Abstract
The main purpose of this study was to determine whether intake of coenzyme Q10, which can potentially act as both an antioxidant and a prooxidant, has an impact on indicators of oxidative stress and the aging process. Mice were fed diets providing daily supplements of 0, 93, or 371 mg CoQ10 /kg body weight, starting at 3.5 months of age. Effects on mitochondrial superoxide generation, activities of oxidoreductases, protein oxidative damage, glutathione redox state, and life span of male mice were determined. Amounts of CoQ9 and CoQ10, measured after 3.5 or 17.5 months of intake, in homogenates and mitochondria of liver, heart, kidney, skeletal muscle, and brain increased with the dosage and duration of CoQ10 intake in all the tissues except brain. Activities of mitochondrial electron transport chain oxidoreductases, rates of mitochondrial O2−· generation, state 3 respiration, carbonyl content, glutathione redox state of tissues, and activities of superoxide dismutase, catalase, and glutathione peroxidase, determined at 19 or 25 months of age, were unaffected by CoQ10 administration. Life span studies, conducted on 50 mice in each group, showed that CoQ10 administration had no effect on mortality. Altogether, the results indicated that contrary to the historical view, supplemental intake of CoQ10 elevates the endogenous content of both CoQ9 and CoQ10, but has no discernable effect on the main antioxidant defenses or prooxidant generation in most tissues, and has no impact on the life span of mice.
Keywords: Coenzyme Q, Ubiquinone, Mitochondria, Aging, Oxidative stress, Antioxidants, Glutathione, Redox state, Free radicals
Introduction
Coenzyme Q (CoQ) or ubiquinone comprises a benzoquinone ring linked to a polyisoprenyl chain of 9 or 10 units in the mammalian species. In cells, CoQ is located in the middle of the phospholipid bilayer of various membranes; however, the relative amount varies in different organelles. The main chemical characteristic of CoQ responsible for its various functions is that it can exist in three alternate redox states [1-3]: the fully oxidized ubiquinone (Q), which upon two sequential additions of hydrogen atoms converts first into a partially reduced, ubisemiquinone (·QH), a free radical, and then into the fully reduced, ubiquinol (QH2) form. In mitochondria, CoQ serves three well-characterized functions: (i) in concert with vitamin E, it acts as an antioxidant to stem lipid peroxidation in the inner mitochondrial membrane [3-6]; (ii) it ferries electrons from complexes I and II to complex III of the electron transport chain (ETC), while releasing protons into the intermembrane region [2,3]; and (iii) autooxidation of ubisemiquinone is the primary intracellular source of O2−· generation [7,8]. Specific functions of CoQ in nonmitochondrial membranes, where its concentration may even exceed that in the mitochondrion, are currently relatively less well defined.
Because CoQ can act as a generator or a quencher of ROS [3] and because it is widely consumed by humans as a dietary supplement, there is a compelling rationale to understand the in vivo effects of intake of CoQ10. Particularly, the relationship between long-term intake of CoQ and the aging process needs to be defined because oxidative stress/damage has been widely postulated to be a primary causal factor underlying the senescent-associated losses in physiological functions (reviewed in [9]). Another major issue requiring further clarification is whether dietary supplementation can augment endogenous CoQ content in a dose- and/or time-dependent manner. The historical view is that CoQ10 intake by rodents increases CoQ content in plasma and liver only, but not in other tissues [10-12]. And yet, CoQ administration has also been widely reported to ameliorate a variety of cardiovascular and neural dysfunctions [13,14]. It is also currently unclear S whether CoQ intake alters the rate of mitochondrial O2−· generation or affects the activities of oxidoreductases in the mitochondrial ETC. Above all, it needs to be established whether long-term intake of CoQ affects mortality as the current information is quite contradictory [15]. For instance, in the nematode, Caenorhabditis elegans, diets lacking CoQ10 are reported to extend life span by up to 59% [16]. Furthermore, clk-1 mutants of C. elegans, which cannot synthesize the endogenous isoform, CoQ9, exhibit an ~40% extension of life span [17]. It was postulated that life span extension in these cases was due to a decrease in mitochondrial ROS generation. Paradoxically, exposure to antimycin A, which blocks S electron transport and enhances the rate of mitochondrial O2−· generation, presumably by enhanced oxidation of ubise-miquinone, also extends the life span of the worm [18]. In rodents, prolonged CoQ10 intake at a relatively low dosage, or if initiated in the second half of life, has been reported to have no effect on longevity [19,20].
In this context, the purpose of this study was to address some of the issues pertaining to the in vivo effects of CoQ10 administration, using a relatively long-lived mouse line as a model. Specifically, the effects of two different dosages of CoQ10, 93 or 371 mg/kg/day, on (i) amounts of CoQ homologues in tissue homogenates and mitochondria, (ii) life span, (iii) activities S of ETC oxidoreductases, (iv) rates of mitochondrial O2−· generation, (v) activities of the antioxidant enzymes superoxide dismutase, catalase, and glutathione peroxidase, (vi) GSH:GSSG ratios, and (vii) protein carbonyl content, were determined.
Materials and methods
Materials
Coenzyme Q10, obtained from Tishcon Corp. (Westbury, NY), was added to Purina diet 5001 (Cat. Nos. 10038 and 10039, respectively, Purina Mills Test Diet, Richmond, IN) to yield two concentrations of CoQ10: 0.72 mg/g or 2.81 mg/g. All solvents used were HPLC grade (Fisher Scientific, Fair Lawn, NJ). CoQ9 and CoQ10 standards were purchased from Sigma Chemical Co.
Animals
Three-month-old, male C57BL/6 mice were obtained from the National Institute on Aging, National Institutes of Health and subsequently maintained in the University of North Texas Health Science Center vivarium until they reached appropriate target ages. Genotyping by The Jackson Laboratory subsequently indicated that all mice surviving to 17 months of age in this study also had one or more non-C57BL/6 markers. Mice were housed individually in clear polycarbonate cages (29.5 × 19.2 × 12.8 cm, modified into two compartments with a stainless-steel divider) and maintained at 23 ± 1°C under a 12-h light:dark cycle that began at 0600 h. After 2 weeks of acclimation, mice were randomly assigned to one of three groups and subsequently fed ad libitum on diets providing 0 (control), 93 (low), or 371 mg (high) of CoQ10/kg body weight/day. Mice were weighed weekly for the first year and then at monthly intervals for the remainder of the study. Food intake over a period of 1 week was determined for a subset of each treatment group prior to supplementation and after 1, 20, or 32 months.
