Abstract
Alpha cells are a type of ganglion cell whose morphology appears to be conserved across a number of mammalian retinas. In particular, alpha cells display the largest somata and dendritic arbors at a given eccentricity and tile the retina as independent on- (ON) and off-center (OFF) subtypes. Mammalian alpha cells also express a variable tracer coupling pattern, which often includes homologous (same cell type) coupling to a few neighboring alpha cells and extensive heterologous (different cell type) coupling to two to three amacrine cell types. Here, we use the gap junction-permeant tracer Neurobiotin to determine the architecture and coupling pattern of alpha cells in the mouse retina. We find that alpha cells show the same somatic and dendritic architecture described previously in the mammal. However, alpha cells show varied tracer coupling patterns related to their ON and OFF physiologies. ON alpha cells show no evidence of homologous tracer coupling but are coupled heterologously to at least two types of amacrine cell whose somata lie within the ganglion cell layer. In contrast, OFF alpha cells are coupled to one another in circumscribed arrays as well as to two to three types of amacrine cell with somata occupying the inner nuclear layer. We find that homologous coupling between OFF alpha cells is unaltered in the connexin36 (Cx36) knockout (KO) mouse retina, indicating that it is not dependent on Cx36. However, a subset of the heterologous coupling of ON alpha cells and all the heterologous coupling of OFF alpha cells are eliminated in the KO retina, suggesting that Cx36 comprises most of the junctions made with amacrine cells.
Indexing terms: connexin, gap junction, amacrine cell
The alpha cell is a morphological type of ganglion cell described first in the cat retina (Boycott and Wässle, 1974) and subsequently in a wide range of mammalian species (Peichl et al., 1987a,b). Alpha cells characteristically display the largest somata and dendritic fields of ganglion cells in the retina and occur as two subpopulations whose dendrites occupy either the inner or outer strata of the inner plexiform layer (IPL), corresponding to their on-(ON) or off-center (OFF) receptive field physiologies, respectively (Nelson et al., 1978; Bloomfield and Miller, 1986). Although typically less than 10% of all ganglion cells, each subpopulation of alpha cell forms a regular mosaic that economically covers the retina (Wässle et al., 1981). It is now clear that the alpha ganglion cells form the morphological correlate of the physiological Y-cells, which show brisk, transient light-evoked responses and nonlinear receptive fields (Cleland et al., 1975; Peichl and Wässle, 1981; Fukuda et al., 1984; Stanford and Sherman, 1984).
Another emerging property of alpha cells is their coupling pattern via gap junctions. In a number of mammals, alpha ganglion cells are coupled homologously to neighboring alpha cells as well as heterologously to at least two types of amacrine cell, suggesting a stereotypic coupling pattern conserved across species (Vaney, 1991; Dacey and Brace, 1992; Penn et al., 1994; Xin and Bloomfield, 1997). However, clear variations in the coupling pattern of alpha cells have been reported. In the rabbit, the OFF alpha cells show the stereotypic coupling pattern, whereas the ON cells are uncoupled (Hu and Bloomfield, 2003); it remains unclear whether this dichotomy occurs in other mammalian species as well. Over 20 years ago, Mastronarde (1983a–c) speculated that alpha cell electrical coupling serves to synchronize the spike activity of neighboring cells. This idea was recently verified by Hu and Bloomfield (2003), who showed that OFF alpha cells maintain correlated activity whereas ON cells do not, indicating that coupling is essential for synchronization of the discharges between neighboring alpha cells. In primates, Jacoby et al. (1996) reported gap junctions between amacrine cells and parasol cells (the homologues of alpha cells) but no junctions between parasol cells in serially reconstructed material. This suggested that alpha cells may not form gap junctions directly with one another, and so the apparent homologous tracer coupling could result from an alpha cell-to-amacrine cell-to-alpha cell circuit. In contrast, a recent study has reported gap junctions between the dendrites of putative alpha ganglion cells in the rat retina, suggesting a possible species difference (Hidaka et al., 2004). Furthermore, immunocytochemical data indicate that connexin36 (Cx36) is incorporated in the gap junctions formed between rat alpha cells, although it is unclear whether these subunits are expressed in the heterologous junctions as well (Hidaka et al., 2004)
Due to the relative ease of genetic manipulation, the mouse has recently become a favorite model for studying retinal physiology and pathology. However, our understanding of the organization of the wild-type mouse retina remains limited and continues to be based to a large extent on inferences from other mammalian species. Here, we describe experiments using the biotinylated tracer Neurobiotin to define clearly the morphology and coupling pattern of alpha cells in the mouse retina. In addition, we have taken advantage of the Cx36 knockout mouse retina to help identify the subunit structure of the underlying gap junctions. Our results indicate clear differences in the coupling pattern of ON and OFF alpha cells in the mouse retina. Whereas we find that both ON and OFF alpha cells are coupled to amacrine cells, the amacrine cell somata are segregated to the ganglion cell layer (GCL) or to the inner nuclear layer (INL), respectively. Moreover, whereas OFF alpha cells show homologous tracer coupling to one another, ON alpha cells do not. The coupling pattern in the Cx36 KO retina indicate that whereas most of the coupling between alpha cells and amacrine cells is dependent on Cx36, the alpha-to-alpha cell coupling is not.
MATERIALS AND METHODS
Preparation
Adult (P30 –90) wild-type and Cx36 knockout mice (Deans et al., 2001) were used for tracer injections. The mice were deeply anesthetized with an intraperitoneal injection of Nembutal (0.08 g/g body weight). Lidocaine hydrochloride (20 mg/ml) was applied locally to the eyelids and surrounding tissue. A flattened retinal-scleral preparation developed for the rabbit by Hu et al. (2000) was adopted and modified for the mouse. Briefly, the eye was removed under dim red illumination and hemisected anterior to the ora serrata. Anterior optics and the vitreous humor were removed, and the resultant retina-eyecup was placed in a superfusion chamber. Several radial incisions were made peripherally, allowing the eyecup to be flattened. The chamber was then mounted in a light-tight Faraday cage and superfused with an oxygenated mammalian Ringer’s solution (pH 7.4, 32°C; Bloomfield and Miller, 1982). Retinas were dark adapted for 1 hour prior to injections. All animal procedures were approved by the Institutional Animal Care and Use Committee at NYU School of Medicine.
Neurobiotin injections
Ganglion cells were visualized by using transscleral illumination with infrared (IR) light and impaled with standard borosilicate glass microelectrodes (Sutter Instrument Co., Novato, CA). The use of IR light allowed us to visualize cells while maintaining retinas in the dark-adapted state. Electrodes were filled at their tips with 4% N-(2-amino-ethyl)-biotinamide hydrochloride (Neurobiotin, Vector, Burlingame, CA), in 0.1 M Tris buffer (pH 7.6) and then backfilled with 4 M potassium chloride. Neurobiotin was iontophoresed into the neurons using a sinusoidal (3-Hz, 0.8-nA peak-to-peak) current for 15 minutes.
