at the dilutions at which the percentage EGFP-positive population and the fold of dilution had linear relationships. The titers of concentrated GRGRS 4C and VSV-G pseudotypes on HUVEC were 1.3 × 108 and 7.5 × 108 EGFP transduction units/ml, respectively.
Discussion
In the present study, we have shown that integrin-targeting peptides can be inserted into our targeting envelope proteins, and that the inserted peptides redirect pseudotyped vectors. The inserted peptides served as receptor-binding regions of the chimeric envelope proteins, which demonstrated that the peptides were exposed on the virion and accessible to the receptors expressed on target cells. The vectors pseudotyped with GRGDS 4C transduced target cells more efficiently than the 2.2-pseudotyped vectors conjugated with anti-integrin antibodies, demonstrating that insertion of the peptides did not hinder the folding of the entire envelope proteins or abrogate their fusion activity. When the RGD-4C peptide with two disulfide bonds surrounding the RGD peptide, which has much higher affinity for integrins than the linear RGD peptides, was inserted into the envelope protein, the pseudotyped vectors transduced five- to tenfold more efficiently than the vectors pseudotyped with the envelope protein with linear RGD peptide insertion (GRGDS3). Thus, the titers of the pseudotyped vectors appear to be dependent on the affinities of the inserted ligands.
The targeting peptides could not only be inserted into E2 amino acid 71, where the ZZ domain was originally inserted, but also into the junction region between the E3 and E2 proteins. Originally, this region was known as a furin-cleavage site. It was reported that this site can bind to heparin sulfate when not cleaved by furin [35]. This binding can mediate attachment of Sindbis virus to the target cells, resulting in enhanced transduction by the virus. We also found that this site can mediate binding of the lentiviral vectors pseudotyped with our targeting envelope protein; thus, mutating this region prevents nonspecific binding of the vectors [24]. We can now exploit the region that originally mediated nonspecific transduction to serve as a new receptor-binding region of the envelope protein.
The vector pseudotyped with the envelope, which contains RGD-4C in the E3-E2 junction and liner RGD in the E2 amino acid 71 region (GRGDS 4C), demonstrated higher titers than those pseudotyped with the envelope proteins containing one RGD-4C peptide, either in the E3-E2 junction (BRGDH) or E2 amino acid 70 (4CRGD). This indicates that multiple insertions of the targeting ligand into the envelope proteins increases the avidity of the pseudotyped vectors for the target cells and increases the titers of the virus. Therefore, finding the optimal site for insertion of targeting ligands could increase the titers of targeting vectors. However, insertion of two RGD-4C peptides in both sites (4CRGD II) reduced the titers of the pseudotyped vector. We also inserted two tandem repeats of RGD-4C peptides in the E2 amino acid 71 region, which had less infectivity than the envelope containing a single peptide (4CRGD) when pseudotyping the vectors (data not shown). This is possibly a result of inaccurate disulfide bond formation, or the two disulfide bonds at each location may interfere with the folding and/or affect the structure of the entire envelope protein. Multiple cyclic RGD were chemically synthesized by several groups. Multimers usually have higher affinity to integrins than monomers [36–39]. Therefore, if the structures of the inserted RGD-4C peptide and envelope protein are maintained, the 4CRGD II pseudotype should have higher titers than the 4CRGD SINDBIS pseudotype.
Many types of viruses have been shown to utilize integrins as their receptors [40]. Herpes viruses contain integrin-binding peptides in their envelope proteins. Binding of their envelope proteins to integrins induces a signal that facilitates entry of the viruses. Hantaviruses, another type of envelope virus, also bind to integrins, which mediates the attachment of virus and signaling. Non-envelope viruses, including adenoviruses, picornaviruses and reoviruses, bind to integrins with the integrin-binding sites in their capsid proteins. Binding to integrins mediates not only attachment of the viruses, but also signaling through integrins, including polymerization of the actin cytoskeleton and activation of kinases and GTPase, which facilitate entry of the viruses into cells. Herpes viruses, adenoviruses, and reoviruses contain linear integrin-binding peptides in their envelope proteins or capsid proteins. None have evolved to have a structure similar to that of the RGD-4C peptide, although RGD-4C has a much higher affinity for integrins than linear RGD peptides. The RGD-4C peptide was created by screening with the phage display system [28]. By contrast, when viruses evolve naturally to acquire new receptor-binding motifs in their structural proteins, the motifs should be selected based not only on their high affinity, but also for a lesser effect on the entire structure and infectivity of the viruses.
