Abstract
Background
Expression of insulin-like growth factor binding protein 5 (IGFBP5) is strongly induced upon activation of hepatic stellate cells and their transdifferentiation into myofibroblasts in vitro. This was confirmed in vivo in an animal model of liver fibrosis. Since IGFBP5 has been shown to promote fibrosis in other tissues, the aim of this study was to investigate its role in the progression of liver fibrosis.
Methods
The effect of IGFBP5 was studied in LX2 cells, a model for partially activated hepatic stellate cells, and in human primary liver myofibroblasts. IGFBP5 signalling was modulated by the addition of recombinant protein, by lentiviral overexpression, and by siRNA mediated silencing. Furthermore, the addition of IGF1 and silencing of the IGF1R was used to investigate the role of the IGF-axis in IGFBP5 mediated effects.
Results
IGFBP5 enhanced the survival of LX2 cells and myofibroblasts via a >50% suppression of apoptosis. This effect of IGFBP5 was not modulated by the addition of IGF1, nor by silencing of the IGF1R. Additionally, IGFBP5 was able to enhance the expression of established pro-fibrotic markers, such as collagen Iα1, TIMP1 and MMP1.
Conclusion
IGFBP5 enhances the survival of (partially) activated hepatic stellate cells and myofibroblasts by lowering apoptosis via an IGF1-independent mechanism, and enhances the expression of profibrotic genes. Its lowered expression may, therefore, reduce the progression of liver fibrosis.
Background
The extensive accumulation of extracellular matrix (ECM) produced by activated hepatic stellate cells (HSC), which normally reside in the space of Disse as the major vitamin A storage site, is a hallmark of liver fibrosis [1,2]. Liver damage induces HSC activation and, upon repeated and/or chronic injury, they transdifferentiate into myofibroblast-like cells [1,3]. These cells migrate to the damaged regions of the liver [4-6] where they play a central role in the pathogenic deposition of ECM [7,8].
In order to identify novel therapeutic targets, we used gene expression profiling at different stages of the pathogenic transdifferentiation of HSC [9]. One of the factors found to be upregulated upon HSC activation and further enhanced upon transdifferentiation into myofibroblasts was IGFBP5 (insulin-like growth factor binding protein 5). This strong induction of IGFBP5 expression was confirmed during the development of liver fibrosis in the Mdr2-/- mice, a well established animal model of liver fibrosis [10]. Expression of IGFBP5 in HSC has been reported to be enhanced by insulin-like growth factor 1 (IGF1) via a post-translational mechanism, while its novel synthesis was decreased by TGFβ1 (transforming growth factor beta 1) [11].
IGFBP5 is a member the IGFBPs that bind IGF1 [12-14]. IGF1 is mainly synthesized by the liver and gets secreted into the circulation bound to IGFBPs, which prolong its half-life and, by modulating its interaction with the IGF1 receptor (IGF1R), control its biological activity [12,15,16]. In advanced liver fibrosis, the IGF1 axis is severely impaired mostly due to a reduced number of healthy IGF1 producing hepatocytes [17]. The decrease in IGF1 signalling seems to provide a pro-fibrotic environment, since the progression of liver fibrosis could be delayed by IGF1 administration [18,19]. As IGFBP5 impairs the binding of IGF1 to the cell-surface receptor IGF1R [20], its increased expression in activated HSC and myofibroblasts may reduce IGF1 signalling and, thus, promote liver fibrosis. In contrast, in another study IGF1 has been reported to exert pro-fibrotic activity [21]. In that case the inhibition of IGF1 signalling by IGFBP5 would impair the pathogenesis of liver fibrosis.
In lung and skin, IGFBP5 has also been shown to induce fibrosis upon epithelial injury [13,22,23]. Induction of IGFBP5 expression initiated the activation and transdifferentiation of resident fibroblasts into myofibroblasts, causing increased ECM production and deposition in these tissues. Moreover, it seemed to cause cellular senescence and epithelial-mesenchymal transition [24].
The aim of this study was to investigate the role of IGFBP5 in liver fibrosis by employing both gain and loss of function approaches. We focused on the effect of IGFBP5 on HSC, using the human LX2 cell line [25], which recapitulates many features of the activated HSC phenotype. In addition, to see if IGFBP5 could play a role in more advanced stages of fibrosis, we analysed its effect on human primary liver myofibroblasts.
Materials and methods
Cell culturing
LX2 cells (kindly provided by Prof. dr. S. Friedman) and human myofibroblasts (obtained as described [9]) were cultured in Dulbecco's modified Eagle's medium (Lonza, Verviers, Belgium), supplemented with 10% fetal calf serum (FCS), 1 mmol/l L-glutamine, 100 IU/ml penicillin and streptomycin. Human recombinant IGFBP5 and IGF1 (rIGFBP5 and rIGF1; Gro-Pep, Reutlingen, Germany) were added in concentration 0.1 ng/μl [26] and 1 ng/μl, respectively, at 24 and 45 h of cell culturing. The cells were used for additional assays 3 h after the last protein additions.
In order to induce apoptosis, the cells were serum deprived for 48 h. For drug induced apoptosis, the cells grown in 10% FCS were incubated with 0.5 μM gliotoxin (Alexis Biochemicals, Lausen, Switzerland) for 3 h. This dose induced caspase activity sixfold 3 h after gliotoxin administration (data not shown).
Lentiviral transduction
A lentiviral vector encoding human IGFBP5 behind the constitutive PGK promoter was generated using the self-inactivating cppt-PGKiresGFP-PRE vector [27]. Sequencing was performed to exclude mutations.
siRNA mediated silencing
IGFBP5, IGF1R and negative control small interfering RNAs (siRNAs) were purchased from Invitrogen (Breda, Netherlands). siRNA was introduced to the cells using Lipofectamine 2000 (Invitrogen, Breda, Netherland) in Optimem (Gibco, Invitrogen, Paisley, UK), according to the manufacturer's instructions. For the downstream applications siRNA transfected cells were grown in 10% FCS and used 48 h after transfection. When apoptosis was induced by serum starvation, the cells were first grown o/n (16 h) in serum containing medium, which was then replaced for 48 h by serum depleted medium.
Cell viability test
Cell Proliferation Reagent WST-1 (Roche Applied Science, Mannheim, Germany) was added (1/10th of the culture volume) to cells grown in 96-well plates. Absorbance was measured at 450 nm (reference at 630 nm) immediately and after 1 h of incubation at 37°C.
Bromo-2'-deoxy-uridine (BrdU) assay
BrdU assay (Roche applied Science, Penzberg, Germany) was performed according to the manufacturer's protocol. The cells grown in 96 well plates were incubated with BrdU during the last 16 h of culturing.
Apoptosis assay
The Apo-One homogeneous Caspase 3/7 assay (Promega, Medison, WI, USA) was performed according to the manufacturer's protocol. The Apo-One Caspase 3/7 reagent was added in a 1:1 ratio with medium and fluorescence was measured using Novostar plate reader at 521 nm.
Reverse transcription and quantitative polymerase chain reaction (qPCR)
Total RNA was isolated using Trizol (Invitrogen, Breda, The Netherlands). RNA concentration was determined spectrophotometrically and the integrity was checked by gel electrophoreses. cDNA was synthesized using an oligo-dT primer and Superscript III (Invitrogen, Breda, The Netherlands). qPCR was performed on a LightCycler using FastStart DNA Master Plus SYBR Green I (Roche, Mannheim, Germany). The relative expression levels were calculated using the LinReg program [28] and for each sample normalized by the expression of housekeeping gene 36B4 (acidic ribosomal phosphoprotein P0). Primer sequences are shown in Table 1.
