Abstract
Background
Targeted gene transduction in vivo is the ultimate preferred method for gene delivery. We previously developed targeting lentiviral vectors that specifically recognize cell surface molecules with conjugated antibodies and mediate targeted gene transduction both in vitro and in vivo. Although effective in some experimental settings, the conjugation of virus with antibodies is mediated by the interaction between protein A and the Fc region of antibodies, which is not as stable as covalent conjugation. We have now developed a more stable conjugation strategy utilizing the interaction between avidin and biotin.
Methods
We inserted the biotin-adaptor-peptide, which was biotinylated by secretory biotin ligase at specific sites, into our targeting envelope proteins, enabling conjugation of the pseudotyped virus with avidin, streptavidin or neutravidin.
Results
When conjugated with avidin-antibody fusion proteins or the complex of avidin and biotinylated targeting molecules, the vectors could mediate specific transduction to targeted cells recognized by the targeting molecules. When conjugated with streptavidin-coated magnetic beads, transduction by the vectors was targeted to the locations of magnets.
Conclusions
This targeting vector system can be used for broad applications of targeted gene transduction using biotinylated targeting molecules or targeting molecules fused with avidin.
Keywords: biotin adaptor peptide, endothelial cells, lentiviral vector, Sindbis virus envelope, targeting vector
Introduction
Retroviral vectors integrate their transgenes into host cell chromosomes, allowing sustained transgene expression, which is favorable for therapy of chronic diseases such as congenital genetic deficiencies and chronic acquired infectious and malignant diseases [1]. Gene therapy using retroviral vectors has been shown to be an effective approach for several genetic diseases such as X-linked severe combined immunodeficiency (SCID) [2,3]. In the case of hematopoietic diseases, such as X-linked SCID, the cells to be transduced are hematopoietic, which can be easily isolated from the body, transduced in vitro, and infused back into the body without losing their physiological functions. However, ex vivo transduction is not suitable for gene transduction of solid organs or most body tissues. An ideal method for gene transduction of solid organs, including tumor tissues, is intravenous administration of gene therapy vectors that specifically home in on and transduce desired cells and tissues in vivo. Such vectors are referred to as ‘targeting vectors’, and the development of effective targeting vectors has been one of the most important issues of gene therapy [4].
Because retroviral vectors are one of the most useful types of gene therapy vectors, many research groups have attempted to create targeting retroviral vectors. One approach is pseudotyping retroviral vectors with chimeric proteins generated between retroviral envelope proteins and targeting molecules, such as single-chain antibodies or growth factors [5–12]. Another approach is conjugating virus with adaptor molecules that specifically recognize desired molecules [13,14]. Each approach has its own advantages and disadvantages. The targeting molecules in the first approach are covalently conjugated to the viral envelope proteins; therefore, conjugation of these molecules is very stable. However, it is necessary to generate new chimeric envelope proteins for each target molecule, and insertion of targeting molecules sometimes destroys the functions and structures of chimeric proteins. The second approach does not require generating new envelope proteins for each target molecule. However, conjugation between virus and adaptor molecules must be of very high affinity and stability to maintain conjugation, especially for in vivo usage. Previously, we have developed targeting retroviral vectors, both lentiviral and oncoretroviral, using the second approach [15–18]. These targeting vectors were conjugated with monoclonal antibodies via the interaction between the Fc-binding region of protein A (ZZ domain) inserted into the envelope protein and the Fc region of antibodies. This targeting system is effective in vitro and in some in vivo experimental settings that do not require highly stable conjugation. However, conjugation between the virus and antibodies may not be sufficiently stable in immune competent animals because serum immunoglobulin will compete with conjugated antibodies for binding to the ZZ domain of the envelope protein [19]. Binding between avidin and biotin is of very high affinity. The dissociation constant (Kd) of binding between these two molecules is 10−15, which is 107–8 less than the Kd of the binding between the ZZ domain and the Fc region of antibodies [20]. Several studies have used this interaction to conjugate adenovirus, adeno-associated virus and baculovirus vectors with targeting molecules [21–25]. They inserted peptides, which are substrates for biotinylation, into structural proteins of adenovirus, adeno-associated virus and baculovirus vectors, and the biotinylated sites bound avidin, neutravidin or streptavidin. Because avidin, neutravidin and streptavidin forms tetramers, and each tetramer has four biotin-binding sites, these molecules can bridge between bitotinylated virus and biotinylated targeting molecules. By conjugating a wide variety of biotinylated molecules, these vectors bound to targeted cell surface molecules and transduced the cells expressing those target molecules. Although particularly effective in in vitro experimental settings, these adenoviral and adeno-associated virus vectors still retain their original native tropism, resulting in transduction of a wide variety of cell types, regardless of the expression of targeted molecules and trapping in untargeted organs before reaching the target cells and tissues [26–28].
We abrogated the native tropisms of our targeting lentiviral vectors, resulting in less trapping, especially in the liver and spleen. The targeting vectors recognized a target molecule in vivo, and mediated specific gene transduction to target cells via the interaction between a conjugated antibody and a target cell surface antigen.
To stabilize the conjugation between targeting molecules and virus in the presence of serum immunoglobin, we used the avidin–biotin interaction to conjugate targeting molecules to lentivirus vectors. Instead of the ZZ domain, we inserted the biotin-adaptor-peptide (BAP) into our targeting envelope protein, allowing biotinylation of the envelope protein at specific sites [29]. The biotinylated envelope proteins were conjugated with targeting molecules via its interaction with avidin or neutravidin, and were utilized for targeted gene transduction.