Insertion of molecules, such as single-chain antibodies covalently inserted into envelope proteins, would broaden the applications of this targeting strategy [41,42]. Maintaining the structure of the envelope protein and inserted targeting molecules will be more challenging when inserting larger molecules. Choosing appropriate insertion sites and the use of amino acid linkers to maintain proper structures of both the inserted ligands and envelope protein will be important.
The ability to create novel envelope proteins bearing covalently inserted ligands is a critical step for the eventual use of such vectors in clinical applications.
Acknowledgements
We thank Dr Airi Harui for supporting experiments and discussion. This work was supported by US National Institute of Health grants, CA-92194, AI039975, AI069350 and AI028697 (UCLA CFAR).
References
- 1.Amado RG, Chen IS. Lentiviral vectors – the promise of gene therapy within reach? Science. 1999;285:674–676. doi: 10.1126/science.285.5428.674. [DOI] [PubMed] [Google Scholar]
- 2.Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- 3.Aiuti A, Slavin S, Aker M, et al. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science. 2002;296:2410–2413. doi: 10.1126/science.1070104. [DOI] [PubMed] [Google Scholar]
- 4.Akporiaye ET, Hersh E. Clinical aspects of intratumoral gene therapy. Curr Opin Mol Ther. 1999;1:443–453. [PubMed] [Google Scholar]
- 5.Follenzi A, Battaglia M, Lombardo A, et al. Targeting lentiviral vector expression to hepatocytes limits transgene-specific immune response and establishes long-term expression of human antihemophilic factor IX in mice. Blood. 2004;103:3700–3709. doi: 10.1182/blood-2003-09-3217. [DOI] [PubMed] [Google Scholar]
- 6.Brown BD, Cantore A, Annoni A, et al. A microRNA-regulated lentiviral vector mediates stable correction of hemophilia B mice. Blood. 2007;110:4144–4152. doi: 10.1182/blood-2007-03-078493. [DOI] [PubMed] [Google Scholar]
- 7.Brown BD, Sitia G, Annoni A, et al. In vivo administration of lentiviral vectors triggers a type I interferon response that restricts hepatocyte gene transfer and promotes vector clearance. Blood. 2007;109:2797–2805. doi: 10.1182/blood-2006-10-049312. [DOI] [PubMed] [Google Scholar]
- 8.Brown BD, Venneri MA, Zingale A, et al. Endogenous microRNA regulation suppresses transgene expression in hematopoietic lineages and enables stable gene transfer. Nat Med. 2006;12:585–591. doi: 10.1038/nm1398. [DOI] [PubMed] [Google Scholar]
- 9.De Palma M, Venneri MA, Roca C, et al. Targeting exogenous genes to tumor angiogenesis by transplantation of genetically modified hematopoietic stem cells. Nat Med. 2003;9:789–795. doi: 10.1038/nm871. [DOI] [PubMed] [Google Scholar]
- 10.Bushman F, Lewinski M, Ciuffi A, et al. Genome-wide analysis of retroviral DNA integration. Nat Rev Microbiol. 2005;3:848–858. doi: 10.1038/nrmicro1263. [DOI] [PubMed] [Google Scholar]
- 11.Waehler R, Russell SJ, Curiel DT. Engineering targeted viral vectors for gene therapy. Nat Rev Genet. 2007;8:573–587. doi: 10.1038/nrg2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boerger AL, Snitkovsky S, Young JA. Retroviral vectors preloaded with a viral receptor-ligand bridge protein are targeted to specific cell types. Proc Natl Acad Sci USA. 1999;96:9867–9872. doi: 10.1073/pnas.96.17.9867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roux P, Jeanteur P, Piechaczyk M. A versatile and potentially general approach to the targeting of specific cell types by retroviruses: application to the infection of human cells by means of major histocompatibility complex class I and class II antigens by mouse ecotropic murine leukemia virus-derived viruses. Proc Natl Acad Sci USA. 1989;86:9079–9083. doi: 10.1073/pnas.86.23.9079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bupp K, Roth MJ. Targeting a retroviral vector in the absence of a known cell-targeting ligand. Hum Gene Ther. 2003;14:1557–1564. doi: 10.1089/104303403322495061. [DOI] [PubMed] [Google Scholar]