Table 1.
Primers sequences used in quantitative polymerase chain reaction.
| Gene | Symbol | Primers (5'→3') |
|---|---|---|
| Insulin-like growth factor binding protein 5 | IGFBP5 | F: GTCACTCCCCAGAGAAGCTG |
| R: CCCCTGCTTAGATTGCCATA | ||
| Insulin-like growth factor 1 receptor | IGF1R | F: GGTTGAGGTGAGAGGTTTGC |
| R: CAAATTGGCCATGTTATTACCTT | ||
| Acidic ribosomal phosphoprotein P0 | 36B4 | F: AGGCGTCCTCGTGGAAGTGA |
| R: GCGGATCTGCTGCATCTGCT | ||
| Matrix metallopeptidase 1 | MMP1 | F: AGCTAGCTCAGGATGACATTGATG |
| R: CTCCCCGAATCGTAGTTATAGCAT | ||
| TIMP metallopeptidase inhibitor 1 | TIMP1 | F: CACCCACAGACGGCCTTCT |
| R: TCTGGTGTCCCCACGAACTT | ||
| Collagen, type I, alpha 1 | COL1A1 | F: GGCGGCCAGGGCTCCGAC |
| R: AATTCCTGGTCTGGGGCACC | ||
| B-cell CLL/lymphoma 2 | BCL2 | F: GTTGGTGGGGTCATGTGTGTGGAGAG |
| R: TAGCTGATTCGACGTTTTGCCTGA | ||
Western blot analysis
Cell extracts were analysed by Western blotting as described [29]. We used antisera against IGFBP5 (1:250; Abcam, Cambridge, UK), IGF1R (1:200; Santa Cruz Biotechnology, CA, USA), poly (ADP-ribose) polymerase (PARP; 1:100; Promega, Madison, USA) and β-actine (1:3000; Sigma, St Louis, USA). Goat anti-mouse (1:2500; Bio-Rad Laboratories, Veenendaal, The Netherlands) and goat anti-rabbit IgG horseradish peroxidase conjugated (1:5000; Santa Cruz Biotechnology, CA, USA) were used as secondary antibodies. Chemiluminescence was quantified on the Lumi-Imager F1 using CDP-Star (Roche, Mannheim, Germany). Amido-black staining and β-actine immuno-blot verified similar protein loading.
Statistical analysis
All cell culturing experiments were performed in quadruplo, and were repeated at least three times. In order to assess the significance of the data, ANOVA analysis and Student's t test were employed. The error bars in the figures represent the standard deviation. Significance threshold was set to P < 0.05 (denoted by the asterisks in all the Figures).
Results
IGFBP5 expression in LX2 cells
In human HSC in vitro, IGFBP5 expression is enhanced twofold upon activation and 200-fold upon transdifferentiation into myofibroblasts [9]. LX2 cells also express IGFBP5 (Table 2). The 30% higher expression in these cells compared to resting HSC confirms reports demonstrating that it is a model for partially activated HSC [25].
Table 2.
Relative gene expression of insulin-like growth factor 1 (IGF1) and insulin-like growth factor binding protein 5 (IGFBP5) in LX2 cells, activated hepatic stellate cells (HSC) and myofibroblasts given as fold changes of basal expression in freshly isolated human HSC.
| LX2 cells | HSC | Activated HSC | Myofibroblasts | |
|---|---|---|---|---|
| IGFBP5 | 1.3 | 1 | 2.3 | 236 |
| IGF1 | 0.7 | 1 | 1.2 | 0.3 |
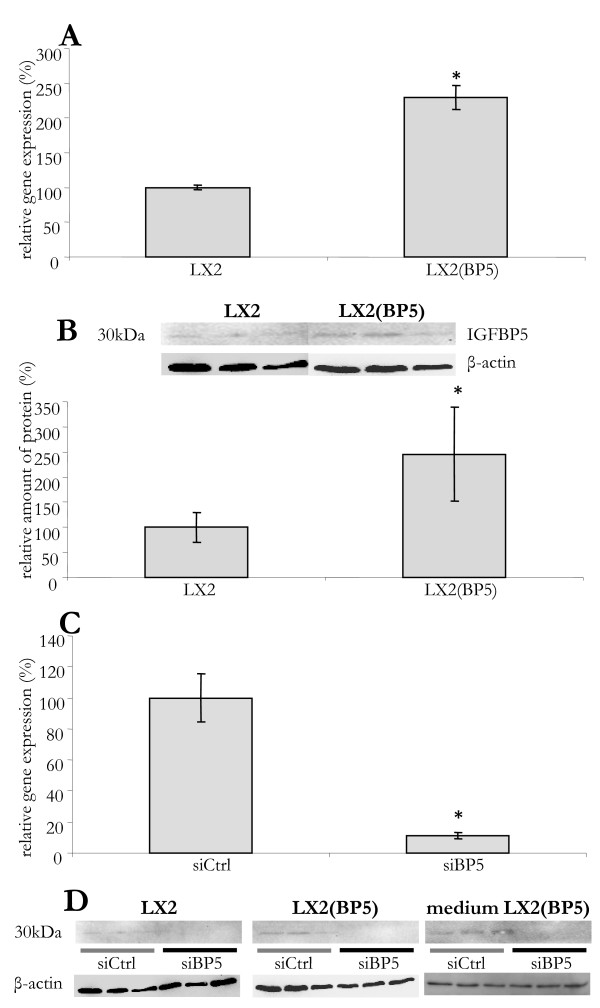
In order to investigate a potential role of IGFBP5 in liver fibrosis, we developed methods to modulate its expression in LX2 cells. The endogenous expression of IGFBP5 mRNA was doubled using lentiviral transduction (Figure 1A). This resulted in a 2.5-fold increase in protein levels (Figure 1B). Transfection with IGFBP5 siRNA reduced IGFBP5 mRNA 80% and led to complete absence of detectable IGFBP5 signal on a Western blot (Figure 1C and 1D, respectively).
Figure 1.
Modulation of IGFBP5 expression in LX2 cells. (A) IGFBP5 mRNA levels in control cells (LX2) and in cells with lentiviral overexpression of IGFBP5 (LX2(BP5)). RNA levels normalized to 36B4 are shown as a percentage of control cells. (B) IGFBP5 protein levels in control (LX2) and transduced (LX2(BP5)) cells as demonstrated on a Western blot (upper panel) and quantified using the LumiImager (lower panel). (C) Silencing of IGFBP5 expression. Cells were transfected with small interfering (si) RNA for IGFBP5 (siBP5) or with a control siRNA (siCtrl). RNA was isolated 48 h later and IGFBP5 mRNA levels were quantified, normalized to 36B4 and given as a percentage of control cells. (D) Proteins were isolated from the cells and the medium 48 h after small interfering RNA (sRNA) transfection. The presence of IGFBP5 protein was visualized by Western blotting in LX2 and LX2(BP5) cells transfected with control siRNA (siCtrl) and IGFBP5 siRNA (siBP5) and in medium obtained from transfected LX2(BP5). The results are given as a percentage of the control cells. The error bars represent standard deviations, with asterisks denoting significant difference (P < 0.05).