Materials and methods
Plasmid, antibodies, proteins and chemicals
2.2 1L1L was constructed from 2.2 by replacing the ZZ domain in the E2 protein with two sets of flexible linker peptides (GGGGS) X2. BAP SINDBIS was constructed by inserting BAP between the two flexible linkers of 2.2 1L1L. BBAPH SINDBIS was constructed by inserting BAP into the junction region of the E3 and E2 proteins of 2.2 1L1L. BAP II SINDBIS was constructed from BAP SINDBIS and BBAPH SINDBIS by combining the BAP insertion of each construct into one construct. Therefore, BAP II SINDBIS contains two BAP insertions at amino acid position 70 of E2 and the junction between E3 and E2. The expression vector of biotin ligase, pBirA, was provided by Dr Michael Barry (Baylor College of Medicine, Houston, TX, USA). The fusion proteins between avidin and anti-rat [anti-rat transferrin receptor (TfR) immunoglobulin (Ig)G3-Av] or human (anti-huTfR IgG3-Av) transferrin receptors were prepared as described previously [30,31]. Neutravidin was purchased from Pierce (Rockford, IL, USA). Biotinylated transferrin was purchased from Molecular Probes (Eugene, OR, USA). Anti-rat and human transferrin receptor antibodies were purchased from BD Bioscience (Bedford, MA, USA).
Cells and viruses
293T cells were cultured in IMDM (Sigma-Aldrich, St Louis, MO, USA) containing 10% fetal calf serum (FCS) and antibiotics. Jurkat and Y3-Ag1.2.3 cells were cultured in RPMI (Invitrogen, Carlsbad, CA, USA) containing 10% FCS. All lentiviral vectors were produced in 293T cells, using the calcium phosphate transfection method as described previously [15]. Briefly, 293T cells (1.8 × 107) were transfected with one of envelope protein expression vectors (10 µg), packaging plasmid, 8.2 delta VPR (12.5 µg), either lentiviral vector, cppt2e [16] or FuhLucW [17] (12.5 µg) and 6 µg of pSec BirA. After transfection, the cells were cultured in Opti-MEM (Invitrogen) containing 2% FCS and 500 mm of biotin. The supernatant was subjected to ultracentrifugation, and the pellet containing the virus was resuspended in Hepes-buffered saline. The resuspended virus was dialysed in phosphate-buffered saline (PBS) for 4 h to eliminate residual biotin. The concentrations of virus were quantified by measuring amounts of viral capsid protein, p24. The dialysed virus was frozen at −70 °C until use.
The viruses (40 ng p24) were conjugated with anti-ratTfR IgG3-Av (5 µg) or anti-huTfR IgG3-Av (5 µg) for 30 min on ice before infection. Jurkat or Y3-Ag1.2.3 cells (5 × 104) were incubated with the viruses for 2 h at 37 °C, and then the viruses were washed away. Enhanced green fluorescent protein (EGFP) transgene expression was analysed by flow cytometry 3 and 10 days post-transduction.
BAP II SINDBIS pesudotype (40 ng p24) was incubated with neutravidin (2 µg) for 30 min, then incubated with biotinylated transferrin (2 µg). Jurkat cells (5 × 104) were incubated with the virus for 2 h. Firefly luciferase transgene expression was assayed by a luminometer 3 days post-infection.
Flow cytometric analysis of transferrin receptor 1 expression on Jurkat and Y3 cells
Jurkat and Y3 cells (5 × 105) were incubated with 100 µl (5 µg/ml) of either mouse anti-rat transferrin receptor 1 (BD Biosciences, San Diego, CA, USA) or mouse antihuman transferrin receptor 1 (BD Biosciences) for 1 h at 4 °C, followed by incubation with 100 µl (200-fold dilution in PBS) of rabbit anti-mouse IgG conjugated with Alexa 488 (Invitrogen) for 1 h at 4 °C. Antibody staining was analysed by flow cytometry.
Western blotting
The amounts of viral vectors were normalized to the amount of HIV p24 (1 mg/ml). The viral vectors were mixed with same volume of electrophoresis loading buffer [20% glycerol, 10% 2-mercaptoethanol, 4% sodium dodecyl sulfate (SDS), 125 mm Tris-HCl (pH 6.8), 0.02% bromophenol blue] and boiled for 5 min. Each sample (15 µl) was subjected to electrophoresis through a SDS 4–20% polyacrylamide gel (Lonza, Rockland, ME, USA). Immunoblot analyses of envelope proteins were performed with rabbit anti-Sindbis virus polyclonal antibody (kindly provided by Dr John M. Polo, Chiron Vaccines, Emeryville, CA, USA) and horseradish peroxidase (HRP)-conjugated goat anti-rabbit polyclonal antibody (Pierce). Biotinylation of envelope proteins was analysed using HRP-conjugated neutravidin (Pierce). The protein bands were visualized by enhanced chemiluminescence (Pierce).
Quantitative analysis of biotinylation of lentiviral vectors
Purified viral vectors (0.5 and 1 µg of p24) were incubated either with 100 µl of magnet beads conjugated with streptavidin (Pierce), or these beads incubated with an excess amount of biotin for 2 h at 4 °C. The beads were then removed using magnets. The amounts of the viral vectors after magnet subtraction were quantified by enzyme-linked immunosorbent assay.
Transduction with BAP II SINDBIS pseudotypes conjugated with streptavidin-coated magnet beads
Human microvascular endothelial cells (HMVEC) were provided by Dr Benhur Lee (UCLA, Los Angeles, CA, USA) and cultured in EBM-2 medium supplemented with EGM-2 (Lonza). HMVEC (8 × 104 cells) were seeded in a chamber slide (Nalge Nunc Intetnational, Naperville, IL, USA) 1 day prior to transduction. BAP II SINDBIS pseudotype (400 ng p24) was incubated with 10 µl of streptavidin-coated magnet beads for 1 h at room temperature. The unbound virus was washed away three times, using a magnetic particle concentrator and PBS (Invitrogen). The magnet of the magnetic particle concentrator was placed under the chamber slides containing cultured HMVEC. The virus conjugated with the beads (resuspended in 2 ml of PBS containing calcium and magnesium) was added to the cells in the chamber with or without application of the magnet. Magnet-conjugated viruses were incubated with HMVEC for various time periods (10 s, 1 and 10 min, and 1 h) with application of a magnet. EGFP expression was analysed 4 and 10 days post-transduction.