Histology
One hour after the labeling of the last cell in an experiment, the retina was fixed in a cold (4°C) solution of 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.3) overnight. Retinas were then washed in phosphate buffer and soaked in a solution of 0.18% hydrogen peroxide in methyl alcohol for an hour. This treatment completely abolished the endogenous peroxidase activity. Retinas were then washed in phosphate buffer and reacted with the Elite ABC kit (Vector) and 1% Triton X-100 in sodium phosphate-buffered saline (9% saline, pH 7.6). Retinas were subsequently processed for peroxidase histochemistry by using 3,3′-diaminobenzidine (DAB). Retinas were then dehydrated and flat mounted for light microscopy.
Alternatively, Neurobiotin injections in some retinas were visualized by using a Cy3-conjugated streptavidin reagent (Sigma, St. Louis, MO). Images of labeled neurons were captured by a cooled CCD camera (Spot 2, Diagnostic Instruments, Sterling Heights, MI) followed by software manipulation of brightness and contrast (Photoshop, Adobe Systems, San Jose, CA). Drawings of cells were made by using a camera lucida microscope attachment and then digitized by scanner.
To determine the level at which dendritic processes stratified in the IPL, we examined Neurobiotin-labeled cells in flat mount under a 100× oil-immersion lens. The borders of the IPL were determined by the location of amacrine and ganglion cell bodies by using Nomarski interference contrast optics. The position of the outer margin of the IPL next to the amacrine cell bodies was defined as 0% depth, whereas the vitreal border of the IPL was defined as 100% depth. The position of cellular processes in the IPL was thereby determined by using a precision micrometer and given a depth value of 0 –100%. Multiple measures were made for a single cell to elucidate any variations in stratifications throughout its extent.
RESULTS
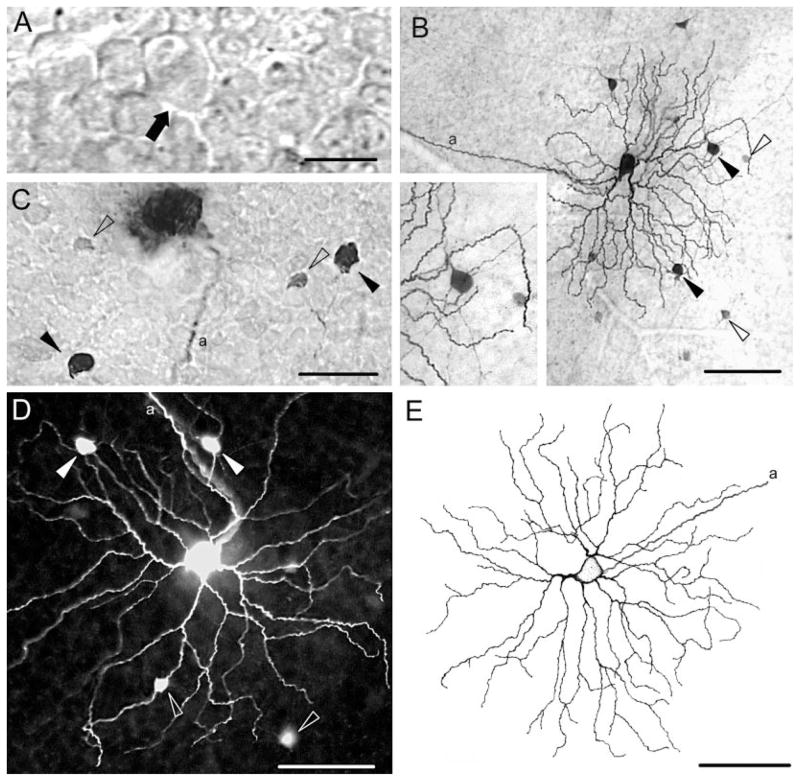
Under IR illumination, we were able to visualize the somata of neurons lying in the GCL of the living mouse retina (Fig. 1A). Because the object of our experiments was to study the morphology of alpha ganglion cells, we initially targeted the largest somata within a particular retinal area and injected them with Neurobiotin. Subsequent histology revealed that most (n = 59) of these neurons showed all the previously defined, soma-dendritic features of alpha ganglion cells in mammalian retinas, including rodents (Peichl et al., 1987b; Peichl, 1989; Penn et al., 1994; Huxlin and Goodchild, 1997; Sun et al., 2002a,b; Pang et al., 2003). These features included: 1) relatively large somata and dendritic fields; 2) stout primary dendrites; 3) dendrites with up to sixth-order radiate branching at acute angles; 4) rare overlap of dendrites; 5) relatively long terminal dendrites; and 6) a narrowly stratified arbor in either sublamina a or b of the inner plexiform layer (IPL).
Fig. 1.
Morphology and tracer coupling pattern of ON alpha cells in the wild-type mouse retina. A: Digital video showing the vitreal surface of a live mouse retina-eyecup preparation illuminated with transscleral infrared light. Outlines of somata in the ganglion cell layer are clearly visible. Arrow points to largest soma in the field, which was impaled with an intracellular electrode and injected with Neurobiotin. B: Neurobiotin-labeled ON alpha cells visualized with DAB histochemistry showing the basic dendritic architecture and tracer coupling patterns. Filled arrowheads indicate tracer-coupled amacrine cells with intensive Neurobiotin label and relatively large somata. Open arrowheads indicate tracer-coupled amacrine cells with lightly labeled, small somata. Inset shows an enlargement of the upper right corner of B detailing the morphology of large and small tracer-coupled amacrine cells. C: Tracer coupling pattern of an injected ON alpha cell in which lightly labeled amacrine cell somata (open arrowhead) are located more proximal to the ganglion cell body than the darkly labeled amacrine cells (black arrowheads). Conventions the same as in B. D: Tracer coupling pattern of an ON alpha cell injected with Neurobiotin and visualized with fluorescent (Cy3-conjugated streptavidine) histochemistry is similar to that seen with DAB histochemistry. Conventions the same as in B. E: Camera lucida drawing of an ON alpha ganglion cell. a, axonal process. Scale bar = 20 μm in A; 75 μm in B (40 μm for inset); 40 μm in C; 50 μm in D,E.
It should be noted that, in some cases, injected cells were found to have morphologies that differed from that of alpha ganglion cells. Although these ganglion cells also possessed large somata and dendritic arbors, they lacked most of the features described above and thus could be easily differentiated from alpha cells. Only those cells displaying the classic features of alpha cells were included in this study.