Preparation of tissue homogenates, isolation of mitochondria, and quantification of CoQ
Mice were killed by cervical dislocation and the tissues were homogenized in 10 vol (w/v) of the indicated tissue-specific isolation buffer. The homogenate was centrifuged for 5 min at 700g at 4°C to sediment unbroken cells and cellular debris and an aliquot of the supernatant was removed for the quantification of CoQ homologues. Mitochondria were isolated by differential centrifugation, according to the procedures indicated previously [21-23].
Extractions of CoQ from tissues were made by the method of Takada et al. [24], as described in detail previously [21]. Briefly, 10 μl Na2 EDTA (10% w/v) and 750 μl of hexane: ethanol (5:2 v/v) were added to 20–200 μl of the sample and the mixture was centrifuged for 3 min at 4000g; 400 μl of the hexane layer was dried under a stream of nitrogen and dissolved in 100 μl of ethanol. Quantification of CoQ and CoQ10 was made by HPLC according to Katayama et al. [25], using a reverse-phase C18 HPLC column (25.0 × 0.46 cm, 5 μm; Supelco Inc., Bellefonte, PA). The mobile phase consisted of 0.7% NaClO4 in ethanol:methanol:70% HClO4 (900:100:1, v/v/v), with a flow rate of 1.2 ml/min, and the eluent was monitored with an electrochemical detector (ESA Coulochem II, ESA Inc., Chelmsford, MA). The settings of the electrochemical detector were guard cell (upstream from the injector), +200 mV; conditioning cell (downstream of the column), −550 mV; analytical cell, +175 mV. The concentrations of CoQ9 and CoQ10 were obtained by comparison of the peak areas with those of solutions of known concentrations. Values represent the sum of the quinone and quinol forms.
Biochemical measurement
Effects of CoQ10 on various biochemical parameters were determined at the specified ages. Most of the procedures used in this study have been described in detail previously and will thus be only referred to or abridged here. Activities of mitochondrial oxidoreductases were determined according to the S protocols described in Kwong and Sohal [26]. Rate of O2−· generation by submitochondrial particles (SMPs) was measured as SOD-inhibitable reduction of acetylated cytochrome c, as described in [27]. Rates of mitochondrial oxygen consumption were determined using a Clark-type oxygen electrode at 30°C. Activities of SOD, catalase, and glutathione peroxidase were determined according to the procedures used in [28-30]. Protein carbonyl content was determined using DNPH, as reported in [31]. GSH and GSSG amounts were measured according to [21].
Statistical analysis
The concentrations of CoQ9 and CoQ10 in each tissue were considered in separate two-way analyses of variance (ANOVA), with Duration of Intake and CoQ Dosage as between-groups factors. Body weights of surviving mice at ages of 50 and 80% mortality were each subjected to a two-way ANOVA with Duration of Intake as a within-groups factor. Biochemical measurements were considered in two-way analyses with Tissue and CoQ Dosage as the factors. Planned individual comparisons of each treatment group with the age-matched control group were made with single degree-of-freedom F tests using pooled error from the overall analysis. For analysis of the effects of different concentrations of CoQ in the diets on longevity, Kaplan-Meier survival distributions were calculated and log-rank, χ2 statistics (Tarone-Ware) were used to compare mortality rates among the three groups. An alpha level of 0.05 was set for all analyses.
Results
Effects of CoQ10 intake on amounts of CoQ homologues in tissue homogenates and mitochondria
The purpose of this experiment was to establish the relationship between the amounts of CoQ10 intake and the augmentation of CoQ homologues, CoQ9 and CoQ10, in the tissues. Accordingly, their content was determined in homogenates and mitochondria of liver, heart, kidney, skeletal muscle, and brain of control and experimental mice, fed relatively low (93 mg) or high (371 mg/kg body weight/day) amounts of CoQ10, starting at 3.5 months of age. Measurements of endogenous CoQ were made at 7 and 21 months of age, i.e., after 3.5 and 17.5 months of CoQ10 intake.
Regardless of age and group, endogenous CoQ9 was the predominant CoQ homologue in homogenates as well as mitochondria (Figs. 1 and 2). In all tissues, the total CoQ (CoQ9 plus CoQ10) content of mitochondria was several-fold higher than that of homogenate. The rank order of endogenous CoQ content was as follows: in homogenates, kidney > heart > brain > liver > skeletal muscle; in mitochondria, heart = kidney > skeletal muscle > brain > liver. In 7-month-old control mice, the percentage ratio of CoQ9 homologues in tissue homogenates was 92% in heart, 96% in liver, 72% in brain, and 73% in skeletal muscle, whereas in mitochondria it was 96% in liver, 92% in skeletal muscle, 89% in heart, and 79% in brain. Furthermore, compared to the tissue homogenates, mitochondrial CoQ9 content per milligram protein was higher by 2.9-fold in heart, 4.5- fold in brain, 5.5-fold in liver, and 320-fold in the skeletal muscle (Figs. 1 and 2).
Fig. 1.