Results
Construction of targeting envelope proteins with insertion of BAP
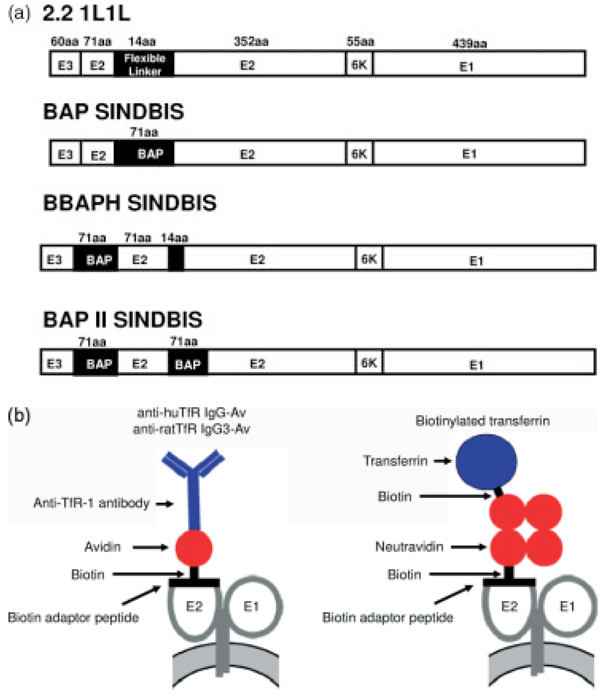
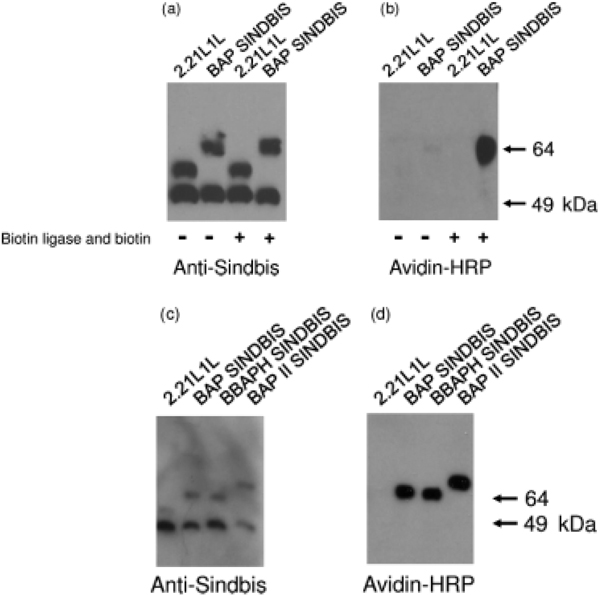
We inserted BAP into our targeting envelope protein, 2.2, instead of the ZZ domain of protein A. The 2.2 envelope protein is derived from the Sindbis virus envelope protein, into which the ZZ domain was inserted into its original receptor-binding region [15], and contains mutations to eliminate its original tropism [17] and to increase infectivity for target cells [16]. To maintain the entire structure of the envelope protein after insertion of BAP, we inserted two flexible linkers, which consisted of four glycine and one serine, at the site of BAP insertion, designated 2.2 1L1L (Figure 1). The envelope protein with insertion of BAP was designated BAP SINDBIS. BAP SINDBIS was used to pseudotype a lentiviral vector, and expression of the envelope was analysed by western blotting (Figure 2a), using anti-Sindbis virus antibody. The lower band is E1 and the upper band is E2 fused with E3 and into which the linker is inserted. In the case of BAP SINDBIS, a higher molecular mass band of 64 kDa represents the E2/E3 BAP fusion protein.
Figure 1.
(a) Schematic representation of chimeric Sindbis virus envelope proteins. 2.2 1L1L was derived from the chimeric 2.2 envelope protein [18]. 2.2 1L1L has two flexible linkers (Gly-Gly-Gly-Gly-Ser) and AVR II-Bst E II cloning sites at amino acid 71 of the E2 protein. BAP Sindbis contains a biotin acceptor peptide (BAP) derived from Escherichia coli biotin holoenzyme synthetase between the two flexible linkers. BBAPH contains BAP at the first amino acid of the E2 protein, and BAP II SINDBIS contains two BAP at both positions of the E2 protein. (b) The schematic strategy to conjugate virus with anti-human or rat transferrin receptor 1 and transferrin. BAP II SINDBIS envelope proteins were covalently conjugated with biotin. Anti-human or rat transferrin receptor antibodies were fused with avidin, designated anti-huTfR IgG-Av or anti-ratTfR IgG3-Av, respectively. Anti-huTfR IgG-Av or anti-ratTfR IgG3-Av can be conjugated with the BAP II SINDBIS envelope protein through the interaction of avidin and the biotin of the envelope protein. Neutravidin has four biotin binding sites. Thus, one neutravidin can bind both the biotinylated BAP II SINDBIS envelope protein and biotinlynated transferrin, which results in bridging the pseudotyped virus with transferrin
Figure 2.