IGFBP5 enhances survival of LX2 cells
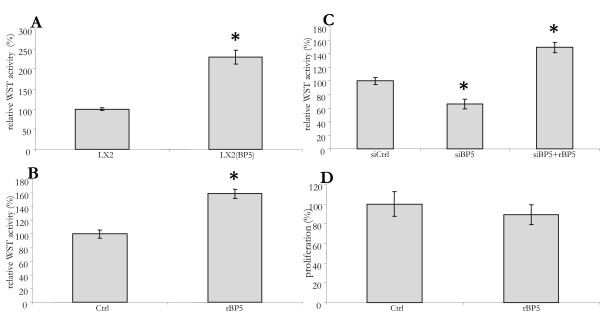
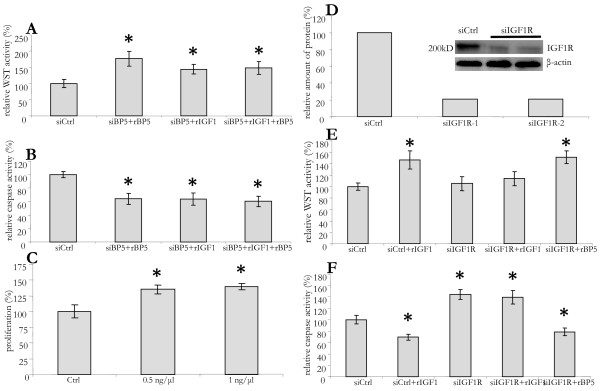
Several studies have shown that IGFBP5 can affect cell survival [30-33]. We therefore compared the viability of control LX2 cells with cells over-expressing IGFBP5. When grown with 10% FCS, cell growth did not differ (data not shown). However, upon culturing in serum free medium for 48 h, a WST assay (for cell viability) indicated 100% higher viability of the transduced cells (Figure 2A). The similar 60% increase in viability seen upon addition of recombinant IGFBP5 (rIGFBP5) confirmed the effect of IGFBP5 on LX2 survival (Figure 2B). In serum free medium, the siRNA mediated reduction of IGFBP5 expression resulted in a >40% decrease in cell viability. This reduction in viability was prevented by the addition of rIGFBP5 to the silenced cells (Figure 2C).
Figure 2.
IGFBP5 increases survival of LX2 cells. (A) The viability of IGFBP5 overexpressing (LX2(BP5)) and control cells (LX2) cultured in serum-free medium for 48 h was measured using WST. (B) The viability of LX2 cells cultured in serum-free medium for 48 h was determined using WST. At t = 24 and 45 h, recombinant IGFBP5 was added (rBP5). (C) LX2 cells were transfected with small interfering control (siCtrl) or IGFBP5 siRNA (siBP5). At 16 h after transfection the medium was replaced by serum-free medium and 48 h later the viability of the cells was measured using WST. rIGFBP5 was added at t = 24 and 45 h of serum-free culturing (siBP5+rBP5). (D) Bromo-2'-deoxy-uridine (BrdU) incorporation was used to determine the proliferation of LX2 cells cultured in serum-free medium for 48 h. rIGFBP5 was added at t = 24 and 45 h (rBP5). BrdU was added at t = 32 h. The results are shown as percentage of the control cells.
In order to investigate if IGFBP5 affected proliferation, we studied its effect on the BrdU incorporation. In the cells grown in serum depleted medium the addition of 0.1 ng/μl rIGFBP5 at 24 h and 45 h of culturing did not effect the incorporation of BrdU (Figure 2D). This indicates that IGFBP5 does not enhance LX2 cell proliferation.
Impact of IGFBP5 on apoptosis in LX2cells
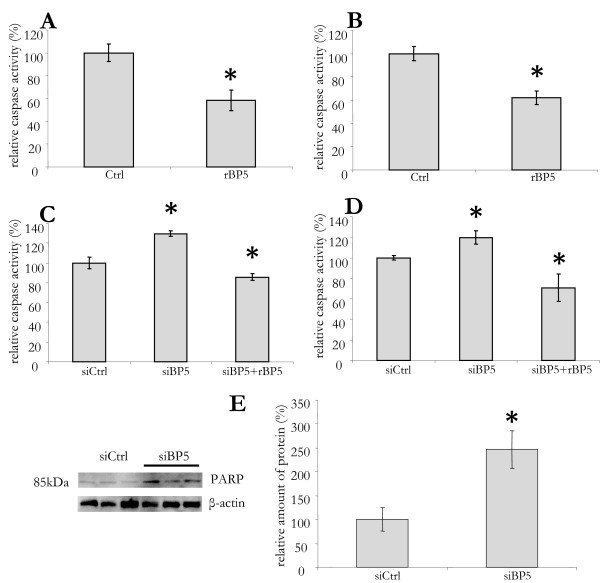
In order to investigate if the effect of IGFBP5 on viability was caused by decreased apoptosis, we determined the caspase activity using a Caspase 3/7 assay. The addition of rIGFBP5 to LX2 cells cultured in serum free medium did reduce the caspase activity by 40% (Figure 3A). In order to substantiate this protective effect, we also tested it in a model of drug induced apoptosis by gliotoxin administration [34]. The addition of rIGFBP5 3 h prior to the addition of 0.5 μM of gliotoxin resulted in a significant (40%) lowering of caspase activity (Figure 3B). In order to investigate if lowering of IGFBP5 would increase apoptosis, we studied the effect of siRNA mediated silencing on its expression. Compared to control-siRNA transfected cells, the caspase activity in silenced cells was increased by 30%. This increase was seen when apoptosis was induced both by serum starvation and gliotoxin. Administration of rIGFBP5 reduced caspase activity in silenced cells to a level significantly lover than that in control cells (Figure 3C and 3D, respectively). The increase in apoptosis in silenced cells was further confirmed by Western blot analysis, which demonstrated a 150% increase of poly (ADP-ribose) polymerase (PARP) protein expression in silenced compared to control cells (Figure 3E).
Figure 3.
IGFBP5 impact on apoptosis in LX2 cells. (A) LX2 cells were cultured in serum-free medium for 48 h. Recombinant IGFBP5 (rBP5) was added at 24 h and 45 h and, at 48 h, a Caspase 3/7 assay was performed. (B) Cells grown in 10% fetal calf serum were treated with rBP5 as above, with the addition of 0.5 μM gliotoxin after 45 h. (C) LX2 cells were transfected with small interfering control (siCtrl) or IGFBP5 siRNA (siBP5). Sixteen hours after transfection, the medium was replaced by serum-free medium and cells were cultured for additional 48 h before performing the caspase assay. To siIGFBP5 transfected cells, recombinant IGFBP5 was added at t = 24 h and 45 h (siBP5+rBP5). (D) To cells treated with si- and r-BP5 as in C, 0.5 μM gliotoxin was added 3 h before performing the caspase assay. All results are shown as a percentage of the control cells. (E) Apoptosis was induced in LX2 cells transfected with control siRNA (siCtrl) or IGFBP5 siRNA (siBP5) by the addition of 0.5 uM gliotoxin at t = 45 h. Proteins were isolated at 48 h and Western blotting was performed for poly(ASP-ribose) polymerase detection (left panel). Quantification is shown in the right panel. β-actin was used as a loading control.
Altogether, these data indicate that IGFBP5 enhances the survival of this model for partially activated HSC by arresting apoptosis.