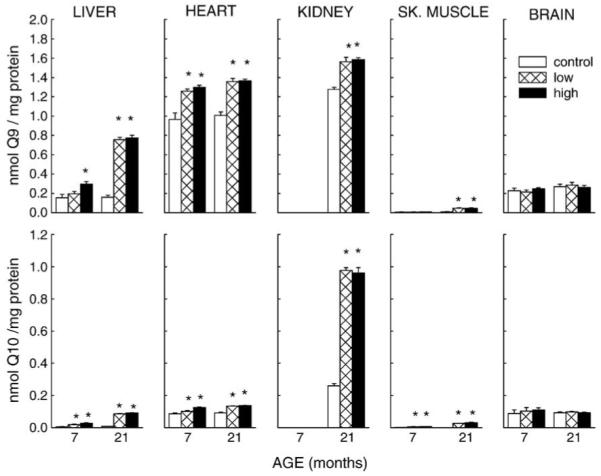
Effect of coenzyme Q10 supplementation on concentrations of CoQ9 and CoQ10 in tissue homogenates of mice. CoQ10 was administered from 3.5 months of age to 7 or 21 months of age. The average daily intake of supplemental CoQ10 was 0 (control), 93 mg/kg body weight (low), or 371 mg/kg body weight (high). All values represent the mean ± SE of 2 – 6 samples. * P < 0.05 when compared with age-matched control.
Fig. 2.
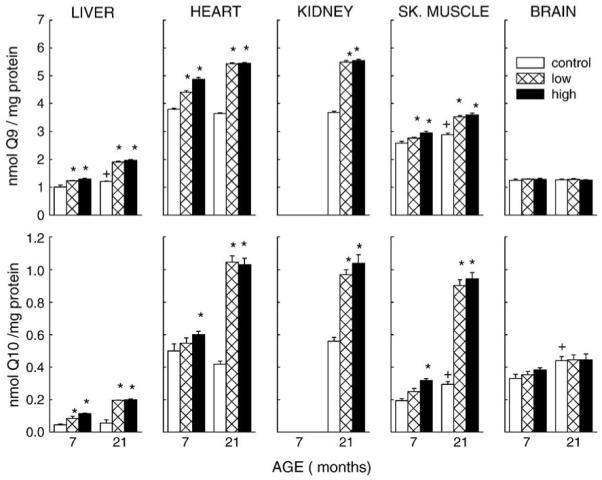
Effect of coenzyme Q10 supplementation on mitochondrial concentrations of CoQ9 and CoQ10. CoQ10 was administered from 3.5 months of age to 7 or 21 months of age. All values represent the mean ± SE of 2 – 6 samples. * P < 0.05 when compared with age-matched control; † P < 0.05 when compared with young control.
Control mice showed no age-related (7 vs 21 months of age) difference in CoQ9 or CoQ10 content in any of the tissue homogenates; however, compared to 7-month-old mice, mitochondrial CoQ9 content in liver and skeletal muscle of 21-month old mice was 20 and 12% higher, respectively, while CoQ10 content was 33 and 52% greater in brain and skeletal muscle, respectively. Mice fed CoQ10 for 3.5 or 17.5 months exhibited augmentation of both CoQ9 and CoQ10 in homogenates of heart, liver, and skeletal muscle, except in the brain, albeit the percentage increases varied in different tissues. Compared to controls, the CoQ9 content of liver homogenate of mice, fed 93 mg (low) and 371 mg CoQ10 /kg/d (high) dosages of CoQ10 was, respectively, 26 and 90% greater after 3.5 months, and 3.7-fold and 3.8-fold higher after 17.5 months of supplementation. CoQ10 content increased 2.6-fold at the low dosage and 4.2-fold at the high dosage after 3.5 months, and by 10-fold following 17.5 months of CoQ10 intake. The dose-related increase in amounts of endogenous CoQ9 and CoQ10 in liver homogenates, observed in the younger but not the older mice, resulted in a significant interaction of Duration of Intake and CoQ Dosage (P < 0.001). In the heart, CoQ9 content increased 30% after 3.5 months and 35% after 17.5 months at both low and high dosages of CoQ10 intake. The amount of CoQ10 increased 17% (low dosage) and 45% (high dosage) after 3.5 months and, at both dosages, 48% after 17.5 months of intake. Statistical analysis indicated a significant Duration of Intake × CoQ Dosage interaction for heart CoQ9 and CoQ10 (P = 0.01). In the kidney, where tissues were available only from 21-month-old mice, there was a 23% increase in CoQ9 and a 2.7- fold rise in CoQ10 content at both of the dosages. In skeletal muscle homogenates, CoQ9 content was unaffected after 3.5 months of CoQ10 administration, but increased 3.5-fold after 17.5 months at low or high dosages, whereas CoQ10 increased at both ages and dosages by more than 2-fold.
CoQ10 intake resulted in the increase of total mitochondrial CoQ content in heart, liver, kidney, and skeletal muscle, but not in the brain. At both low and high dosages, liver mitochondria showed a 25 and 60% increase, respectively, in CoQ9 content after 3.5 and 17.5 months of CoQ10 intake, whereas CoQ10 amounts increased 87% at low dosage and 1.5-fold at the high dosage after 3.5 months. The increase in CoQ10 content after 17.5 months of treatment was 2.5-fold at both dosages. In heart, CoQ9 amount was increased by 11% at low dosage and 28% at high dosage after 3.5 months of CoQ10 intake, and by 50% at both dosages following 17.5 months of intake. CoQ10 content increased 20% at high dosage at the younger age and 1.5 fold at the older age at both low and high dosages of CoQ10 intake. In kidney mitochondria, there was an increase of 50% in CoQ9 and of 80% in CoQ10 content for both dosages. In skeletal muscle, CoQ9 amount increased 7% at low dosage and by 14% at high dosage in younger mice and by 23% in older mice for both dosages, whereas CoQ10 was elevated by 65% in mice fed the high dosage for 3.5 months and by 2-fold in the mice receiving either dosages of CoQ10 for 17.5 months. Except for the brain, a Duration of Intake × CoQ Dosage interaction was found for mitochondria from all tissues studied for both CoQ homologues (P < 0.001).