SDS-polyacrylamide gel electrophoresis and western blotting analysis of chimeric Sindbis virus envelope proteins. Lentiviral vectors pseudotyped with 2.2 1L1L or BAP SINDBIS, produced in the presence or absence of pSec BirA and biotin (500 mM), were analysed using: (a) rabbit anti-Sindbis virus antibody and goat anti-rabbit IgG antibody conjugated with horseradish peroxidase; or (b) neutravidin conjugated with horseradish proxidase. Lentiviral vectors pseudotyped with 2.2 1L1L, BAP SINDBIS, BBAPH SINDBIS, or BAP II SINDBIS, produced in the presence of pSec BirA and biotin, were analysed using: (c) rabbit anti-Sindbis virus antibody and goat anti-rabbit IgG antibody conjugated with horseradish peroxidase; or (d) neutravidin conjugated with horseradish peroxidase
To investigate biotinylation of the inserted BAP, we performed western blotting and immunostaining with streptavidin conjugated with HRP (Figure 2b). BAP SINDBIS was barely biotinylated in the conditions previously described for production of 2.2 pseudotypes. Both secreted biotin ligase and adequate amounts of biotin in culture medium are required for efficient biotinylation of secretory proteins and viral envelope proteins [29]. Therefore, to enhance the biotinylation of BAP, we generated BAP SINDBIS pseudotypes in the presence of secreted biotin ligase and greater amounts of biotin in culture medium. Under these conditions, the BAP SINDBIS protein was biotinylated, which is consistent with a previous study [29] (Figure 2b).
To further increase the efficiency of biotinylation of the targeting envelope, we inserted an additional BAP into the envelope protein. We previously found that the site between the E2 and E3 proteins serves as a receptor-binding site, and other studies have successfully inserted targeting molecules into this site [32]. We therefore inserted BAP into this site, and designated the envelope BBAPH SINDBIS (Figure 1a). BBAPH SINDBIS was expressed, pseudotyped with a lentiviral vector, and was shown to be biotinylated as efficiently as BAP SINDBIS (Figures 2c and 2d). Accordingly, we created two BAP insertions in one envelope, one at the junction between E2 and E3 and the other at the same location as the ZZ domain. The combined envelope protein was designated BAP II SINDBIS (Figure 1a). BAP II SINDBIS envelope had the expected molecular mass in western blot analysis, and expressed and pseudotyped a lentiviral vector as efficiently as single BAP insertions (Figure 2d). As expected, streptavidin-HRP bound BAP II SINDBIS more than the single BAP insertions into envelopes, such as BAP SINDBIS and BBAPH SINDBIS (Figure 2d). We also investigated the percentage of virions bearing biotinylation of BAP II SINDBIS pseudotypes using streptavidin-coated magnetic beads, followed by panning with magnets. Using this method, the percentages of the virus subtracted by magnets would represent the minimum percentage of virus bearing biotinylation. Streptavidin-coated beads pre-bound with saturating amounts of biotin were used as a negative control for virus subtraction. The majority of virus (90%) was shown to bind to streptavidin-coated beads (Table 1).
Table 1.
Percentage of biotinylated virus
| Total virusa | StAv-magnetic beadsb | StAv-magnetic beads pre-treated with biotinc |
Bound virus in StAv–biotin interactiond |
Minimum percentage of biotinylated viruse |
|---|---|---|---|---|
| 1000 | 948 | 33 | 915 | 91 |
| 500 | 480 | 30 | 450 | 90 |
Total virus is the amount of virus (ng HIV p24) subjected to binding with 100 µl of streptavidin (StAv)-conjugated magnetic beads.
Virus bound to StAv-magnetic beads (ng HIV p24) is calculated as: total virus amounta minus amount of remaining virus of in sample incubated with StAv-magnet.
Virus bound to StAv-magnetic beads pre-treated with biotin (ng HIV p24) is calculated as: total virus amounta minus amount of unbound virus in sample incubated with biotin-saturated StAv magnetic beads.
Targeted infection with virion-conjugated targeting molecules
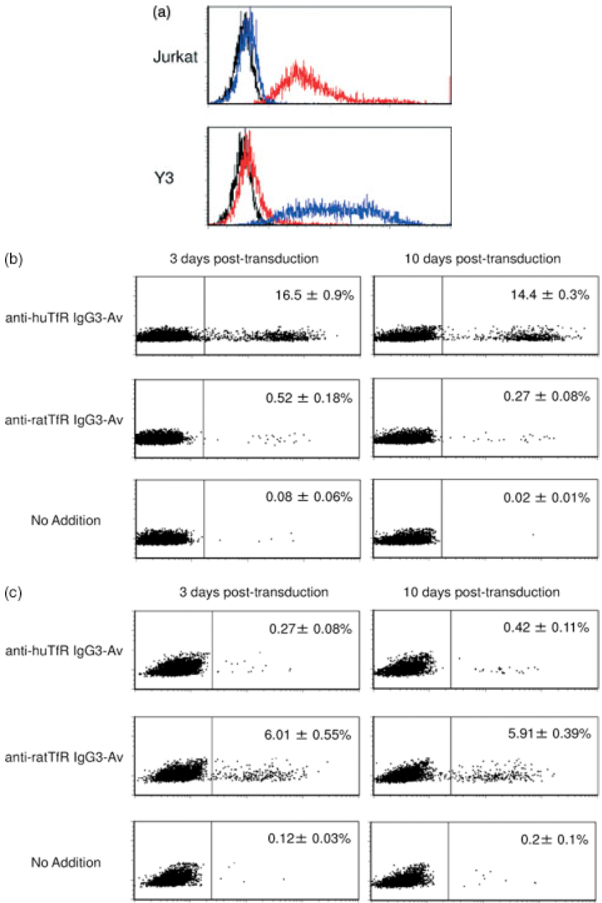
We tested the utility of the biotin/avidin targeting strategy by using human and rat transferrin receptor 1 as our target for virus binding (Figure 1b). The targeting ligands were a fusion between avidin and anti-human transferrin receptor 1 antibody, designated anti-huTfR IgG3-Av, and a fusion between avidin and anti-rat transferrin receptor 1 antibody, designated anti-ratTfR IgG3-Av [30,31]. Because the human and rat transferrin receptor antibodies do not cross react with the heterologous species, these reagents also allow us to test specificity of targeting (Figure 3a). Either anti-huTfR IgG3-Av or anti-ratTfR IgG3-Av was conjugated with a BAP II SINDBIS pseudotype, and cells expressing human or rat transferrin receptor 1 were infected with these viruses (Figures 3b and 3c). Human Jurkat cells were transduced with conjugates of anti-huTfR IgG3-Av and not with conjugates of anti-ratTfR IgG3-Av. The reciprocal patterns were observed when rat Y3-Ag1.2.3. cells were used as the target. The expression levels of EGFP did not differ 10 days post-transduction, eliminating the possibility of pseudo-transduction and expression from unintegrated vectors. The titers of BAPII pseudotypes conjugated with anti-human or rat transferrin receptor-avidin fusion protein are 2 × 105 and 6 × 104 EGFP-transducing units/1 µg HIV p24. These results demonstrated that infection with BAP II SINDBIS pesudotypes conjugated with targeting molecules was specifically mediated by the interactions between the conjugated targeting molecules and targeted molecules on cells.