IGFBP5 also enhances survival of human myofibroblasts
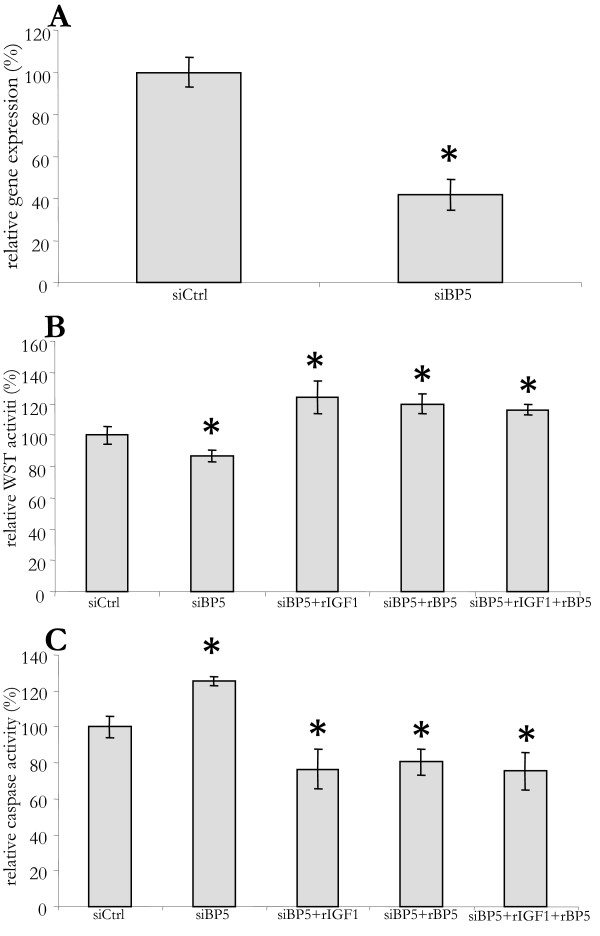
Activated HSC transdifferentiate into myofibroblasts in advanced stages of liver fibrosis. This transdifferentiation results in a further induction of IGFBP5 expression [9]. Given the effects on LX2 cells, IGFBP5 may also affect the survival of liver myofibroblasts. In order to investigate this, we performed siRNA mediated silencing of IGFBP5 expression in primary human myofibroblasts (Figure 4A). Forty-eight hours after transfection, IGFBP5 mRNA levels in primary myofibroblasts were decreased by 60%. Although the silencing was not as effective as that in LX2 cells, it did cause a small, but significant, decrease in survival, which could be prevented by the addition of both rIGF1 and rIGFBP5 (Figure 4B). As in LX2 cells, this decrease in survival was due to an increase in caspase activity that could be avoided by the addition of rIGF1 and rIGFBP5 (Figure 4C). Thus, IGFBP5 also increases the survival of human liver myofibroblasts by reducing apoptosis.
Figure 4.
In human myofibroblasts IGFBP5 silencing decreases survival and promotes apoptosis. (A) Primary human myofibroblasts were transfected with small interfering (si) RNA for IGFBP5 (siBP5) or with a control siRNA (siCtrl). At 16 h after transfection the medium was replaced by serum-free medium. Cells were cultured for an additional 48 h. Then RNA was isolated and IGFBP5 mRNA level determined by quantitative polymerase chain reaction and normalized with 36B4. (B) Recombinant IGFBP5 (rBP5), IGF1 (rIGF1), or both (rIGF1+ rBP5), where added at t = 24 h and 45 h, after replacing the medium, to siBP-5 transfected cells. At 48 h the WST assay was performed. (C) To cells treated as above but grown in 10% fetal calf serum, 0,5 μM gliotoxin was added at 45 h. Caspase 3/7 assay was performed at 48 h. Results are given as a percentage of control cells.
Involvement of BCL2
In order to investigate the mechanism involved in the anti-apoptotic effect of IGFBP5 we studied its effect on the expression of several pro- and anti-apoptotic genes. The enhanced expression of IGFBP5 in transduced cells resulted in a significant induction of B-cell CLL/lymphoma 2 (BCL2) mRNA levels (Figure 5). Also, the addition of rIGFBP5 to IGFBP5 silenced LX2 cells resulted in a clear increase in BCL2 expression. The lack of effect of IGFBP5 silencing on BCL2 expression is unexpected and seems to suggest that, in presence of low levels of IGFBP5, other factors do determine (basal) BCL2 expression. BCL2 mRNA induction by high IGFBP5 levels seems to suggest that it could play a role in the anti-apoptotic effect of IGFBP5, but additional studies such as BCL2 silencing are needed in order to establish this possibility.
Figure 5.
IGFBP5 influences BCL2 expression. LX2 cells were transfected with control small interfering RNA (siCtrl) or IGFBP5 siRNA (siBP5). Sixteen hours after transfection the medium was replaced by serum-free medium. Recombinant IGFBP5 was added at t = 24 h and 45 h, after replacing the medium, to siIGFBP5 transfected cells (siBP5+rBP5). After 48 h RNA was isolated from control (siCtrl), silenced (siBP5), silenced treated with rIGFBP5 (siBP5+rBP5) and LX2 cells overexpression IGFBP5 (LX2(BP5)) and used for quantitative polymerase chain reaction in order to determine BCL2 mRNA. The BCL2 mRNA levels were normalized to 36B4 and given as a percentage of control cells.
Increased cell survival by IGFBP5 seems independent of IGF1
IGFBP5 binds IGF1 with high affinity. Therefore, its protective role in LX2 cells and myofibroblasts could be due to modulation of the pro-survival effects of IGF1 signalling [20,35]. In order to investigate if IGFBP5 exerts its action via IGF1, we also determined the effect of IGF1 on LX2 cells viability. As with IGFBP5, the addition of IGF1 induced a 70% increase in survival of LX2 cells (Figure 6A), and lowering of apoptosis by 40% (Figure 6B). In order to investigate if IGF1 effect is modulated by IGFBP5, we studied the effect of the two factors added together. The response in viability and apoptosis were similar when IGF1 and IGFBP5 were added, alone or in combination (Figure 6A and 6B). However, in contrast to IGFBP5, IGF1 was able to enhance proliferation of LX2 cells for about 40% (Figure 6C), indicating that the two factors exert different effects on LX2 cells.
Figure 6.
Cell survival by IGFBP5 is not affected by IGF1 or mediated by IGF1R. (A) LX2 cells were transfected with IGFBP5 small interfering RNA (siBP5). Sixteen hours after transfection the medium was replaced by serum-free medium for 48 h and then the viability was determined by performing a WST assay. Recombinant IGFBP5 (rBP5), IGF1 (rIGF1), or both (rIGF1+ rBP5), were added at 24 hand 45 h. (B) To cells treated as above, but cultured in 10% fetal calf serum, 0.5 μM gliotoxin was added at 45 h followed by a Caspase 3/7 assay 3 h later. (C) LX2 cells were cultured in serum-free medium for 48 h. rIGF1 was added in different concentrations at 24 h and 45 h, bromo-2'-deoxy-uridine (BrdU) was added at 32 h. Cells were lysed 16 h later (at 48 h) and assayed for BrdU incorporation. (D) LX2 cells were transfected with control (siCtrl) or siRNA for IGF1R (siIGF1R). Sixteen hours after transfection the medium was replaced by serum-free medium and cells were cultured for additional 48 h. The cells were then lysed and used for Western blot analysis in order to detect IGF1R. (E) Cells were transfected and cultured as in D. rIGF1 or rIGFBP5 (rBP5) was added at t = 24 h and 45 h after replacing medium. After 48 h a WST assay was performed. (F) 0.5 μM gliotoxin was added at 45 h, after replacing the medium. Caspase activity was measured 3 h later. All results are given as a percentage control cells.