In initial studies, we recorded the light-evoked responses of alpha cells and, as expected from many prior studies (Bloomfield and Miller, 1986; Kolb and Nelson, 1993; Nelson et al., 1993), cells stratifying in sublamina a showed off-center receptive fields, whereas those stratifying in sublamina b displayed on-center responses. Because we did not record from all cells in this study, descriptions below of alpha cells as ON or OFF are based on the level of dendritic stratification in the IPL.
ON alpha ganglion cells
Morphology
ON alpha cells displayed large, spherical somata with diameters of 15–18 μm, depending on eccentricity (Fig. 1B–D). Primary dendrites were stout and smooth, whereas higher order branches showed slight waviness. Dendrites characteristically branched at acute angles and showed almost no overlap. Dendritic arbors displayed diameters of 175–200 μm and were unistratified within sublamina b at the 71–77% level of the IPL. The morphology of these ganglion cells coincides with that described previously for the ON alpha cells in mouse (Sun et al., 2002b; Pang et al., 2003) and other mammalian retinas (Peichl et al., 1987a,b; Peichl, 1989; Huxlin and Goodchild, 1997; Rockhill et al., 2002; Sun et al., 2002a).
Tracer coupling
The use of Neurobiotin enabled us to determine the coupling pattern of alpha cells, presumably via gap juctions. ON alpha cells (n = 17) were typically tracer coupled to 4–14 medium-sized cell bodies found mainly in the GCL but with a few occupying the proximal edge of the INL (Figs. 1B–D, 2). These tracer-coupled cells displayed a number of morphological features characteristic of amacrine cells. First, they showed relatively small somata compared with the ganglion cell subtypes described in the mouse retina (Sun et al., 2002b). Second, they showed very thin dendrites, including the primary branches (Fig. 1B, inset). Third, each dendritic branch ran a relatively long distance between branchpoints (Fig. 2B). Fourth, these cells showed no evidence of axons, which are typically visible, even in ganglion cells poorly labeled with Neurobiotin (Xin and Bloomfield, 1997). Finally, even though the tracer-coupled cells were incompletely labeled, their dendritic fields were still far larger than those of the neighboring alpha ganglion cells. Thus, ON alpha cells never showed homologous coupling to their alpha cell neighbors.
Fig. 2.
Details of the tracer coupling pattern of an ON alpha cell in the wild-type mouse retina. A: Camera lucida drawing shows the Neurobiotin-injected ON alpha cell from Figure 1B as well as the array of tracer-coupled amacrine cell somata. B: Same injection as in A; the dendritic architecture of coupled amacrine cells with large somata is highlighted. These amacrine cells typically showed two to three primary dendrites bifurcating relatively close to the soma at right angles. Note that the arbor of the coupled amacrine cell was not completely filled. The soma of the injected cell is depicted as an outline in the figure. C: Composite of drawings in A and B, comparing the arbors of the tracer-coupled amacrine cell processes (gray) and injected ON cell (black). a, axonal process. Scale bar = 100 μm in A (applies to A–C).
The difference in soma sizes and labeling intensity and the irregular arrays formed by the tracer-coupled neurons indicated that they included more than one type of amacrine cell (Figs. 1B–D, 2). One population of putative amacrine cells had small, asymmetric somata (6–8 μm in diameter) located in the GCL. These cells were typically very lightly labeled, making it difficult to visualize dendritic branches (Fig. 1B–D). The second population of amacrine cell was typically darker and displayed larger spherical somata (9–12 μm in diameter) that lay in the GCL (Fig. 1B–D). The relatively small size and weak labeling of one type was not a reflection of distance from the injected ON cell, as these cells were often found more proximal to the injected alpha cell body than their more darkly labeled counterparts (Fig. 1C).
The darkly labeled amacrine cells displayed two to four primary dendrites that typically bifurcated at right or obtuse angles within a relatively close distance to the soma (<100 μm) and showed no further branching (Fig. 2). The dendritic branches of well-labeled cells could often be followed for several hundred microns before gradually fading from view (Fig. 2). The dendrites of these amacrine cells were always found to co-stratify with the dendrites of the injected ON alpha cell. Although the complete dendritic arbor of these amacrine cells could not be visualized, their morphological characteristics were reminiscent of wide-field amacrine cells described previously in other mammalian species (Bloomfield, 1992; Dacheux and Raviola, 1995; MacNeil and Masland, 1998).
It should be mentioned that, on rare occasions, we could visualize two to four very lightly labeled amacrine cells in the GCL following injections of ON as well as OFF alpha cells in both wild-type and KO retinas. However, the somata of one of these lightly labeled cells were always juxtaposed to the injection site and therefore were probably labeled inadvertently by tracer leakage from the advancing microelectrode. The label may then have been passed to the few other cells via gap junctions. Therefore, we believe that these rarely seen, lightly labeled amacrines cells reflect a technical artifact. In contrast, the tracer-coupled amacrine cells (and ganglion cells reported below for OFF cells) we describe in detail were visualized in all injections.
OFF alpha ganglion cells
Morphology
Like the on-center subtype, OFF alpha cells (n = 42) displayed relatively large, spherical somata (15–18 μm in diameter) and stout, smooth primary dendrites (Fig. 3). However, in contrast to ON cells, the dendritic arbors of OFF alpha cells displayed more varicose branches that formed a denser and more asymmetric field. Their relatively large dendritic arbors (170 –190 μm in diameter) were unistratified within the 30 –38% level of the IPL, corresponding to part of sublamina a.
Fig. 3.
Morphology and tracer coupling patterns of Neurobiotin-injected OFF alpha ganglion cells in the wild-type mouse retina. A,B: OFF alpha cells were visualized with either DAB or fluorescent (Cy3-conjugated streptavidine) histochemistry, respectively. Both techniques revealed similar basic dendritic architecture and tracer coupling patterns. Asterisks mark cell bodies of homologously tracer-coupled OFF alpha ganglion cells. Filled arrowheads indicate tracer-coupled amacrine cells with intensive label and large cell bodies. Open arrowheads indicate lightly labeled amacrine cells with small cell bodies. C: Camera lucida drawing of an OFF alpha ganglion cell showing the asymmetry in its dendritic arbor. D: Photomicrograph showing long dendritic processes (black arrowhead) of amacrine cells that are heterologously tracer-coupled to the injected OFF alpha cell. E–G: Photomicrograph enlargements showing details of somata and primary dendrites, intermediate dendrites, and terminal endings, respectively, of heterologously tracer-coupled amacrine cells. a, axonal process. Scale bar = 50 μm in A,C,E–G; 75 μm in B,D.