- 15.Funke S, Maisner A, Muhlebach MD, et al. Targeted cell entry of lentiviral vectors. Mol Ther. 2008;16:1427–1436. doi: 10.1038/mt.2008.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kasahara N, Dozy AM, Kan YW. Tissue-specific targeting of retroviral vectors through ligand–receptor interactions. Science. 1994;266:1373–1376. doi: 10.1126/science.7973726. [DOI] [PubMed] [Google Scholar]
- 17.Marin M, Noel D, Valsesia-Wittman S, et al. Targeted infection of human cells via major histocompatibility complex class I molecules by Moloney murine leukemia virus-derived viruses displaying single-chain antibody fragment-envelope fusion proteins. J Virol. 1996;70:2957–2962. doi: 10.1128/jvi.70.5.2957-2962.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin F, Chowdhury S, Neil S, et al. Envelope-targeted retrovirus vectors transduce melanoma xenografts but not spleen or liver. Mol Ther. 2002;5:269–274. doi: 10.1006/mthe.2002.0550. [DOI] [PubMed] [Google Scholar]
- 19.Nilson BH, Morling FJ, Cosset FL, et al. Targeting of retroviral vectors through protease-substrate interactions. Gene Ther. 1996;3:280–286. [PubMed] [Google Scholar]
- 20.Somia NV, Zoppe M, Verma IM. Generation of targeted retroviral vectors by using single-chain variable fragment: an approach to in vivo gene delivery. Proc Natl Acad Sci USA. 1995;92:7570–7574. doi: 10.1073/pnas.92.16.7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valsesia-Wittmann S, Drynda A, Deleage G, et al. Modifications in the binding domain of avian retrovirus envelope protein to redirect the host range of retroviral vectors. JVirol. 1994;68:4609–4619. doi: 10.1128/jvi.68.7.4609-4619.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morizono K, Bristol G, Xie YM, et al. Antibody-directed targeting of retroviral vectors via cell surface antigens. J Virol. 2001;75:8016–8020. doi: 10.1128/JVI.75.17.8016-8020.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morizono K, Ringpis GE, Pariente N, et al. Transient low pH treatment enhances infection of lentiviral vector pseudotypes with a targeting Sindbis envelope. Virology. 2006;355:71–81. doi: 10.1016/j.virol.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 24.Morizono K, Xie Y, Ringpis GE, et al. Lentiviral vector retargeting to P-glycoprotein on metastatic melanoma through intravenous injection. Nat Med. 2005;11:346–352. doi: 10.1038/nm1192. [DOI] [PubMed] [Google Scholar]
- 25.Pariente N, Mao SH, Morizono K, et al. Efficient targeted transduction of primary human endothelial cells with dual-targeted lentiviral vectors. J Gene Med. 2008;10:242–248. doi: 10.1002/jgm.1151. [DOI] [PubMed] [Google Scholar]
- 26.Pariente N, Morizono K, Virk MS, et al. A novel dual-targeted lentiviral vector leads to specific transduction of prostate cancer bone metastases in vivo after systemic administration. Mol Ther. 2007;15:1973–81. doi: 10.1038/sj.mt.6300271. [DOI] [PubMed] [Google Scholar]
- 27.Ruoslahti E, Pierschbacher MD. New perspectives in cell adhesion: RGD and integrins. Science. 1987;238:491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- 28.Koivunen E, Wang B, Ruoslahti E. Phage libraries displaying cyclic peptides with different ring sizes: ligand specificities of the RGD-directed integrins. Biotechnology. 1995;13:265–270. doi: 10.1038/nbt0395-265. [DOI] [PubMed] [Google Scholar]