In order to establish a possible IGF1-independent effect of IGFBP5, we chose to silence IGF1R, the receptor that mediates IGF1 signalling. Transfection of LX2 cells with siRNA for IGF1R resulted in a 90% drop of IGF1R protein expression after 48 h (Figure 6D). This large decrease effectively inhibited the pro-survival effect of IGF1, but not that of IGFBP5 (Figure 6E). The possibility of IGF1-independent action of IGFBP5 was further confirmed by the addition of rIGFBP5 to IGF1R-silenced LX2 cells, which caused a decrease in caspase activity, even to the level below that in the control cells. The addition of IGF1, on the other hand, was not able to overcome the effect of IGF1R silencing and its effect on caspase activity was lacking (Figure 6F). Thus, in contrast to IGF1, the effect of IGFBP5 on LX2 cells survival is not mediated by the IGF1R, suggesting that the effect it exerts on partly activated stellate cells is not by modulation of IGF1 signalling.
IGFBP5 and fibrotic markers
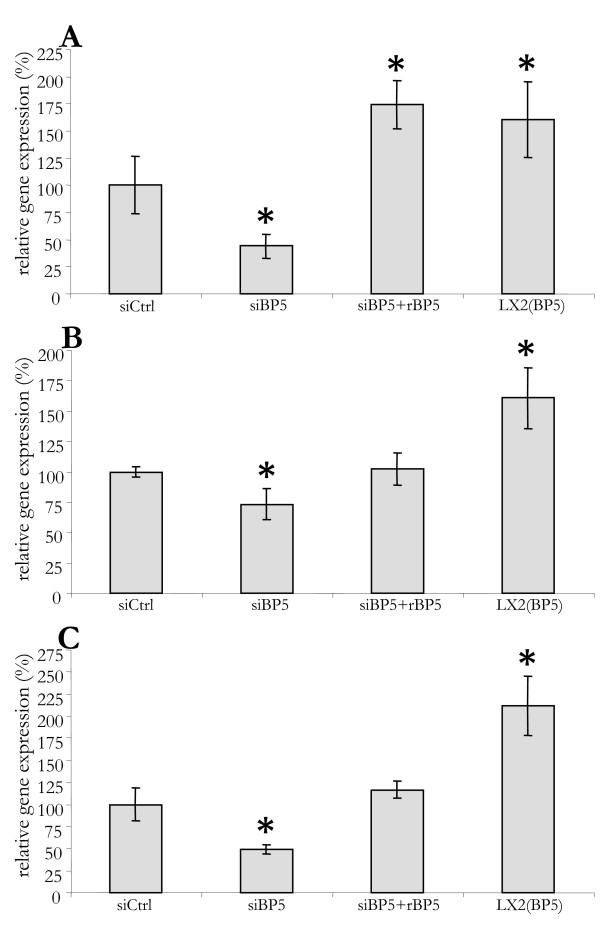
In tissues such as lung and skin, IGFBP5 was reported to enhance the expression of established pro-fibrotic markers, such as αSMA, collagen and fibronectin. As changed expression of these markers could further establish the (pro-fibrotic) role of IGFBP5 in liver fibrosis, we examined its influence on their expression in LX2 cells. As shown in Figure 7, overexpression of IGFBP5 induced the mRNA level of collagen, type I, alpha 1 (COL1A1) by 70%, tissue inhibitor of metalloproteinase (TIMP) metallopeptidase inhibitor 1 (TIMP1) by 70% and matrix metallopeptidase 1 (MMP1) by 100% in LX2 cells (Figure 7A-C). Silencing of IGFBP5 reduced the expression of these genes, which could be recuperated by the addition of recombinant IGFBP5. This indicates that IGFBP5 may not only enhance fibrosis by promoting the survival of activated HSC and myofibroblasts, but also by stimulating the expression of pro-fibrotic genes in these cells.
Figure 7.
IGFBP5 influences the expression of genes involved in fibrogenesis. LX2 cells were transfected with small interfering control (siCtrl) or IGFBP5 siRNA (siBP5). Sixteen hours after transfection, medium was replaced by serum-free medium. Recombinant IGFBP5 (rIGFBP5) was added at 24 h and 45 h, after replacing the medium, to siIGFBP5 transfected cells (siBP5+rBP5). After 48 h of culturing on serum-free medium RNA was isolated from control, transfected and transfected treated with rIGFBP5. For comparison, RNA was isolated from LX2 cells overexpressing IGFBP5 cultured in serum-free medium (LX2(BP5)). Collagen 1α1 (A),TIMP1 (B) and MMP1 (C) mRNA levels were measured with quantitative polymerase chain reaction, normalized to 36B4 and given as a percentage of control cells.
Discussion
We have previously shown that IGFBP5 expression was strongly upregulated during HSC transdifferentiation in vitro and during development of liver fibrosis in vivo [9]. The aim of this study was to scrutinize the role of IGFBP5 in (partially) activated and transdifferentiated hepatic stellate cells.
IGFBP5 expression is increased in fibrotic lung, skin and liver [9,10,22,23]. The effects of IGFBP5 depend on cell type and tissue. For instance, in the process of mammary gland involution, IGFBP5 promotes apoptosis of epithelial cells in vivo and in vitro [36-39]. In contrast, an anti-apoptotic role of IGFBP5 has been reported, for instance, in myogenesis, in breast cancer cells grown in vitro, and in gingival epithelial cells [40-43].
In liver fibrosis, the level of apoptosis is reduced in activated HSC that play a pivotal role in the initiation and perpetuation of this pathological process [44]. Since IGFBP5 is induced in these cells upon activation and can promote survival, it may play a role in increasing the numbers of these activated cells seen in fibrotic liver. In order to study the effects of IGFBP5 in vitro, we used primary human myofibroblasts and LX2 cells, an established human model cell line for partially activated HSC [45-47]. The expression of IGFBP5 in LX2 cells was 30% higher than in quiescent normal HSC. For comparison, in cultured (that is, partially activated) HSC, IGFBP5 expression was doubled, while in hepatic myofibroblasts expression went up more than 200-fold. We used lentiviral transduction of LX2 cells to enhance the IGFBP5 expression to the levels seen in activated HSC. Upon serum depletion, both IGFBP5 over-expression and the addition of rIGFBP5 promoted the survival of LX2 cells by lowering the apoptosis. This suggests that the increased IGFBP5 expression could play a role in the reduction of apoptosis seen in activated HSC in the fibrotic liver. In accordance, lowered IGFBP5 expression by RNA silencing decreased the viability of LX2 cells. IGFBP5 silencing also decreased survival in human primary liver myofibroblasts. In both cell types this was due to increased apoptosis, demonstrating that IGFBP5 functions as an anti-apoptotic, pro-survival factor in these two pro-fibrotic cell types. Two recent studies reported a high expression of IGFBP5 in hepatocellular carcinoma (HCC) and intra-hepatic cholangiocarcinoma (CC), suggesting that it has a similar role in vivo by promoting the survival of cancer cells [48,49]. We demonstrated that IGFBP5 expression strongly increased during development of liver fibrosis in Mdr2-/- mice [9]. This model of liver fibrosis not only spontaneously develops portal fibrosis, but is also prone to HCC formation [50,51]. The presence of IGFBP5 in this animal model and in patients with HCC or CC suggests that, in addition to a role in the pathogenesis of liver fibrosis, IGFBP5 may also contribute to tumour formation. Lowering IGFBP5 expression may, therefore, not only impair the development of liver fibrosis but also reduce the risk of tumour formation.