Tracer coupling
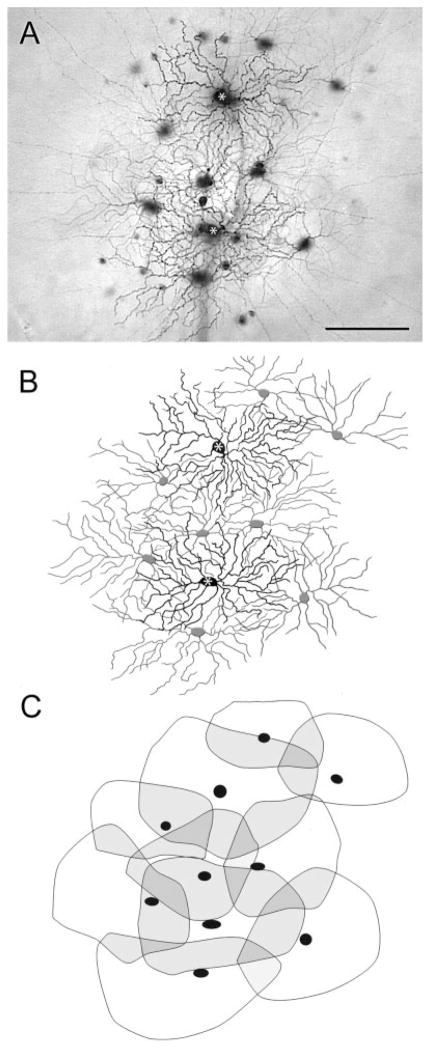
OFF alpha cells showed a more complex tracer coupling pattern than that of ON alpha cells (Figs. 3–5). They were typically coupled to a symmetrical group of three to eight well-labeled, neighboring ganglion cells. The soma-dendritic morphology of these neighbors was identical to that of the injected cell, suggesting homologous coupling between neighboring OFF alpha cells. Dendrites of neighboring cells showed 30 –50% overlap of their arbors (Figs. 4, 5), comparable to that described for alpha cell arrays in cat (Wässle et al., 1981), rabbit (Peichl et al., 1987a), and rat (Peichl, 1989) retinas. This extensive overlap resulted in large retinal areas covered by two to three alpha cell arbors and only very small areas covered by only a single cell (Fig. 5C). Interestingly, the coupled array of neighboring alpha cells was typically skewed to the side on which the dendritic arbor of the injected cell lay (Fig. 4B). This coupling asymmetry was detected in even the best labeled specimens, indicating that it was not an artifact of incomplete labeling. This finding suggests not only an asymmetry in the arbors of OFF alpha cells but also a corresponding anisotropy in the local coupled network they form.
Fig. 5.
Details of the dendritic arbors of an array of coupled OFF alpha cells following injection of Neurobiotin into a pair of second-tier neighbors. A: Photomicrograph showing the overlapping arrays of coupled OFF alpha ganglion cell bodies focusing on the dendritic processes in the IPL. The somata of the injected OFF cells are indicated by asterisks. B: Drawing of the same coupled arrays detailing the arbors of the two injected OFF alpha cells (black; asterisks indicate somata) and those of the homologously tracer-coupled ganglion cells (gray). C: Arbors of labeled alpha cells in the arrays are outlined to show overlap of dendritic territories. Lightly shaded areas represent territories covered by the dendritic arbors of two OFF alpha cells. Darkly shaded areas indicate territories covered by overlapping arbors of three OFF alpha cells. Scale bar = 100 μm in A.
Fig. 4.
Series of camera lucida drawings showing details of the tracer-coupled array of cells following injection of Neurobiotin into a single OFF alpha ganglion cell. A: Complex meshwork of dendritic processes of tracer-coupled amacrine cells and ganglion cells. B: Same tracer-coupled array as in A showing the morphology of the injected OFF alpha cell (black) and that of four tracer-coupled ganglion cells (gray). C: Drawing of the same array as in previous panels detailing the dendritic arbors of heterologously tracer-coupled amacrine cells with large somata (black). Coupled alpha cells are in light gray. D: Drawing of the completely labeled arbor of a tracer-coupled amacrine cell with large soma. This wide-field amacrine cell displays long, straight radially running dendrites that ended several hundred microns from the cell body. Scale bar = 100 μm in A (applies to A–C); 200 μm for D.
OFF alpha cells also showed heterologous coupling to two populations of cells with somata lying within the proximal border of the INL (Figs. 3, 4). Based on the criteria described above for ON cells, we conclude that these tracer-coupled neurons are amacrine cells. One type had relatively small, round cell bodies (6–8 μm in diameter) in the proximal INL, but due to weak labeling their dendrites could not be visualized. The other subtype had larger, spherical somata (8–11 μm) located in the INL. This subtype was darkly labeled, allowing for visualization of nearly the entire dendritic arbor (Figs. 3, 4). These cells displayed two to three very thin primary dendrites that branched proximally up to two times before emitting continuous distal branches running several hundred microns before forming hook-like terminals (Figs. 3G, 4D). Processes of these wide-field amacrine cells largely co-stratified with OFF alpha cell dendrites, but they occasionally meandered vertically within the 20–60% levels of the IPL. Overall, the soma-dendritic morphology of these wide-field amacrine cells differed from those coupled to ON alpha cells.
Cx36 KO mouse retina
It has been shown that Cx36 comprises gap junctions found throughout the mammalian retina. Although Cx36 has been localized to AII amacrine cell dendrites and bipolar cell axon terminals (Feigenspan et al., 2001; Güldenagel et al., 2001; Mills et al., 2001; Deans et al., 2002), its wide distribution throughout the IPL suggests that Cx36 may comprise other gap junctions as well. Indeed, Cx36 has been reported to comprise the homologous gap junctions found between alpha cells in the rat retina (Hidaka et al., 2004). To test whether Cx36 is expressed in alpha cell gap junctions in the mouse, we compared the tracer-coupling pattern in the wild-type retina described above with that found in the Cx36 KO mouse retina.