Effect of CoQ10 intake on body weight, food intake, and life span of mice
To determine the effect of CoQ10 intake on body weight and longevity, 50 mice from each of the three treatment groups were left undisturbed, except for measurement of body weights, until dead or moribund. The effect of age on mean body weights of control, low, and high CoQ intake groups is shown in the top panel of Fig. 3. In all groups, body weights increased about 30 to 35%, reaching a peak at approximately 20 months of age, which was followed by a gradual 15% decline. ANOVA revealed neither a significant main effect of the CoQ Dosage (P = 0.92) nor an interaction of CoQ Dosage with Duration of Intake. Food intake by mice, measured 1, 20, and 30 months after the initiation of supplementation, also showed no effect of CoQ Dosage, Duration of Intake, or their interaction. Thus, CoQ10 intake remained quite stable over the life span and was estimated to be 93 and 371 mg/kg/day for the low and high CoQ intake groups, respectively.
Fig. 3.
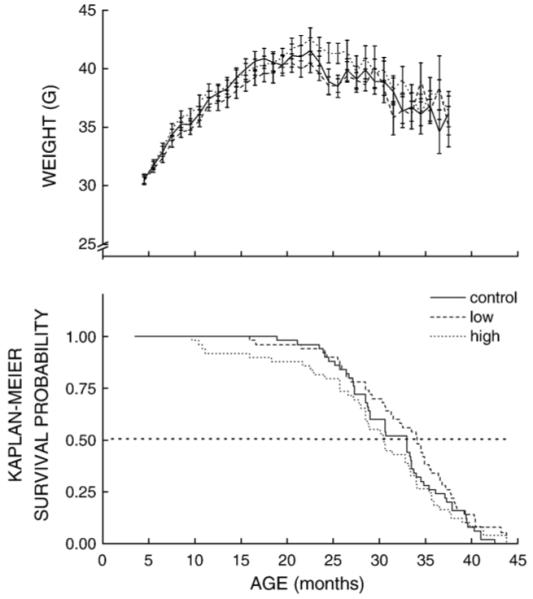
(Top panel) Body weight (g ± SE) of mice as a function of age. Groups of 50 mice were fed a control diet or one of two different diets containing either low (0.72 mg/g of food) or high (2.81 mg/g) amounts of coenzyme Q10 starting at 3.5 months of age. Top panel shows mean body weights of mice surviving at each time point until the point of 90% mortality. (Bottom panel) Kaplan-Meier survival plot of mice receiving control, low, or high amounts of coenzyme Q10.
Analysis of survivorship of control and experimental mice, expressed as Kaplan-Meier probability, revealed that neither of the two dosages of CoQ10 had a significant effect on survival of the mice (P > 0.065, Tarone-Ware) (see Fig. 3, lower panel). Except for a trend toward early death in mice fed the high CoQ10 dose, there was no apparent or statistically significant difference between this group and the controls. Similarly, there was a trend in the direction of an increase in the median life span of the mice supplemented with the low CoQ10 dosage, relative to the control group, but this effect was not statistically significant (P > 0.191).
Activities of catalase, glutathione peroxidase, and superoxide dismutase
The effects of relatively long-term CoQ10 supplementation on the activity of these antioxidative enzymes were determined in liver, kidney, skeletal muscle, and brain of 19-month-old mice, i.e., after 16.5 months of CoQ10 intake. As indicated in Table 1, there was a marked difference in enzyme activities among different tissues, but CoQ10 intake had no apparent effect. Analyses of data for catalase, glutathione peroxidase, and superoxide dismutase activities did not reveal a significant effect or interaction between Tissue and CoQ Dosage (P > 0.403).
Table 1.
Effect of CoQ10 intake on antioxidative enzyme activities in tissue homogenates of 19-month-old mice fed CoQ10 starting at 3.5 months of age
| Tissue | Diet | Catalase (U/mg protein) |
GSH-Px (U/mg protein) |
SOD (U/mg protein) |
|---|---|---|---|---|
| Liver | Control | 330.4 ± 48.0 | 374.9 ± 30.8 | 543.4 ± 54.7 |
| LoQ | 405.0 ± 39.1 | 425.5 ± 61.4 | 467.9 ± 76.8 | |
| HiQ | 478.8 ± 129.1 | 381.7 ± 25.7 | 557.2 ± 35.4 | |
| Kidney | Control | 710.2 ± 46.0 | 359.8 ± 20.1 | 432.9 ± 20.1 |
| LoQ | 781.7 ± 62.2 | 322.6 ± 37.4 | 418.2 ± 7.7 | |
| HiQ | 752.1 ± 69.9 | 384.9 ± 37.0 | 409.6 ± 14.0 | |
| Skeletal muscle |
Control | 5.1 ± 0.6 | 3.0 ± 0.3 | 133.4 ± 4.9 |
| LoQ | 7.0 ± 1.3 | 3.6 ± 0.3 | 145.4 ± 6.8 | |
| HiQ | 10.0 ± 1.1 | 3.0 ± 0.2 | 107.8 ± 5.3 | |
| Brain | Control | 13.1 ± 1.1 | 11.7 ± 0.5 | 195.7 ± 12.2 |
| LoQ | 14.5 ± 1.1 | 12.4 ± 1.7 | 183.4 ± 21.0 | |
| HiQ | 13.3 ± 1.2 | 10.3 ± 0.4 | 164.7 ± 19.3 |
Data are presented as means ± SE (N = 3–5).
Activities of mitochondrial oxidoreductases, rates of oxygen consumption, and superoxide radical generation
The effects of CoQ10 intake on activities of NADH-ferricytochrome c reductase (complex I/III) and ferrocytochrome c oxidase (complex IV) were determined in mitochondria from liver, heart, kidney, skeletal muscle, and brain of 25-month-old mice that had been fed 93 or 371 mg/CoQ10/kg/day since the age of 3.5 months (Table 2). There was no effect on the activity of these complexes in any of the tissues at either of the two dosages of CoQ10 (P > 0.19).
Table 2.