The role of IGFBP5 in IGF1 signalling is well established. IGFBP5 binds IGF1 with high affinity and protects it from rapid degradation [20] but, at the same time, prevents induction of the IGF1R and thereby inhibits pro-survival signalling by IGF1. The observed effects of IGFBP5 may therefore be due to its role in IGF1 signalling - that is to its disturbance of the IGF1 axis observed in liver fibrosis [19]. Our in vitro studies show, however, that, in contrast to IGFBP5, IGF1 increases proliferation of LX2 cells. Moreover, no effect of IGF1 on IGFBP5 action, nor of IGFBP5 on IGF1 signalling, was detected. Therefore, it seems that the two factors exert their effect on LX2 cells via different routes. In line with this, silencing of IGF1R resulted in loss of the pro-survival effects of IGF1 but did not interfere with the pro-survival effects of IGFBP5. This strongly supports the notion that IGFBP5 promotes survival of activated HSC by lowering apoptosis in an IGF1-independent manner. Several mechanisms, such as activation of TGFβ1, modulation of the coagulation cascade and integrin activation may be involved in the IGF1-independent effect of IGFBP5 [52-54]. In addition, the existence of an IGFBP5 receptor has been proposed to explain the IGF1-independent actions of IGFBP5 [53,54].
The finding that IGFBP5 promoted survival without inducing proliferation suggests that it may induce cellular senescence. Overexpression of IGFBP5 is shown to induce senescence in HUVEC cells by induction of the tumour suppressor p53 [26]. The induction of senescence plays a promoting role in the development of pulmonary fibrosis [24]. On the other hand, senescence in activated HSC was associated with impediment of liver fibrosis [55], leaving the role of IGFBP5 in the development of senescence in liver fibrogenesis opened for further inquiries. Additionally, adenovirally mediated overexpression of IGFBP5 induced the expression pro-fibrotic genes and ECM deposition in lung and skin [22,23]. We showed that IGFBP5 also enhanced the expression of genes directly involved in liver fibrogenesis such as collagen1α1, TIMP1 and MMP1 in LX2 cells. Its effect on these pro-fibrotic genes in a model for activated HSC indicates that IGFBP5 may also have a direct effect on the ECM deposition in liver fibrosis. Increase of both TIMP1 and MMP1 expression, and thus exertion of both fibrotic and matrix-degrading effects, could be seen in the light of a necessity to establish a balance between matrix deposition and restructuring during the process of fibrogenesis. However, since our data only demonstrate changes on the mRNA level, it seems possible that the changes in the expression of these opposing factors may not have occurred on the protein level due to posttranscriptional and posttranslational modifications.
In conclusion, our data show that IGFBP5 improves the survival of (partially) activated HSC and liver myofibroblasts by lowering the level of apoptosis via an IGF1-independent mechanism. In addition, IGFBP5 increases the expression of genes involved in ECM deposition. Accordingly, lowering expression of this apparently pro-fibrotic factor may impair the progression of liver fibrosis.
Abbreviations
BCL2: B-cell CLL/lymphoma 2; BrdU: bromo-2'-deoxy-uridine; CC: cholangiocarcinoma; ECM: extracellular matrix; FCS: fetal calf serum; HCC: hepatocellular carcinoma; HSC: hepatic stellate cells; IGF1: insulin-like growth factor 1; IGF1R: insulin-like growth factor 1 receptor; IGFBP5: insulin-like growth factor binding protein 5; MMP1: matrix metallopeptidase 1; PARP: poly (ADP-ribose) polymerase; PGK: phosphoglycerate; qPCR: quantitative polymerase chain reaction; rIGFBP5: human recombinant insulin-like growth factor binding protein 5; siRNA: small interfering RNA; TGFβ1: transforming growth factor, beta 1; TIMP1: tissue inhibitor of metallopeptidase inhibitor 1.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
AS carried out the experimental part of the study and prepared the manuscript. MS took part in the experiments and writing. WB provided an insight into the SAGE database. ROE and PB supervised the study.
Contributor Information
Aleksandar Sokolović, Email: a.sokolovic@amc.uva.nl.
Milka Sokolović, Email: m.sokolovic@amc.uva.nl.
Willem Boers, Email: w.boers@amc.uva.nl.
Ronald PJ Oude Elferink, Email: r.p.oude-elferink@amc.uva.nl.
Piter J Bosma, Email: p.j.bosma@amc.uva.nl.
Acknowledgements
The study was supported by a MLDS grant CG 102001. We thank Prof. dr. S. Friedman for kindly providing us with the LX2 cell line.
References
- Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol. 2003;38(Suppl 1):S38–S53. doi: 10.1016/S0168-8278(02)00429-4. [DOI] [PubMed] [Google Scholar]
- Blomhoff R, Wake K. Perisinusoidal stellate cells of the liver: important roles in retinol metabolism and fibrosis 6. FASEB J. 1991;5:271–277. doi: 10.1096/fasebj.5.3.2001786. [DOI] [PubMed] [Google Scholar]
- Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Wakahara T, Wang YQ, Kadoya H, Kawada N, Kaneda K. In vitro migratory potential of rat quiescent hepatic stellate cells and its augmentation by cell activation. Hepatology. 1999;29:1760–1767. doi: 10.1002/hep.510290640. [DOI] [PubMed] [Google Scholar]
- Kinnman N, Hultcrantz R, Barbu V, Rey C, Wendum D, Poupon R, Housset C. PDGF-mediated chemoattraction of hepatic stellate cells by bile duct segments in cholestatic liver injury. Lab Invest. 2000;80:697–707. doi: 10.1038/labinvest.3780073. [DOI] [PubMed] [Google Scholar]
- Marra F, Romanelli RG, Giannini C, Failli P, Pastacaldi S, Arrighi MC, Pinzani M, Laffi G, Montalto P, Gentilini P. Monocyte chemotactic protein-1 as a chemoattractant for human hepatic stellate cells. Hepatology. 1999;29:140–148. doi: 10.1002/hep.510290107. [DOI] [PubMed] [Google Scholar]
- Gressner AM. Liver fibrosis: perspectives in pathobiochemical research and clinical outlook. Eur J Clin Chem Clin Biochem. 1991;29:293–311. [PubMed] [Google Scholar]
- Schuppan D, Ruehl M, Somasundaram R, Hahn EG. Matrix as a modulator of hepatic fibrogenesis. Semin Liver Dis. 2001;21:351–372. doi: 10.1055/s-2001-17556. [DOI] [PubMed] [Google Scholar]
- Boers W, Aarrass S, Linthorst C, Pinzani M, Elferink RO, Bosma P. Transcriptional profiling reveals novel markers of liver fibrogenesis: gremlin and insulin-like growth factor-binding proteins. J Biol Chem. 2006;281:16289–16295. doi: 10.1074/jbc.M600711200. [DOI] [PubMed] [Google Scholar]