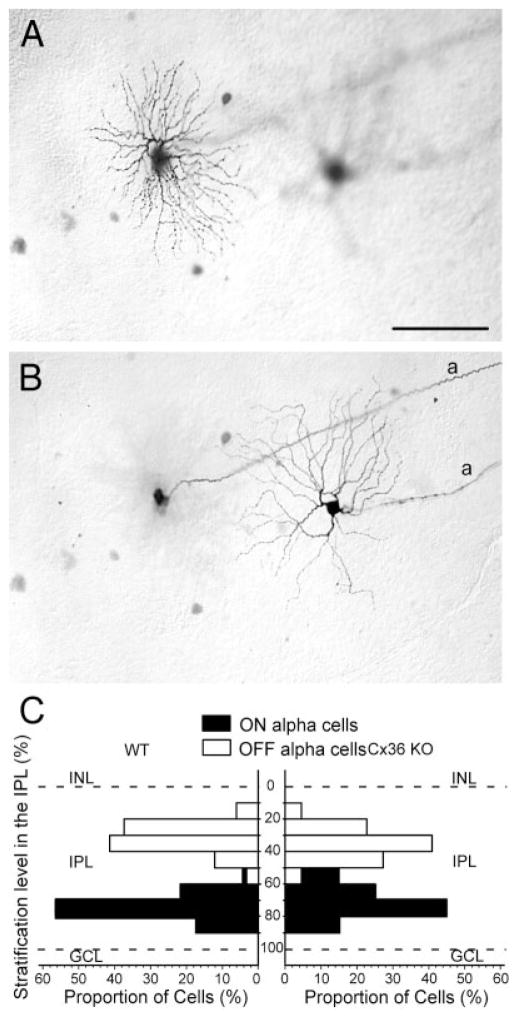
As in the wild type, injection of Neurobiotin into large ganglion cell bodies in the Cx36 KO mouse retina most often produced labeled ganglion cells with soma-dendritic morphology corresponding to that of alpha cells (Figs. 6, 7). Furthermore, we found that dendrites of these cells in the KO retina stratified at exactly the same levels of the IPL as observed for alpha cells in the wild type (Fig. 6C). These data indicate that the basic morphology of alpha ganglion cells is conserved in the Cx36 KO retina.
Fig. 6.
Dendritic architecture of ON and OFF alpha ganglion cells is preserved in the Cx36 KO retina. A,B: Photomicrographs showing two planes of focus on the arbors of an OFF (left) and an ON (right) alpha ganglion cell illustrating the different levels of stratification in sublaminae a and b, respectively. a, axonal process. C: Histogram comparing the level of inner plexiform layer (IPL) stratification of dendrites of ON and OFF alpha ganglion cells in the Cx36 KO and wild-type (WT) mouse retinas. Dashed lines represent the vitreal (100%) and scleral (0%) borders of the IPL. INL, inner nuclear layer. Scale bar = 100 μm in A (applies to A,B).
Fig. 7.
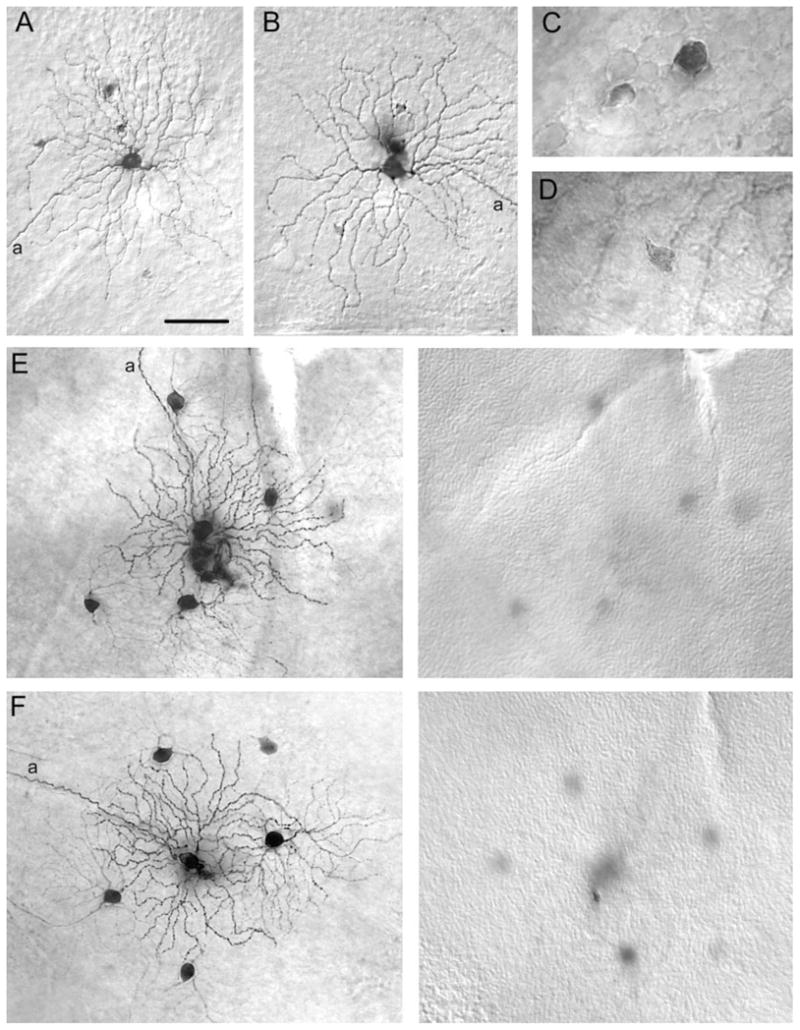
Tracer-coupling pattern of changes in Neurobiotin-injected alpha cells in the Cx36 KO animals. A,B: Photomicrographs showing that ON alpha cells in the Cx36 KO retina have heterologous tracer coupling to only a few amacrine cells with small, lightly labeled somata. C: High-magnification micrograph showing the somata of the two subtypes of amacrine cell tracer coupled to ON alpha cells in the wild-type retina. D: High magnification of soma of an amacrine cell tracer coupled to an ON alpha cell in the Cx36 KO retina. The size, shape, and labeling intensity of this cell is very similar to that of the lightly labeled amacrine cell in C. E,F: Photomicrographs of pairs of Neurobiotin-injected OFF alpha cells. Left panels show arbors of injected and tracer-coupled alpha cells with somata overlaid for clarity. Right panels illustrate plane of focus on the amacrine cell layer in the proximal edge of the INL. Whereas homologous coupling of OFF alpha cells is evident in the Cx36 KO retina, all coupling to amacrine cells is eliminated. a, axonal process. Scale bar = 75 μm in A (applies to A,B,E,F); 20 μm for C,D.
In contrast, we found that the tracer-coupling pattern of ON alpha cells (n = 13) was significantly changed in the Cx36 KO mouse. In contrast to wild-type retinas, ON alpha cells in the KO displayed only a few lightly labeled amacrine cell somata laying within the INL (Fig. 7A,B). Due to the poor labeling of these coupled cells, we were unable to visualize their dendrites. However, the size, shape, and location of their cell bodies, together with their light labeling, was very similar to that found for the first subtype of tracer-coupled amacrine cell seen in wild-type retinas (Fig. 7C,D). In contrast, the large, well-labeled amacrine cells found coupled to ON alpha cells in wild-type retinas were never seen in the Cx36 KO (Fig. 7A–D).