Figure 3.
Gene transduction mediated by fusion proteins between avidin and anti-human or transferrin receptor antibody fusion proteins. (a) Expression of human and rat transferrin receptor 1 on Jurkat (human T-cell line) and Y3 (rat myeloma cell line) cells. Each cell type was stained either with isotype control control antibody (black line), mouse anti-human (red line), or rat (blue line) transferrin receptor 1 antibody, followed by staining with Alexa 488-conjugated secondary antibody. (b) Jurkat, was infected with the BAP II SINDBIS pseudotype in the presence or absence of fusion protein between avidin and either anti-human (anti-huTfR IgG-Av) or anti-rat (anti-ratTfR IgG3-Av) transferrin receptor 1 antibody. EGFP expression was analysed by flow cytometry 3 and 10 days post-transduction. Representive flow cutometric profiles are shown. Percent EGFP-positive is shown as the mean ± SD of triplicate experiments. (c) Y3-Ag 1.2.3, was infected with the BAP II pseudotype in the presence or absence of anti-huTfR IgG-Av or anti-ratTfR IgG3-Av. EGFP expression was analysed by flow cytometry 3 and 10 days post-transduction. Representive flow cutometric profiles are shown. Percent EGFP-positive is shown as the mean ± SD of triplicate experiments
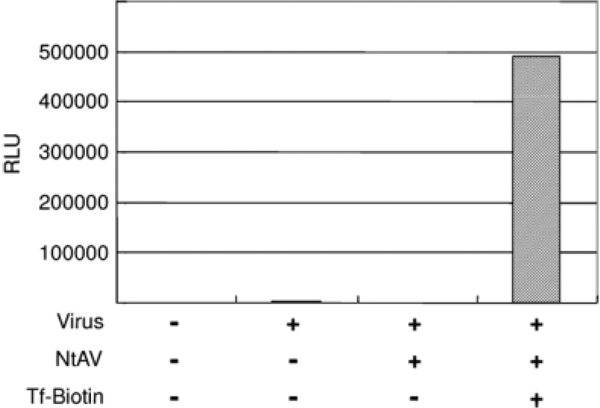
We next attempted to conjugate ligands with BAP II SINDBIS pseudotypes by using neutravidin to bridge BAP-containing viruses and biotinylated ligands molecules (Figure 2b). Because avidin has four biotin-binding sites, avidin can bridge four biotinylated molecules. We incubated the BAP II SINDBIS pseudotyped virus with neutravidin, an avidin derivative with lower nonspecific binding as a result of mutations at charged amino acid and N-glycan residues, followed by incubation with chemically biotinylated transferrin [33]. The conjugated biotinylated transferrin increased the transduction of BAP II SINDBIS pseudotypes for Jurkat cells (Figure 4), demonstrating that the bridged molecules can mediate the binding and transduction by BAP II SINDBIS pseudotypes.
Figure 4.
Gene transduction mediated by biotinylated transferrin. Jurkat cells were transduced with BAP II SINDBIS pseudotype either without conjugation, conjugated with neutravidin, or conjugated with neutravidin and biotinylated transferrin. Firefly luciferase was used as a reporter gene, and gene transduction was analysed by measuring the relative luciferase units (RLU) of transduced cells. Representative data of three independent experiments are shown
Targeting BAP II SINDBIS pseudotypes by magnetic force
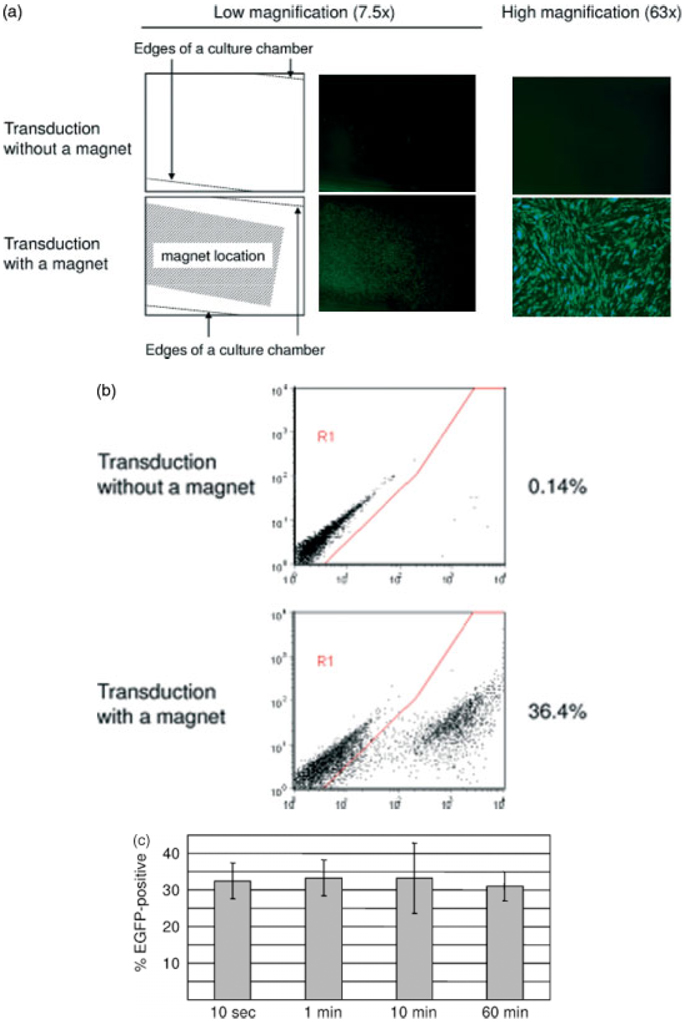
Because BAP II SINDBIS pseudotypes can bind streptavidin-coated magnetic beads, we hypothesized that magnetic force could be used to control the distribution of viruses. HMVEC in chamber slides, under which a magnet was attached, were infected with BAP II SINDBIS pseudotypes conjugated with streptavidin-coated magnetic beads. Expression of the transgene, EGFP, was visualized with a fluorescent microscope. As shown in Figure 5a, transgene expression was drastically increased in a localized area with application of a magnet force. This increase in transduction was confirmed by flow cytometry (Figure 5b). HMVEC in the magnetized area transduced preferentially and almost all cells in the center of the magnetic field were transduced. We attempted to optimize the incubation time of the magnet-conjugated virus with target cells (Figure 5c). We incubated the cells with magnet-conjugated virus for 10 s, 1 and 10 min, and 1 h. We found that incubation for 10 s is sufficient to obtain maximal transduction efficiency. The levels of transgene expression did not decrease 10 days post-transduction and no obvious cytotoxicity was observed during this culture period.
Figure 5.
Gene transduction mediated by magnetic force. (a) HMVEC cultured in slide chambers with or without a magnet applied underneath were infected with viruses conjugated with streptavidin-coated magnet beads. EGFP expression was analysed by a fluorescent microscope at low (×7.5) and high (×63) magnification. The magnet was placed in the area indicated by the white dotted line. (b) EGFP expression was analysed by flow cytometry. (c) The effect of incubation time of magnet-conjugated virus with HMVEC on transduction efficiency. Magnet-conjugated viruses were incubated with HMVEC for a variety of time periods (10 s, 1 and 10 min, and 1 h) with application of a magnet. EGFP expression was analysed 4 days post-transduction. Percent EGFP-positive is shown as the mean ± SD of triplicate experiments