- Van Nieuwkerk CM, Elferink RP, Groen AK, Ottenhoff R, Tytgat GN, Dingemans KP, Weerman MA Van Den Bergh, Offerhaus GJ. Effects of Ursodeoxycholate and cholate feeding on liver disease in FVB mice with a disrupted mdr2 P-glycoprotein gene. Gastroenterology. 1996;111:165–171. doi: 10.1053/gast.1996.v111.pm8698195. [DOI] [PubMed] [Google Scholar]
- Gentilini A, Feliers D, Pinzani M, Woodruff K, Abboud S. Characterization and regulation of insulin-like growth factor binding proteins in human hepatic stellate cells. J Cell Physiol. 1998;174:240–250. doi: 10.1002/(SICI)1097-4652(199802)174:2<240::AID-JCP11>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Schneider MR, Wolf E, Hoeflich A, Lahm H. IGF-binding protein-5: flexible player in the IGF system and effector on its own. J Endocrinol. 2002;172:423–440. doi: 10.1677/joe.0.1720423. [DOI] [PubMed] [Google Scholar]
- Pilewski JM, Liu L, Henry AC, Knauer AV, Feghali-Bostwick CA. Insulin-like growth factor binding proteins 3 and 5 are overexpressed in idiopathic pulmonary fibrosis and contribute to extracellular matrix deposition. Am J Pathol. 2005;166:399–407. doi: 10.1016/S0002-9440(10)62263-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemmons DR, Jones JI, Busby WH, Wright G. Role of insulin-like growth factor binding proteins in modifying IGF actions. Ann N Y Acad Sci. 1993;692:10–21. doi: 10.1111/j.1749-6632.1993.tb26201.x. [DOI] [PubMed] [Google Scholar]
- Jones JI, Clemmons DR. Insulin-like growth factors and their binding proteins: biological actions. Endocr Rev. 1995;16:3–34. doi: 10.1210/edrv-16-1-3. [DOI] [PubMed] [Google Scholar]
- Twigg SM, Baxter RC. Insulin-like growth factor (IGF)-binding protein 5 forms an alternative ternary complex with IGFs and the acid-labile subunit 1. J Biol Chem. 1998;273:6074–6079. doi: 10.1074/jbc.273.11.6074. [DOI] [PubMed] [Google Scholar]
- Scharf JG, Schmitz F, Frystyk J, Skjaerbaek C, Moesus H, Blum WF, Ramadori G, Hartmann H. Insulin-like growth factor-I serum concentrations and patterns of insulin-like growth factor binding proteins in patients with chronic liver disease. J Hepatol. 1996;25:689–699. doi: 10.1016/S0168-8278(96)80240-6. [DOI] [PubMed] [Google Scholar]
- Conchillo M, de Knegt RJ, Payeras M, Quiroga J, Sangro B, Herrero JI, Castilla-Cortazar I, Frystyk J, Flyvbjerg A, Yoshizawa C, Jansen PL, Scharschmidt B, Prieto J. Insulin-like growth factor I (IGF-I) replacement therapy increases albumin concentration in liver cirrhosis: results of a pilot randomized controlled clinical trial. J Hepatol. 2005;43:630–636. doi: 10.1016/j.jhep.2005.03.025. [DOI] [PubMed] [Google Scholar]
- Sanz S, Pucilowska JB, Liu S, Rodríguez-Ortigosa CM, Lund PK, Brenner DA, Fuller CR, Simmons JG, Pardo A, Martínez-Chantar ML, Fagin JA, Prieto J. Expression of insulin-like growth factor I by activated hepatic stellate cells reduces fibrogenesis and enhances regeneration after liver injury. Gut. 2005;54:134–141. doi: 10.1136/gut.2003.024505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie J, Allan GJ, Lochrie JD, Flint DJ. Insulin-like growth factor-binding protein-5 (IGFBP-5): a critical member of the IGF axis. Biochem J. 2006;395:1–19. doi: 10.1042/BJ20060086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen JT, Spiteri MA. Growth factors in idiopathic pulmonary fibrosis: relative roles. Respir Res. 2002;3:13. doi: 10.1186/rr162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuoka H, Jukic DM, Zhou Z, Choi AM, Feghali-Bostwick CA. Insulin-like growth factor binding protein 5 induces skin fibrosis: A novel murine model for dermal fibrosis. Arthritis Rheum. 2006;54:3001–3010. doi: 10.1002/art.22084. [DOI] [PubMed] [Google Scholar]
- Yasuoka H, Zhou Z, Pilewski JM, Oury TD, Choi AM, Feghali-Bostwick CA. Insulin-like growth factor-binding protein-5 induces pulmonary fibrosis and triggers mononuclear cellular infiltration. Am J Pathol. 2006;169:1633–1642. doi: 10.2353/ajpath.2006.060501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan GJ, Beattie J, Flint DJ. Epithelial injury induces an innate repair mechanism linked to cellular senescence and fibrosis involving IGF-binding protein-5. J Endocrinol. 2008;199:155–164. doi: 10.1677/JOE-08-0269. [DOI] [PubMed] [Google Scholar]
- Xu L, Hui AY, Albanis E, Arthur MJ, O'Byrne SM, Blaner WS, Mukherjee P, Friedman SL, Eng FJ. Human hepatic stellate cell lines, LX-1 and LX-2: new tools for analysis of hepatic fibrosis. Gut. 2005;54:142–151. doi: 10.1136/gut.2004.042127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KS, Seu YB, Baek SH, Kim MJ, Kim KJ, Kim JH, Kim JR. Induction of cellular senescence by insulin-like growth factor binding protein-5 through a p53-dependent mechanism. Mol Biol Cell. 2007;18:4543–4552. doi: 10.1091/mbc.E07-03-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppen J, Rijnberg M, Cooreman MP, Oude Elferink RP. Lentiviral vectors for efficient transduction of isolated primary quiescent hepatocytes. J Hepatol. 2002;36:459–465. doi: 10.1016/S0168-8278(01)00308-7. [DOI] [PubMed] [Google Scholar]
- Ramakers C, Ruijter JM, Deprez RHL, Moorman AFM. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neuroscience Letters. 2003;339:62–66. doi: 10.1016/S0304-3940(02)01423-4. [DOI] [PubMed] [Google Scholar]
- Keijzer R, Blommaart PJ, Labruyère WT, Vermeulen JL, Doulabi BZ, Bakker O, Tibboel D, Lamers WH. Expression of thyroid hormone receptors A and B in developing rat tissues; evidence for extensive posttranscriptional regulation. J Mol Endocrinol. 2007;38:523–535. doi: 10.1677/jme.1.02125. [DOI] [PubMed] [Google Scholar]
- Johnson SK, Haun RS. Insulin-like growth factor binding protein-5 influences pancreatic cancer cell growth. World J Gastroenterol. 2009;15:3355–3366. doi: 10.3748/wjg.15.3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows KA, Holly JM, Stewart CE. Tumor necrosis factor-alpha-induced apoptosis is associated with suppression of insulin-like growth factor binding protein-5 secretion in differentiating murine skeletal myoblasts. J Cell Physiol. 2000;183:330–337. doi: 10.1002/(SICI)1097-4652(200006)183:3<330::AID-JCP5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Miyake H, Nelson C, Rennie PS, Gleave ME. Overexpression of insulin-like growth factor binding protein-5 helps accelerate progression to androgen-independence in the human prostate LNCaP tumor model through activation of phosphatidylinositol 3'-kinase pathway. Endocrinology. 2000;141:2257–2265. doi: 10.1210/en.141.6.2257. [DOI] [PubMed] [Google Scholar]