Homologous tracer coupling between neighboring OFF alpha cells (n = 18) appeared to be unaffected in the Cx36 KO retina (Fig. 7E,F). Groups of well-labeled, coupled alpha cells were seen in the KO retinas just as described above in the wild type. In contrast, heterologous tracer coupling between OFF alpha cells and the two types of amacrine cell commonly found in control retinas was completely missing in the Cx36 KO mouse retina. That is, neither the small amacrine cells nor the large wide-field amacrine cells could be detected in the inner retinas of Cx36 KO animals (Fig. 7E,F). Overall, these data indicate that heterologous coupling of OFF alpha cells is eliminated in the Cx36 KO mouse retina.
DISCUSSION
Alpha ganglion cell morphology is conserved in the mouse retina
Our strategy of performing Neurobiotin tracer injections into the largest cell bodies in the ganglion cell layer to yield labeled alpha cells actually produced a heterogenous population of ganglion cells including a number of different subtypes. However, the majority of these cells showed the stereotypic soma-dendritic architecture of mammalian alpha cells, which included stout primary dendrites, up to sixth-order radiate branching at acute angles, rare overlap of dendrites, and a narrow stratification of the dendritic arbor in either sublamina a or b of the IPL (Boycott and Wassle, 1974; Peichl et al., 1987a,b; Peichl, 1989; Huxlin and Goodchild, 1997; Sun et al., 2002a). Our data thus indicate that the stereotypic ON and OFF alpha cell morphologies occur in the mouse retina, confirming recent studies of mouse retinal anatomy using different cell labeling techniques (Sun et al., 2002b; Pang et al., 2003).
The remainder of the ganglion cells labeled in our study showed soma-dendritic and axonal dimensions on the scale of alpha cells, but their architecture was clearly different. These cells resembled either “giant” cells (Bunt, 1976; Huxlin and Goodchild, 1997; Sun et al., 2002a,b) or delta cells (Peichl, 1989) described in the rodent retina. Thus, as suggested previously by Peichl (1987a, 1989) for a number of mammalian retinas, our results indicate that the soma sizes of alpha cells in the mouse overlap extensively with other large ganglion cell types. Thus, these data indicate that unequivocal identification of alpha cells in the mouse cannot be made based strictly on soma morphology but requires labeling and visualization of dendritic architecture.
Tracer coupling pattern of alpha ganglion cells
Prior studies have reported a tracer coupling pattern for alpha cells in a number of species consisting of limited homologous coupling to a ring of alpha-cell nearest neighbors and a more extensive heterologous coupling to two to three amacrine cell types (Vaney, 1991, 1994; Dacey and Brace, 1992; Penn et al., 1994; Ghosh et al., 1996; Huxlin and Goodchild, 1997; Xin and Bloomfield, 1997; Hu and Bloomfield, 2003). Interestingly, Hu and Bloomfield (2003) showed that only OFF alpha cells in the rabbit retina show this coupling pattern, whereas ON cells are uncoupled. We also found a difference in the coupling pattern of ON and OFF alpha cells in the mouse. Whereas OFF alpha cells showed the stereotypic homologous and heterologous pattern expressed in other species, the ON cells in mouse showed only heterologous coupling. These findings are consistent with a recent report by Schubert et al. (2005).
In primate, the parasol cells are thought to be the homologue of the alpha ganglion cells in other species (Leventhal et al., 1981; Rodieck et al., 1985; Dacey and Brace, 1992). Interestingly, whereas both ON and OFF parasol cells show the typical mixed homologous/heterologous tracer coupling pattern displayed by other alpha cells (Dacey and Brace, 1992), Jacoby et al. (1996) reported that ON parasol ganglion expressed gap junctions with amacrine cells but found no evidence for direct junctions with each other. Taken together, these results across a number of mammalian species suggest that whereas OFF alpha cells show a stereotypic coupling pattern, the ON alpha cells express more interspecies variability in their tracer coupling patterns and the underlying distribution of gap junctions. It is interesting to note, however, that the homology of parasol cells with alpha cells has recently been called into question with the discovery of a new large, monostratified ganglion cell type in the primate (Dacey, 2004; Yamada et al., 2005). It will of course be of interest to determine the coupling pattern of this newly discovered ganglion cell.
Role of Cx36 in alpha cell gap junctions
Our data suggest a complexity in the distribution of Cx36 in alpha cell gap junctions and the amacrine cells to which they are coupled. Tracer labeling of neighboring OFF alpha cells was equally robust in KO and wild-type retinas. In contrast, OFF alpha cell-to-amacrine cell coupling was lost in the KO retina. ON alpha cell-to-amacrine cell coupling was also affected in the Cx36 KO, but, whereas coupling to the large amacrine cell type was completely lost, coupling to the small amacrine cells was unaffected. Taken together, our results suggest that whereas most coupling between alpha and amacrine cells is dependent on Cx36, alpha-to-alpha cell coupling is not.
Our finding that alpha-to-alpha cell coupling is not dependent on Cx36 appears to conflict with recent immunocytochemical studies reporting Cx36 expression in alpha ganglion cells in both rat (Hidaka et al., 2004) and mouse (Schubert et al., 2005) retinas. However, this discrepancy could reflect the presence of an additional connexin in alpha cell junctions that provides possibly redundant functionality. In favor of this idea, many gap junctions contain multiple connexins (Altevogt and Paul, 2004), and additional connexins are expressed in the mammalian retina (Deans and Paul, 2001; Guldenagel et al., 2000). In this model, OFF alpha cells express Cx36 and at least one additional connexin, which allows the maintenance of homologous coupling in the Cx36 KO, whereas the gap junctions that couple OFF alpha cells to amacrine cells express Cx36. Multiple connexin expression in ON alpha cells would explain the differential loss and retention of coupling with the two amacrine cell types in the KO.
Our results are in clear disagreement with a very recent study by Schubert et al. (2005), who found that both homologous and heterologous coupling of alpha cells is lost in a different Cx36 KO mouse model. This conflict may reflect some disparity in the retina of the KO strain used by Schubert et al. from the one we employed. Another possibility is that they relied on fluorescent histology to visualize coupling, whereas we used a more sensitive peroxidase amplification reaction. In any event, further work is called for to reconcile this discrepancy.
The retention of coupling between OFF alpha cells in the KO when all OFF alpha cell-to- amacrine cell coupling is lost strongly suggests that murine OFF alpha cells establish gap junctions with each other directly. These data are thus consistent with the observation of dendro-dendritic junctions between alpha cells in the rat (Hidaka et al., 2004).
Finally, it has been suggested that the homologous coupling between alpha cell neighbors underlies short-latency synchrony of impulse activity, whereas the heterologous coupling between alpha and amacrine cells results in a broader, correlated activity (Mastronarde, 1983a, b–c; Meister et al., 1995; Brivanlou et al., 1998; DeVries, 1999; Meister and Berry, 1999; Hu and Bloomfield, 2003). Our data show that, in combination, alpha cells in the wild-type and Cx36 KO retinas express mixed homologous/heterologous, pure homologous, or pure heterologous coupling. Thus, the mouse retina should serve as an important experimental model to examine the functional roles played by the different combinations of gap junctions expressed by amacrine and ganglion cells in the inner mammalian retina.