These results successfully demonstrate targeting via a magnetic force.
Discussion
The successful application of gene therapy would be greatly accelerated through the development of effective and specific vectors that can target and express therapeutic molecules and/or genes to specified cells, tissues and organs. We previously developed a targeting lentiviral vector, which, for the first time, allowed effective targeting through intravenous injection. A number of modifications to the pseudotype Sindbis virus envelope were necessary to maintain infectivity at the same time as decreasing nonspecific transduction through utilization of the native Sindbis virus receptors. This vector construct relied upon an embedded ZZ domain in the Sindbis envelope to conjugate with antibodies directed to specific cell surface molecules. However, the use of antibody conjugates limits application because serum immunoglobulin in immunocompetent animals can compete for binding of the target specific antibodies to the virions. In the present study, we took advantage of the high affinity between avidin and biotin to create targeting vectors that are not limited by potential competition with serum immunoglobulin. To achieve biotinylation of the virus, we introduced the bacterial BAP peptide into the Sindbis envelope. Biotinylation occurred during production of virus from cells and the biotinylated vector was then utilized to conjugate with avidin fusion molecules. We demonstrated the effectiveness and specificity of this technique using human and rat transferrin receptors as target cell surface molecules.
One further modification of the conjugates with avidin fusion proteins was to use avidin as a bridge between biotinylated virus and biotinylated ligands. Targeting vector was generated using a ‘sandwich’ technique, whereby neutravidin, a mutant form of avidin, was used to bridge between biotinylated virus and biotinylated ligand. One advantage of such an approach is to facilitate the formation of conjugates with the appropriate ligand in that the laborious construction of chimeric fusion molecules between avidin and the ligand molecule is not required. The ligands could simply be biotinylated and utilized with avidin as a bridge. We utilized neutravidin, a mutant form of avidin, bearing point mutations at N-glycan residues and charged amino acids [33]. This mutant form of avidin was shown to have less nonspecific interactions with charged cell surface molecules, such as heparan sulfate, as well as lectins present on many cell types that can interact with N-glycans. Because each ligand molecule is likely to have its own structural properties, the choice of whether to construct a fusion molecule or bridge with neutravidin is likely to depend empirically upon the nature of the ligand, the stability of the conjugates, and the transduction efficiency of the resulting virions.
One novel application of this targeting vector is the ability to utilize commercially available streptavidin-coated magnetic beads to form viral conjugates, which can then be directed with a magnetic field. There have been reports of clinical applications using magnets to direct chemotherapeutic reagents to liver tumors [34]. Other studies have utilized magnetic force to draw magnetic nanoparticle-loaded endothelial cells to the surface of steel stents transplanted in rat carotid arteries [35]. Another group delivered magnetic aerosol droplets to the lungs of mice, using an external magnetic field. Both studies demonstrated successful targeting [36]. Viruses and their components were also utilized for magnet-assisted targeted gene delivery in vivo. Iron oxide nanoparticles coated with hemagglutinating virus of Japan envelopes were targeted to the brains of mice by placing magnets on their heads [37]. Adenoviral vectors associated with magnetic nanoparticles were also successfully used to transduce to the stomachs of mice when an external magnetic field was applied to the stomach area [38]. Because these viruses and their components maintain their original tropisms, systemic injection of such vectors could still transduce untargeted organs [21,27,38]. Applications of magnetic targeting by viral vectors will be facilitated if the original tropisms of vectors are abrogated. The appropriate placement of a magnetic field could direct a targeting vector to a localized organ within the body.