- Perks CM, McCaig C, Holly JM. Differential insulin-like growth factor (IGF)-independent interactions of IGF binding protein-3 and IGF binding protein-5 on apoptosis in human breast cancer cells. Involvement of the mitochondria. J Cell Biochem. 2000;80:248–258. doi: 10.1002/1097-4644(20010201)80:2<248::AID-JCB140>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Hagens WI, Olinga P, Meijer DK, Groothuis GM, Beljaars L, Poelstra K. Gliotoxin non-selectively induces apoptosis in fibrotic and normal livers. Liver Int. 2006;26:232–239. doi: 10.1111/j.1478-3231.2005.01212.x. [DOI] [PubMed] [Google Scholar]
- Butt AJ, Firth SM, Baxter RC. The IGF axis and programmed cell death. Immunol Cell Biol. 1999;77:256–262. doi: 10.1046/j.1440-1711.1999.00822.x. [DOI] [PubMed] [Google Scholar]
- Flint DJ, Boutinaud M, Tonner E, Wilde CJ, Hurley W, Accorsi PA, Kolb AF, Whitelaw CB, Beattie J, Allan GJ. Insulin-like growth factor binding proteins initiate cell death and extracellular matrix remodeling in the mammary gland. Domest Anim Endocrinol. 2005;29:274–282. doi: 10.1016/j.domaniend.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Tonner E, Allan G, Shkreta L, Webster J, Whitelaw CB, Flint DJ. Insulin-like growth factor binding protein-5 (IGFBP-5) potentially regulates programmed cell death and plasminogen activation in the mammary gland. Adv Exp Med Biol. 2000;480:45–53. doi: 10.1007/0-306-46832-8_5. full_text. [DOI] [PubMed] [Google Scholar]
- Lochrie JD, Phillips K, Tonner E, Flint DJ, Allan GJ, Price NC, Beattie J. Insulin-like growth factor binding protein (IGFBP)-5 is upregulated during both differentiation and apoptosis in primary cultures of mouse mammary epithelial cells. J Cell Physiol. 2006;207:471–479. doi: 10.1002/jcp.20587. [DOI] [PubMed] [Google Scholar]
- Ning Y, Hoang B, Schuller AG, Cominski TP, Hsu MS, Wood TL, Pintar JE. Delayed mammary gland involution in mice with mutation of the insulin-like growth factor binding protein 5 gene. Endocrinology. 2007;148:2138–2147. doi: 10.1210/en.2006-0041. [DOI] [PubMed] [Google Scholar]
- Cobb LJ, Salih DA, Gonzalez I, Tripathi G, Carter EJ, Lovett F, Holding C, Pell JM. Partitioning of IGFBP-5 actions in myogenesis: IGF-independent anti-apoptotic function. J Cell Sci. 2004;117:1737–1746. doi: 10.1242/jcs.01028. [DOI] [PubMed] [Google Scholar]
- Perks CM, Bowen S, Gill ZP, Newcomb PV, Holly JM. Differential IGF-independent effects of insulin-like growth factor binding proteins (1-6) on apoptosis of breast epithelial cells. J Cell Biochem. 1999;75:652–664. doi: 10.1002/(SICI)1097-4644(19991215)75:4<652::AID-JCB11>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- McCaig C, Perks CM, Holly JM. Intrinsic actions of IGFBP-3 and IGFBP-5 on Hs578T breast cancer epithelial cells: inhibition or accentuation of attachment and survival is dependent upon the presence of fibronectin. J Cell Sci. 2002;115:4293–4303. doi: 10.1242/jcs.00097. [DOI] [PubMed] [Google Scholar]
- Hung PS, Kao SY, Liu CJ, Tu HF, Wu CH, Lin SC. Insulin-like growth factor binding protein-5 enhances the migration and differentiation of gingival epithelial cells. J Periodontal Res. 2008;43:673–80. doi: 10.1111/j.1600-0765.2007.01070.x. [DOI] [PubMed] [Google Scholar]
- Iredale JP. Hepatic stellate cell behavior during resolution of liver injury. Semin Liver Dis. 2001;21:427–436. doi: 10.1055/s-2001-17557. [DOI] [PubMed] [Google Scholar]
- Wang XM, Yu DM, McCaughan GW, Gorrell MD. Fibroblast activation protein increases apoptosis, cell adhesion, and migration by the LX-2 human stellate cell line. Hepatology. 2005;42:935–945. doi: 10.1002/hep.20853. [DOI] [PubMed] [Google Scholar]
- Anan A, Baskin-Bey ES, Bronk SF, Werneburg NW, Shah VH, Gores GJ. Proteasome inhibition induces hepatic stellate cell apoptosis. Hepatology. 2006;43:335–344. doi: 10.1002/hep.21036. [DOI] [PubMed] [Google Scholar]
- Shi YF, Fong CC, Zhang Q, Cheung PY, Tzang CH, Wu RS, Yang M. Hypoxia induces the activation of human hepatic stellate cells LX-2 through TGF-beta signaling pathway. FEBS Lett. 2007;581:203–210. doi: 10.1016/j.febslet.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Umemura A, Itoh Y, Itoh K, Yamaguchi K, Nakajima T, Higashitsuji H, Onoue H, Fukumoto M, Okanoue T, Fujita J. Association of gankyrin protein expression with early clinical stages and insulin-like growth factor-binding protein 5 expression in human hepatocellular carcinoma. Hepatology. 2008;47:493–502. doi: 10.1002/hep.22027. [DOI] [PubMed] [Google Scholar]
- Nishino R, Honda M, Yamashita T, Takatori H, Minato H, Zen Y, Sasaki M, Takamura H, Horimoto K, Ohta T, Nakanuma Y, Kaneko S. Identification of novel candidate tumour marker genes for intrahepatic cholangiocarcinoma. J Hepatol. 2008;49:207–216. doi: 10.1016/j.jhep.2008.03.025. [DOI] [PubMed] [Google Scholar]
- Mauad TH, van Nieuwkerk CM, Dingemans KP, Smit JJ, Schinkel AH, Notenboom RG, Weerman MA van den Bergh, Verkruisen RP, Groen AK, Oude Elferink RP. Mice with homozygous disruption of the mdr2 P-glycoprotein gene. A novel animal model for studies of nonsuppurative inflammatory cholangitis and hepatocarcinogenesis. Am J Pathol. 1994;145:1237–1245. [PMC free article] [PubMed] [Google Scholar]
- Popov Y, Patsenker E, Fickert P, Trauner M, Schuppan D. Mdr2 (Abcb4)-/- mice spontaneously develop severe biliary fibrosis via massive dysregulation of pro- and antifibrogenic genes. J Hepatol. 2005;43:1045–1054. doi: 10.1016/j.jhep.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Andress DL. Comparison studies of IGFBP-5 binding to osteoblasts and osteoblast-derived extracellular matrix. Prog Growth Factor Res. 1995;6:337–344. doi: 10.1016/0955-2235(95)00008-9. [DOI] [PubMed] [Google Scholar]
- Andress DL. Heparin modulates the binding of insulin-like growth factor (IGF) binding protein-5 to a membrane protein in osteoblastic cells. J Biol Chem. 1995;270:28289–28296. [PubMed] [Google Scholar]
- Andress DL. Insulin-like growth factor-binding protein-5 (IGFBP-5) stimulates phosphorylation of the IGFBP-5 receptor. Am J Physiol. 1998;274:E744–E750. doi: 10.1152/ajpendo.1998.274.4.E744. [DOI] [PubMed] [Google Scholar]
- Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]