- 29.Dmitriev I, Krasnykh V, Miller CR, et al. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. JVirol. 1998;72:9706–9713. doi: 10.1128/jvi.72.12.9706-9713.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harui A, Roth MD, Vira D, et al. Adenoviral-encoded antigens are presented efficiently by a subset of dendritic cells expressing high levels of alpha(v)beta3 integrins. JLeukocBiol. 2006;79:1271–1278. doi: 10.1189/jlb.1105694. [DOI] [PubMed] [Google Scholar]
- 31.Shi W, Bartlett JS. RGD inclusion in VP3 provides adeno-associated virus type 2 (AAV2)-based vectors with a heparan sulfate-independent cell entry mechanism. Mol Ther. 2003;7:515–525. doi: 10.1016/s1525-0016(03)00042-x. [DOI] [PubMed] [Google Scholar]
- 32.Vigne E, Mahfouz I, Dedieu JF, et al. RGD inclusion in the hexon monomer provides adenovirus type 5-based vectors with a fiber knob-independent pathway for infection. J Virol. 1999;73:5156–5161. doi: 10.1128/jvi.73.6.5156-5161.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wickham TJ, Tzeng E, Shears LL, II, et al. Increased in vitro and in vivo gene transfer by adenovirus vectors containing chimeric fiber proteins. JVirol. 1997;71:8221–8229. doi: 10.1128/jvi.71.11.8221-8229.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klimstra WB, Williams JC, Ryman KD, et al. Targeting Sindbis virus-based vectors to Fc receptor-positive cell types. Virology. 2005;338:9–21. doi: 10.1016/j.virol.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 35.Klimstra WB, Heidner HW, Johnston RE. The furin protease cleavage recognition sequence of Sindbis virus PE2 can mediate virion attachment to cell surface heparan sulfate. JVirol. 1999;73:6299–6306. doi: 10.1128/jvi.73.8.6299-6306.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao Q, Li ZB, Chen K, et al. Evaluation of biodistribution and anti-tumor effect of a dimeric RGD peptide-paclitaxel conjugate in mice with breast cancer. Eur J Nucl Med Mol Imaging. 2008;35:1489–1498. doi: 10.1007/s00259-008-0744-y. [DOI] [PubMed] [Google Scholar]
- 37.Dijkgraaf I, Rijnders AY, Soede A, et al. Synthesis of DOTA-conjugated multivalent cyclic-RGD peptide dendrimers via 1,3-dipolar cycloaddition and their biological evaluation: implications for tumor targeting and tumor imaging purposes. Org Biomol Chem. 2007;5:935–944. doi: 10.1039/b615940k. [DOI] [PubMed] [Google Scholar]
- 38.Mousa SA. Alpha v integrin affinity/specificity and antiangio-genesis effect of a novel tetraaza cyclic peptide derivative, SU015, in various species. J Cardiovasc Pharmacol. 2005;45:462–467. doi: 10.1097/01.fjc.0000159044.27618.be. [DOI] [PubMed] [Google Scholar]
- 39.Thumshirn G, Hersel U, Goodman SL, et al. Multimeric cyclic RGD peptides as potential tools for tumor targeting: solid-phase peptide synthesis and chemoselective oxime ligation. Chemistry. 2003;9:2717–2725. doi: 10.1002/chem.200204304. [DOI] [PubMed] [Google Scholar]
- 40.Stewart PL, Nemerow GR. Cell integrins: commonly used receptors for diverse viral pathogens. Trends Microbiol. 2007;15:500–507. doi: 10.1016/j.tim.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 41.Gao Y, Whitaker-Dowling P, Watkins SC, et al. Rapid adaptation of a recombinant vesicular stomatitis virus to a targeted cell line. J Virol. 2006;80:8603–8612. doi: 10.1128/JVI.00142-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aires da Silva F, Costa MJ, Corte-Real S, et al. Cell type-specific targeting with sindbis pseudotyped lentiviral vectors displaying anti-CCR5 single-chain antibodies. Hum Gene Ther. 2005;16:223–234. doi: 10.1089/hum.2005.16.223. [DOI] [PubMed] [Google Scholar]