The development of specific targeting vectors would facilitate the application of gene therapy for many diseases. In the future, targeting vectors that home to specific cells would allow earlier therapeutic intervention in the case of progressive diseases, such as cancer [39], or perhaps target residual cells in chronic and latent infections by infectious agents, such as HIV-1. The use of targeting vectors enhances safety of gene therapy by restricting the number of cells to only those that require therapeutic intervention [40]. The refinements described in the present study extend the utility of targeting vectors beyond that which we previously reported utilizing antibody conjugates. Further enhancements eventually may allow us to achieve our goal of specific targeting to localized tissues in order to effectively treat that disease within a diseased individual.
Acknowledgements
We thank Dr Micheal Barry for providing pSec BirA plasmid, Dr John Polo for providing antibody against Sinbdbis virus, Tomo-o Ishikawa for supporting magnetic transduction, and Rina Lee for manuscript preparation. This work was supported by US National Institute of Health grants, CA92194 (I.S.Y.C.), AI039975 (I.S.Y.C.), AI069350 (I.S.Y.C.), AI028697 (UCLA CFAR), CA107023 (M.L.P.), CA107023-02S1 (M.L.P.), CA057152-13S1 (M.L.P), and T32-CA009120 (M.L.P.).
References
- 1.Amado RG, Chen IS. Lentiviral vectors – the promise of gene therapy within reach? Science. 1999;285:674–676. doi: 10.1126/science.285.5428.674. [DOI] [PubMed] [Google Scholar]
- 2.Cavazzana-Calvo M, Hacein-Bey S, de Saint Basile G, et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science. 2000;288:669–672. doi: 10.1126/science.288.5466.669. [DOI] [PubMed] [Google Scholar]
- 3.Aiuti A, Slavin S, Aker M, et al. Correction of ADA-SCID by stem ell gene therapy combined with nonmyeloablative conditioning. Science. 2002;296:2410–2413. doi: 10.1126/science.1070104. [DOI] [PubMed] [Google Scholar]
- 4.Waehler R, Russell SJ, Curiel DT. Engineering targeted viral vectors for gene therapy. Nat Rev Genet. 2007;8:573–587. doi: 10.1038/nrg2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martin F, Chowdhury S, Neil S, et al. Envelope-targeted retrovirus vectors transduce melanoma xenografts but not spleen or liver. Mol Ther. 2002;5:269–274. doi: 10.1006/mthe.2002.0550. [DOI] [PubMed] [Google Scholar]
- 6.Kasahara N, Dozy AM, Kan YW. Tissue-specific targeting of retroviral vectors through ligand–receptor interactions. Science. 1994;266:1373–1376. doi: 10.1126/science.7973726. [DOI] [PubMed] [Google Scholar]
- 7.Marin M, Noel D, Valsesia-Wittman S, et al. Targeted infection of human cells via major histocompatibility complex class I molecules by Moloney murine leukemia virus-derived viruses displaying single-chain antibody fragment-envelope fusion proteins. J Virol. 1996;70:2957–2962. doi: 10.1128/jvi.70.5.2957-2962.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nilson BH, Morling FJ, Cosset FL, et al. Targeting of retroviral vectors through protease–substrate interactions. Gene Ther. 1996;3:280–286. [PubMed] [Google Scholar]
- 9.Somia NV, Zoppe M, Verma IM. Generation of targeted retroviral vectors by using single-chain variable fragment: an approach to in vivo gene delivery. Proc Natl Acad Sci USA. 1995;92:7570–7574. doi: 10.1073/pnas.92.16.7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valsesia-Wittmann S, Drynda A, Deleage G, et al. Modifications in the binding domain of avian retrovirus envelope protein to redirect the host range of retroviral vectors. J Virol. 1994;68:4609–4619. doi: 10.1128/jvi.68.7.4609-4619.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Funke S, Maisner A, Muhlebach MD, et al. Targeted cell entry of lentiviral vectors. Mol Ther. 2008;16:1427–1436. doi: 10.1038/mt.2008.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bupp K, Roth MJ. Targeting a retroviral vector in the absence of a known cell-targeting ligand. Hum Gene Ther. 2003;14:1557–1564. doi: 10.1089/104303403322495061. [DOI] [PubMed] [Google Scholar]
- 13.Boerger AL, Snitkovsky S, Young JA. Retroviral vectors preloaded with a viral receptor-ligand bridge protein are targeted to specific cell types. Proc Natl Acad Sci USA. 1999;96:9867–9872. doi: 10.1073/pnas.96.17.9867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roux P, Jeanteur P, Piechaczyk M. A versatile and potentially general approach to the targeting of specific cell types by retroviruses: application to the infection of human cells by means of major histocompatibility complex class I and class II antigens by mouse ecotropic murine leukemia virus-derived viruses. Proc Natl Acad Sci USA. 1989;86:9079–9083. doi: 10.1073/pnas.86.23.9079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morizono K, Bristol G, Xie YM, et al. Antibody-directed targeting of retroviral vectors via cell surface antigens. J Virol. 2001;75:8016–8020. doi: 10.1128/JVI.75.17.8016-8020.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morizono K, Ringpis GE, Pariente N, et al. Transient low pH treatment enhances infection of lentiviral vector pseudotypes with a targeting Sindbis envelope. Virology. 2006;355:71–81. doi: 10.1016/j.virol.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 17.Morizono K, Xie Y, Ringpis GE, et al. Lentiviral vector retargeting to P-glycoprotein on metastatic melanoma through intravenous injection. Nat Med. 2005;11:346–352. doi: 10.1038/nm1192. [DOI] [PubMed] [Google Scholar]