Effect of CoQ10 intake on mitochondrial superoxide production, carbonyl content, and activities of oxidoreductases of 25-month-old mice
| Tissue | Diet | Superoxide (nmol/min/mg protein) |
Carbonyl (nmol/mg protein) |
Complex I/III (nmol/min/mg protein) |
Complex IV (μmol/min/mg protein) |
|---|---|---|---|---|---|
| Liver | Control | N.D. | 3.03 ± 0.10 | 819.8 ± 81.2 | 2050.4 ± 67.9 |
| LoQ | N.D. | 3.09 ± 0.15 | 822.5 ± 85.7 | 2232.5 ± 41.1 | |
| HiQ | N.D. | 3.40 ± 0.26 | 748.8 ± 125.6 | 2280.9 ± 87.5 | |
| Heart | Control | 10.89 ± 0.51 | N.D. | 665.2 ± 4.1 | 10,560.1 ± 1073.3 |
| LoQ | 11.93 ± 0.48 | N.D. | 726.4 ± 27.0 | 14,234.0 ± 1056.5 | |
| HiQ | 11.28 ± 0.39 | N.D. | 939.8 ± 123.7 | 13,112.4 ± 1403.6 | |
| Kidney | Control | 3.70 ± 0.14 | 3.12 ± 0.15 | 1000.7 ± 76.4 | 3086.9 ± 309.1 |
| LoQ | 3.48 ± 0.15 | 2.75 ± 0.11 | 1032.8 ± 80.7 | 3723.3 ± 181.9 | |
| HiQ | 3.34 ± 0.17 | 2.52 ± 0.12 | 1072.9 ± 53.9 | 3587.1 ± 86.1 | |
| Skeletal muscle | Control | 5.02 ± 0.43 | N.D. | 1257.1 ± 53.2 | 3104.8 ± 398.9 |
| LoQ | 5.50 ± 0.23 | N.D. | 1026.6 ± 175.6 | 2744.7 ± 321.7 | |
| HiQ | 5.64 ± 0.38 | N.D. | 1270.1 ± 126.8 | 3167.0 ± 38.7 | |
| Brain | Control | N.D. | 2.59 ± 0.28 | 1735.9 ± 138.1 | 4628.2 ± 64.7 |
| LoQ | N.D. | 2.39 ± 0.22 | 1552.1 ± 249.0 | 4309.4 ± 128.0 | |
| HiQ | N.D. | 2.18 ± 0.18 | 1688.5 ± 135.1 | 4511.3 ± 161.7 |
Mice were fed CoQ10 starting at 3.5 months of age. Data are presented as means ± SE (N = 3–6).
N.D., not determined.
The effect of CoQ10 intake on rate of oxygen consumption was examined in liver mitochondria because CoQ augmentation in the experimental groups was the highest in this tissue. The rates of glutamate/malate- and succinate-supported state 3 (ADP stimulated) respiration were similar in controls and experimental mice (data not shown).
The rate of O2−· generation was determined as SOD-inhibitable reduction of acetylated ferricytochrome c in submitochondrial particles from heart, kidney, and skeletal muscle of 25-month-old mice fed the low or the high dosages of CoQ10 since the age of 3.5 months (Table 2). The rates of O2−· by SMPs varied about 3-fold in these tissues with the rank order: heart > skeletal muscle > kidney. However, there were no significant differences between the control and the experimental mice (P > 0.756).
Protein carbonyl content was measured in the mitochondrial fraction of liver, kidney, heart, and brain of experimental and control mice at 25 months of age (Table 2); however, there were no significant differences between the groups. GSH: GSSG ratios are widely believed to be sensitive indicators of redox state. A comparison among control and experimental groups (Table 3), conducted at 25 months of age, also showed no notable effect of CoQ10 intake on GSH:GSSG ratios in tissue homogenates (P > 0.06).
Table 3.
Effect of CoQ10 intake on amounts of glutathione (GSH), glutathione disulfide (GSSG), and the GSH:GSSG ratio in tissue homogenates of 19-month-old mice, fed CoQ10 starting at 3.5 months of age
| Tissue | Diet | GSH (nmol/mg protein) |
GSSG (nmol/mg protein) |
GSH:GSSG ratio |
|---|---|---|---|---|
| Liver | Control | 26.70 ± 0.33 | 0.491 ± 0.008 | 54 ± 0.4 |
| LoQ | 27.55 ± 0.39 | 0.491 ± 0.027 | 57 ± 3.1 | |
| HiQ | 27.69 ± 0.39 | 0.483 ± 0.008 | 57 ± 1.0 | |
| Heart | Control | 15.60 ± 0.24 | 0.478 ± 0.033 | 33 ± 2.0 |
| LoQ | 15.26 ± 0.31 | 0.448 ± 0.009 | 34 ± 0.9 | |
| HiQ | 14.74 ± 0.24 | 0.476 ± 0.034 | 31 ± 1.9 | |
| Kidney | Control | 2.64 ± 0.05 | 0.084 ± 0.001 | 32 ± 0.5 |
| LoQ | 2.73 ± 0.05 | 0.088 ± 0.007 | 32 ± 2.8 | |
| HiQ | 2.61 ± 0.03 | 0.104 ± 0.003 | 25 ± 0.8 | |
| Brain | Control | 15.14 ± 0.22 | 0.071 ± 0.003 | 213 ± 7.2 |
| LoQ | 14.76 ± 0.20 | 0.067 ± 0.003 | 222 ± 10.6 | |
| HiQ | 14.80 ± 0.29 | 0.074 ± 0.002 | 202 ± 4.8 |
Data are presented as means ± SE (N = 4).
Discussion
Results of this study indicate that, with the exception of brain, endogenous amounts of CoQ10, as well as CoQ9, were elevated in tissue homogenates and mitochondria in response to dietary supplementation with CoQ10. However, CoQ9 content was augmented by a relatively greater amount than the CoQ10 amount, suggesting that the latter was endogenously processed to generate the former. In general, the highest endogenous augmentations of CoQ content (CoQ9 + CoQ10) were achieved with the duration of intake rather than the dosages alone, thereby indicating that the administered dosages of CoQ10 were not a limiting factor in obtaining the maximal endogenous accrual of CoQ. Nevertheless, different tissues varied in their capacity for CoQ accretion, with liver and skeletal muscle exhibiting the highest elevation and the brain showing none. Although the specific reasons for such intertissue variations are unclear, an analysis of various relevant studies suggests that factors such as duration of intake, dosage, and formulation [32,33] are among the main factors. For instance, in contrast to the present study, in which CoQ10 powder was added to the food, in previous studies where a water-miscible CoQ10 formulation (Q-Gel) was administered, significant augmentation of CoQ9 and CoQ10 was achieved in mouse and rat brain, following 11–13 weeks of supplementation [32,33]. As also discussed previously [32,33], the historical view that CoQ10 intake does not elevate endogenous CoQ levels except in plasma and liver [10-12] seems to be based on studies where relatively low dosages of CoQ10 were administered for short durations. The present study firmly contradicts this long-accepted notion by demonstrating that membrane CoQ content can indeed be altered in vivo by dietary administration of CoQ10, thereby providing the rationale for future studies exploring the potential in vivo functional role of CoQ in different cell organelles.