Acknowledgments
National Institutes of Health; Grant number: EY07360 (to S.A.B.); Grant number: GM37751; Grant number: EY14127 (to D.L.P.); Grant sponsor: Research to Prevent Blindness, Inc. (unrestricted grant to the Department of Ophthalmology, New York University School of Medicine).
LITERATURE CITED
- Altevogt BM, Paul DL. Four classes of intercellular channels between glial cells in the CNS. J Neurosci. 2004;24:4313–4323. doi: 10.1523/JNEUROSCI.3303-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield SA. Relationship between receptive and dendritic field size of amacrine cells in the rabbit retina. J Neurophysiol. 1992;68:711–725. doi: 10.1152/jn.1992.68.3.711. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA, Miller RF. A physiological and morphological study of the horizontal cell types of the rabbit retina. J Comp Neurol. 1982;208:288–303. doi: 10.1002/cne.902080306. [DOI] [PubMed] [Google Scholar]
- Bloomfield SA, Miller RF. A functional organization of ON and OFF pathways in the rabbit retina. J Neurosci. 1986;6:1–13. doi: 10.1523/JNEUROSCI.06-01-00001.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boycott BB, Wässle H. The morphological types of ganglion cells of the domestic cat’s retina. J Physiol Lond. 1974;286:397–419. doi: 10.1113/jphysiol.1974.sp010616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brivanlou IH, Warland DK, Meister M. Mechanisms of concerted firing among retinal ganglion cells. Neuron. 1998;20:527–539. doi: 10.1016/s0896-6273(00)80992-7. [DOI] [PubMed] [Google Scholar]
- Bunt AH. Ramification patterns of ganglion cell dendrites in the retina of the albino rat. Brain Res. 1976;103:1–8. doi: 10.1016/0006-8993(76)90682-x. [DOI] [PubMed] [Google Scholar]
- Cleland BG, Levick WR, Wässle H. Physiological identification of a morphological class of cat retinal ganglion cells. J Physiol. 1975;248:151–171. doi: 10.1113/jphysiol.1975.sp010967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dacey DM. Origins of perception: retinal ganglion cell diversity and the creation of parallel visual pathways. In: Gazzaniga MS, editor. The cognitive neurosciences III. Cambridge, MA: MIT Press; 2004. pp. 281–301. [Google Scholar]
- Dacey DM, Brace S. A coupled network for parasol but not midget ganglion cells in the primate retina. Vis Neurosci. 1992;9:279–290. doi: 10.1017/s0952523800010695. [DOI] [PubMed] [Google Scholar]
- Dacheux RF, Raviola E. Light responses from one type of ON-OFF amacrine cells in the rabbit retina. J Neurophysiol. 1995;74:2460–2468. doi: 10.1152/jn.1995.74.6.2460. [DOI] [PubMed] [Google Scholar]
- Deans MR, Paul DL. Mouse horizontal cells do not express connexin26 or connexin36. Cell Commun Adhes. 2001;8:361–366. doi: 10.3109/15419060109080754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deans MR, Gibson JR, Sellitto C, Connors BW, Paul DL. Synchronous activity of inhibitory networks in neocortex requires electrical synapses containing connexin36. Neuron. 2001;31:477–85. doi: 10.1016/s0896-6273(01)00373-7. [DOI] [PubMed] [Google Scholar]
- Deans MR, Völgyi B, Goodenough DA, Bloomfield SA, Paul DL. Connexin36 is essential for transmission of rod-mediated visual signals in the mammalian retina. Neuron. 2002;36:1–20. doi: 10.1016/s0896-6273(02)01046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries SH. Correlated firing in rabbit retinal ganglion cells. J Neurophysiol. 1999;81:908–920. doi: 10.1152/jn.1999.81.2.908. [DOI] [PubMed] [Google Scholar]
- Feigenspan A, Teubner B, Willecke K, Weiler R. Expression of neuronal connexin36 in AII amacrine cells of the mammalian retina. J Neurosci. 2001;21:230–239. doi: 10.1523/JNEUROSCI.21-01-00230.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda Y, Hsiao CF, Watanabe M, Ito H. Morphological correlates of physiologically identified Y-, X-, and W-cells in cat retina. J Neurophysiol. 1984;52:999–1013. doi: 10.1152/jn.1984.52.6.999. [DOI] [PubMed] [Google Scholar]
- Ghosh KK, Goodchild AK, Sefton AE, Martin PR. Morphology of retinal ganglion cells in a new world monkey, the marmoset Callithrix jacchus. J Comp Neurol. 1996;366:76–92. doi: 10.1002/(SICI)1096-9861(19960226)366:1<76::AID-CNE6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Güldenagel M, Ammermüller J, Feigenspan A, Teubner B, Degen J, Söhl G, Willecke K, Weiler R. Visual transmission deficits in mice with targeted disruption of the gap junction gene connexin36. J Neurosci. 2001;21:6036–6044. doi: 10.1523/JNEUROSCI.21-16-06036.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güldenagel M, Sohl G, Plum A, Traub O, Teubner B, Weiler R, Willecke K. Expression patterns of connexin genes in mouse retina. J Comp Neurol. 2000;425:193–201. [PubMed] [Google Scholar]
- Hidaka S, Akahori Y, Kurosawa Y. Dendrodendritic electrical synapses between mammalian retinal ganglion cells. J Neurosci. 2004;24:10553–10567. doi: 10.1523/JNEUROSCI.3319-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu EH, Bloomfield SA. Gap junctional coupling underlies the short-latency spike synchrony of retinal α ganglion cells. J Neurosci. 2003;23:6768–6777. doi: 10.1523/JNEUROSCI.23-17-06768.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu EH, Dacheux RF, Bloomfield SA. A flattened retina-eyecup preparation suitable for electrophysiological studies of neurons visualized with trans-scleral infrared illumination. J Neurosci Methods. 2000;103:209–216. doi: 10.1016/s0165-0270(00)00319-8. [DOI] [PubMed] [Google Scholar]
- Huxlin KR, Goodchild K. Retinal ganglion cells in the albino rat: revised morphological classification. J Comp Neurol. 1997;385:309–323. [PubMed] [Google Scholar]