- 18.Pariente N, Morizono K, Virk MS, et al. A novel dual-targeted lentiviral vector leads to specific transduction of prostate cancer bone metastases in vivo after systemic administration. Mol Ther. 2007;15:1973–1981. doi: 10.1038/sj.mt.6300271. [DOI] [PubMed] [Google Scholar]
- 19.Yang L, Bailey L, Baltimore D, et al. Targeting lentiviral vectors to specific cell types in vivo. Proc Natl Acad Sci USA. 2006;103:11479–11484. doi: 10.1073/pnas.0604993103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laitinen OH, Hytonen VP, Nordlund HR, et al. Genetically engineered avidins and streptavidins. Cell Mol Life Sci. 2006;63:2992–3017. doi: 10.1007/s00018-006-6288-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stachler MD, Chen I, Ting AY, et al. Site-specific modification of AAV vector particles with biophysical probes and targeting ligands using biotin ligase. Mol Ther. 2008;16:1467–1473. doi: 10.1038/mt.2008.129. [DOI] [PubMed] [Google Scholar]
- 22.Kaikkonen MU, Viholainen JI, Narvanen A, et al. Targeting and purification of metabolically biotinylated baculovirus. Hum Gene Ther. 2008;19:589–600. doi: 10.1089/hum.2007.177. [DOI] [PubMed] [Google Scholar]
- 23.Pereboeva L, Komarova S, Roth J, et al. Targeting EGFR with metabolically biotinylated fiber-mosaic adenovirus. Gene Ther. 2007;14:627–637. doi: 10.1038/sj.gt.3302916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campos SK, Parrott MB, Barry MA. Avidin-based targeting and purification of a protein IX-modified, metabolically biotinylated adenoviral vector. Mol Ther. 2004;9:942–954. doi: 10.1016/j.ymthe.2004.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parrott MB, Adams KE, Mercier GT, et al. Metabolically biotinylated adenovirus for cell targeting, ligand screening, and vector purification. Mol Ther. 2003;8:688–700. doi: 10.1016/s1525-0016(03)00213-2. [DOI] [PubMed] [Google Scholar]
- 26.Waddington SN, McVey JH, Bhella D, et al. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell. 2008;132:397–409. doi: 10.1016/j.cell.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 27.Shayakhmetov DM, Gaggar A, Ni S, et al. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J Virol. 2005;79:7478–7491. doi: 10.1128/JVI.79.12.7478-7491.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perabo L, Goldnau D, White K, et al. Heparan sulfate proteoglycan binding properties of adeno-associated virus retargeting mutants and consequences for their in vivo tropism. J Virol. 2006;80:7265–7269. doi: 10.1128/JVI.00076-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parrott MB, Barry MA. Metabolic biotinylation of secreted and cell surface proteins from mammalian cells. Biochem Biophys Res Commun. 2001;281:993–1000. doi: 10.1006/bbrc.2001.4437. [DOI] [PubMed] [Google Scholar]
- 30.Penichet ML, Kang YS, Pardridge WM, et al. An antibody-avidin fusion protein specific for the transferrin receptor serves as a delivery vehicle for effective brain targeting: initial applications in anti-HIV antisense drug delivery to the brain. J Immunol. 1999;163:4421–4426. [PubMed] [Google Scholar]
- 31.Ng PP, Dela Cruz JS, Sorour DN, et al. An anti-transferrin receptor-avidin fusion protein exhibits both strong proapoptotic activity and the ability to deliver various molecules into cancer cells. Proc Natl Acad Sci USA. 2002;99:10706–10711. doi: 10.1073/pnas.162362999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klimstra WB, Williams JC, Ryman KD, et al. Targeting Sindbis virus-based vectors to Fc receptor-positive cell types. Virology. 2005;338:9–21. doi: 10.1016/j.virol.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 33.Marttila AT, Laitinen OH, Airenne KJ, et al. Recombinant NeutraLite avidin: a non-glycosylated, acidic mutant of chicken avidin that exhibits high affinity for biotin and low non-specific binding properties. FEBS Lett. 2000;467:31–36. doi: 10.1016/s0014-5793(00)01119-4. [DOI] [PubMed] [Google Scholar]
- 34.Hafeli UO. Magnetically modulated therapeutic systems. Int J Pharm. 2004;277:19–24. doi: 10.1016/j.ijpharm.2003.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Polyak B, Fishbein I, Chorny M, et al. High field gradient targeting of magnetic nanoparticle-loaded endothelial cells to the surfaces of steel stents. Proc Natl Acad Sci USA. 2008;105:698–703. doi: 10.1073/pnas.0708338105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dames P, Gleich B, Flemmer A, et al. Targeted delivery of magnetic aerosol droplets to the lung. Nat Nanotechnol. 2007;2:495–499. doi: 10.1038/nnano.2007.217. [DOI] [PubMed] [Google Scholar]
- 37.Flexman JA, Cross DJ, Lewellen BL, et al. Magnetically targeted viral envelopes: a PET investigation of initial biodistribution. IEEE Trans Nanobioscience. 2008;7:223–232. doi: 10.1109/TNB.2008.2002288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scherer F, Anton M, Schillinger U, et al. Magnetofection: enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther. 2002;9:102–109. doi: 10.1038/sj.gt.3301624. [DOI] [PubMed] [Google Scholar]
- 39.Burton JB, Johnson M, Sato M, et al. Adenovirus-mediated gene expression imaging to directly detect sentinel lymph node metastasis of prostate cancer. Nat Med. 2008;14:882–888. doi: 10.1038/nm.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hacein-Bey-Abina S, Von Kalle C, Schmidt M, et al. LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1. Science. 2003;302:415–419. doi: 10.1126/science.1088547. [DOI] [PubMed] [Google Scholar]