Results indicated that CoQ10 intake had no discernible impact on a number of mitochondrial functions. For instance, a comparison of the activities of mitochondrial ETC oxidoreductases, NADH-cytochrome c oxidoreductase (Complexes I–III) and cytochrome c oxidase (Complex IV), between control and experimental groups in liver, heart, kidney, skeletal muscle, and brain, showed no effect of CoQ10 intake at either the low or the high dosage. Furthermore, rates of glutamate- and succinate-supported ADP-stimulated (state 3) oxygen consumption by liver mitochondria were unaffected by CoQS10 intake. Similarly, there was no alteration in the rate of O2−· generation by SMPs from liver, kidney, and skeletal muscle, even though mitochondrial CoQ content was elevated in these tissues in the experimental animals. It would thus seem that O2−· generation is not simply a linear function of mitochondrial CoQ content. This inference is corroborated by the finding that neither protein carbonyl content of mitochondria nor the GSH:GSSG ratios in tissue homogenates were affected by CoQ10 supplementation. Thus, the speculation that CoQ10 intake may boost O2−· production and exacerbate oxidative stress is not borne out by the present data. Similarly, CoQ10 intake was found not to have any impact on isoprostane content, produced by lipid peroxidation in the plasma in human [34,35].
This inference is further buttressed by comparisons among different mammalian species, which indicated that rates of mitochondrial O2−· generation were correlated with the CoQ pool bound to the inner mitochondrial membrane proteins rather than the total CoQ content of the mitochondrial membranes [36]. Previous experimental studies involving in vitro mitochondrial CoQ depletion/repletion indicated that rates of NADH- and succinate-supported oxygen consumption (state 3) and of O2−· generation by SMPs decreased in correspondence to depletion of CoQ, whereas reconstitution of CoQ-depleted SMPs with increasing amounts of CoQ9 or CoQ10 caused an initial steep increase in the rate of oxygen consumption and O2−· generation, which was followed by a plateau [37]. The main finding of this study was that within the physiological range, variations in mitochondrial CoQ content had no significant effect on the rate of oxygen consumption or O2−· generation. Thus, results of the present ex vivo studies tend to confirm the findings of this previous in vitro investigation.
The general lack of an effect of prolonged CoQ10 intake (16.5 to 21.5 months) on antioxidative enzyme activity and mitochondrial oxidant generation, observed in the current study, differs from that reported previously, where CoQ10 was administered to 14-month-old rats for 13 weeks [33]. A decrease in skeletal muscle carbonyl content as well as a reductive shift in glutathione redox state was observed in this investigation. This discrepancy is hypothesized to be due to differences among species, age of the animals, or duration and dosage of CoQ10 supplementation. It is also possible that the ability of CoQ10 supplementation to modify oxidative stress is a transient phenomenon.
Since administration of low molecular weight antioxidants can potentially affect the activities of antioxidant enzymes, it was considered relevant to examine the possibility that CoQ10 elevation in the tissues may suppress the activities of antioxidative enzymes such as SOD, catalase, or glutathione peroxidase. The absence of any significant difference in activity of any of these enzymes in any of the tissues examined between the control and the experimental mice suggests that exogenous CoQ10 does not interfere with the main antioxidant defenses against O2−·/H2O2. This finding together with a similar absence of an effect on GSH:GSSG ratios is consistent with the view that endogenous CoQ augmentation does not significantly interfere with redox homeostasis.
Results of previous studies on the effects of CoQ10 intake on life span seem to be quite contradictory and species specific. For instance, life span of the worm, C. elegans, has been reported to be prolonged by CoQ supplementation [38], dietary deficiency [16], or the inability to synthesize the normal CoQ homologue [15,17]. On the other hand, no effect has been noted in Drosophila [39] or in mice and rats [19,20]. One explanation may be that in C. elegans, a deficiency of CoQ induces a hypometabolic or dauer-like state, thereby prolonging the life span of the worm. Such a response may also explain why even antimycin A treatment, which increases mitochondrial ROS generation, prolongs rather than shortens the nematode life span [18]. The relevance of this phenomenon observed in the nematode to the mammals, where antimycin A is highly toxic, is currently unclear.
Two previous studies have been conducted on the effect of CoQ10 administration on the life span of mice and/or rat. Both involved dosages that were lower than the highest dose used in this study. In one investigation, the diet of mice and rats was supplemented with 10 mg CoQ10/kg body weight/day, starting at 2 months of age, but no effect on life span was observed [19]. In the other study, mice were fed diets supplemented with 100 mg CoQ10/kg of food (35% of the high dosage administered in the present study) starting at 14 months of age, which also had no effect on life span [20]. Results of the current study are in general accord with these findings, which leads to the overall inference that CoQ10 supplementation has indeed no demonstrable effect on longevity of rodents over a wide range of dosages.
Studies on the pattern of gene expression in mice in response to CoQ10 supplementation have shown elevated expression of some genes, including those encoding the subunits of cytochrome c oxidase, DNA repair enzymes, and glutathione S- transferase, among others [20]. CoQ10 intake also suppressed the expression of an array of genes, such as those involved in protein synthesis and turnover, stress responses, intracellular vascular trafficking, and ketone body metabolism. Although changes in mRNA levels do not, a priori, imply corresponding changes in protein levels and function, they suggest that the effects of CoQ10 on cells are highly diverse and complex.