- Jacoby R, Stafford D, Kouyama N, Marshak D. Synaptic inputs to ON parasol ganglion cells in the primate retina. J Neurosci. 1996;16:8041–8056. doi: 10.1523/JNEUROSCI.16-24-08041.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb H, Nelson R. OFF-alpha and OFF-beta ganglion cells in cat retina: II. Neural circuitry as revealed by electron microscopy of HRP stains. J Comp Neurol. 1993;329:85–110. doi: 10.1002/cne.903290107. [DOI] [PubMed] [Google Scholar]
- Leventhal AG, Rodieck RW, Dreher B. Retinal ganglion cell classes in the Old World monkey: morphology and central projections. Science. 1981;213:1139–1142. doi: 10.1126/science.7268423. [DOI] [PubMed] [Google Scholar]
- MacNeil MA, Masland RH. Extreme diversity among amacrine cells: implications for function. Neuron. 1998;20:971–982. doi: 10.1016/s0896-6273(00)80478-x. [DOI] [PubMed] [Google Scholar]
- Mastronarde DN. Correlated firing of cat retinal ganglion cells. I. Spontaneously active inputs to X- and Y-cells. J Neurophysiol. 1983a;49:303–324. doi: 10.1152/jn.1983.49.2.303. [DOI] [PubMed] [Google Scholar]
- Mastronarde DN. Correlated firing of cat retinal ganglion cells. II. Responses of X- and Y-cells to single quantal events. J Neurophysiol. 1983b;49:325–349. doi: 10.1152/jn.1983.49.2.325. [DOI] [PubMed] [Google Scholar]
- Mastronarde DN. Interactions between ganglion cells in the cat retina. J Neurophysiol. 1983c;49:350–365. doi: 10.1152/jn.1983.49.2.350. [DOI] [PubMed] [Google Scholar]
- Meister M, Berry MJ. The neuronal code of the retina. Neuron. 1999;22:435–450. doi: 10.1016/s0896-6273(00)80700-x. [DOI] [PubMed] [Google Scholar]
- Meister M, Legnado L, Baylor DA. Correlated signaling by retinal ganglion cells. Science. 1995;270:1207–1210. doi: 10.1126/science.270.5239.1207. [DOI] [PubMed] [Google Scholar]
- Mills SL, O’Brien JJ, Li W, O’Brien J, Massey SC. Rod pathways in the mammalian retina use connexin 36. J Comp Neurol. 2001;436:336–350. [PMC free article] [PubMed] [Google Scholar]
- Nelson R, Famiglietti EV, Kolb H. Intracellular staining reveals different levels of stratification for on- and off-center ganglion cells in cat retina. J Neurophysiol. 1978;41:472–483. doi: 10.1152/jn.1978.41.2.472. [DOI] [PubMed] [Google Scholar]
- Nelson R, Kolb H, Freed MA. OFF-alpha and OFF-beta ganglion cells in cat retina. I: Intracellular electrophysiology and HRP stains. J Comp Neurol. 1993;329:68–84. doi: 10.1002/cne.903290106. [DOI] [PubMed] [Google Scholar]
- Pang J-J, Gao F, Wu SM. Light-evoked excitatory and inhibitory synaptic inputs to ON and OFF α ganglion cells. J Neurosci. 2003;23:6063–6073. doi: 10.1523/JNEUROSCI.23-14-06063.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peichl L. Alpha and delta ganglion cells in the rat retina. J Comp Neurol. 1989;286:120–139. doi: 10.1002/cne.902860108. [DOI] [PubMed] [Google Scholar]
- Peichl L, Wässle H. Morphological identification of on- and off-centre brisk transient (Y) cells in the cat retina. Proc R Soc Lond B Biol Sci. 1981;212:139–153. doi: 10.1098/rspb.1981.0030. [DOI] [PubMed] [Google Scholar]
- Peichl L, Buhl EH, Boycott BB. Alpha ganglion cells in the rabbit retina. J Comp Neurol. 1987a;263:25–41. doi: 10.1002/cne.902630103. [DOI] [PubMed] [Google Scholar]
- Peichl L, Ott H, Boycott BB. Alpha ganglion cells in mammalian retinae. Proc R Soc Lond B. 1987b;231:169–197. doi: 10.1098/rspb.1987.0040. [DOI] [PubMed] [Google Scholar]
- Penn AA, Wong RO, Shatz CJ. Neuronal coupling in the developing mammalian retina. J Neurosci. 1994;14:3805–3815. doi: 10.1523/JNEUROSCI.14-06-03805.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockhill RL, Daly FJ, MacNeil MA, Brown SP, Masland RH. The diversity of ganglion cells in a mammalian retina. J Neurosci. 2002;22:3831–3843. doi: 10.1523/JNEUROSCI.22-09-03831.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodieck RW, Binmoeller KF, Dineen J. Parasol and midget ganglion cells of the human retina. J Comp Neurol. 1985;233:115–132. doi: 10.1002/cne.902330107. [DOI] [PubMed] [Google Scholar]
- Schubert T, Degen J, Willwcke K, Hormuzdi SG, Monyer H, Weiler R. Connexin36 mediates gap junctional coupling of alpha-ganglion cells in mouse retina. J Comp Neurol. 2005;485:191–201. doi: 10.1002/cne.20510. [DOI] [PubMed] [Google Scholar]
- Stanford LR, Sherman SM. Structure/function relationships of retinal ganglion cells in the cat. Brain Res. 1984;297:381–386. doi: 10.1016/0006-8993(84)90580-8. [DOI] [PubMed] [Google Scholar]
- Sun W, Li X, He S. Large-scale morphological survey of rat retinal ganglion cells. Vis Neurosci. 2002a;19:483–493. doi: 10.1017/s0952523802194107. [DOI] [PubMed] [Google Scholar]
- Sun W, Li X, He S. Large-scale morphological survey of mouse retinal ganglion cells. J Comp Neurol. 2002b;451:115–126. doi: 10.1002/cne.10323. [DOI] [PubMed] [Google Scholar]
- Vaney DI. Many diverse types of retinal neurons show tracer coupling when injected with biocytin or Neurobiotin. Neurosci Lett. 1991;125:187–190. doi: 10.1016/0304-3940(91)90024-n. [DOI] [PubMed] [Google Scholar]
- Vaney DI. Territorial organization of direction-selective ganglion cells in rabbit retina. J Neurosci. 1994;14:6301–6316. doi: 10.1523/JNEUROSCI.14-11-06301.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wässle H, Peichl L, Boycott BB. Dendritic territories of cat retinal ganglion cells. Nature. 1981;292:344–345. doi: 10.1038/292344a0. [DOI] [PubMed] [Google Scholar]
- Xin D, Bloomfield SA. Tracer coupling pattern of amacrine and ganglion cells in the rabbit retina. J Comp Neurol. 1997;383:512–528. doi: 10.1002/(sici)1096-9861(19970714)383:4<512::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Yamada ES, Bordt AS, Marshak DW. Wide field ganglion cells in macaque retina. Vis Neurosci. 2005 doi: 10.1017/S095252380522401X. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]