To conclude, results of this study suggest that dietary supplementation with CoQ10 does indeed augment the endogenous CoQ content in tissue homogenates and mitochondria; however, there is no indication of a discernable effect reflecting enhanced antioxidative capacity, prooxidant potentiation, or altered life span.
Acknowledgment
This research was supported by the Grant R01 AG17526 from National Institute on Aging-National Institutes of Health.
Abbreviations
- ETC
electron transport chain
- GSH
glutathione
- GSSG
glutathione disulfide
- SMPs
submitochondrial particles
References
- [1].Battino M, Ferri E, Gorini A, Federico Villa R, Rodriguez Huertas JF, Fiorella P, Genova ML, Lenaz G, Marchetti M. Natural distribution and occurrence of coenzyme Q homologues. Membr. Biochem. 1990;9:179–190. doi: 10.3109/09687689009025839. [DOI] [PubMed] [Google Scholar]
- [2].Ernster L, Dallner G. Biochemical, physiological and medical aspects of ubiquinone function. Biochim. Biophys. Acta. 1995;1271:195–204. doi: 10.1016/0925-4439(95)00028-3. [DOI] [PubMed] [Google Scholar]
- [3].James AM, Smith RAJ, Murphy MP. Antioxidant and prooxidant properties of mitochondrial coenzyme CoQ. Arch. Biochem. Biophys. 2004;423:47–56. doi: 10.1016/j.abb.2003.12.025. [DOI] [PubMed] [Google Scholar]
- [4].Kagan V, Serbinova E, Packer L. Antioxidant effects of ubiquinones in microsomes and mitochondria are mediated by tocopherol recycling. Biochem. Biophys. Res. Commun. 1990;169:851–857. doi: 10.1016/0006-291x(90)91971-t. [DOI] [PubMed] [Google Scholar]
- [5].Forsmark-Andree P, Dallner G, Ernster L. Endogenous ubiquinol prevents protein modification accompanying lipid peroxidation in beef heart submitochondrial particles. Free Radic. Biol. Med. 1995;19:749–757. doi: 10.1016/0891-5849(95)00076-a. [DOI] [PubMed] [Google Scholar]
- [6].Lass A, Sohal RS. Electron transport-linked ubiquinone-dependent recycling of alpha-tocopherol inhibits autooxidation of mitochondrial membranes. Arch. Biochem. Biophys. 1998;352:229–236. doi: 10.1006/abbi.1997.0606. [DOI] [PubMed] [Google Scholar]
- [7].Boveris A, Cadenas E, Stoppani AO. Role of ubiquinone in the mitochondrial generation of hydrogen peroxide. Biochem. J. 1976;156:435–444. doi: 10.1042/bj1560435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Turrens JF, Alexandre A, Lehninger AL. Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch. Biochem. Biophys. 1985;237:408–414. doi: 10.1016/0003-9861(85)90293-0. [DOI] [PubMed] [Google Scholar]
- [9].Sohal RS, Mockett RJ, Orr WC. Mechanisms of aging: an appraisal of the oxidative stress hypothesis. Free. Radic. Biol. Med. 2002;33:575–586. doi: 10.1016/s0891-5849(02)00886-9. [DOI] [PubMed] [Google Scholar]
- [10].Zhang Y, Aberg F, Appelkvist EL, Dallner G, Ernster L. Uptake of dietary coenzyme Q supplement is limited in rats. J. Nutr. 1995;125:446–453. doi: 10.1093/jn/125.3.446. [DOI] [PubMed] [Google Scholar]
- [11].Zhang Y, Turunen M, Appelkvist EL. Restricted uptake of dietary coenzyme Q is in contrast to the unrestricted uptake of α-tocopherol into rat organs and cells. J. Nutr. 1996;126:2089–2097. doi: 10.1093/jn/126.9.2089. [DOI] [PubMed] [Google Scholar]
- [12].Bentinger M, Dallner G, Choinacki T, Sweiezewsk E. Distribution and breakdown of labelled coenzyme Q10 in rat. Free Radic. Biol. Med. 2003;34:563–575. doi: 10.1016/s0891-5849(02)01357-6. [DOI] [PubMed] [Google Scholar]
- [13].Rowland MA, Nagley P, Linnane AW, Rosenfeldt FL. Coenzyme Q10 treatment improves the tolerance of the senescent myocardium to pacing stress in the rat. Cardiovasc. Res. 1998;40:165–173. doi: 10.1016/s0008-6363(98)00132-1. [DOI] [PubMed] [Google Scholar]
- [14].Matthews RT, Yang L, Browne S, Baik M, Beal MF. Coenzyme Q10 administration increases brain mitochondrial concentrations and exerts neuroprotective effects. Proc. Natl. Acad. Sci. USA. 1998;95:8892–8897. doi: 10.1073/pnas.95.15.8892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Asencio C, Rodriguez-Aguilera JC, Ruiz-Ferrer M, Vela J, Navas P. Silencing of ubiquinone biosynthesis genes extends life span in Caenorhabditis elegans. FASEB J. 2003;17:1135–1137. doi: 10.1096/fj.02-1022fje. [DOI] [PubMed] [Google Scholar]
- [16].Larsen P, Clarke C. Extension of life-span in Caenorhabditis elegans by a diet lacking Coenzyme Q. Science. 2002;295:120–123. doi: 10.1126/science.1064653. [DOI] [PubMed] [Google Scholar]
- [17].Branicky R, Benard C, Hekimi S. clk-1, mitochondria and physiological rates. Bio essays. 2000;22:48–56. doi: 10.1002/(SICI)1521-1878(200001)22:1<48::AID-BIES9>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- [18].Dillin A, Hsu AL, Arantes-Oliveira N, Lehrer-Graiwer J, Hsin H, Fraser AG, Kamath RS, Ahringer J, Kenyon C. Rates of behavior and aging specified by mitochondrial function during development. Science. 2002;298:2398–2401. doi: 10.1126/science.1077780. [DOI] [PubMed] [Google Scholar]
- [19].Lonnrot K, Holm P, Lagerstedt A, Huhtala H, Alho H. The effects of lifelong ubiquinone Q10 supplementation on the Q9 and Q10 tissue concentrations and life span of male rats and mice. Biochem. Mol. Biol. 1998;44:727–737. doi: 10.1080/15216549800201772. [DOI] [PubMed] [Google Scholar]
- [20].Lee CK, Pugh TD, Klopp RG, Edwards J, Allison DB, Weindruch R, Prolla TA. The impact of alpha-lipoic acid, coenzyme Q10 and caloric restriction on life span and gene expression patterns in mice. Free Radic. Biol. Med. 2004;36:1043–1057. doi: 10.1016/j.freeradbiomed.2004.01.015. [DOI] [PubMed] [Google Scholar]
- [21].Rebrin I, Kamzalov S, Sohal RS. Effect of age and caloric restriction on glutathione redox couple in mice. Free Radic. Biol. Med. 2003;35:626–635. doi: 10.1016/s0891-5849(03)00388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Rebrin I, Sohal RS. Comparison of thiol redox state of mitochondria and homogenates of various tissues between two strains of mice with different longevities. Exp. Gerontol. 2004;39:1513–1519. doi: 10.1016/j.exger.2004.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Yarian CS, Rebrin I, Sohal RS. Aconitase and ATP synthase are targets of malondialdehyde modification and undergo age-related decrease in activity in mouse heart mitochondria. Biochem. Biophy. Res. Commun. 2005;330:150–156. doi: 10.1016/j.bbrc.2005.02.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Takada M, Ikenoya S, Yuzuriha T, Katayama K. Simultaneous determination of reduced and oxidized ubiquinones. Methods Enzymol. 1984;105:147–155. doi: 10.1016/s0076-6879(84)05020-5. [DOI] [PubMed] [Google Scholar]
- [25].Katayama K, Takada M, Yuzuriha T, Abe K, Ikenoya S. Simultaneous determination of ubiquinone-10 and ubiquinol-10 in tissues and mitochondria by high-performance liquid chromatography. Biochem. Biophys. Res. Commun. 1980;95:971–977. doi: 10.1016/0006-291x(80)91568-5. [DOI] [PubMed] [Google Scholar]
- [26].Kwong LK, Sohal RS. Age-related changes in activities of mitochondrial electron transport complexes in various tissues of the mouse. Arch. Biochem. Biophys. 2000;373:16–22. doi: 10.1006/abbi.1999.1495. [DOI] [PubMed] [Google Scholar]
- [27].Ku H-H, Brunk UT, Sohal RS. Relationship between mitochondrial superoxide and hydrogen peroxide production and longevity of mammalian species. Free Radic. Biol. Med. 1993;15:621–627. doi: 10.1016/0891-5849(93)90165-q. [DOI] [PubMed] [Google Scholar]
- [28].Spitz DR, Oberley LW. An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal. Biochem. 1989;179:8–18. doi: 10.1016/0003-2697(89)90192-9. [DOI] [PubMed] [Google Scholar]
- [29].Lück H. Catalase. In: Bergmeyer H, editor. Methods of enzymatic analysis. Academic Press; New York: 1965. pp. 885–894. [Google Scholar]
- [30].Beutler E. Glutathione peroxidase. In: Beulter E, editor. Red cell metabolism, a manual of biochemical methods. Grune & Stratton; New York: 1971. pp. 66–68. [Google Scholar]
- [31].Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman E. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- [32].Kamzalov S, Sumien N, Forster MJ, Sohal RS. Coenzyme Q intake elevates the mitochondrial and tissue levels of coenzyme Q and a-tocopherol in young mice. J. Nutr. 2003;133:3175–3180. doi: 10.1093/jn/133.10.3175. [DOI] [PubMed] [Google Scholar]
- [33].Kwong L, Kamzalov S, Rebrin I, Bayne ACV, Jana CK, Morris P, Forster MJ, Sohal RS. Effects of coenzyme Q10 administration on its tissue concentrations, mitochondrial oxidant generation, and oxidative stress in the rat. Free Radic. Biol. Med. 2002;33:627–638. doi: 10.1016/s0891-5849(02)00916-4. [DOI] [PubMed] [Google Scholar]
- [34].Hodgson JM, Watts GF, Playford DA, Burke V, Kroft KD. Coenzyme Q10 improves blood pressure and glycaemic control: a controlled trial in subjects with type 2 diabetes. J. Clin. Nutr. 2002;56:1137–1142. doi: 10.1038/sj.ejcn.1601464. [DOI] [PubMed] [Google Scholar]
- [35].Playford DA, Watts GF, Kroft KD, Burke V. Combined effect of coenzyme Q10 and fenofibrare on forearm microcirculatory function in type 2 diabetes. Atherosclerosis. 2003;168:169–179. doi: 10.1016/s0021-9150(02)00417-3. [DOI] [PubMed] [Google Scholar]
- [36].Lass A, Sohal RS. Comparisons of coenzyme Q bound to mitochondrial membrane proteins among different mammalian species. Free Radic. Biol. Med. 1999;27:220–226. doi: 10.1016/s0891-5849(99)00085-4. [DOI] [PubMed] [Google Scholar]
- [37].Lass A, Agarwal S, Sohal RS. Mitochondrial ubiquinone homologues, superoxide radical generation and longevity in different mammalian species. J. Biol. Chem. 1997;272:19199–19204. doi: 10.1074/jbc.272.31.19199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ishii N, Senoo-Matsuda N, Miyake K, Yasuda K, Ishii T, Hartman PS, Furukawa S. Coenzyme Q10 can prolong C. elegans lifespan by lowering oxidative stress. Mech. Ageing Dev. 2004;125:41–46. doi: 10.1016/j.mad.2003.10.002. [DOI] [PubMed] [Google Scholar]
- [39].Palmer MR, Sackton TB. The effects of dietary coenzyme Q on Drosophila life span. Aging Cell. 2003;2:335–339. doi: 10.1046/j.1474-9728.2003.00065.x. [DOI] [PubMed] [Google Scholar]


